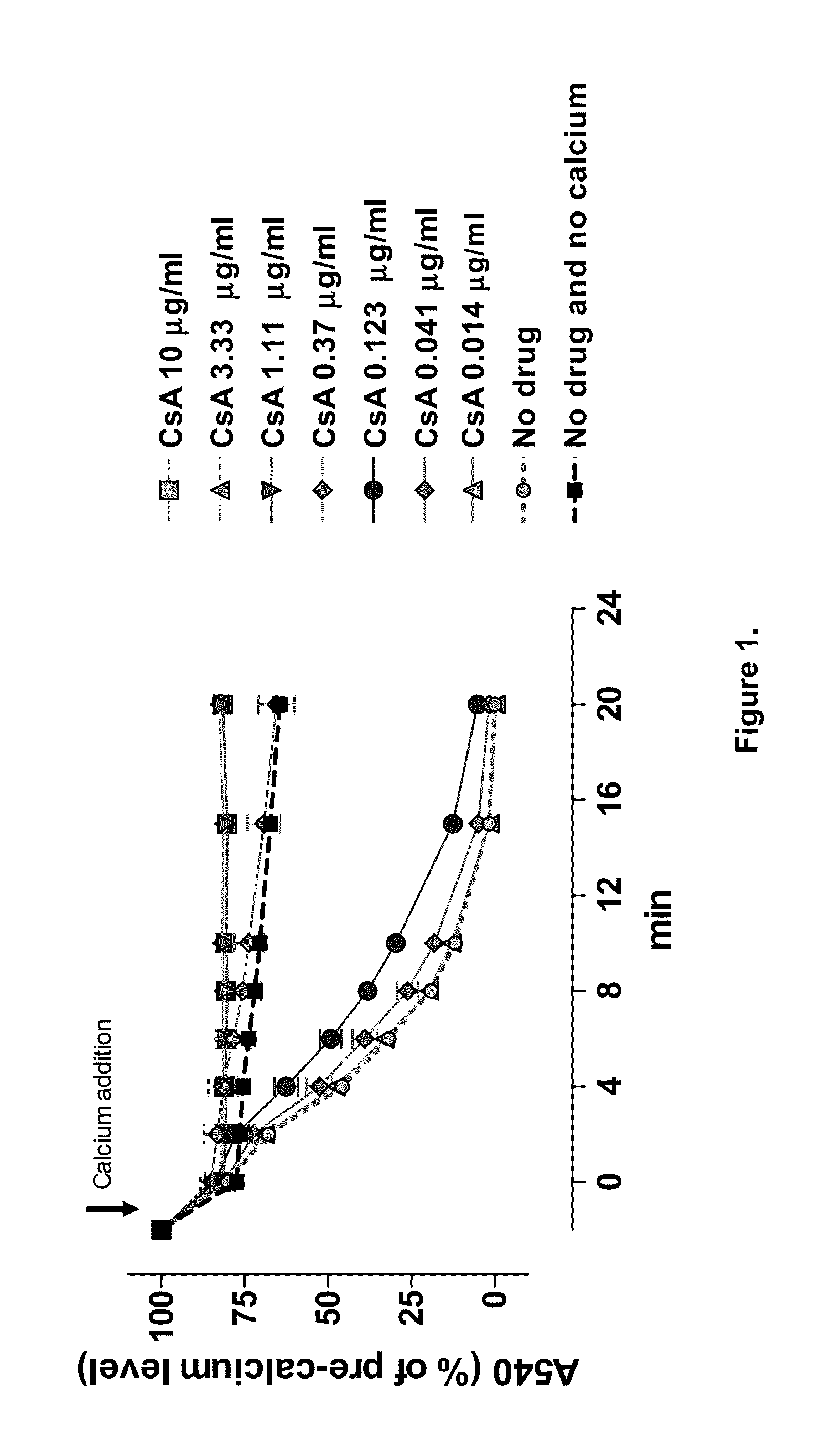
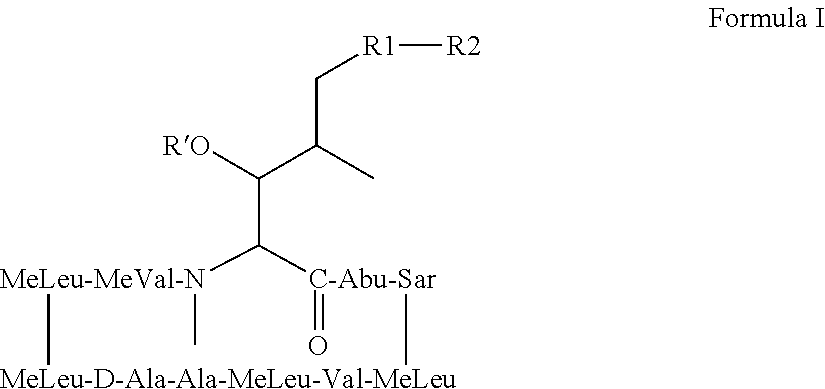
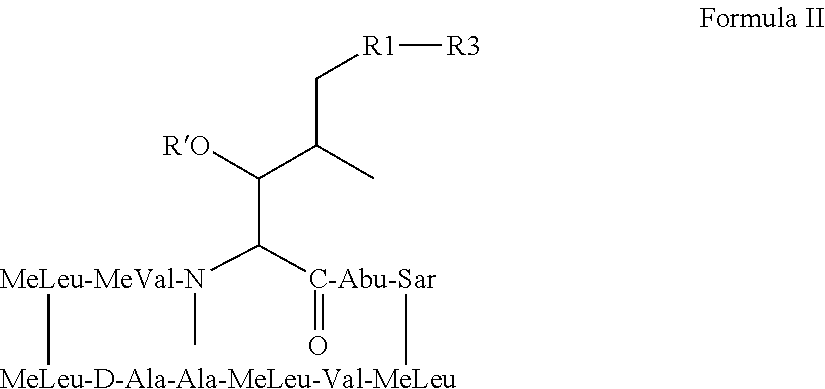
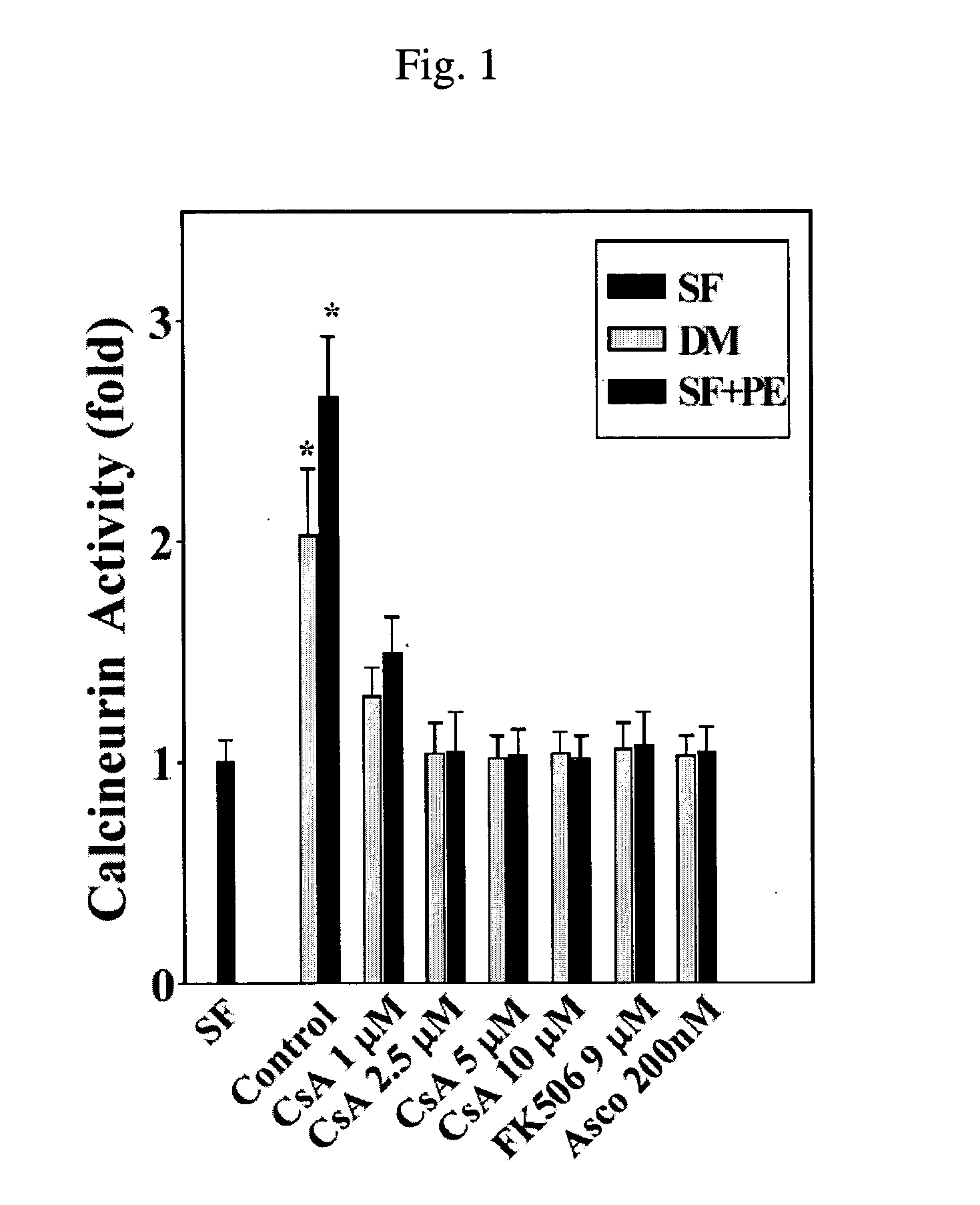
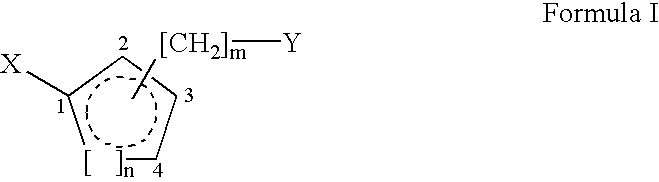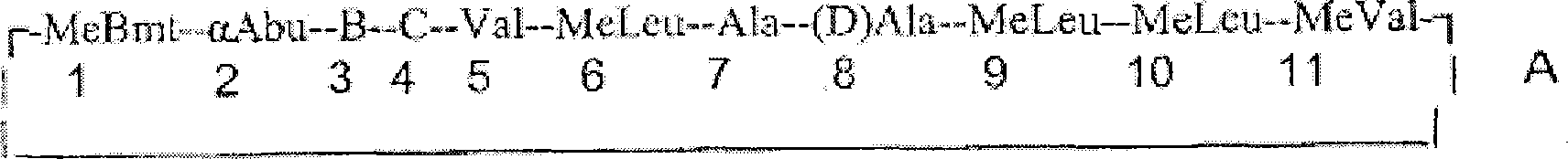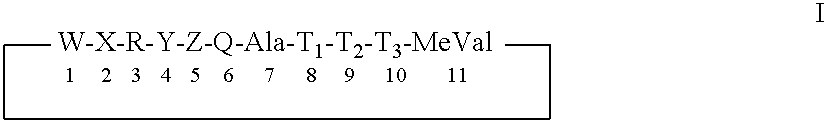Patents
Literature
78 results about "Cyclophilin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
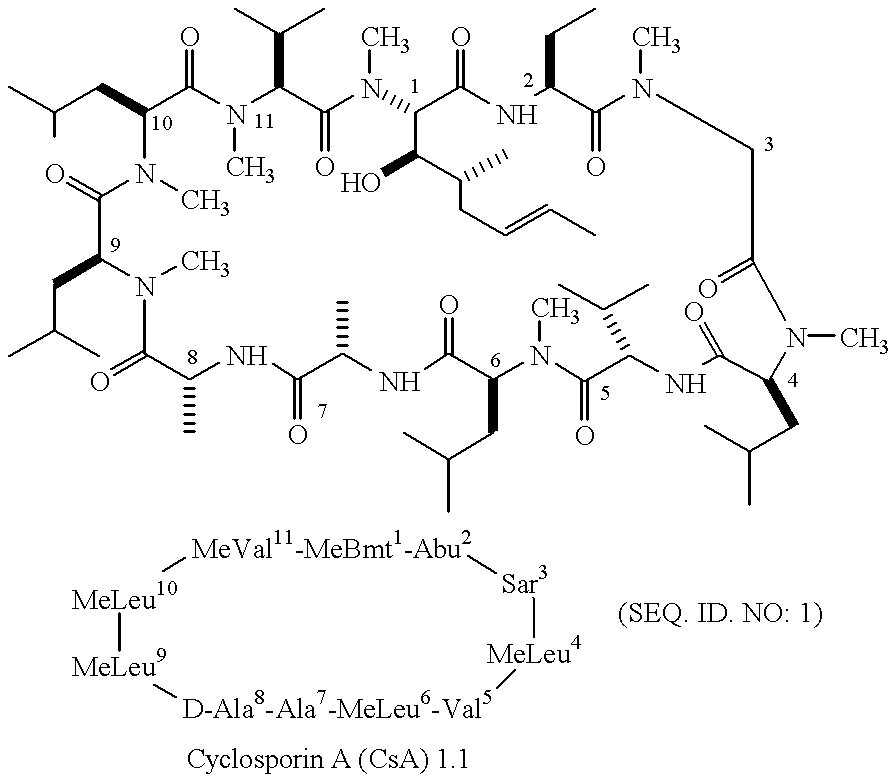
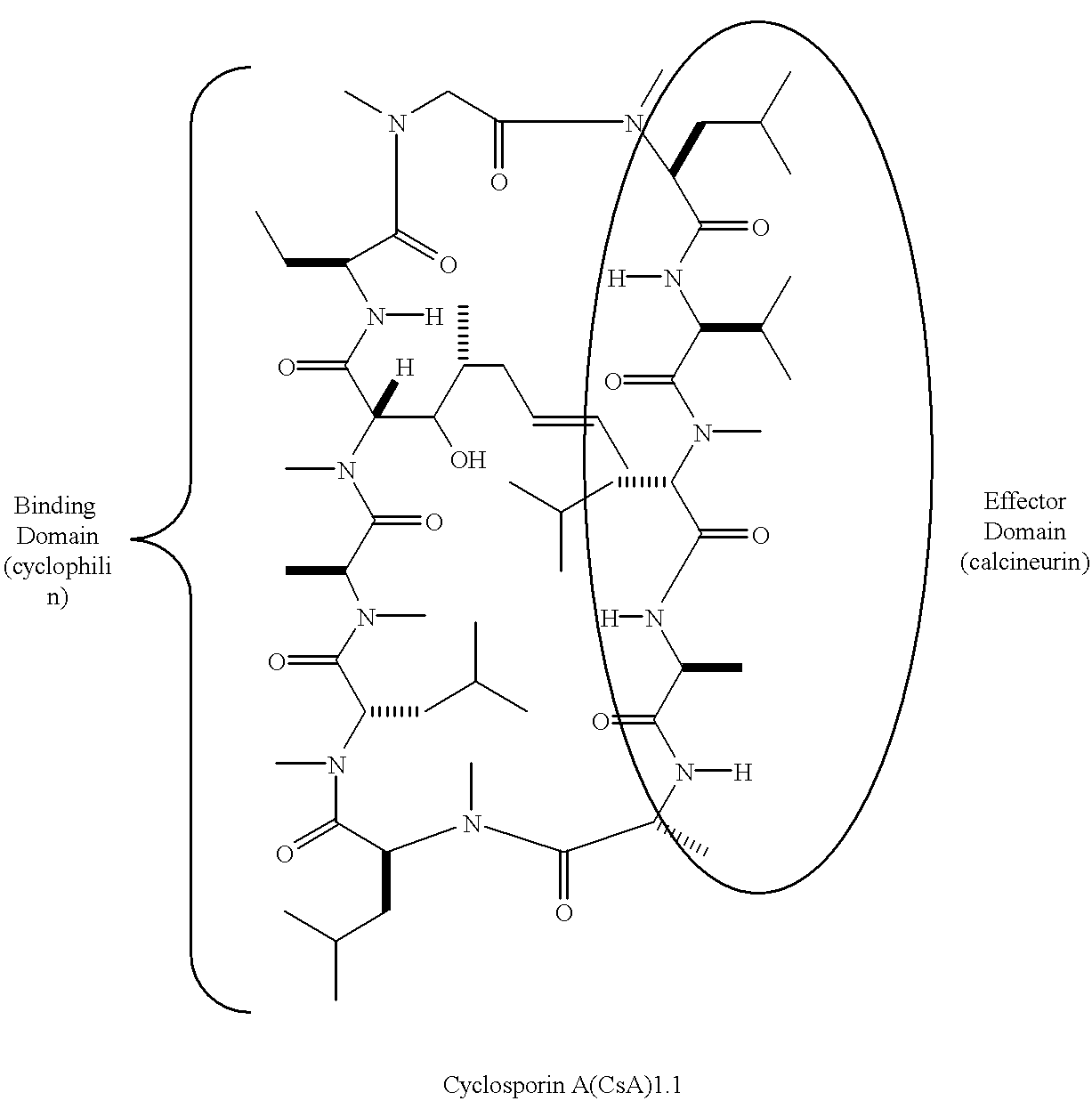
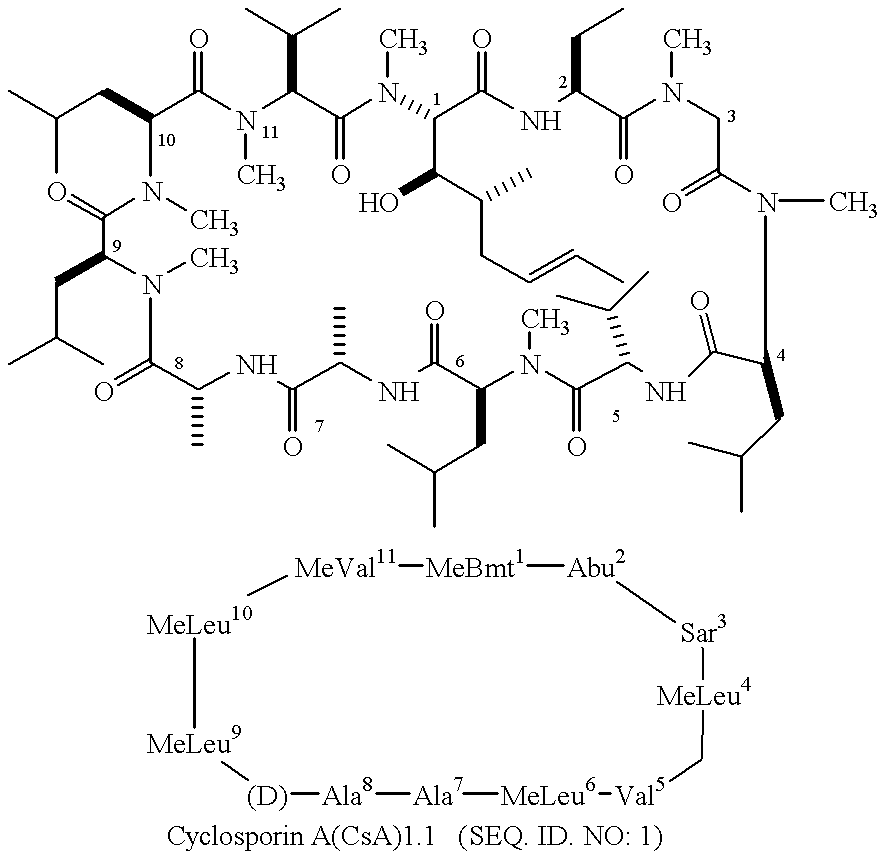
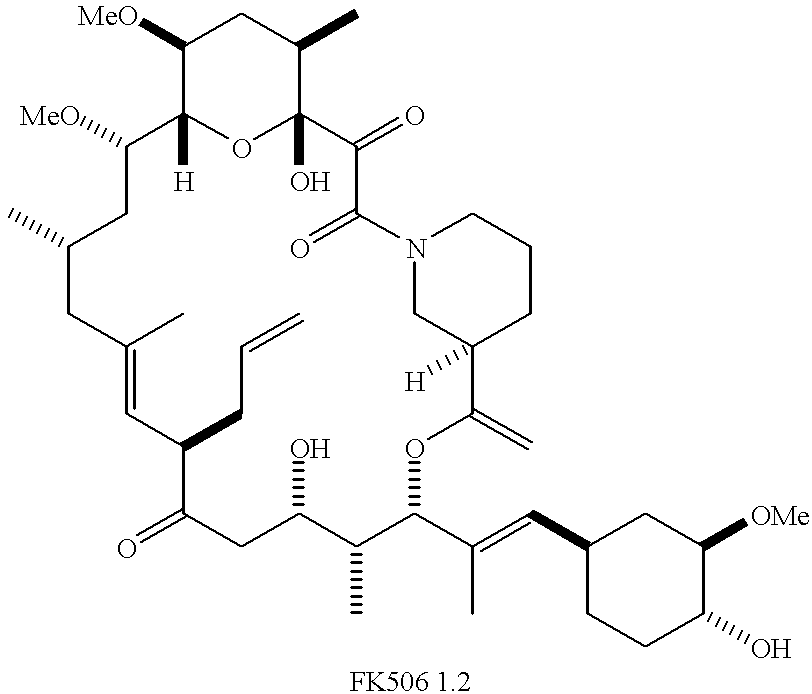
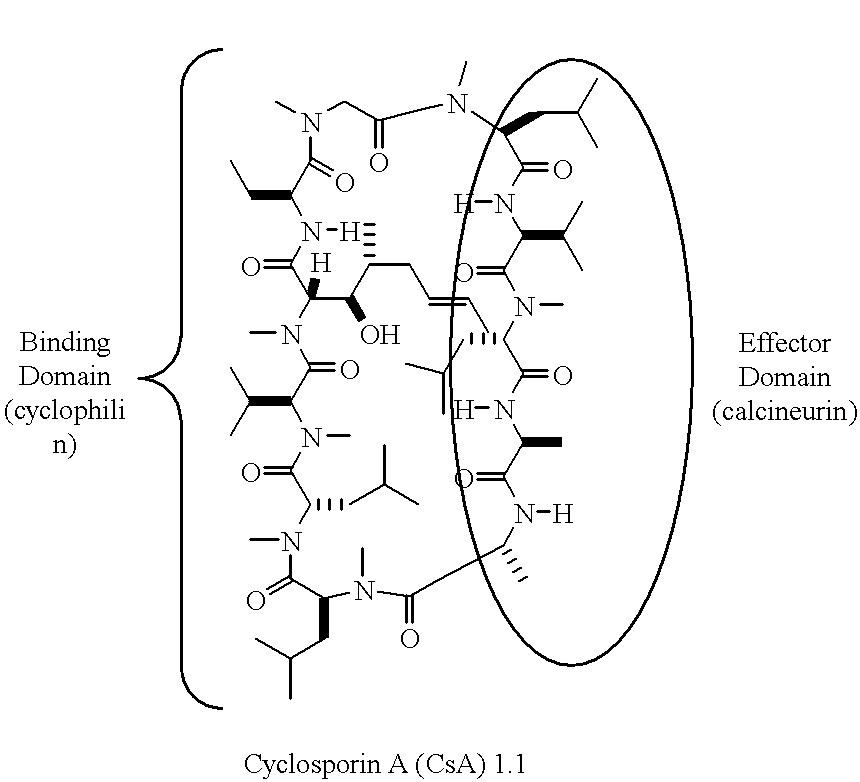
Cyclophilins (CYPs) are a family of proteins named after their ability to bind to ciclosporin (cyclosporin A), an immunosuppressant which is usually used to suppress rejection after internal organ transplants. They are found in all domains of life. These proteins have peptidyl prolyl isomerase activity, which catalyzes the isomerization of peptide bonds from trans form to cis form at proline residues and facilitates protein folding.
Cyclosporin a conjugates and uses therefor
InactiveUS6316405B1Highly effective to treat or prevent neurological disordersAct synergisticallyNervous disorderMetabolism disorderAmyotrophic lateral sclerosisAmyloid
Disclosed are conjugates of Abeta-binding peptides and CsA analogs and conjugates of Abeta-binding peptides and FK506 Binding Peptide inhibitors. These conjugates chemically induce dimerization of either cyclophilin or FK506 Binding Peptide with Abeta peptide, a major component of amyloid plaques found in neurological disorders such as Alzheimer's disease, multiple sclerosis, and amyotrophic lateral sclerosis. The conjugates are useful in the treatment of neurological diseases involving the formation of amyloid plaques because they inhibit and / or prevent the aggregation and deposition of Abeta peptide into plaques.
