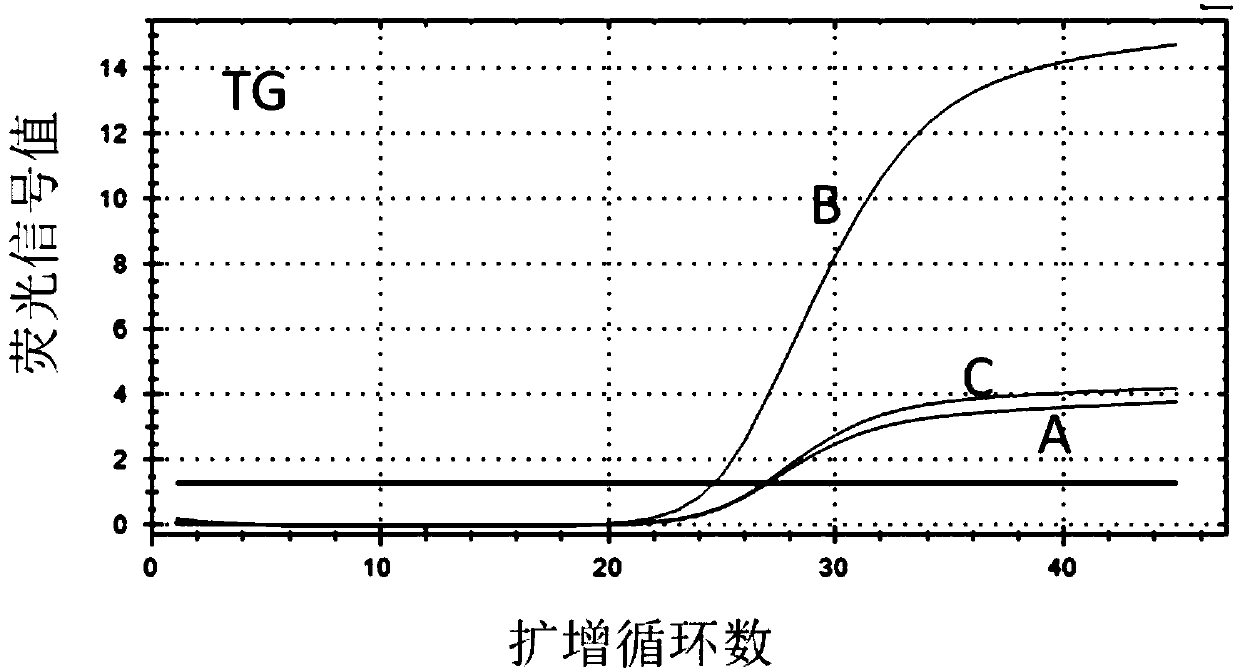
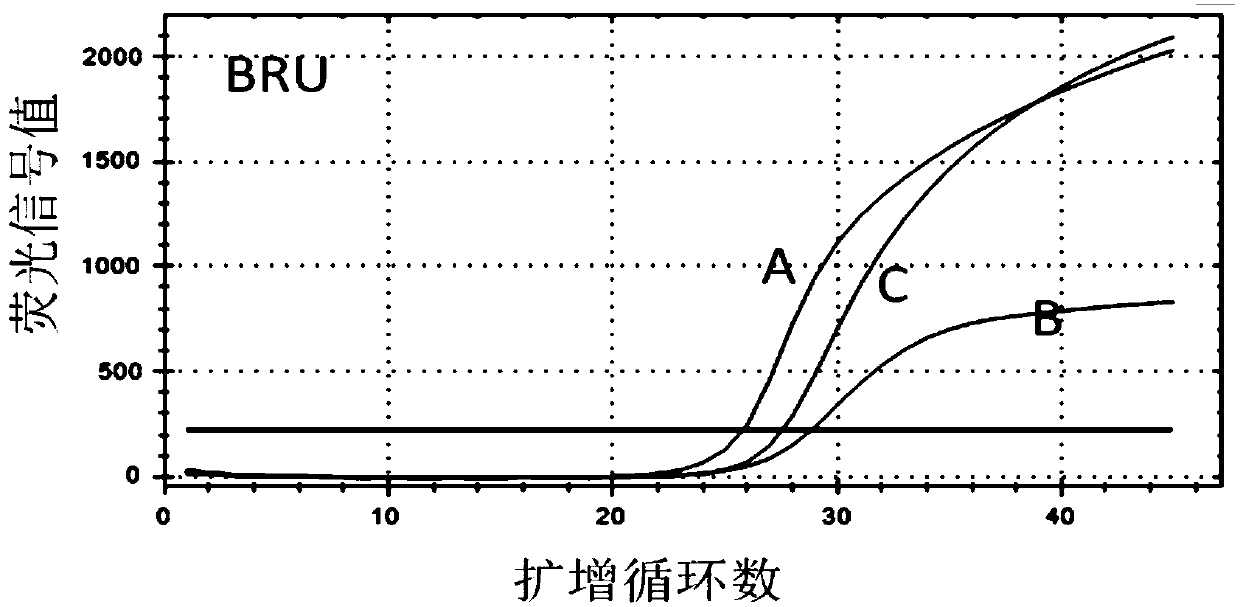
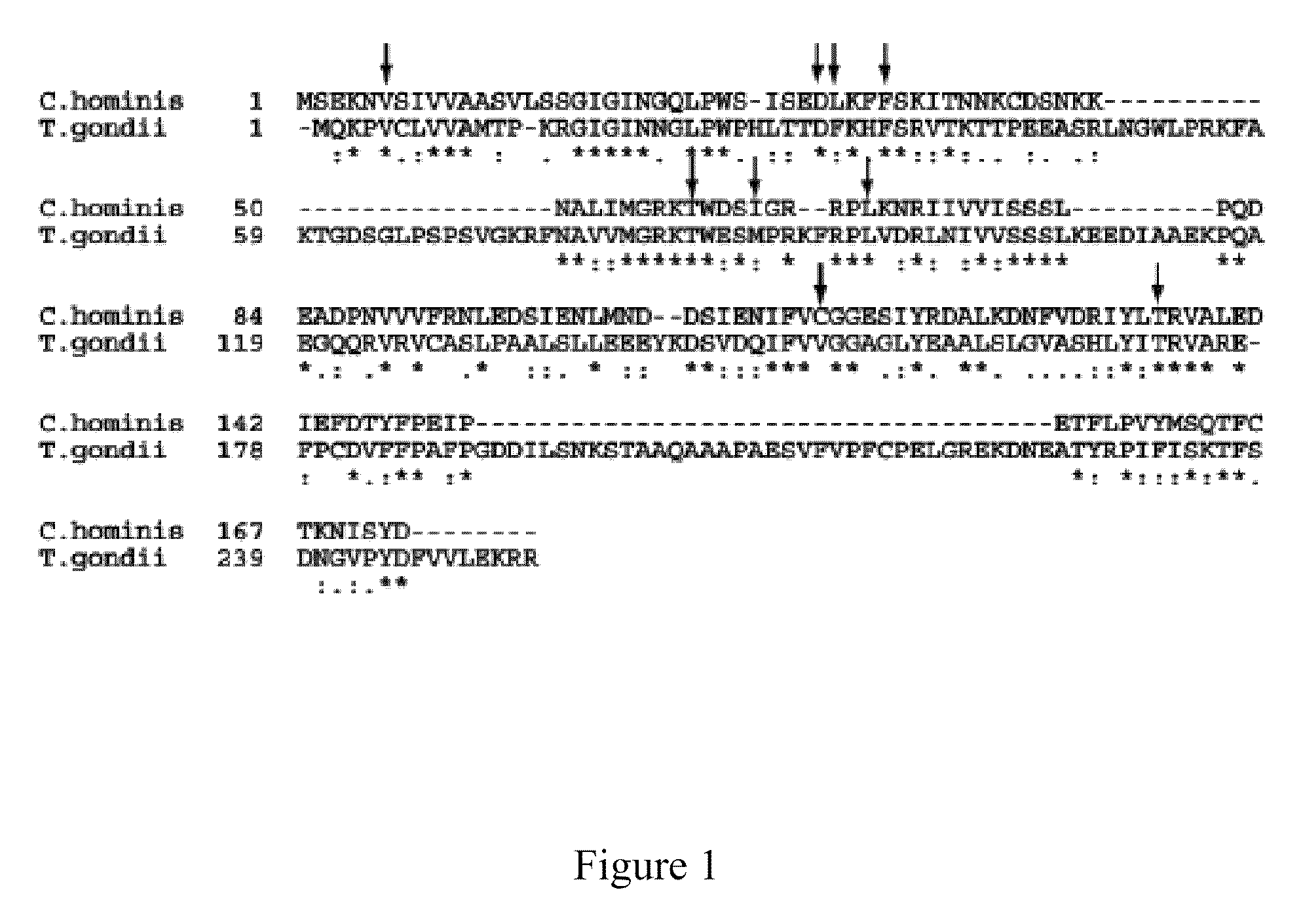
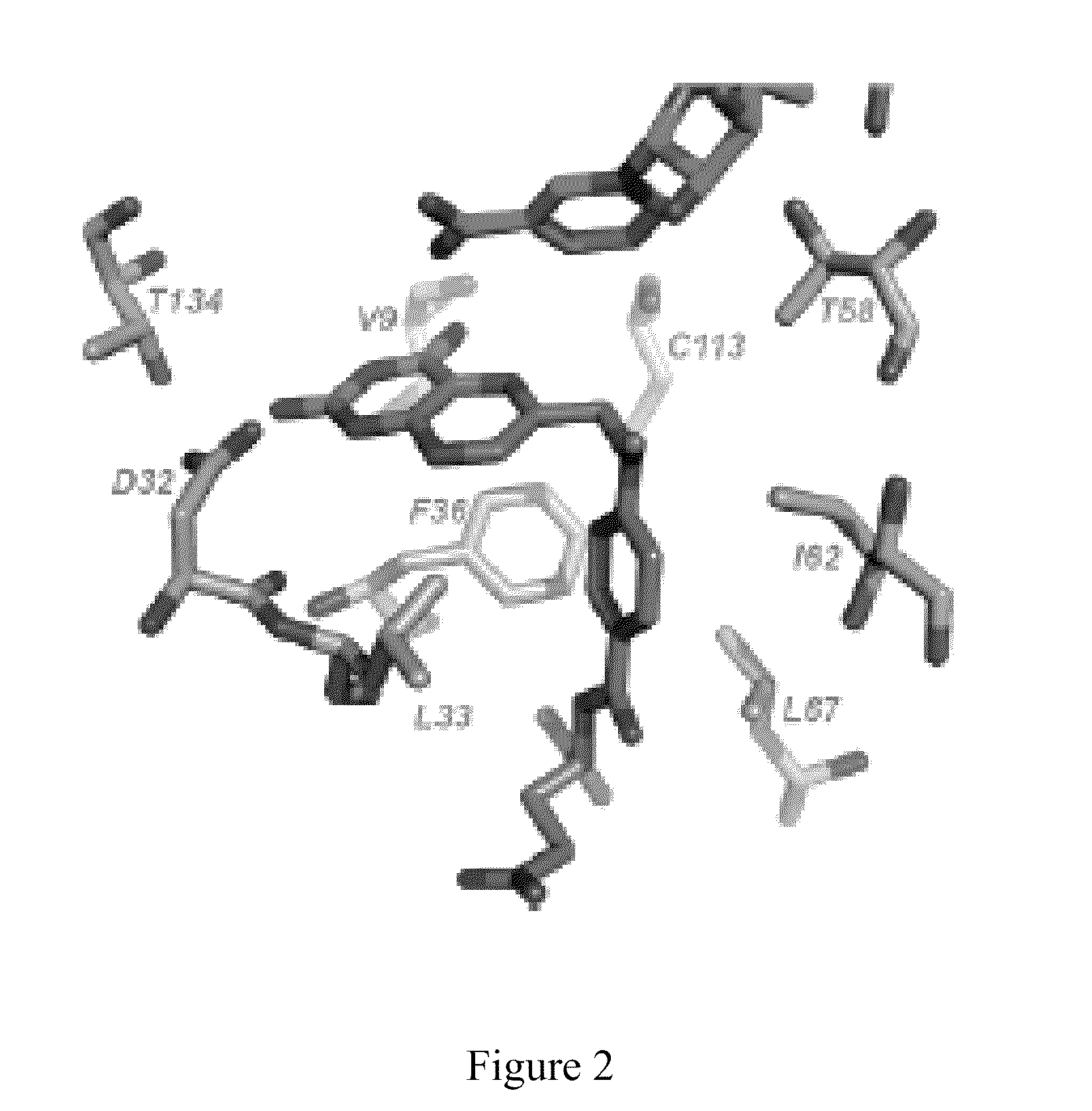
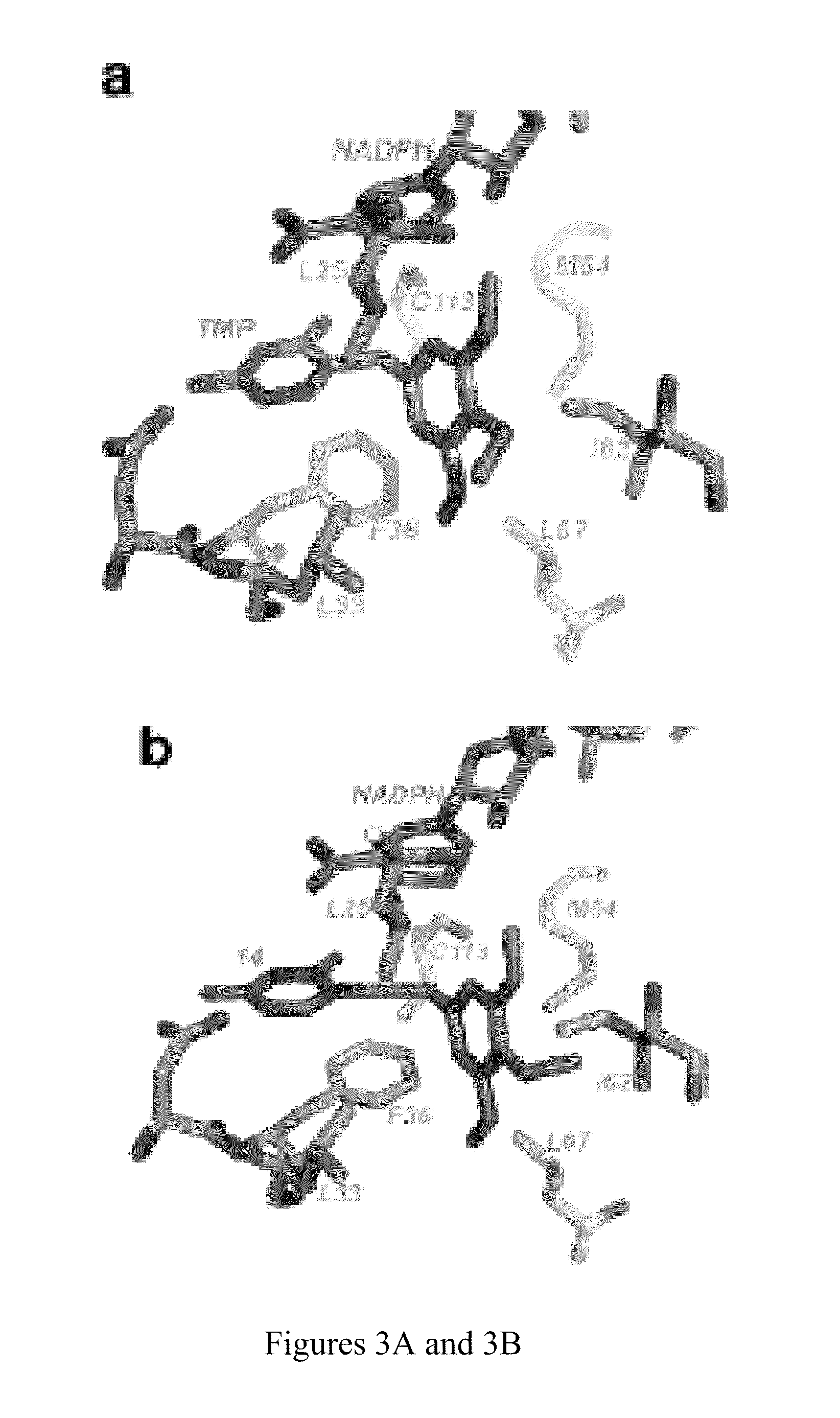
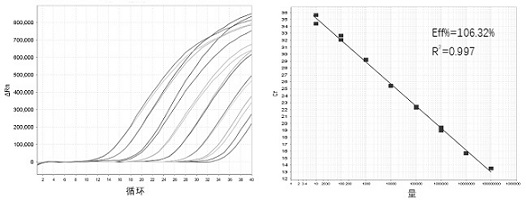
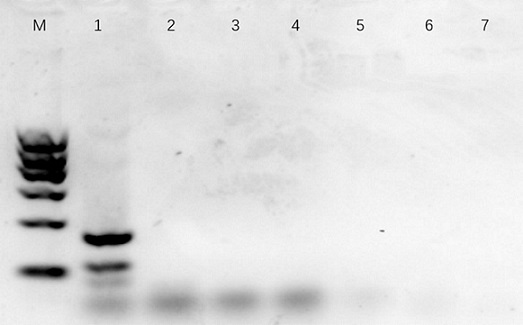
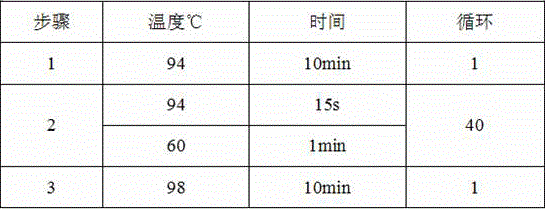
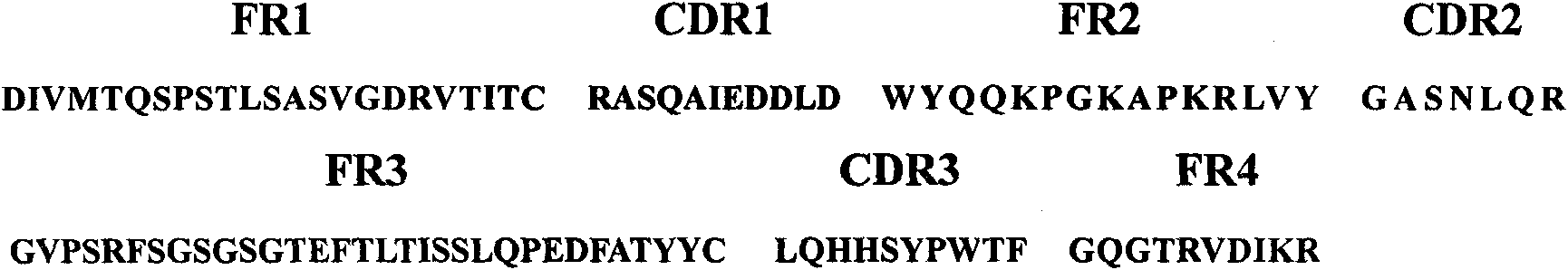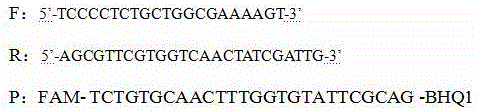Patents
Literature
226 results about "Gondii toxoplasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Toxoplasma gondii (/ˈtɒksoʊplæzmə ˈɡɒndiaɪ/) is an obligate intracellular, parasitic alveolate that causes the disease toxoplasmosis. Found worldwide, T. gondii is capable of infecting virtually all warm-blooded animals, but felids such as domestic cats are the only known definitive hosts in which the parasite may undergo sexual reproduction.
Methods and compositions for treating Toxoplasma
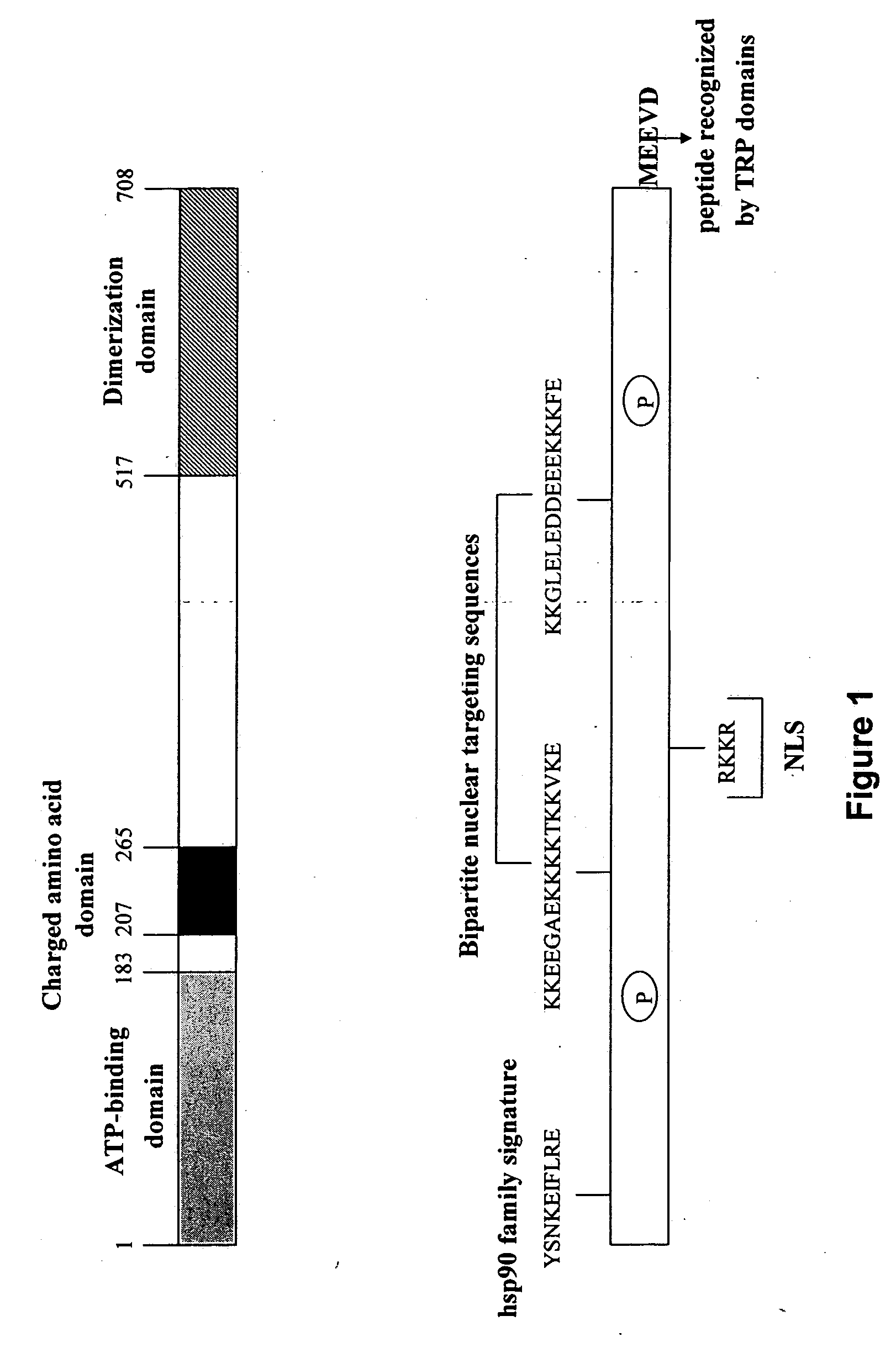
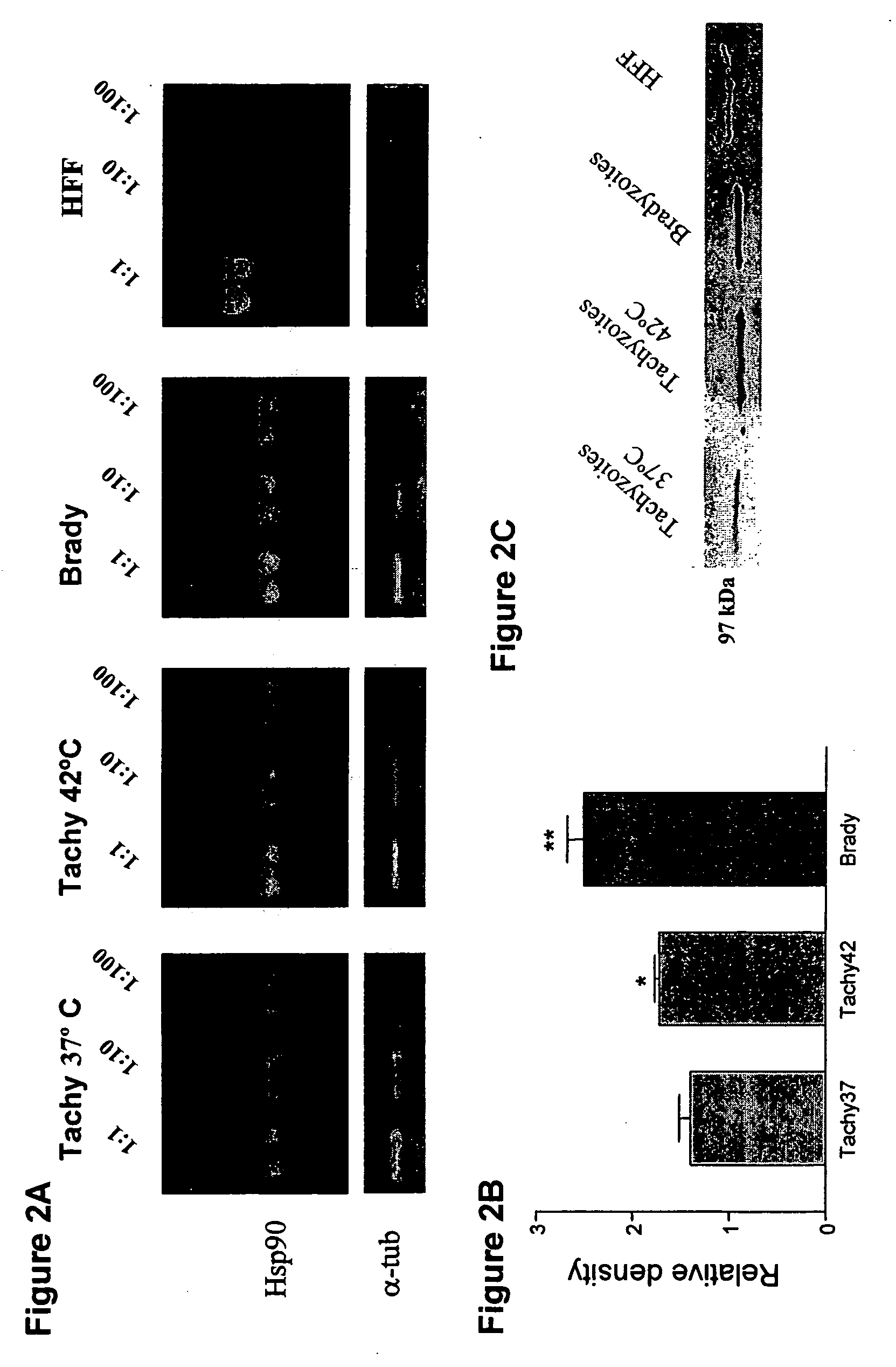
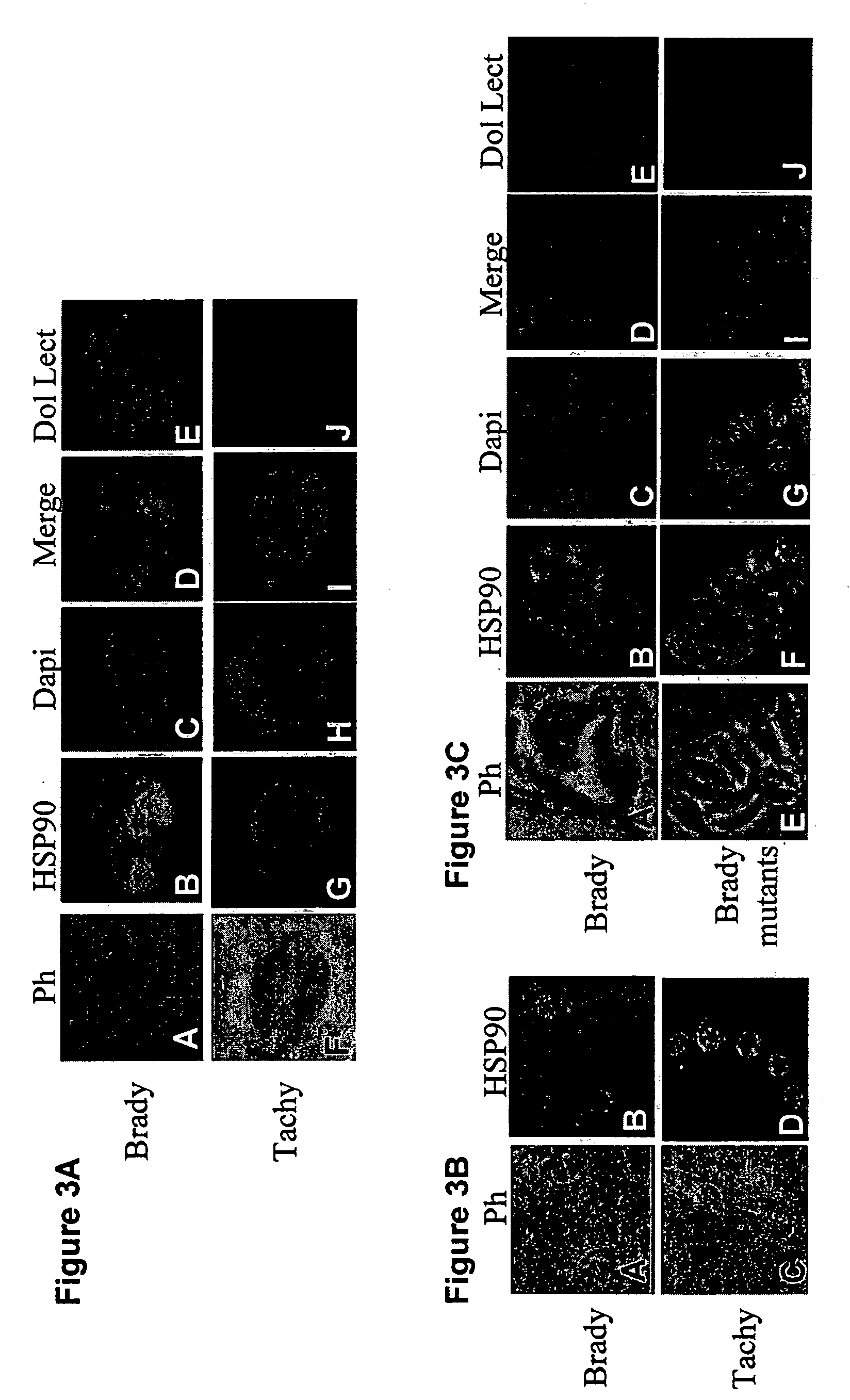
This invention relates to a method for treatment of latent Toxoplasma gondii infection. The invention provides for the use of Hsp90 inhibitors for treatment of latent Toxoplasma gondii infection, particularly in an immunocompromised subject. Also provided is a screening method for identifying compounds useful for treating latent Toxoplasma gondii infection.
Owner:UNIVERSITY OF VERMONT
Chemiluminescence immune analysis determination reagent kit for detecting Toxoplasma Gondi IgM antibody
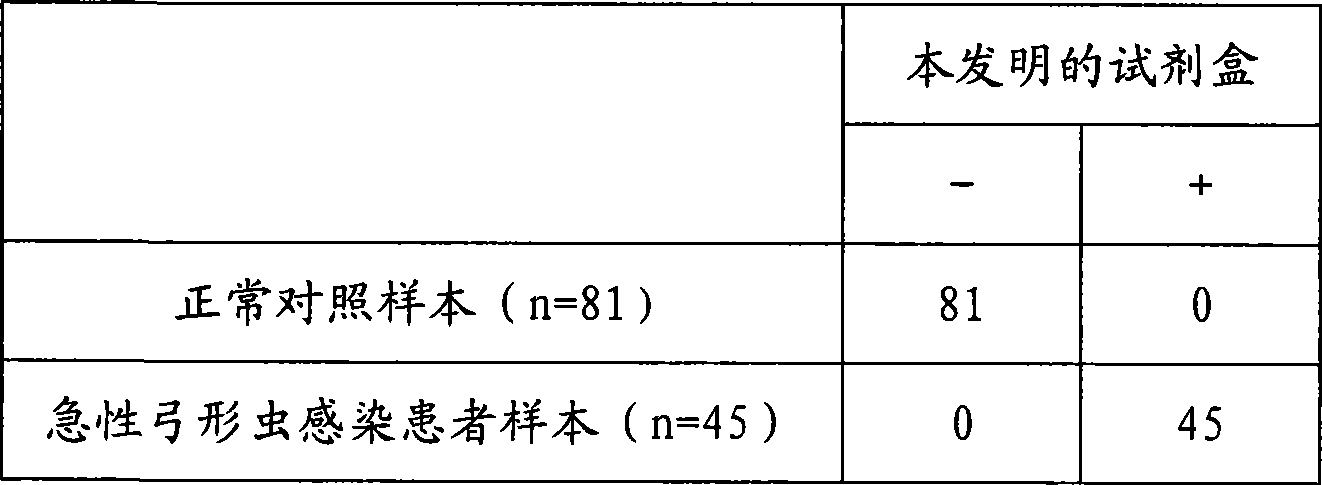
