Patents
Literature
52 results about "Infections site" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medicament composition for eyes or nose, and uses thereof
The present invention provides a combination of medicines for eyes or ears and nose, which comprises levofloxacin and loteprednol carbon ester; wherein, the weight ratio of the loteprednol carbon ester to the levofloxacin is between 1 to 0.2 and 1 to 5. The combination of medicine for eyes or ears and nose of the present invention is used for curing conjunctivitis, keratitis, blepharitis, dacryocystitis, hordeolum, corneal ulcer and eye infection with inflammation of eyes or even inflammation of tissue around eyes. The combination is also used for preventing bacterial infection risk after ophthalmology operation or eye injury and inflammation of the infected region, or the combination is used for curing or alleviating bacterial infection and tissue inflammation of the infected region after ophthalmology operation or eye injury, or cure tympanitis, otitis externa and infectious rhinitis.
Owner:SHENZHEN REGOO LAB
Bacterial efflux pump inhibitors for the treatment of ophthalmic and otic infections
Efflux pump inhibitors are co-administered with antimicrobial agents for the treatment of ophthalmic or otic infections. The agents may be co-administered directly to the site of infection (e.g., the eye or ear).
Owner:REMPEX PHARM INC
Netrin compositions and methods of using the same
InactiveUS20060025335A1Reduce inflammationPromote migrationSenses disorderPeptide/protein ingredientsInfections siteInflammatory cell
The present invention provides methods and compositions to modulate inflammation and inflammatory responses using Netrin polypeptides and Netrin receptors. Methods of the present invention comprise the use of Netrin polypeptides and Netrin receptors to decrease migration of inflammatory cells of the immune system to a site of injury or infection.
Owner:THE GENERAL HOSPITAL CORP
Method for treating local infection
InactiveUS20150045720A1Good treatment effectRemarkable synergistic disinfecting effectElectrotherapyPhotodynamic therapyMedicineInfections site
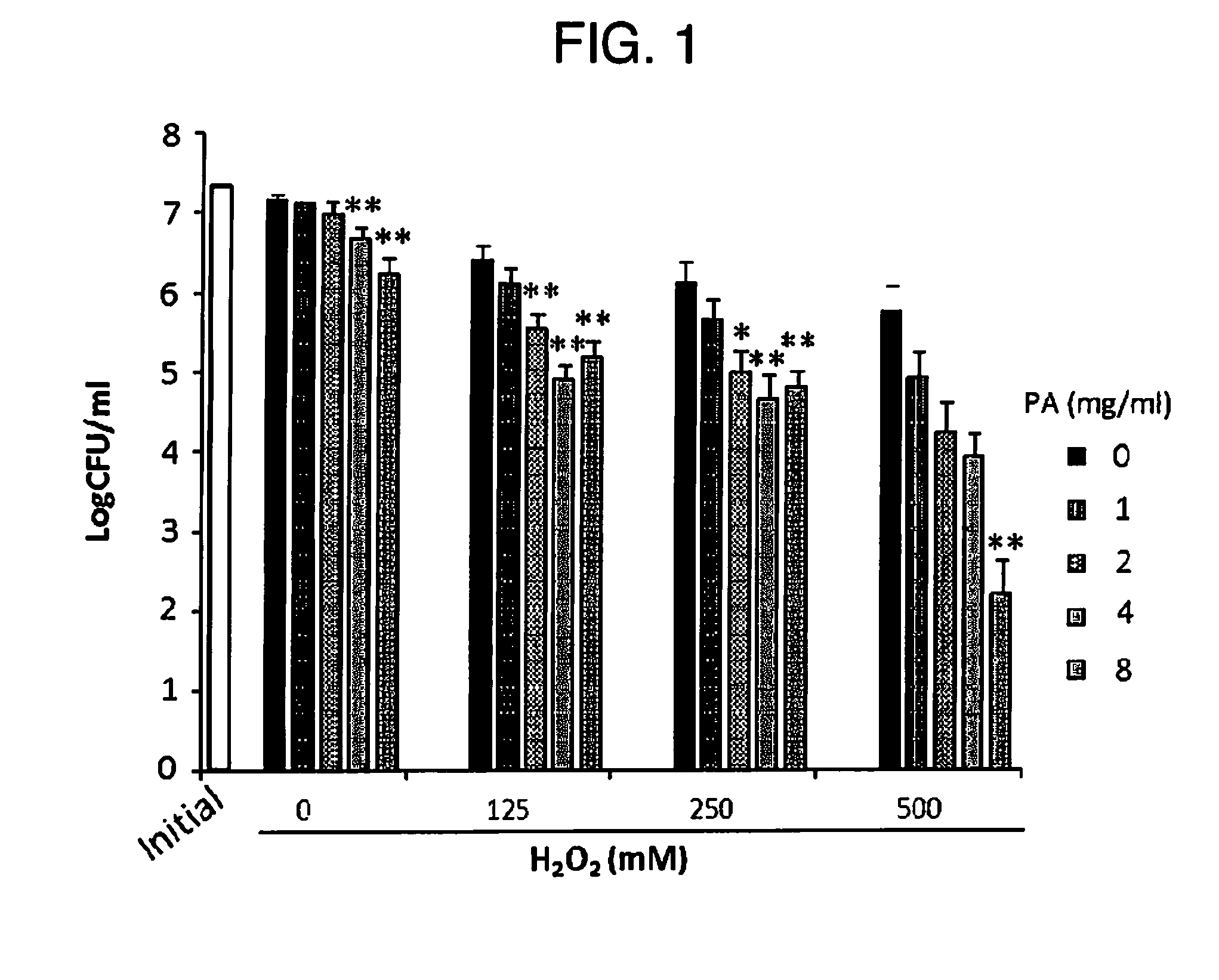
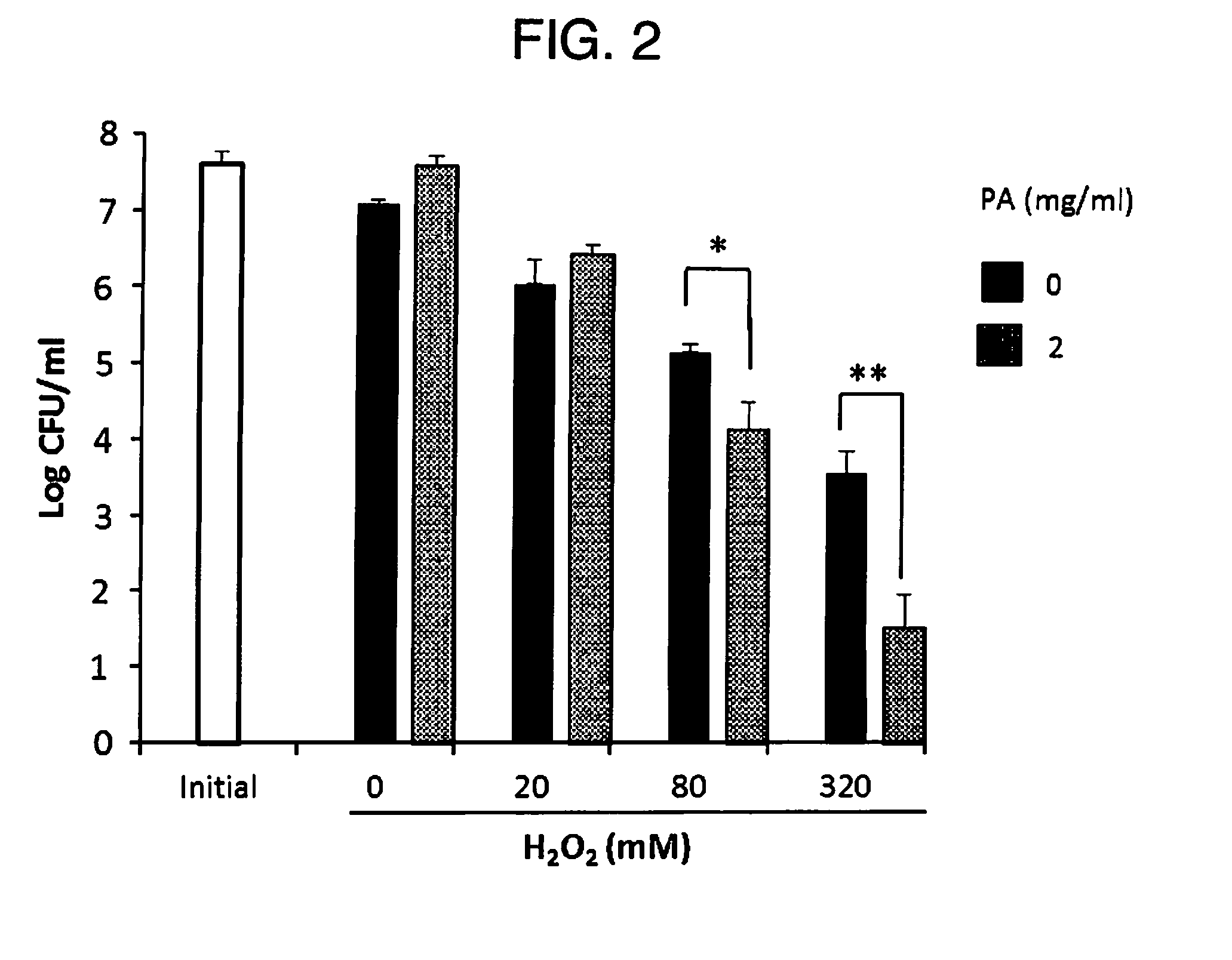
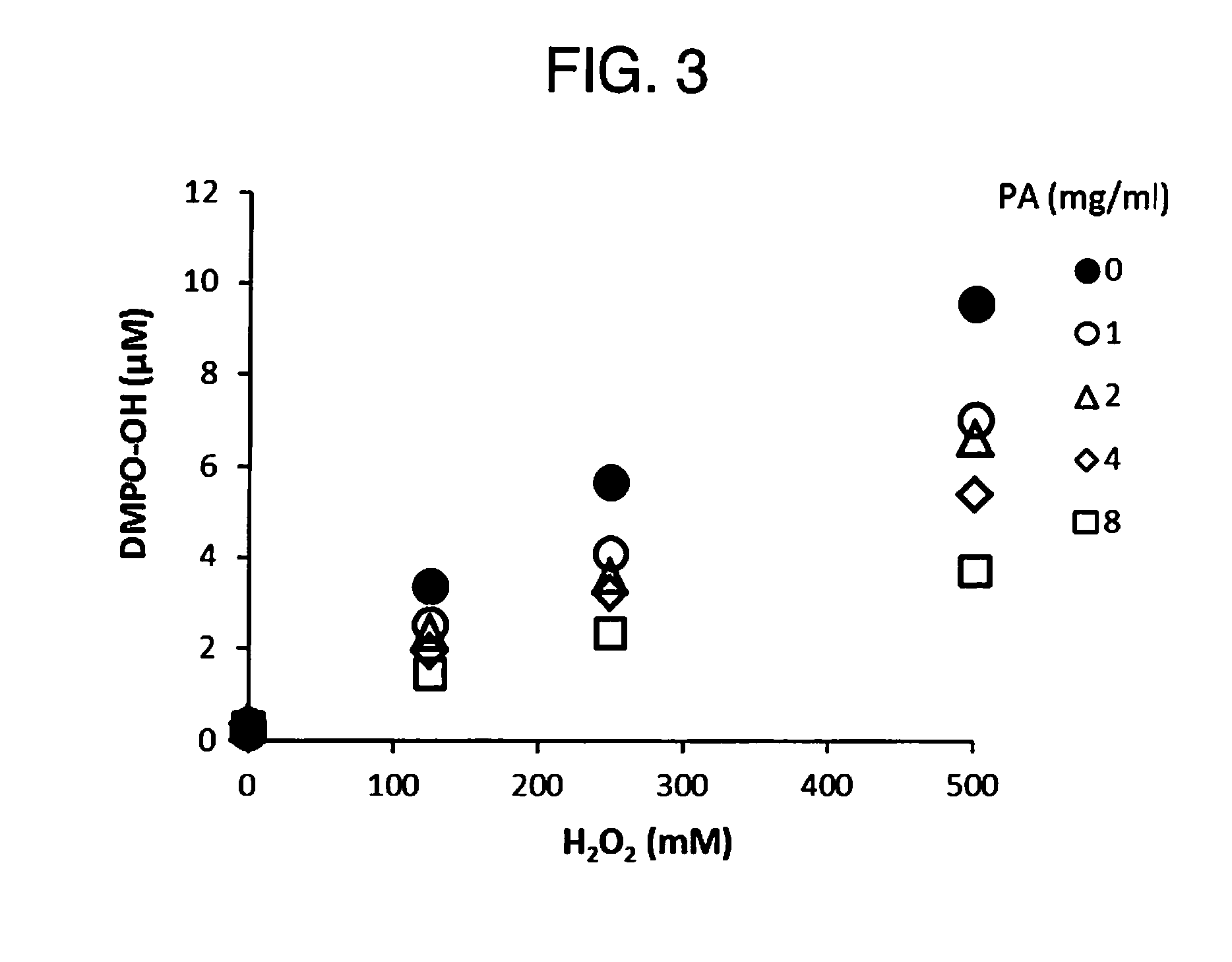
The present invention relates to a method for treating a local infection, for example, a periodontal disease, peri-implantitis or dermartitis, comprising applying a composition containing hydrogen peroxide and a polyphenol such as catechins to an infected site, for example, inside oral cavity or skin, and irradiating the site with light for a predetermined period of time.
Owner:A-Z
Combination laser therapy apparatus
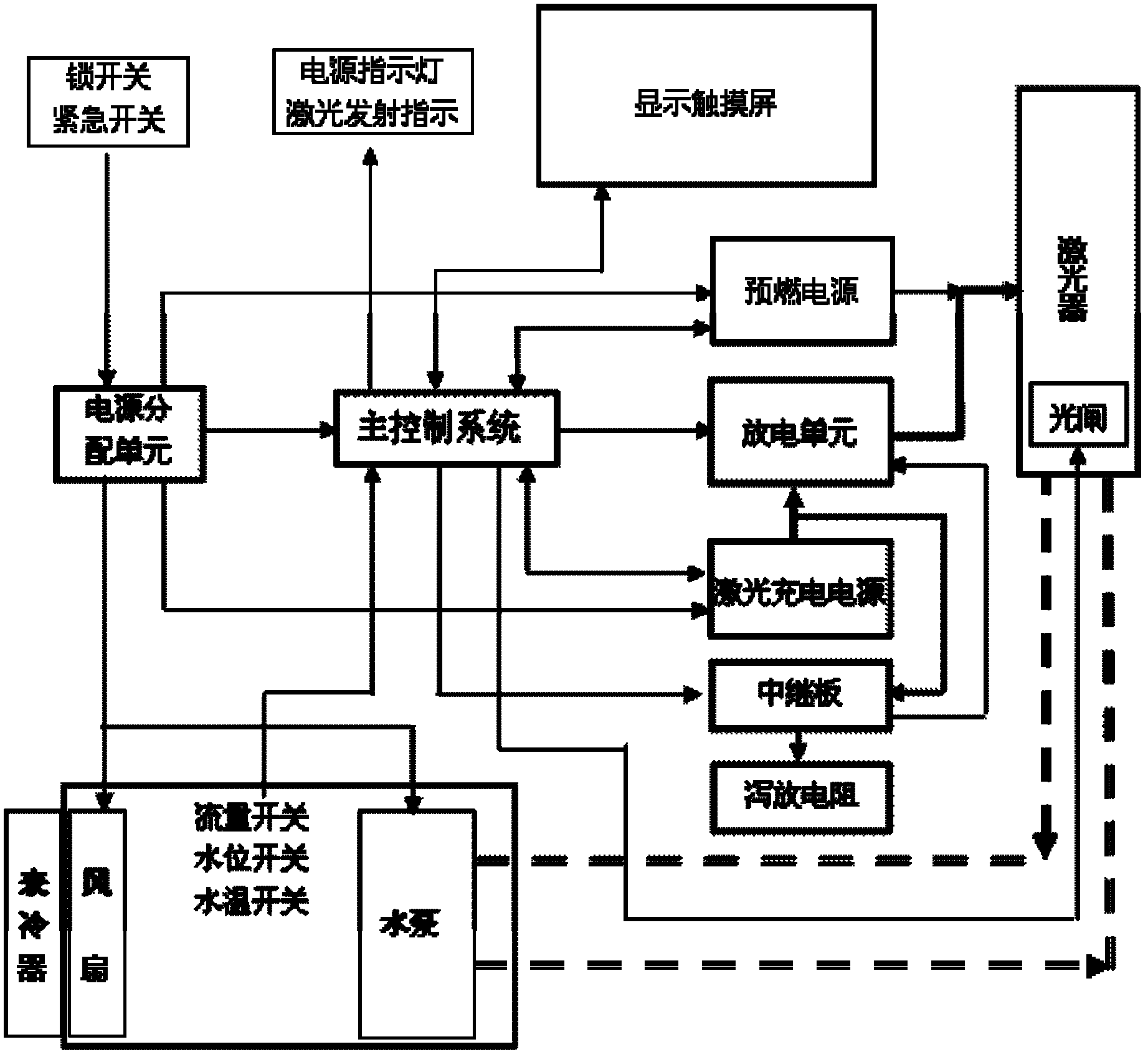
ActiveCN103182149AHigh cure rateLow recurrence rateSurgical instrument detailsRadiation therapyInfections siteMedical product
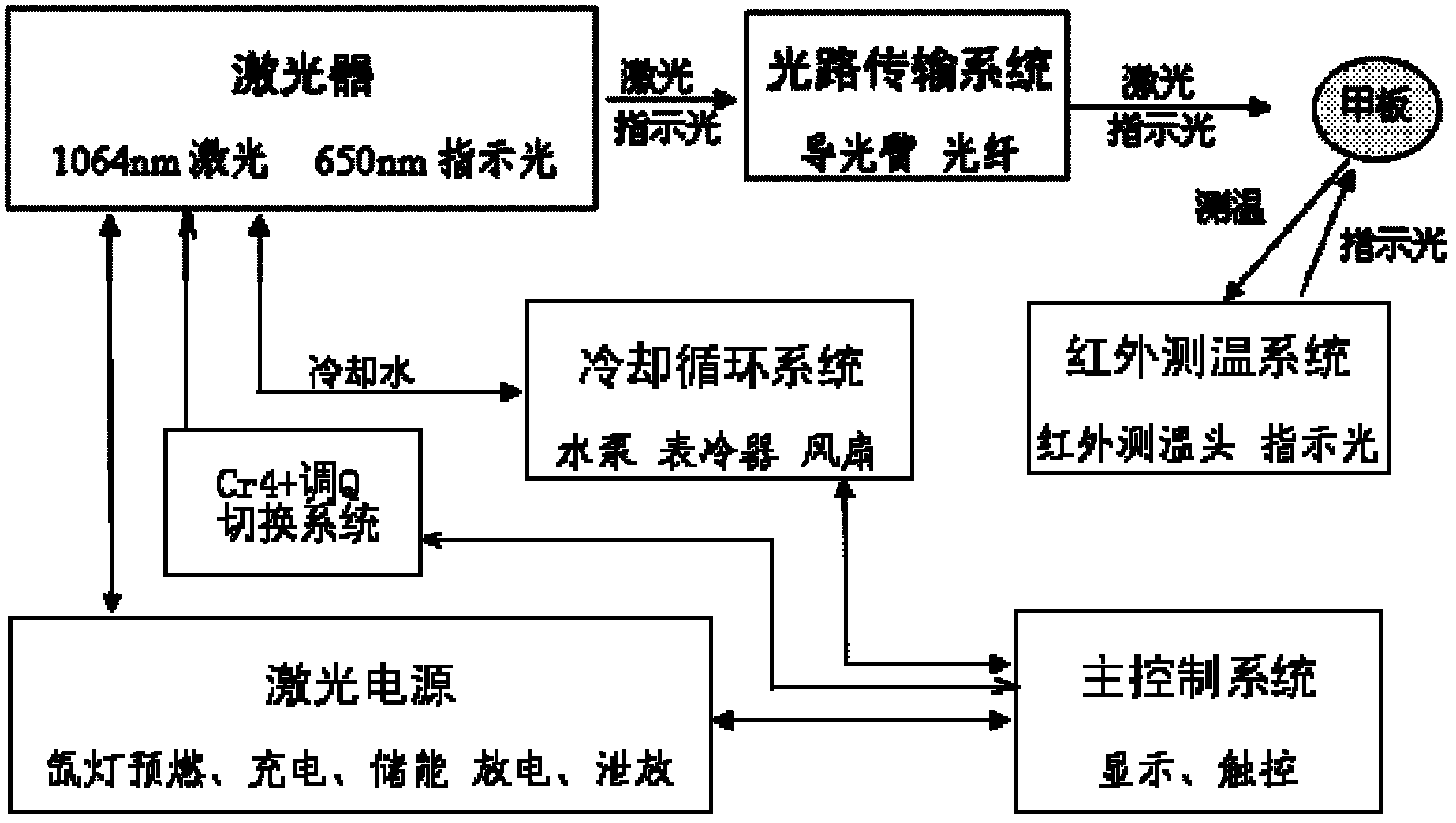
The invention relates to laser medical products and discloses a combination laser therapy apparatus which comprises a laser device with the wavelength of 1064nm, a laser device with the wavelength of 650nm, a Cr4 + Q-switching switch system, a light path transmission system, a laser power supply, an infrared temperature measurement system, a cooling circulating system, a master control system and a display touch screen. The laser device with the wavelength of 650nm is located in the direction of output light of the laser device with the wavelength of 1064nm. The light path transmission system is located on the output light path of the laser device with the wavelength of 650nm. The master control system is connected with the laser power supply, the infrared temperature measurement system, the cooling circulating system, the display touch screen and the Cr4 + Q-switching switch system, and the cooling circulating system is connected with the laser power supply. Laser of the combination laser therapy apparatus can directly reach a fungal infection site, heats fungus and enables the fungus to be deprived of ability of replication and reproduction, and therefore the therapy purpose is achieved. The combination laser therapy apparatus has the advantages of being high in cure rate, low in recurrence rate and free of drug side effects.
