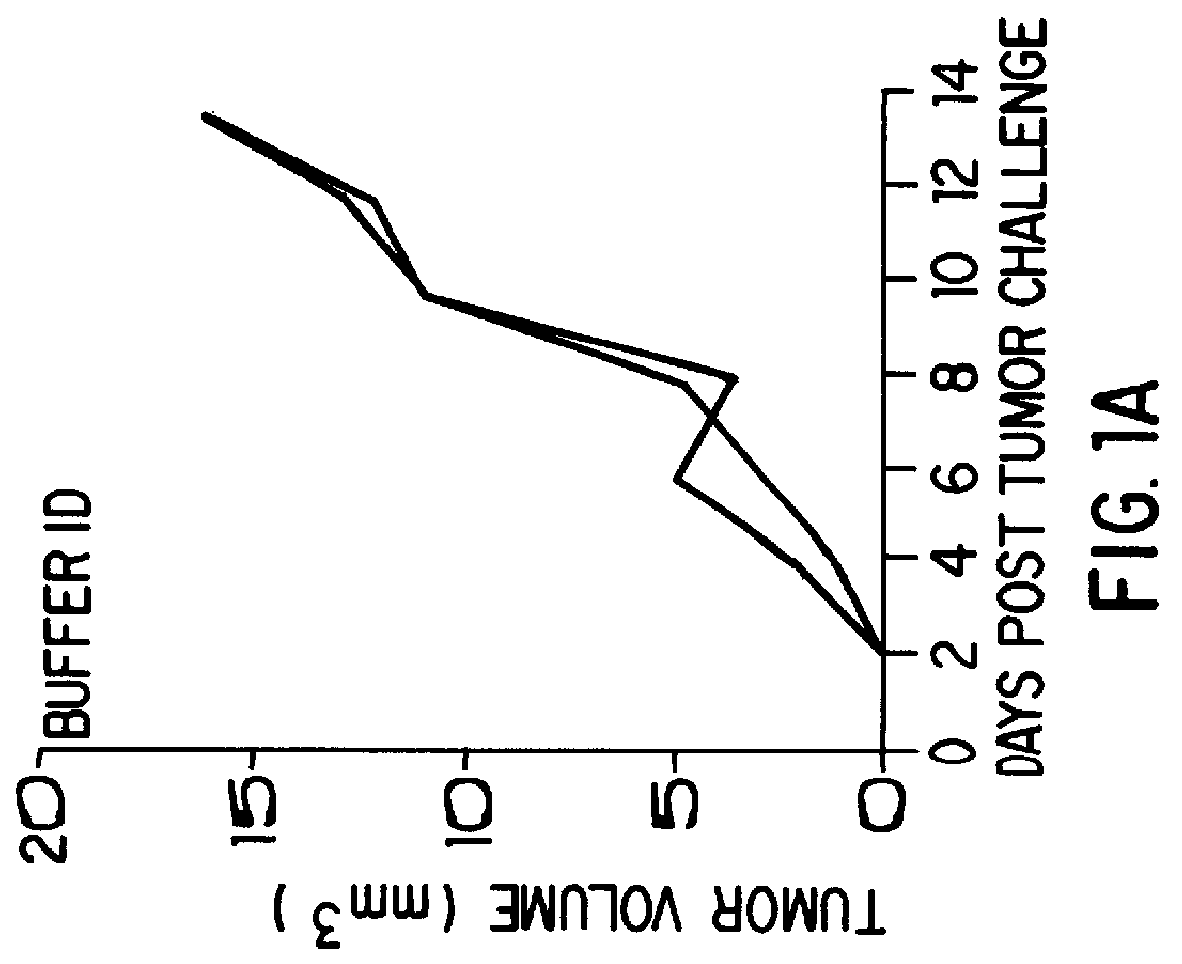
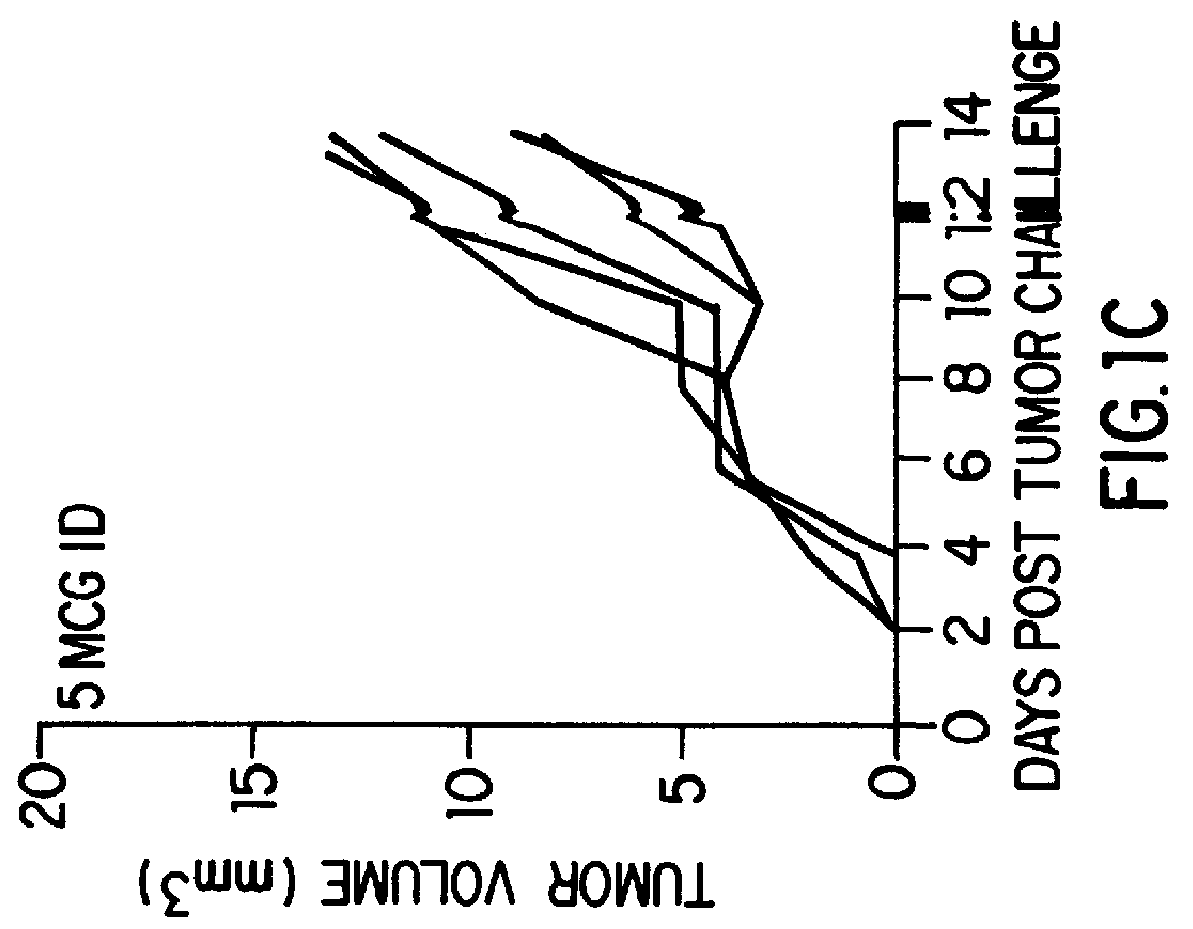
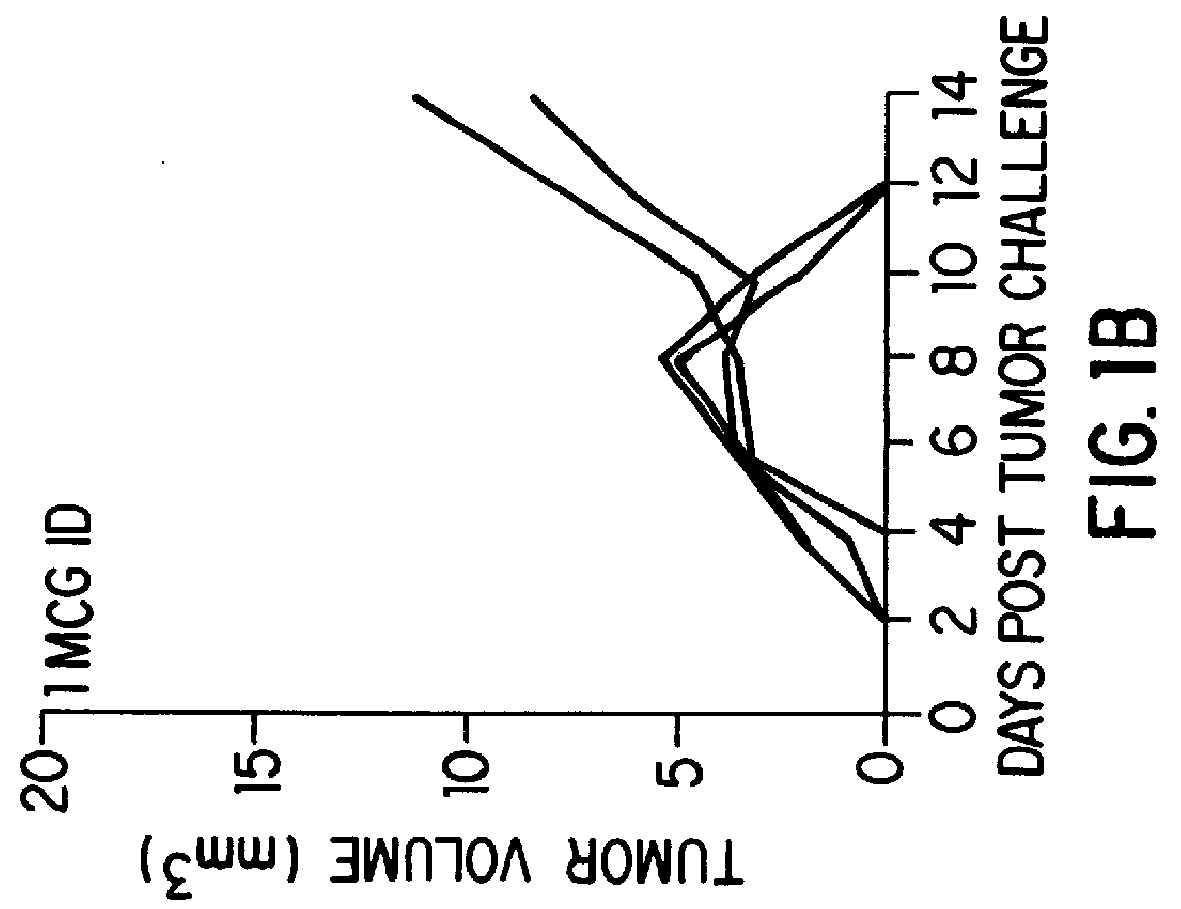
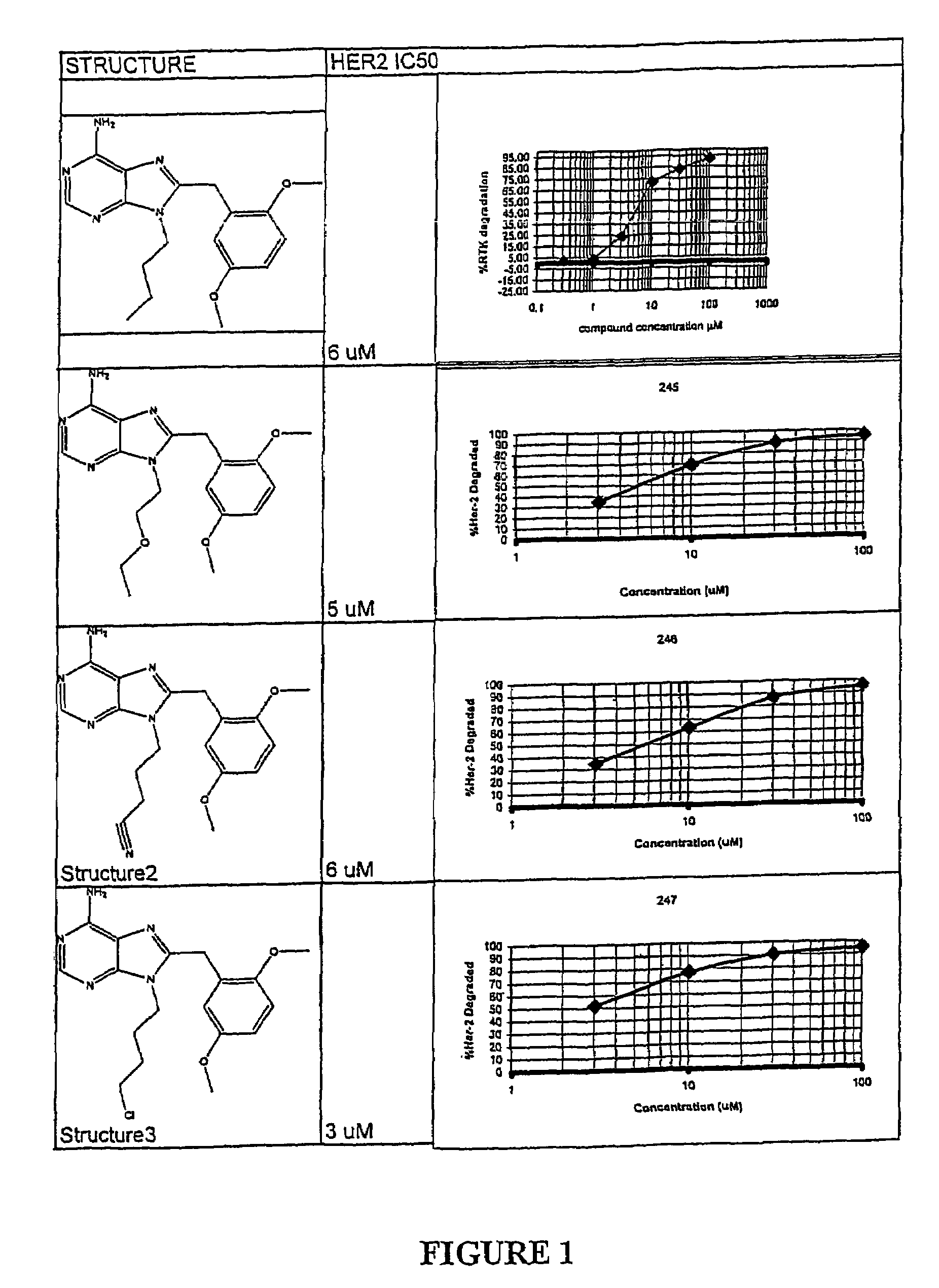
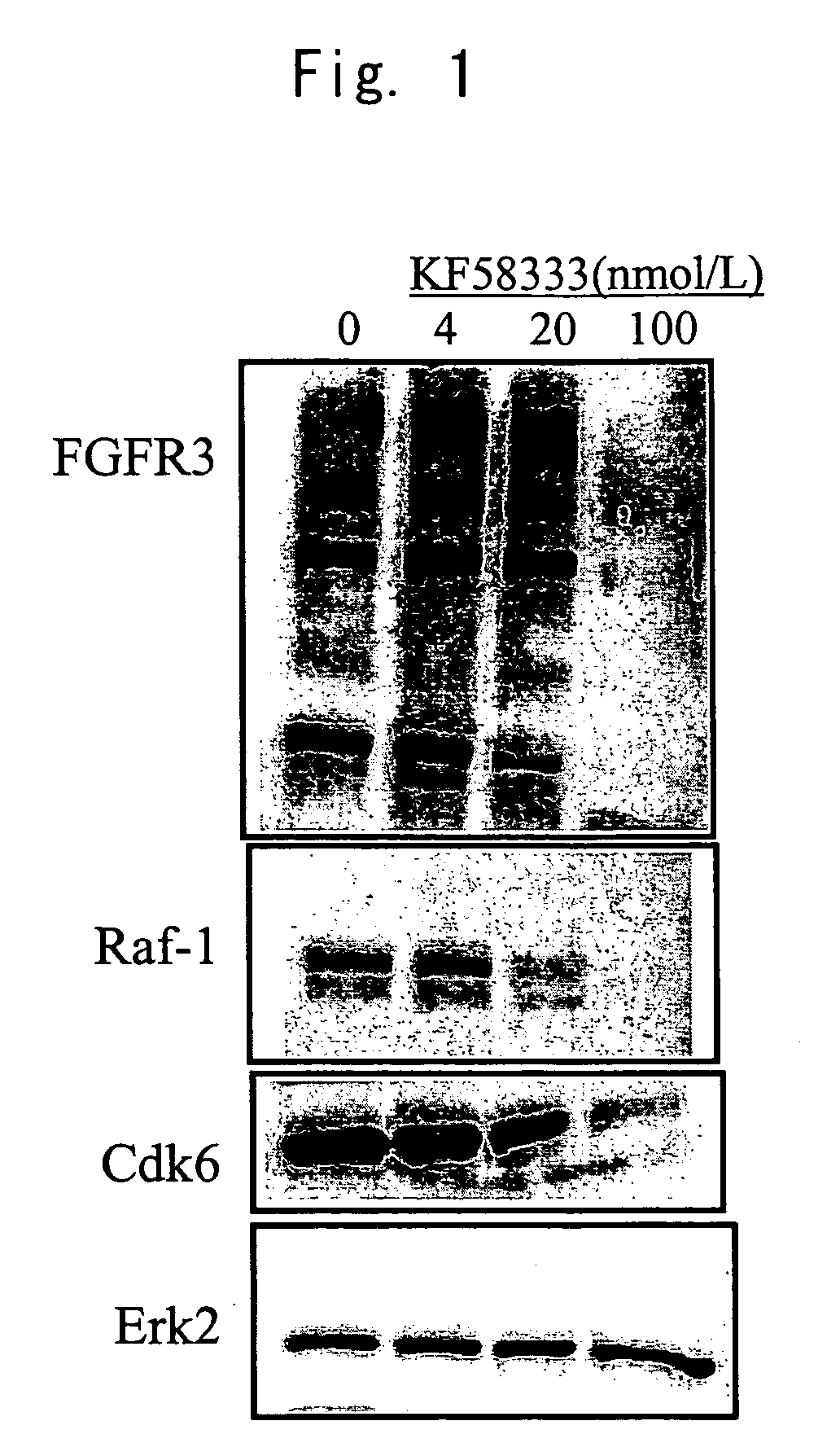
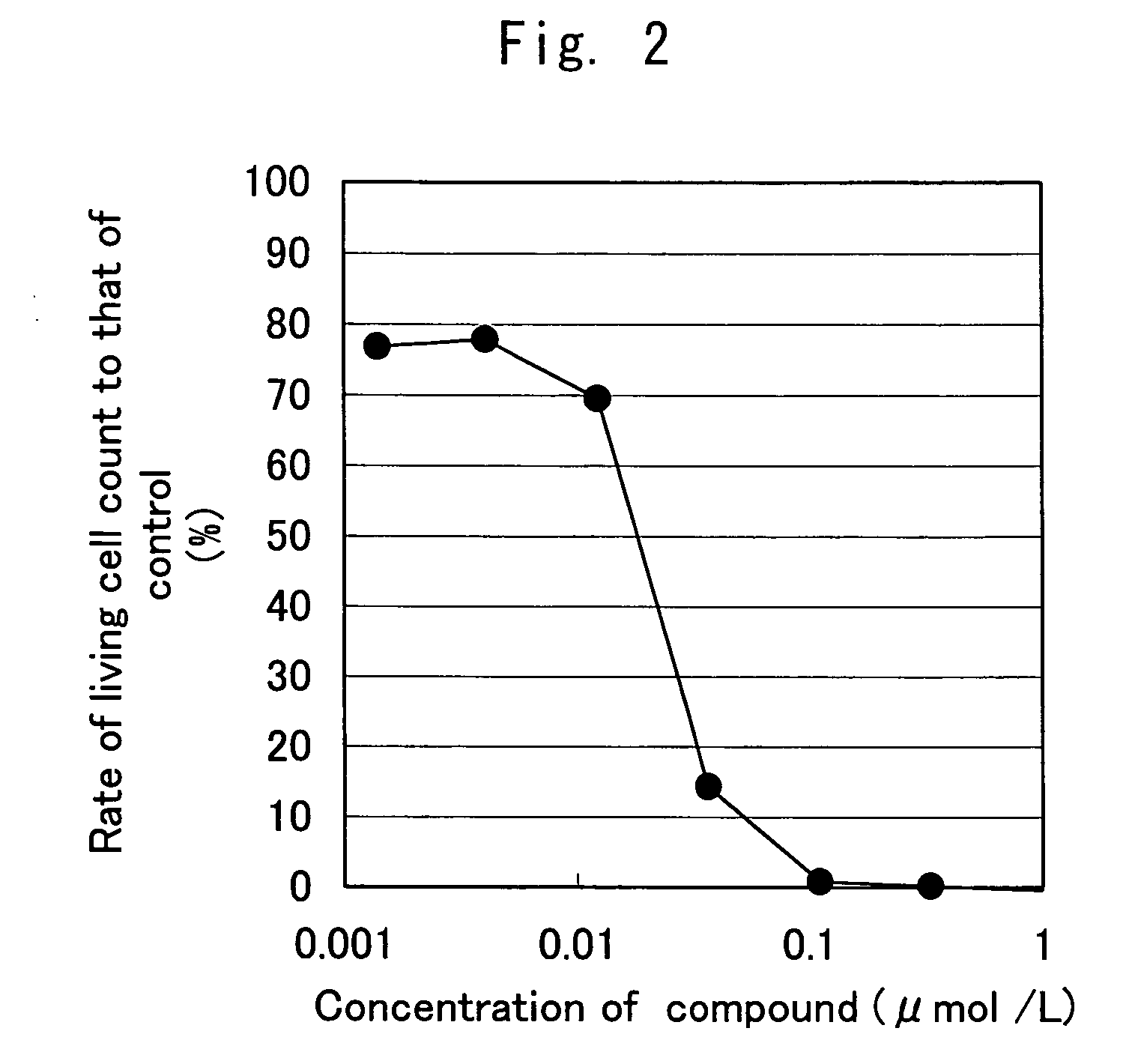
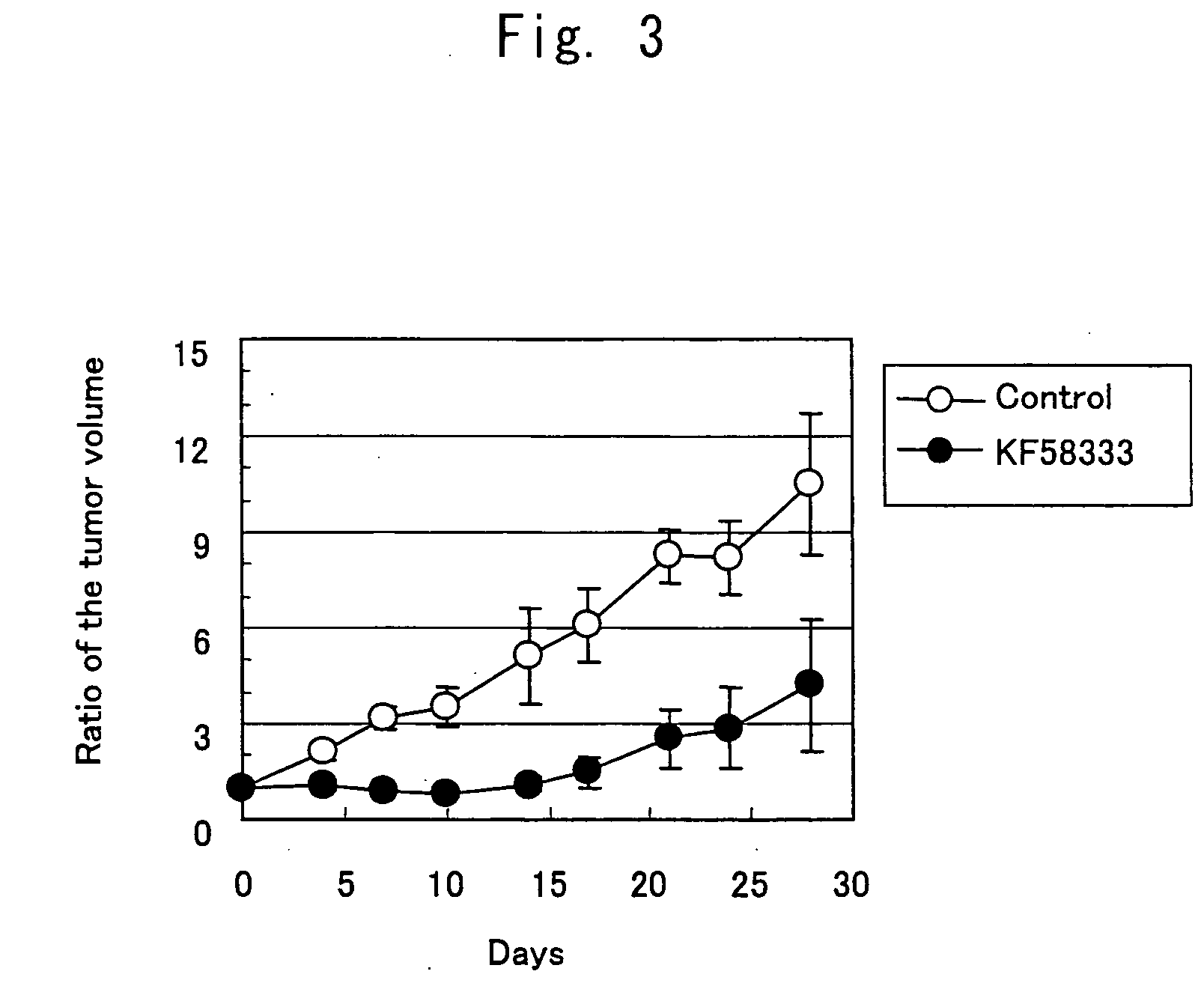
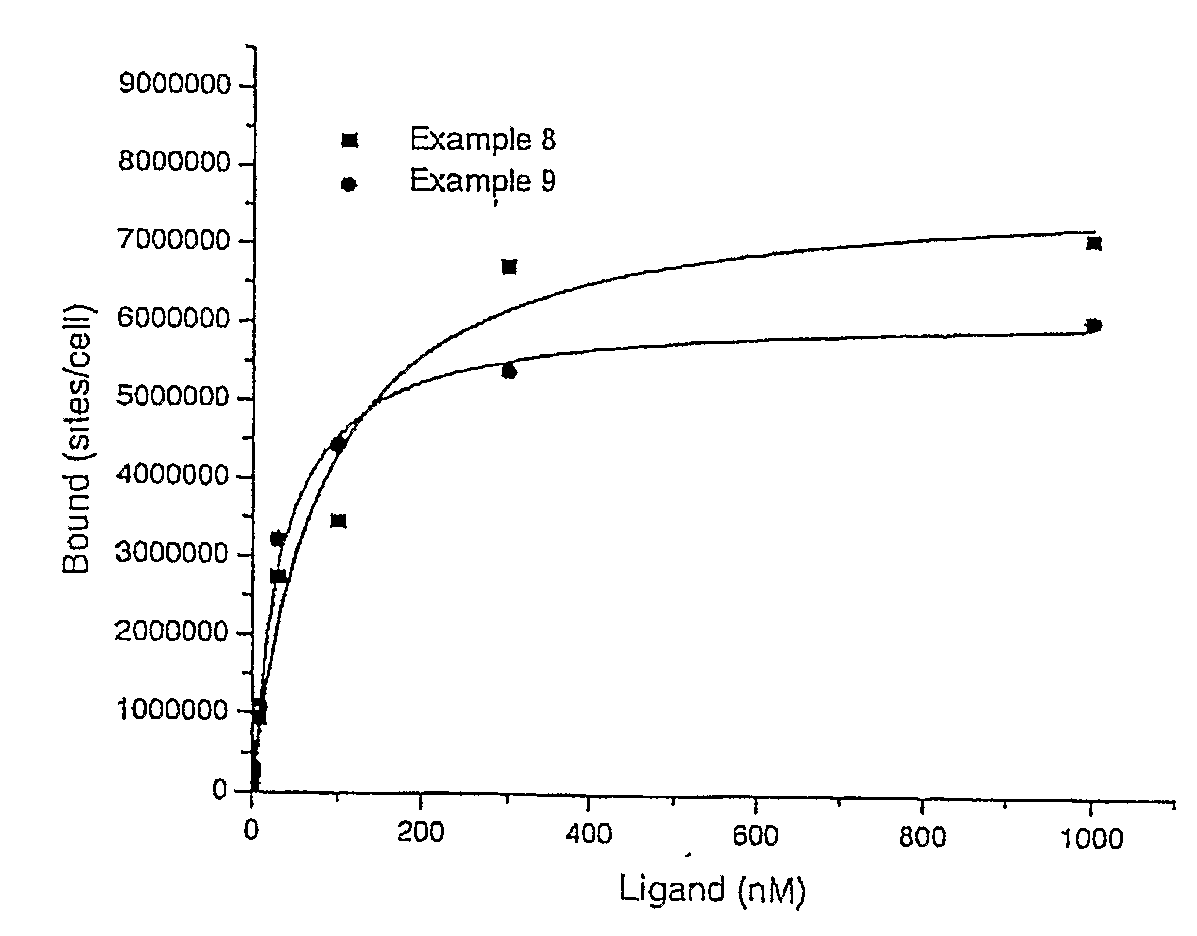