The invention discloses a toxoplasma gondii IgM antibody detection kit combined with the FITC-anti-FITC indirect coating technology and the chemiluminescent immunoassay technology, and a preparation method thereof. The kit of the invention is composed of a negative control, a positive control, solid-phase vectors for anti-FITC antibodies, anti-human Mu-chain monoclonal antibodies of FITC markers, toxoplasma gondii antigens which are marked by horse radish peroxidase, chemiluminescent substrates and concentrated washing solutions. The kit of the invention can be used as the aided detection index for prenatal prepotency diagnosis, and has vital significances for improving the birth population quality and doing the family planning and the prepotency well.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Monoclonal antibody of toxoplasma gondii resistant MIC3 protein and application monoclonal antibody
ActiveCN103333864AHigh potencyStrong specificityTissue cultureImmunoglobulinsHybridoma cellToxoplasma gondii
The invention provides a monoclonal antibody of a toxoplasma gondii resistant MIC3 protein and an application of the monoclonal antibody, and relates to the technical field of biology. The preservation number of a hybridoma cell strain D3 of secreting the monoclonal antibody of the toxoplasma gondii resistant MIC3 protein is CCTCCC (China Center For Type Culture Collection) 201383. The invention also discloses the monoclonal antibody of the toxoplasma gondii resistant MIC3 protein secreted by the hybridoma cell strain D3, and an application of the monoclonal antibody of the toxoplasma gondii resistant MIC3 protein in preparation of a kit and a colloidal gold test strip for detecting the toxoplasma gondii. The monoclonal antibody of the toxoplasma gondii resistant MIC3 protein can be