Owner:WUHAN MIRACLE LASER SYST
Method for treatment of fingernail and toenail microbial infections using infrared laser heating and low pressure
Owner:NEW STAR LASERS
Compositions and methods for delivering an agent to a wound
ActiveUS8993540B2Reduced survivalReduce proliferationAntibacterial agentsOrganic active ingredientsPoint of careClinical settings
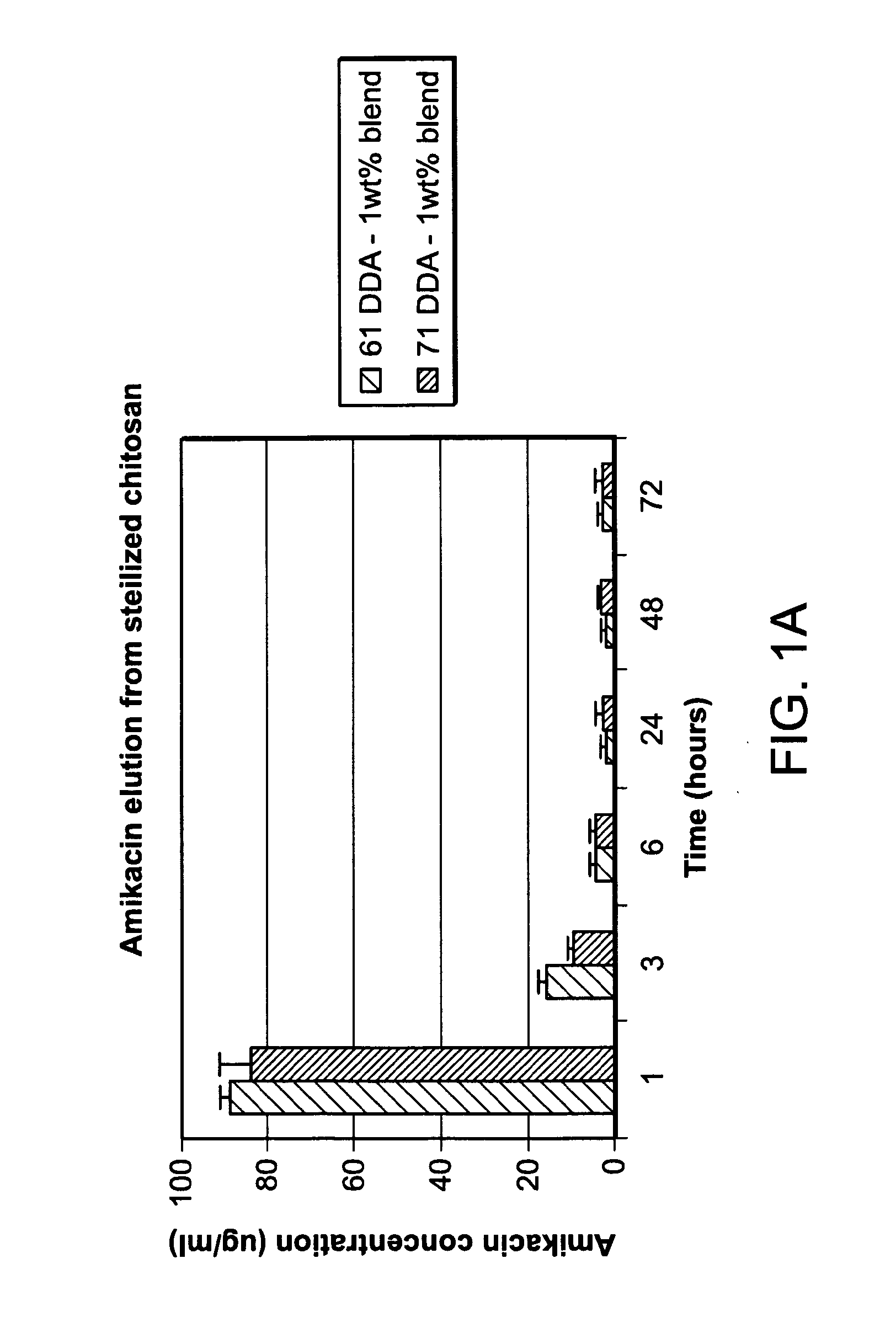
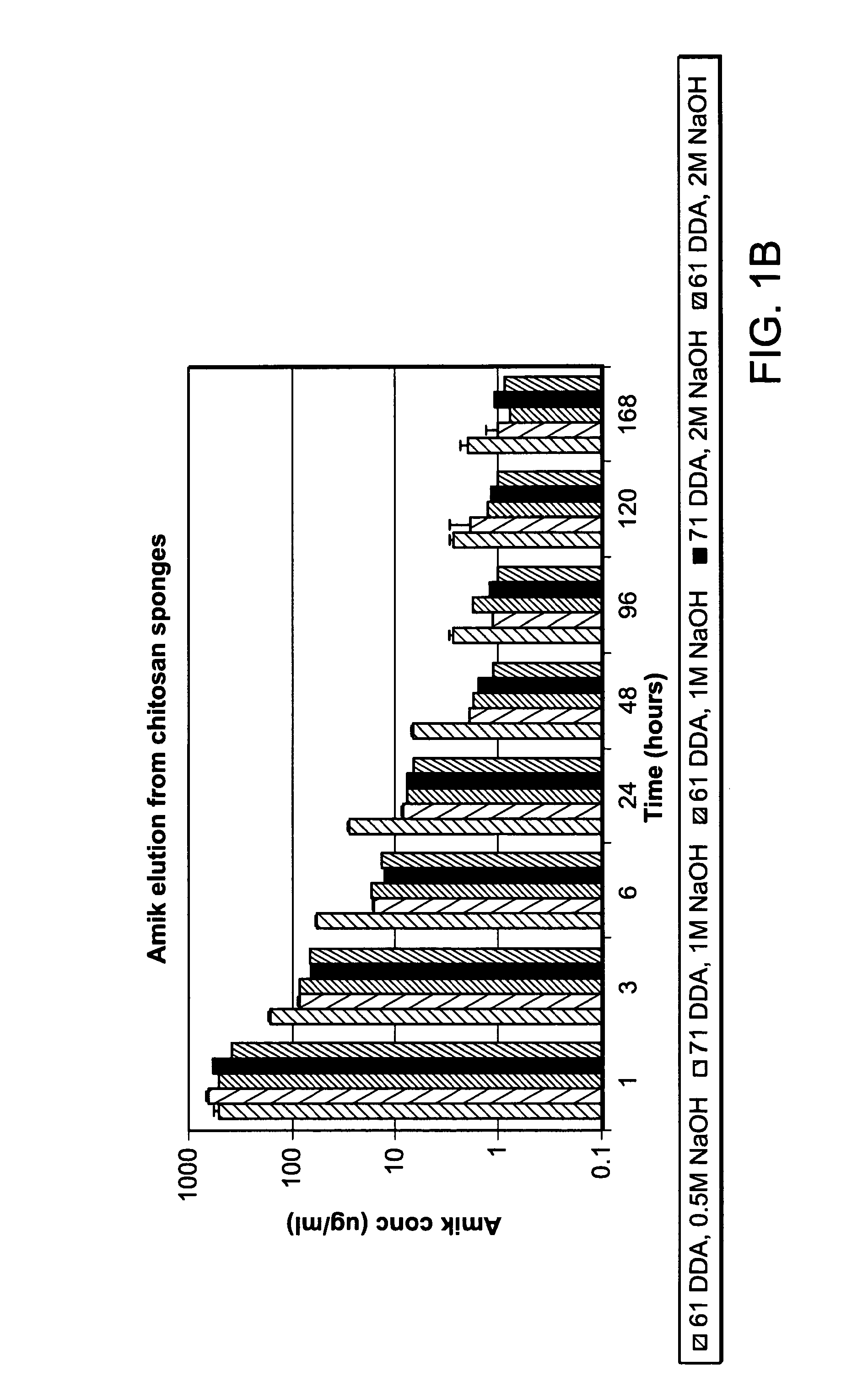
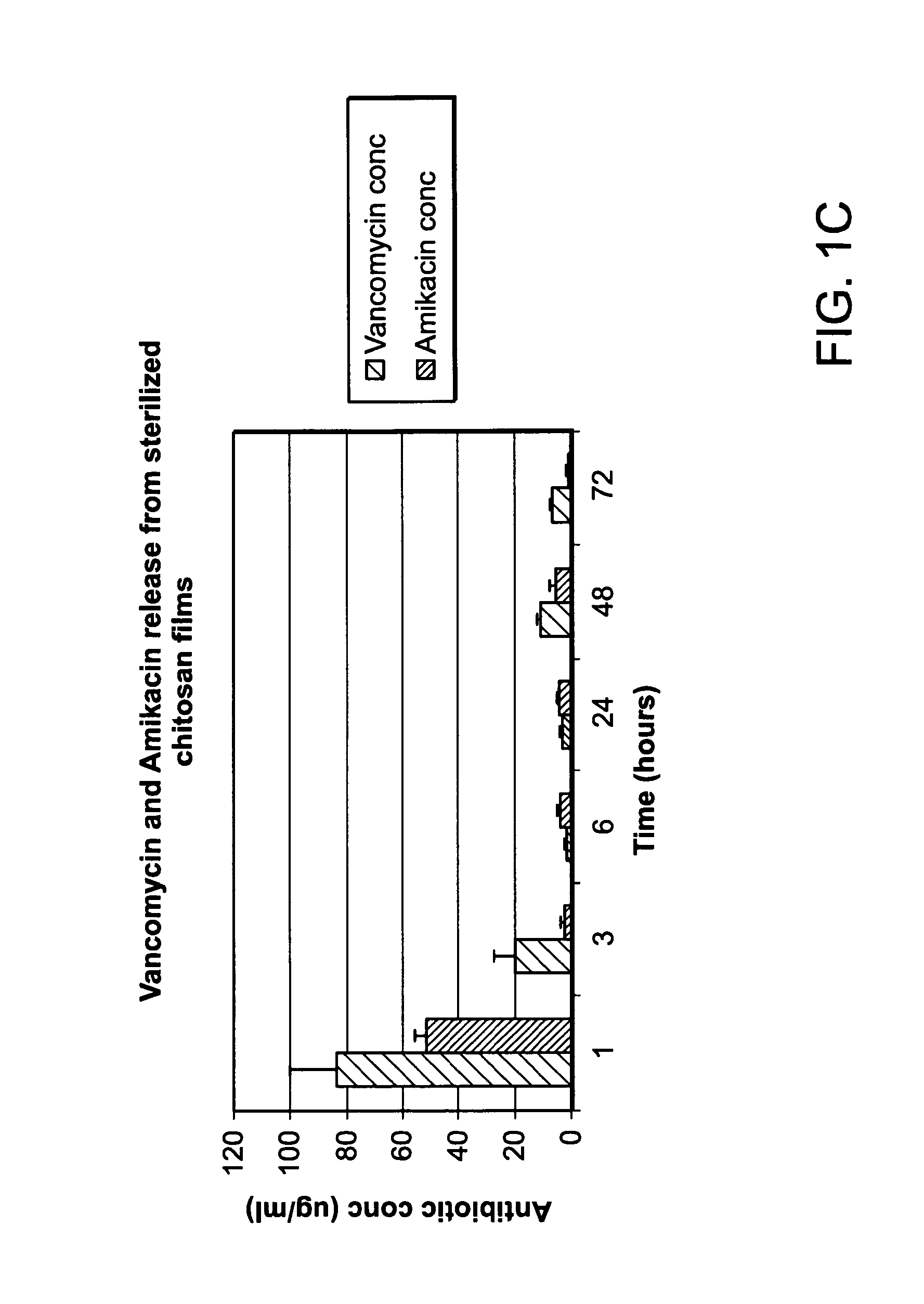
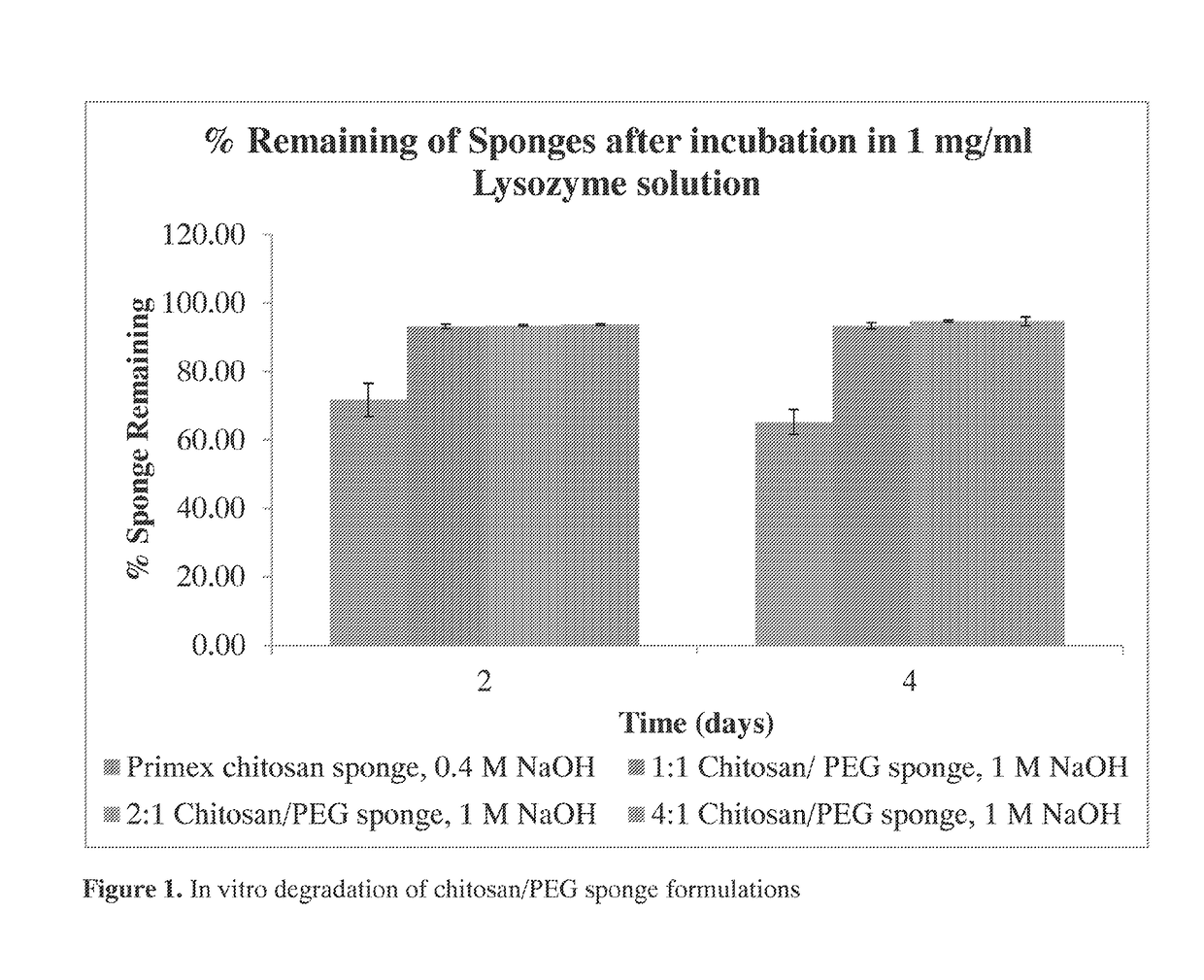
The invention provides compositions featuring chitosan and methods for using such compositions for the local delivery of biologically active agents to an open fracture, complex wound or other site of infection. Advantageously, the degradation and drug elution profiles of the chitosan compositions can be tailored to the needs of particular patients at the point of care (e.g., in a surgical suite, clinic, physician's office, or other clinical setting).