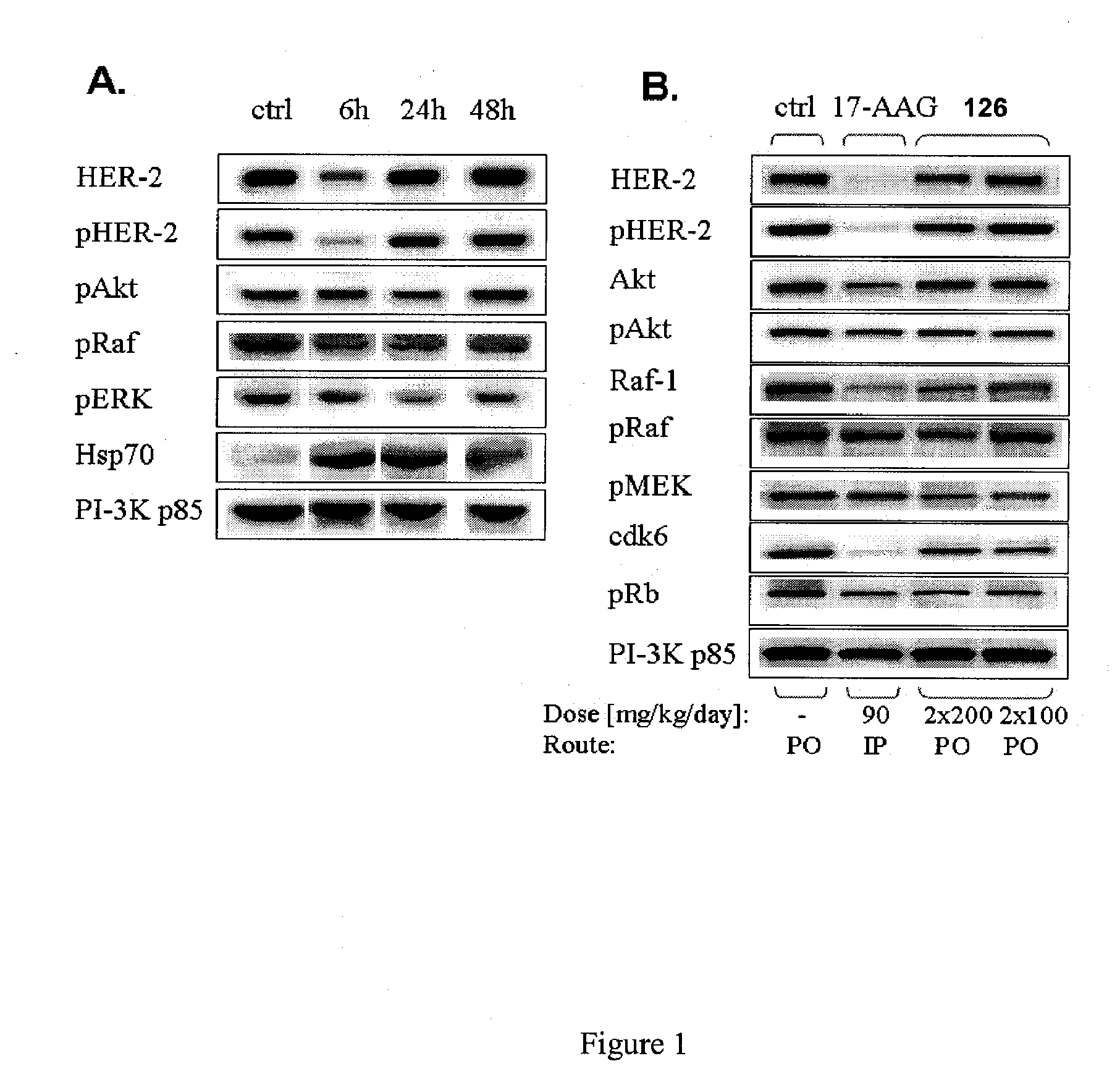
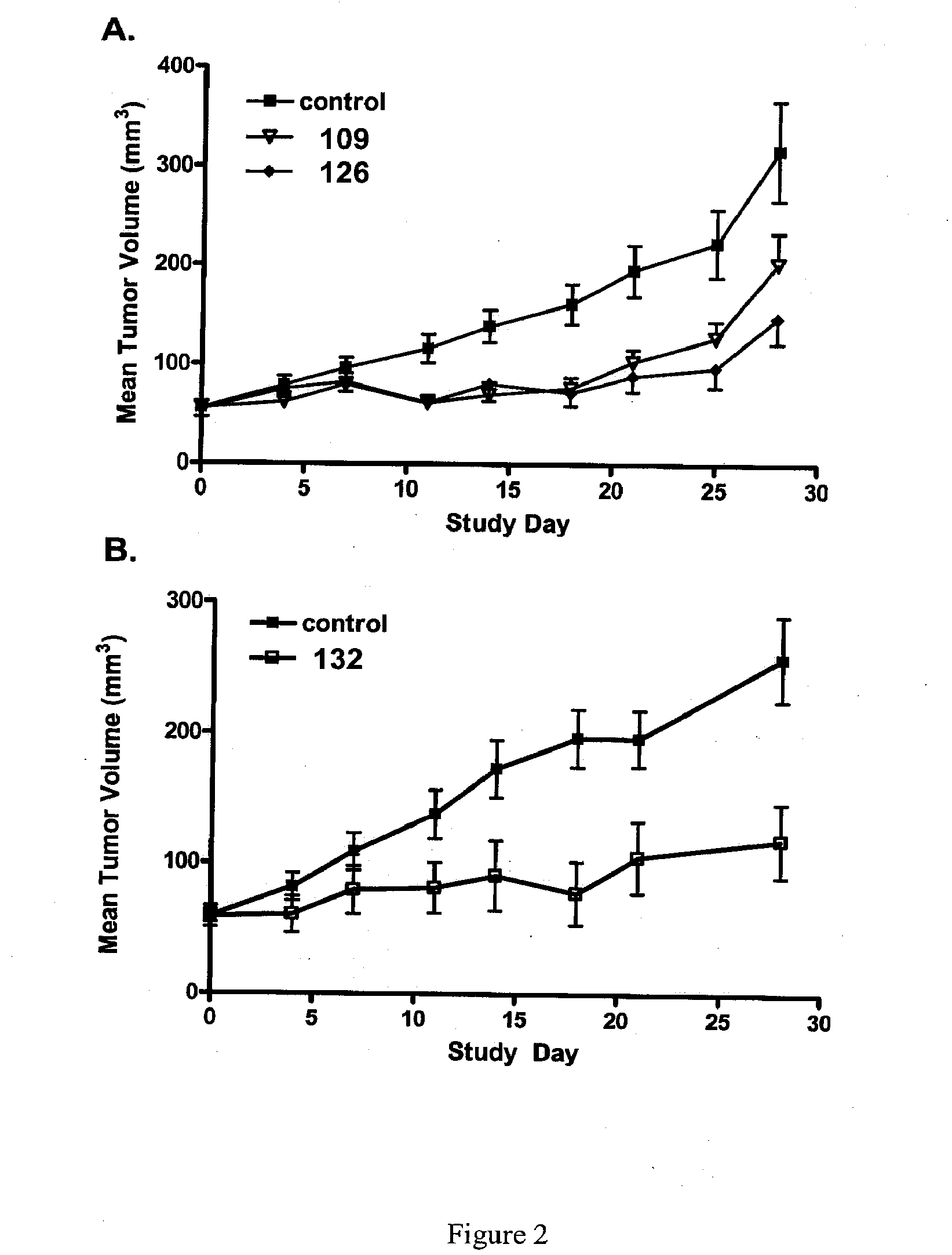
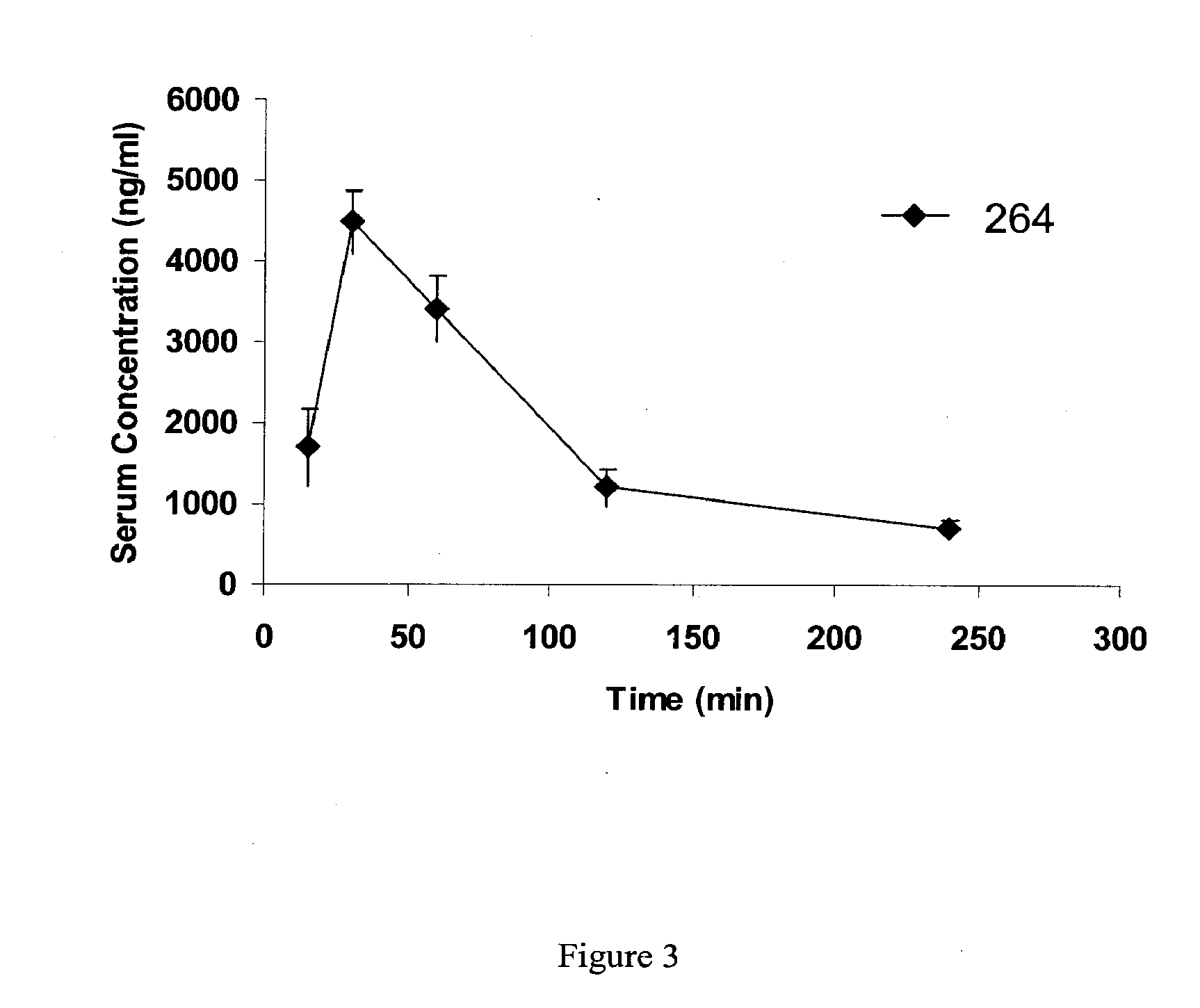
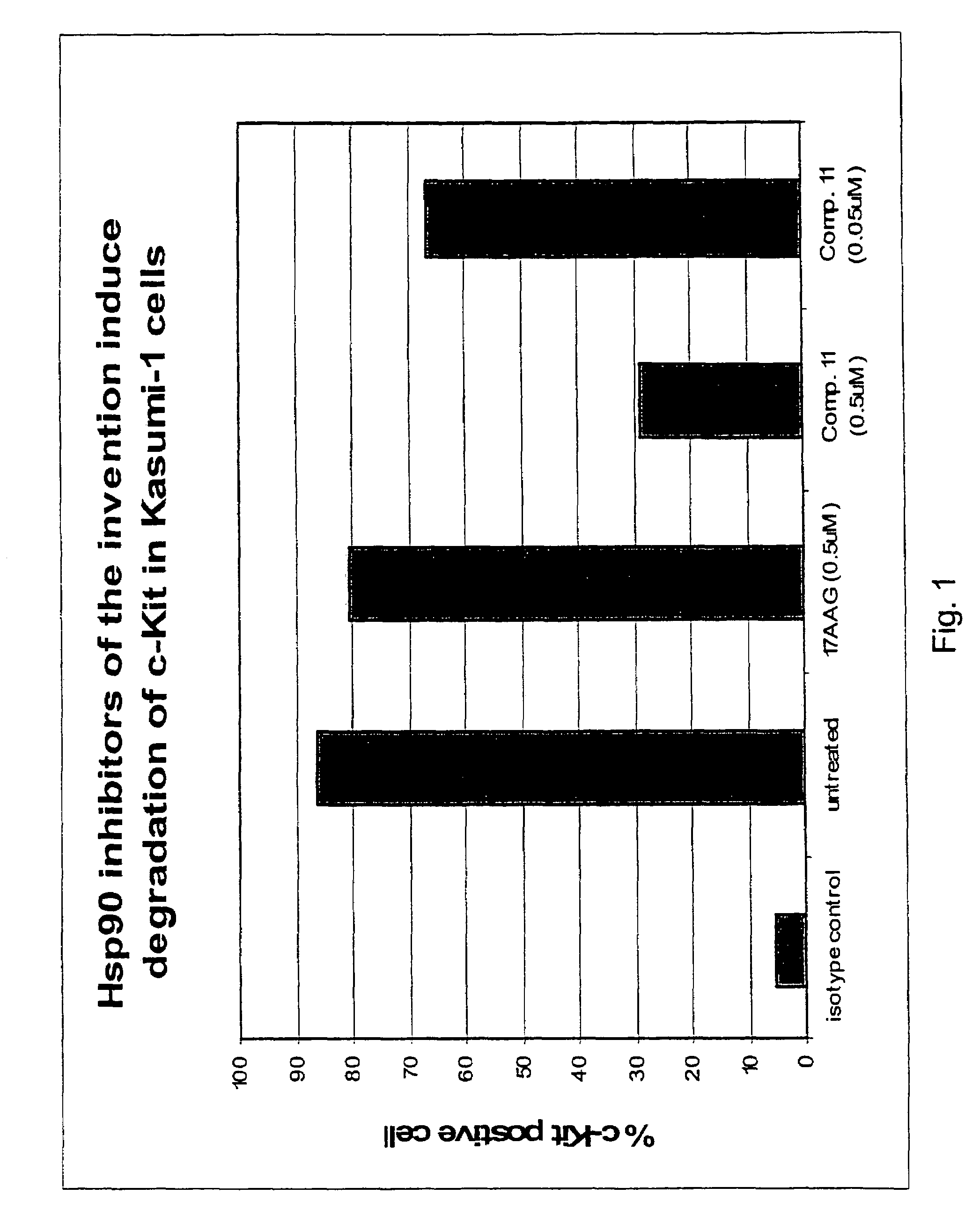
Patents
Literature
153 results about "HSP90 Heat-Shock Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hsp90 (heat shock protein 90) is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation.