Owner:WISCONSIN ALUMNI RES FOUND
Bisubstituted carbocyclic cyclophilin binding compounds and their use
InactiveUS20020127605A1Quick identificationAntibacterial agentsUrea derivatives preparationMedical disorderDisease
The present invention relates to novel, non-peptidic small organic compounds having an affinity for cyclophilin (CyP)-type immunophilin proteins. In the compounds of this invention, at least two carbo- or heterocyclic groups are attached to a central saturated, partially saturated, or aromatic 5-6 membered carbocyclic ring by a combination of straight or branched linker chains. The invention further relates to pharmaceutical compositions comprising one or more of the said compounds, and to the uses of these compounds and compositions for binding CyP-type proteins, inhibiting their peptidyl-prolyl isomerase activity, and for research, development, and therapeutic applications in a variety of medical disorders, such as neurological disorders, hair loss disorders, ischemic disorders, and disorders caused by viral or protozoan infection.
Owner:GUILFORD PHARMACEUTICALS INC
Gene therapy by cell specific targeting
InactiveUS6982082B1Selectively inhibiting proliferationPrevent proliferationBiocideCyclosporinsCell specificDisease
This invention is directed to a modified cyclosporin A and to a modified, genetically engineered version of its receptor, cyclophilin. This invention is further directed to a method for treating host versus graft disease following blood marrow transplantation by transfecting stem cells so that after introduction into a patient the stem cells will express the modified cyclophilin, and, as necessary, administer the modified cyclosporin A to the patient.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Cyclosporine analogue molecules modified at amino acid 1 and 3
ActiveUS9200038B2Little and reduced immunosuppressive activityLimited utilityAntibacterial agentsNervous disorderCyclophilin GCyclosporins
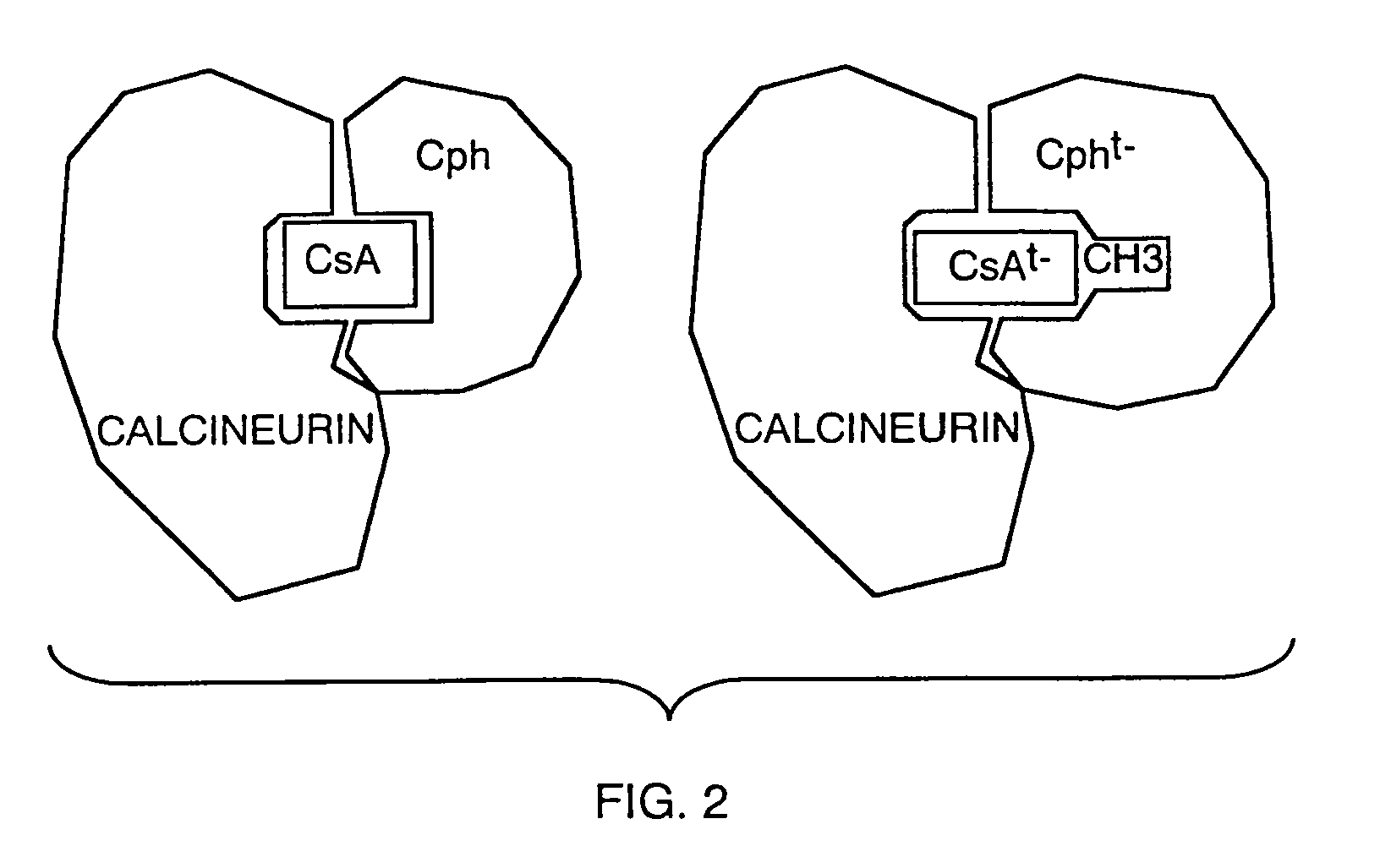
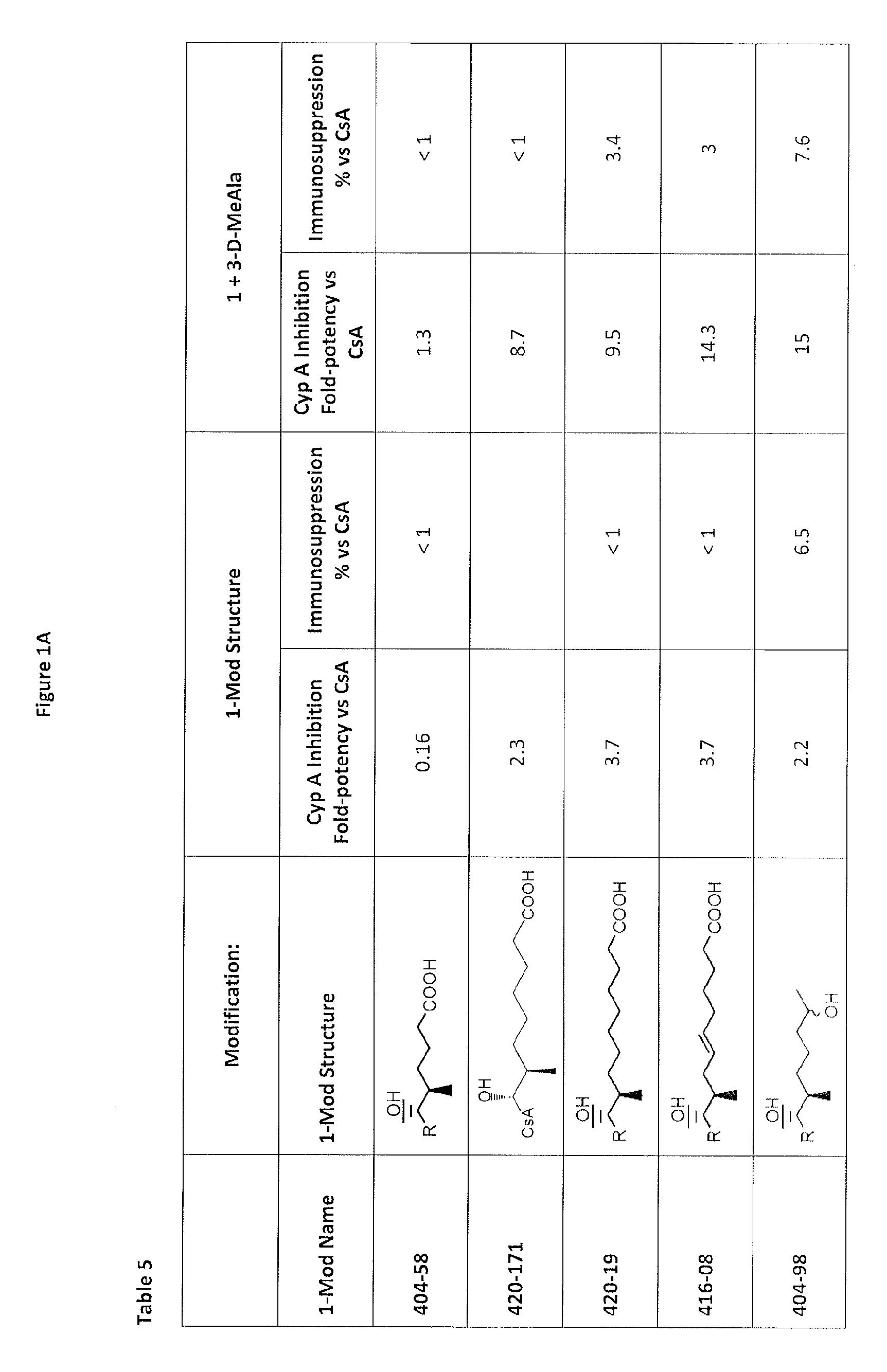
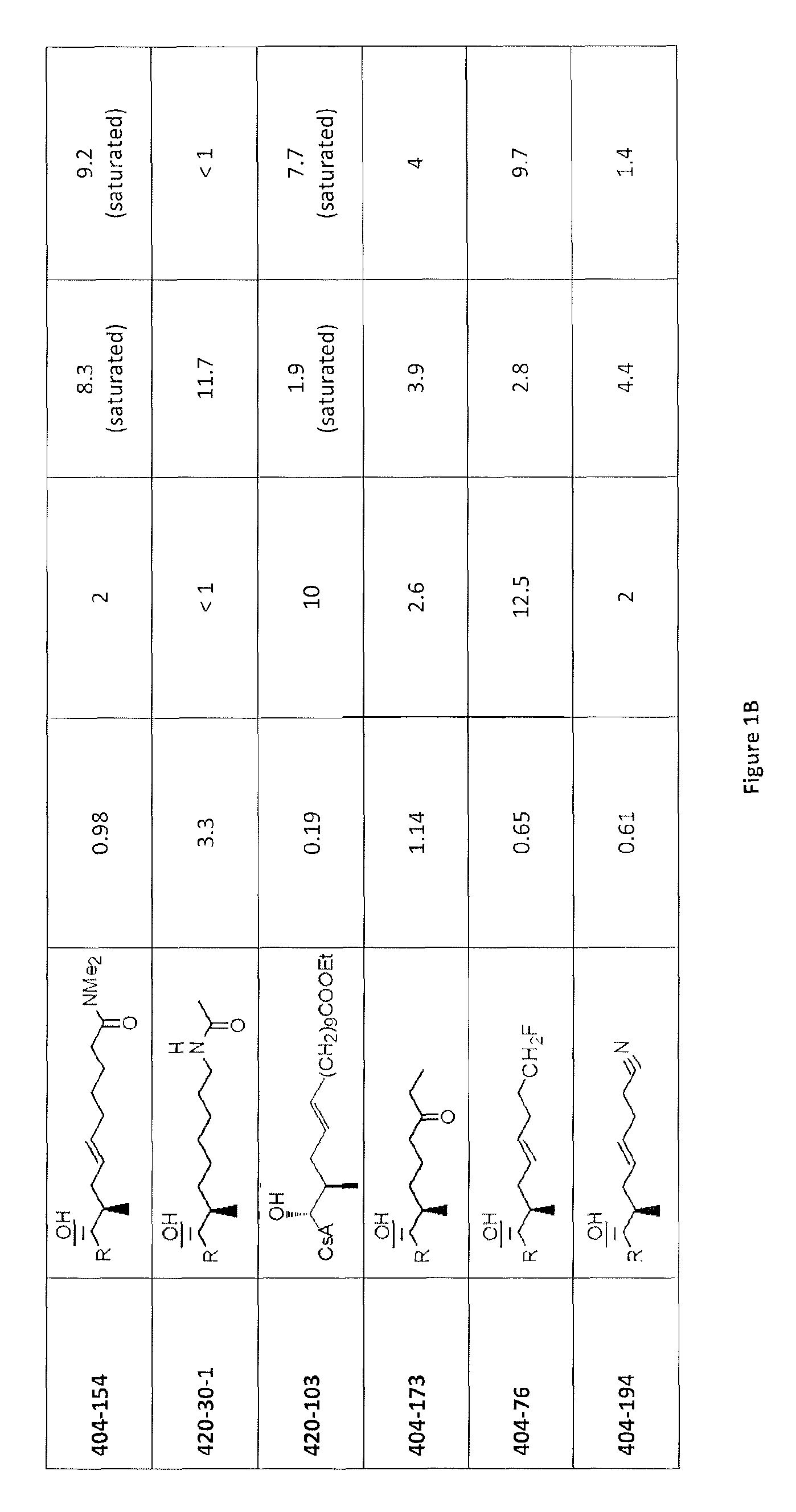
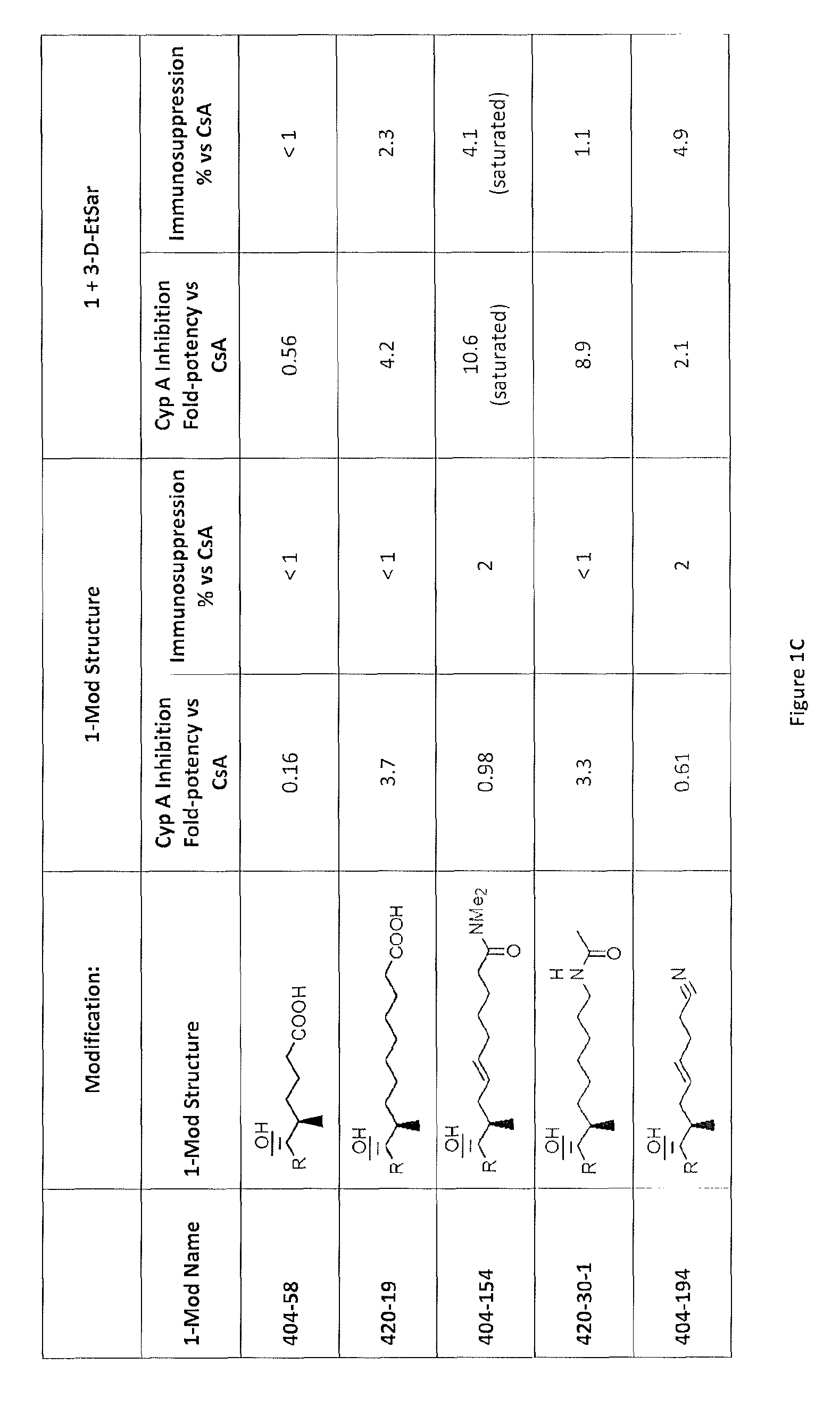
Analogs of cyclosporin-A are disclosed comprising modifications of the substituents as the positions of amino acids 1 and 3, according to the following Formula. The disclosed compounds include compounds having affinity for cyclophilin, including cyclophilin-A, and reduced immunosuppressivity in comparison with cyclosporin-A and analogs thereof modified solely at position 1.
Owner:CONTRAVIR PHARMA
Application of cyclophilin inhibitor
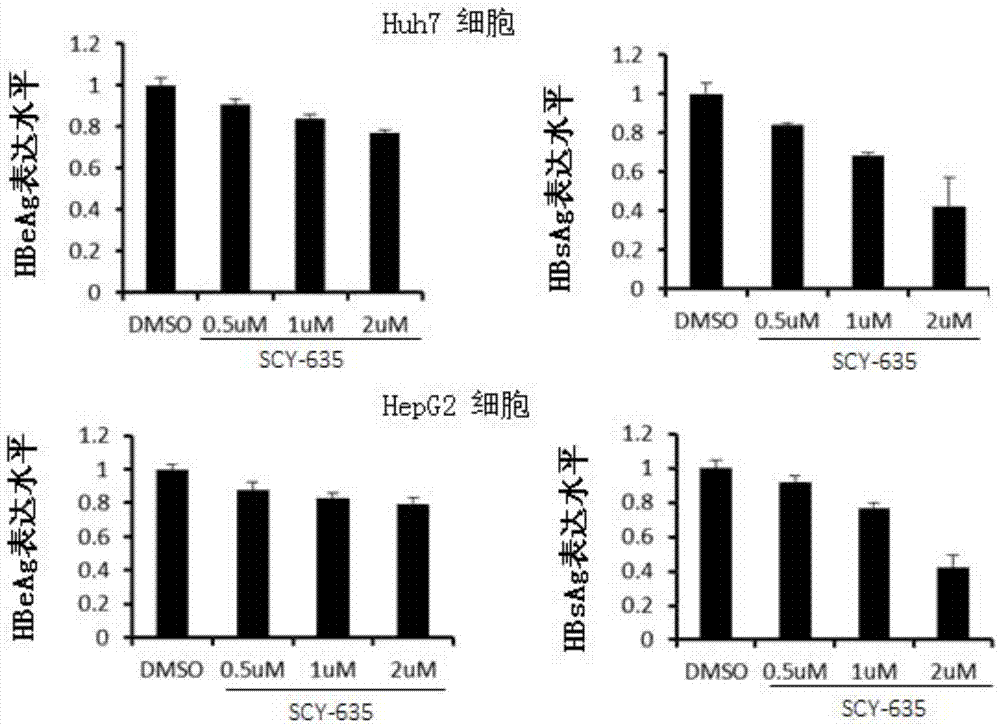
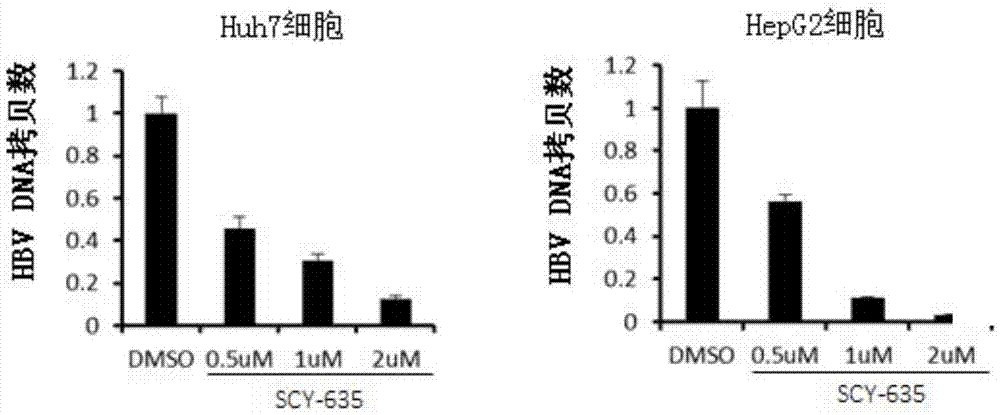
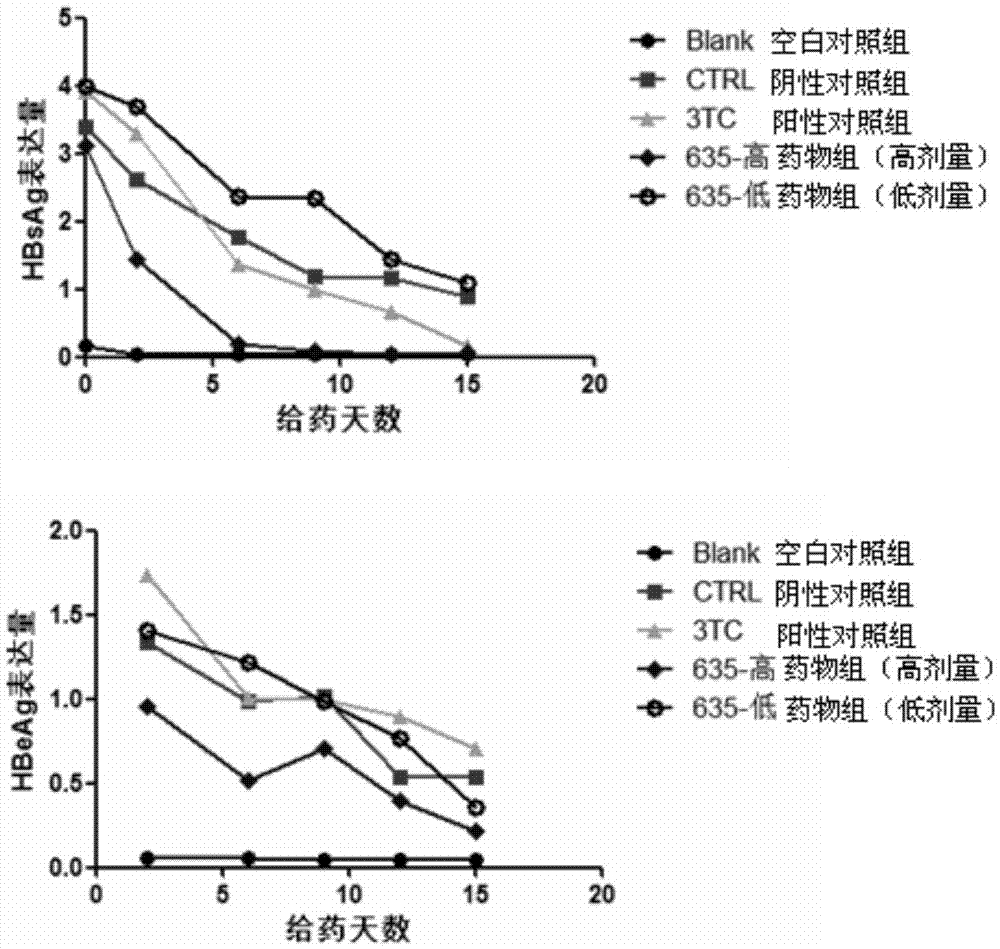
The invention relates to application of a cyclophilin inhibitor, and particularly provides application of a cyclophilin inhibitor or pharmaceutically acceptable salt or solvate thereof in preparation of medicines. The medicines are used for treating and / or preventing diseases caused by hepatitis B virus infection.
