Post processing method for preparing levo-ofloxacin
A levofloxacin and crude product technology, which is applied in the post-treatment field of levofloxacin preparation, can solve the problems related to the increase of impurity content, achieve the effects of increasing yield, avoiding environmental problems, and reducing the unit consumption of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
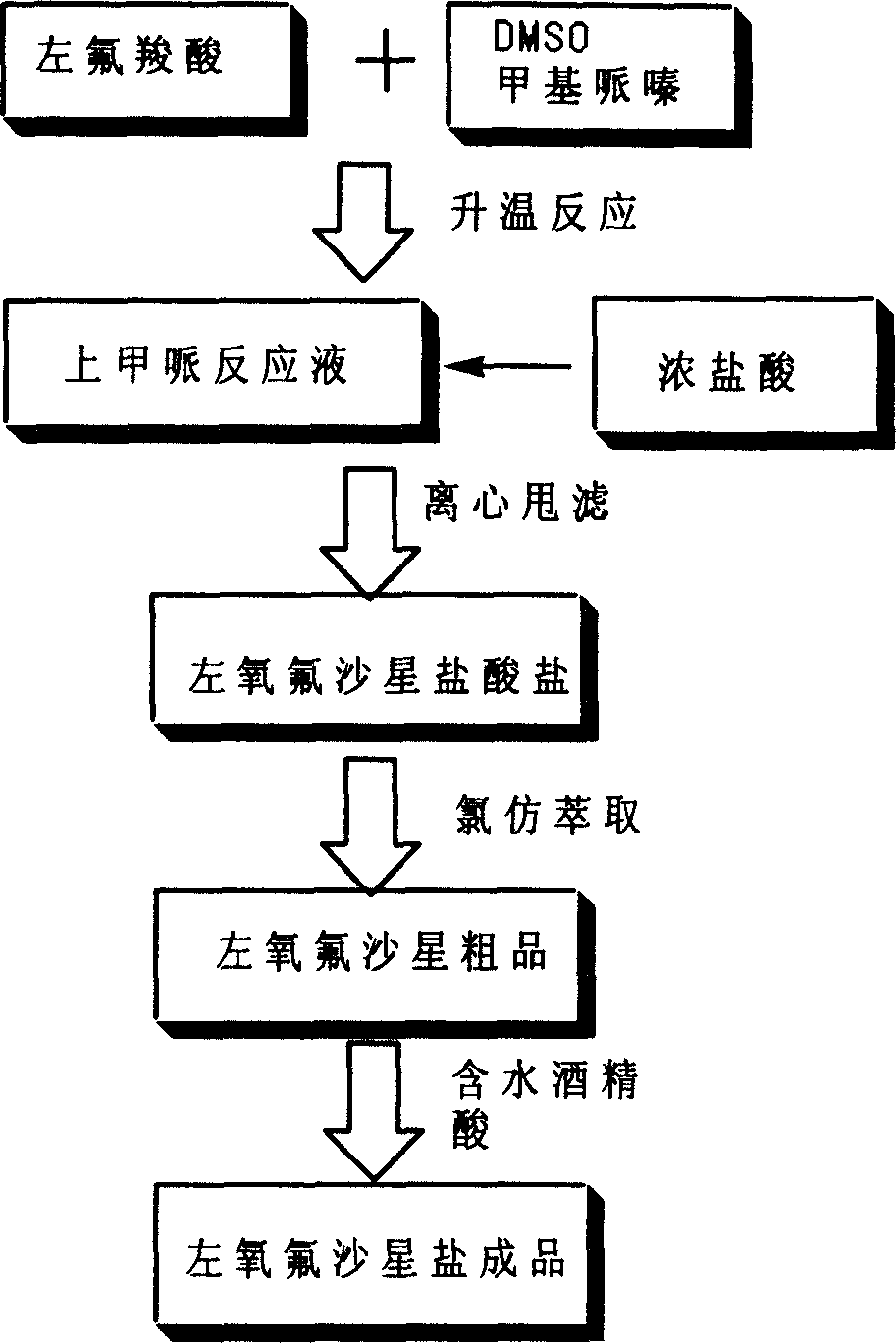
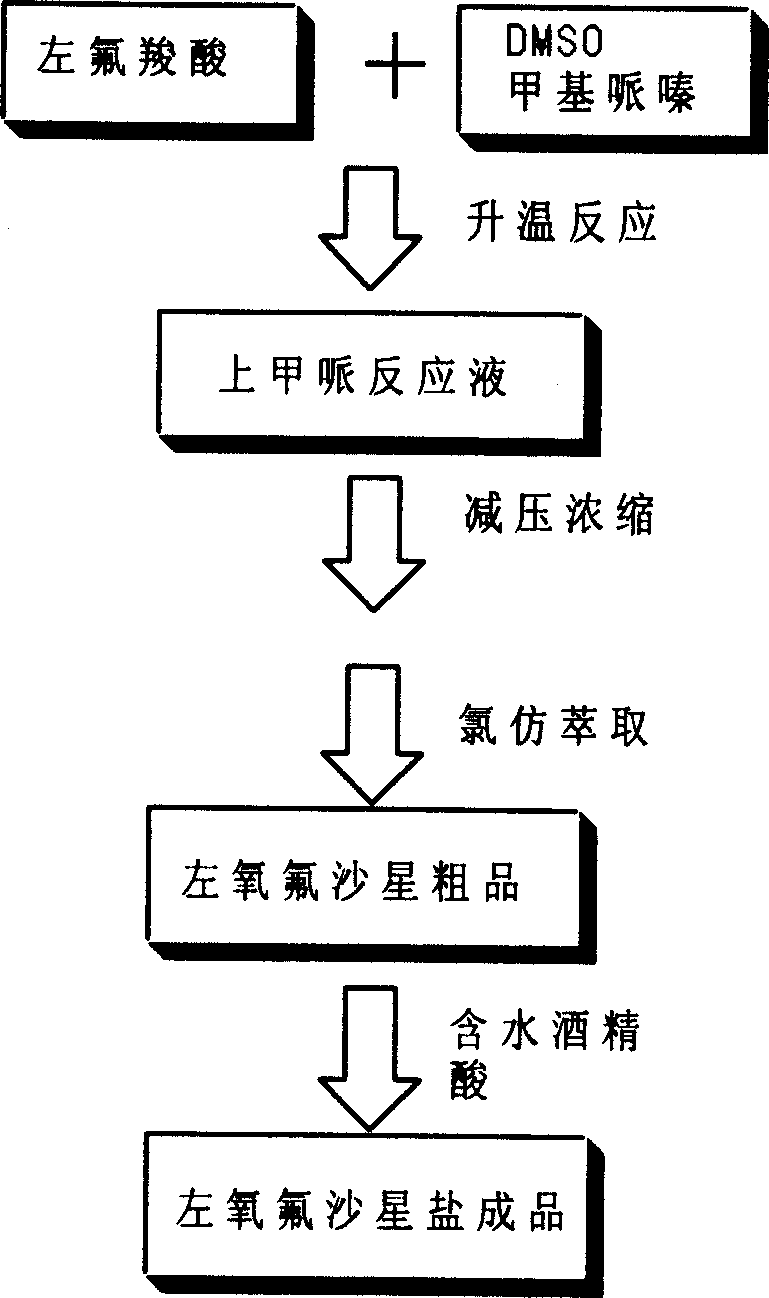
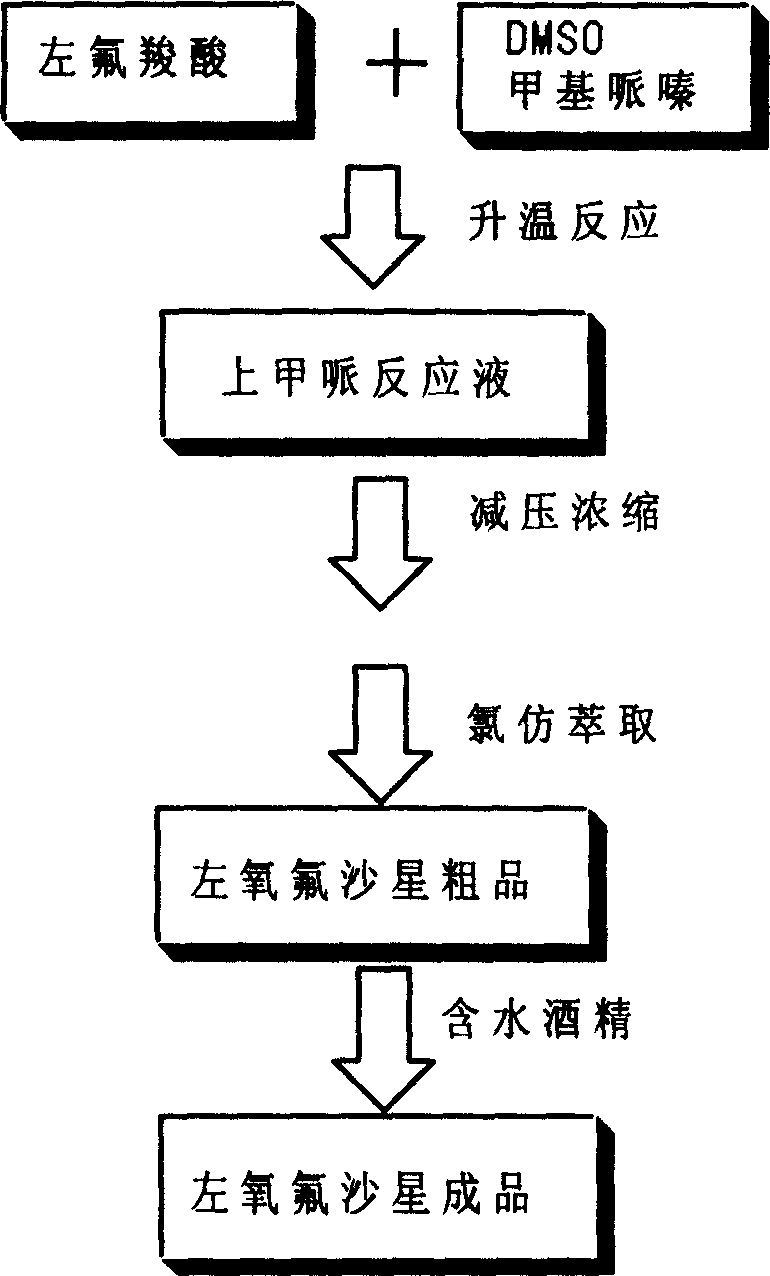
[0026] Put 100 grams of levofluorocarboxylic acid into the reaction flask, then add 400 milliliters of dimethyl sulfoxide and 100 milliliters of N-methylpiperazine, heat and control the inner temperature of 85 ° C for 5 hours; after TLC analysis is qualified, recover N -Methylpiperazine and dimethyl sulfoxide, keep the internal temperature below 100°C. Add 400 milliliters of water to the obtained product to adjust the pH=6.0 to 6.5, add 1.5 grams of activated carbon after dissolving and keep stirring for 30 minutes to carry out acid-dissolving and refining. Then filter, heat to 65-75°C, adjust the pH to 9.5-10.0 with sodium hydroxide solution, add 1.5 grams