stably and efficiently secreted by the hybridoma cell strain D3; and the monoclonal antibody has high titer and good specificity on the toxoplasma gondii MIC 3 protein. Therefore, the monoclonal antibody can be used for preparing the kit and the colloidal gold test strip for detecting the toxoplasma gondii. The monoclonal antibody is simple in preparation method, simple in anti-body purification process, high in efficiency and low in cost.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Toxoplasma gondii detection kit
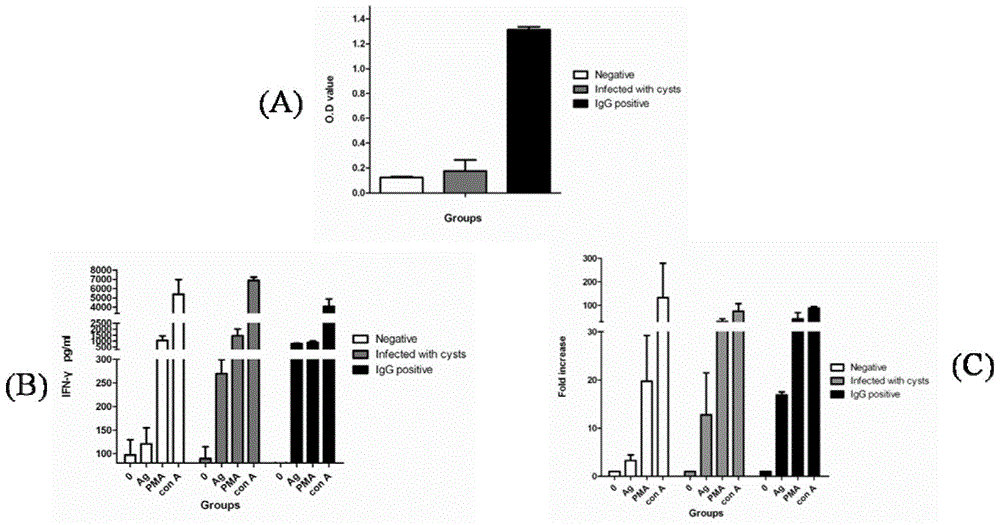
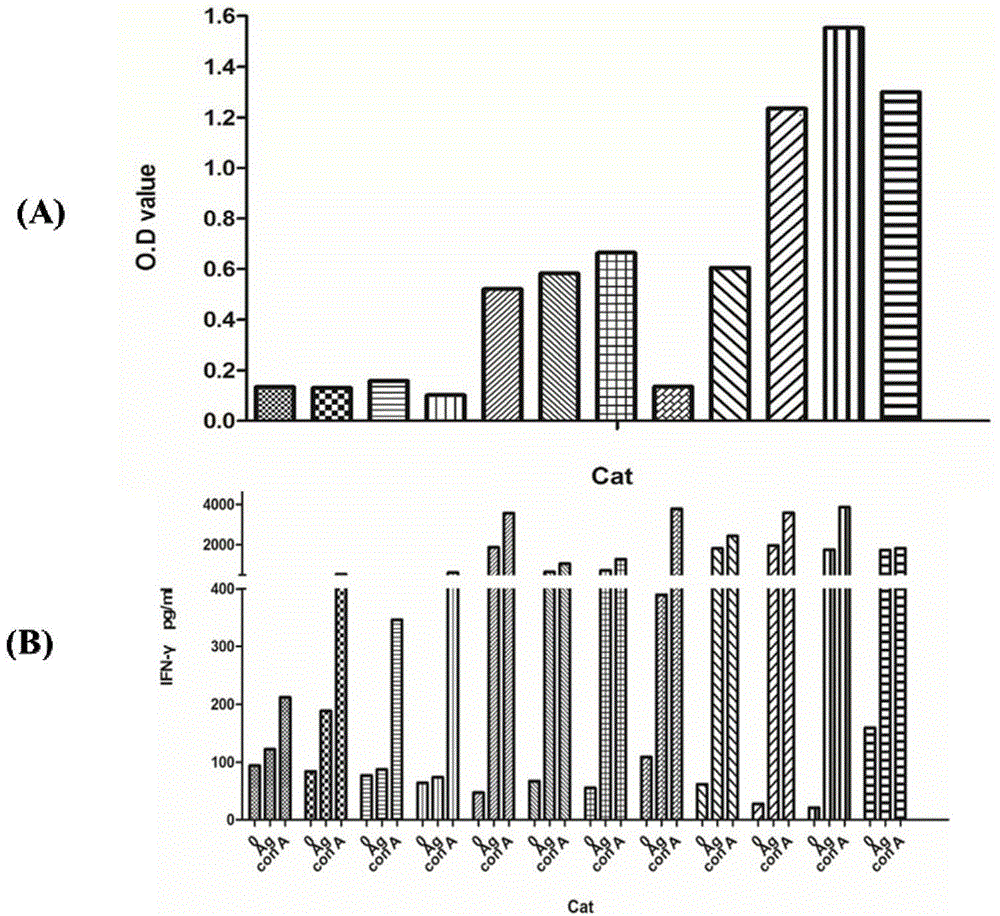
The invention provides a toxoplasma gondii detection kit, and is characterized in that the kit includes a toxoplasma gondii antigen, an IFN-gamma capture antibody, an IFN-gamma standard substance, a biotin-labeled IFN-gamma detection antibody, a cell culture medium, a positive control, a negative control, a coating liquid, a confining liquid, an HRP-labeled streptavidin, a developing solution and a stop solution. The kit has high sensitivity and specificity, and overcomes common shortcomings of poor detection rate and high misdiagnosis rate of a conventional toxoplasma gondii antibody detection kit.
Owner:CHINA AGRI UNIV
Various important aquagenic zoonoses protozoa simultaneous assay kit and preparation method thereof
InactiveCN102154496AAccurate detectionIncreased sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaGondii toxoplasma
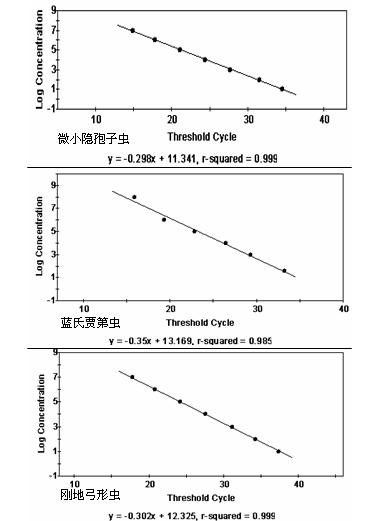
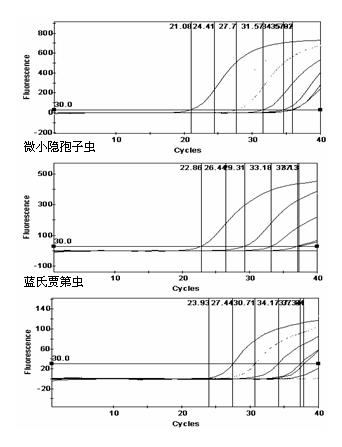