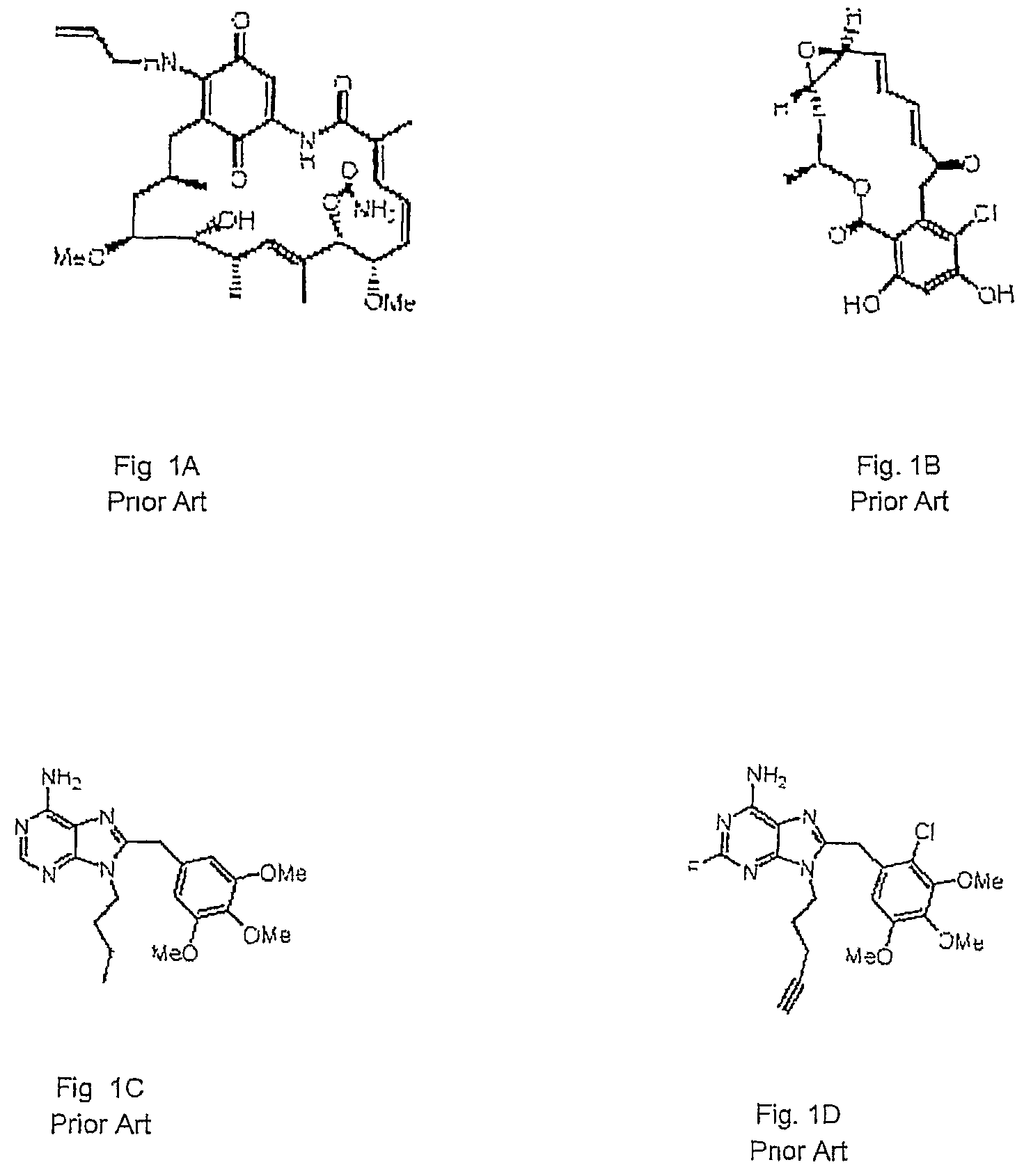
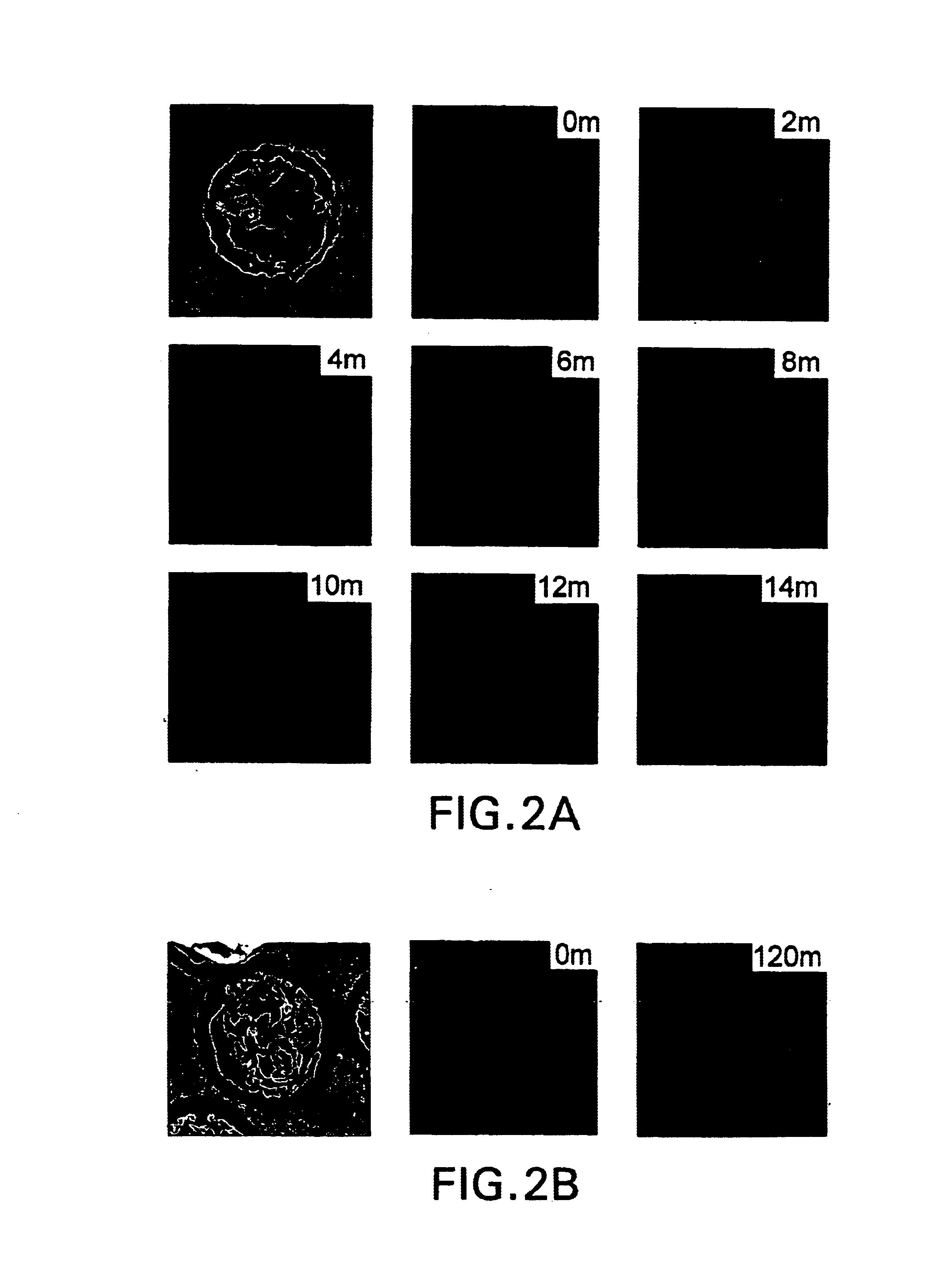
Prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases with heat shock/stress protein-peptide complexes
InactiveUS6017540AEnhancing host 's immunocompetenceHigh activityBiocidePeptide/protein ingredientsStress ProteinsIn vivo
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. Optionally, the methods further comprise administering antigen presenting cells sensitized with complexes of hsps noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. In a specific embodiment, the effective amounts of the complex are in the range of 0.1 to 9.0 micrograms for complexes comprising hsp70, 5 to 49 micrograms for hsp90, and 0.1 to 9.0 micrograms for gp96.
Owner:FORDHAM UNIVERSITY
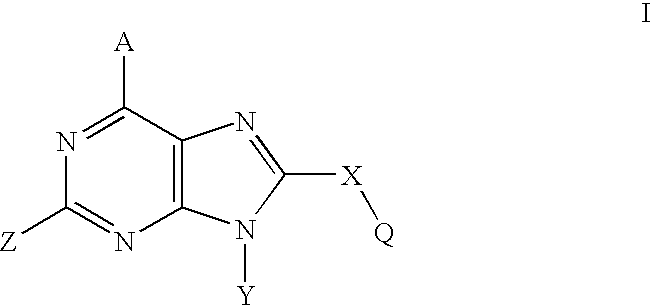
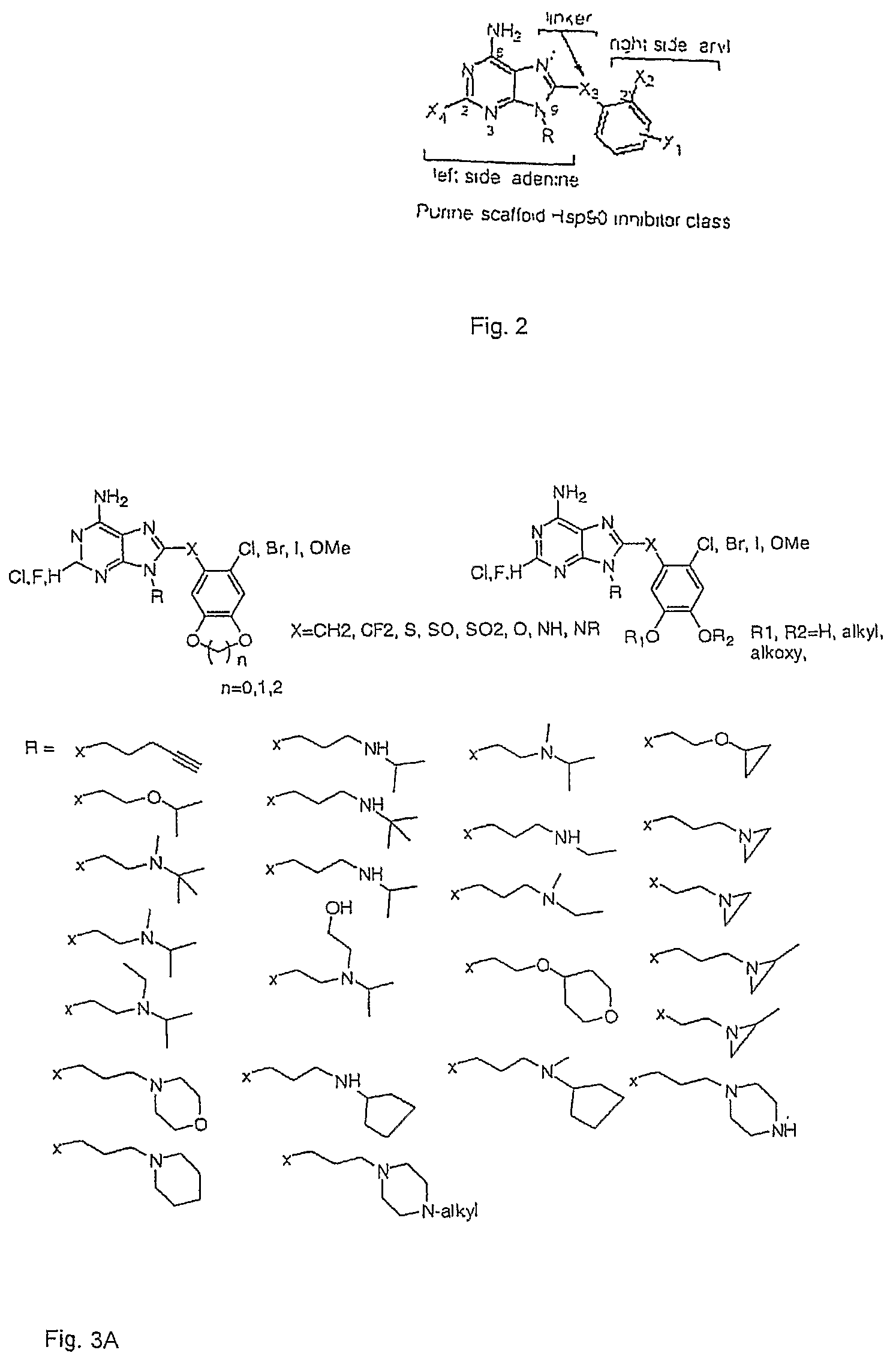
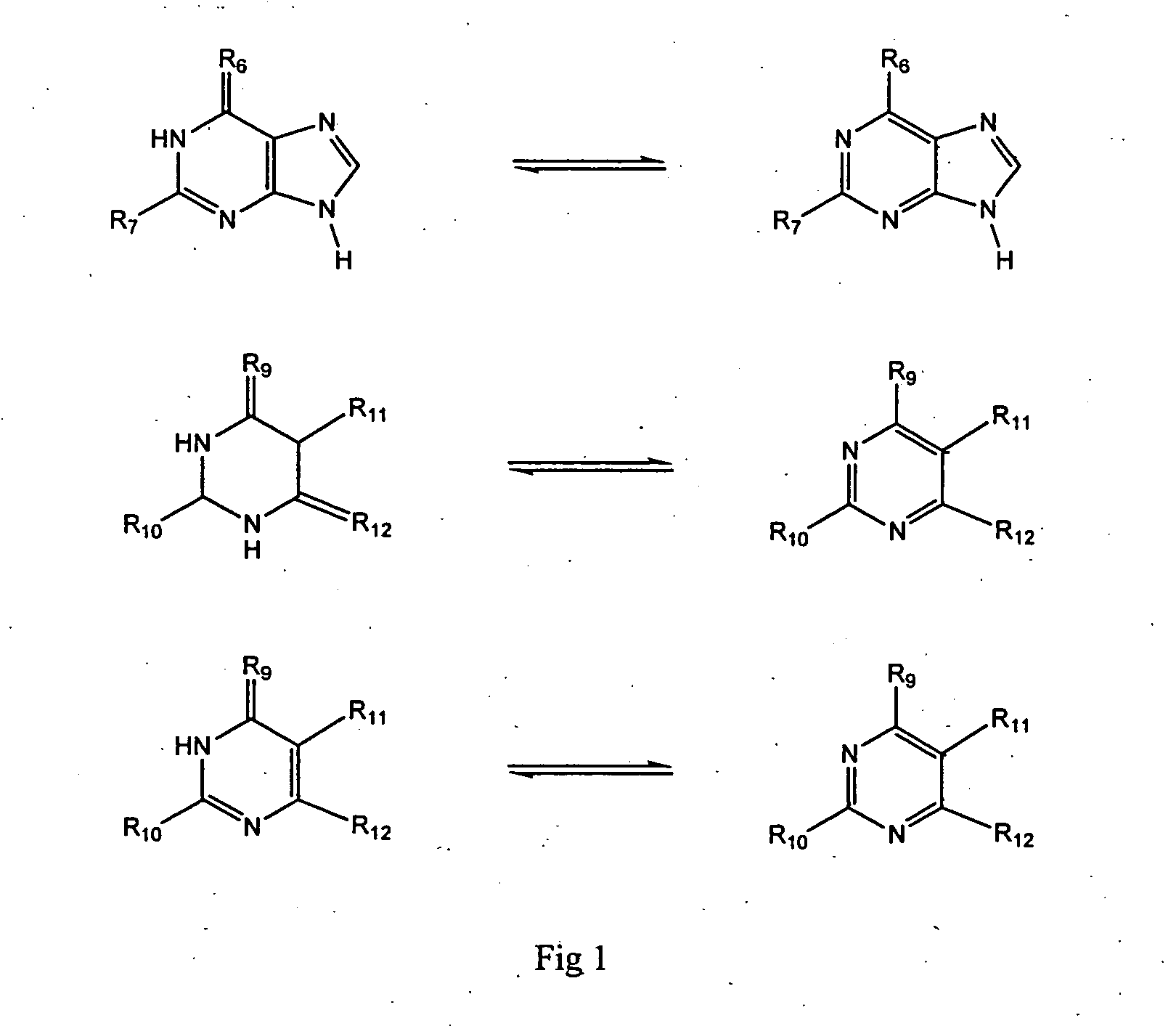
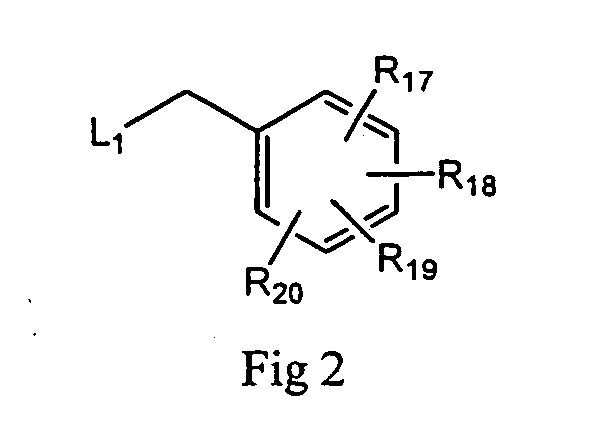
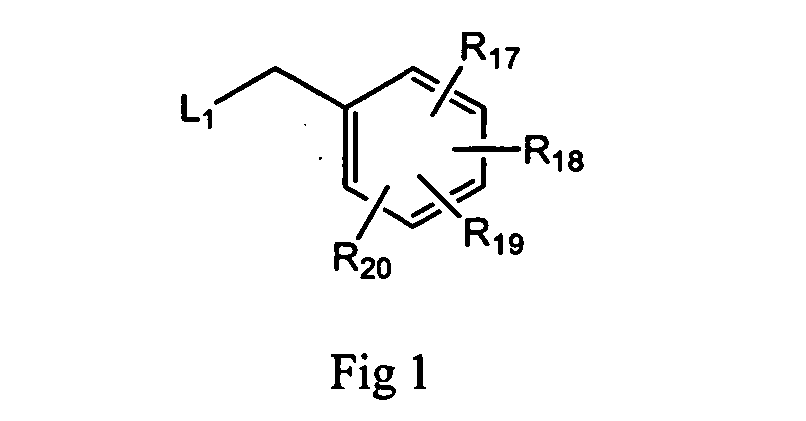
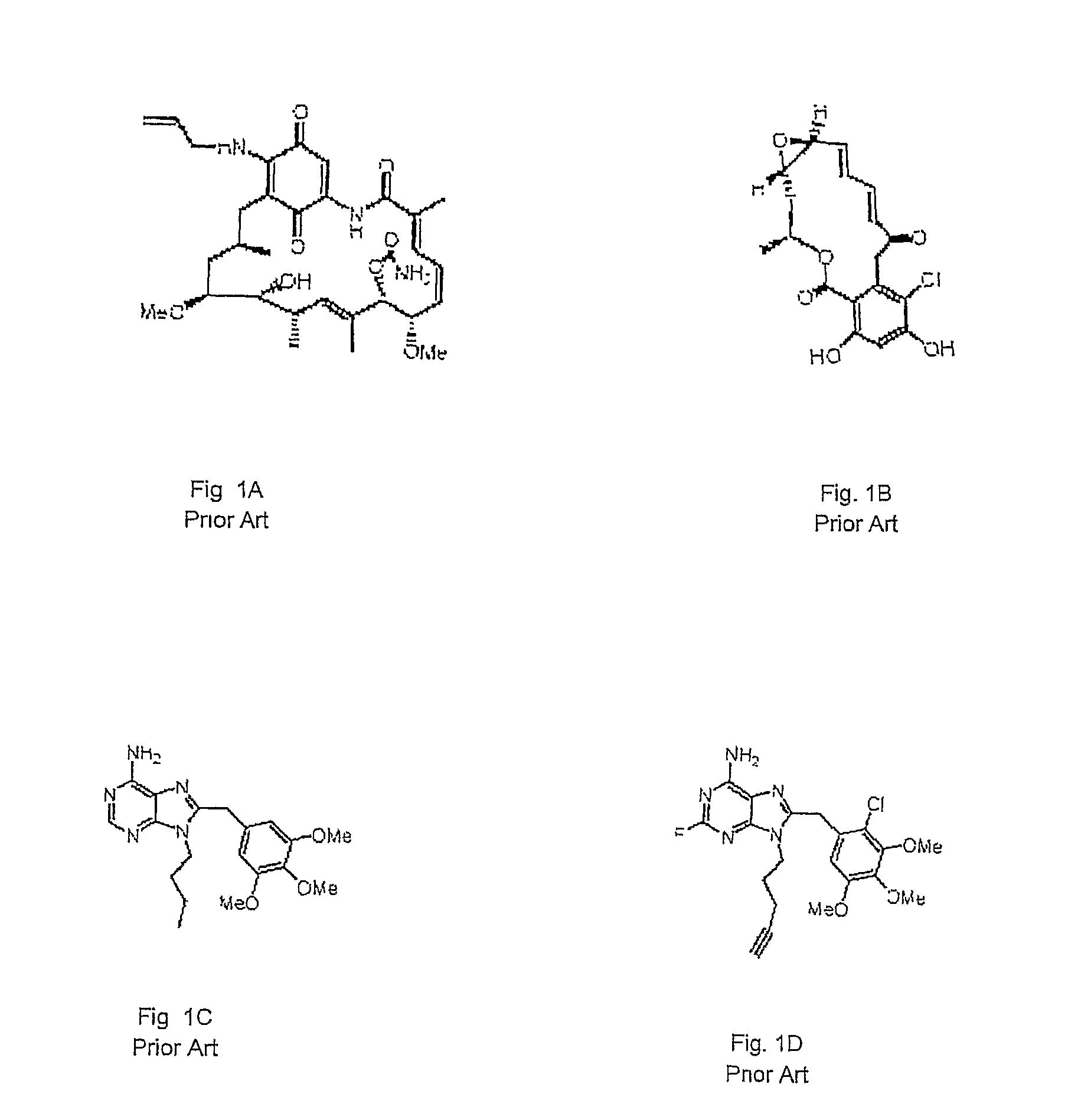
Purine analogs having HSP90-inhibiting activity
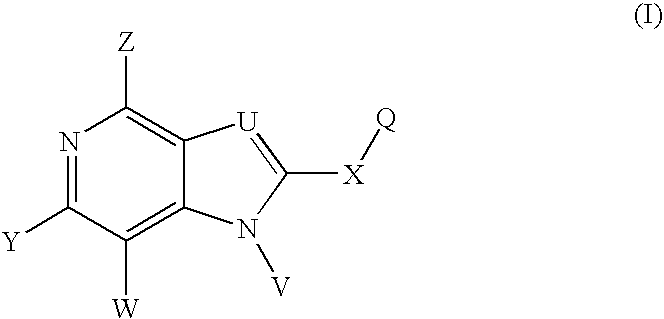
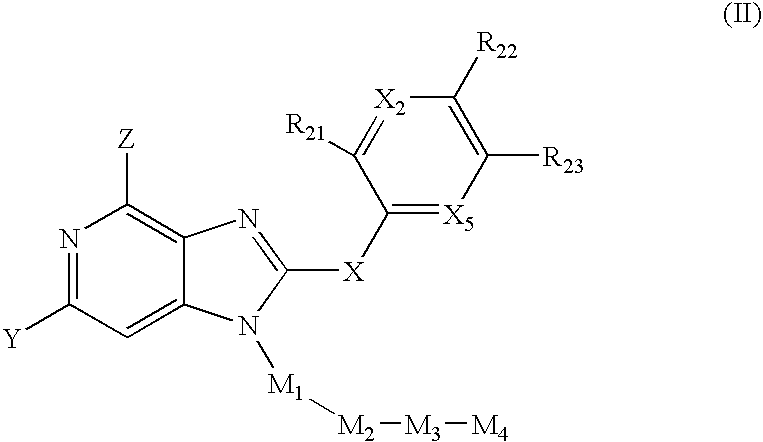
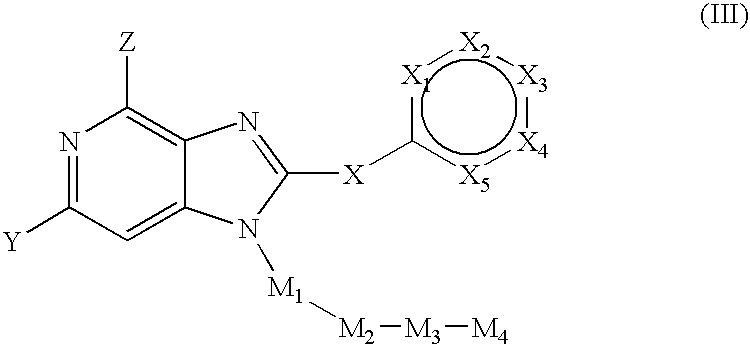
Novel purine compounds of Formula I.and tautomers, pharmaceutically acceptable salts, and prodrugs thereof, wherein X is S, S(O), or S(O)2; and O is selected from alkyl, cycloalkyl, arylalkyl, aryl, heteroaryl, and heterocyclic, all optionally substituted, are described, as are pharmaceutical compositions comprising the same, complexes comprising the same, e.g., HSP90 complexes, and methods of using the same.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Pyrrolopyrimidines and related analogs as HSP90-inhibitors
ActiveUS20050107343A1Broad utilityWide range of usesBiocideOrganic active ingredientsDiseaseHsp Inhibitor
Pyrrolopyrimidines and related analogs are described and demonstrated to have utility as Heat Shock Protein 90 (HSP90) inhibiting agents used in the treatment and prevention of various HSP90 mediated disorders, e.g., proliferative disorders. Methods of synthesis and use of such compounds are also described and claimed.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Treatment of Neurodegenerative Diseases Through Inhibition of HSP90