Owner:WATERSTONE PHARMA WUHAN
Non-Imuunosuppressive cyclosporins and their use in the prevention and treatment of HIV infection
InactiveUS6270957B1Potent inhibitorHighly effective inhibitor of HIV replicationPeptide/protein ingredientsMicrobiological testing/measurementDiseaseProteinase activity
Disclosed are cyclosporin analogs having amino acid residue substitutions at positions 1, 3, or 7 of the cyclosporin peptide backbone. Also disclosed are conjugates of these cyclosporin analogs in which an HIV protease inhibitor moiety is conjugated to the position-7 amino acid residue of the cyclosporin. These compounds simultaneously bind to and inhibit cyclophilin and HIV protease. The compounds have good bioavailability and potent HIV inhibitory activity. They are useful in the treatment and prevention of HIV-mediated disorders, including AIDS.
Owner:WISCONSIN ALUMNI RES FOUND
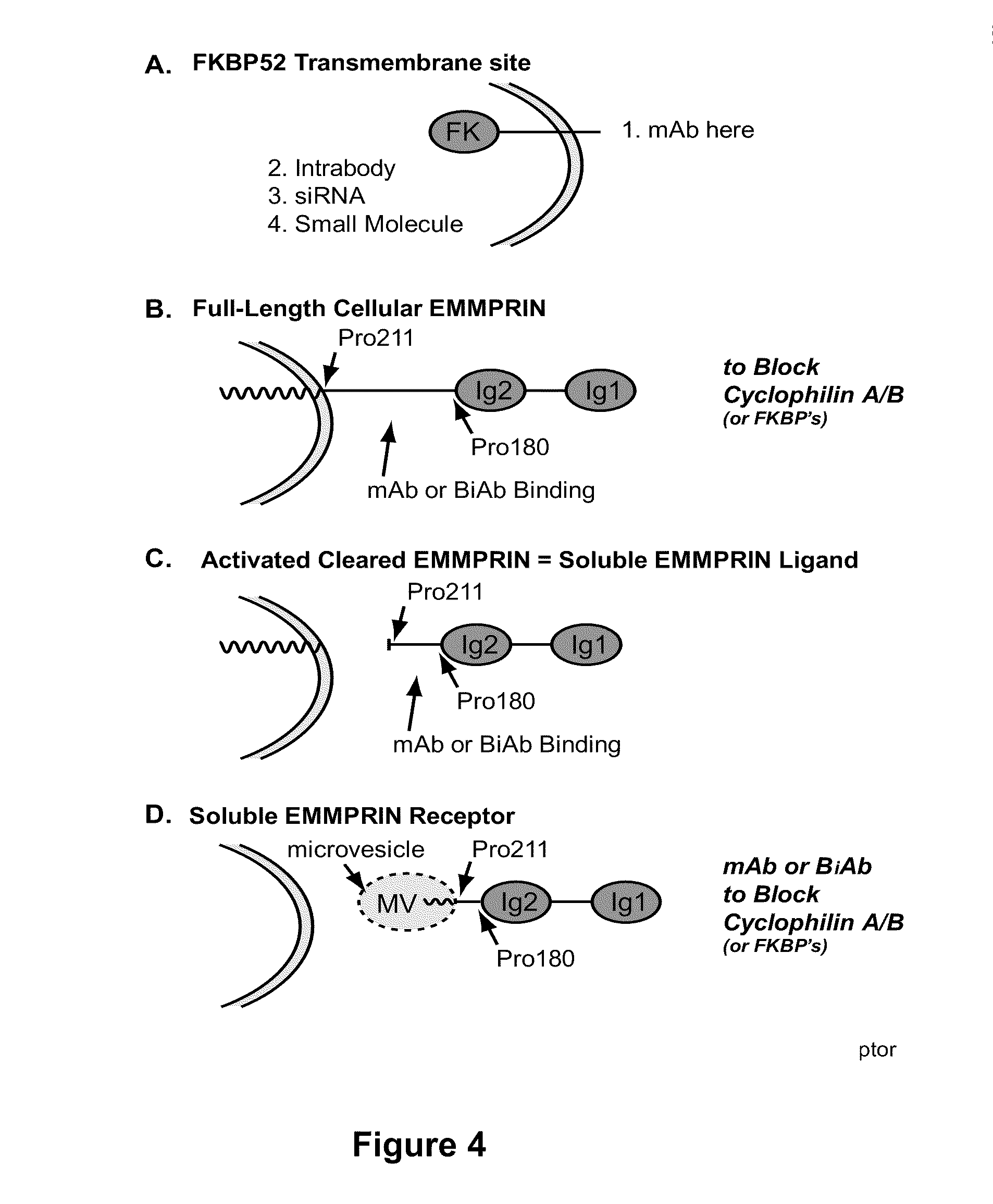
Dual inhibition of immunophilin/cyclophilin family members and emmprin immunoglobulin receptor superfamily members
InactiveUS20100278829A1Inhibit productionInhibitory activityOrganic active ingredientsAntipyreticImmunophilinsImmunity response
The present disclosure provides methods and compositions for modulating an adaptive immune response without significantly affecting an innate immunity response in a subject by modulating a combination of an Immunophilin / Cyclophilin Family Member and an EMMPRIN Immunoglobulin Receptor Superfamily Member. The present invention also provides methods and compositions for treating a variety of clinical conditions by modulating an Immunophilin / Cyclophilin Family Member and an EMMPRIN Immunoglobulin Receptor Superfamily Member.
Owner:SICHUAN HUIYU PHARMA
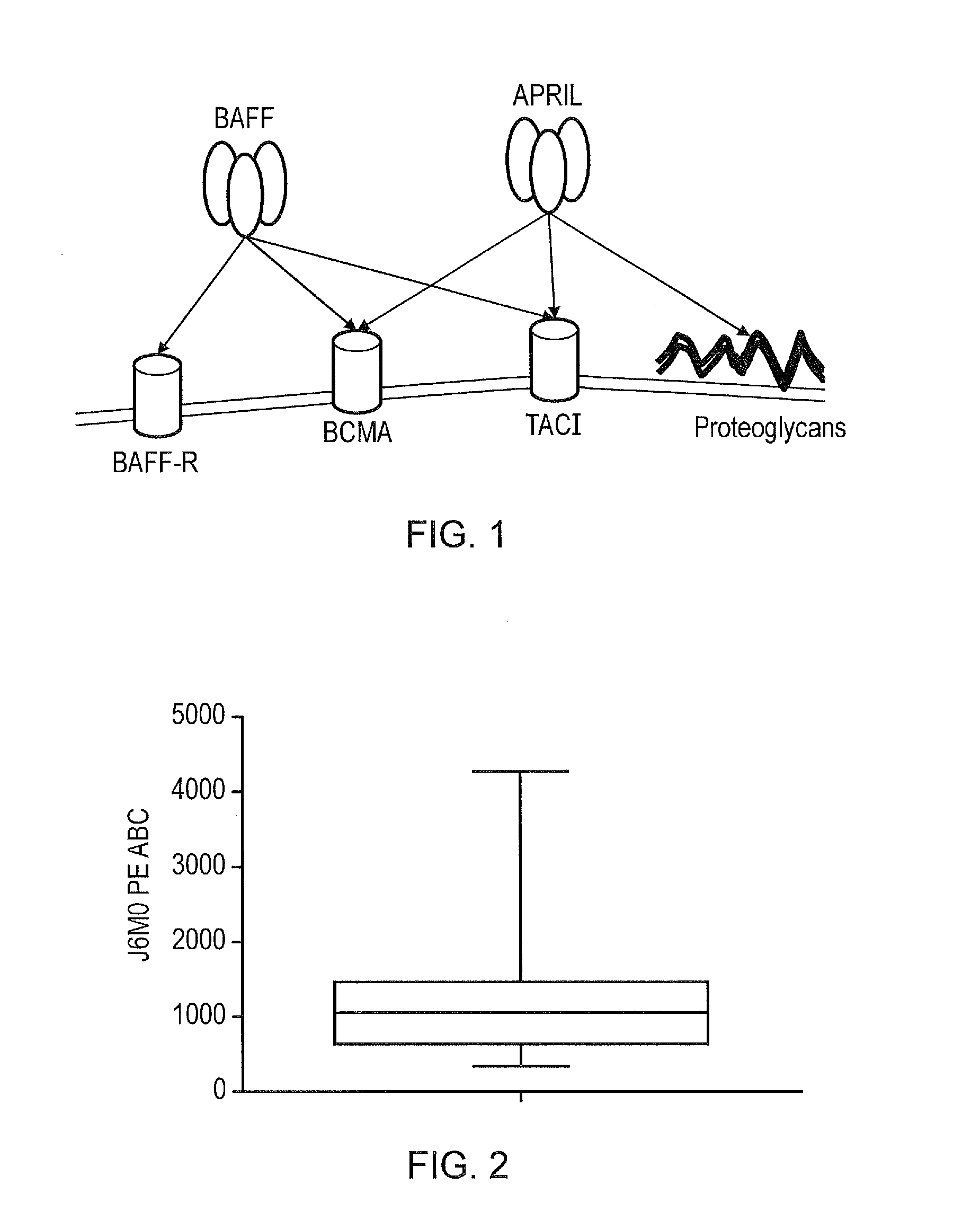
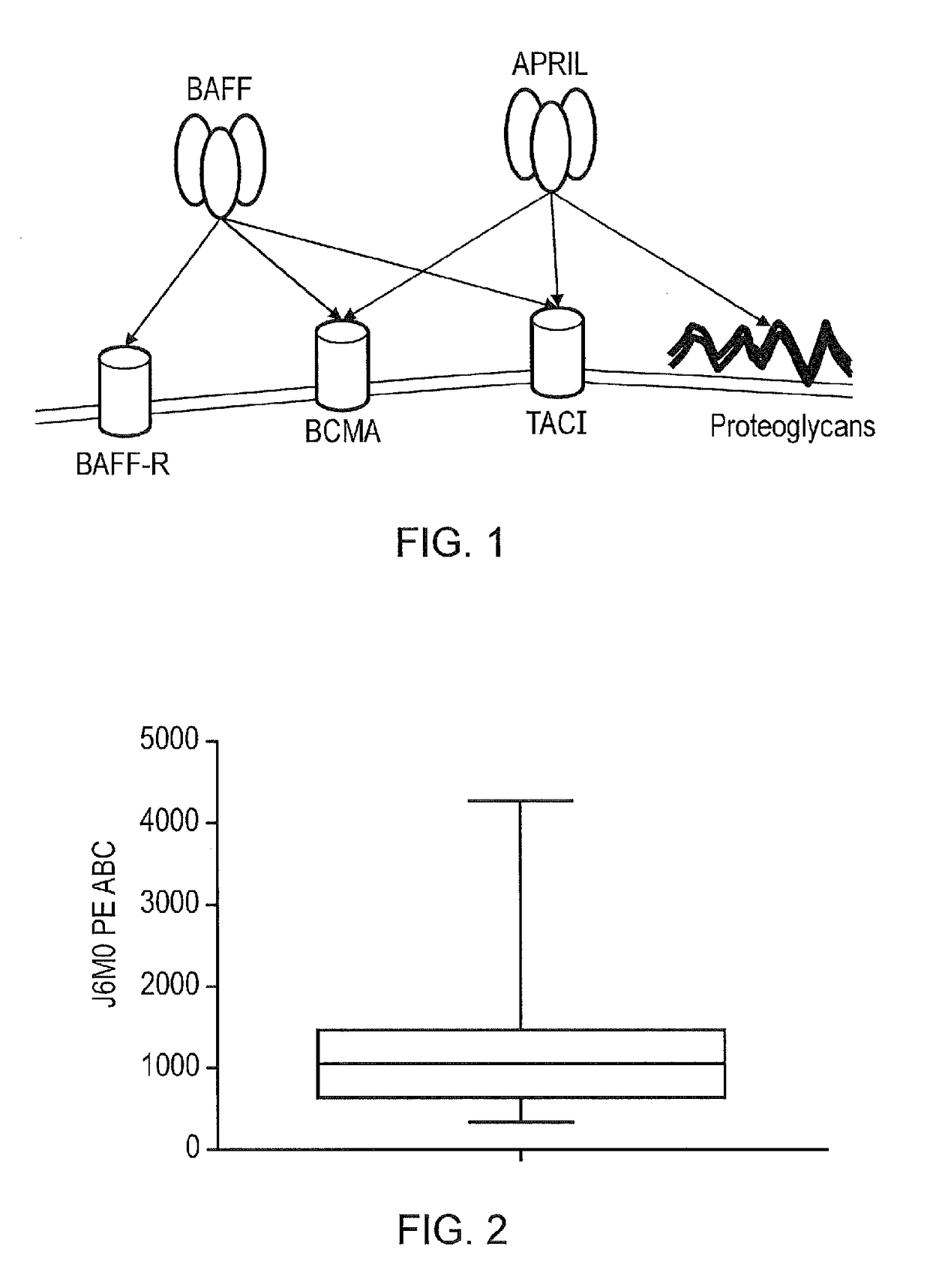
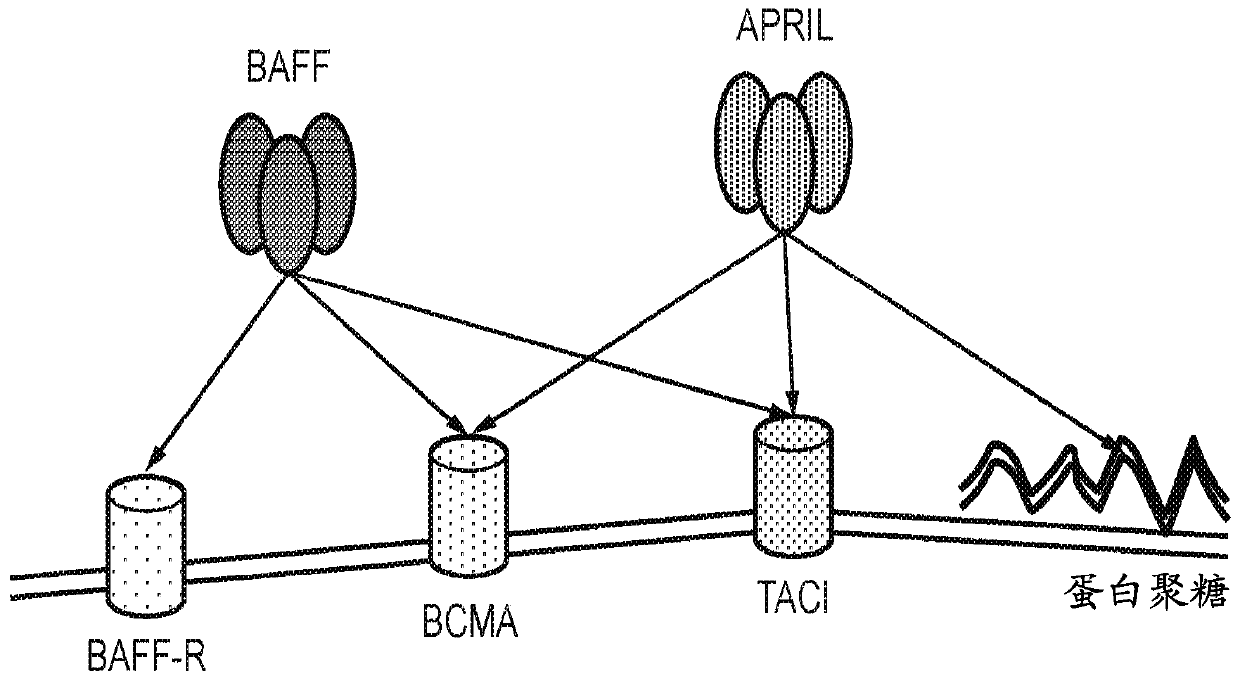
TACI-immunoglobulin fusion proteins
InactiveCN1612750ANervous disorderAntibody mimetics/scaffoldsChemistryTumor necrosis factor receptor
Molecules that interfere with the binding of a tumor necrosis factor receptor with its ligand, such as a soluble receptor, have proven usefulness in both basic research and as therapeutics. The present invention provides improved soluble transmembrane activator and calcium modulator and cyclophilin ligand-interactor (TACI) receptors.
