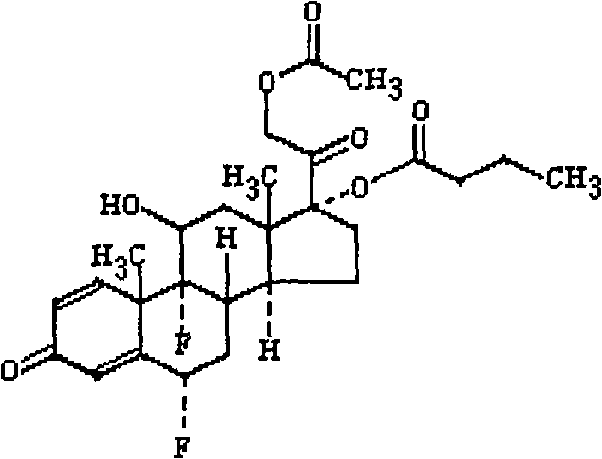
Ophthalmic or otic and nasal composition containing difluprednate and lavo-ofloxacin and application thereof
A technology of levofloxacin and difluprednate, which is applied in the field of ophthalmic or ear-nasal pharmaceutical compositions, can solve problems such as inability to speculate on the compatibility effect of levofloxacin and difluprednate, incomparability of chemical properties and physical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Difluprednate 0.05g
[0039] Levofloxacin 0.5g
[0040] Castor oil 5.0g
[0041] Tween 80 4.0g
[0042] Glycerin 2.0g
[0043] Sodium acetate 0.01g
[0044] Boric acid 0.1g
[0045] Disodium ethylenediaminetetraacetic acid 0.02g
[0046] Sorbic acid 0.1g
[0047] Sodium hydroxide amount
[0048] Sterile distilled water, add water to 100ml
[0049] Heat sterile distilled water to 70°C, then add levofloxacin, Tween 80, medicinal glycerin, sodium acetate, boric acid, disodium edetate and sorbic acid of the above formula and dissolve them. The pH is adjusted to 6.0 with sodium hydroxide to obtain an aqueous phase. In addition, the castor oil is heated to about 70° C., and difluprednisolone is added and stirred to dissolve to obtain an oil phase. A homomixer is used to stir the water phase while adding the oil phase to obtain the emulsified crude oil, and finally the emulsified crude oil is split in the microfluidizer, and finally, the pharmaceutical composition of the present i...
example 2
[0051] Difluprednate 0.005g
[0052] Levofloxacin 0.3g
[0053] Castor oil 1.0g
[0054] Tween 80 0.5g
[0055] Glycerin 2.2g
[0056] Sodium acetate 0.05g
[0057] Hydroxypropyl methylcellulose 0.1g
[0058] Chlorobutanol 0.3g
[0059] Sorbic acid 0.1g
[0060] Hydrochloric acid
[0061] Sterile distilled water, add water to 100ml
[0062] Heat sterile distilled water to about 70°C, then add levofloxacin, Tween 80, medicinal glycerin, hydroxypropyl methylcellulose, sodium acetate and chlorobutanol in the above formula and dissolve them. The pH was adjusted to 4.0 with hydrochloric acid to obtain an aqueous phase. In addition, the castor oil is heated to 70° C., difluprednisolone is added, and the mixture is stirred and dissolved to obtain an oil phase. While mixing the water phase with a homomixer, the oil phase is added to obtain the emulsified crude oil. Finally, the emulsified crude oil is split in a microfluidizer, and finally, the pharmaceutical composition of the present in...
example 3
[0064] Difluprednate 0.01g
[0065] Levofloxacin 0.3g
[0066] Miglyol 10.0g
[0067] Polysorbate 5.0g
[0068] Concentrated glycerin 2.2g
[0069] Amino boric acid 0.1g
[0070] Chlorhexidine Gluconate 0.005g
[0071] Sodium hydroxide amount
[0072] Sterile purified water is added to the total amount of 100ml
[0073] Heat sterile purified water to about 70°C, then add levofloxacin, polysorbate, concentrated glycerin, aminoboronic acid and chlorhexidine gluconate of the above formula and dissolve them, and adjust the pH to 5.5 with sodium hydroxide to obtain water box. In addition, Miglyol was heated to about 70°C, and difluprednisolone was added and dissolved to obtain an oil phase. While mixing the water phase with a homomixer, the oil phase is added to obtain the emulsified crude oil. Finally, the emulsified crude oil is split in a microfluidizer, and then filtered and sterilized to obtain the composition of the present invention. The average particle size of the oil droplets...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com