The invention discloses a various important aquagenic zoonoses protozoa simultaneous assay kit, and further provides a preparation method of the kit. A primer and a probe are designed according to the special gene sequence of the giardia lamblia, the cryptosporidium parvum and the toxoplasma gondii by a multi-fluorescence quantitative PCR (polymerase chain reaction) specific assay method, the sensitivity is improved by optimizing the fluorescence quantitative PCR reaction system and condition, the amplification can be performed, and an amplification result can be directly and timely observed.The invention can be used for fast and exactly assaying three important aquagenic zoonoses protozoa, and has the characteristics of being precise in design, simple and easy to operate, high in sensitivity, high in specificity, exact and objective in judgment, and the like.
Owner:JILIN UNIV
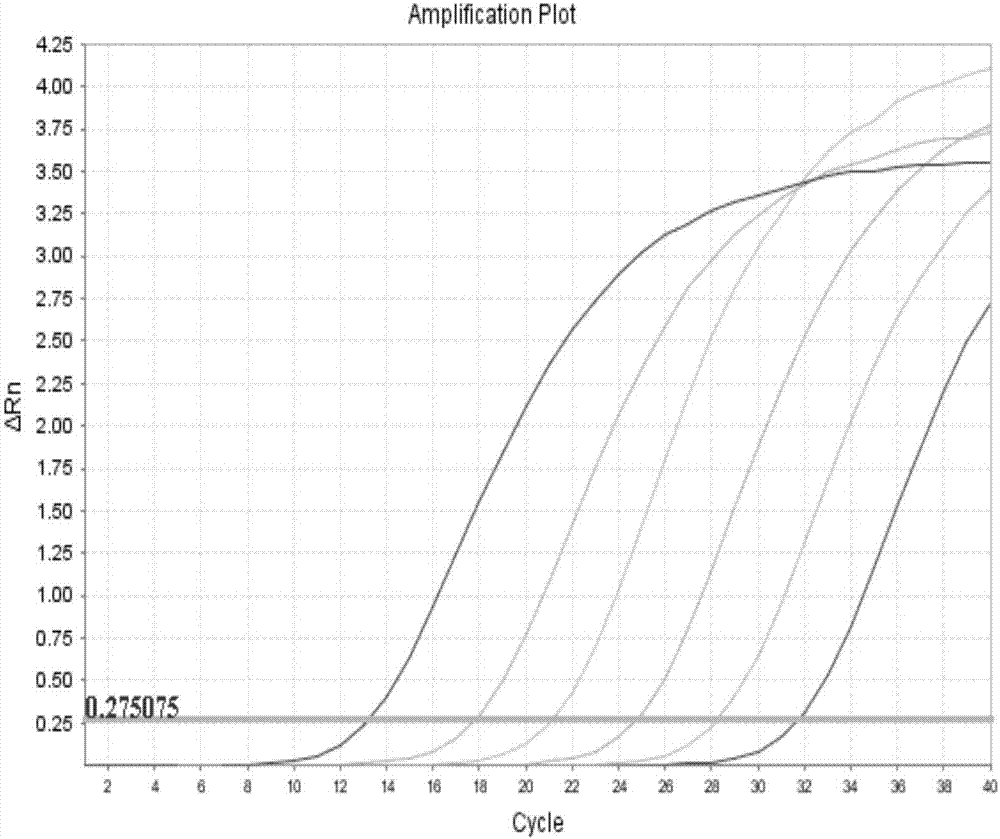
Fluorescent PCR (polymerase chain reaction) method and kit for specifically detecting Toxoplasma gondii nucleic acid
PendingCN107012237AGuaranteed validityLarge-scale usability guaranteeMicrobiological testing/measurementMicroorganism based processesForward primerGondii toxoplasma
The invention relates to a fluorescent PCR (polymerase chain reaction) kit for specifically detecting Toxoplasma gondii nucleic acid, comprising a specific primer pair and a probe; the specific primer pair includes a forward primer: 5'-CCGGGTGAAACAATAGAGAGTACTG-3' and a reverse primer: 5'-GGTCTACGTCGATGGCATGA-3'; the probe is 5'-AACGTCGCCGCTACTGCCCAGTT-3'. The kit of the invention comprises the specific primer pair and probe, has the advantages of high sensitivity, high specificity and high reaction efficiency, can provide qualitative detection for Toxoplasma gondii, and may act as an effective auxiliary detection tool.