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Skin-permeable selective cyclooxygenase-2 inhibitor composition
A dermally deliverable pharmaceutical composition comprises at least one selective cyclooxygenase-2 (COX-2) inhibitory drug or prodrug thereof solubilized in a pharmaceutically acceptable carrier that comprises a low molecular weight monohydric alcohol, and exhibits a skin permeation rate of the therapeutic agent at least equal to that exhibited by a reference solution of the therapeutic agent in 70% aqueous ethanol. A method of effecting targeted delivery of a selective COX-2 inhibitory drug to a site of pain and / or inflammation in a subject comprises topically administering such a composition to skin of the subject, preferably at a locus overlying or adjacent to the site of pain and / or inflammation. A method of effecting systemic treatment of a subject having a COX-2 mediated disorder comprises transdermally administering such a composition, preferably by contacting the composition with an area of skin of the subject not greater than about 400 cm2.
Owner:PHARMACIA CORP
Ophthalmic or otic and nasal composition containing difluprednate and lavo-ofloxacin and application thereof
InactiveCN101564395AAntibacterial agentsOrganic active ingredientsInfective rhinitisInfectious Rhinitis
The invention provides an ophthalmic or otic and nasal pharmaceutical composition, which comprises lavo-ofloxacin and salts thereof and difluprednate, wherein the weight ratio of the difluprednate to the lavo-ofloxacin is 1:1-1:10. The ophthalmic or otic and nasal medicament composition is applied to treating conjunctivitis, keratitis, eyelid inflammation, dacryocystitis, hordeolum, corneal ulcer and eye infections with inflammations of eyes or surrounding tissues, or preventing increased risk of bacterial infections and tissue inflammations of infected parts after ophthalmic surgeries or eye injuries, or treating or relieving the bacterial infections combined with tissue inflammations of the infected parts after the ophthalmic surgeries, or treating otitis media, otitis externa and infectious rhinitis.
Owner:SHANDONG INST OF PHARMA IND
Spray-dried collectin compositions and process for preparing the same
ActiveUS20070154406A1Excellent characteristicsImprove propertiesAntibacterial agentsPowder deliveryCollectinFungal microorganisms
The present invention relates to a spray-dried composition comprising as an active ingredient at least one member protein of the collectin family or its functional equivalent for treating and preventing microbial infectious diseases. The present invention also relates to a method for producing the same composition. The composition produced by the method of the present invention is effective in suppressing infections caused by viruses, bacteria, fungi, and parasites. Since the composition is developed in a form suitable for inhalation, it can directly provide the active ingredient to the sites of infection from these microbes, and thus treat and prevent respiratory infections and external wounds.
Owner:CHA VACCINE RES INST CO LTD
Therapeutic agent for infectious skin or mucosal disease
Owner:TAIKO PHARMA
Treatment of Microbial Infections Using Hot and Cold Thermal Shock and Pressure
InactiveUS20110172586A1Effective treatmentReduce water contentElectrotherapyMedical devicesMedicineInfections site
A method of treating microbial infections, which consists of the steps of sequentially and repeatedly irradiating the microbe with continuous or pulsed infrared radiation and continuous or pulsed cooling such that heat and cold alternatively penetrates to the site of the infection in order to inactivate the pathogen.
Owner:NEW STAR LASERS
Methods for Treating Bacterial Infection
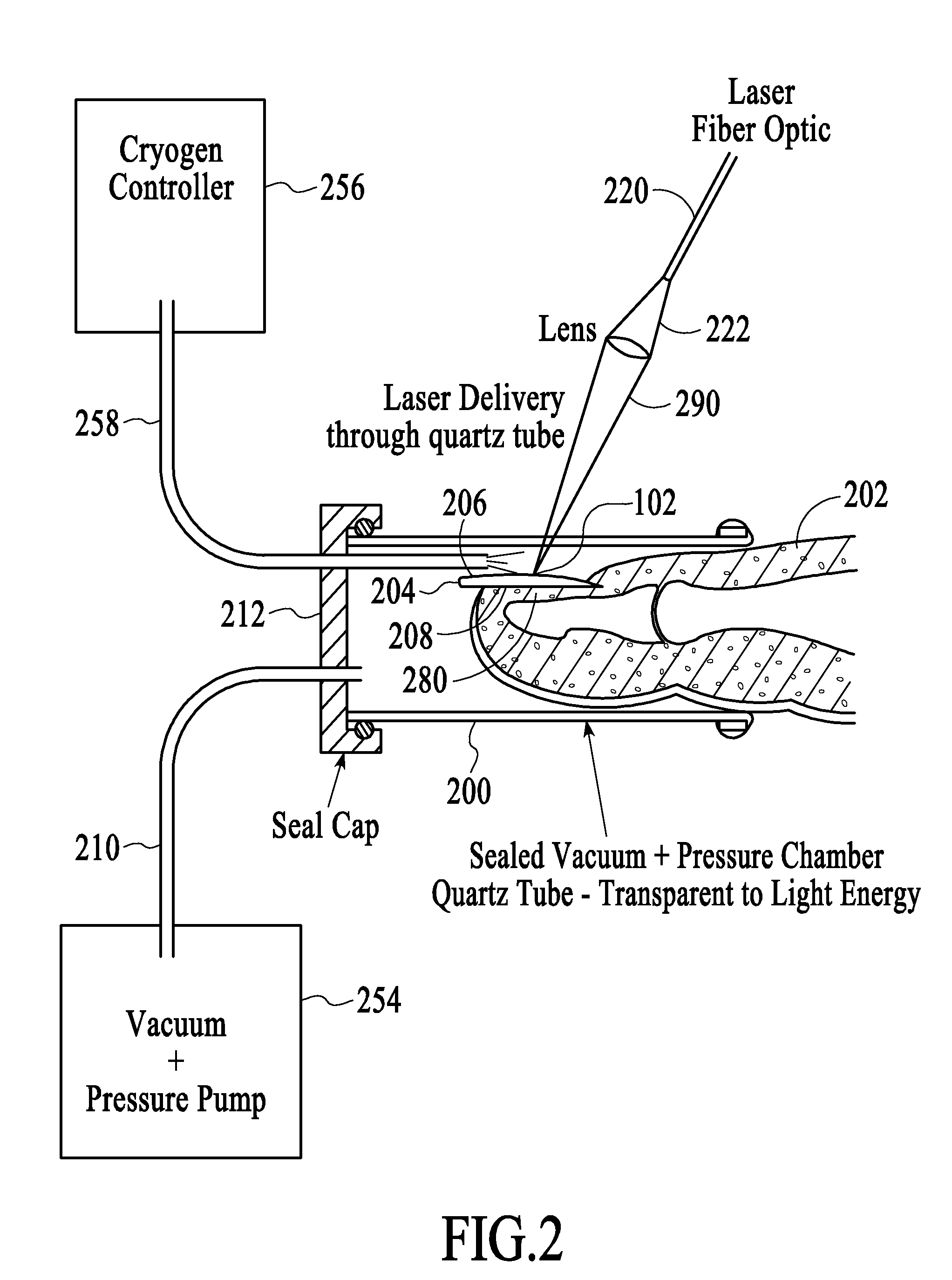
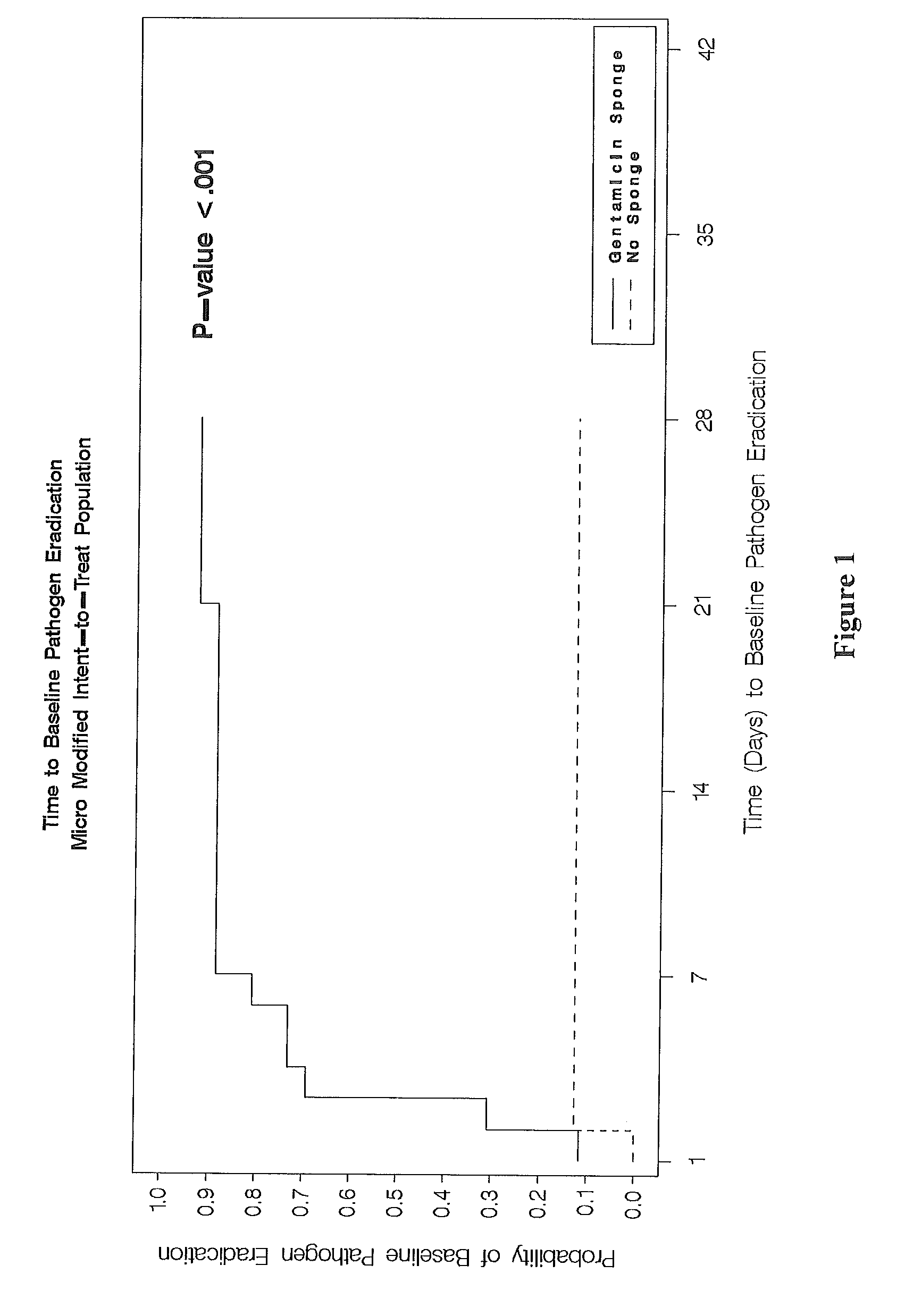
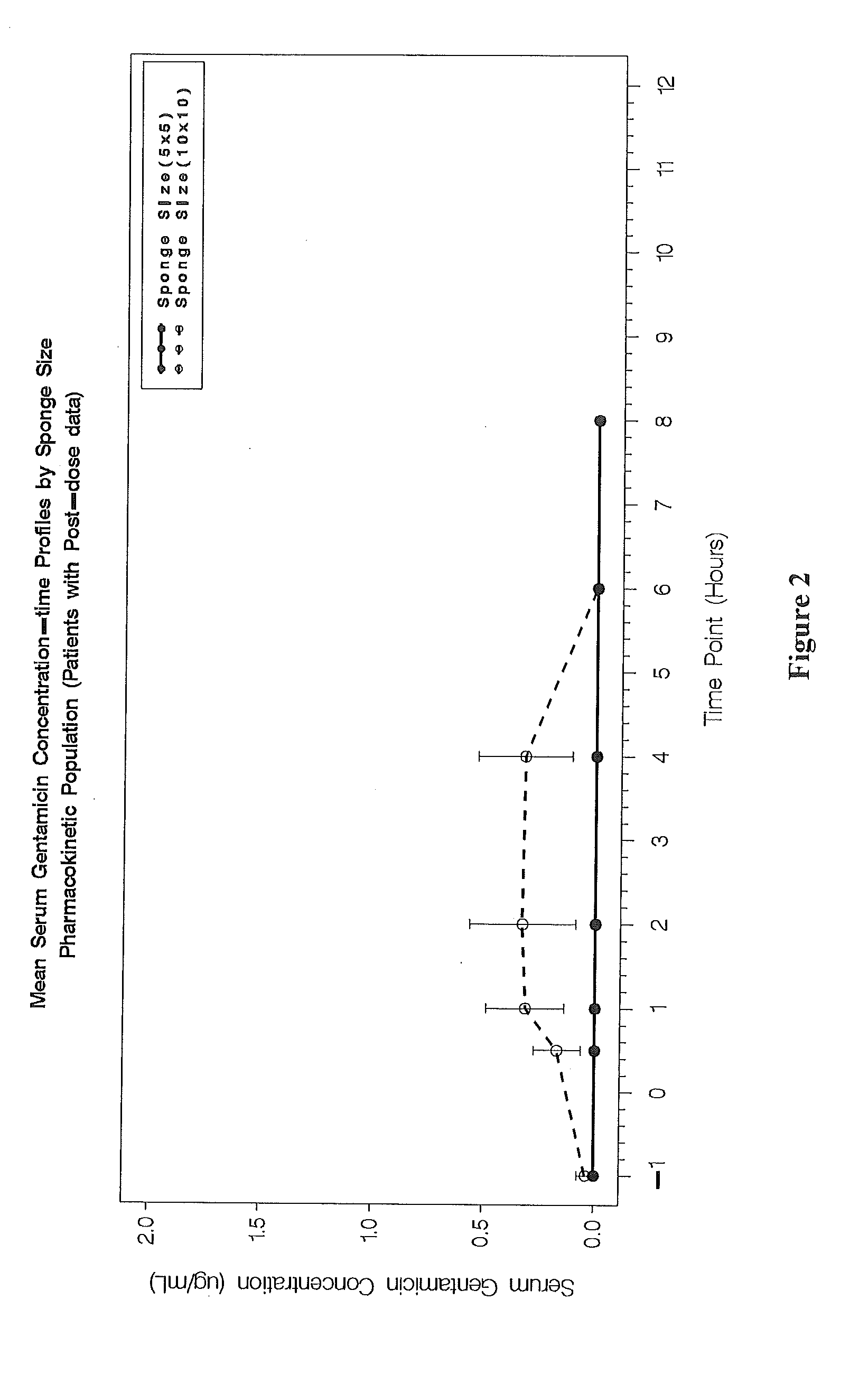
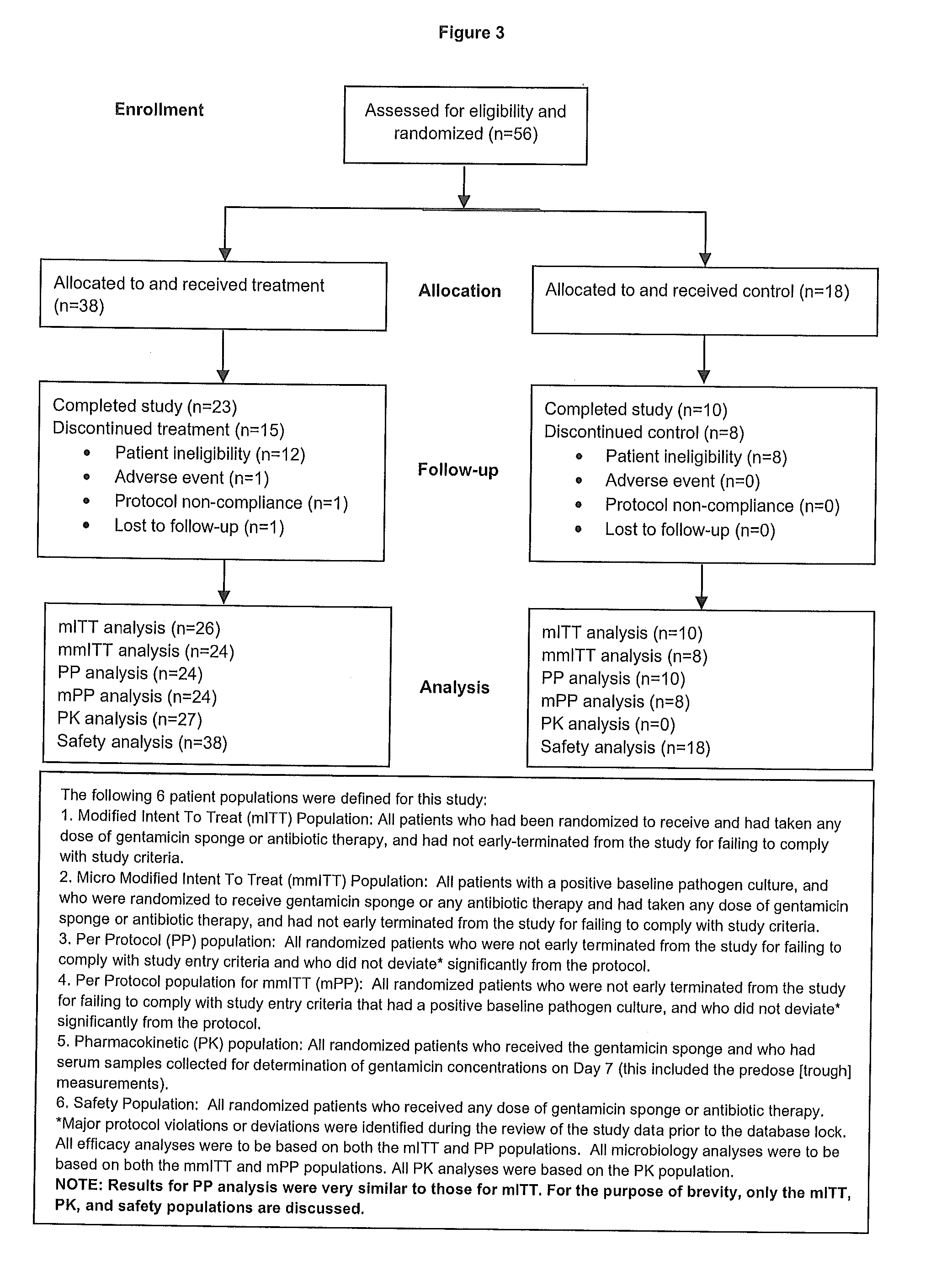
This invention relates to methods for treating bacterial infection, which methods find utility in the treatment of, for example, infected ulcers, optionally infected diabetic ulcers. In particular, this invention relates to treating bacterial infection, for example, infected diabetic ulcers by topical administration of at least one aminoglycoside antibiotic at the site of infection, in combination with at least one antibacterial agent, which antibacterial agent is administered remote from the site of infection, preferably administered systemically. In a particular embodiment, the present invention relates to a composition for use in treating bacterial infection, the composition comprising gentamicin sulphate (a water-soluble broad-spectrum aminoglycoside antibiotic) uniformly dispersed in a type-I collagen matrix; in combination with at least one systemically-administered antibacterial agent. The present invention provides bactericidal activity against most strains of aerobic gram-negative and gram-positive and facultative anaerobic gram-negative pathogens, including methicillin-resistant Staphylococcus aureus.
