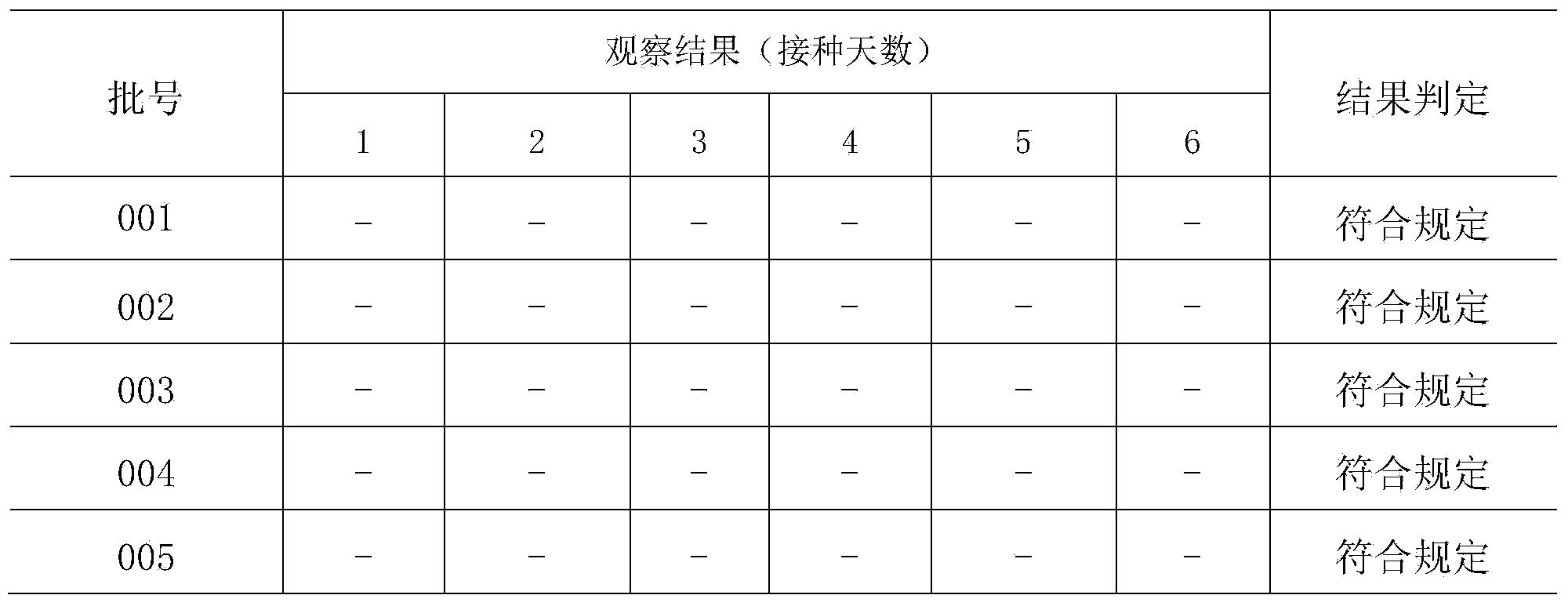
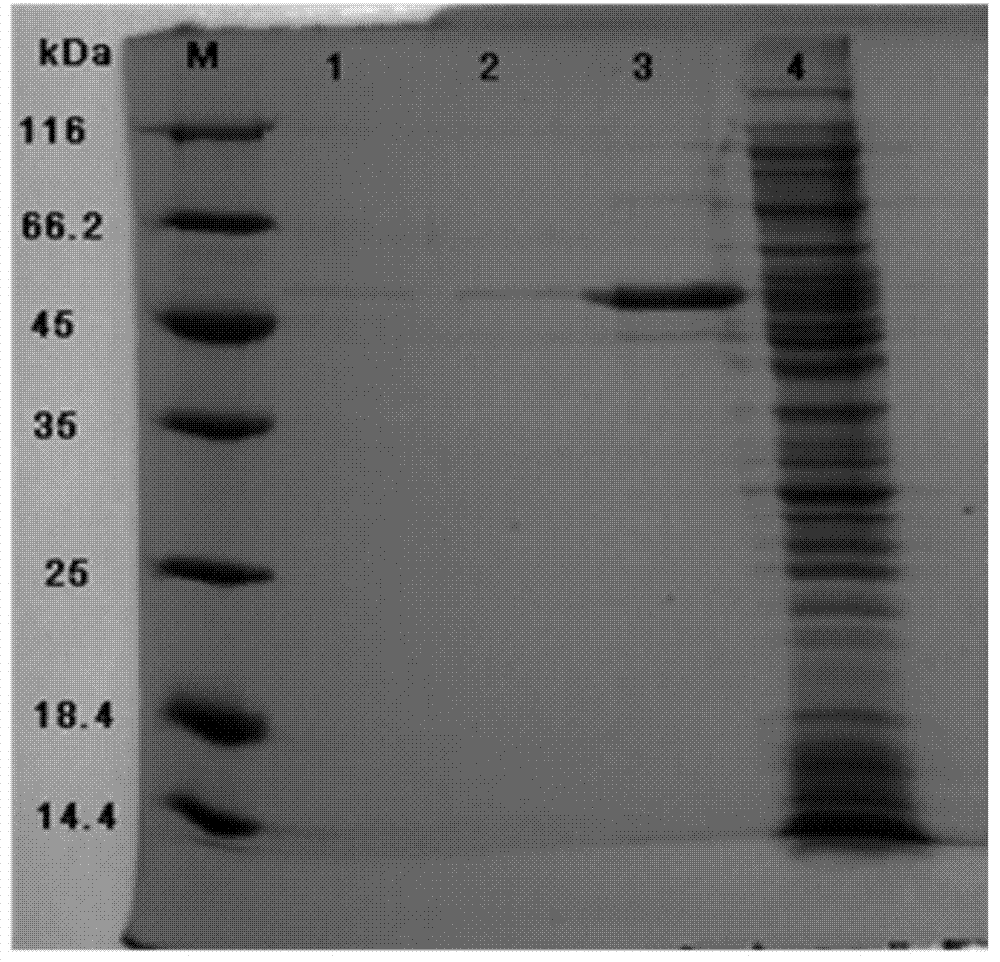
Patents
Literature
246 results about "Brucella" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Brucella is a genus of Gram-negative bacteria, named after David Bruce (1855–1931). They are small (0.5 to 0.7 by 0.6 to 1.5 µm), nonencapsulated, nonmotile, facultatively intracellular coccobacilli. Brucella spp. are the cause of brucellosis, which is a zoonosis transmitted by ingesting contaminated food (such as unpasteurized milk products), direct contact with an infected animal, or inhalation of aerosols. Transmission from human to human, for example through