Owner:ZHANGJIAGANG LANSU BIOLOGICAL ENG
Fluorescent quantitative PCR (FQ-PCR) kit for rapidly detecting Toxoplasma gondii
InactiveCN101613751AQuantitatively accurateThe detection process is fastMicrobiological testing/measurementMicroorganism based processesEtiologyBiology
The invention discloses a fluorescent quantitative PCR (FQ-PCR) kit for rapidly detecting Toxoplasma gondii, relating to gene detection technology of zoonosis pathogen, and being applicable to qualitative and quantitative detection of Toxoplasma gondii. The invention comprises DNA extracting solution, a standard positive template, a fluorescent quantitative PCR reaction solution, Toxoplasma gondii B1 gene specific primer and a negative quality control standard product. The invention is accurate in quantifying, rapid in detection speed, good in specificity, high in sensitivity, simple in use steps and high in repeatability and can replace traditional etiology detection method.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Screening and application method of small-molecule inhibitor capable of inhibiting proliferation of toxoplasma gondii
InactiveCN107884587APrevent proliferationR&D cost economyBiological testingScreening methodAccelerant
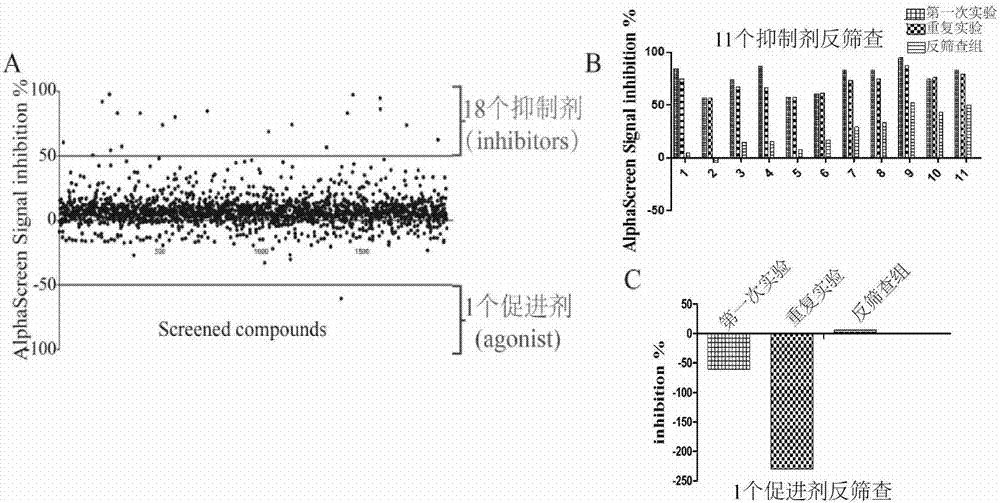
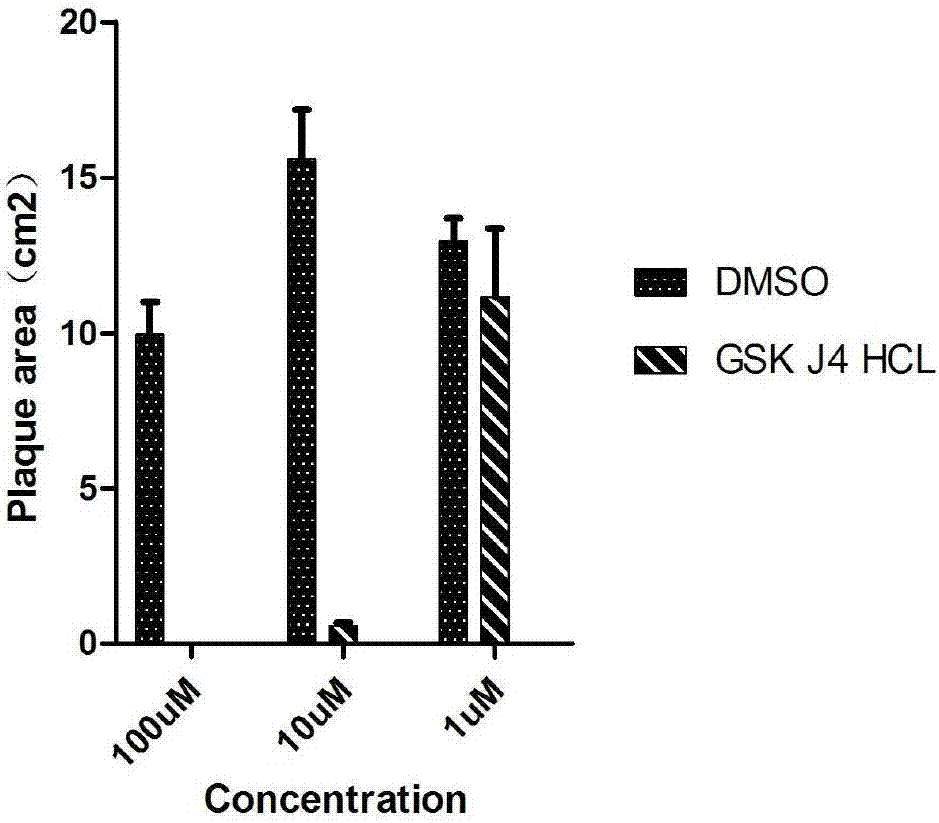
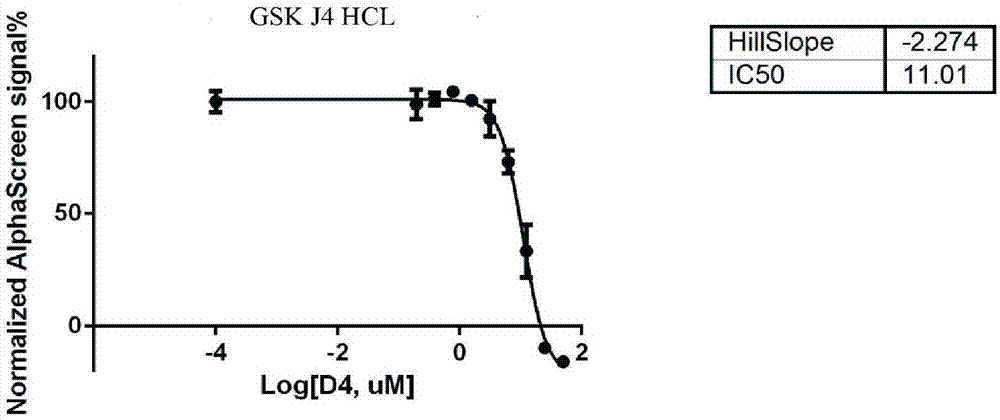
The invention discloses a screening method of small-molecule inhibitor capable of inhibiting proliferation of toxoplasma gondii. The method utilizes an Alphascreen experiment to primarily select 11 small-molecule inhibitors and one small-molecule accelerant, and the 12 drugs are used for clinical treatment. The 12 drugs are further screened at the cellular level, a plaque experiment proves that GSK J4HCL apparently inhibits the proliferation of toxoplasma gondii, and the value EC50 of the GSK J4HCL to toxoplasmagondii is determined to be about 4.5 muM by virtue of intracellular proliferation experiment. The AlphaScreen competitive inhibition experiment evaluates that the value IC50 of the GSK J4HCL for inhibiting the interaction of TgAtg8 to TgAtg3 is 11.01 muM. The CCK8 experiment is usedfor proving that the value IC50 of the drug for inhibiting the growth of a host cell is 34.359 muM which is far greater than the value EC50 of the drug for the insect, which illustrates that the GSKJ4HCL is a low-toxicity and high-efficiency drug for resisting the toxoplasma gondii.
Owner:WENZHOU MEDICAL UNIV
Real-time recombinase-mediated isothermal amplification nucleic acid kit for rapid detection of toxoplasma gondii and application thereof
InactiveCN111139309AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesGondii toxoplasmaRecombinase
The invention discloses a real-time recombinase-mediated isothermal amplification nucleic acid kit for rapid detection of toxoplasma gondii and an application thereof. The kit comprises standards. Thestandards are a positive plasmid containing a 529 gene sequence, specific primers and probes designed for 529 gene of the toxoplasma gondii. The primers designed based on a specific conservative target sequence of the 529 gene of the toxoplasma gondii for qualitatively detecting the 529 gene of the toxoplasma gondii in tissues, feces or blood samples comprise an upstream primer and a downstream primer. According to the present invention, a real-time fluorescent RAA technology is used to establish the method for rapid detection of the toxoplasma gondii. Compared with fluorescent quantitative PCR, the method has the advantages of low cost, simpleness and fastness, can conduct detection at constant temperature of 36 DEG C within 20min, and directly read detection results by a portable instrument. The method of the present invention achieves minimum detection size for toxoplasma gondii genomes up to 102 copies, has sensitivity comparable to the traditional fluorescent quantitative PCR, and is suitable for rapid diagnosis of clinical samples and laboratories.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Indirect ELISA (enzyme-linked immunosorbent assay) detection kit based on toxoplasma gondii matrix protein 1
InactiveCN104155443AConvenient sourceDetect infectionBiological material analysisAssayGondii toxoplasma
The invention provides an indirect ELISA (enzyme-linked immunosorbent assay) detection kit based on toxoplasma gondii matrix protein 1. The indirect ELISA detection kit consists of a 96-hole elisa plate, a substrate coating substance, a standard substance, a reference substance, a substrate solution, skim milk powder, a sample diluent, washing liquid, reaction liquid, color developing liquid and stopping liquid. The indirect ELISA detection kit can identify IgGs of a variety of mammals such as humans, rabbits, pigs, dogs, cats, monkeys, mice, etc., can detect infection of toxoplasma gondii relatively rapidly, is high in specificity and sensitivity, and especially in large-scale clinical detection, can greatly improve the detection efficiency; in a detection method, an expensive PCR detector and other equipment are not required, but only an UV spectrophotometer, a thermostat and other equipment are needed; the indirect ELISA detection kit is simple in operation, high in specificity, good in repeatability and clear and stable in result; a sample to be detected is very easy to obtain.
Owner:ZHEJIANG UNIV
Multiplex PCR primer probes and kit for detecting pet-derived zoonotic pathogens
ActiveCN109609665ASimplify detection workloadShorten detection timeMicrobiological testing/measurementDNA/RNA fragmentationSequence analysisCompanion animal
The present invention provides multiplex PCR primer probes and a kit for detecting pet-derived zoonotic pathogens. Bartonella henselae, toxoplasma gondii and brucella belonging to pet-derived zoonoticpathogens are subjected to gene sequence analysis and comparison, triple fluorescent PCR detection primers and probes suitable for single reaction and simultaneous detection of the three pathogens are designed, and nucleotide sequences of the primers and probes are shown in SEQ ID NO.1-9, respectively. The present invention also discloses a method and a detection kit for detecting the above 3 pathogens. The detection method simplifies detection workload of 2 / 3 or more of the original, and shortens detection time by about 1 hour, and detection sensitivities of the bartonella henselae, toxoplasma gondii and brucella are 5, 5 and 50 copies, respectively. The multiplex PCR primer probes have no non-specific amplification of 23 common pathogens and canine and cat chromosomes, and show good specificity.
Owner:西安博睿康宁生物科技有限公司
Inhibitors of dihydrofolate reductase with antibacterial antiprotozoal, antifungal and anticancer properties
ActiveUS8426432B2Increase capacityStraightforward to synthesizeAntibacterial agentsBiocideProtozoaBacteroides
Owner:UNIV OF CONNECTICUT
Indirect enzyme-linked immuno sorbent assay (ELISA) kit for detecting canine and feline Toxoplasma gondii antibodies
InactiveCN103837688AStrong specificityGood repeatabilityBiological material analysisBiological testingAntigenInfectious Disorder
The invention discloses an indirect enzyme-linked immuno sorbent assay (ELISA) kit for detecting canine and feline Toxoplasma gondii antibodies, relates to an immunological detection technology for human and animal infectious diseases and is applicable to qualitative detection of canine and feline Toxoplasma gondii antibodies. The kit comprises a Toxoplasma gondii recombinant antigen GRA7 pre-coated ELISA plate, wherein the sample diluent is 0.01 mol / L of PBS (with the pH of 7.2) containing 0.5 percent of BSA and 0.05 percent of NaN3; the enzyme bonder is a horse radish peroxidase (HRP)-rabbit anti-dog / cat IgG bonder; the substrate developing solution is a TMB developing solution; the stopping solution is a 2mol / L of sulfuric acid solution, positive control and negative control. The kit is high in sensitivity, high in specificity and high in repeatability and can serve as a method for detecting canine and feline Toxoplasma gondii antibodies.
