Primer, probe and kit for detecting pneumocystis carinii and toxoplasma gondii and detection method thereof
A kit and Toxoplasma gondii technology, applied in biochemical equipment and methods, microbiological determination/inspection, DNA/RNA fragments, etc., can solve the problems of missed diagnosis and low sensitivity of microscopic examination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
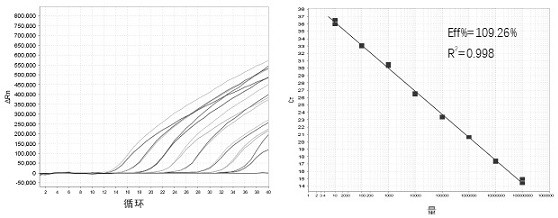
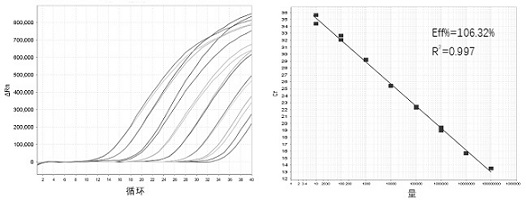
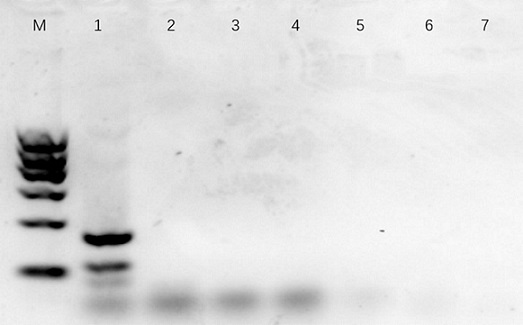
Image
Examples
Embodiment 1
[0076] Example 1: Extraction of total DNA from human sputum or alveolar lavage fluid samples:
[0077] 1. Pretreatment of sputum or alveolar lavage fluid:
[0078](1) Add NaOH solution (1M) into sputum or alveolar lavage fluid (sputum: NaOH solution = 1:1, lavage fluid: NaOH solution = 2:1), and observe after 1 hour in an incubator at 60°C Whether the sputum or alveolar lavage fluid is liquefied, if it is not completely liquefied, the time can be appropriately extended until the liquefaction is complete, if it has been liquefied, the following steps can be continued;
[0079] (2) Centrifuge the liquefied sputum or alveolar lavage fluid at 8000rpm / min for 5 minutes, and remove the supernatant after centrifugation;
[0080] (3) Add 1ml of normal saline, pipette to mix, centrifuge at 8000rpm / min for 5 minutes, and remove the supernatant;
[0081] (4) Repeat step (3).
[0082] 2. Extraction of total DNA in human sputum or alveolar lavage fluid:
[0083] (1) Add 700 μl of 0.1%-...
Embodiment 2
[0092] Example 2: Primer and probe design
[0093] The DNA sequences of the mitochondrial ribosomal small subunit genes of different Pneumocystis carinii were compared to determine the conserved region, and primers and probes were designed according to the conserved region. The sequences of the primers and probes are as follows, and the length of the amplified fragment is 124bp:
[0094] Primer PJ-F: 5'-TTATGAAGTGGGCTACAGAC-3' (SEQ ID No: 1);
[0095] Primer PJ-R: 5'- CTTCAAAGAGCCGAGTTCC -3' (SEQ ID No: 2);
[0096] Probe PJ-Probe: 5'-FAM-TTTGAACGGGATTTCTGCACCCAT-TAMRA-3' (SEQ ID No: 3).
[0097] The 529bp repeat sequence of different Toxoplasma gondii was compared to determine the conserved region, and primers and probes were designed according to the conserved region. The sequences of the primers and probes are as follows, and the length of the amplified fragment of Toxoplasma gondii is 233bp:
[0098] Primer TG-F: 5'- GACTACAGACGCGATGCC -3' (SEQ ID No: 4);
[0099] Prime...
Embodiment 3
[0101] Embodiment 3: the preparation of plasmid standard
[0102] 1. Preparation and purification of target fragments:
[0103] (1) Establish a PCR amplification reaction system: 2×GoTaq Green Master Mix 10μl, primer-F 200nM, primer-R 200nM, DNA sample (the nucleotide sequences of which are shown in SEQ ID No: 13 and 14 respectively) 20-50ng , supplemented to 20 μl with sterile double distilled water.
[0104] (2) PCR amplification reaction conditions: 95°C, 5min; 95°C for 15s, 60°C for 15s, 72°C for 20s, a total of 35 cycles; 72°C for 5min, 4°C ∞.
[0105] (3) Amplified product recovery: 2% agarose gel electrophoresis was used to detect the amplified product, and the general DNA purification and recovery kit of Tiangen Bio was used to cut the gel and recover the target fragment.
[0106] 2. Digestion and purification of plasmid vectors:
[0107] (1) EcoRI and HindⅢ digestion plasmid vector pUC19 linearization enzyme digestion reaction system: HindⅢ 1μl, EcoRI 1μl, 10×M buf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com