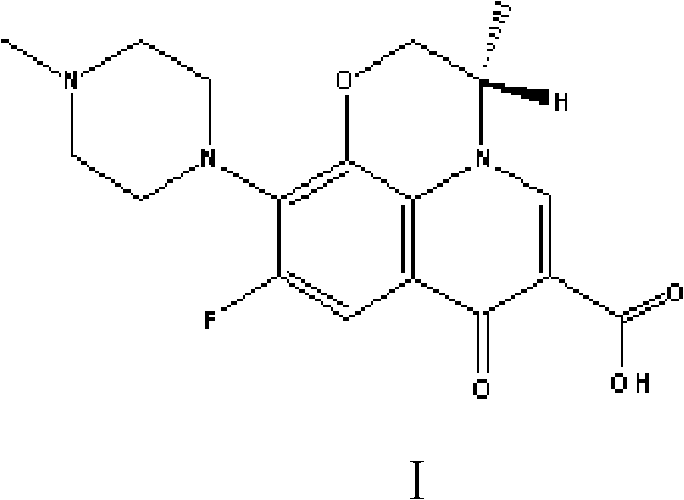
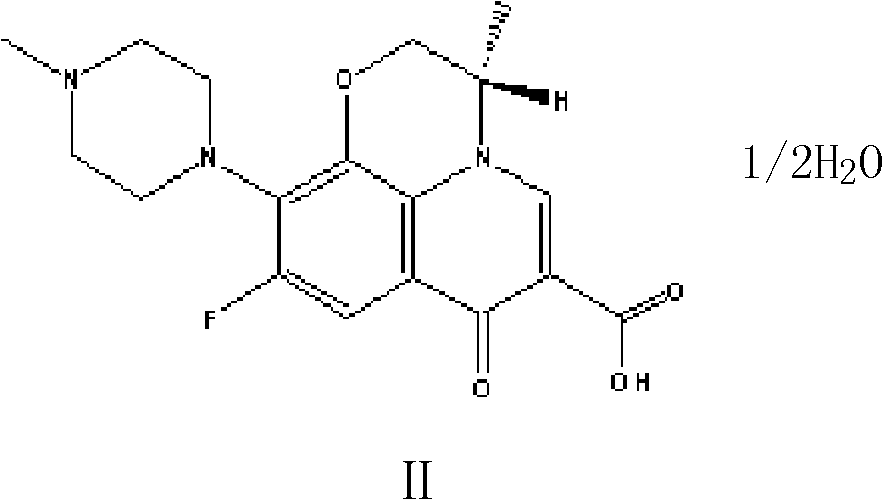
Preparation method of high-purity Levofloxacin semihydrate
A levofloxacin and hemihydrate technology, which is applied in the field of drug synthesis, can solve the problems of low purity of levofloxacin hemihydrate finished products, difficult microbial degradation, and many solvent residues, and achieve the effects of good quality and appearance, high purity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Put 100 grams of raw material levoxyfluorocarboxylic acid into a 1000ml four-necked flask, then add N-methylpiperazine, solvent DMSO and triethylamine to react with piperazine at 85℃ for 6 hours, and recover the solvent DMSO under reduced pressure And triethylamine and N-methylpiperazine; after concentrating to dryness, add 400 grams of pure water, increase the temperature to 75°C until the solids are completely dissolved, add ammonia water dropwise at this temperature until the pH of the solution is 7.0, and cool to After keeping the temperature at 30~35°C for half an hour, filter and rinse the filter cake with a little pure water to obtain crude levofloxacin I.
[0034] Add the crude levofloxacin I obtained above and 700ml of ethanol into a 1000ml four-necked flask, raise the temperature to 70°C until the solids are completely dissolved, add hydrochloric acid dropwise to pH 3.0, raise the temperature to reflux for half an hour, lower the temperature to 30°C, and filter to...
Embodiment 2
[0038] Put 100 grams of levoxyfluorocarboxylic acid as raw material into a 1000ml four-necked flask, then add N-methylpiperazine and solvent DMF to react with piperidine at 90℃ for 6 hours, and recover solvent DMF and N-methyl under reduced pressure. Piperazine; after concentrating to dryness, add 300 grams of pure water, increase the temperature to 80°C until the solid is completely dissolved, add ammonia water dropwise at this temperature, until the pH of the solution is 7.0, reduce the temperature to 30-35°C, and keep it warm for half an hour After filtering, the filter cake is rinsed with a little pure water to obtain crude levofloxacin I.
[0039] Add the crude levofloxacin I obtained above and 600ml of ethanol into a 1000ml four-necked flask, raise the temperature to 75°C until the solids are completely dissolved, add hydrochloric acid dropwise to pH 3.0, raise the temperature to reflux for half an hour, cool to 20°C, and filter to obtain levofloxacin salt Acid salt.
[0040...
Embodiment 3
[0043] Put 100 grams of raw material levoxyfluorocarboxylic acid into a 1000ml four-necked flask, then add N-methylpiperazine, solvent DMSO, and react with piperazine at 95℃ for 6 hours, and recover the solvent DMSO and N-methyl under reduced pressure. Piperazine; After concentrating to dryness, add 500 grams of pure water, increase the temperature to 70°C until the solid is completely dissolved, add ammonia water dropwise at this temperature, until the pH of the solution is 7.0, reduce the temperature to 20-30°C, and keep it warm for half an hour After filtering, the filter cake is rinsed with a little pure water to obtain crude levofloxacin I.
[0044] Add the crude levofloxacin I obtained above and 800ml of ethanol into a 1000ml four-necked flask, raise the temperature to 78°C until the solids are completely dissolved, add hydrochloric acid dropwise to pH 2.0, heat and reflux for half an hour, cool to 10°C, and filter to obtain levofloxacin salt Acid salt.
[0045] Add 3 times ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com