Owner:JILIN AGRICULTURAL UNIV
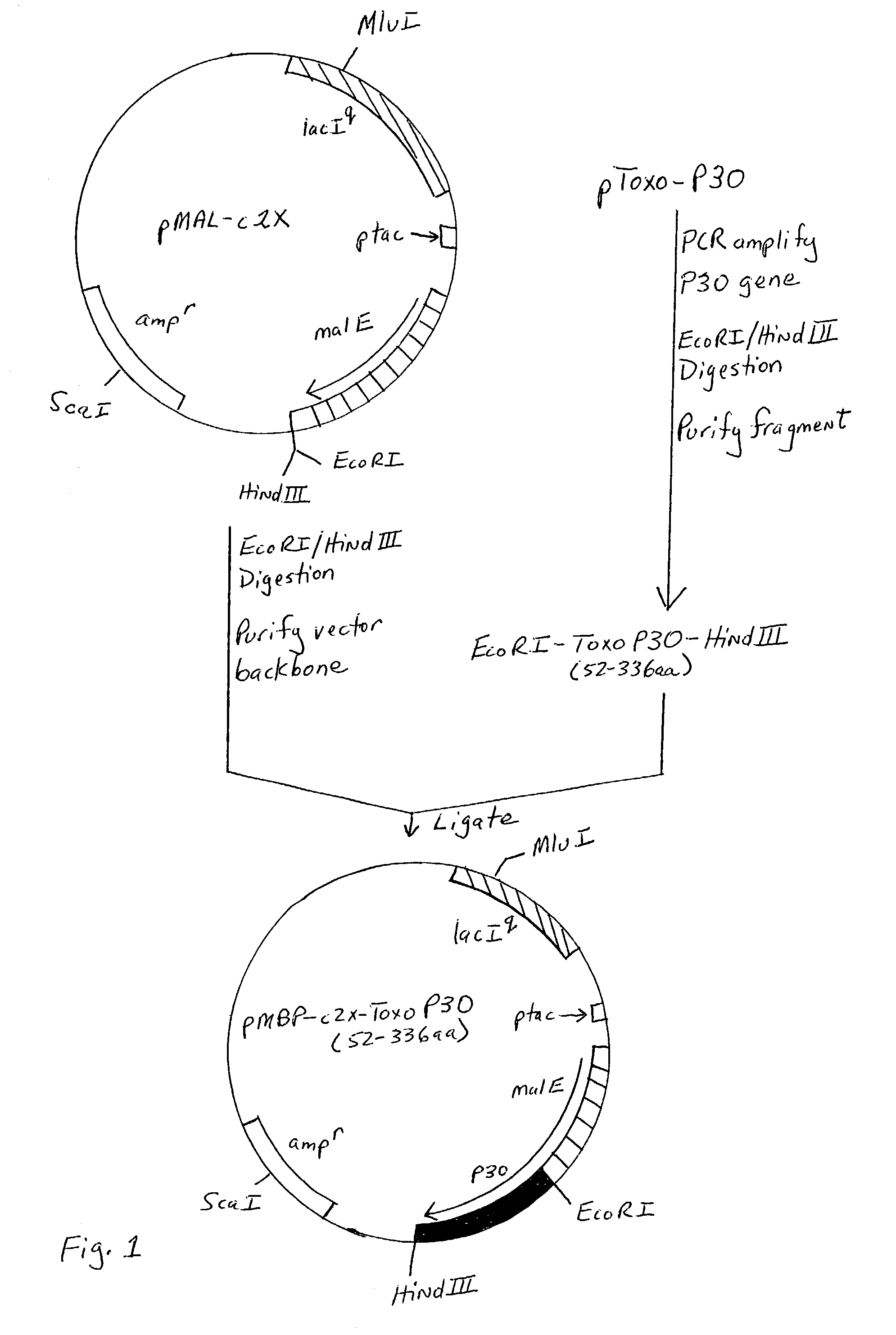
Genetically engineered P30 antigen, improved antigen cocktail, and uses thereof
The present invention relates to a genetically engineered P30 antigen and a combination or mixture of antigens (e.g., the genetically engineered P30 antigen and P35) that may be used in the detection of IgM and / or IgG antibodies to Toxoplasma gondii. Furthermore, the present invention also relates to methods of using this genetically engineered P30 antigen and combination of antigens, antibodies raised against this genetically engineered P30 antigen and combination of antigens, as well as kits and vaccines containing the genetically engineered P30 antigen and antigens present in the combination.
Owner:ABBOTT LAB INC
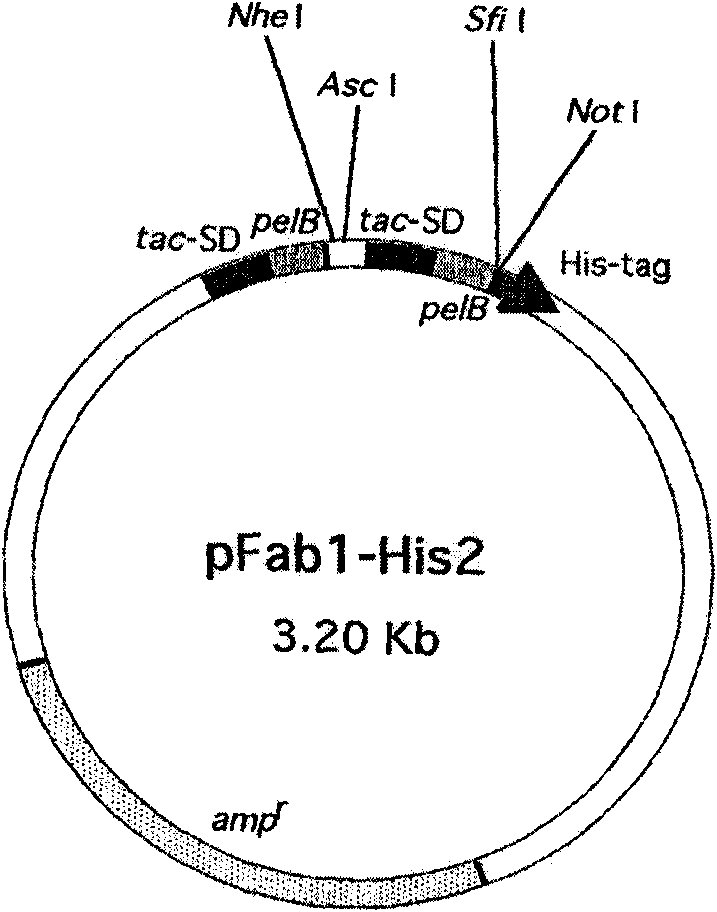
Surface antigen 1 of Toxoplasma gondii human antibody Fab fragment and encoded gene thereof
The present invention belongs to the field of biotechnology, and relates to a surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment, encoded gene and use thereof. According to the invention, the surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is filtered from a base through establishing a Toxoplasma gondii human immunoglobulin, ELISA, diluting the prothrombin time, sequencing analysis, etc. Through expression purifying and authenticating, the human antigen Fab fragment is authenticated to specifically identify the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii and have higher affinity with the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii, for being identified with the specificity of Toxoplasma gondii tachyzoite-bradyzoite. The human antigen Fab fragment of the invention does not contain Fc segment and does not activate the alexin or cause the histopathological damages of human immune response, etc. when the function of restricting the invasion of Toxoplasma gondii to the host cell is exerted. The surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is safe and reliable when applied for the human body. The antigen medicine for treating toxoplasmosis or the antigen targeted medicine can be prepared.
Owner:FUDAN UNIV
Monoclonal antibody to toxoplasma gondii ROP (rhoptry protein) 18, cell strain secreting monoclonal antibody and application thereof
The invention discloses a monoclonal antibody to toxoplasma gondii ROP (rhoptry protein) 18, a cell strain secreting the monoclonal antibody and an application thereof. The cell strain of the monoclonal antibody of the toxoplasma gondii ROP18 is a hybrid tumor cell strain T2 and is preserved by the typical culture preservation center in China, the address is Wuhan University, Wuhan, China, the preservation number is CCTCC, NO: C2018115, and the preservation date is July, 20, 2018. The monoclonal antibody of the toxoplasma gondii ROP18 is secreted by the hybrid tumor cell strain T2 and can be used to prepare an antigen reagent for detecting the toxoplasma gondii ROP18; the mouse-derived cell strain T2 of the monoclonal antibody of the toxoplasma gondii ROP18 is prepared through a cell fusion technique, and the monoclonal antibody secreted by the cell strain is screened by indirect competitive elisa and preliminarily identified by epitope, which lays a foundation for further clinical application.
Owner:ANHUI MEDICAL UNIV
Inhibitors of Dihydrofolate Reductase With Antibacterial Antiprotozoal, Antifungal and Anticancer Properties
The compositions and methods described herein discloses the design, synthesis and testing of compounds that act as inhibitors of DHFR. The basic scaffold of these inhibitors includes a 2,4-diaminopyrimidine ring with a propargyl linker to another substituted aryl, bicyclo or heteroaryl ring. These DHFR inhibitors are potent and selective for many different pathogenic organisms, including the DHFR enzyme from bacteria such as Bacillus anthracis and methicillin-resistant Staphylococcus aureus, fungi such as Candida glabrata, Candida albicans and Cryptococcus neoformans and protozoa such as Cryptosporidium hominis and Toxoplasma gondii. These compounds and other similar compounds are also potent against the mammalian enzyme and may be useful as anti-cancer therapeutics.
Owner:UNIV OF CONNECTICUT
Dog toxoplasma gondii antibody indirect ELISA detection kit
InactiveCN104965086AImproving immunogenicityImprove protectionBiological material analysisBiological testingGondii toxoplasmaEpidemiologic survey
The invention discloses a dog toxoplasma gondii antibody indirect ELISA detection kit, and belongs to the technical field of biological inspection and quarantine. According to the dog toxoplasma gondii antibody indirect ELISA detection kit, toxoplasma gondii ROP5 recombinant protein is taken as a detection antigen. According to the dog toxoplasma gondii antibody indirect ELISA detection kit, toxoplasma gondii recombinant protein ROP5 is obtained via gene cloning and prokaryotic expression; and a detection method comprises following steps: establishment of an indirect ELISA detection method, specificity testing, sensitivity testing, and comparison with other dog toxoplasma gondii detection kits. Large-scale standardized production of an envelope antigen of the dog toxoplasma gondii antibody indirect ELISA detection kit can be realized; detection specificity is high; sensitivity is high; repeatability is high; result determination is objective; operation process is simple; and large-scale detection can be realized. The dog toxoplasma gondii antibody indirect ELISA detection kit is suitable for dog toxoplasmosis epidemiological investigation, and detection and clinical diagnosis of dog toxoplasma gondii infection; and a rapid, simple, and specific detection method is provided for diagnosis and prevention, and epidemiological investigation of dog toxoplasmosis.
Owner:SOUTH CHINA AGRI UNIV
Toxoplasma gondii apical membrane antigen-1
InactiveUS20050210535A1Reduce the possibilityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenToxoplasmosis
The invention provides polypeptide fragments derived from TgAMA-1, nucleic acids that encode the polypeptide fragments, and TgAMA-binding polypeptides such as antibodies. Methods for using the polypeptide and nucleic acid molecules to produce vaccines are also provided. In addition the invention provides methods involving use of the polypeptides, nucleic acids, and binding polypeptides, such as antibodies, for the prevention and treatment of Toxoplasmosis.
