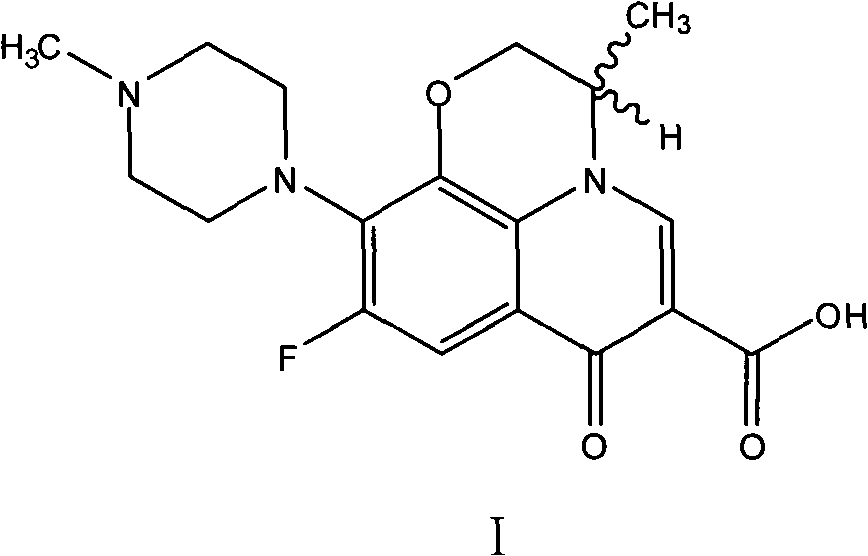
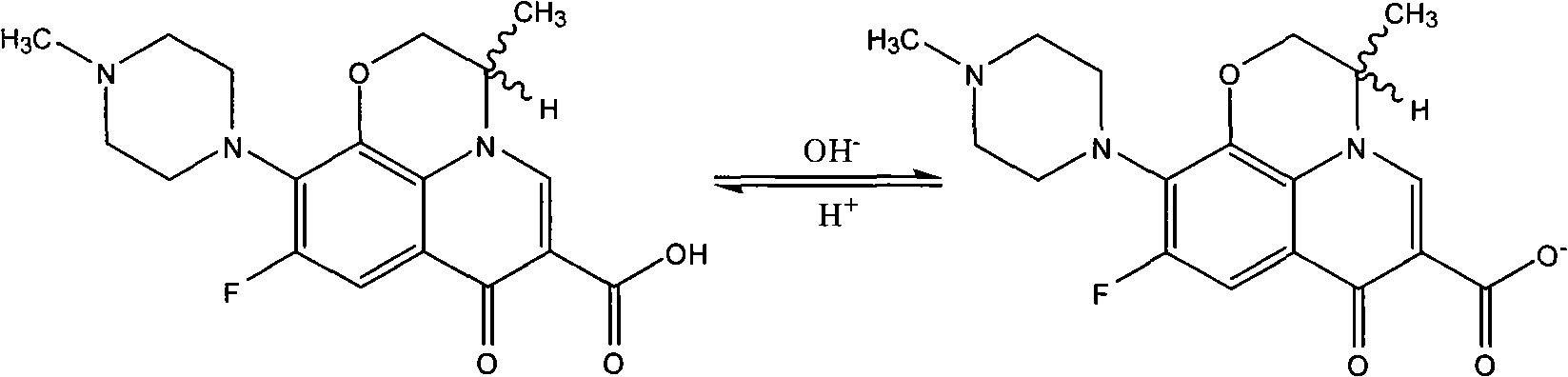
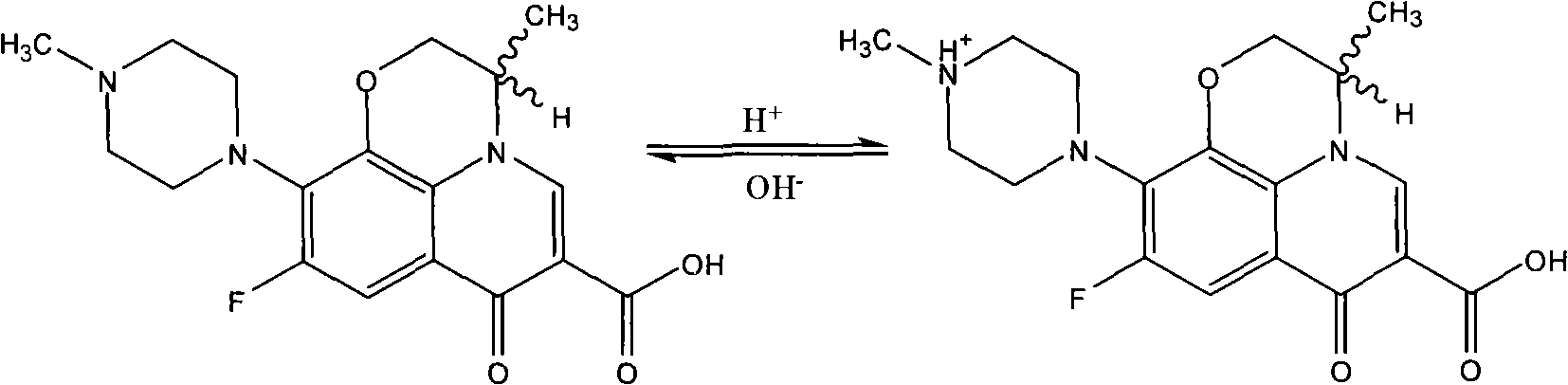
Green synthesizing process for ofloxacin
A technology of ofloxacin, green synthesis, applied in organic chemistry and other directions, can solve the problems of heavy odor, raw material unit consumption and energy consumption increase, and achieve the effects of good comprehensive effect, good quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Weigh 30g of oxyfluorocarboxylic acid (0.1067 moles), 70g of N-methylpiperazine (0.6989 moles), 0.2g of N,N-carbonyldiimidazole (0.0012 moles) and 55g of water (3.0556 moles), and put them into 250ml of reaction Stir in the bottle, heat up to 85°C, and keep the reaction at 85±2°C for 9 hours, and monitor the completion of the reaction by HPLC. After shrinking the piperidine, recover N-methylpiperazine aqueous solution under reduced pressure under the conditions of 90~110°C and -0.09~-0.095MPa until there is basically no flow, then add 180ml tetrahydrofuran to the reaction bottle, heat and reflux for 0.5 hours, and cool down to At about 25°C, keep warm at 25±1°C for 1 hour, filter with suction, rinse the filter cake with tetrahydrofuran until the filtrate is colorless, and then drain it. Put the obtained light yellow solid in a 500ml flask, add 160g of purified water, stir and beat for 30 minutes, then add 30% liquid caustic soda dropwise to the bottle, stir until clear a...
Embodiment 2
[0031] Weigh 30g of oxyfluorocarboxylic acid (0.1067 moles), 60g of N-methylpiperazine (0.5990 moles), 0.15g of cerium trichloride heptahydrate (0.0004 moles) and 40g of water (2.2222 moles), and put into 250ml reaction Stir in the bottle, heat up to 83°C, and keep the reaction at 83±2°C