Treatment of neurodegenerative diseases is achieved using small molecule purine scaffold compounds that inhibit Hsp90 and that possess the ability to cross the blood-brain barrier or are other wise delivered to the brain.
Owner:THE ROCKEFELLER UNIV +1
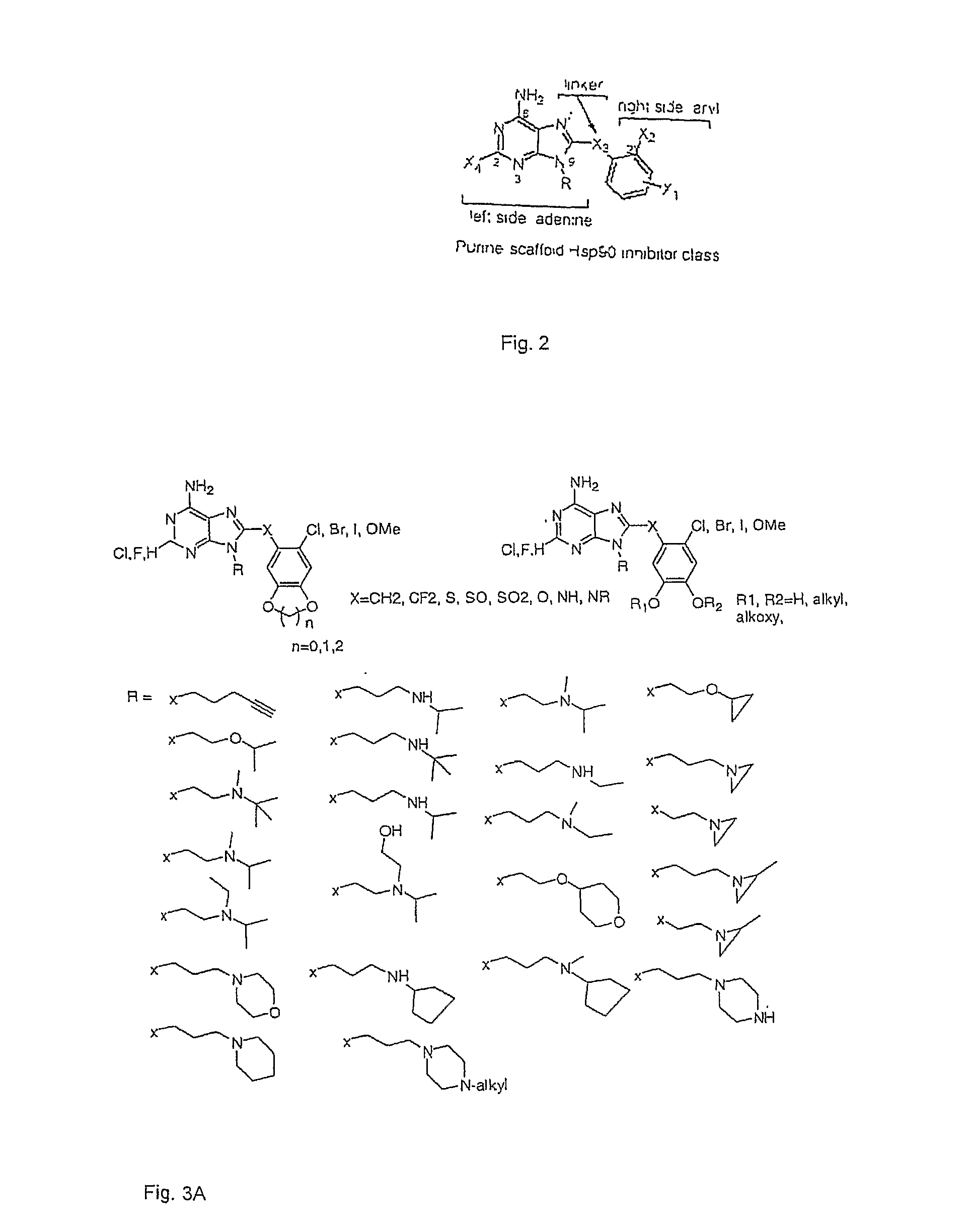
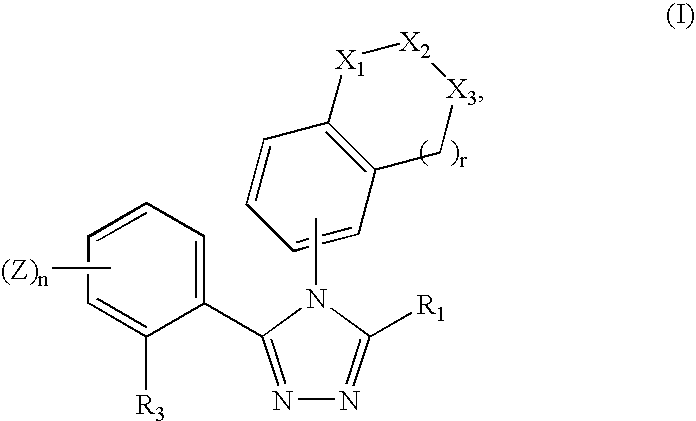
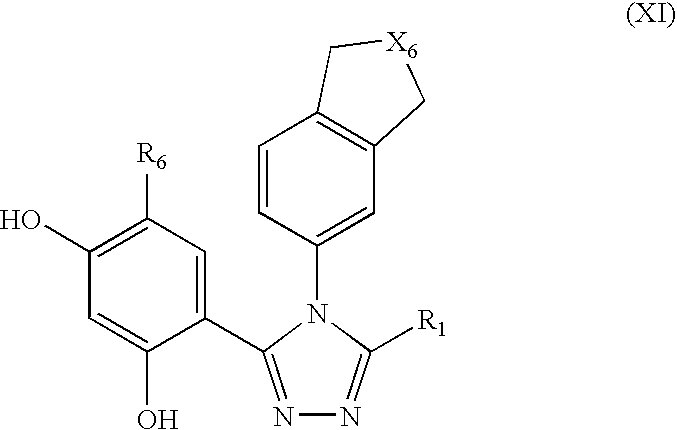
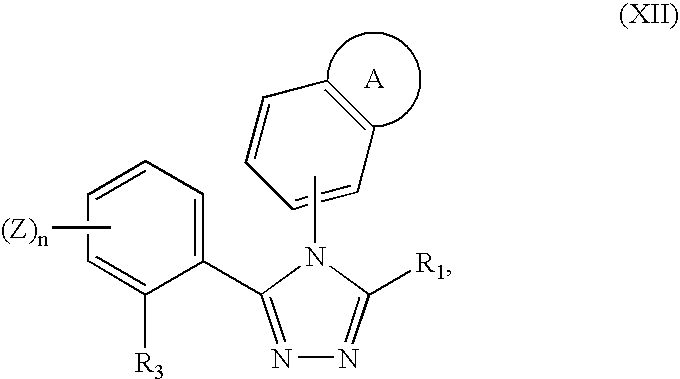
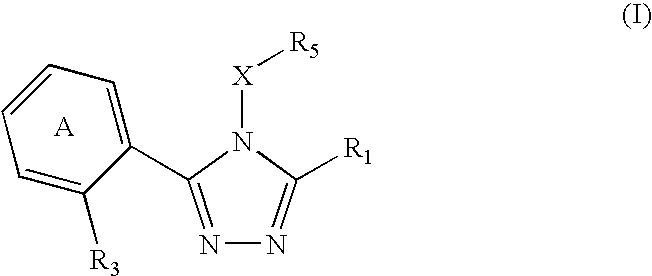
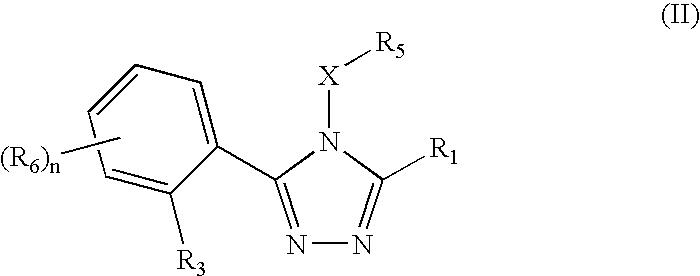
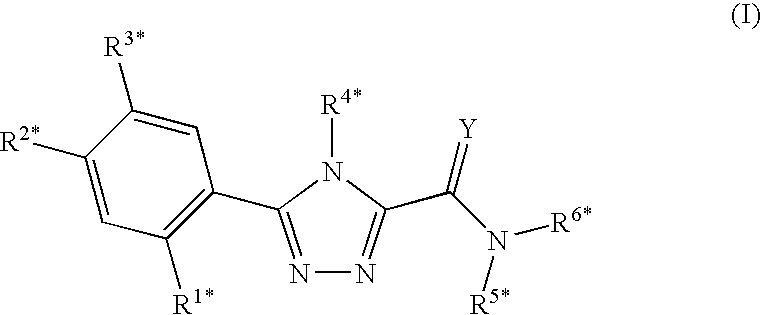
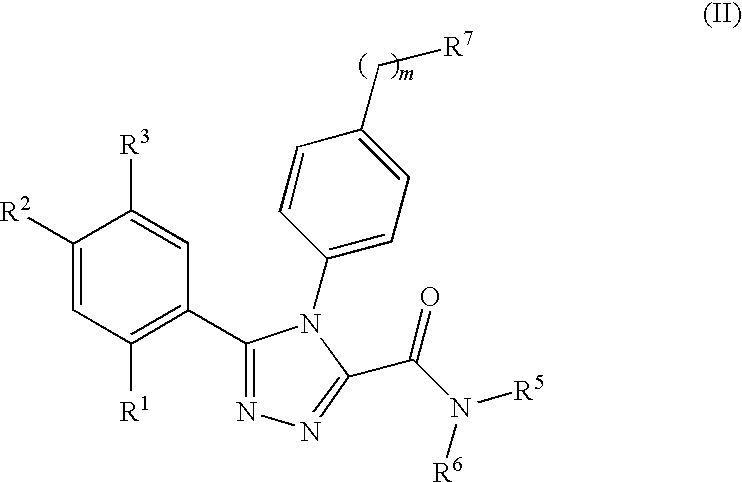
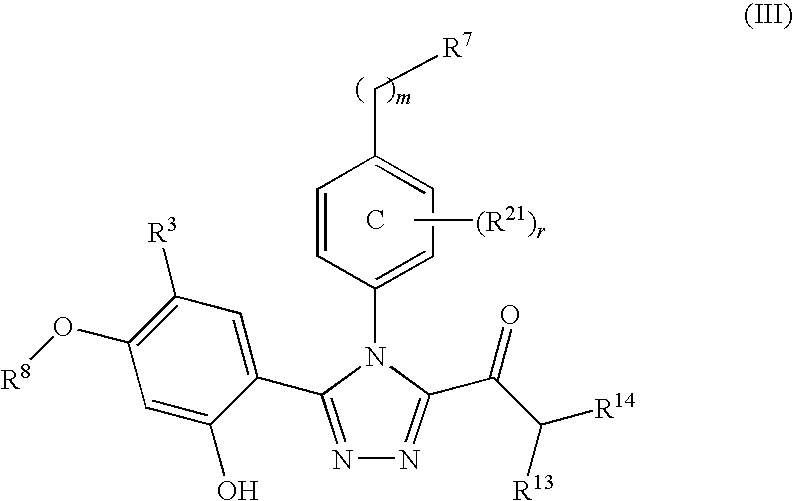
Triazole compounds that modulate Hsp90 activity
ActiveUS20060167070A1Reduced growth rateInhibit tumor growthBiocideOrganic chemistryTriazole antifungalsHSP90 Heat-Shock Proteins
The present invention relates to substituted triazole compounds and compositions comprising substituted triazole compounds. The invention further relates to methods of inhibiting the activity of Hsp90 in a subject in need thereof and methods for preventing or treating hyperproliferative disorders, such as cancer, in a subject in need thereof comprising administering to the subject a substituted triazole compound of the invention, or a composition comprising such a compound.
Owner:SYNTA PHARMA CORP
Small-molecule Hsp90 inhibitors
Hsp90 inhibitors are provided having the formula:with a 2′,4′,5′-substitution pattern on the right-side aryl moiety. X1 represents two substituents, which may be the same or different, disposed in the 4′ and 5′ positions on the aryl group, wherein X1 is selected from halogen, alkyl, alkoxy, halogenated alkoxy, hydroxyalkyl, pyrollyl, optionally substituted aryloxy, alkylamino, dialkylamino, carbamyl, amido, alkylamido dialkylamido, acylamino, alkylsulfonylamido, trihalomethoxy, trihalocarbon, thioalkyl, SO2−alkyl, COO-alkyl, KH2, OH, CN, SO2X5, NO2, NO, C═SR2 NSO2X5, C═OR2, where X5 is F, NH2, alkyl or H, and R2 is alkyl, NH2, NH-alkyl or O-alkyl, C1 to C6 alkyl or alkoxy; or wherein X1 has the formula —O—(CH2)n—O—, wherein n is an integer from 0 to 2, preferably 1 or 2, and one of the oxygen is bonded at the 5′-position and the other at the 4′-position of the aryl ring. The compounds are useful in cancer therapy and as radioimaging ligands.