Owner:INNOCOLL PHARMACEUTICALS LTD
Treatment of inflammatory, autoimmune, or other disorders, using agents that reduce the sequestering of zinc by calprotectin
InactiveUS20070275095A1Reduce activity levelReduce concentrationBiocideNervous disorderDiseaseWhole body
Treatments are disclosed for inflammatory, autoimmune, or other disorders characterized by excessive activity of calprotectin, a protein that normally defends against microbial infections by sequestering available zinc, at a site of infection. Excessive calprotectin activity, which can cause zinc deficiencies in localized tissues, can create or aggravate various disorders. However, ingestion of systemic (oral) zinc supplements tends to activate offsetting mechanisms, and such supplements therefore usually are ineffective. Accordingly, targeted treatments are disclosed herein for suppressing and controlling excessive calprotectin activity, in local tissues. Such methods include targeted injections of zinc solutions, and plasmapheresis treatment. Screening tests also are described for identifying non-protein drugs that can either (i) bind specifically to the zinc-binding sites of calprotectin, or (ii) suppress the release of calprotectin by neutrophil cells.
Owner:KOSSOR DAVID C
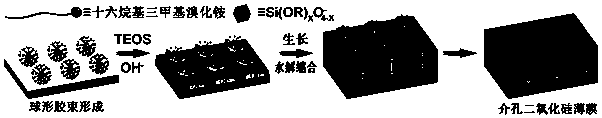
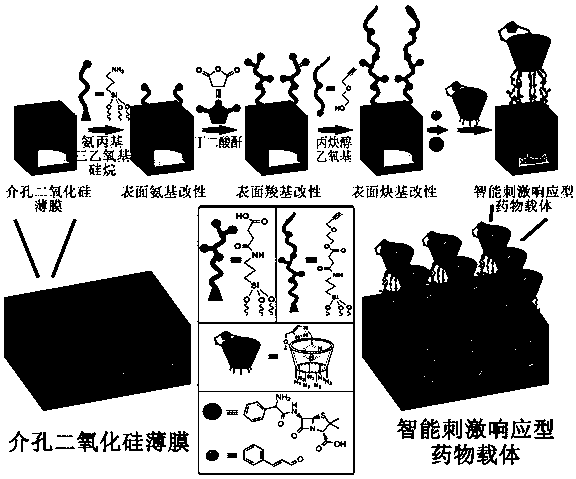
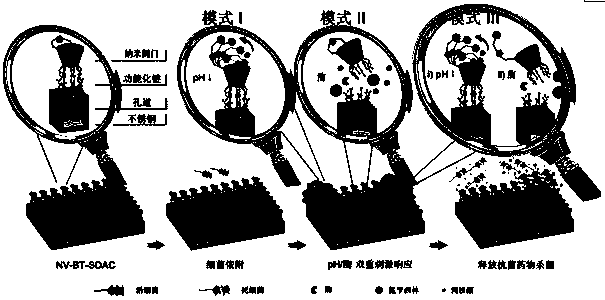
Preparation method of bacterium-sensitive intelligent antibacterial coating and application
ActiveCN107596452AReduce the likelihood of drug resistanceHigh sensitivitySilicaCoatingsSide effectAntibiotic resistance
The invention discloses a preparation method of a bacterium-sensitive intelligent antibacterial coating and an application, and belongs to the field of composite antibacterial materials. The preparation method comprises the steps as follows: firstly, a mesoporous silica film with two-dimensional pore channels perpendicular to a substrate is synthesized; then, organic functional modification is performed on the surface of the silica film; finally, the modified film and macrocyclic molecules form a nano-valve, and antibacterial drugs are encapsulated. When a host is infected with bacteria, the bacteria secrete a series of pathogenic factors to form special bacterial infection microenvironments, the special microenvironments are used as stimulating factors to prompt a drug-loading nano-container to release a drug, and selective delivery of antibiotics is achieved. With adoption of the strategy, selectivity and efficacy of the antibiotics can be increased significantly, toxic and side effects of the drug are reduced, the infection site is maintained at a higher drug concentration, and antibiotic resistance is overcome.
Owner:JIANGSU GUGELANSHAN PROTECTIVE FACILITIES CO LTD +1
Bacterial efflux pump inhibitors for the treatment of ophthalmic and otic infections
Efflux pump inhibitors are co-administered with antimicrobial agents for the treatment of ophthalmic or otic infections. The agents may be co-administered directly to the site of infection (e.g., the eye or ear).
Owner:REMPEX PHARM INC
Compositions and methods for delivering an agent to a wound
InactiveUS20170258967A1Reduce probabilityIncrease or decrease weightSuture equipmentsOrganic active ingredientsPoint of careClinical settings
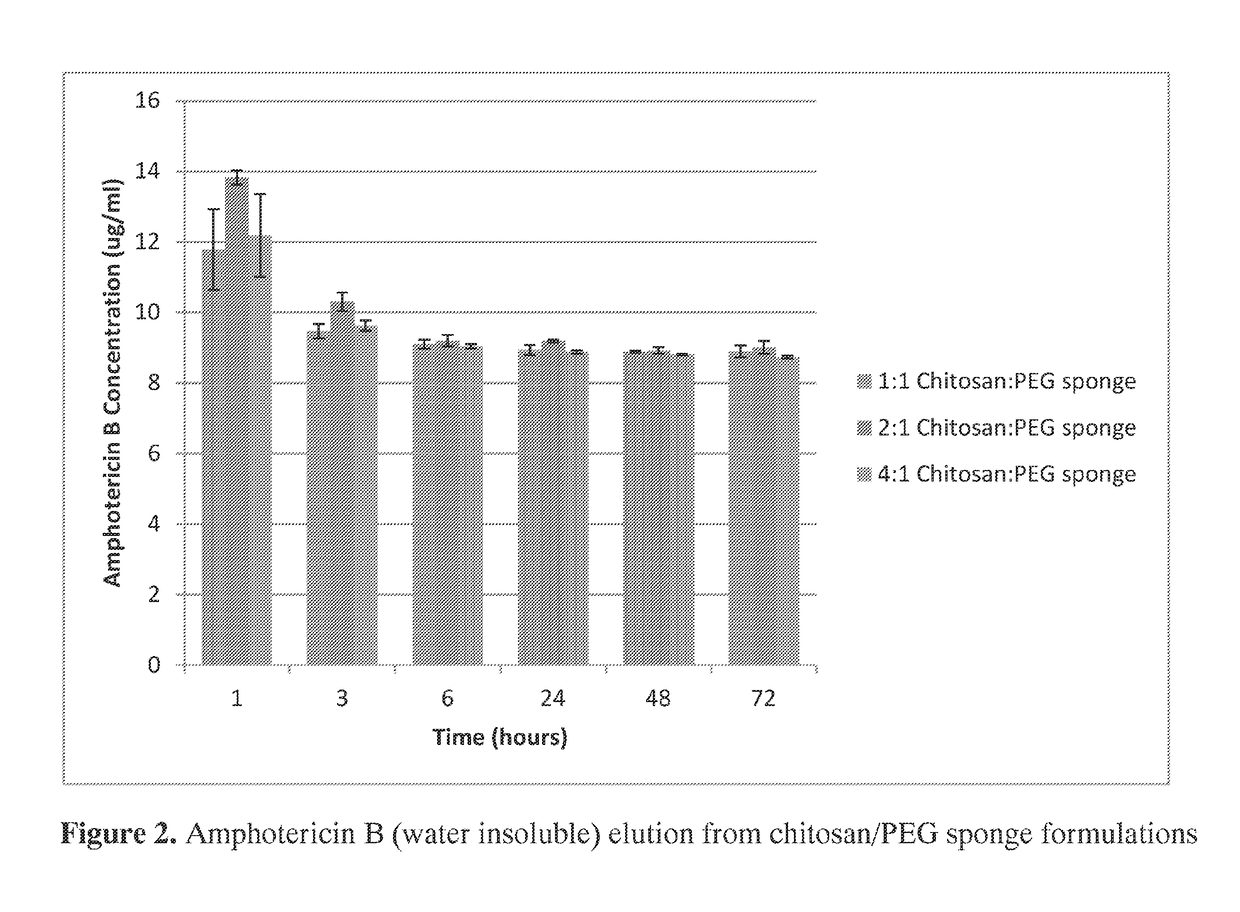
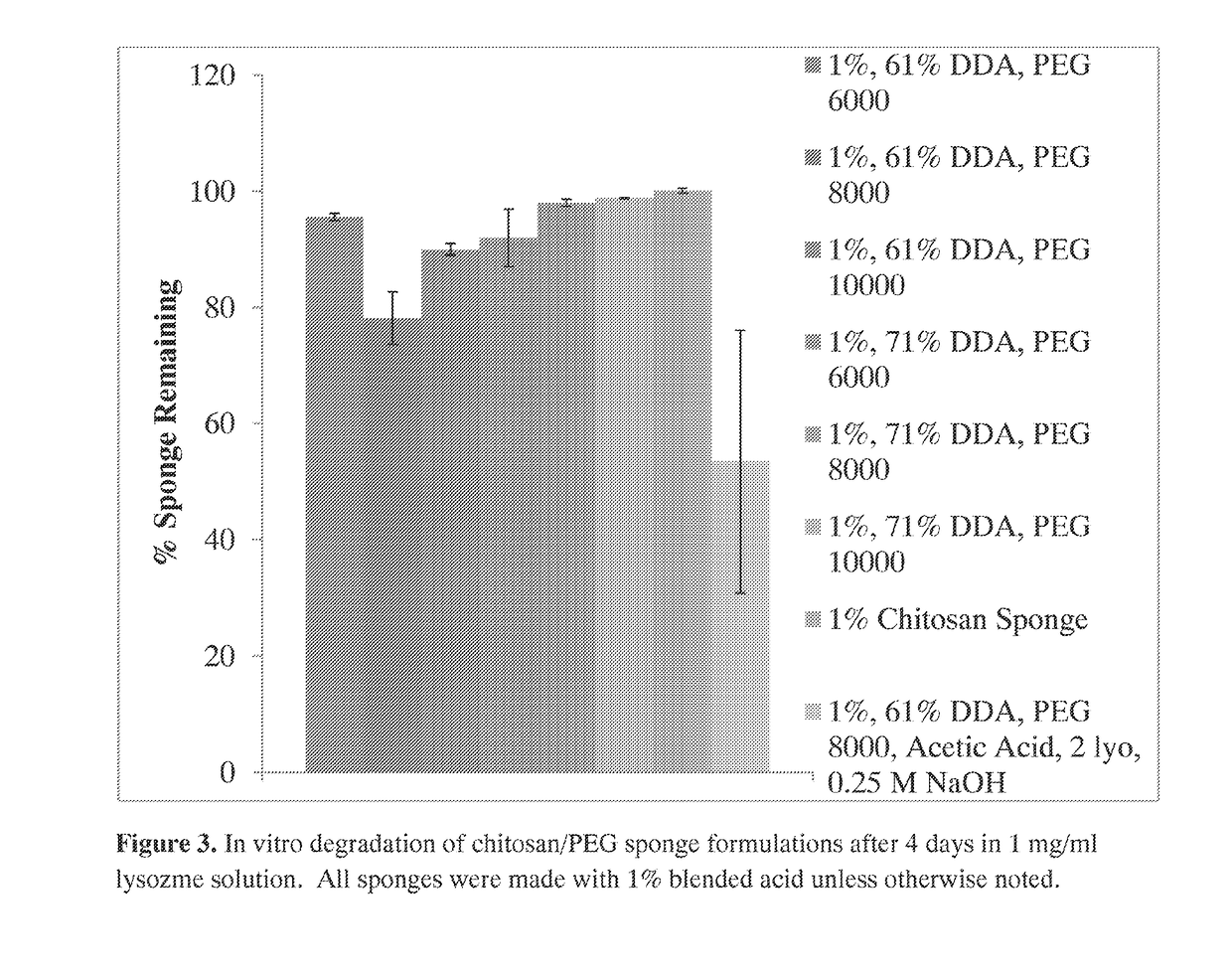
The invention provides compositions featuring chitosan and polyethylene glycol and methods for using such compositions for the local delivery of biologically active agents to an open fracture, complex wound or other site of infection. Advantageously, the chitosan-PEG compositions can be loaded with one or more antimicrobial agents, including hydrophobic agents, and can be tailored to the needs of particular patients at the point of care (e.g., in a surgical suite, clinic, physician's office, or other clinical setting).
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Application of autovaccine preparation containing TGF beta 1
InactiveCN101780272AAvoid it happening againEliminate side effectsGenetic material ingredientsAntiinfectivesAdjuvantRegulatory T cell
The invention discloses application of autovaccine preparation containing TGF beta 1. Adjuvant is added in the autovaccine preparation to prepare medicinal preparation for treating continuous pathogen infection of livestock. The vaccine preparation is fusion protein obtained by the way that part or total of the TGF beta 1 or part or total of mutant or total of similar gene sequences is recombined with at least one helper T cell capable of improving immunogenicity or the gene sequence of carrier protein to construct eukaryon or pronucleus expression plasmid and transform host cell for expression, or is directly prepared by eukaryon expression plasmid obtained by construction. The vaccine preparation can be directly used for preparing medicinal preparation for treating the continuous pathogen infection of livestock, induce domestic animals to generate antibody capable of neutralizing self TGF beta 1, and remove immunodepression function caused by too high level of TGF beta 1 in continuous process of infection; simultaneously, the vaccine preparation can depress the generation of inducible regulatory T cell at the persistent infection part of an organism, thus being beneficial to breaking the immune tolerance state of in-vivo pathogen by the organism and promoting the removing function of the organism on the continuously infectious pathogen.