Owner:ZYMOGENETICS INC
April variants
ActiveUS20160362467A1High BCMA : TACI binding ratioStrong specificityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyBinding ratioWild type
The present invention provides a variant proliferation-inducing ligand (APRIL), which has a higher binding affinity to BCMA than wild-type APRIL; and / or altered binding kinetics compared with wild-type APRIL, and / or a higher BCMA:TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor) binding ratio than wild-type APRIL and which comprises mutations at one or more of the following positions: A125, V174, T175, M200, P201, S202, H203, D205 and R206.
Owner:UCL BUSINESS PLC
Use of selected cyclosporins for the treatment of hepatitis C infection
This invention relates to the use in the treatment of HCV infection, either as single active agents or in combination with another active agent, of a cyclosporin having increased cyclophilin binding activity and essentially lacking immunosuppressive activity.
Owner:SCALFARO PIETRO +3
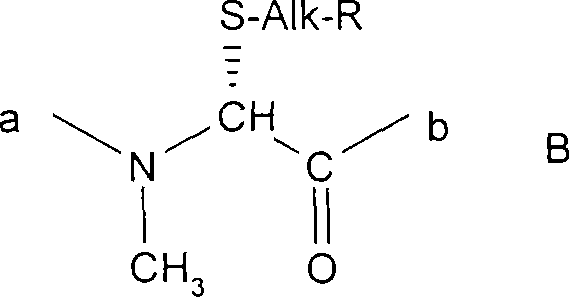
Nonimmunosuppressive cyclosporine analogue molecules
InactiveUS20130190223A1Limited utilityLittle and reduced immunosuppressive activityBiocideNervous disorderSide chainKetone
The compounds of the present invention are non-immunosupressive cyclosporine analogue molecules that are able to bind cyclophilin. Said compounds include a modified side chain of amino acid I of cyclosporin A, consisting of an oxyalkyl having substituents R′, R1 and R2, where R′ is H or Acetyl; R1 is a saturated or unsaturated straight chain or branched aliphatic carbon chain; and R2 may be a hydrogen; a unsubstituted, N substituted or NN disubstituted amide; a N substituted or unsubstituted acyl protected amine; a carboxylic acid; a N substituted or unsubstituted amine; a nitrile; a ester; a ketone; a hydroxy, dihydroxy, trihydroxy or polyhydroxy alkyl; or a substituted or unsubstituted aryl.
Owner:CICLOFILIN PHARMA CORP
Use of cyclophilin as antioxidant and prevention of cyclosporin a-induced toxicity in cell transplantation by overexpression of cyclophilin
InactiveUS20050074438A1Reduce the impactLow toxicityBiocidePeptide/protein ingredientsAntioxidantTransplant rejection
Disclosed is a cyclophilin protein with PPIase activity functioning as an antioxidant. When being overexpressed in transplanted cells, the cyclophilin protein remarkably reduces the cytotoxicity induced by cyclosporin A or its analogues so that it can greatly improve the success rate in transplantation. Also, disclosed is a composition useful to prevent transplant rejection, comprising a recombinant expression vector which can over-express a cyclophilin protein with PPIase activity. The recombinant expression vector is introduced into cells which are thus transformed to be resistant to cyclosporin A and its analogues. A method of preparing such cells is also disclosed.
Owner:KIM SUNG SOO
Protozoan derived compositions and methods for treating autoimmune disease
The instant invention relates to methods for treating a subject suffering from or susceptible to an autoimmune disease or disorder, or a disease or disorder having an autoimmune component, comprising administering to the subject an effective amount of cyclophilin or a biologically active fragment thereof.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Novel Neospora caninum Vaccine
InactiveUS20090208519A1Reduce severityPrevent and to decrease severityProtozoa antigen ingredientsViral antigen ingredientsBALB/cBuprestis novemmaculata
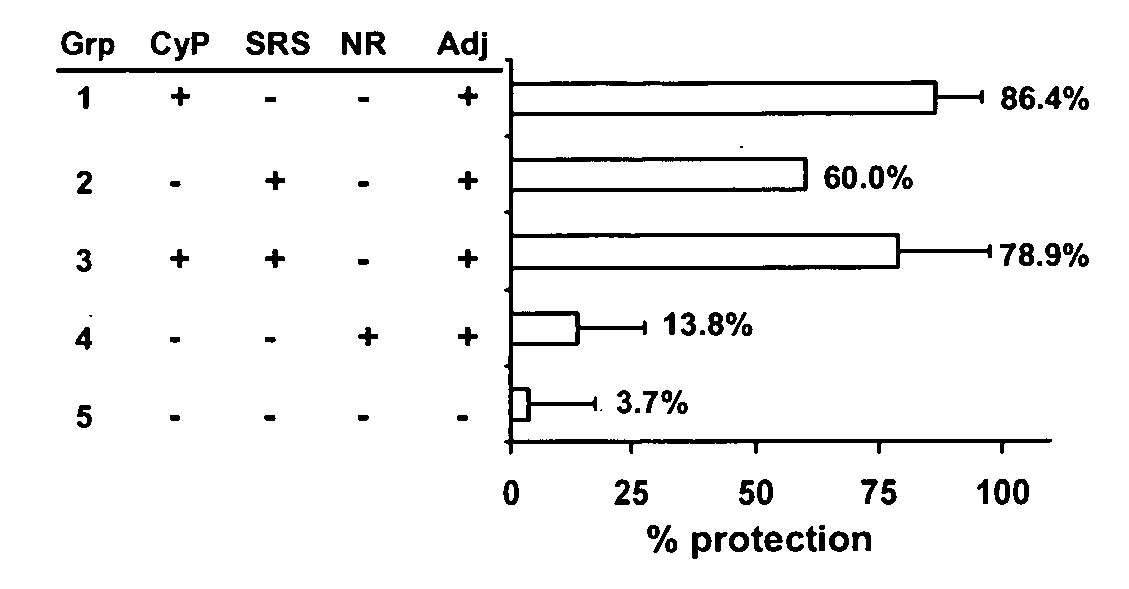
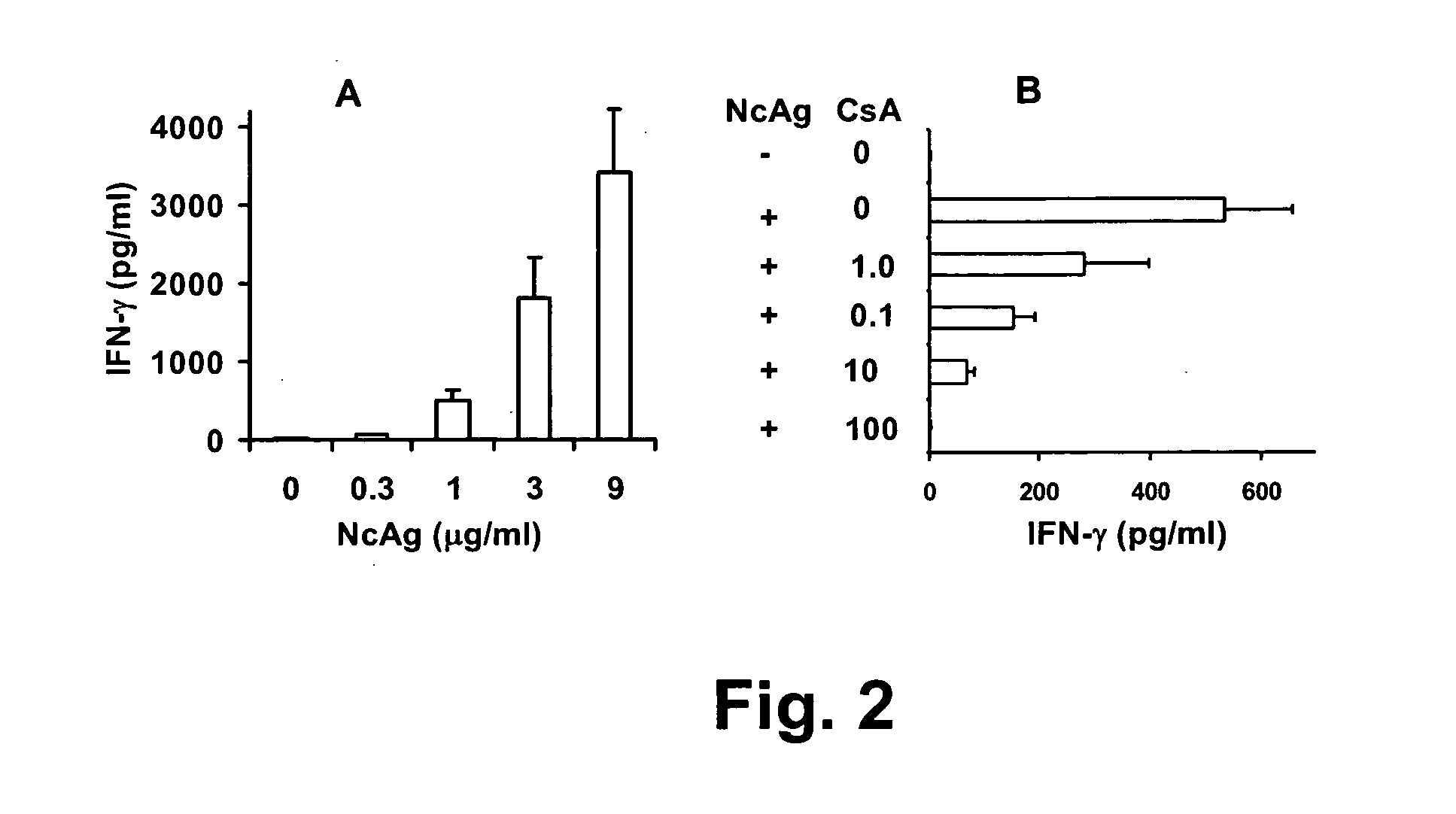
Neospora caninum is the causal agent of bovine neosporosis which results in high levels of abortion. The present study determined the protective efficacy of two Neospora antigens—Neospora cyclophilin (NcCyP) and NcSRS2. The ability of native NcCyP to upregulate mouse IFNγ was also confirmed in this study. Recombinant NcCyP or NcSRS2 were tested either alone or in combination and formulated with adjuvant ImmuMax-SR and CpG. Female BALB / c mice (n=15) of 10-12 weeks of age were immunized s.c. twice in a 2-week interval with vaccines containing either NcCyP alone, NcSRS2 alone, NcCyP plus NcSRS2, or non-recombinant bacterial antigen (NR) in 2 separate trials. All mice were challenge-infected 3 weeks following the booster immunization and necropsied 3 weeks after the challenge infection. Brain and serum were collected and Nc-specific DNA sequence in brain tissue and antibodies in serum were analyzed by PCR or ELISA / Western blotting. Results showed that mice vaccinated with rNcCyP, rNcSRS2, or both rNcCyP and rNcSRS2 responded with high levels of NcCyP or NcSRS2 specific antibodies. Overall, mice received vaccines formulated with either rNcCyP or rNcCyP and rNcSRS2 had a higher (p<0.01) percent protection when compared to the mock- or non-vaccinated mice. The groups immunized with rNcSRS2 alone exhibited slightly lower levels of protection, which was higher (p<0.05) than that of the non-vaccinated group but did not differ (p=0.06) from that of the mock-vaccinated group. The results of the present study indicate that NcCyP is a highly efficacious vaccine candidate which may be useful in protection against Neospora infection.
Owner:UNITED STATES OF AMERICA
Cyclosporins to treat alzheimer's disease
InactiveCN1984670APromote secretionIncreased and stable secretionNervous disorderCyclic peptide ingredientsCyclosporinsSecretion
Non-immunosuppressive, cyclophilin-binding cyclosporins, are useful as neuroprotective agents, e.g. in the prevention or treatment of pathological conditions associated with A beta secretion and / or production.