Owner:UNIVERSITY OF VERMONT +1
TgVP1 extracellular region antigen polypeptide, anti-TgVP1 polyclonal antibody and application of polyclonal antibody
The invention discloses a toxoplasma gondii TgVP1 extracellular region antigen polypeptide, an anti-toxoplasma gondii TgVP1 polyclonal antibody and the application of the polyclonal antibody. The amino acid sequence of the toxoplasma gondii TgVP1 extracellular region antigen polypeptide is as shown in SEQ ID NO: 1 or a sequence as shown in SEQ ID NO: 1 of which the N end is connected with a cysteine. A New Zealand rabbit is immunized by the toxoplasma gondii TgVP1 extracellular region antigen polypeptide as an immunogen so that the anti-toxoplasma gondii TgVP1 polyclonal antibody can be obtained; an identification result indicates that the polyclonal antibody is capable of specifically identifying the toxoplasma gondii TgVP1 protein and can be applied to the detection of the toxoplasma gondii TgVP1 protein in tests such as ELISA, Western blot and immunofluorescence. A powerful tool is provided for the fundamental research of the protein functions and the research of the protein as a potential anti-toxoplasma gondii drug target; in short, the polyclonal antibody is wide in application prospect.
Owner:SOUTHERN MEDICAL UNIVERSITY
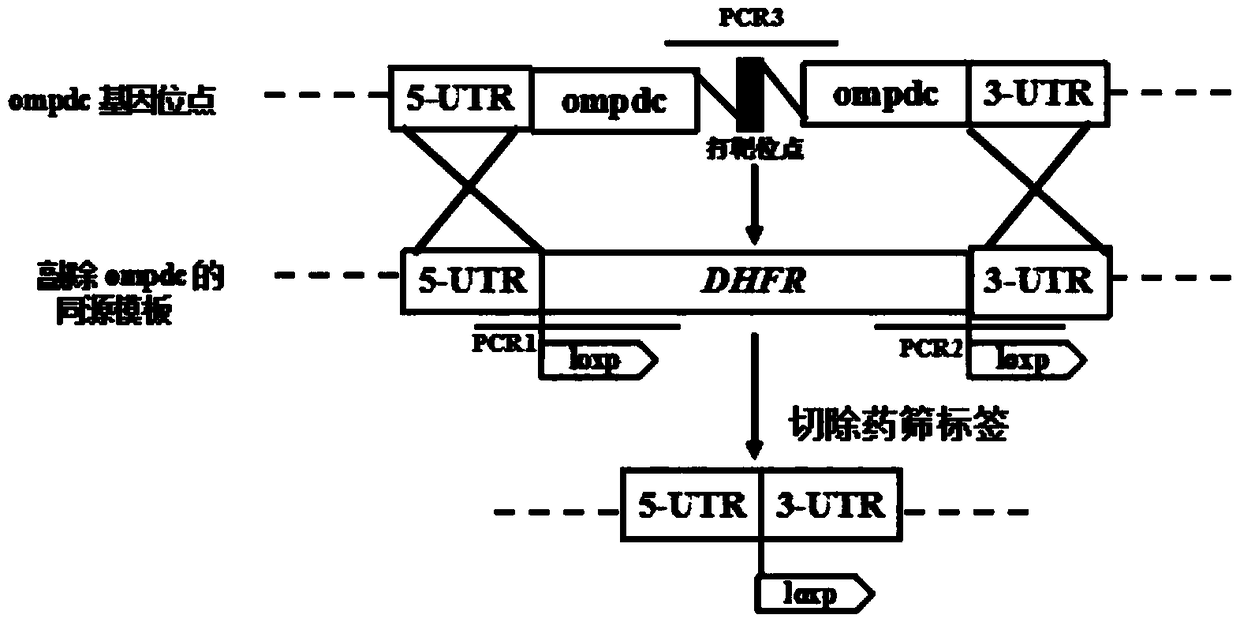
Toxoplasma gondii attenuated live vaccine with deficiency of OMPDC and LDH1 genes
ActiveCN108434447APrevention of acute and chronicPrevent congenital infectionProtozoa antigen ingredientsStable introduction of DNALactate dehydrogenaseGondii toxoplasma
The invention discloses an attenuated live vaccine capable of preventing toxoplasma gondii infection. The vaccine contains a toxoplasma gondii vaccine strain with simultaneous deficiency of the orotidine-5-phosphate decarboxylase gene and the lactic dehydrogenase 1 gene, wherein the nucleotide sequence of the orotidine-5-phosphate decarboxylase gene is as shown in SEQ ID NO:1, and the nucleotide sequence of the lactic dehydrogenase 1 gene is as shown in SEQ ID NO:2. The attenuated live vaccine provided by the invention has the advantages that basically no virulence exists, the immunocompetencefor toxoplasma gondii of animals can be improved, the acute and chronic as well as congenital infection of toxoplasma gondii can be prevented, the vaccine strain cuts off the drug resistance screening label, and thus the vaccine strain has the potential of being prepared into the genetically engineered vaccine for resisting toxoplasma gondii.
Owner:HUAZHONG AGRI UNIV
Nano antibody for resisting toxoplasma gondii SAG1 as well as coding gene and application thereof
ActiveCN108503707AEfficient detectionGood water solubilityImmunoglobulinsFermentationSolubilityAntigen
The invention discloses a nano antibody for resisting toxoplasma gondii SAG1 as well as a coding gene and application thereof. A VHH chain amino acid sequence of the nano antibody is as shown by SEQ ID NO. 4. The nano antibody comprises two VHH chains. The nano antibody for resisting the toxoplasma gondii SAG1 is obtained by immunizing a camel by virtue of toxoplasma gondii SAG1 antigen, obtainingan anti-SAG1 nano antibody library, and selecting a nano antibody with good performance from the nano antibody library. The nano antibody has high solubility and conformation stability and high antigen affinity and excellent tissue penetration capability, and has high affinity with SAG1 antigen, the affinity constant KD value is 1.66nM, the toxoplasma gondii can be efficiently detected, and the nano antibody can be used for preparing toxoplasma gondii detection kit.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
Compositions and methods for treatment of Toxoplasma gondii and other apicomplexans
Pyrimidine auxotroph mutants of apicomplexans are provided which are mutated in one of six enzymes of the de novo pyrimidine biosynthesis pathway. Also provided are methods of protecting an animal against infection by apicomplexans by administering a pyrimidine auxotroph mutant and methods for screening for inhibitors of pyrimidine salvage enzymes in apicomplexans.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Monoclonal antibody for anti-toxoplasma gondii rhoptry protein4(ROP4) and preparation method and application of monoclonal antibody
PendingCN110527668ASample handling is convenientLow costAntibody mimetics/scaffoldsHybrid cell preparationAntigenTiter
The invention relates to the field of biotechnology, in particular to a monoclonal antibody for anti-toxoplasma gondii rhoptry protein4(ROP4) and a preparation method and application of the monoclonalantibody. The monoclonal antibody for anti-ROP4 is produced by secretion of hybridoma cell strain 3B-7, and can be used for detecting toxoplasma gondii circulating antigen of serum and plasma of dogs, cats, pigs and chooks, and the lowest limit of the monoclonal antibody for toxoplasma gondii protein detection is 17.71 ng / mL. According to the monoclonal antibody for anti-toxoplasma gondii rhoptryprotein4(ROP4) and the preparation method and application of the monoclonal antibody, the murine hybridoma cell strain 3B-7 obtained through a cell fusion technique can stably secrete monoclonal antibody for anti-ROP4, it is discovered through determination that the monoclonal antibody has the advantages that the titer is high, and the specificity is high, and a foundation is laid for further application of the monoclonal antibody to detection of toxoplasma gondii pathogeny in clinic.
Owner:SOUTH CHINA AGRI UNIV
Toxoplasma gondii IgG antibody immunoblotting kit and preparation method thereof
Toxoplasma gondii IgG antibody immunoblotting kit and preparation method thereof relate to a kit. The kit is provided with a carrier plate, a nitrocellulose membrane, a toxoplasma IgG antibody detection line and a control line; the toxoplasma IgG antibody detection line and the control line are sequentially arranged on the nitrocellulose membrane; Toxoplasma recombinant antigen, coated with human IgG antibody at the control line; horseradish peroxidase-labeled anti-human γ-chain antibody. Preparation of Toxoplasma recombinant antigen; spotting of nitrocellulose membrane; preparation of anti-human IgG specific fragment γ chain monoclonal antibody; anti-human IgG specific fragment γ chain monoclonal antibody labeled with horseradish peroxidase; preparation of western blot Reagent test kit. When testing, the required sample volume is extremely small, no special equipment is required, and the results can be directly interpreted with the naked eye. The detection is simple and fast, with strong specificity, high sensitivity, accuracy and reliability, low cost, and wide application.