Owner:HUNAN AGRICULTURAL UNIV
Liposome encapsulated poly-iclc method to prophylactically treat an avian influenza viral infection
InactiveUS20090214638A1Enhancing immunologicalImprove biological activityOrganic active ingredientsAntiviralsInfections siteProphylactic treatment
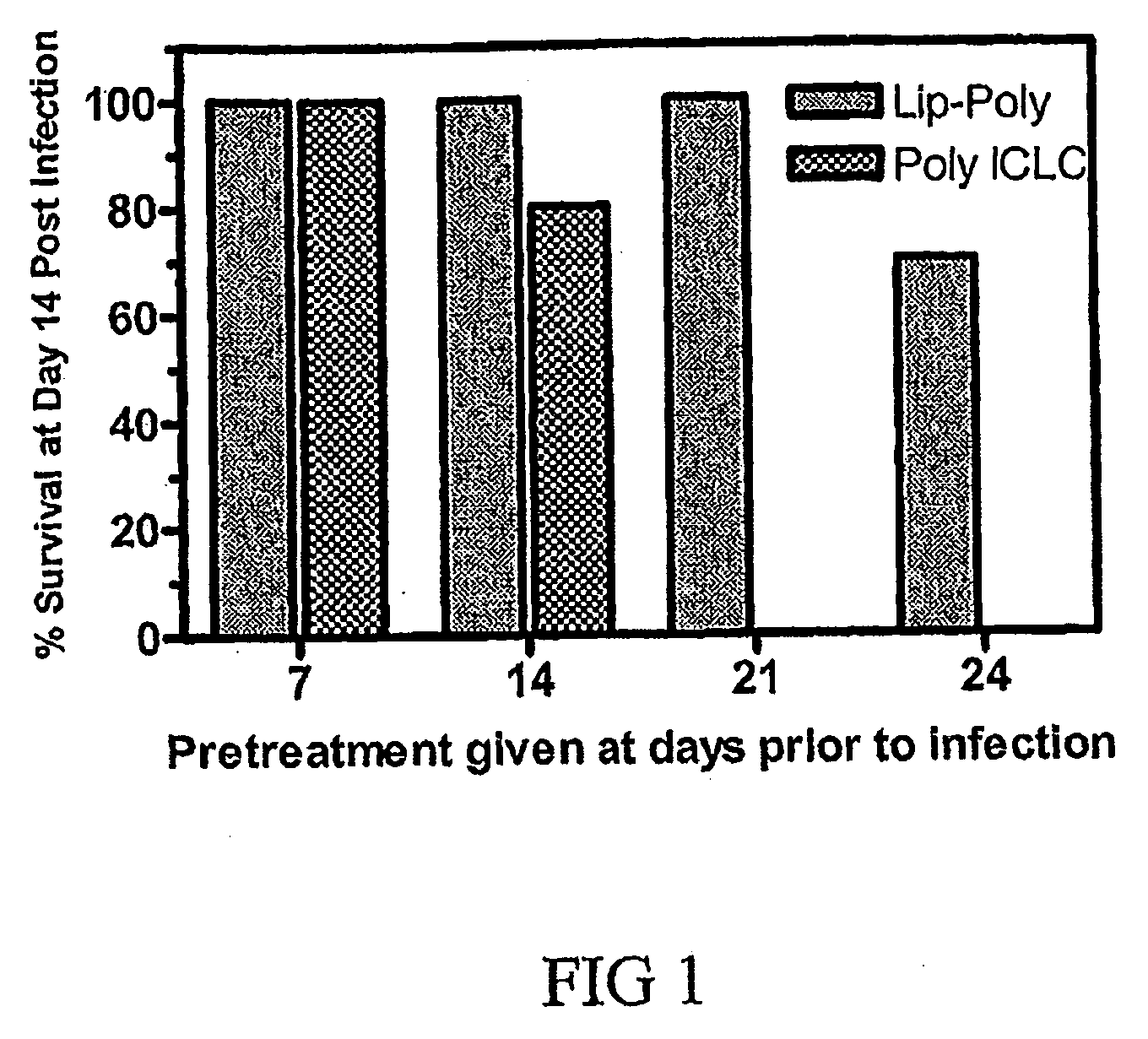
A method of treating an avian influenza viral infection using a poly ICLC formulation with improved therapeutic efficacy is disclosed. The poly ICLC is encapsulated within liposomes which provides a drug delivery system with slow sustained release characteristic and which has the ability to target the drug to sites of viral infection without causing systemic burden to normal tissues, thereby enhancing the immunological and biological activities of poly ICLC. This poly ICLC formulation has been found to be particularly effective in treating an avian influenza viral infection and in particular, an avian H5N1 influenza viral infection.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Device for in situ production and topical administration of allicin
InactiveUS20110027341A1Useful in treatmentAntibacterial agentsAntimycoticsInfections siteTopical treatment
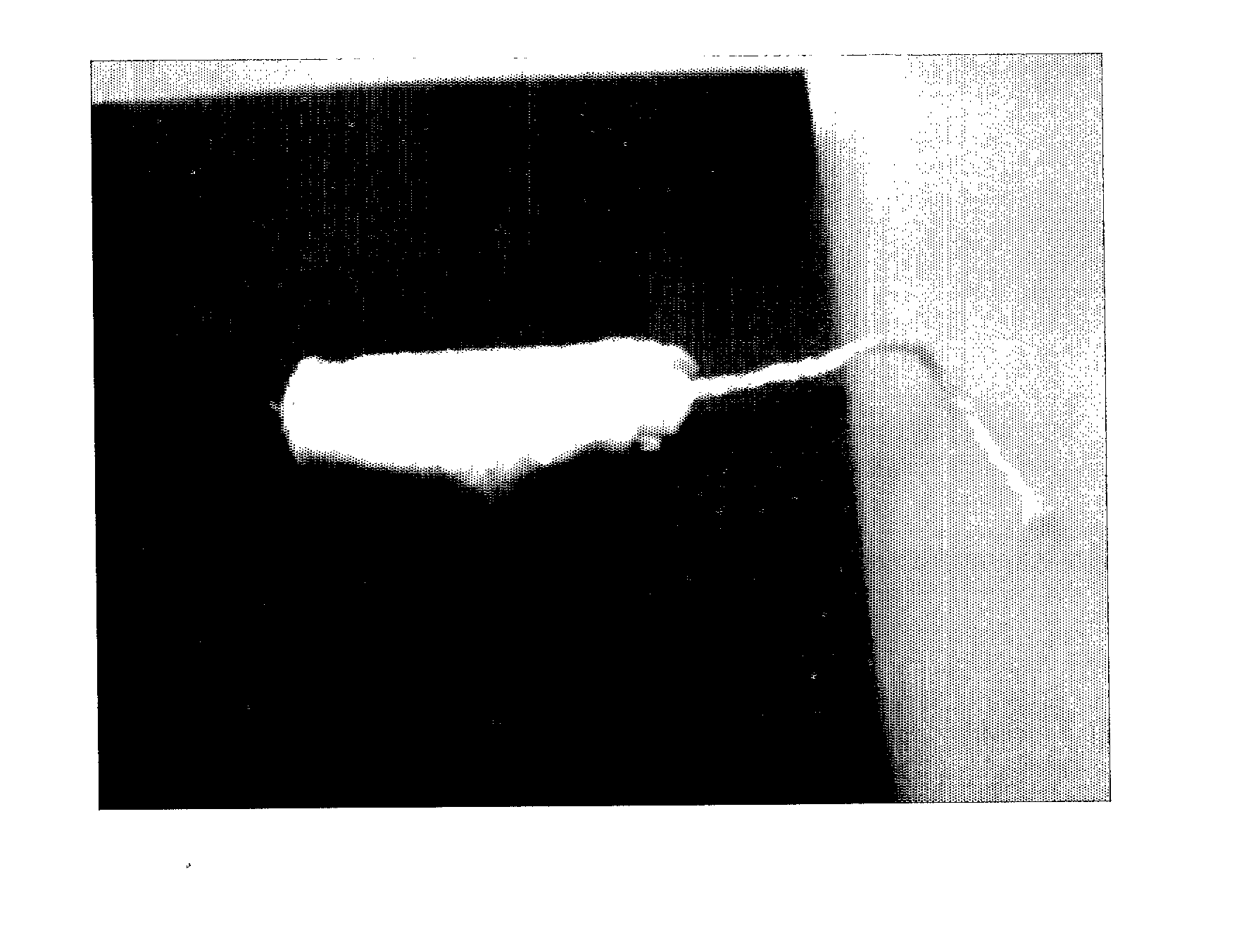
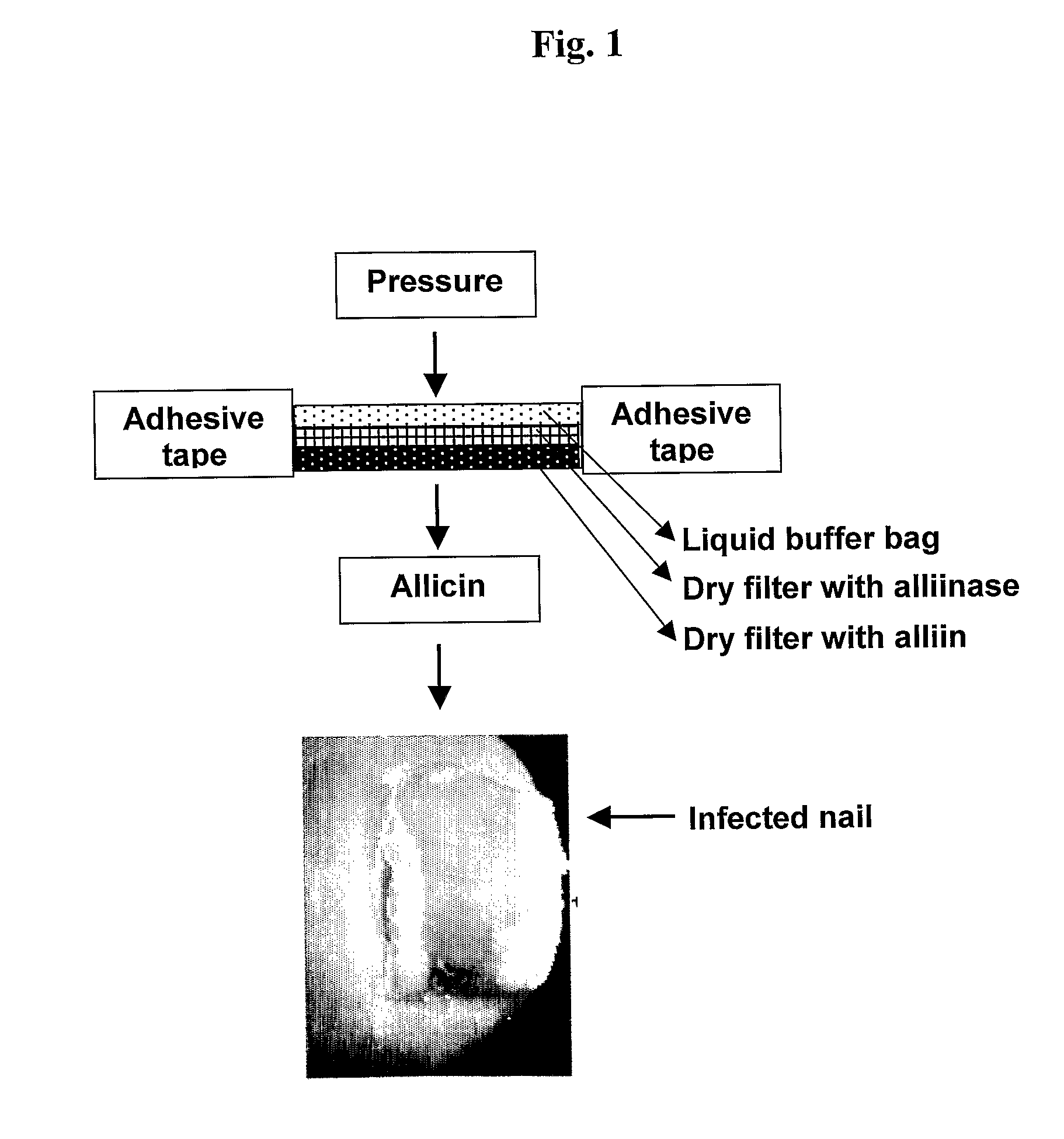
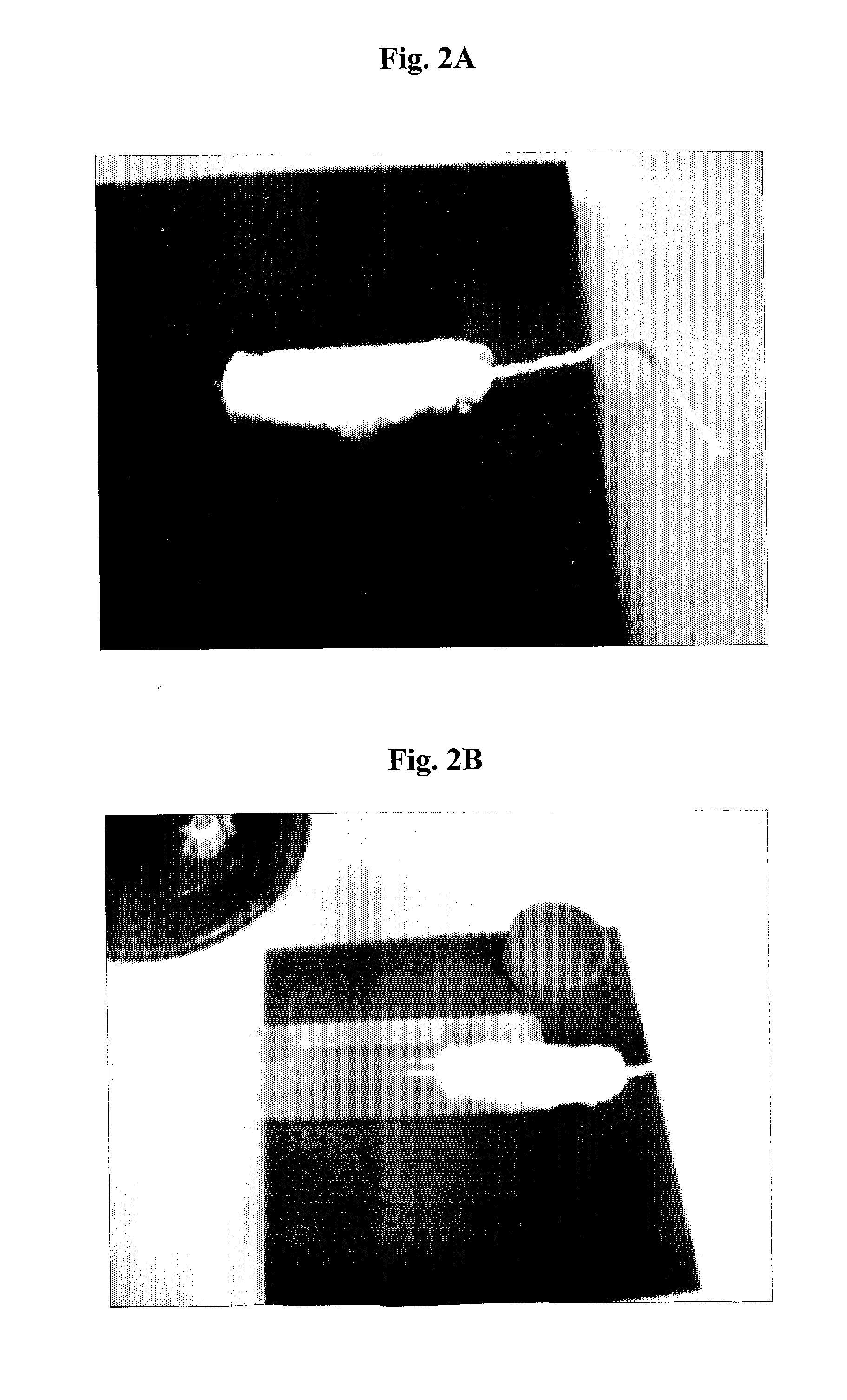
The present invention relates to a drug delivery device that is useful for topical treatment of various infections such as skin and nail, or vaginal infections. More specifically, the invention provides a device for topical administration of allicin to an infection site, comprising either one solid carrier or two adjacent solid carriers, dry alliin and dry alliinase, wherein either a mixture of said dry alliin and dry alliinase is contained within said one solid carrier or dry alliin and dry alliinase are each separately contained within each one of said two adjacent solid carriers, whereby in contact with the infection site and a wetting agent, the alliinase acts on the alliin and allicin is produced in situ and administered to the infection site
Owner:YEDA RES & DEV CO LTD
Wound infection nursing apparatus
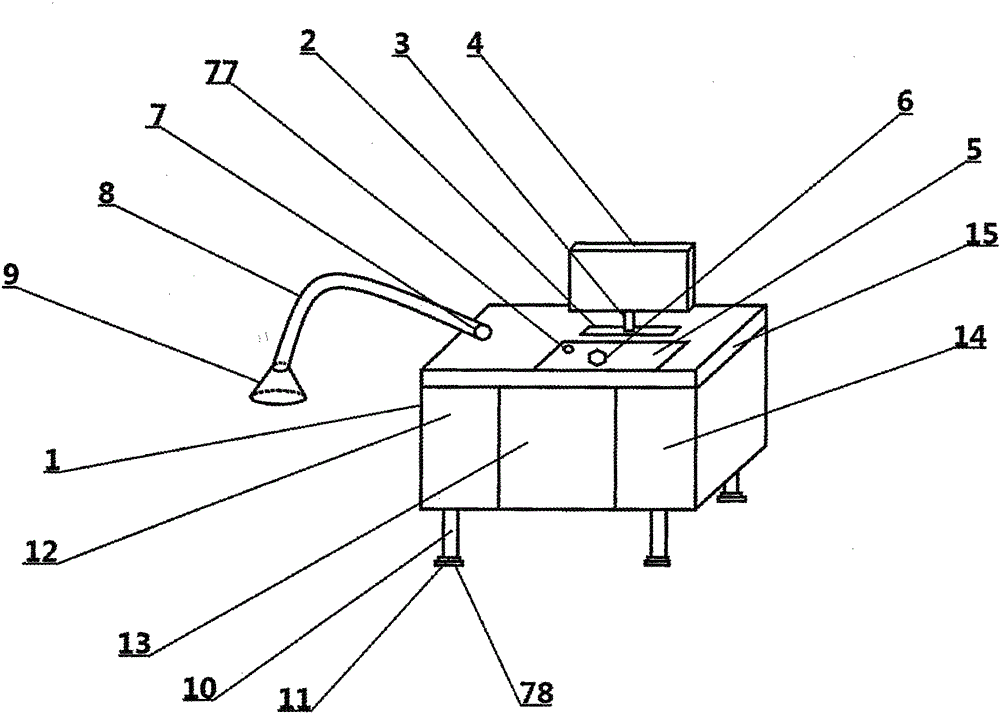
InactiveCN104997583ADetect RecoveryTimely processingNon-surgical orthopedic devicesMedical equipmentInfections site
The invention relates to a wound infection nursing apparatus, and belongs to the technical field of medical equipment. The wound infection nursing apparatus comprises a nursing apparatus main body. A display support frame pedestal is arranged on the nursing apparatus main body; a display support frame is arranged on the display support frame pedestal; a display is arranged on the display support frame; a master control panel is arranged in front of the display support frame pedestal; a master power switch is arranged on the master control panel; a liquid medicine tube port is arranged on the left side of the display support frame pedestal; and an external infusion tube is arranged on the liquid medicine tube port. The wound infection nursing apparatus has complete functions, is convenient to use and flexible to operate when performing wound infection nursing, can effectively detect recovery conditions of infection sites, can timely deal with the infection sites of a patient, and reduces burdens of medical workers.