Owner:SLOAN KETTERING INST FOR CANCER RES
2-Aminopurine analogs having HSP90-inhibiting activity
2-Aminopurine analogs are described and demonstrated or predicted to have utility as Heat Shock Protein 90 (HSP90) inhibiting agents in the treatment and prevention of various HSP90 mediated disorders, e.g., proliferative disorders. Method of synthesis and use of such compounds are also described and claimed.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Compositions and methods of use of targeting peptides for diagnosis and therapy of human cancer
The present invention concerns compositions comprising and methods of identification and use of targeting peptides selective for cancer tissue, particularly prostate or ovarian cancer tissue. The method may comprise identifying endogenous mimeotopes of such peptides, such as GRP78, IL-11Rα and hsp90. Antibodies against such targeting peptides or their mimeotopes may be used for detection, diagnosis and / or staging of prostate or ovarian cancer. In other embodiments, the compositions and methods concern novel type of gene therapy vector, known as adeno-associated phage (AAP). AAP are of use for targeted delivery of therapeutic agents to particular tissues, organs or cell types, such as prostate or ovarian cancer. In still other embodiments, targeting peptides selective for low-grade lipomas may be used for detection, diagnosis and targeted delivery of therapeutic agents.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
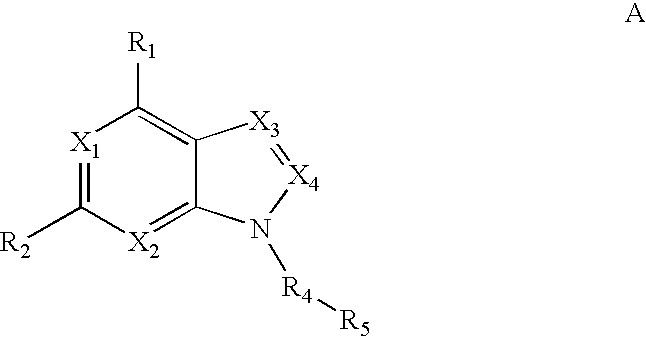
Fused amino pyridine as hsp90 inhibitors
The present invention relates to HSP90 inhibitors containing fused amino pyridine core that are useful as inhibitors of HSP90 and their use in the treatment of HSP90 related diseases and disorders such as cancer, an autoimmune disease, or a neurodegenerative disease.
Owner:CURIS INC
Compositions comprising heat shock proteins or alpha(2) macroglobulin, antigenic molecules and saponins, and methods of use thereof
InactiveUS20020037290A1Growth inhibitionEffective amountBiocideSenses disorderAutoimmune ReactionsAutoimmune responses
The present invention relates to pharmaceutical compositions and methods for the prevention and treatment of autoimmune diseases, infectious diseases, neurodegenerative diseases, and primary and metastatic neoplastic diseases. In the practice of the invention, the compositions are employed comprising: (a) a heat shock protein (hsp) or an alpha(2)macroglobulin (alpha2M); (b) a saponin; and, optionally, (c) an antigenic molecule. The antigenic molecule displays the antigenicity of an antigen of: (a) a cell that elicits an autoimmune response; (b) an agent of an infectious disease; (c) a cancerous cell; or (d) a cell or structure associated with a neurodegenerative or amyloid disease. The hsps that can be used in the practice of the invention include but are not limited to hsp70, hsp90, gp96, calreticulin, hsp 110, grp 170, and PDI, alone or in combination with each other. The antigenic molecule can be covalently or noncovalently bound to the hsp or alpha2M, free in solution, and / or covalently bound to the saponin. The compositions of the invention can be administered alone or in combination with the administration of antigen presenting cells sensitized with an hsp- or alpha2M-antigenic molecule complex.
Owner:ANTIGENICS
Purification of heat shock/stress protein cell surface receptors and their use as immunotherapeutic agents
InactiveUS6797480B1Improve isolationBiocidePeptide/protein ingredientsAntigenImmunotherapeutic agent
The present invention relates to receptors for heat shock proteins (HSPs), such as gp96, Hsp70 and Hsp90. The heat shock receptor is associated with the cell membranes of a subset of antigen presenting cells, such as macrophages and dendritic cells. The present invention relates to the use of the heat shock protein receptor positive cells, heat shock protein receptor protein, and heat shock protein receptor genes in methods for screening a molecule for the ability to modulate heat shock protein levels or activities.
Owner:CONNECTICUT HEALTH CENT UNIV OF
Triazolopyrimidines and related analogs as HSP90-inhibitors
Triazolopyrimidines and related compounds are described and demonstrated or predicted to have utility as Heat Shock Protein 90 (HSP90) inhibiting agents in the treatment and prevention of various HSP90 mediated disorders, e.g., proliferative disorders. Method of synthesis and use of such compounds are also described and claimed.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Remedy for diseases associated with immunoglobulin gene translocation
InactiveUS20070004674A1Easy to produceEasy to implementBiocideOrganic chemistryHSP90 Heat-Shock ProteinsBULK ACTIVE INGREDIENT