sexual intercourse or from mother to child, is exceedingly rare, but possible. Minimum infectious exposure is between 10 and 100 organisms.
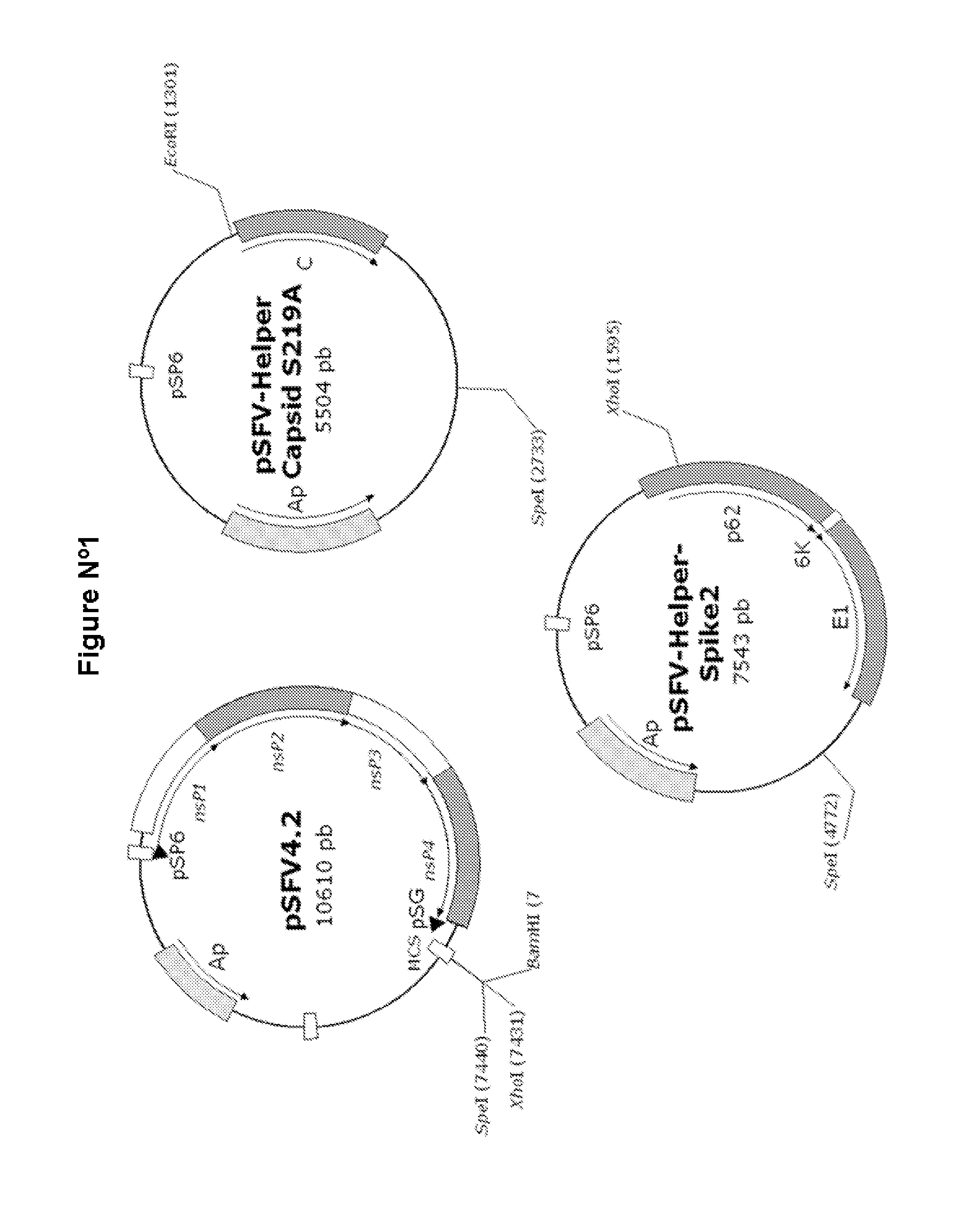
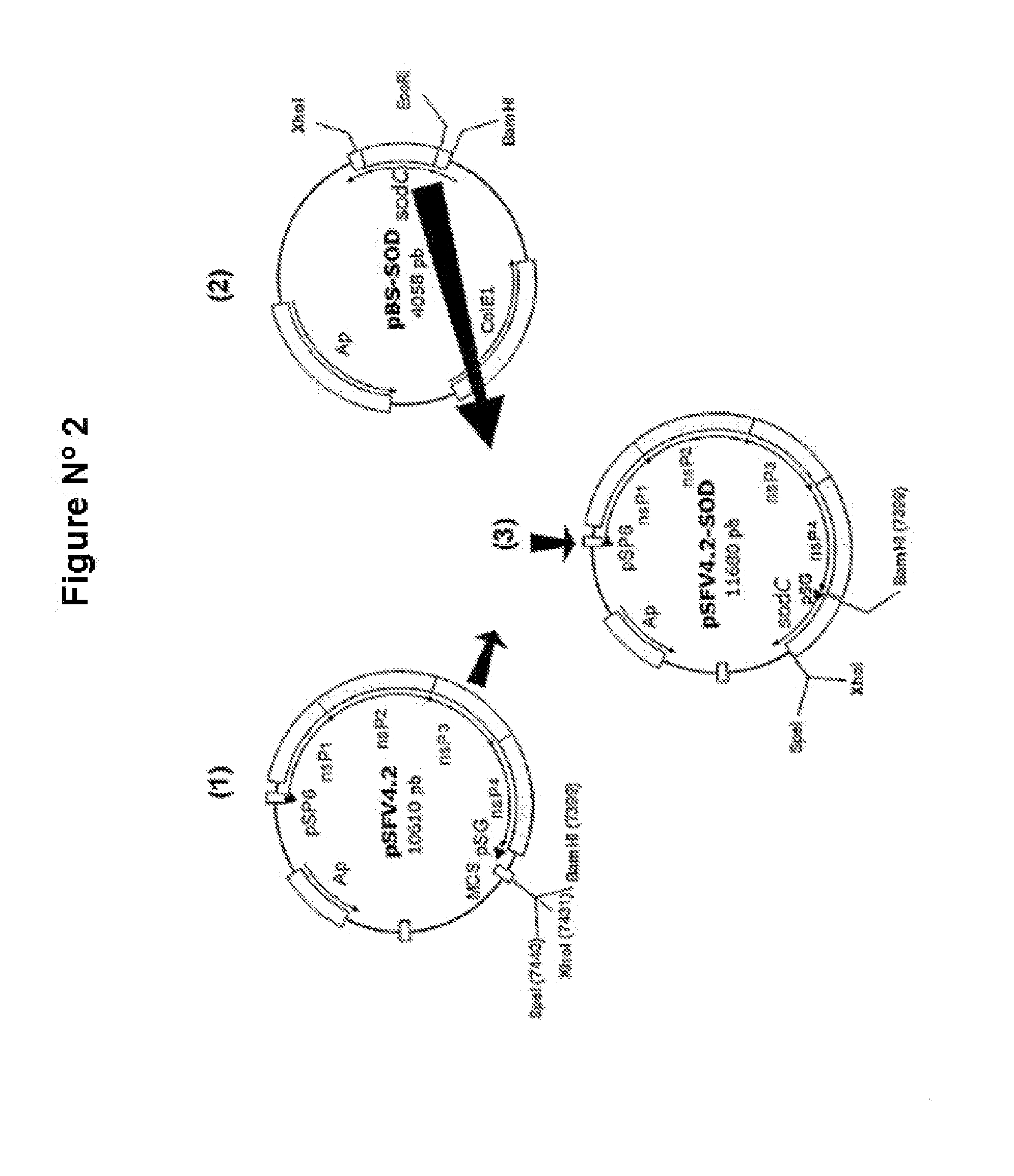
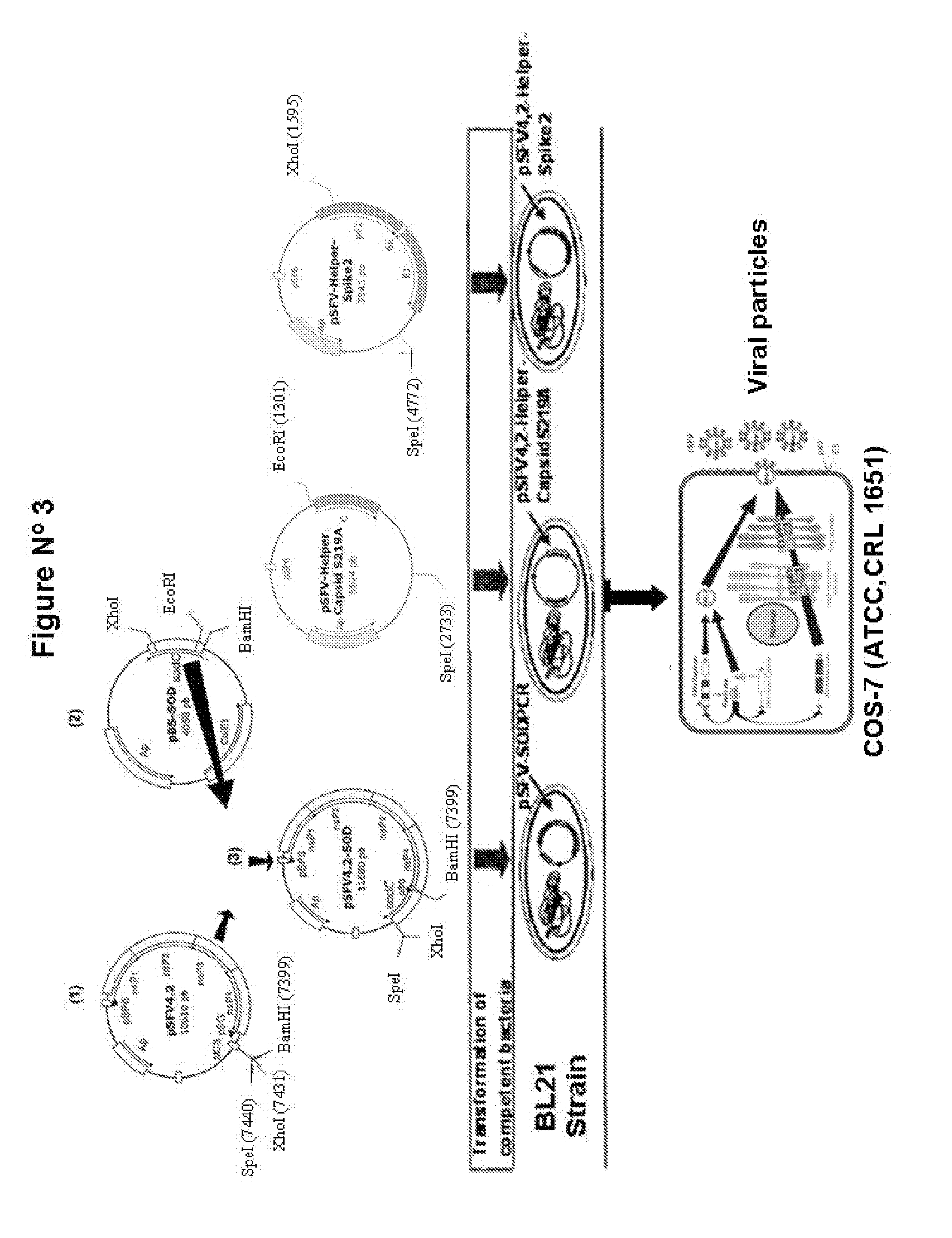
Veterinary pharmaceutical formulacion that comprises an RNA recombinant particle that encodes for a cu/zn superoxide dismutase protein of ruminant pathogenic bacteria and at least one RNA alphavirus belonging to the semliki forest virus family
InactiveUS20110200667A1Improve efficacyProtective efficacyAntibacterial agentsOrganic active ingredientsBrucella abortusZn superoxide dismutase