Owner:黄蕊萍
Compositions and methods for diagnosis and treatment of chronic inflammatory diseases
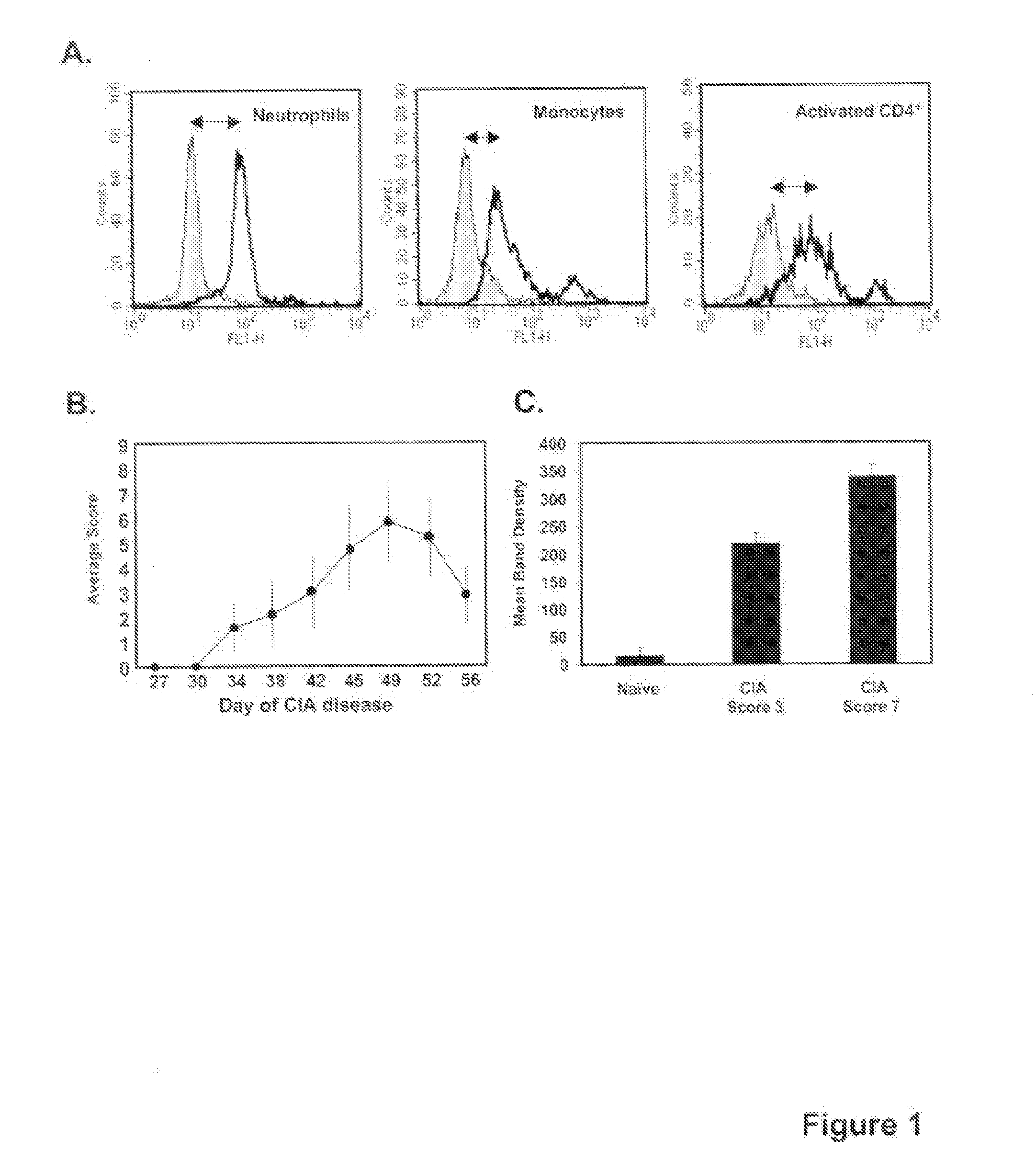
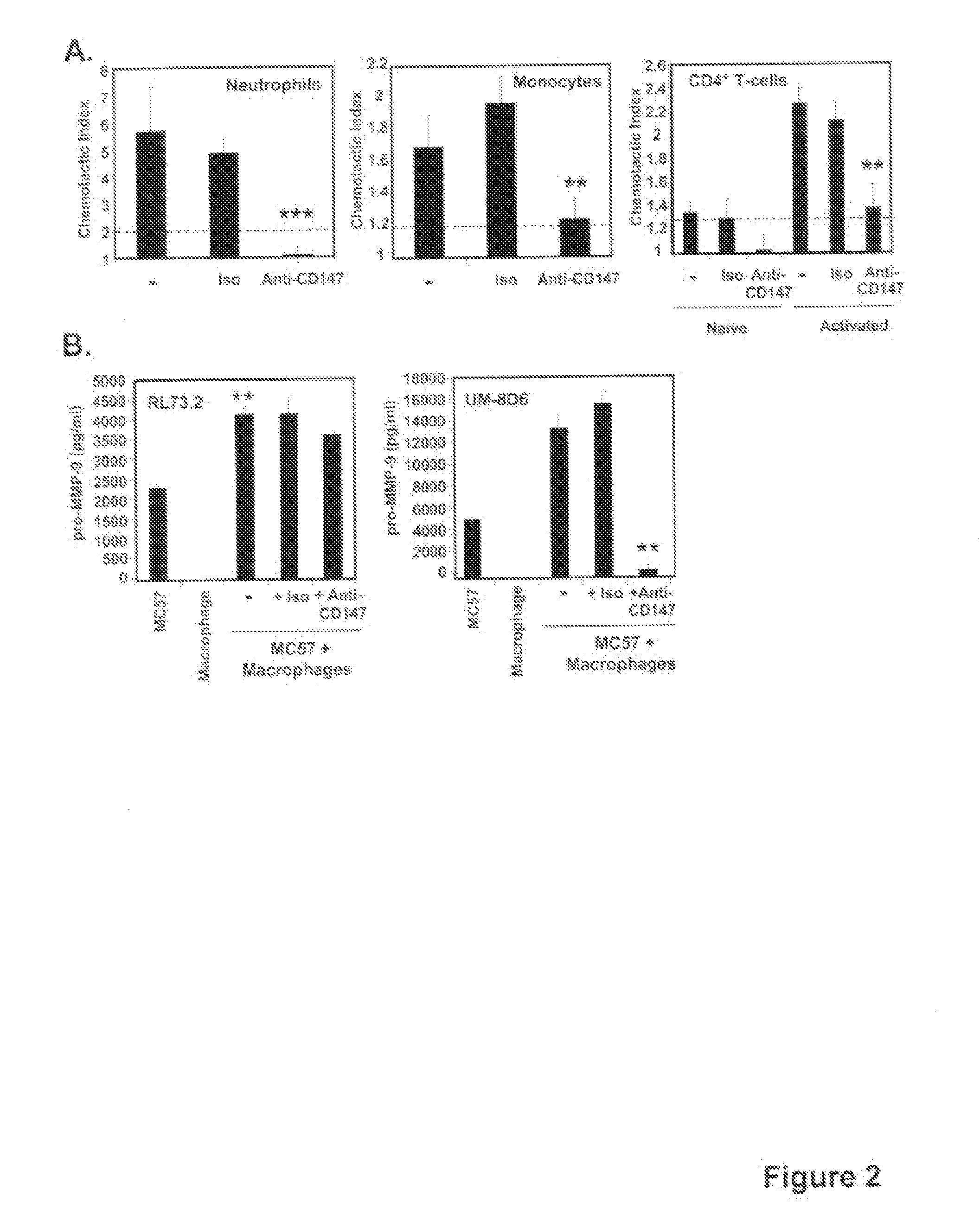
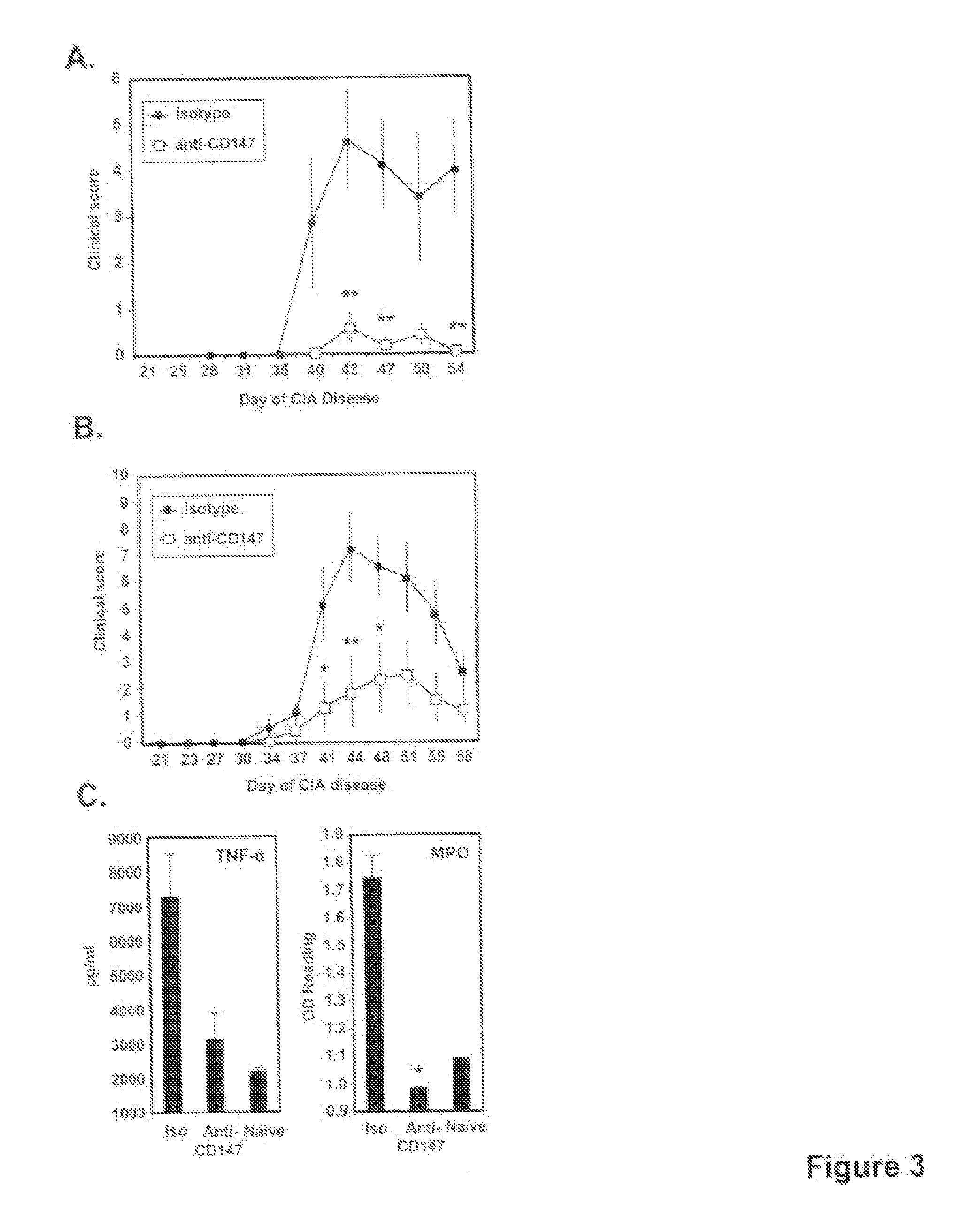
This invention relates to methods and compositions for diagnosis and treatment of chronic inflammatory diseases by blocking CD147 interaction with extracellular cyclophilin. Specifically, the methods and compositions of this invention regulate recruitment of leukocyte to the infection site by specifically blocking the CD147 domain involved with the chemotactic function without blocking the CD147 domain involved with EMMPRIN function.
Owner:THE GEORGE WASHINTON UNIV
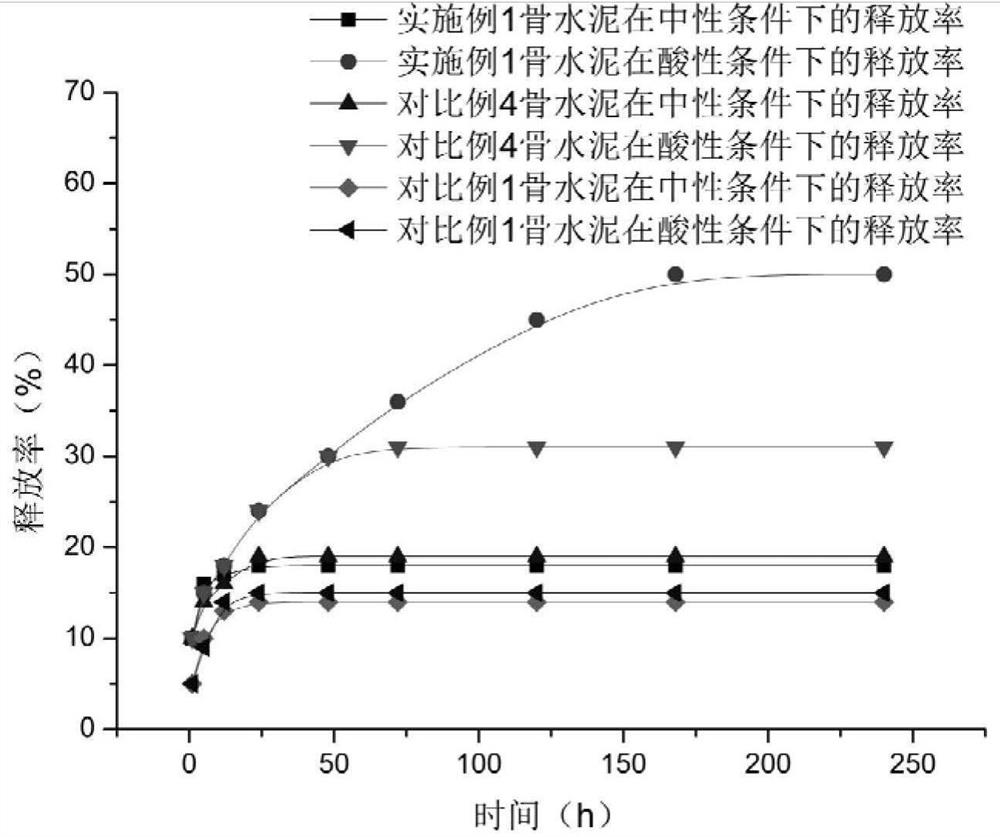
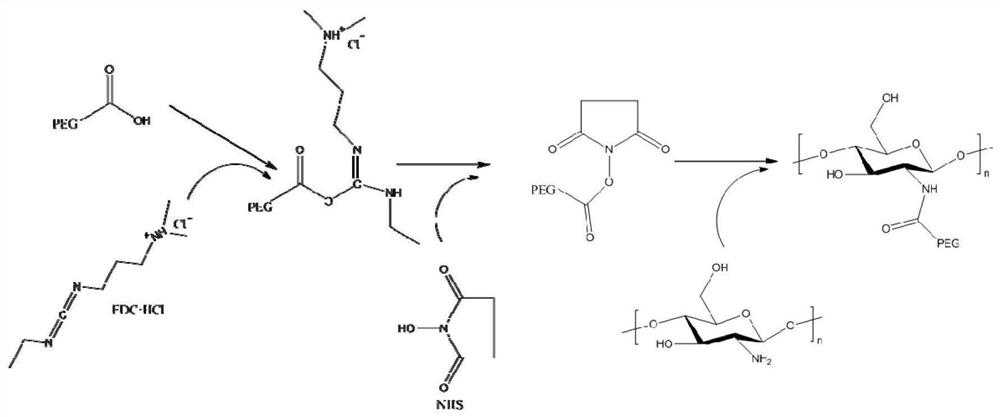
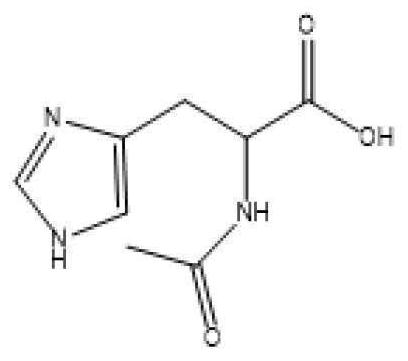
Intelligent polymethylacrylate drug bearing microsphere with pH response, polymethylacrylate and preparation method and use method thereof
ActiveCN111821509AGood antibacterial effectGuaranteed mechanical propertiesTissue regenerationMicrocapsulesMicrosphereInfections site
The invention provides an intelligent polymethylacrylate drug bearing microsphere with pH response, polymethylacrylate and a preparation method and a use method thereof, and relates to the technical field of biomedical materials. The intelligent polymethylacrylate drug bearing microsphere with pH response takes a PEG-chitosan-histidine polymer microsphere as a drug carrier, and the drug includes antibiotics, wherein the antibiotic part is partially embedded inside the PEG-chitosan-histidine polymer microsphere and partially loaded on the surface of the PEG-chitosan-histidine polymer microsphere. The invention solves the problem of sudden release and unsustainable and inefficient release of antibiotics in the antibiotics-loaded polymethylacrylate. In the early stage of implantation, the antibiotics are partially released to play a role of preventing infection. If infection occurs, and becomes serious gradually, the polymethylacrylate continuously releases a large amount of antibiotics at the infection site to achieve the purpose of treating infection. The addition of microspheres not only ensures an antibacterial effect of the polymethylacrylate, but also ensures mechanical properties of the polymethylacrylate within the standard range.
Owner:BEIJING BONSCI TECH CO LTD
Traditional Chinese medicine compound preparation for treating foot-and-mouth disease, and preparation method, usage method and applications thereof
InactiveCN104127697AImprove efficacyClear Orange AntihistamineHydroxy compound active ingredientsClimate change adaptationAdditive ingredientInfections site
The invention relates to a traditional Chinese medicine compound preparation for treating a foot-and-mouth disease, and a preparation method, a usage method and applications thereof, belonging to the technical field of biomedicine. According to the traditional Chinese medicine compound preparation, one or more of cortex dictamni, radix sanguisorbae and fructus cnidii are especially added; the preparation method comprises the following steps: processing various ingredients into medicinal powder according to certain processing technique and mixing, and soaking for seven days with 9-degree acetin; the usage method of the traditional Chinese medicine compound preparation comprises the following steps: smearing the traditional Chinese medicine compound preparation to infection sites of livestock daily for three to four times for two to three days; and the traditional Chinese medicine compound preparation is applied to treat the foot-and-mouth disease, not only can the pharmaceutical effect be improved, but also the price is low, so that the traditional Chinese medicine compound preparation is suitable for popularization and use, as well as good in safety.