Owner:NOVARTIS AG
Clone, expression and biological activity of stingray caudal spine cyclophilin A gene
InactiveCN1428348AGood application prospectHigh industrial development valueSugar derivativesMicrobiological testing/measurementCDNA libraryEscherichia coli
The present invention utilizes the construction of dasytis akajei caudal spine cDNA library and DNA sequencing and designs the primer according to the obtained (CyP) EST 3' end and carrier nucleotide sequence, and uses PCR method to screen the dasytis akajei caudal spine cDNA library and clone the cyclophilin A total strength gene. The tength of new gene is 656 bp for coding mature peptide of 167 amino acids, the isoelectric point of the expressed protein is 8.34, and the molecular weight is 18,021 Dalton. The dasytis akajei caudal spine cyclophilin A gene is cloned in the uxA gene 3' tail end of thioredoxing gene fusion expression vector pTRX and the fusion gene meeting reading frame is construcled, said fusion protein is existed in colibacillus, its expression amount can be up to 60 mg / L.
Owner:北京博奥环宇生物技术有限公司
Ligand
ActiveUS20170334964A1High BCMA : TACI binding ratioStrong specificityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyBinding ratioWild type
The present invention provides a variant proliferation-inducing ligand (APRIL), which has a higher binding affinity to BCMA than wild-type APRIL; and / or altered binding kinetics compared with wild-type APRIL, and / or a higher BCMA:TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor) binding ratio than wild-type APRIL and which comprises mutations at one or more of the following positions: A125, V174, T175, M200, P201, S202, H203, D205 and R206.
Owner:UCL BUSINESS PLC
Methods for inhibiting growth of prolactin-responsive cancer cells with cyclosporine A or other cyclophilin inhibitors
ActiveUS20060094646A1Growth inhibitionBlock enzymatic activityHormone peptidesDepsipeptidesCancer cellCell growth
Methods are provided for inhibiting growth of prolactin-responsive cancer cells and treating prolactin-responsive malignancies via administration an agent such as cyclosporine A which directly inhibits an enzymatic activity of a cyclophilin.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
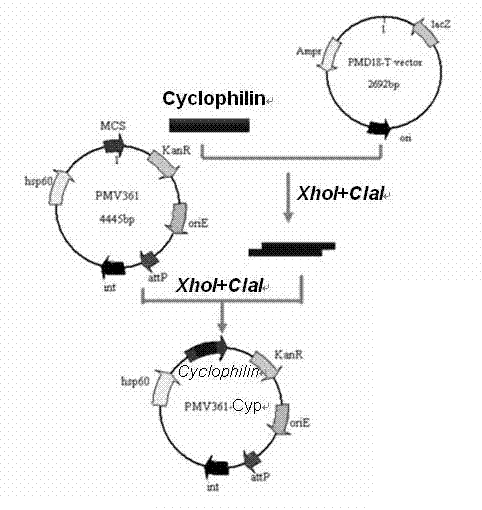
Recombinant bacillus calmette guerin vaccine for toxoplamasis and preparation method thereof
InactiveCN102198267AImprove thermal stabilityEasy transportationProtozoa antigen ingredientsMicroorganism based processesEscherichia coliGondii toxoplasma
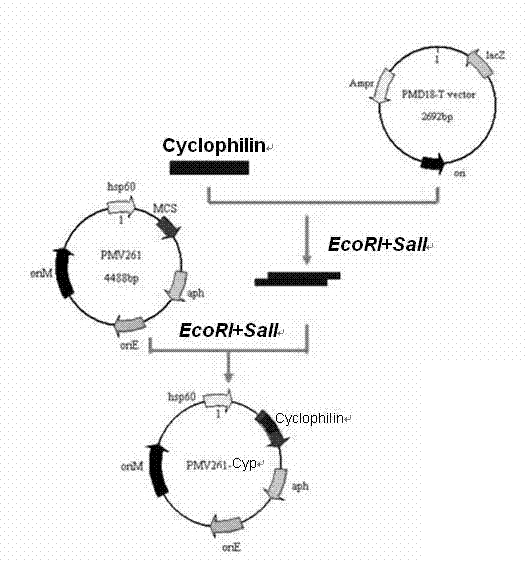
The invention provides a recombinant bacillus calmette guerin (BCG) vaccine for toxoplamasis and a preparation method thereof. The preparation method for the recombinant bacillus calmette guerin vaccine comprises the following steps of: performing target (TA) cloning on an obtained toxoplasma gondii cyclophilin gene; sequencing and identifying correctly, performing enzyme cutting and recovering target fragments; connecting the target fragments with an Escherichia coli-mycobacterium shuttle expression vector pMV261 and an integrated expression vector pMV361 which are subjected to enzyme cutting reaction respectively; and converting recombinant plasmids to BCG, and screening by resistance and polymerase chain reaction (PCR) to obtain the recombinant bacillus calmette guerin vaccine for the positive toxoplasma gondii. The vaccine is high in heat stability and easy to transport, store and produce, is not needed to be purified and can be directly used for immune protection tests, and a complex process of protein aftertreatment is avoided, so the cost is reduced greatly, and the recombinant bacillus calmette guerin vaccine is suitable for vast rural areas.
Owner:JILIN UNIV
Compositions for HCV treatment
The present invention concerns a pharmaceutical combination comprising a) a first agent which is a non-immunosuppressive cyclophilin-binding cyclosporine, e.g., a compound of formula I and b) a co-agent. Co-agents include, but are not limited to, interferons, a conjugate of interferon, antiviral agents, helicase inhibitors, protease inhibitors, polymerase inhibitors and nucleoside analogs. The instant pharmaceutical combination may be used, e.g., in treating subjects having a flaviviridae infection, e.g., a Hepatitis C infection.
Owner:NOVARTIS AG
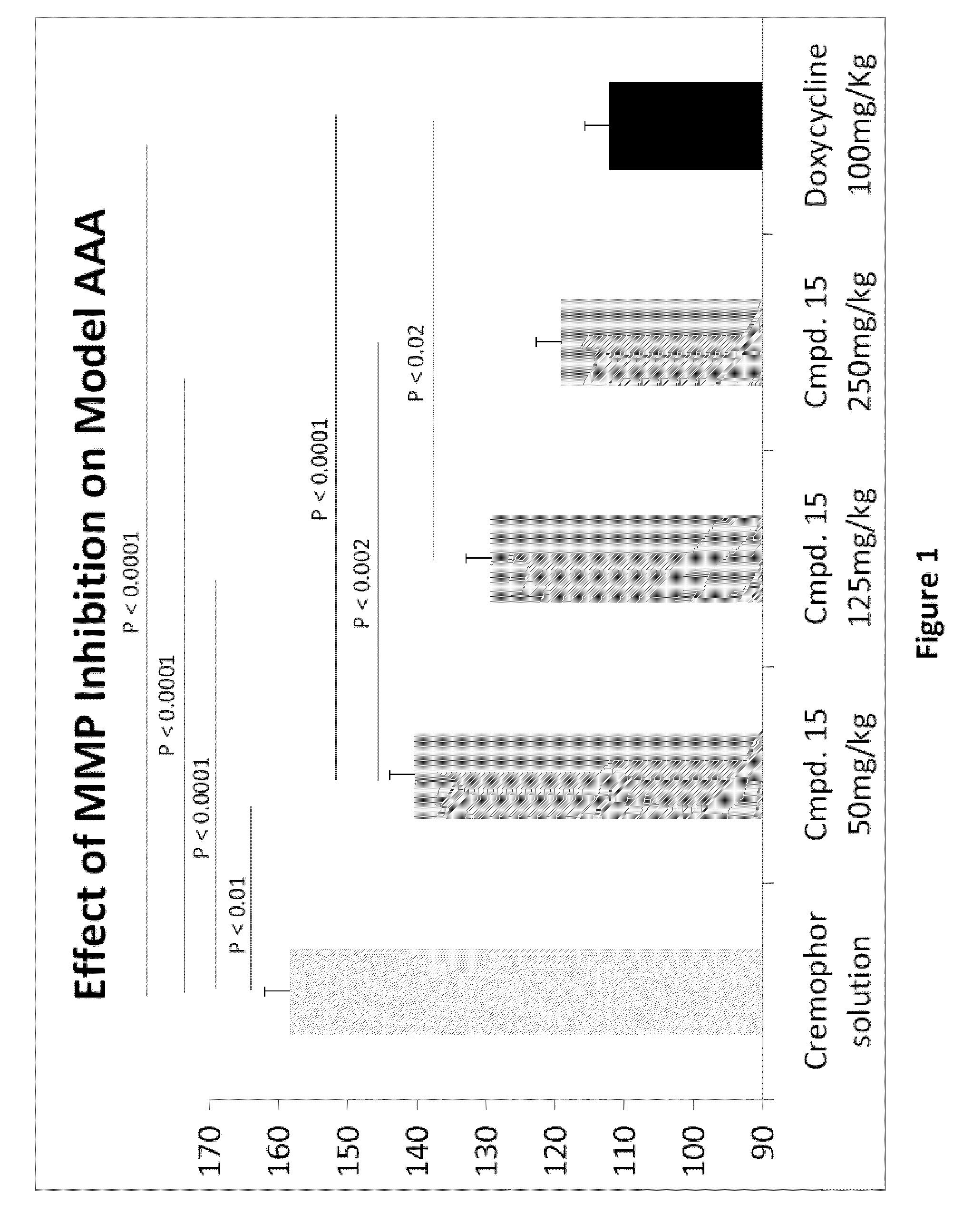
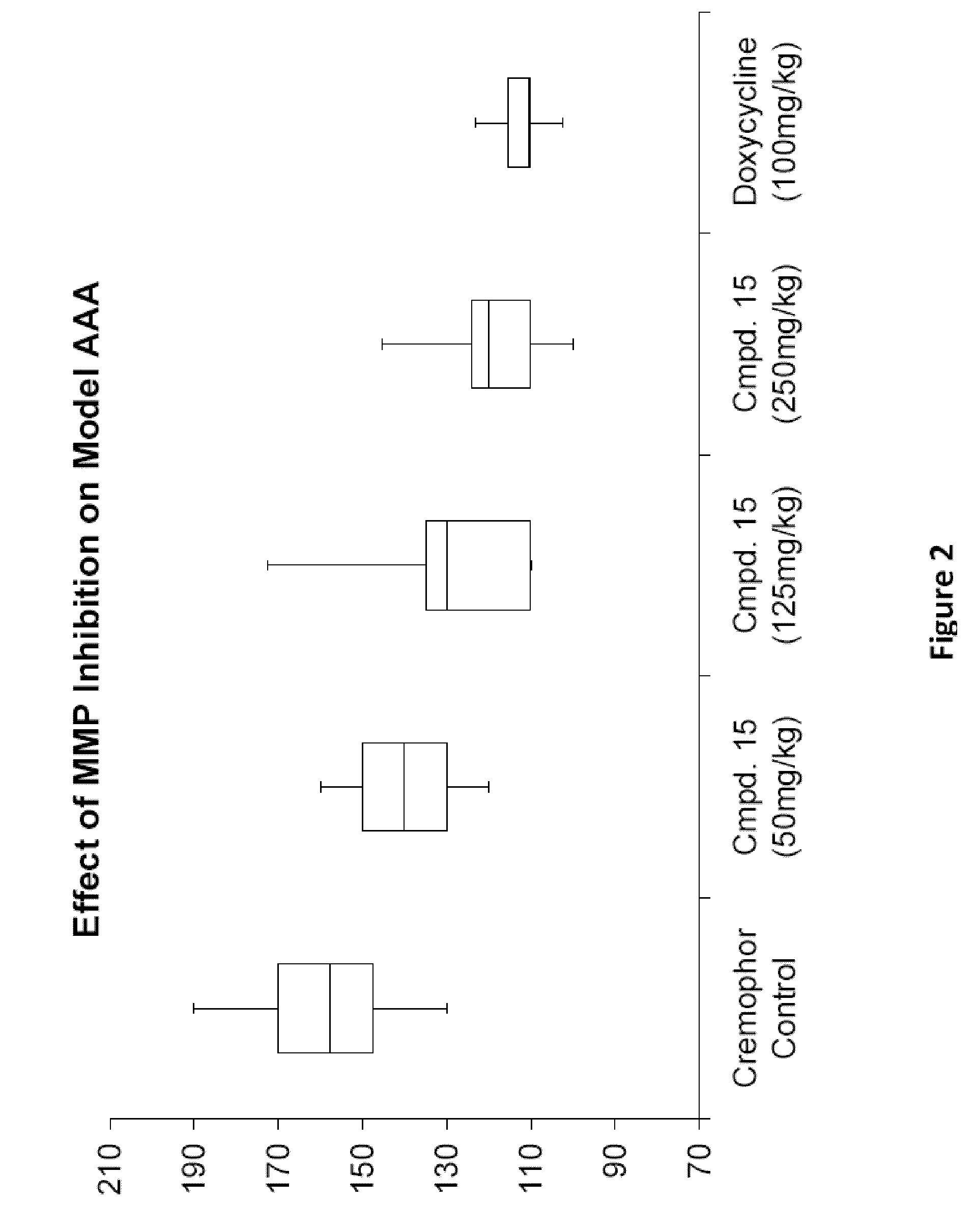
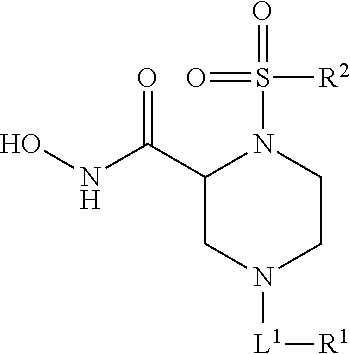
Methods of Treating Aneurysmal Dilatation, Blood Vessel Wall Weakness and Specifically Abdominal Aortic and Thoracic Aneurysm Using Matrix Metalloprotease-2 Inhibitors
InactiveUS20110082114A1Little effectIncreased riskBiocideAnimal repellantsAngiotensin Receptor BlockersIn vitro study
The present invention provides methods of treating aneurysmal dilatation, blood vessel wall weakness, and specifically abdominal aortic aneurysm and thoracic aneurysm by inhibiting MMPs and ADAM-10. Such compounds are useful in the in vitro study of the role of MMPs and ADAM-10 (and its inhibition) in biological processes. The present invention also comprises pharmaceutical compositions comprising one or more MMPs or ADAM-10 inhibitors according to the invention in combination with a pharmaceutically acceptable carrier. Such compositions are useful for the treatment of aneurysmal dilatation or blood vessel wall weakness, for example abdominal aortic aneurysm and thoracic aneurysm. The invention also comprises methods of treating aneurysmal dilatation or blood vessel wall weakness, for example abdominal aortic aneurysm and thoracic aneurysm utilizing the compounds of the invention in conjunction with inhibitors of angiotensin II, including angiotensin II receptor blockers and angiotensin converting enzyme inhibitors, and cyclophilin inhibitors.