The technology is a veterinary pharmaceutical formulation of two vaccines, one from an RNA viral vector system constituted by an RNA recombinant particle that codifies for a Cu / Zn superoxide dismutase protein of Brucella abortus, and the other based on naked RNA constituted by a recombinant molecule of naked RNA that carries a sequence for the synthesis of at least one recombinant Cu / Zn superoxide dismutase protein of Brucella abortus and some Semliki Forest virus genes. An expression system based on the Semliki Forest virus and a use of this system, in addition to a method for the preparation of the pharmaceutical formulations.
Owner:UNIV DE CONCEPCION
Gene chip for high-flux detection of pathogens and application thereof
InactiveCN102534013AStrong specificityDetermine the typeMicrobiological testing/measurementAgainst vector-borne diseasesYersinia pestisBrucella
The invention relates to a gene chip for high-flux detection of pathogens and application thereof. The gene comprises (1) a combination of 174 oligonucleotide probes of pathogen variety specific genes, toxin genes and drug-resistant genes; and (2) a probe array, which is formed by curing the oligonucleotide probes on a carrier material by arm molecules. The gene chip comprises 174 gene probes, namely 32 pathogen variety specific gene probes of the following 8 pathogens of Burkholderia mallei, Burkholderia pseudomallei, Brucella, salmonella, Yersinia pestis, Bacillus anthracis, comma bacillus and the like, 25 toxin gene probe of the following 7 toxins of diphtheria toxin, Shiga toxin, staphylococcus enterotoxin, choleratoxin and the like, and 117 drug-resistant gene probes of 17 drug-resistant genes of extended-spectrum beta-lactamase, cephalosporinase, carbapenemase, integrase gene, common gene engineering carrier drug-resistant gene and the like. The gene chip can be used to detect multiple pathogen variety specific genes, toxin genes and drug-resistant genes.