Owner:刚宏林
Chronic inflammation and transplantation
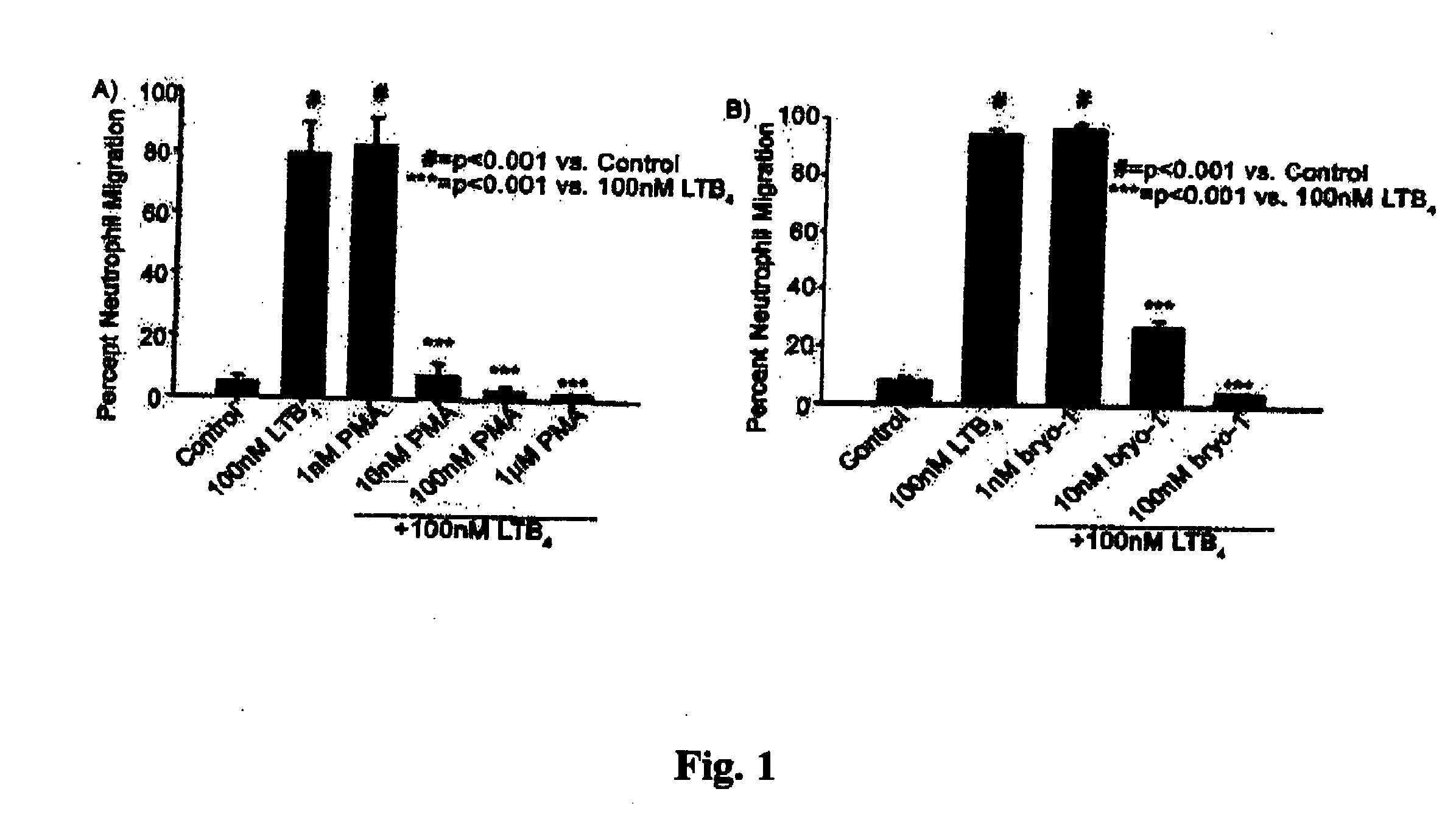
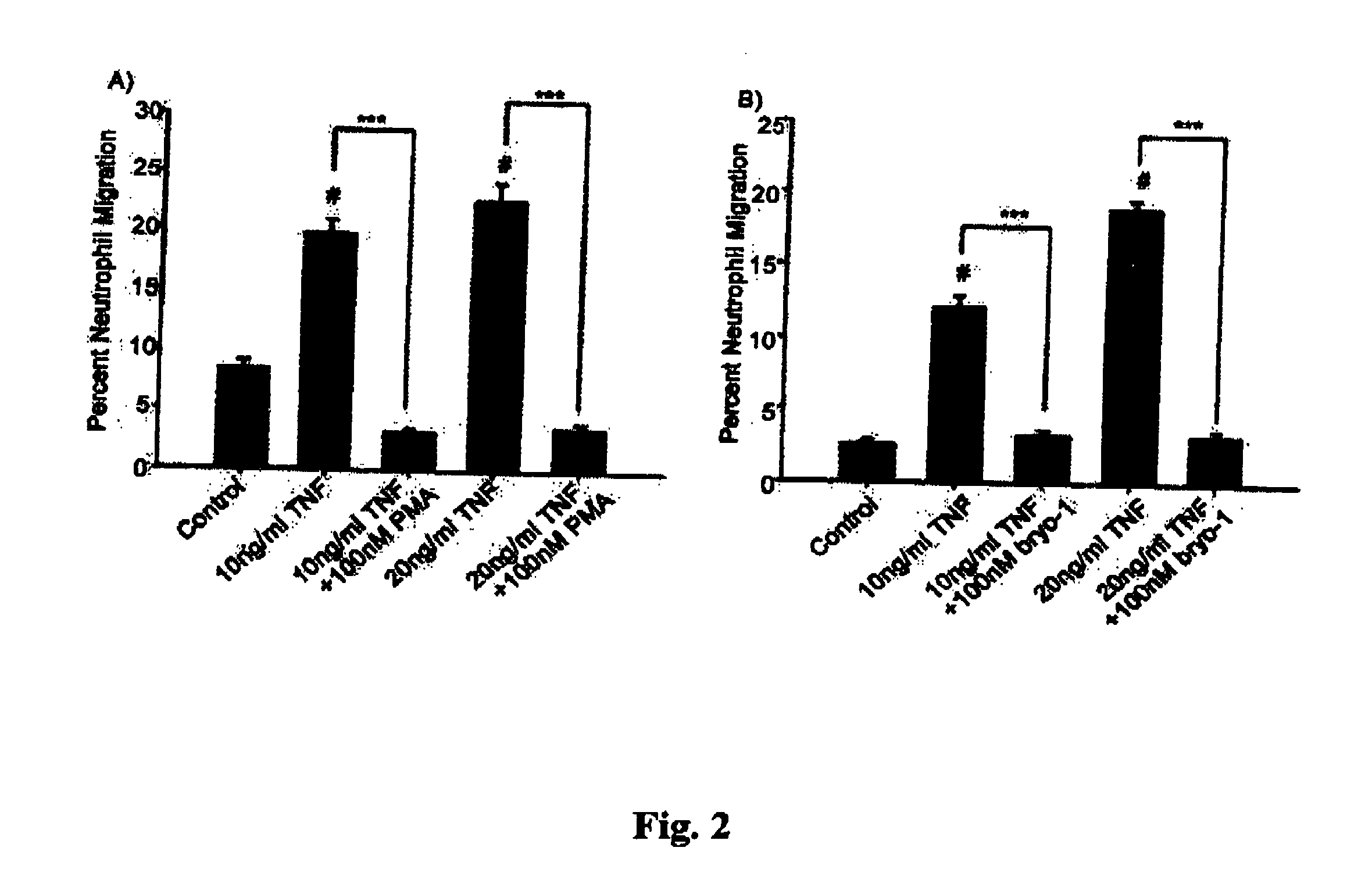
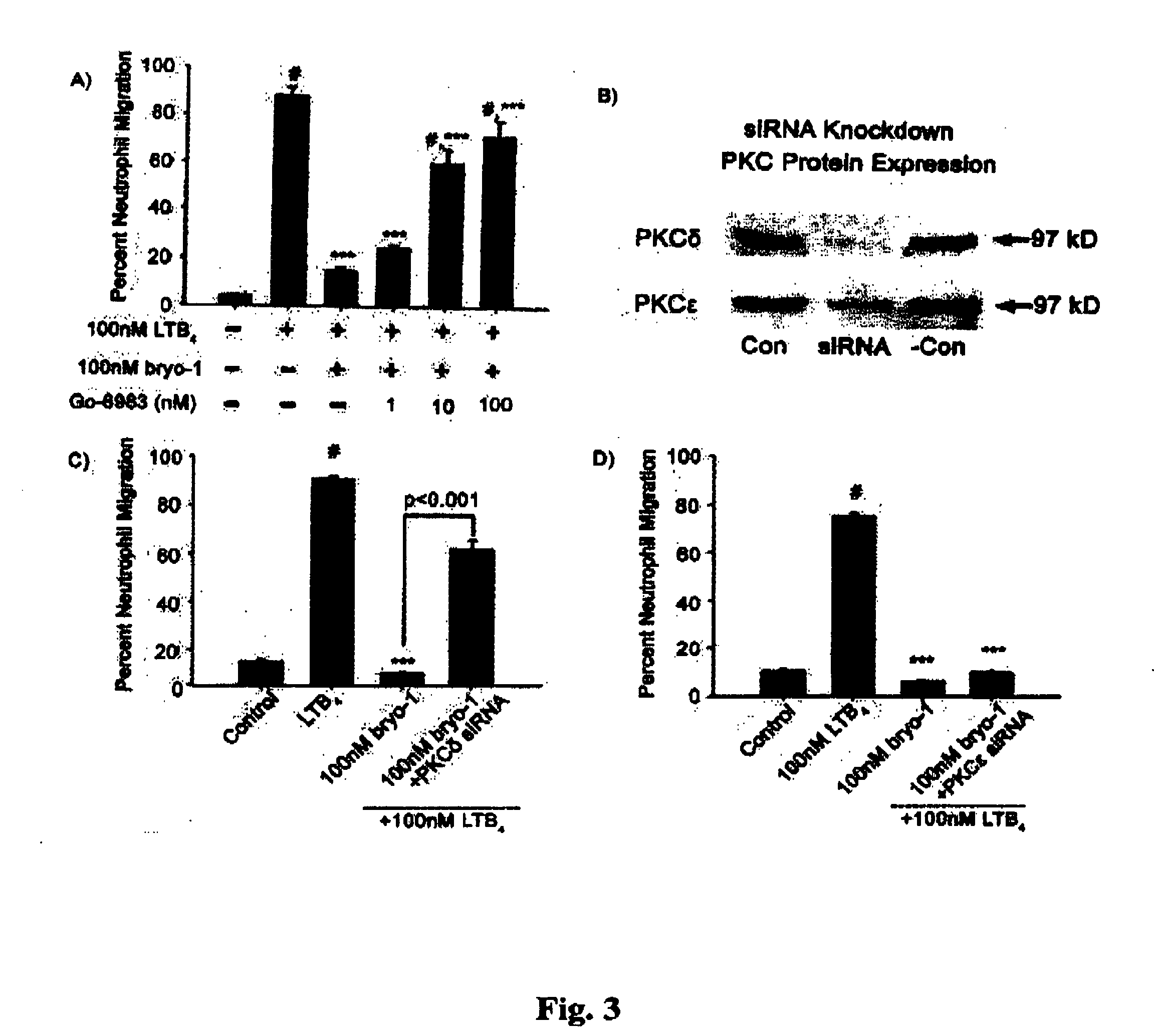
Neutrophils (PMN) can migrate along gradients of chemoattractants across endothelial monolayers to sites of inflammation and infection. This chemotaxis through endothelial cell borders is involved in several acute and chronic inflammatory diseases, however our understanding of the role of endothelial second messengers in the regulation of leukocyte emigration is still incomplete. We investigated this using an in vitro model of neutrophil migration across human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells (HMECs) on cell culture inserts. We report that activation of endothelial protein kinase C (PKC) by both phorbol myristate acetate (PMA) and Bryostatin-1 (a potent PKCδ and c activator) can completely abolish neutrophil migration mediated by both endothelial TNF-α stimulation and a leukotriene B4 (LTB4) gradient. PMA protected against LTB4 induced PMN transmigration for at least 24 hours in HMECs and HUVECs. Bryostatin-1 protected PMN migration for at least 24 hours in HMECs and at least 48 hours in HUVECs. Pretreatment with Go-6983 (PKCα, β, and δ inhibitor) before the addition of Bryostatin-1 restored the loss of LTB4 induced neutrophil migration, while pretreatment with GO-6976 (PKCα and β inhibitor) did not. In addition using PKCδ and ε specific small interfering RNA, we were able to show that PKCδ, but not ε was at least mostly responsible for the loss of neutrophil migration in response to LTB4. Taken together, these observations suggest that activation of endothelial PKCδ could be therapeutic in the treatment of various inflammatory disorders characterized by enhanced neutrophil infiltration.This invention relates to pharmaceutical compositions, particularly pharmaceutical compositions comprising bryostatin-1 and substituted derivatives of bryostatin-1, thereof as pharmaceuticals for inhibition of inflammation, and for use in combating arteriosclerosis, diseases of the cardiovascular system, of the central nervous system and prior to / following organ transplantation, ischemia. The invention relates to methods for treating leukocyte dependent injury in chronic inflammatory diseases, and injury from transplantation mediated organ stress. The method involves injecting bryostatin-1 into patients with the inflammatory condition, treating the skin with bryostatin-1, or perfusing organs with bryostatin-1 prior to transplantation / cold storage. Activation of protein kinase Cd (PKCd) results in a near complete blockade of leukocyte infiltration which is the result of stabilization of the microvascular (endothelial) barrier.
Owner:LOUSNA STATE UNIV & AGRI & MECHANICAL COLLEGE
Molecular markers for five important pathogens and application thereof
InactiveCN106047993AAccurate identificationHigh resolutionMicrobiological testing/measurementAgainst vector-borne diseasesNucleotideYersinia pestis
The invention discloses a method for using nucleotide DNA fragments as molecular markers for identification of five important pathogens, i.e., Yersinia pestis, Plasmodium falciparum, Vibrio cholera, Leishmania donovani and Trypanosoma brucei. The nucleotide DNA fragments can be used as effective genetic markers for rapid and accurate identification of pathogens, are beneficial for accurate and rapid discrimination of populations migrated from an original infection site and provide bases for molecular epidemiological study of pathogens.
Owner:SHANGHAI QY BIOTECH CO LTD
Method for treating otic infections after tympanostomy tube placement