A medicament comprising a compound having an inhibitory action on Hsp90 or a pharmaceutically acceptable salt thereof as an active ingredient is used as a therapeutic agent for diseases associated with immunoglobulin gene translocations. In diseases associated with immunoglobulin gene translocations, abnormal enhancement of the expression of a partner gene in immunoglobulin gene translocation participates in the development of the diseases and the progress of the symptoms. By administering the compound having an inhibitory action on Hsp90 or a pharmaceutically acceptable salt thereof, degradation of protein encoded by the partner gene is promoted and the diseases can be treated.
Owner:KYOWA HAKKO KIRIN CO LTD
Small-Molecule Hsp90 Inhibitors
Hsp90 inhibitors are provided having the formula:with a 2′,4′,5′-substitution pattern on the right-side aryl moiety. X1 represents two substituents, which may be the same or different, disposed in the 4′ and 5′ positions on the aryl group, wherein X1 is selected from halogen, alkyl, alkoxy, halogenated alkoxy, hydroxyalkyl, pyrollyl, optionally substituted aryloxy, alkylamino, dialkylamino, carbamyl, amido, alkylamido dialkylamido, acylamino, alkylsulfonylamido, trihalomethoxy, trihalocarbon, thioalkyl, SO2. alkyl, COO-alkyl, KH2, OH, CN, SO2X5, NO2, NO, C═SR2 NSO2X5, C═OR2, where X5 is F, NH2, alkyl or H, and R2 is alkyl, NH2, NH-alkyl or O-alkyl, C1 to C6 alkyl or alkoxy; or wherein X1 has the formula -0-(CH2)n-0-, wherein n is an integer from O to 2, preferably 1 or 2, and one of the oxygens is bonded at the 5′-position and the other at the 4′-position of the aryl ring. The compounds are useful in cancer therapy and as radioimaging ligands.
Owner:SLOAN KETTERING INST FOR CANCER RES
Triazole compounds that modulate HSP90 activity
ActiveUS20080090887A1Less developmentAntibacterial agentsBiocideTriazole antifungalsHSP90 Heat-Shock Proteins
The present invention relates to substituted triazole compounds and compositions comprising substituted triazole compounds. The invention further relates to methods of inhibiting the activity of Hsp90 in a subject in need thereof and methods for preventing or treating hyperproliferative disorders, such as cancer, in a subject in need thereof comprising administering to the subject a substituted triazole compound of the invention, or a composition comprising such a compound.
Owner:SYNTA PHARMA CORP
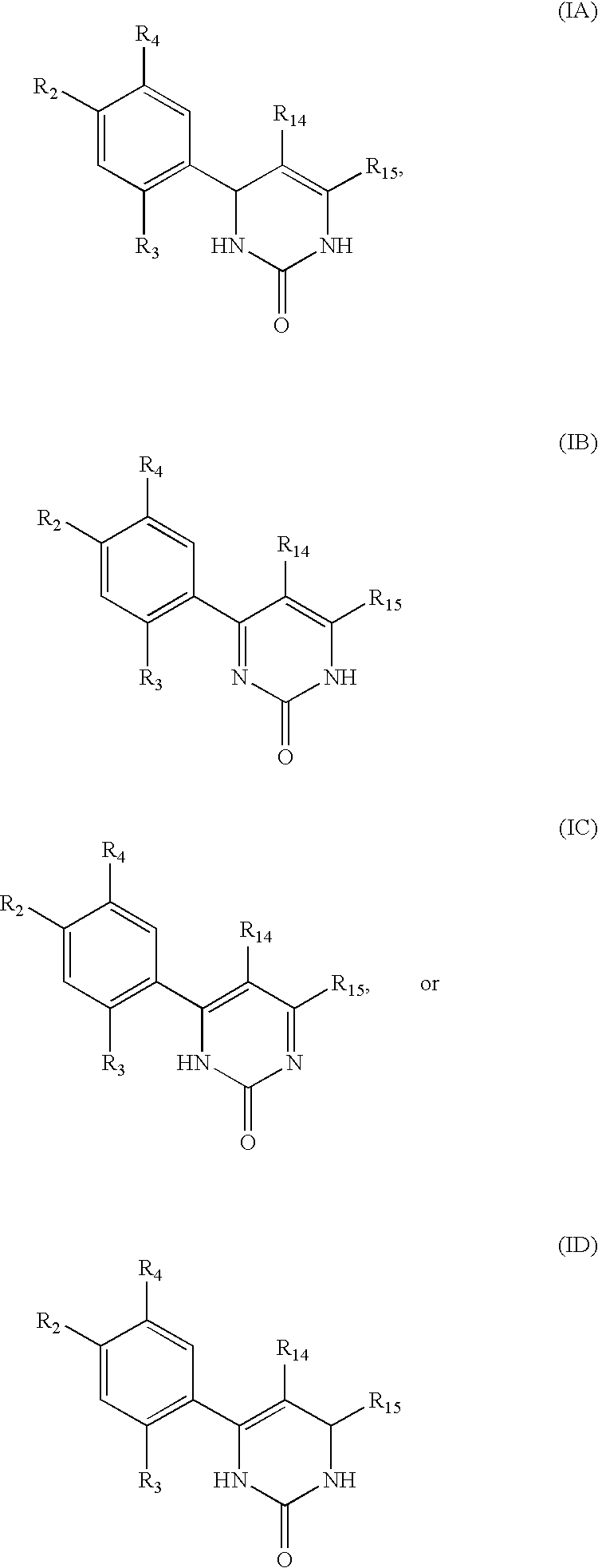
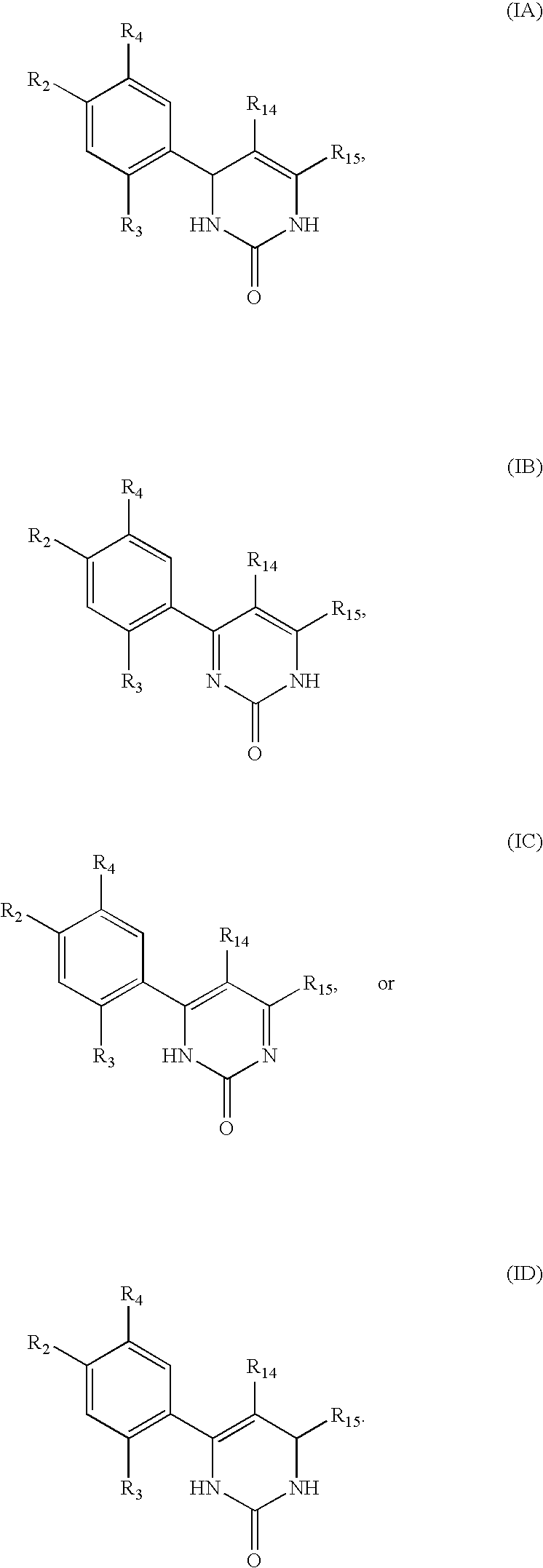
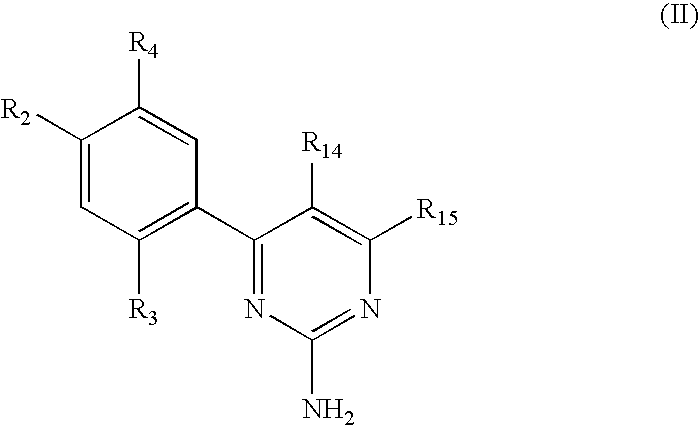
Triazinone and diazinone derivatives useful as hsp90 inhibitors
The present invention relates to compounds according to formulae (IA) to (ID) and compositions that inhibit the activity of Hsp90. The invention further relates to methods of inhibiting the activity of Hsp90 in a subject in need thereof and methods for preventing or treating hyperproliferative disorders, such as cancer, in a subject in need thereof comprising administering to the subject a compound of the invention, or a composition comprising such a compound.
Owner:SYNTA PHARMA CORP
Orally Active Purine-Based Inhibitors of Heat Shock Protein 90
Novel purine compounds and tautomers and pharmaceutically acceptable salts thereof are described, as are pharmaceutical compositions comprising the same, complexes comprising the same, e.g., HSP90 complexes, and methods of using the same. Methods of using the novel purine compounds of the invention, and tautomers and pharmaceutically acceptable salts thereof, include their use in inhibiting heat shock protein 90's (HSP90's) to thereby treat or prevent HSP90-dependent diseases, e.g., proliferative disorders such as breast cancer.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Triazole compounds that modulate HSP90 activity
Owner:SYNTA PHARMA CORP
Compositions and methods of use of targeting peptides for diagnosis and therapy of human cancer
The present invention concerns compositions comprising and methods of identification and use of targeting peptides selective for cancer tissue, particularly prostate or ovarian cancer tissue. The method may comprise identifying endogenous mimeotopes of such peptides, such as GRP78, IL-11Rα and hsp90. Antibodies against such targeting peptides or their mimeotopes may be used for detection, diagnosis and / or staging of prostate or ovarian cancer. In other embodiments, the compositions and methods concern novel type of gene therapy vector, known as adeno-associated phage (AAP). AAP are of use for targeted delivery of therapeutic agents to particular tissues, organs or cell types, such as prostate or ovarian cancer. In still other embodiments, targeting peptides selective for low-grade lipomas may be used for detection, diagnosis and targeted delivery of therapeutic agents.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Inhibitors of extracellular Hsp90
ActiveUS20060073151A1Increased toxicityEasy to synthesizePowder deliveryBiocideExtracellularCancer cell
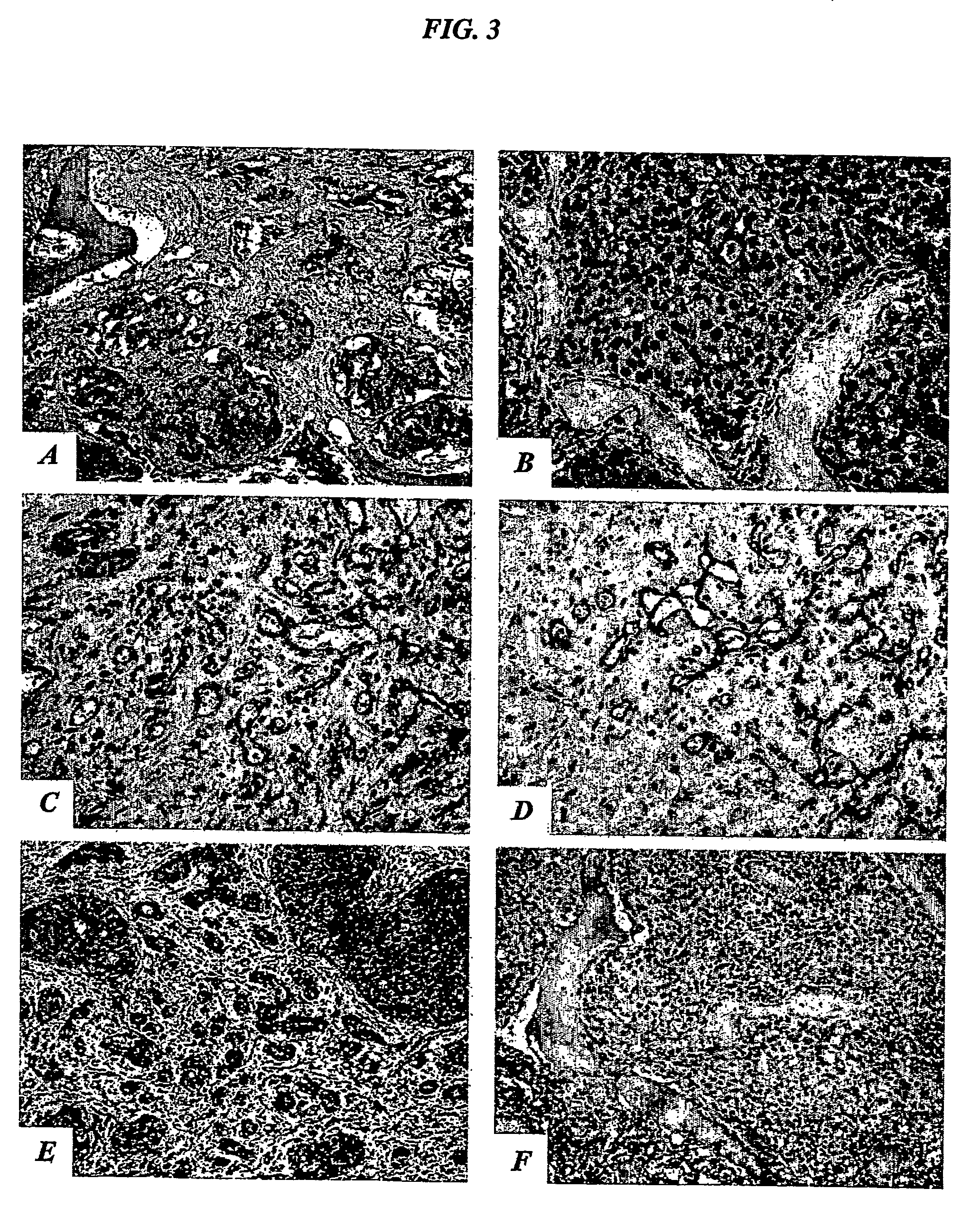
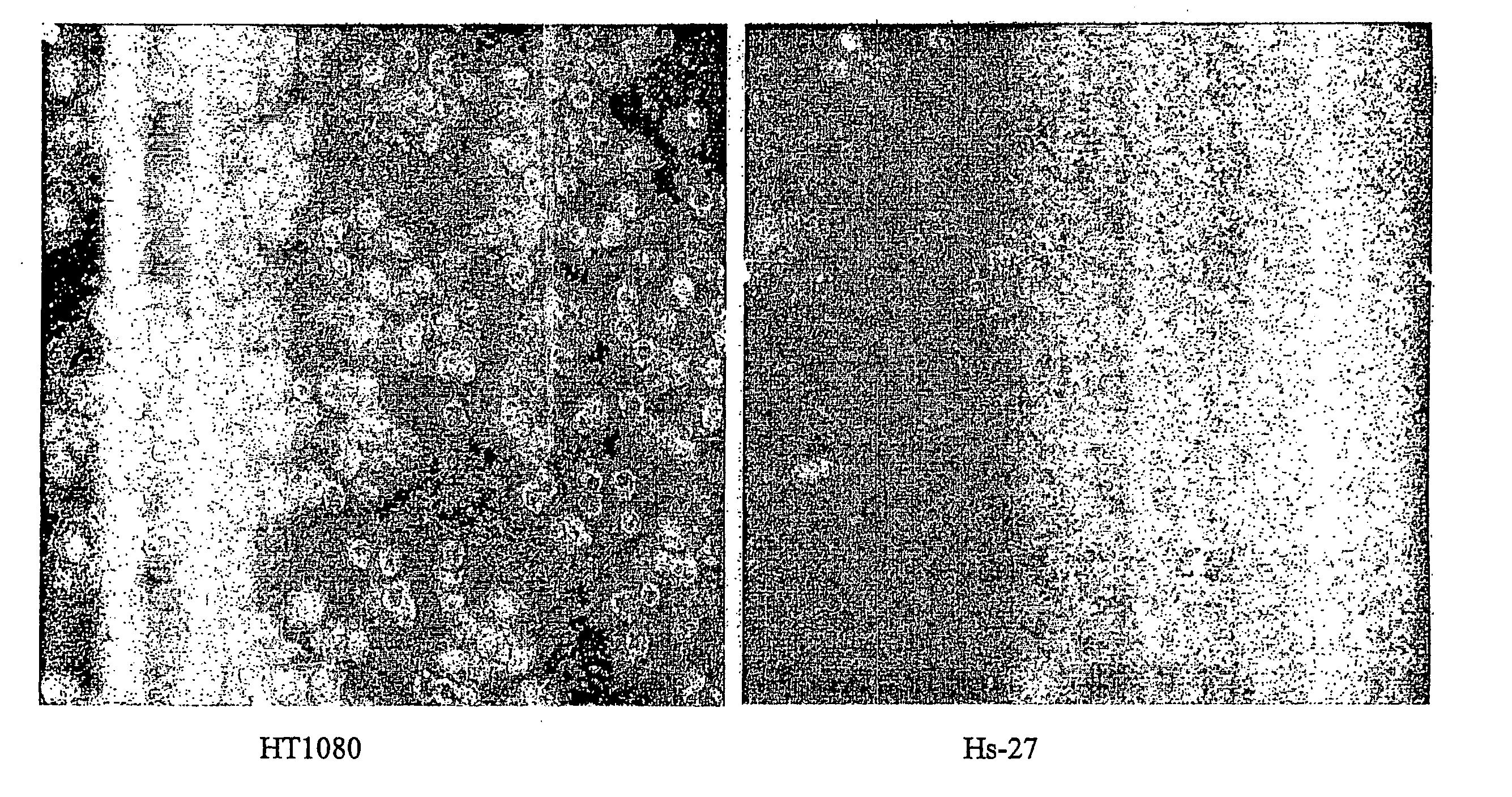
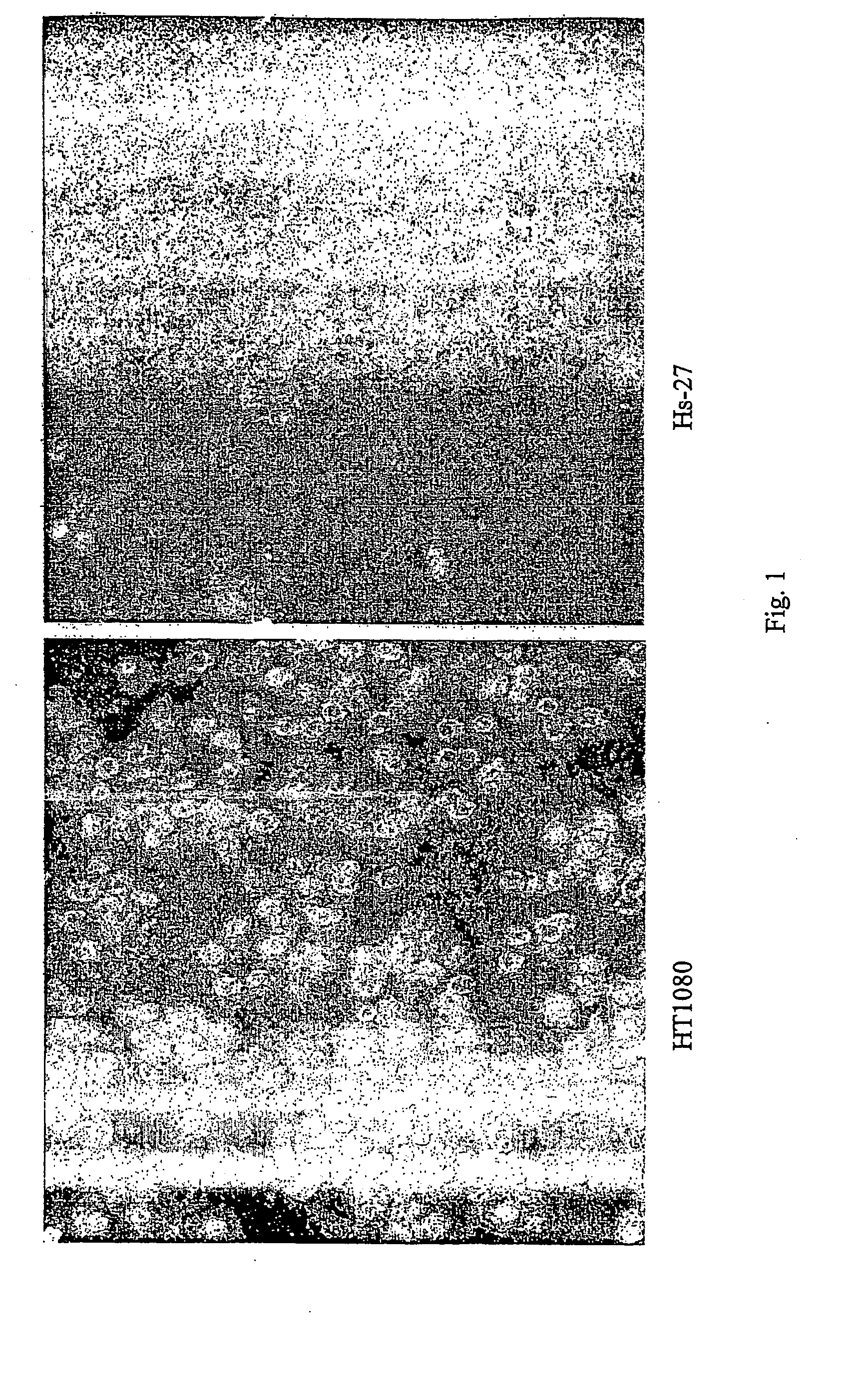
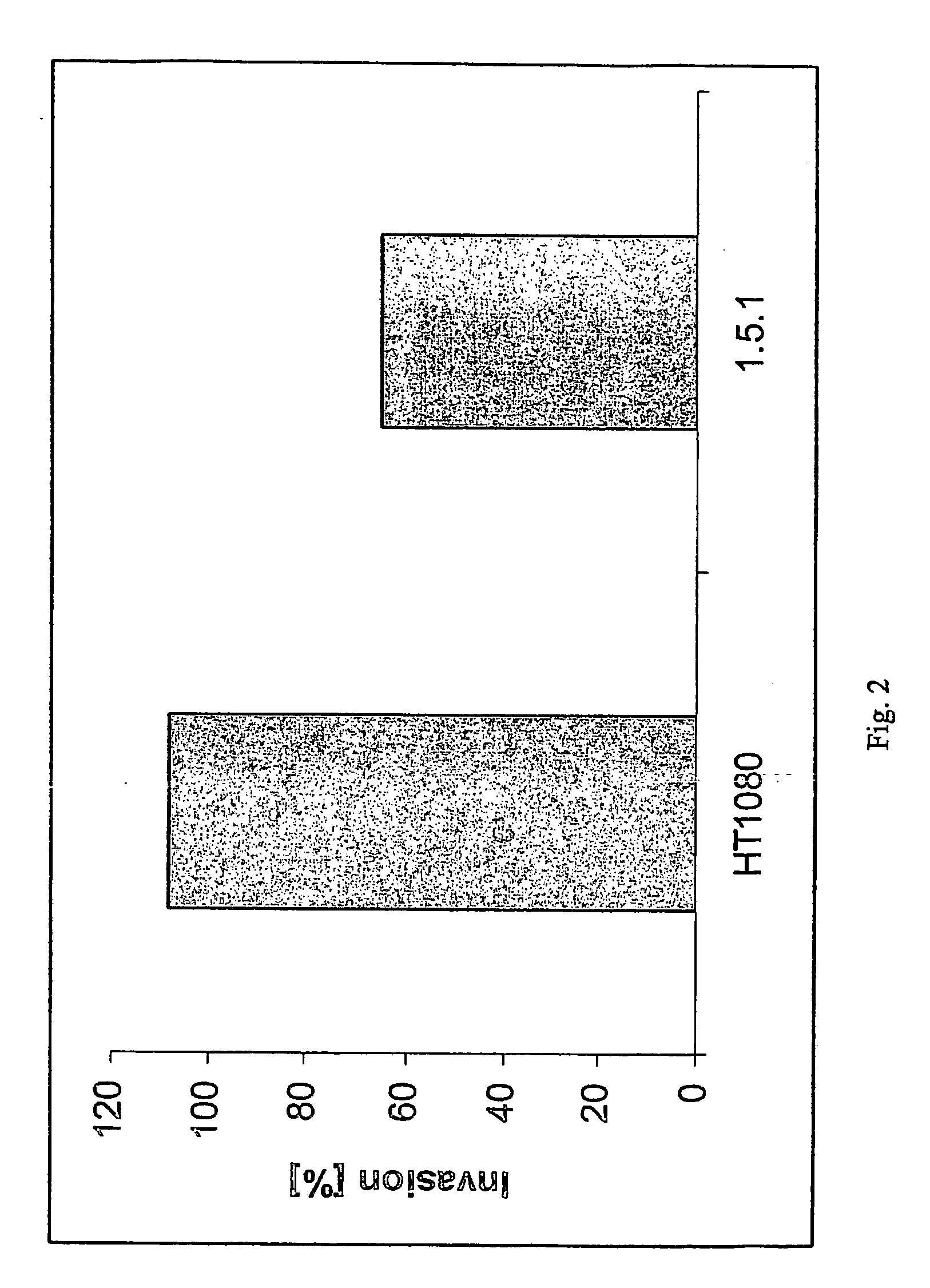
The present invention describes inhibitors of extracellular Hsp90. The inhibition of extracellular Hsp90 leads to a reduction of the invasiveness of the tumor cells. Furthermore, the invention relates to the use of molecules inhibiting extracellular Hsp90 function for the manufacture of a medicament for the treatment or prevention of invasion and / or metastatic potential of cancer cells.