Owner:李越希
Kit for recognizing Brucella wild strain and vaccine strains A19 and S2
ActiveCN105018489AEasy to distinguishStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBacteroidesBrucella Vaccine
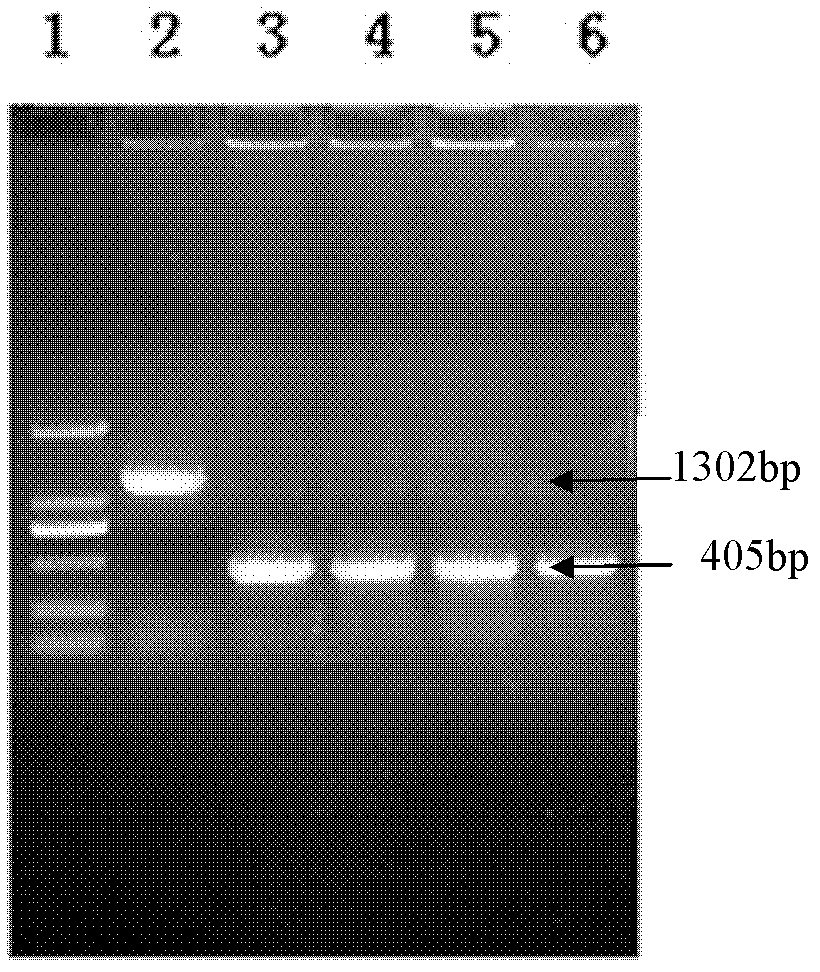
The invention relates to a kit for recognizing a Brucella wild strain and vaccine strains A19 and S2. The kit comprises a special primer pair SEQ ID No.1 and SEQ ID No.2 for recognizing the Brucella vaccine strain A19 and a special primer pair SEQ ID No.3 and SEQ ID No.4 for recognizing the Brucella vaccine strain S2. The kit is used for conducting further sequencing analysis on a PCR amplification product after the Brucella and other conventional bacterial strains are distributed on a PCR amplification-electrophoresis detection area, and the Brucella A19 and S2 vaccine strains in a clinic sample can be rapidly and effectively recognized and diagnosed according to the sequencing result.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Oligonucleotide probe kit for detecting common intestine trac kpathogenic bacteria and its use
InactiveCN1683565AQuick checkAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesAntigenBio engineering
The present invention belongs to the field of microbe detecting technology. The oligonucleotide probe for detecting common intestinal tract pathogenic bacteria is designed on 16S rRNA and 23S rRNA of bacteria, ipaH of dysentery bacillus giant plasmid, VipR of Salmonella typhi and other gene sequence, has length of 25-50 bp, and relatively high sensitivity and specificity. The oligonucleotide probe is suitable for detection based on nucleic acid hybridization principle, especially detection based on gene chip principle. Under certain use condition, it can detect Listeria, parahemolutic vibrio, Campylobacter, etc. It may be used in many aspects, such as disease diagnosis, environment detection, food poisoning detection, etc.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
Reagent for detecting brucella and complex probe fluorescence quantitative PCR (polymerase chain reaction) brucella detection method
InactiveCN102146466ASimple and efficient operationGuaranteed specificityMicrobiological testing/measurementFluorescence/phosphorescenceQuenchingFluorescence
The invention provides a reagent for detecting brucella. The reagent comprises an upstream primer, a downstream primer, a fluorescence probe and a quenching probe; the gene sequence of the upstream primer is 5'-caagggcaaggtggaagatt-3'; the gene sequence of the downstream primer is 5'-ctgcgaccgatttgatgttt-3'; the gene sequence of the fluorescence probe is 5'-fam-atcgtttccgggtaaagcgtcgcca-P-3'; and the gene sequence of the quenching probe is 5'-cgctttacccggaaacga-Dabcyl-3'. The invention also provides a complex probe fluorescence quantitative polymerase chain reaction (PCR) brucella detection method using the reagent. The method is simple and convenient in operation, efficient, quick and specific; the detection time of the brucella is greatly shortened; the quantitative detection of a sample can be completed in about 2 hours; and the reagent and the method have significance for early diagnosis of brucella disease.
