Recombinant bacillus calmette guerin vaccine for toxoplamasis and preparation method thereof
A technology of recombinant BCG and Toxoplasma gondii, which is applied in the field of preparation of Toxoplasma recombinant BCG vaccine, can solve the problems of weak anti-infection ability and inapplicability to human beings, and achieve easy transportation and storage, strong humoral immune adjuvant effect, thermal good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Toxoplasma gondii recombinant BCG vaccine of the present invention
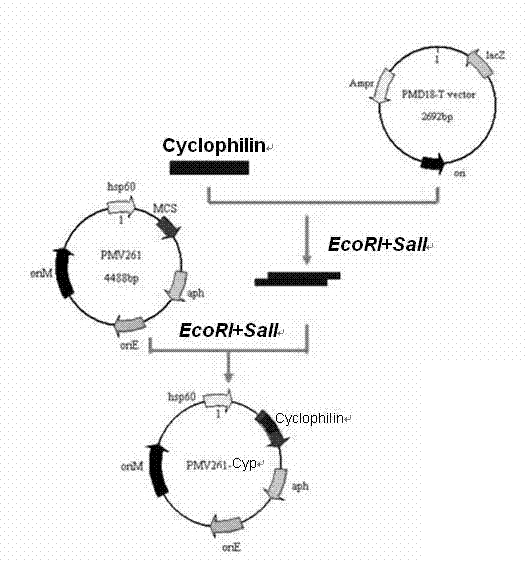
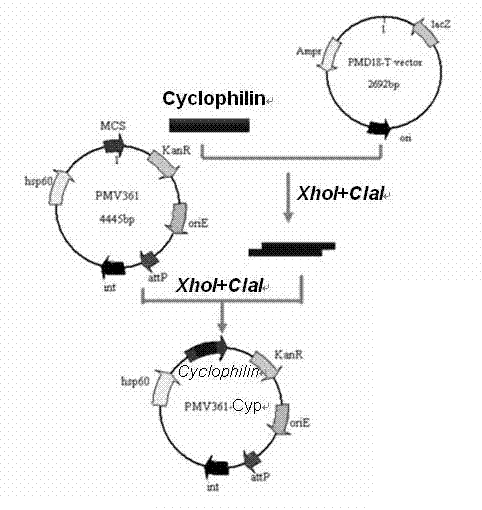
[0026] Taking the protective antigen Cyclophilin gene of Toxoplasma gondii as an example, the shuttle expression vector and the integrated expression vector were constructed.
[0027] 1. The preparation steps of the recombinant BCG vaccine of the shuttle expression vector Toxoplasma gondii:
[0028] According to the Cyclophilin gene DNA sequence and the physical map of the shuttle vector pMV261, two pairs of primers were designed and restriction restriction sites were introduced.
[0029] Upstream primer QF1: 5'- GAATTCATGAAGCTCGTGCTGTTTTTCCT -3'; the 5' end contains an EcoRI site;
[0030] Downstream primer QR: 5'- GTCGACTTACTCCAACAAACCAATGTCCGT -3'; the 5' end contains a SalI site.
[0031] The PCR purified product was cloned into the pMD18-T vector and identified by PCR, enzyme digestion and sequencing. The fragment recovered from the gel was ligated with the shuttle expression vect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com