Owner:SYMPHONY EVOLUTION
Compositions and methods for diagnosis and treatment of chronic inflammatory diseases
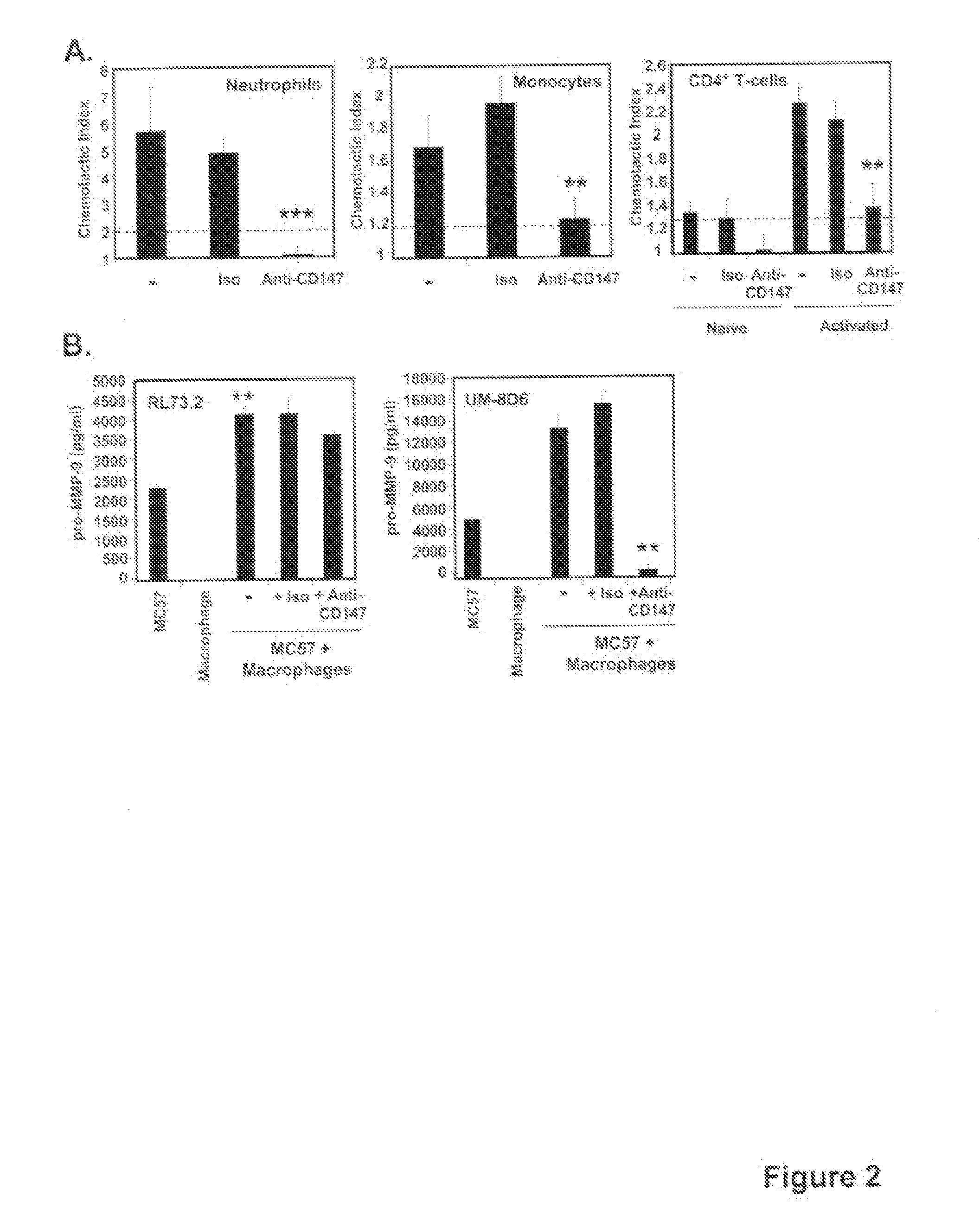
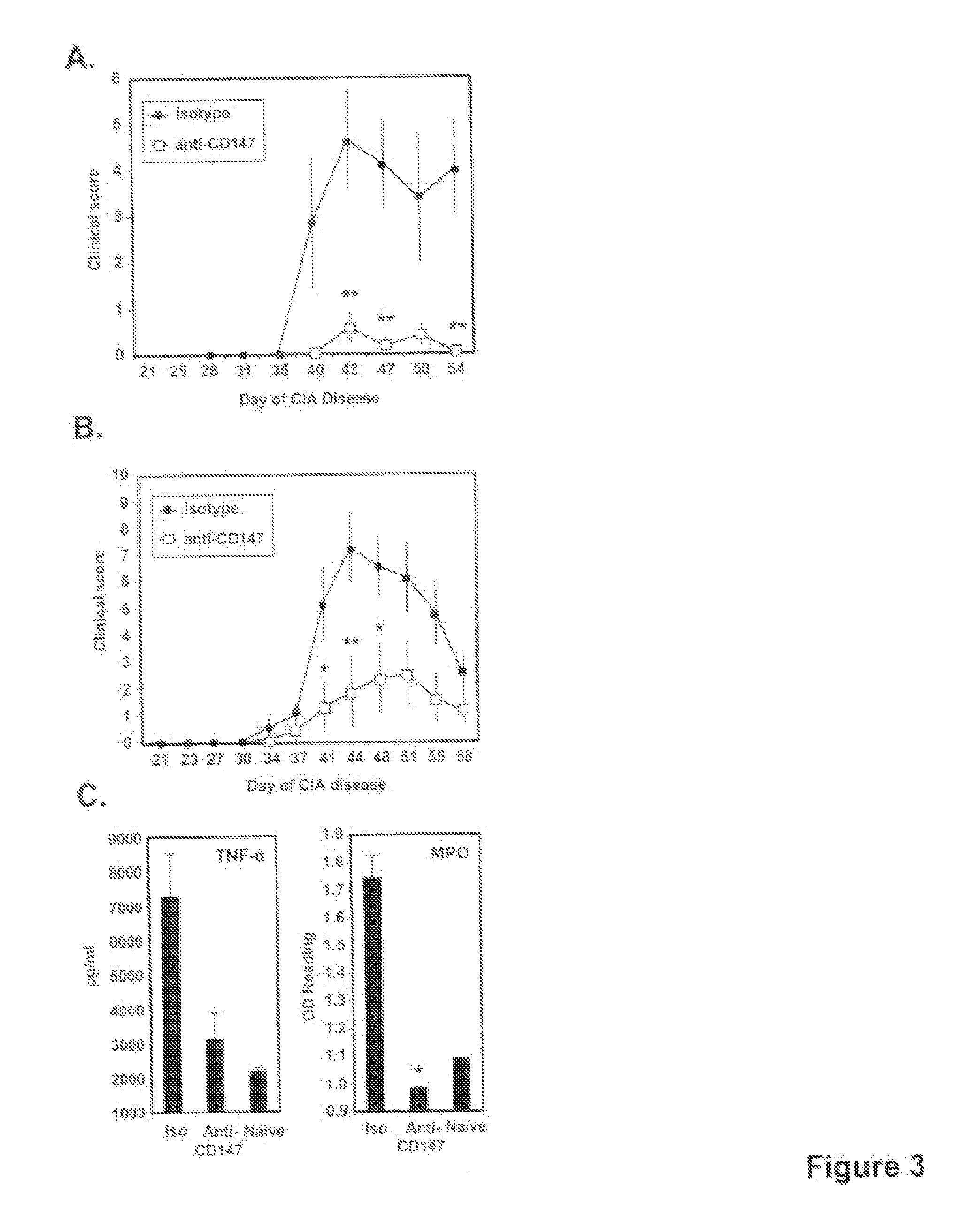
This invention relates to methods and compositions for diagnosis and treatment of chronic inflammatory diseases by blocking CD147 interaction with extracellular cyclophilin. Specifically, the methods and compositions of this invention regulate recruitment of leukocyte to the infection site by specifically blocking the CD147 domain involved with the chemotactic function without blocking the CD147 domain involved with EMMPRIN function.
Owner:THE GEORGE WASHINTON UNIV
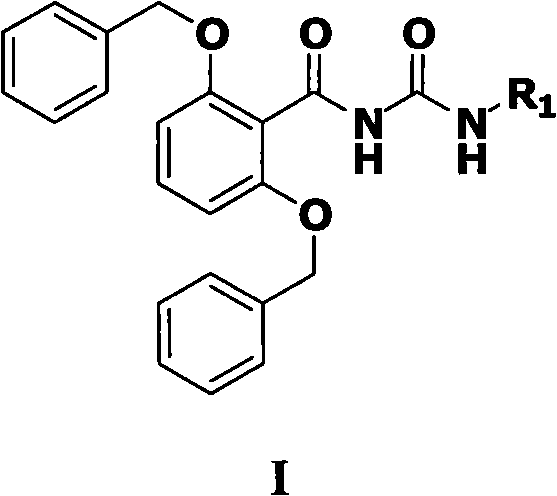
N-(2,6-dibenzyloxy benzoyl)-N'-substituted urea compound and preparation method and application thereof
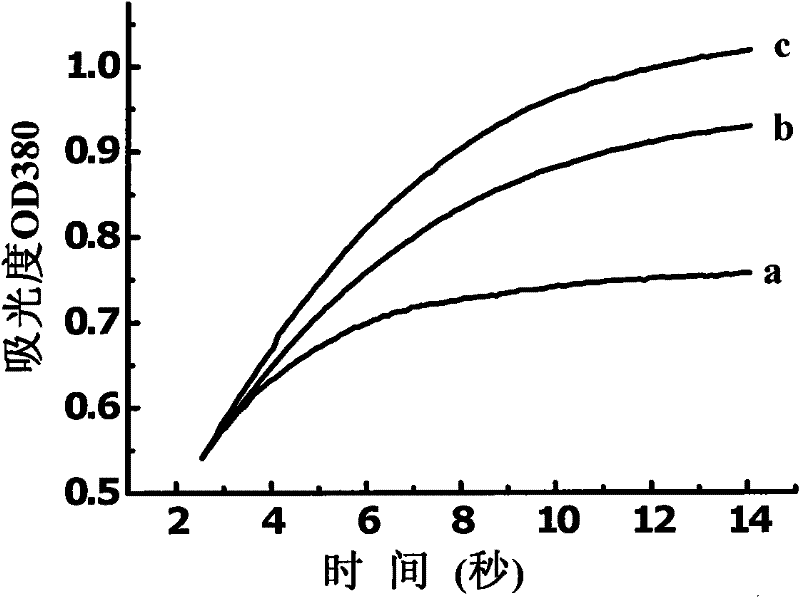
The invention relates to a novel N-(2,6-dibenzyloxy benzoyl)-N'-substituted urea compound and a preparation method and an application thereof. Structural general formula of the compound is shown as a formula (I), wherein R1 is a C5-C25 cyclic alkyl, aryl or heterocyclic group, or a substituted C1-C6 straight-chain or branch-chain alkyl (shown in the specification). The current study shows that cyclophilin A (CypA) participates in multiple physiological and pathological processes of a human body. Thus, the compound provided by the invention can be applied to preparation of a cyclophilin A (CypA) excitant or preparation of drugs used for preventing or treating diseases mediated by CypA.
Owner:EAST CHINA UNIV OF SCI & TECH
Methods for inhibiting growth of prolactin-responsive cancer cells with cyclosporine A or other cyclophilin inhibitors
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Fusion protein, and canine toxoplasma subunit vaccine and vaccine composition thereof
InactiveCN111234035AStimulate humoral immune responseHigh expressionProtozoa antigen ingredientsAntibody mimetics/scaffoldsVaccine StabilityCellular antigens
The invention provides a fusion protein and a nucleotide sequence thereof. The fusion protein comprises a toxoplasma cyclophilin protein subunit and a T cell epitope. The invention also provides a vaccine for preventing toxoplasmosis of dogs by using the fusion protein and a vaccine composition of the vaccine. The invention has the beneficial effects that the fusion protein has the characteristicsof high expression quantity and high immunogenicity; and the vaccine and the vaccine composition can effectively prevent canine toxoplasmosis and have high vaccine stability.
Owner:海木集团有限公司 +1
Vaccine for preventing dog Toxoplasma gondii infection and preparation method of vaccine
ActiveCN108822200AMaintain immunogenicityPreventing Canine ToxoplasmosisProtozoa antigen ingredientsBacteriaEscherichia coliEnzyme digestion
The invention provides protein with Toxoplasma gondii immunogenicity. The protein is cyclophilin mutant protein and consists of the amino acid sequence shown in SEQ ID 2. The invention further provides nucleic acid capable of encoding the protein with Toxoplasma gondii immunogenicity and consists of the nucleic acid sequence shown in SEQ ID 1. The invention also provides a vaccine. A Toxoplasma gondii antigen gene is subjected to double enzyme digestion, then linked to prokaryotic expression vectors such as pET28a and the like and transformed into prokaryotic expression engineering bacteria such as BL21 (DE3) and the like to be induced to be highly expressed, protein obtained after purification is soluble protein, and the specific immunogenicity of the protein is maintained. The sequence of the cyclophilin protein is optimized, so that the expression amount of the cyclophilin protein in prokaryotic Escherichia coli is remarkably increased. The vaccine can be prepared by engineering strains by adding vaccine adjuvants, unique immunogenicity of the vaccine is maintained, and the vaccine is suitable for industrial production.
Owner:HAIMU ANIMAL HEALTH PROD (SHANDONG) CO LTD
Application of anti-BASIGIN humanized antibody in preparation of drugs for treating COVID-19
PendingCN111420048AInhibition of chemotaxisAchieve healingAntiviralsAntibody ingredientsReceptorHumanized antibody
The invention discloses an application of an anti-human BASIGIN humanized antibody in preparation of drugs for treating COVID-19. The amino acid sequence of a light chain variable region of the anti-human BASIGIN humanized antibody is shown as SEQ ID NO:1, and the amino acid sequence of a heavy chain is shown as SEQ ID NO:3; and the nucleotide sequence of the light chain variable region is shown as SEQ ID NO:2, and the nucleotide sequence of the heavy chain variable region is shown as SEQ ID NO:4. The antibody can specifically recognize and bind a virus invasion host epithelial cell co-receptor CD147, block the interaction of the CD147 and COVID-19 S protein, and interaction of the CD147 and cyclophilin A (CyPA), thereby inhibiting the COVID-19 from infecting host cells.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Use of modified cyclosporins for the treatment of HCV disorders
PendingUS7968518B2Eliminate infectionAlleviate or eliminate oneBiocideDigestive systemDiseaseCyclosporins
Disclosed are non-immunosuppressive cyclophilin-binding cyclosporins, e.g., of formula (I, Ia or II) as defined herein, having useful properties in the prevention of Hepatitis C infections.
Owner:NOVARTIS AG
Taci-immunoglobulin fusion proteins
Molecules that interfere with the binding of a tumor necrosis factor receptor with its ligand, such as a soluble receptor, have proven usefulness in both basic research and as therapeutics. The present invention provides improved soluble transmembrane activator and calcium modulator and cyclophilin ligand-interactor (TACI) receptors.