Owner:浙江国际旅行卫生保健中心
Kit for identifying Brucella S2 vaccine strain and wild strain
InactiveCN105002173AEasy to distinguishStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBacteroidesSequence analysis
The invention relates to a kit for identifying a Brucella S2 vaccine strain and a wild strain. The kit comprises a specific primer pair SEQ ID No.1 and SEQ ID No.2 for identifying the Brucella vaccine strain S2. Sequencing analysis is further performed on a PCR amplification product after the kit is used for distributing Brucella and other conventional bacterial strains through a PCR amplification-electrophoresis detection area, and the Brucella S2 vaccine strain in a clinic sample can be rapidly and effectively identified and diagnosed according to the sequencing result.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Preparation of common antigen monoclonal antibody of Brucella sLPS (lipopolysaccharides) and establishment of c-ELISA (competitive enzyme-linked immuno sorbent assay)method
InactiveCN103864928AImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliSorbent
The invention provides a monoclonal antibody against smooth Brucella (Brucella) lipopolysaccharides (sLPS), and preferably provides 14F4. Cross reaction tests show that the monoclonal antibody 14F4 has no cross reaction with Escherichia coli (O:157), Salmonella Dublin (C79-86) whole thallus and the LPS. On the basis, a competitive ELISA (c-ELISA) test method using the monoclonal antibody 14F4 as a competition antibody is established, and the method is suitable for testing large-scale clinical samples, and has the characteristics of being rapid, high-throughput, high-sensitivity and high-specificity.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Primer sequence for detecting Brucella based on dual priming oligonucleotide (DPO) primer, and detection kit thereof
InactiveCN103215372AHigh detection specificityVerify reliabilityMicrobiological testing/measurementMicroorganism based processesNucleotideBrucella
The invention discloses a polymerase chain reaction (PCR) method for detecting Brucella based on a dual priming oligonucleotide (DPO) primer, and a DPO primer sequence thereof. The method comprises the following steps of: selecting a Brucella Omp25 gene as a target gene; through BLAST analysis of the gene sequence, selecting a conserved region of the target gene sequence, and designing and synthesizing the DPO primer, wherein the nucleotide sequence of the DPO primer is as follows: BO-DPOF: 5'-CTTTTGCTGCCGACGCCATCCIIIIIAGGAACAGC-3', and BO-DPOR: 5'-TTCGTCGTCCAAGCCGTTGTTAAIIIIIGCTTGATCT-3'; and establishing a DPO-PCR detection method so as to perform precise qualitative detection on the Brucella. The invention also relates to a detection kit which has the advantages of high detection specificity, high accuracy and high sensitivity.