Owner:TUFTS UNIV
Compounds that modulate HSP90 activity and methods for identifying same
InactiveUS20080027047A1Less developmentBiocideCompound screeningHSP90 Heat-Shock ProteinsStereochemistry
The present invention relates to compositions and methods related to inhibitors of Hsp90, including substituted triazole compounds and compositions comprising substituted triazole compounds. The invention further relates to methods of inhibiting the activity of Hsp90 in a subject in need thereof and methods for preventing or treating hyperproliferative disorders, such as cancer, in a subject in need thereof comprising administering to the subject a substituted triazole compound of the invention, or a composition comprising such a compound. The invention further provides methods for designing and identifying inhibitors of Hsp90.
Owner:SYNTA PHARMA CORP
Triazole compounds that modulate HSP90 activity
The present invention relates to substituted triazole compounds and compositions comprising substituted triazole compounds. The invention further relates to methods of inhibiting the activity of Hsp90 in a subject in need thereof and methods for preventing or treating hyperproliferative disorders, such as cancer, in a subject in need thereof comprising administering to the subject a substituted triazole compound of the invention, or a composition comprising such a compound.
Owner:SYNTA PHARMA CORP
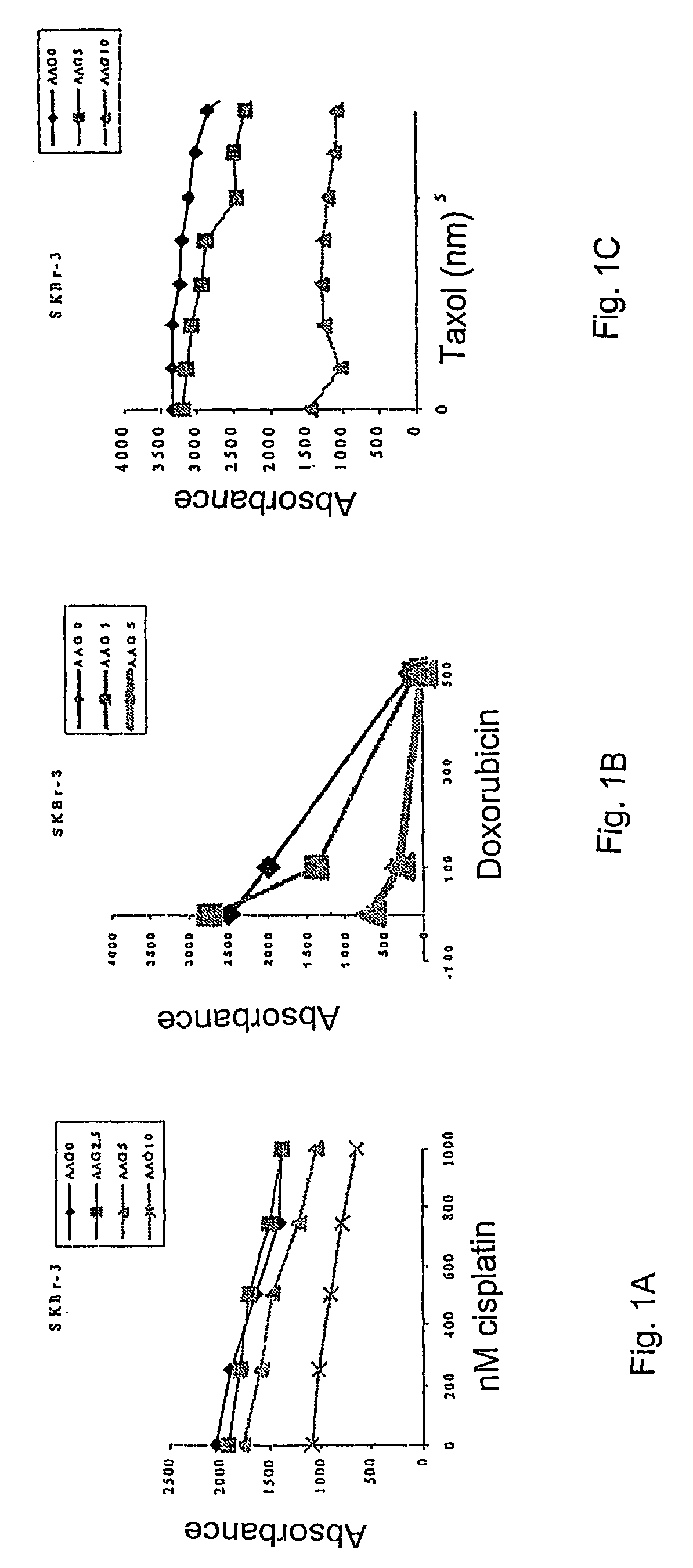
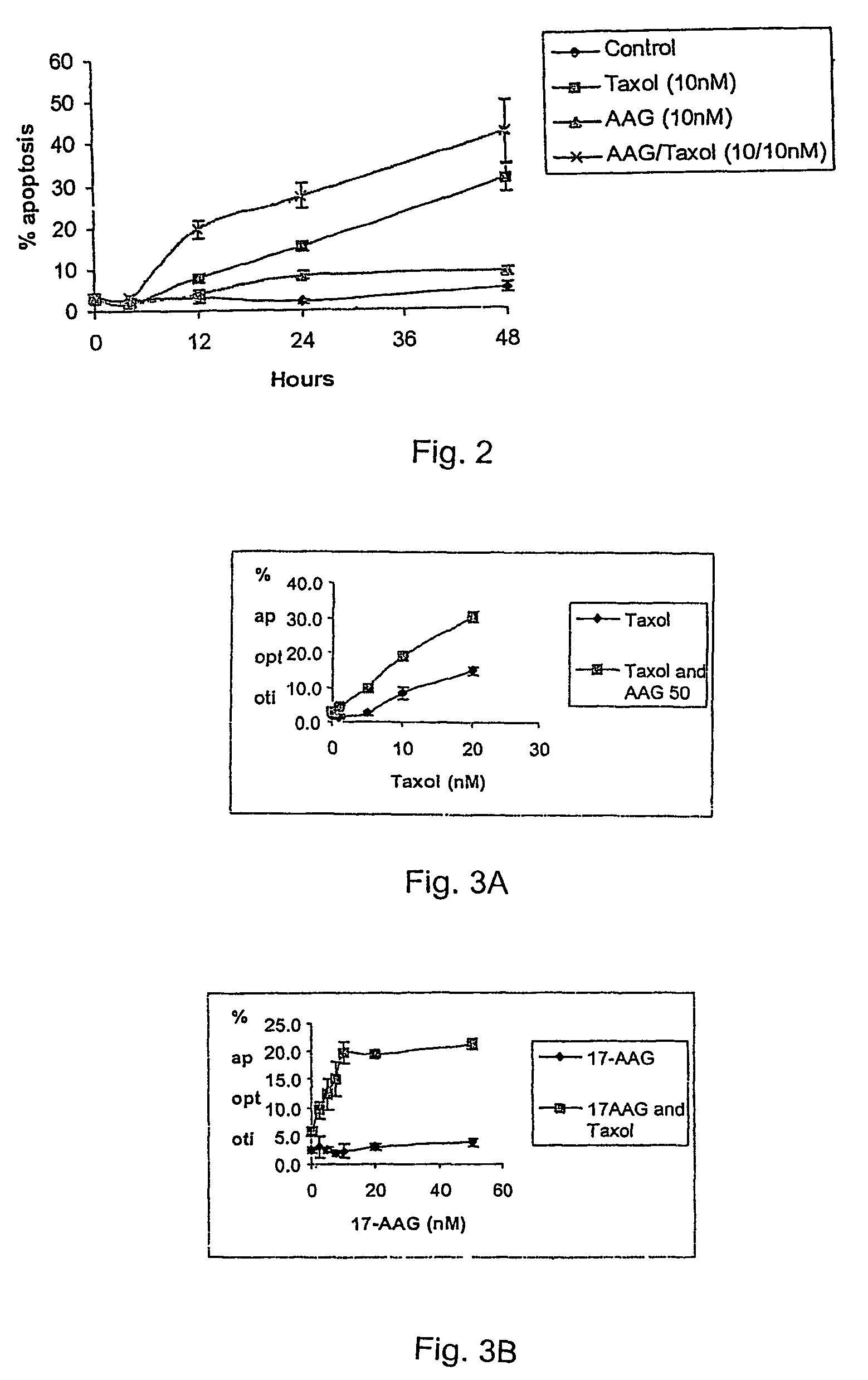
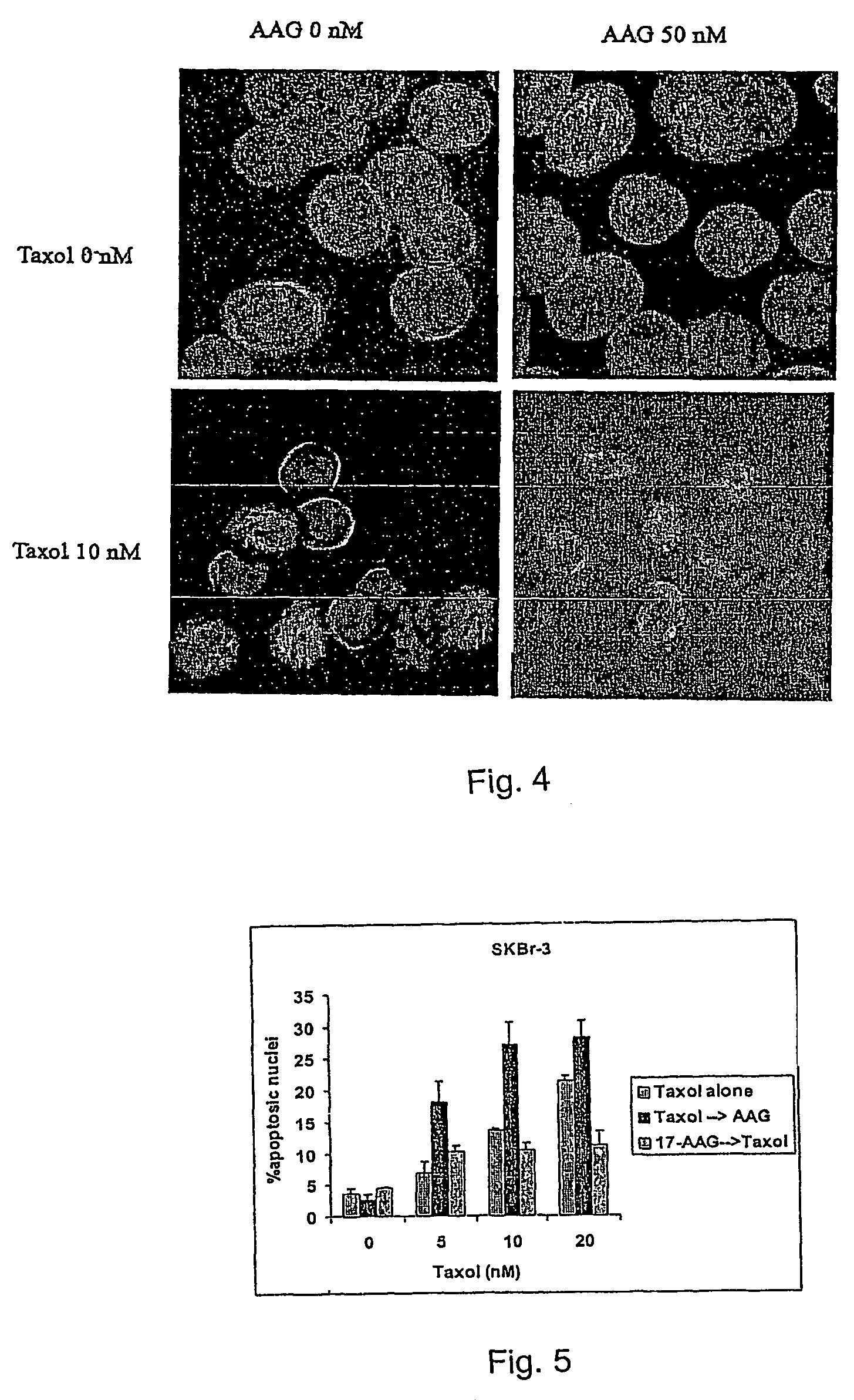
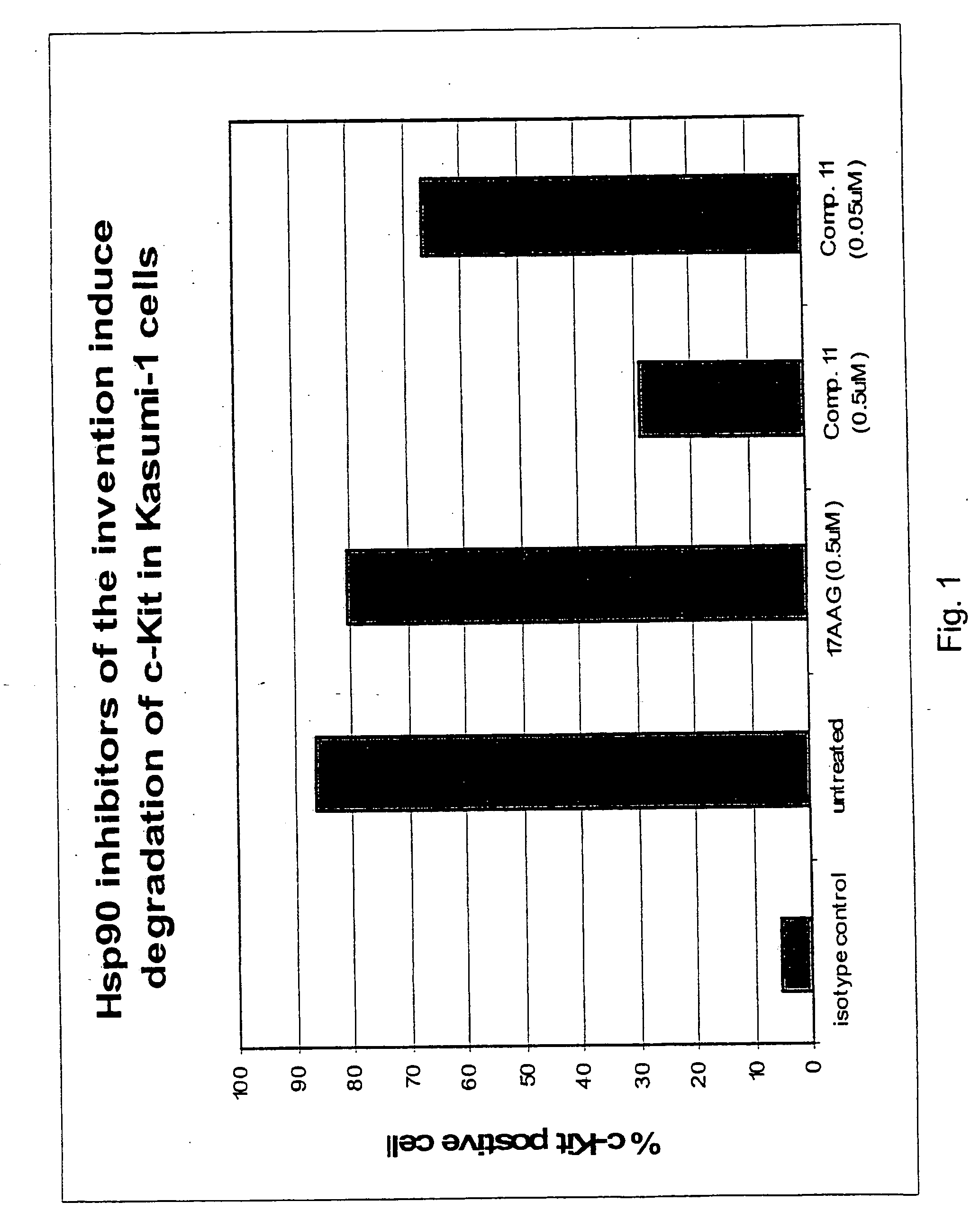
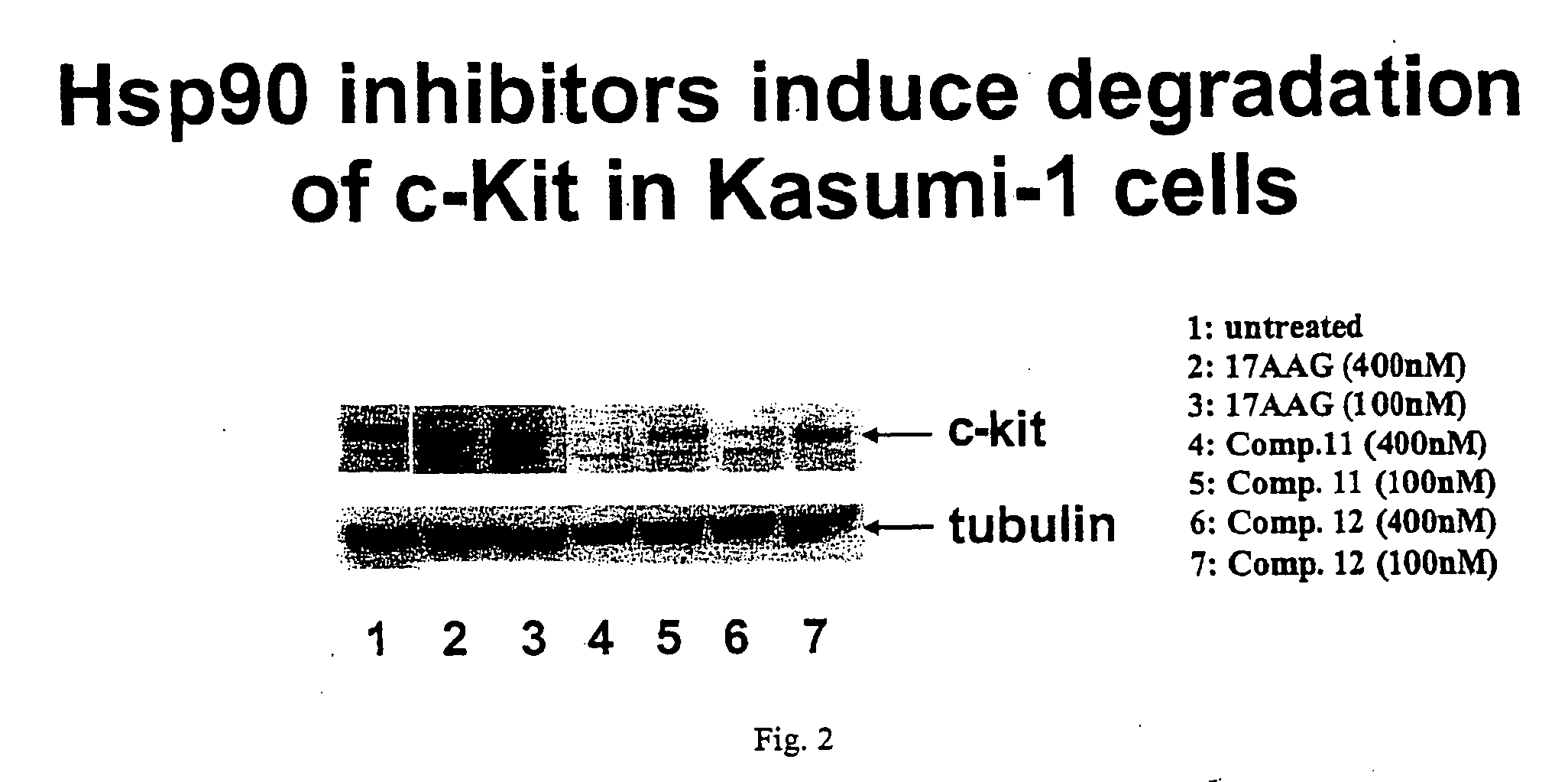
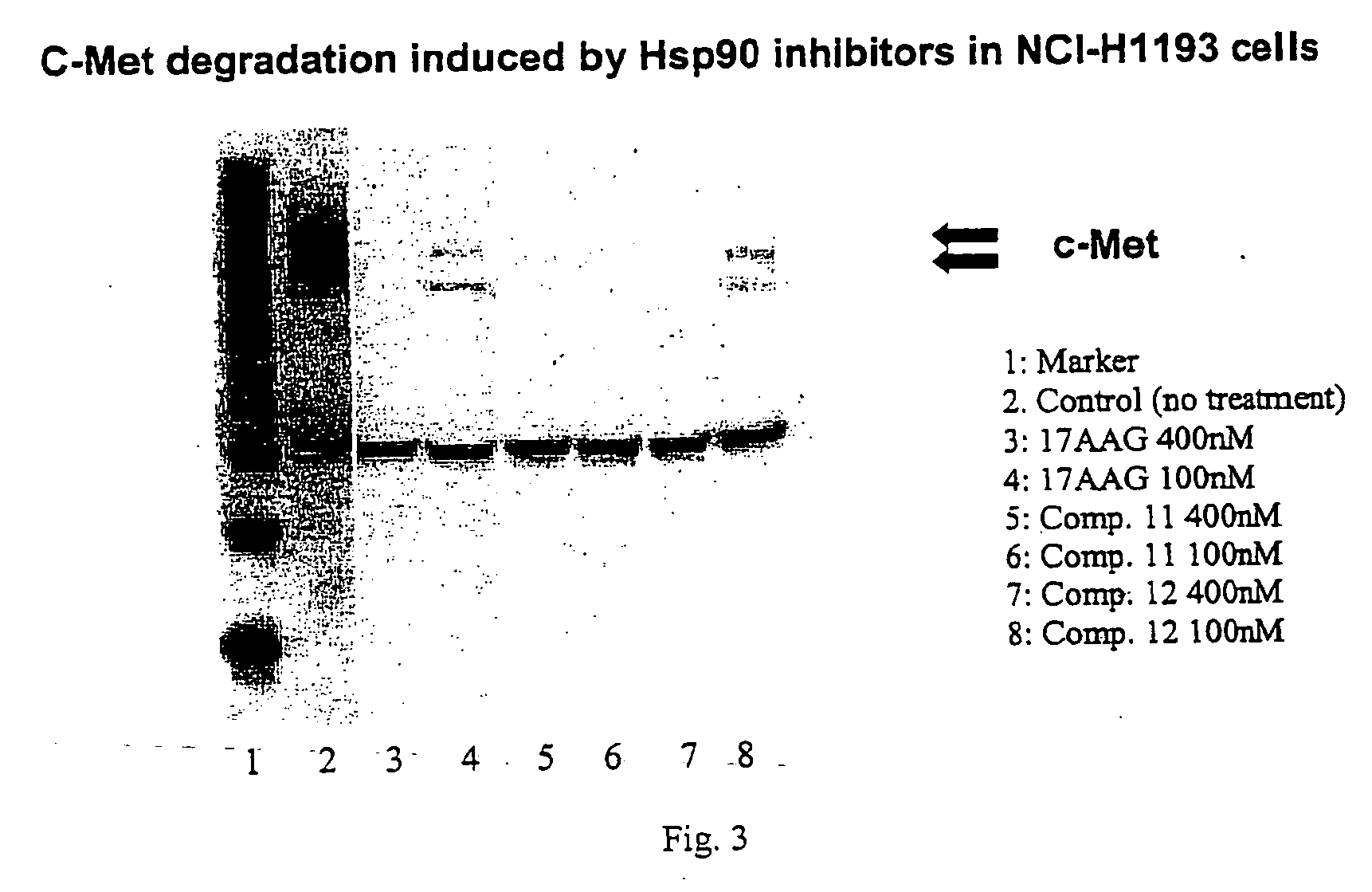
Methods for enhancing the efficacy of cytotoxic agents through the use of HSP90 inhibitors
InactiveUS7211562B2Avoid or reduce their respective toxicity to patientsGrowth inhibitionHeavy metal active ingredientsPeptide/protein ingredientsBinding siteRadicicol
The administration of cytotoxic agents followed by the administration of heat shock protein 90 inhibitors, such as ansamycins, has a synergistic effect on the growth inhibition of cells. This synergy occurs at doses of each cytotoxic agent that normally only causes minimal growth inhibition of cells. Such combination therapy thus allows one to use lower doses of cytotoxic agents to avoid or reduce their respective toxicity to patients without compromising their growth inhibitory effects. Thus, these combinations can be used for the treatment of an animal, preferably a mammal, that has a cell proliferative disorder, whether the cells have wild-type Rb or are Rb deficient or Rb negative. One such method, directed to treating cell proliferative disorders includes the step of administering a therapeutic effective amount of a cytotoxic agent followed by administering a therapeutic effective amount of a heat shock protein 90 inhibitor. The cytotoxic agent may be a microtubule-affecting agent, topoisomerase II inhibitor, a platinum complex, paclitaxel, or a paclitaxel derivative. The HSP90 inhibitor may be an ansamycin, radicicol or a synthetic compound that binds to the ATP-binding site of HSP90.
Owner:SLOAN KETTERING INST FOR CANCER RES
Triazole compounds that modulate HSP90 activity
ActiveUS20070155809A1Less developmentBiocideOrganic chemistryTriazole antifungalsHSP90 Heat-Shock Proteins
The present invention relates to substituted triazole compounds and compositions comprising substituted triazole compounds. The invention further relates to methods of inhibiting the activity of Hsp90 in a subject in need thereof and methods for preventing or treating hyperproliferative disorders, such as cancer, in a subject in need thereof comprising administering to the subject a substituted triazole compound of the invention, or a composition comprising such a compound.
Owner:SYNTA PHARMA CORP
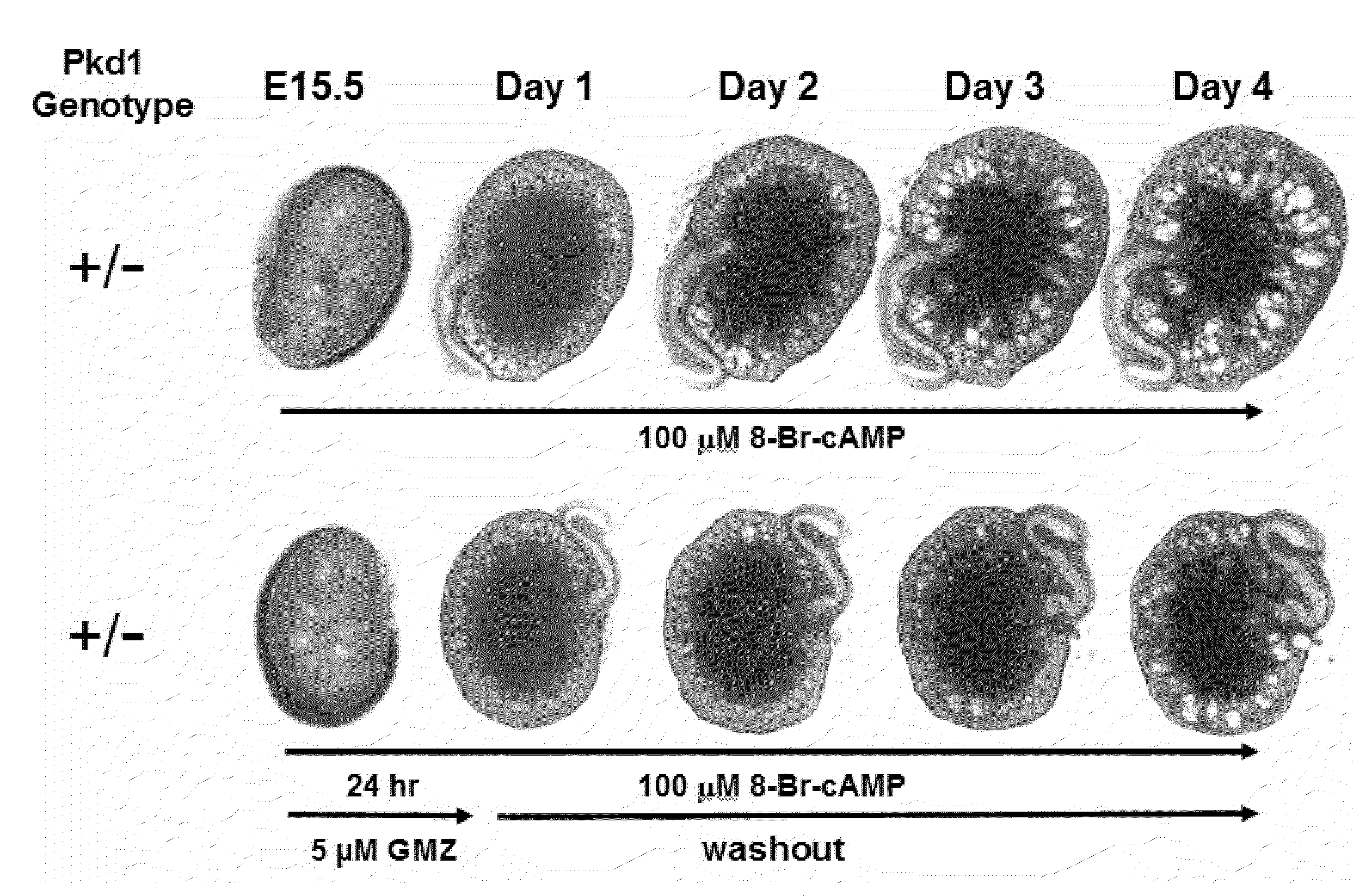
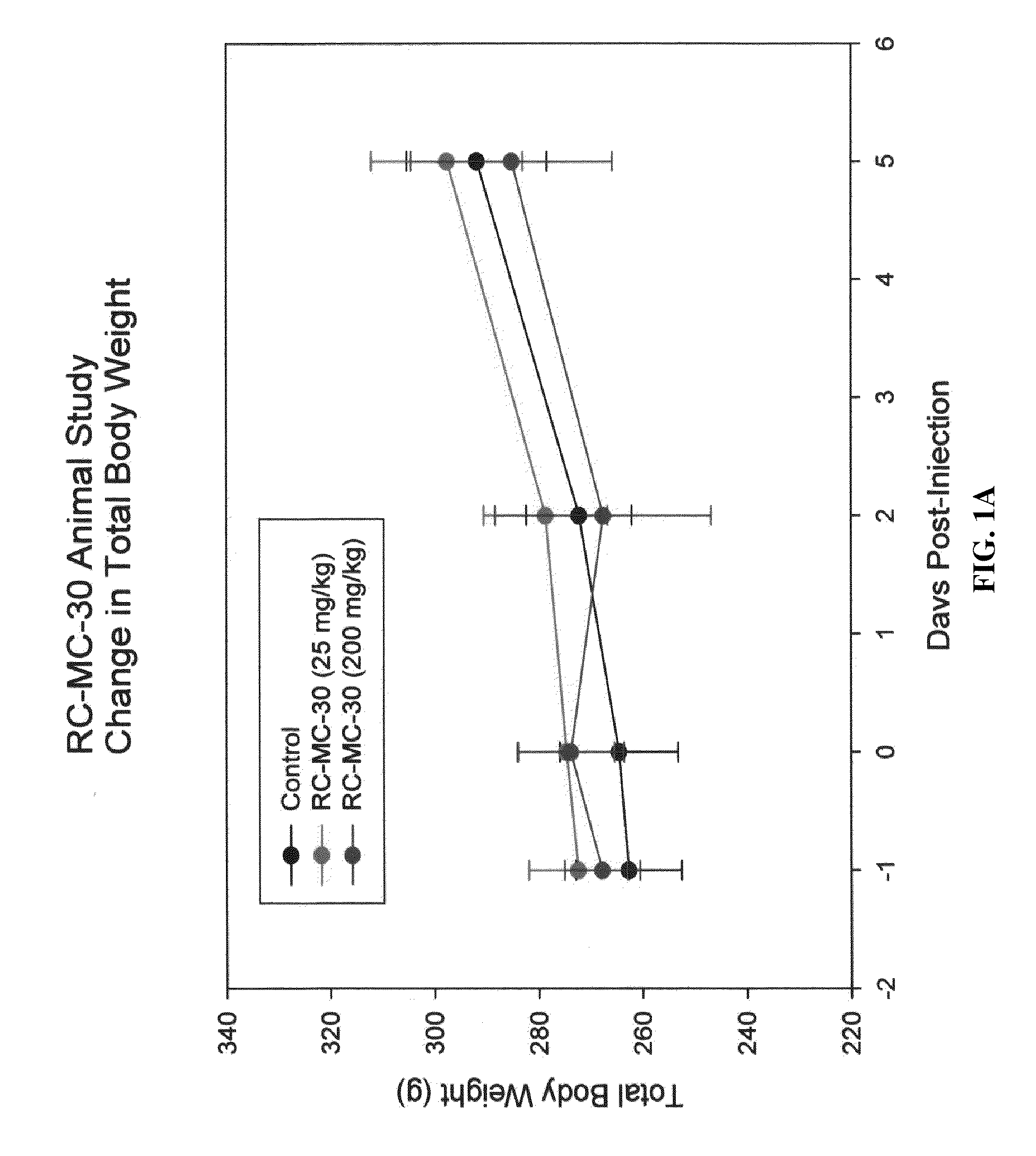
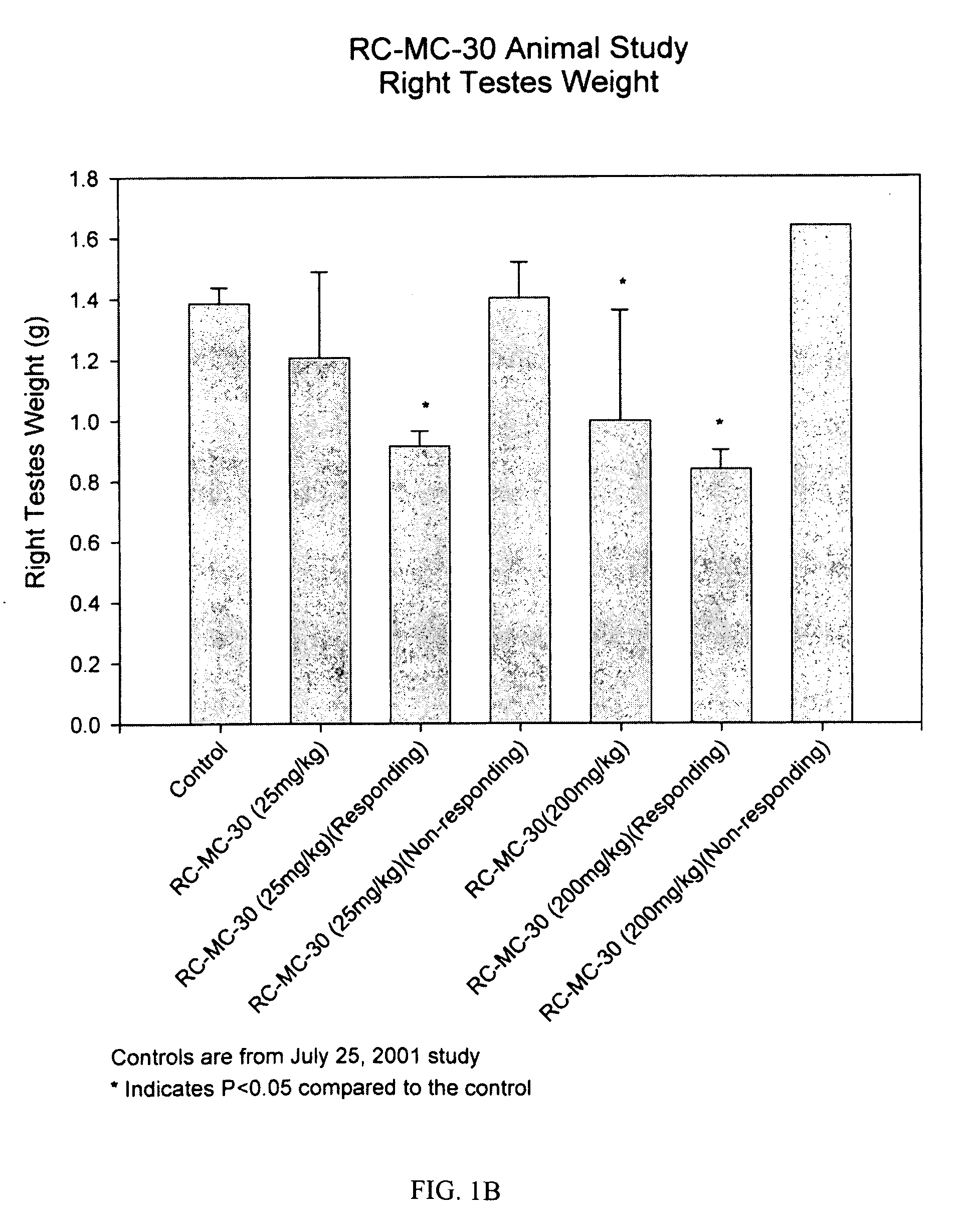
Lonidamine analogues and treatment of polycystic kidney disease
Lonidamine derivatives can be useful in methods of treating, inhibiting, and / or preventing polycystic kidney disease (PKD). Accordingly, lonidamine derivatives can be administered in a therapeutically effective amount for inhibiting, and / or preventing polycystic kidney disease (PKD) in the subject. This can include administering a therapeutically effective amount of the lonidamine derivatives for inhibiting CFTR and / or Hsp90 or biological pathway thereof. Also, the method can include administering the lonidamine derivatives in a therapeutically effective amount for inhibiting ErbB2, Src, Raf-1, B-Raf, MEK, Cdk4, NKCC1, or combinations thereof. For example, the therapeutically effective amount of the lonidamine derivatives can be configured so as to provide a concentration in or adjacent to a kidney cell of about 0.25 uM or more or less.