InactiveUS20150045739A1Fast timeEffective treatmentAntibacterial agentsOrganic active ingredientsInfections siteIntensive care medicine
Owner:NOVARTIS AG
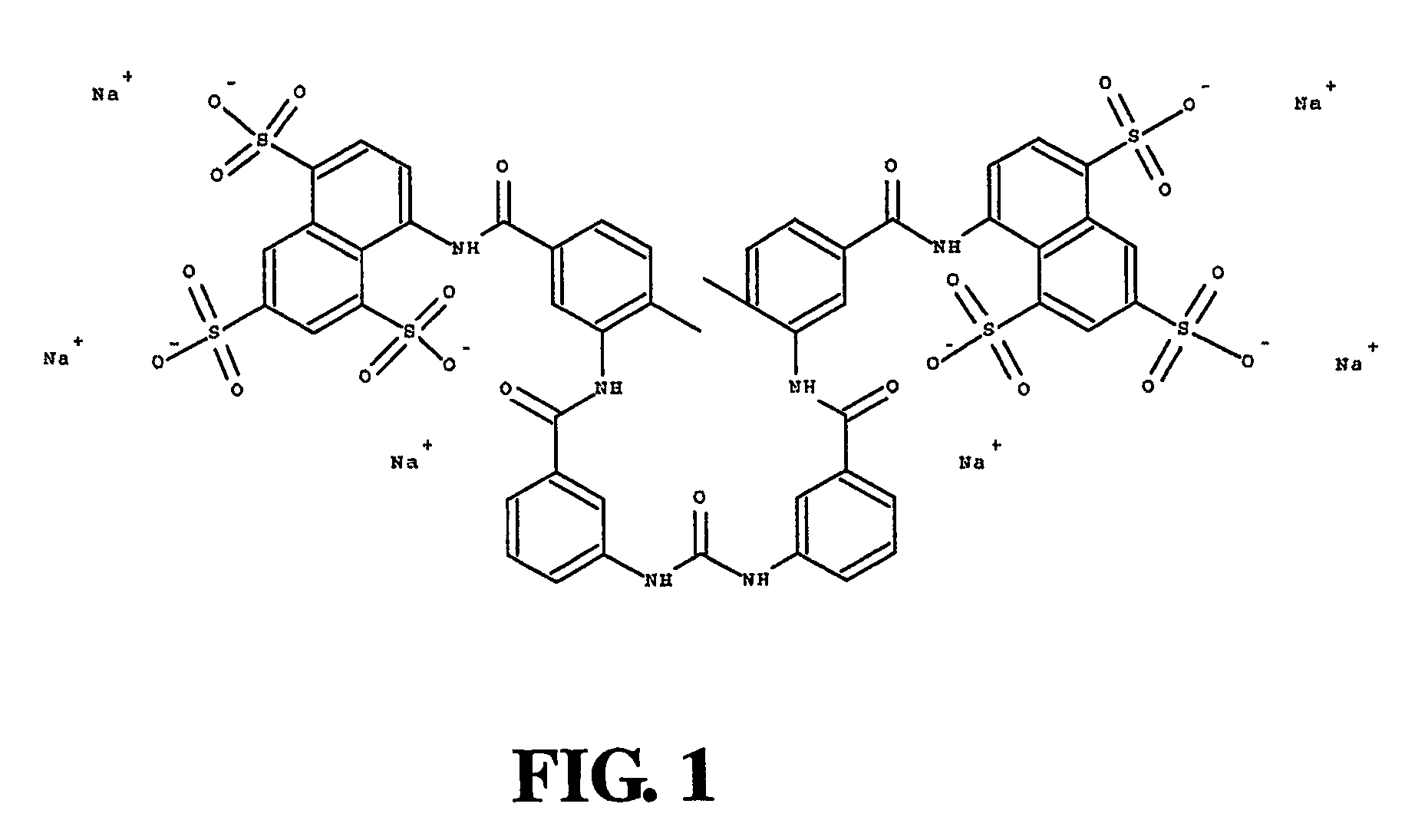
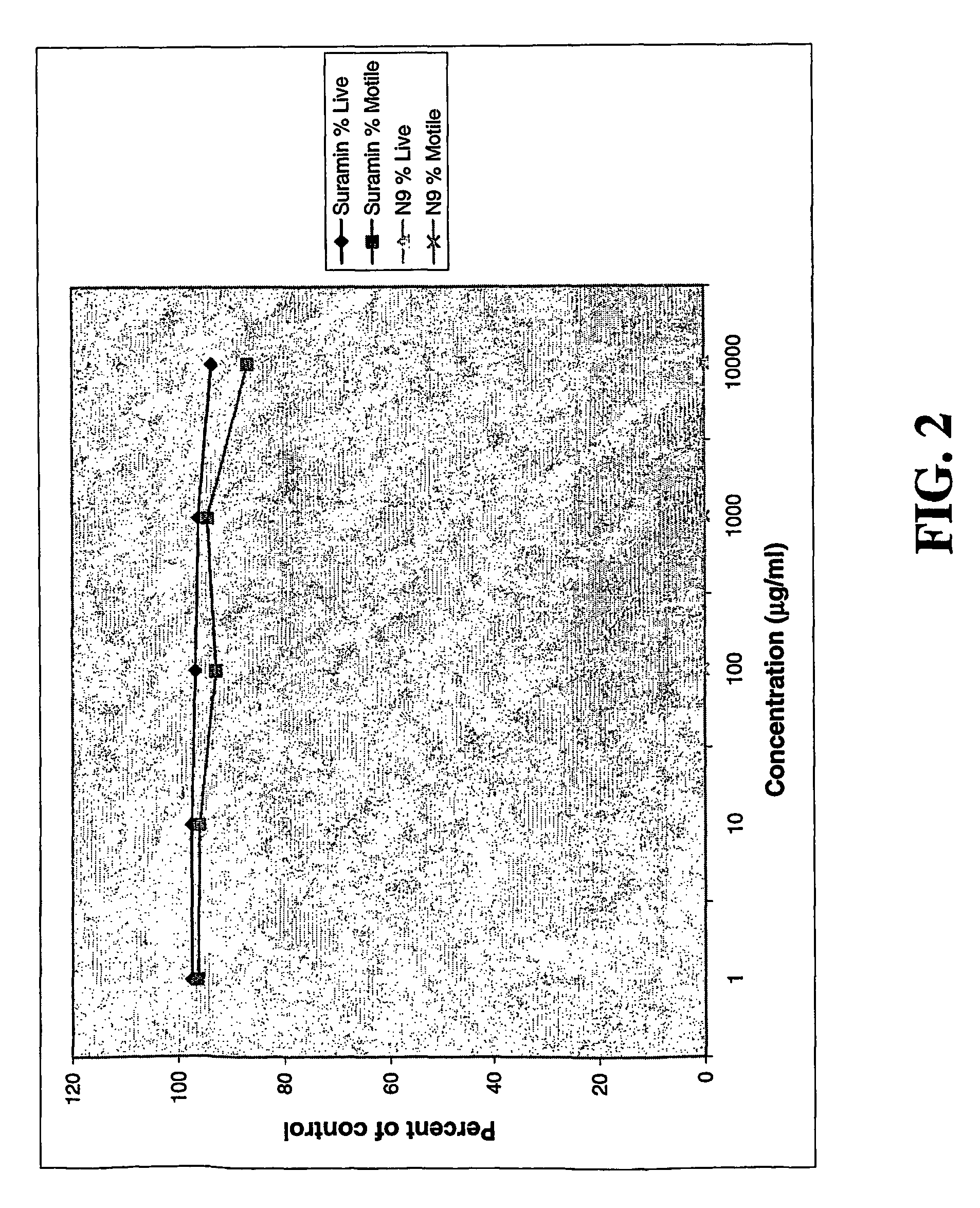
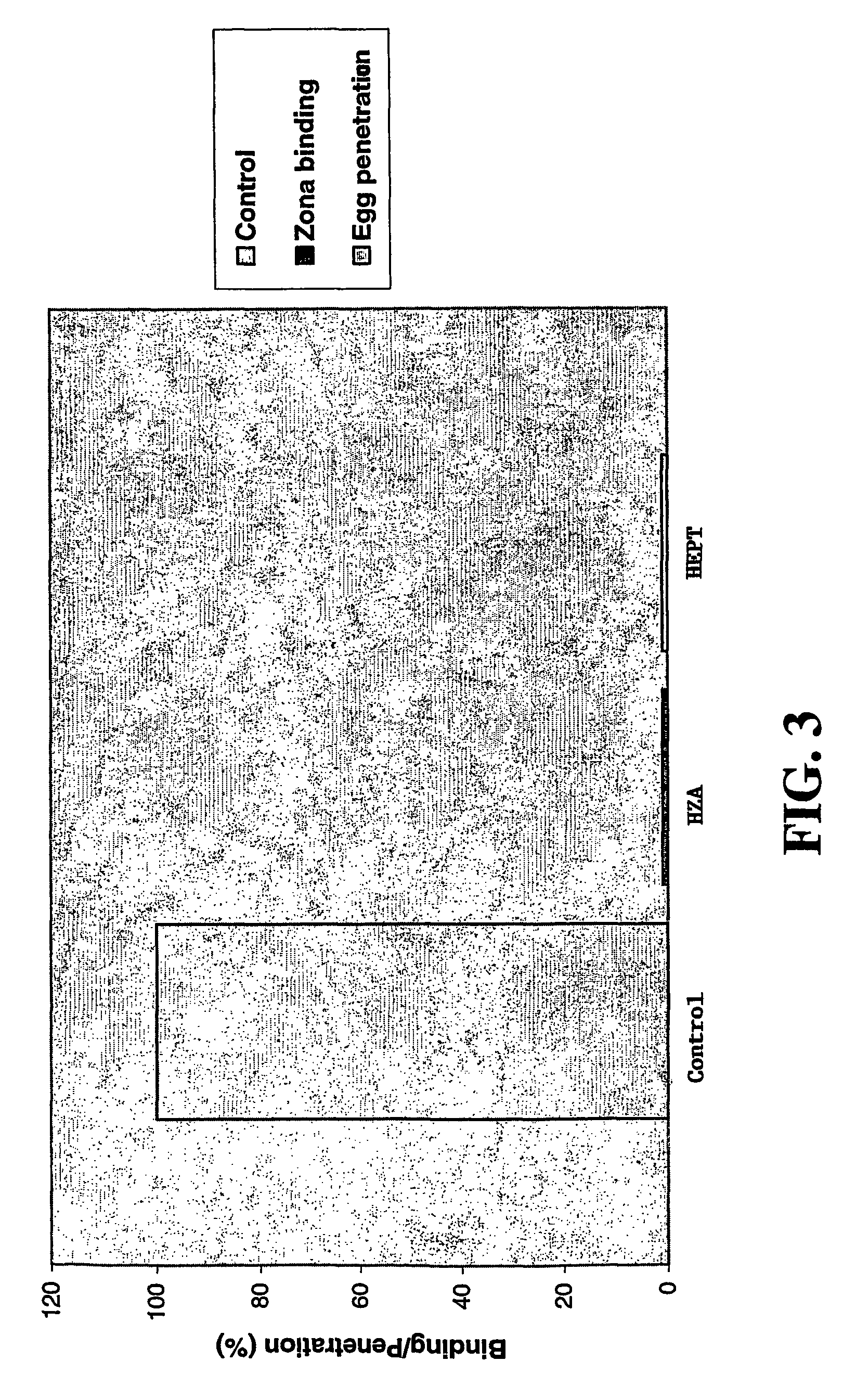
Suramin and derivatives thereof as topical microbicide and contraceptive
The present invention is directed to methods of inhibiting STDs by topically administering suramin or a derivative thereof to actual or potential sites of infection, and methods of preventing pregnancy by topically applying suramin or a derivative thereof intravaginally. Suramin compositions that include an antimicrobial agent and / or a sperm-function inhibitor are also provided and may advantageously be used in the methods of the invention. A method of simultaneously inhibiting STDs and preventing pregnancy is also provided. Devices impregnated or coated with the topical suramin compositions are further disclosed and may be used to apply the compositions described herein.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
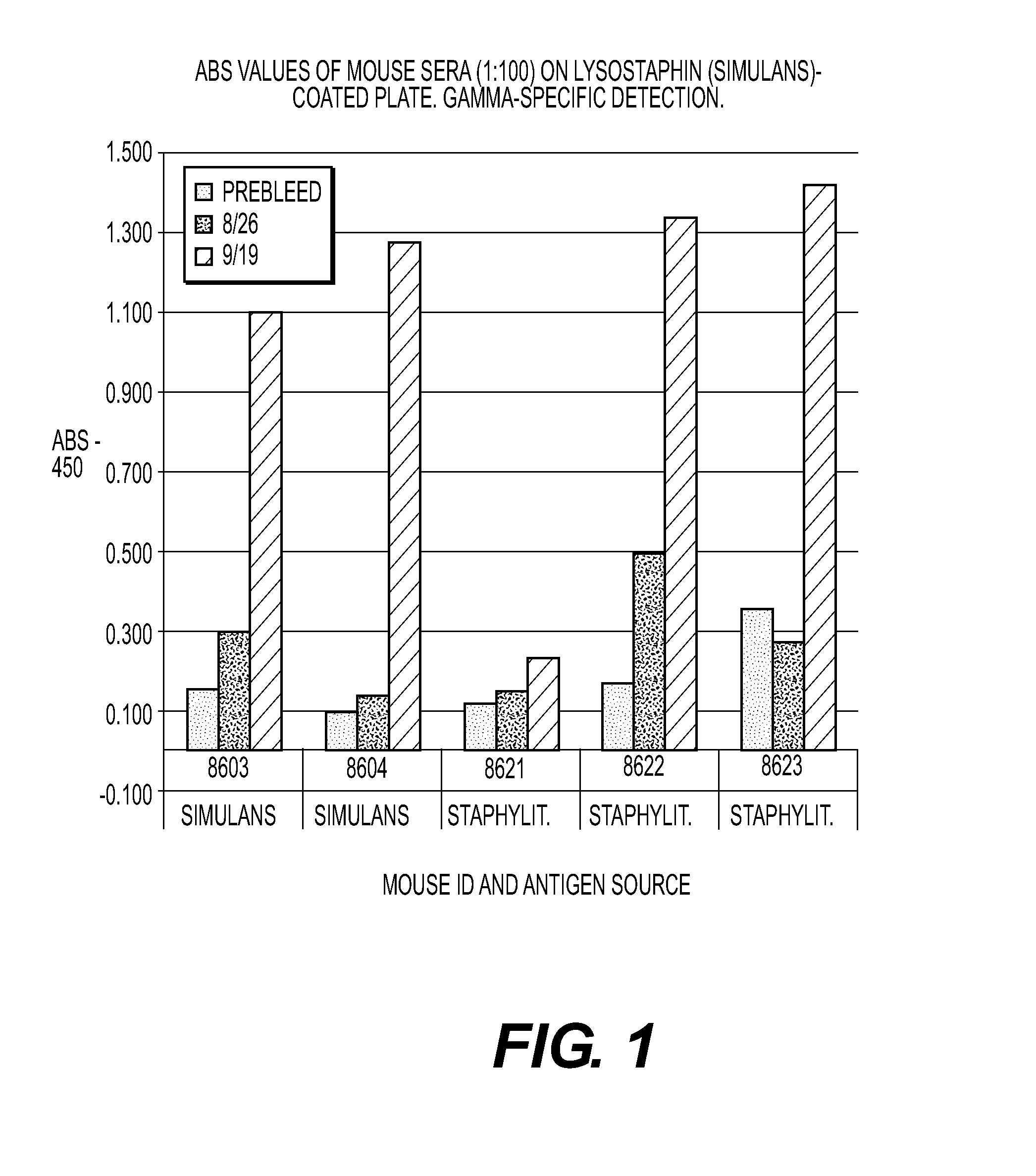
Multimodal Antimicrobial Therapy
ActiveUS20150182606A1Significant positive effectReduce negative impactAntibacterial agentsPowder deliveryDiseaseInfections site
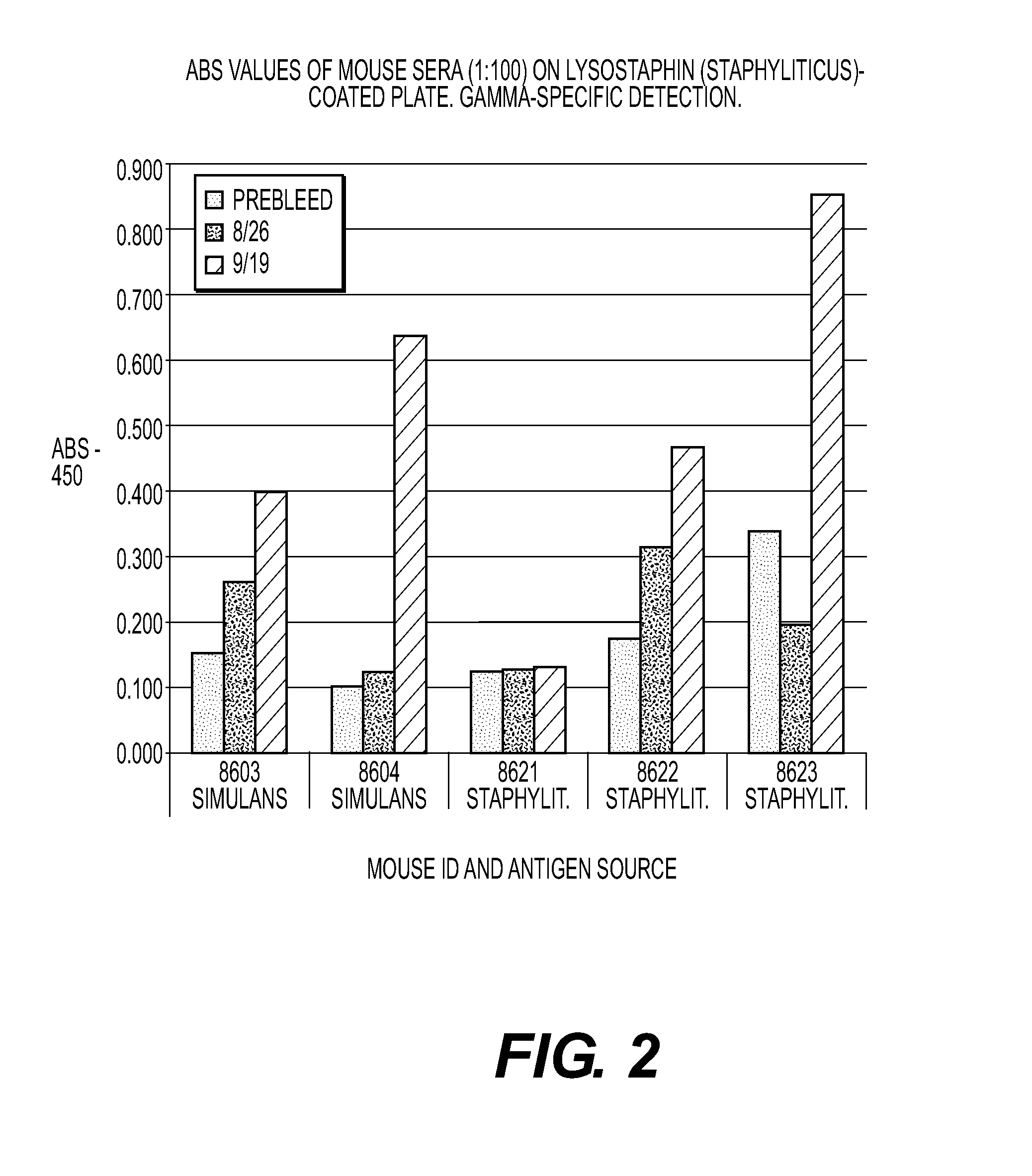
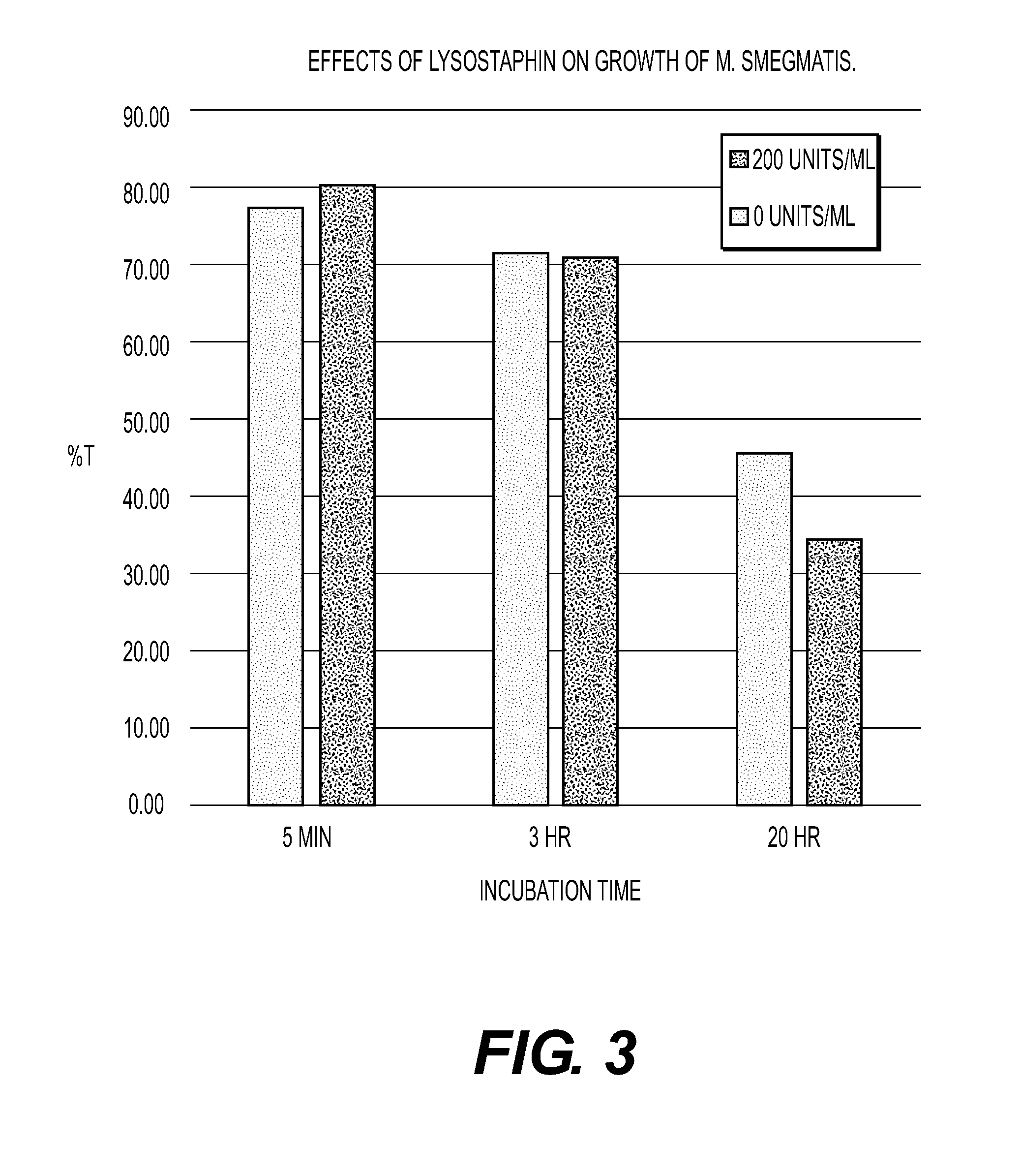
The present invention is directed to compositions and methods for preventing and / or treating diseases and disorders of patients caused by non-Staphylococcal microorganisms. In particular, compositions and methods contain lysostaphin, altered forms of lysostaphin as compared to wild-type, and synergistic combinations of lysostaphin plus additional conventional treatments such as other enzyme, antibiotic and / or antibody treatment. The invention is also directed to detecting and identifying altered forms of lysostaphin that possess increased efficacy against infections as compared to wild-type lysostaphin, and forms that generate a minimal or no immune response in a patient. The invention is also directed to method of manufacturing lysostaphin and altered forms of lysostaphin, and compositions that direct the lysostaphin to the site of the infection such as aerosolized nanoparticles.
Owner:LONGHORN VACCINES & DIAGNOSTICS LLC
Treatment of inflammatory and/or bacterial conditions with particles of microstructure
InactiveUS20100104648A1Efficient use ofSmall sizeAntibacterial agentsHeavy metal active ingredientsOsteitisInfections site
The present invention refers to a method for treatment of an inflammatory and / or bacterial condition, wherein particles of microstructure comprising titanium, titanium alloy, at least one titanium oxide or a combination thereof, and having a surface with at least a substantial part consisting of at least one type of titanium oxide, are brought into contact with at least one infected site in a human or animal body by insertion, injection or implantation, which at least one infected site exhibits the inflammatory and / or bacterial condition. Moreover, the present invention refers to an injectable suspension comprising the particles according to the invention and a fluid vehicle for use as a medicament. Finally, the present invention also defines use of the particles of microstructure according to the invention for the manufacture of a medicament in the form of an injectable suspension. Examples of conditions being treated with the injectable suspension according to the present invention are periodontitis, periimplantitis, and osteitis.
Owner:TIGRAN TECH AB (PUBL)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com