Owner:ZYMOGENETICS INC
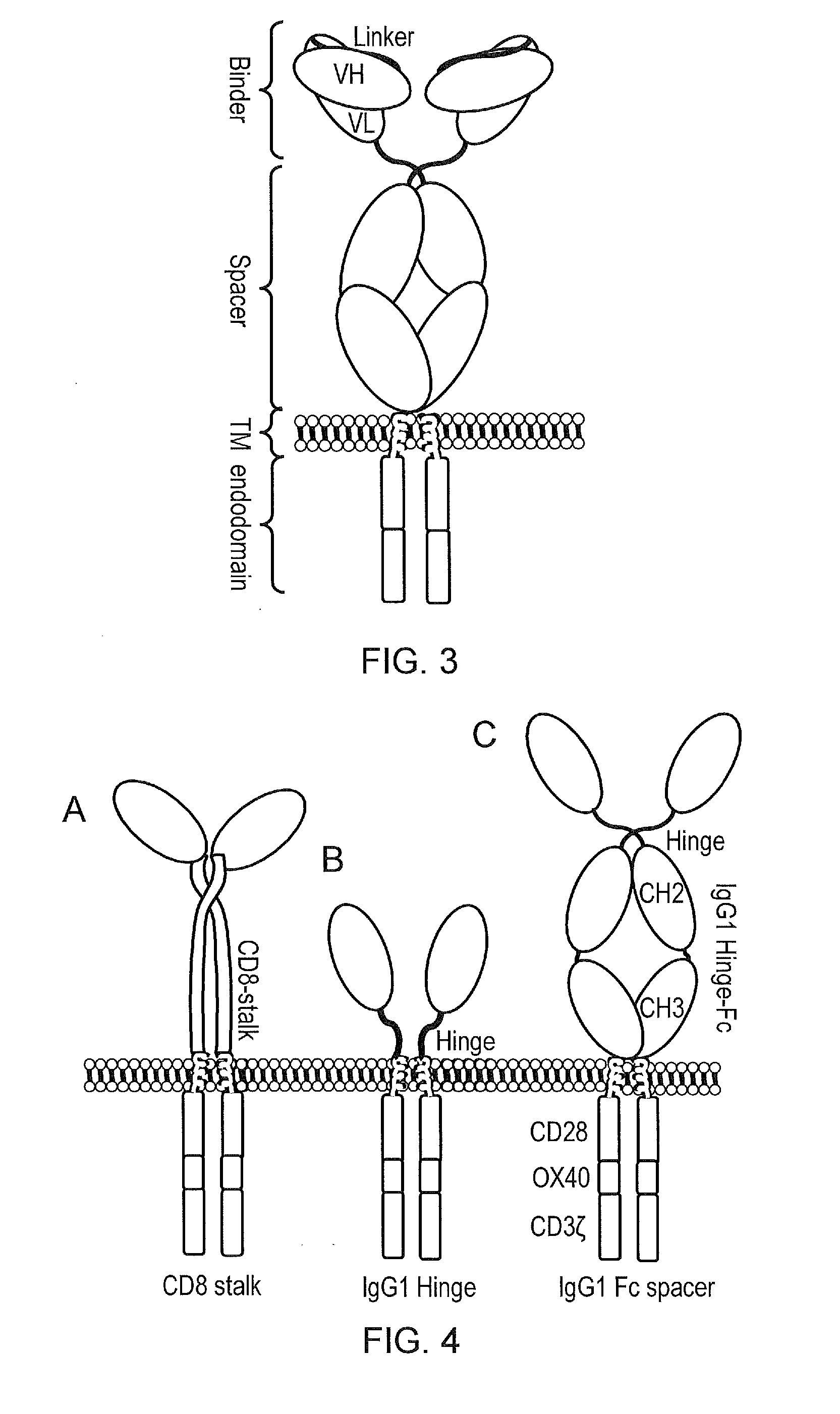
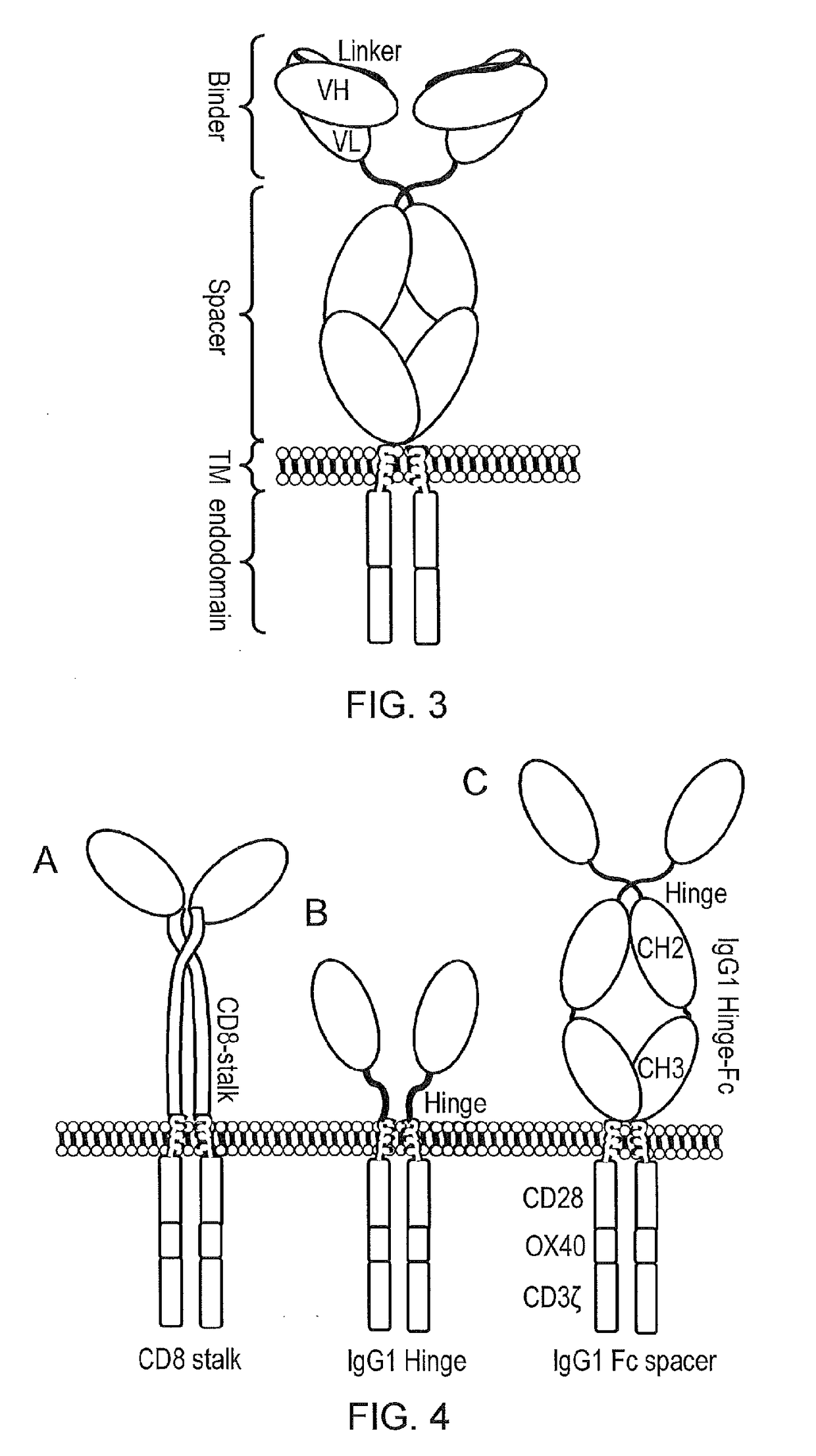
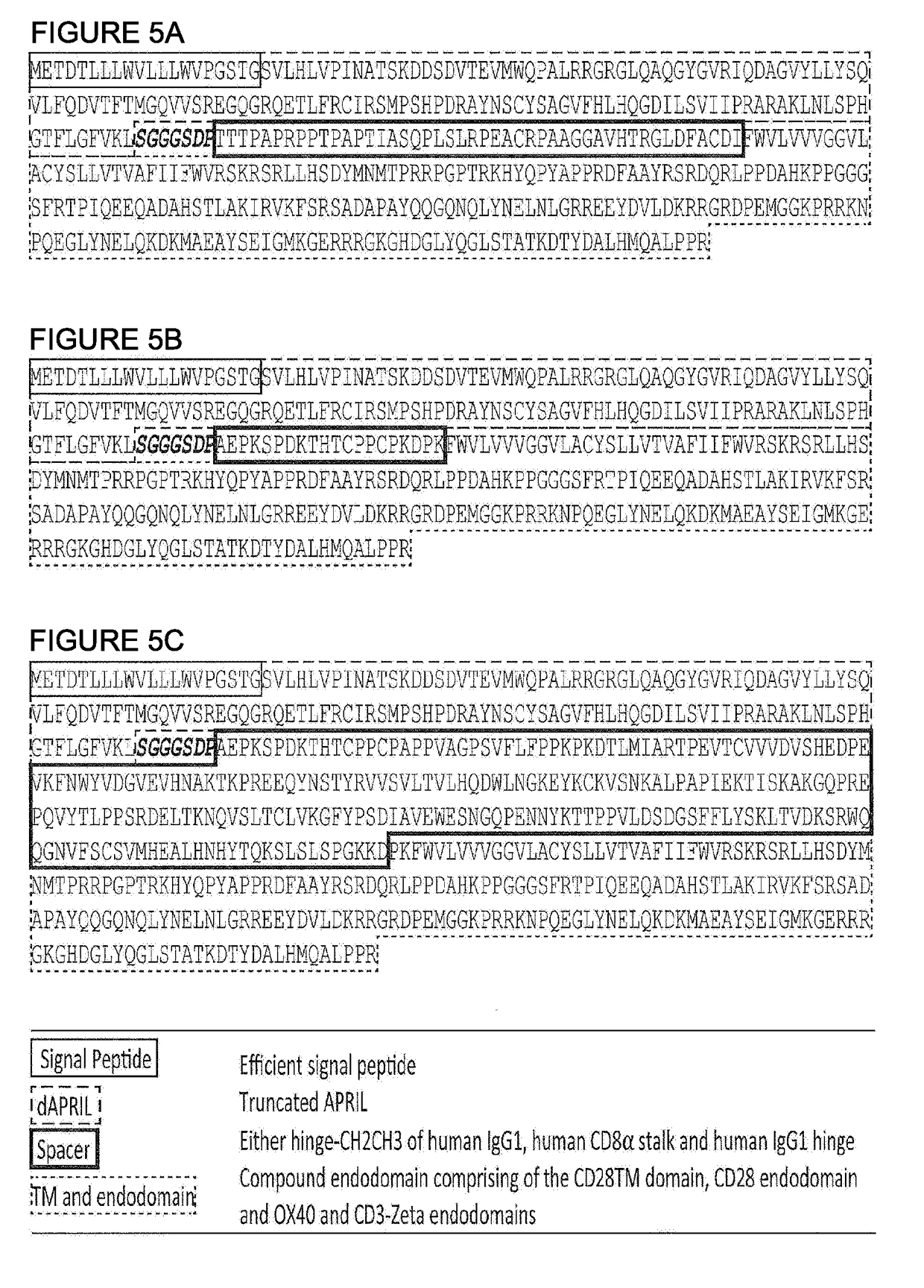
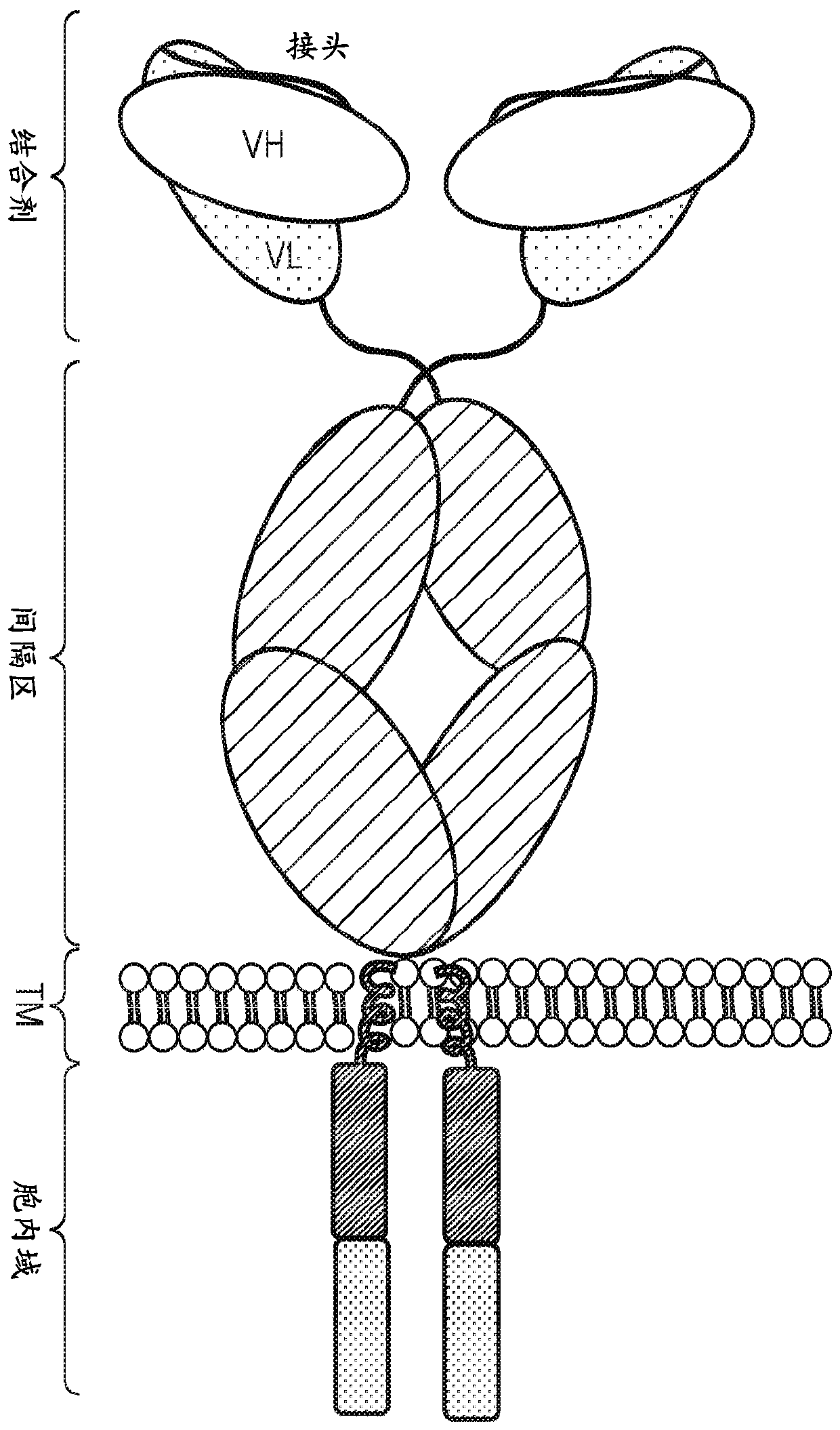
Chimeric antigen receptor
InactiveCN110114371APolypeptide with localisation/targeting motifImmunoglobulins against cell receptors/antigens/surface-determinantsCyclophilin GDisease
The present invention provides a cell comprising first and second chimeric antigen receptors (CARs), which bind to different antigens, wherein the first CAR binds to Transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI). The present invention also provides a cell comprising a tanCAR comprising first and second antigen-binding domains which bind to differentantigens, wherein the first antigen binding domain binds the antigen Transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI). The present invention further provides corresponding nucleic acid sequences and / or constructs, kits and vectors comprising said nucleic acid sequences and / or constructs, molecules and methods for making such cells. The cells may be used in cellular immunotherapy approaches for treating diseases such as multiple myeloma.
Owner:AUTOLUS LIMIED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com