of activated carbon and keep stirring for 30 minutes to carry out alkali-dissolving refinement, and then filter. Add an appropriate amount of hydrochloric acid to adjust the pH to 7.0-8.0 while stirring, add 200 ml of chloroform for extraction, repeat the chloroform extraction for 6-10 times, combine the extracts, recover t...
Embodiment 2
[0030] 100 grams of levofloxacin was fed, and the levofloxacin crude product was prepared as described in Example 1, with a wet weight of 163 grams.
[0031] Add 600 ml of absolute ethanol to the levofloxacin wet product, heat up in a water bath, and after the dissolution is complete, add a small amount of EDTA and activated carbon, keep warm at 60-75°C for 30 minutes, filter, add about 30 ml of 15% sulfuric acid to the filtrate, cool and crystallize , filtered to obtain levofloxacin sulfate, with a wet weight of 185 grams.
[0032] Add 550 ml of absolute ethanol and 25 ml of 5% ammonia water to the levofloxacin hydrochloride sulfate wet product, heat up in a water bath, and after the dissolution is complete, add a small amount of EDTA and activated carbon, keep warm at 60-75°C for 30 minutes, filter, and the filtrate is cooled and crystallized , filter the obtained crystals and dry under reduced pressure with a water pump for 2.0 to 3.0 hours at a temperature of 50 to 55° C. ...
Embodiment 3
[0034] 100 grams of levofloxacin was fed, and the levofloxacin crude product was prepared as described in Example 1, with a wet weight of 167 grams.
[0035] Add 600 ml of absolute ethanol to the wet product of levofloxacin, heat up in a water bath, and after the dissolution is complete, add a small amount of EDTA and activated carbon, keep warm at 60-75°C for 30 minutes, filter, add about 30 ml of concentrated hydrochloric acid to the filtrate, cool and crystallize, Filter to obtain levofloxacin hydrochloride, wet weight 170 grams.
[0036] Add 550 ml of absolute ethanol to the wet product of levofloxacin hydrochloride, adjust the pH to 7.5-8.0 with saturated sodium bicarbonate solution, heat up in a water bath, and after the dissolution is complete, add a small amount of EDTA and activated carbon, and keep warm at 60-75°C for 30 minutes. Filtrate, cool the filtrate to crystallize, and dry the obtained crystals with a water pump under reduced pressure for 2.0 to 3.0 hours at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com