Owner:厦门市湖里区妇幼保健院
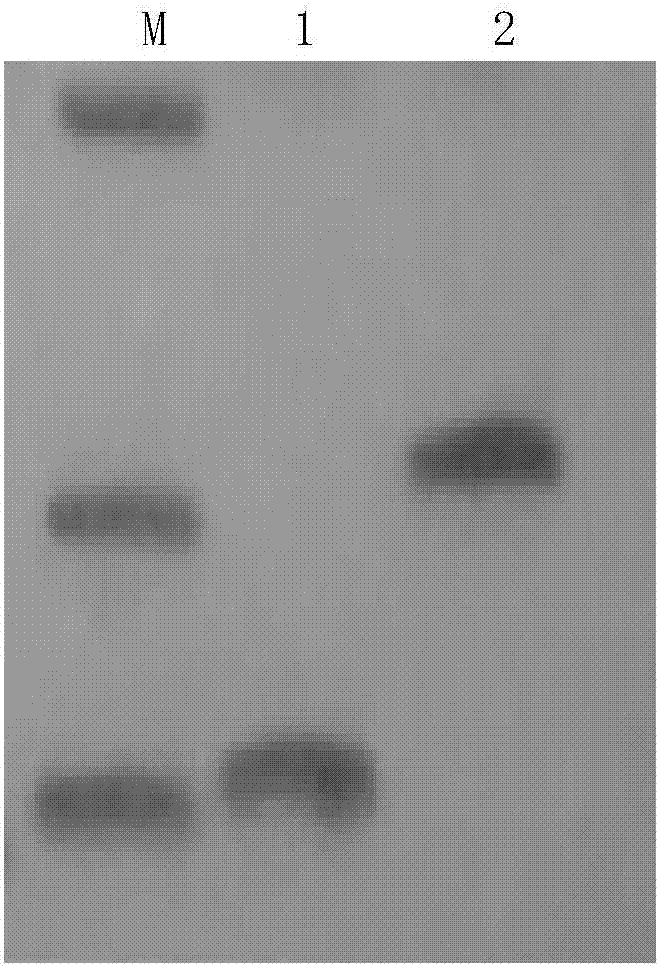
Specific primers, probes, kit and chip for gene detection of Toxoplasma gondii
PendingCN110982880AConvenient diagnosis and treatmentEasy to monitorMicrobiological testing/measurementDNA/RNA fragmentationGondii toxoplasmaVirology
The invention relates to the field of molecular biology, and provides specific primers for detecting Toxoplasma gondii. The sequence of an upstream primer in the specific primers is as shown in SEQ IDNo. 1; the sequence of a downstream primer in the specific primers is as shown in SEQ ID No. 2. The invention also provides probes for detecting Toxoplasma gondii. The sequences of the probes are asshown in SEQ ID No. 3-6. The invention further provides a kit for detecting Toxoplasma gondii. The kit comprises the primers and the probes for detecting Toxoplasma gondii. The invention further provides a chip for detecting Toxoplasma gondii. The chip comprises the probes for detecting Toxoplasma gondii. According to the invention, gene chip detection is carried out on Toxoplasma gondii by usingthe primers and the probes, and differential diagnosis can be specifically carried out on Toxoplasma gondii infection conditions.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
Polymerase chain reaction (PCR) method for identifying four pathogens in prenatal and postnatal care examination through single tube and kit thereof
ActiveCN104120195AAccurate detectionReliable detectionMicrobiological testing/measurementDNA/RNA fragmentationReaction tubeToxoplasma gondii
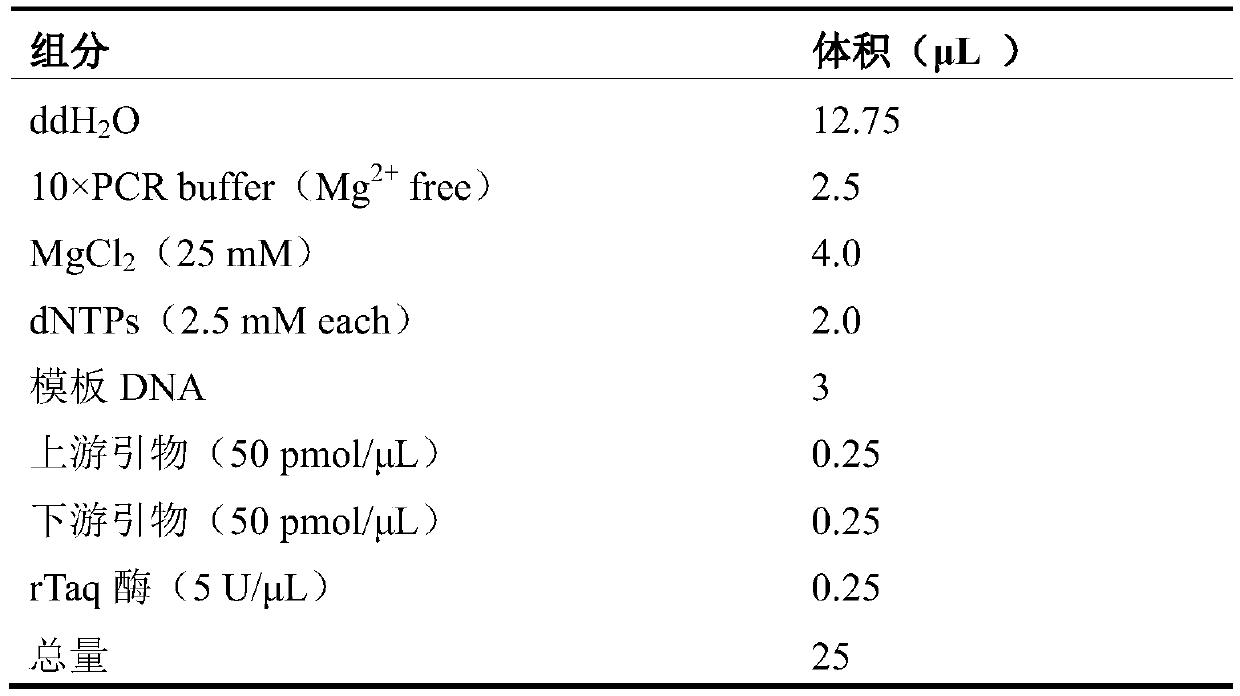
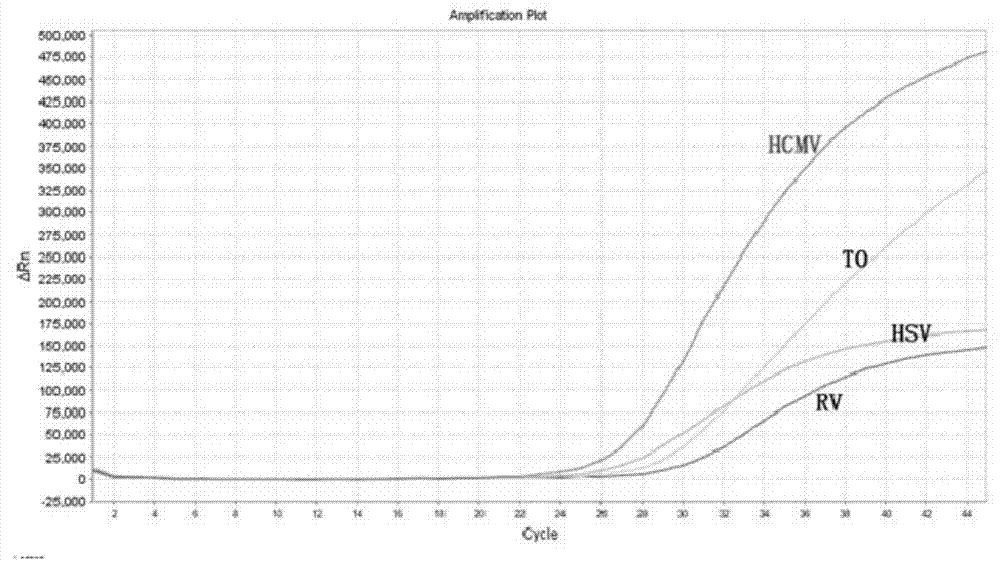
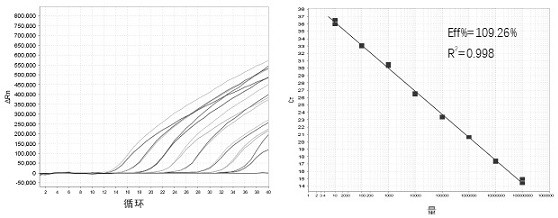
The invention discloses a real-time fluorescent polymerase chain reaction (PCR) method for simultaneously detecting four targeting nucleic acids in a single PCR reaction tube. The method is used for detecting toxoplasma gondii, rubella virus, cytomegalovirus and herpes simplex virus in samples. The method is fast in operation, is finished through one step and is high in accuracy and sensitivity, and false positive and false negative results are not discovered in practice detection. In addition, the invention also relates to a reagent involved in the PCR method, as well as a detection kit for the method and preparation and application of the detection kit.
Owner:苏州华益美生物科技有限公司
Primer, probe and kit for detecting pneumocystis carinii and toxoplasma gondii and detection method thereof
ActiveCN111926007APrevention and Control of TransmissionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationGondii toxoplasmaPharmaceutical drug
The invention discloses a primer, a probe, a kit and a detection method for detecting pneumocystis carinii and toxoplasma gondii, and particularly relates to a kit and a detection method for double fluorescent quantitative PCR detection of pneumocystis carinii and toxoplasma gondii. According to the invention, the primer pair and the probe are designed by utilizing conserved regions of pneumocystis carinii and toxoplasma gondii, the primer pair and the probe which can specifically amplify pneumocystis carinii and toxoplasma gondii are screened out, the sensitivity is high, the two pairs of primers and the two probes do not interfere with each other, and a sample only containing 1 copy can be accurately detected; by utilizing the primer pair, the probe and the detection method, the contentsof the pneumocystis carinii and the toxoplasma gondii can be absolutely quantified; the kit is simple in structure, safe in detection, rapid and convenient, can be used for detecting the pneumocystiscarinii and the toxoplasma gondii and tracking and monitoring the treatment effect of medicines of a patient, and is beneficial to carrying out epidemiological investigation and preventing and controlling propagation of the pneumocystis carinii and the toxoplasma gondii.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD +1
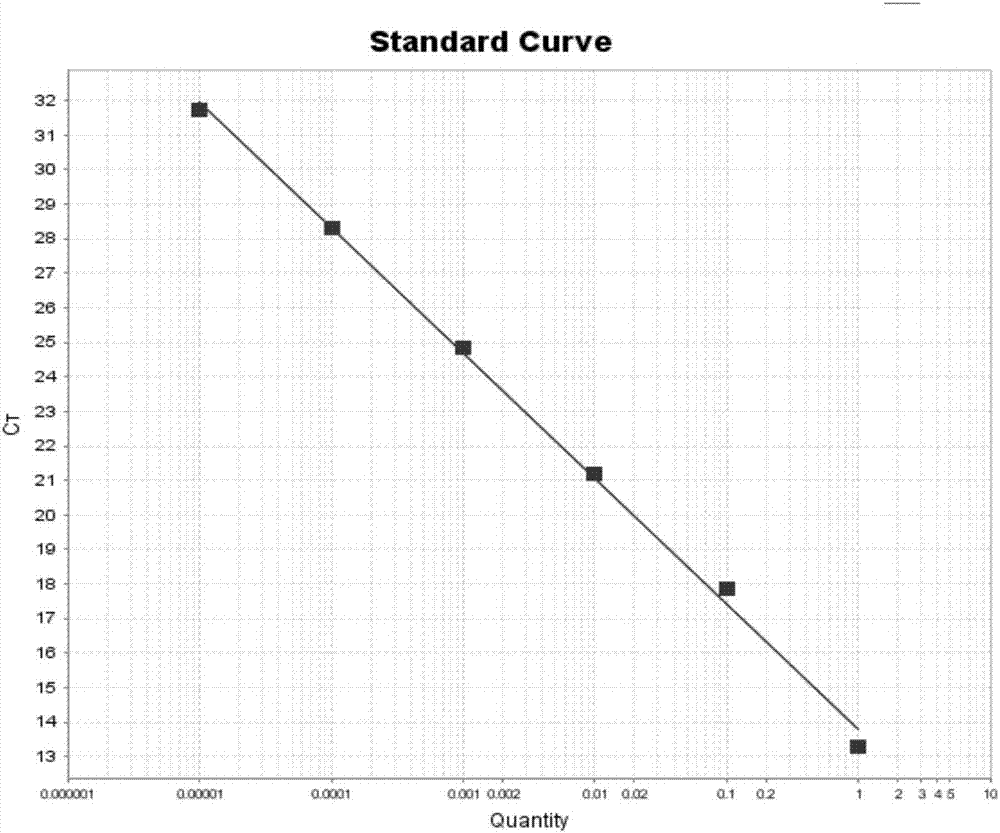
Kit for absolutely quantitatively detecting Toxoplasma gondii based on digital PCR and detection method thereof
InactiveCN106434992AEfficient detectionEasy to determineMicrobiological testing/measurementMicroorganism based processesTest sampleMicrobiology
The invention relates to a kit for absolutely quantitatively detecting Toxoplasma gondii based on digital PCR and a detection method thereof; the kit is composed of a microdrop digital PCR kit and a plurality of microdrop generator sheets; the detection method is characterized by comprising: (1) extracting total DNA of a test sample; (2) using the total DNA as a template to carry out PCR amplification; (3) placing the PCR production and microdrop reader to read signals, analyzing experimental data to obtain an absolute content of Toxoplasma gondii in the sample. The kit and method can provide direct quantitation in Toxoplasma gondii detection, have no need for standard curves, have the advantages, such as good operational convenience, high speed and efficiency, good specific sensitivity and low cost, etiological diagnosis on Toxoplasma gondii can be effectively carried out, detection rate is increased, the common problems of existing Toxoplasma gondii detection kits, such as low sensitivity and high misdiagnosis rate, are solved, and the kit and method are suitable for clinical investigations and large-scale disease detection and supervision, related preventive and control measures can be collected in time, and economic loss is decreased.
Owner:吉林省畜牧兽医科学研究院
Kit for simultaneously detecting multiple cat pathogens as well as detection method and application of kit
ActiveCN113957172AReduce usageStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesFeline calicivirus infectionGondii toxoplasma
The invention discloses a kit for simultaneously detecting multiple cat pathogens as well as a detection method and application of the kit. A specific primer group and a probe group are obtained through design, so that rapid and accurate detection and identification on feline plague viruses, feline calicivirus, feline infectious peritonitis viruses, feline toxoplasma gondii, feline herpes viruses, feline chlamydia and feline mycoplasma in one reaction are realized at the same time. The detection kit obtained on the basis of the design is high in specificity and sensitivity, the feline pathogens with the concentration of 1.7 * 10 <2> copies / mL can be effectively detected, repeated detection is not needed, the actual application accuracy is high, and the detection kit can be widely applied to epidemic situation monitoring, prevention and control of feline virus infection.
Owner:广州蔚捷生物医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com