for 7 hours, and monitor the completion of the reaction by HPLC. After shrinking the piperidine, recover the N-methylpiperazine aqueous solution under reduced pressure under the conditions of 90~110°C and -0.09~-0.095MPa until there is basically no flow, then add 220ml of methanol to the reaction bottle, heat and reflux for 0.5 hours, and cool down to At about 25°C, keep warm at 25±1°C for 1 hour, filter with suction, rinse the filter cake with methanol until the filtrate is colorless, and then drain it. Put the obtained light yellow solid in a 500ml flask, add 210g of purified water, stir and beat for 30 minutes, then add 30% liquid caustic soda dropwise to the bottle, stir at 35±1°C until cl...
Embodiment 3
[0033] Weigh respectively 30g of oxyfluorocarboxylic acid (0.1067 moles), 45g of N-methylpiperazine (0.4493 moles), 0.1g of cerium trichloride (0.0004 moles) and 35g of water (1.9444 moles), and drop them into a 250ml reaction flask successively, Stir, heat up to 90°C, and keep the reaction at 90±2°C for 6.5 hours, and monitor the completion of the reaction by HPLC. After shrinking the piperidine, recover the N-methylpiperazine aqueous solution under reduced pressure under the conditions of 90~110°C and -0.09~-0.095MPa until there is basically no flow, then add 250ml of petroleum ether to the reaction bottle, heat and reflux for 0.5 hours, and cool down Heat to about 25°C, keep warm at 25±1°C for 1 hour, filter with suction, rinse the filter cake with petroleum ether until the filtrate is colorless, and then drain it. Put the obtained light yellow solid in a 500ml flask, add 190g of purified water, stir and beat for 30 minutes, then add 30% liquid caustic soda dropwise to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com