Owner:UNIVERSITY OF KANSAS
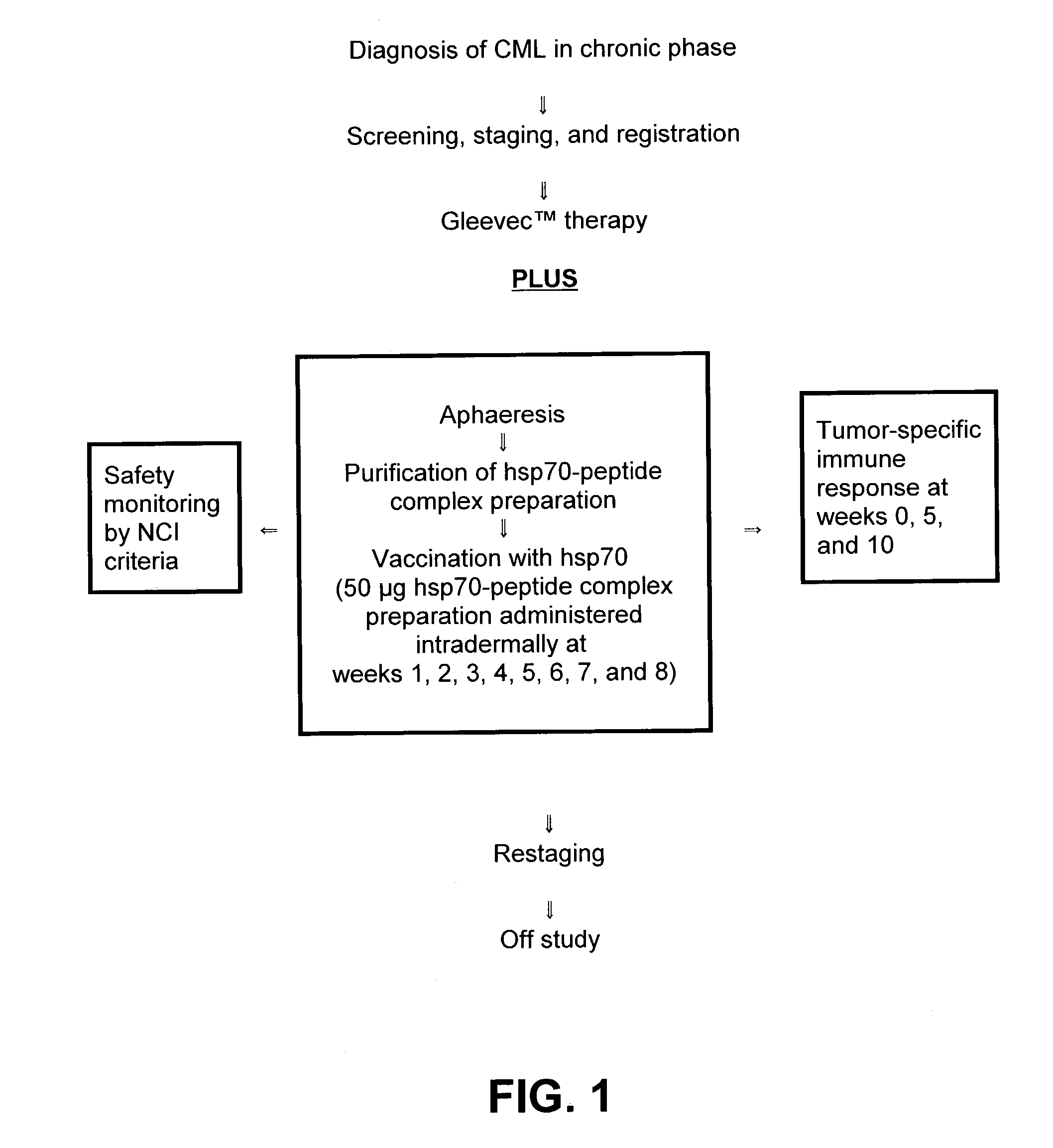
Using heat shock proteins to improve the therapeutic benefit of a non-vaccine treatment modality
InactiveUS6984389B2Enhance and improve benefitImproved profileBiocidePeptide/protein ingredientsAntigenHeat shock
The present invention relates to methods of improving a treatment outcome comprising administering a heat shock protein (HSP) preparation or an α-2-macroglobulin (α2M) preparation with a non-vaccine treatment modality. In particular, an HSP preparation or an α2M preparation is administered in conjunction with a non-vaccine treatment modality for the treatment of cancer or infectious diseases. In the practice of the invention, a preparation comprising HSPs such as but not limited to, hsp70, hsp90 and gp96 alone or in combination with each other, noncovalently or covalently bound to antigenic molecules or α2M, noncovalently or covalently bound to antigenic molecules is administered in conjunction with a non-vaccine treatment modality.
Owner:CONNECTICUT HEALTH CENT UNIV OF
Triazole compounds that modulate HSP90 activity
The present invention relates to substituted triazole compounds and compositions comprising substituted triazole compounds. The invention further relates to methods of inhibiting the activity of Hsp90 in a subject in need thereof and methods for treating hyperproliferative disorders, such as cancer, in a subject in need thereof comprising administering to the subject a substituted triazole compound of the invention, or a pharmaceutical composition comprising such a compound.
Owner:SYNTA PHARMA CORP
Small molecule compositions for binding to hsp90
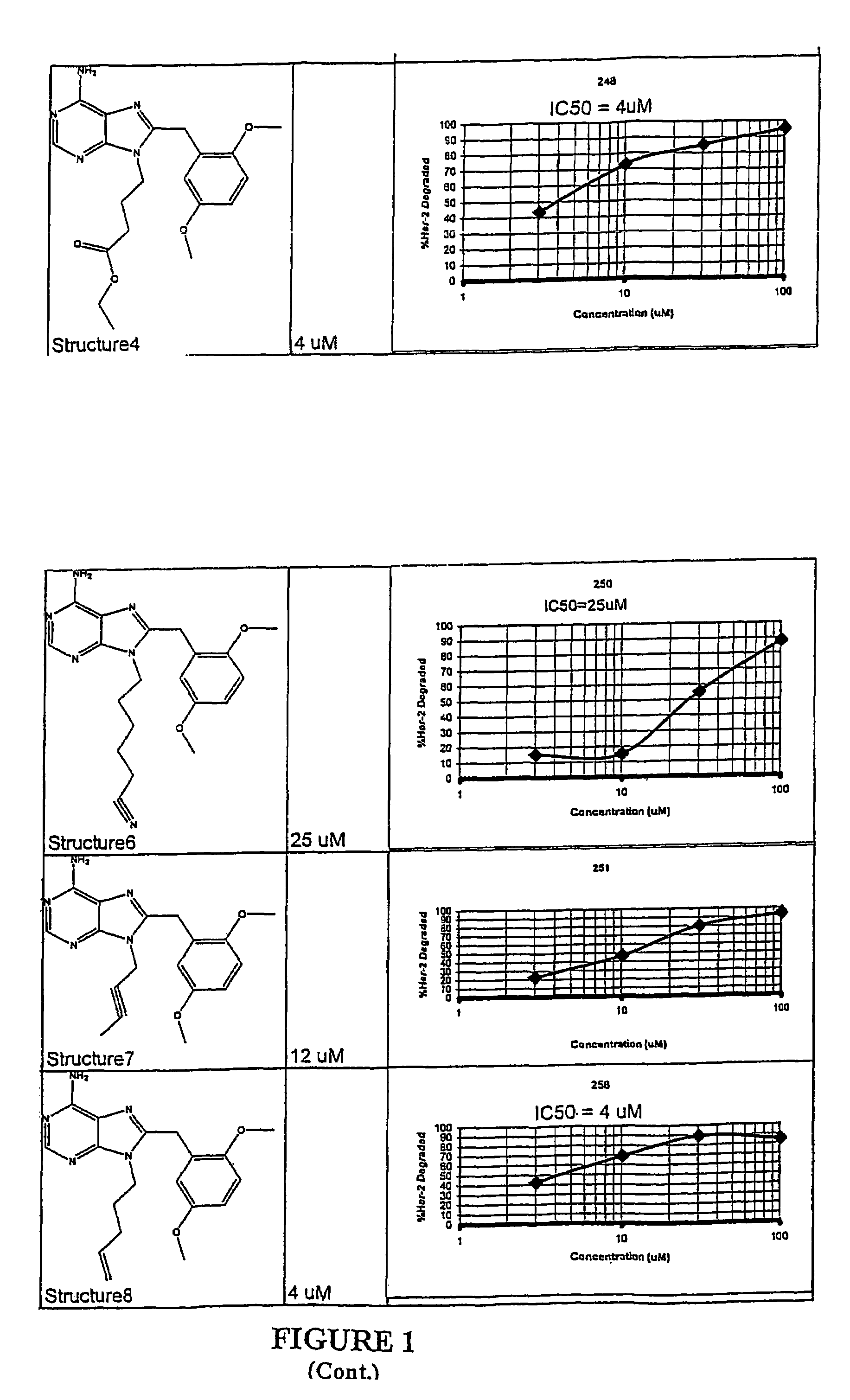
Structural differences in binding pockets of members of the HSP90 family can be exploited to achieve differential degradation of kinases and other signaling proteins through the use of designed small molecules which interact with the N-terminal binding pocket with an affinity which is greater than ADP and different from the ansamycin antibiotics for at least one species of the HSP90 family. Moreover, these small molecules can be designed to be soluble in aqueous media, thus providing a further advantage over the use of ansamycin antibiotics. Pharmaceutical compositions can be formulated containing a pharmaceutically acceptable carrier and a molecule that includes a binding moiety which binds to the N-terminal pocket of at least one member of the HSP90 family of proteins. Such binding moieties were found to have antiproliferative activity against tumor cells which are dependent on proteins requiring chaperones of the HSP90 family for their function. Different chemical species have different activity, however, allowing the selection of, for example Her2 degradation without degradation of Raf kinase. Thus, the binding moieties possess an inherent targeting capacity. In addition, the small molecules can be linked to targeting moieties to provide targeting of the activity to specific classes of cells. Thus, the invention further provides a method for treatment of diseases, including cancers, by administration of these compositions. Dimeric forms of the binding moieties may also be employed.
Owner:SLOAN KETTERING INST FOR CANCER RES
Using heat shock proteins to improve the therapeutic benefit of a non-vaccine treatment modality
InactiveUS20030203846A1Enhance and improve benefitBetter therapeutic profileBiocidePeptide/protein ingredientsAntigenHeat shock
The present invention relates to methods of improving a treatment outcome comprising administering a heat shock protein (HSP) preparation or an alpha-2-macroglobulin (alpha2M) preparation with a non-vaccine treatment modality. In particular, an HSP preparation or an alpha2M preparation is administered in conjunction with a non-vaccine treatment modality for the treatment of cancer or infectious diseases. In the practice of the invention, a preparation comprising HSPs such as but not limited to, hsp70, hsp90 and gp96 alone or in combination with each other, noncovalently or covalently bound to antigenic molecules or alpha2M, noncovalently or covalently bound to antigenic molecules is administered in conjunction with a non-vaccine treatment modality.
Owner:CONNECTICUT HEALTH CENT UNIV OF
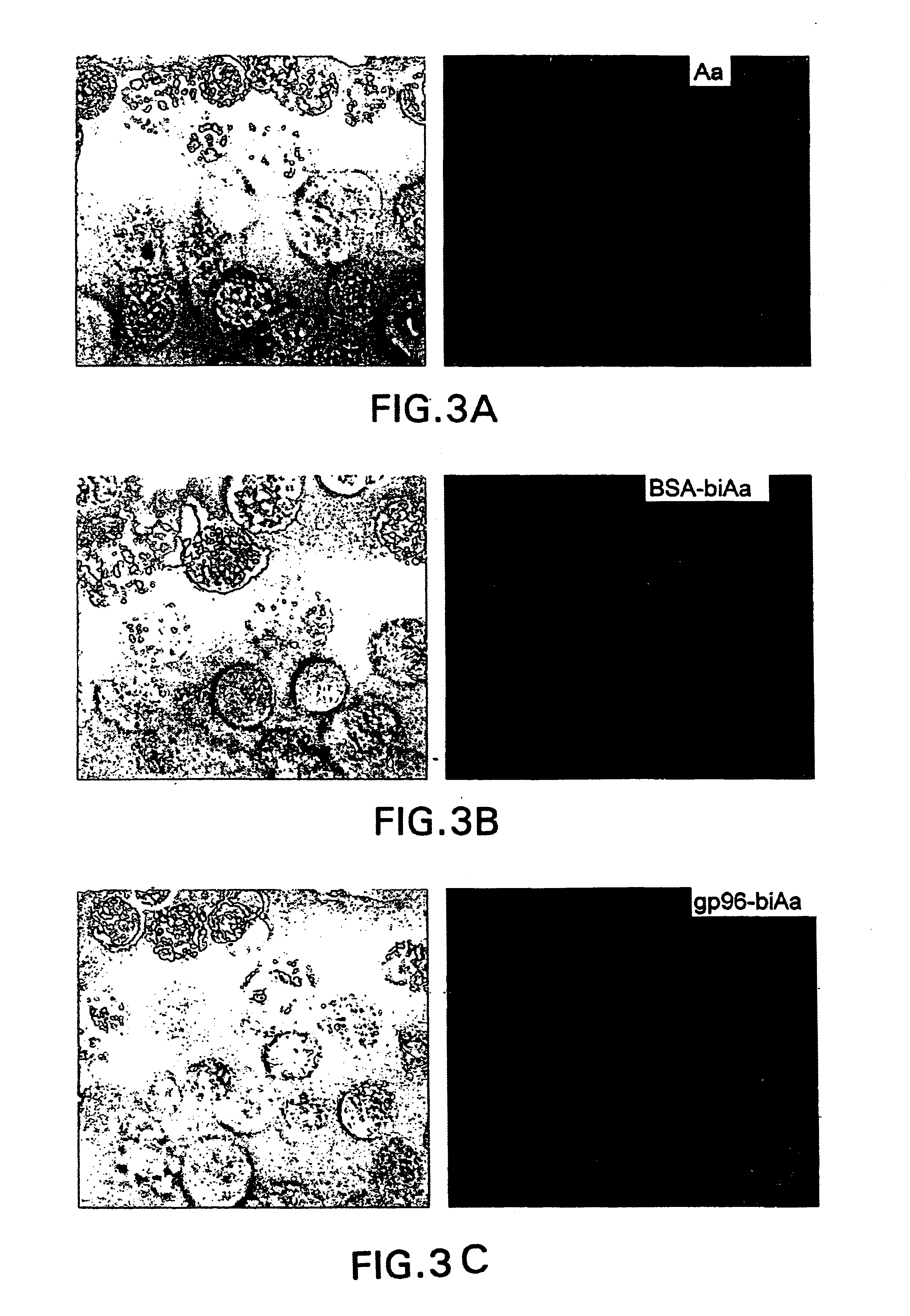
Compositions and methods using complexes of heat shock protein gp96 and antigenic molecules for the treatment and prevention of infectious diseases
InactiveUS6143299AElicit immune responseOrganic active ingredientsPeptide/protein ingredientsMHC class IAntigenic valence
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp70, 50-1000 micrograms for hsp90, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in vivo in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp70-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com