Owner:哈尔滨海关技术中心
Human and animal brucella antibody immunochromatography test paper and preparation method thereof
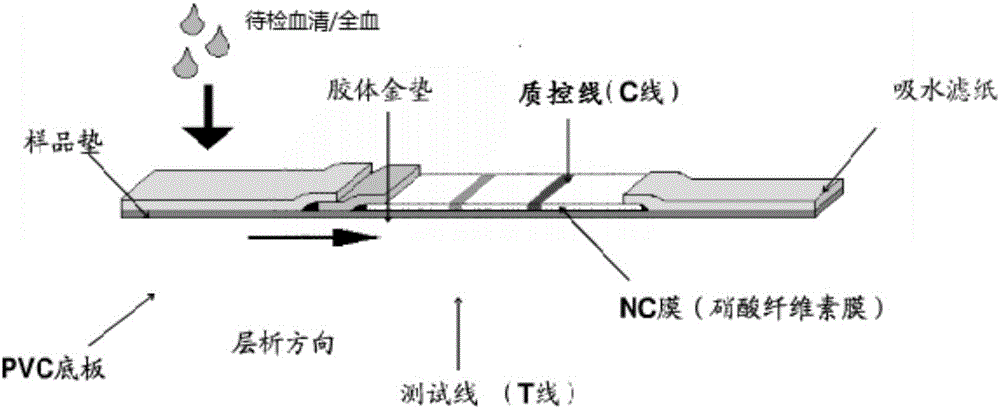
The invention relates to the field of zoonosis immune diagnosis and discloses a brucellosis antibody detection immunochromatography test strip based on colloidal gold as a marking material and a preparation method of the test strip. In a quick brucellosis antibody detection technology, the 40-nm colloidal gold labeled with staphylococcus aureus protein A (SPA) is sprayed on glass fibers to form a gold labeled pad. Genes OMP31 and BP26 are cloned from a brucella genome, form prokaryotic expression recombinant plasmids and are transformed in escherichia coli to express proteins omp31 and bp26, the two proteins as coating antigens are respectively coated on a nitrocellulose membrane to serve as detection lines, and the detection lines, the gold labeled pad, a specially treated sample loading pad and water absorption paper are assembled into an immunochromatography detection device. The test strip has the characteristics of strong specificity, high sensitivity, convenience, simplicity, economy and the like, can be applied to typing detection of brucellosis antibodies of sheep and cattle, and has very important meaning and practical application value for brucellosis monitoring and prevalence control.
Owner:SHIHEZI UNIVERSITY
Lactobacillus plantarum nitrite reductase gene, and encoded protein and application of the same
The invention discloses a Lactobacillus plantarum nitrite reductase gene, a recombinant vector containing the gene, a host converted by the vector, an expression product produced by utilization of the converted body, and the application of the product. Staphylococcus aureus, pseudomonas aeruginosa, brucella, and nitrite reductase gene of escherichia coli serve as references for a primer design, genome DNA extracted by Lactobacillus plantarum serves as a template, and the gene segments of Lactobacillus plantarum nitrite reductase are amplified via the PCR technique; the segments are connected to a pET-32a(+) expression vector and then transferred to escherichia coli BL21(DE3) cells; and after IPTG induction, expression of Lactobacillus plantarum nitrite reductase (NiRL) protein is carried out, finally obtaining Lactobacillus plantarum nitrite reductase protein.
Owner:SHANGHAI INST OF TECH
Immunogenic compositions including rough phenotype Brucella host strains and complementation DNA fragments
InactiveUS20050142151A1Sufficient attenuationEasy to operateBiocideBacterial antigen ingredientsHeterologousHeterologous Antigens
Live attenuated vaccines against brucellosis and infection by other diseases are described. It has been discovered that trans complementation of the Brucella wboA gene can be used to maintain an expression vector in an attenuated Brucella host cell in a vaccinee. Further, heterologous antigens can be expressed using this Brucella platform, thus effecting a multivalent vaccine against Brucella and the disease corresponding to the heterologous antigen.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Cattle Brucella recombination strain S19-delta bp 26-BL and preparation method and application thereof
ActiveCN103289986AImprove the effect of prevention and controlDifferentiating natural infectionAntibacterial agentsBacterial antigen ingredientsCompetent cellBrucella
The invention discloses a cattle Brucella recombination strain S19-delta bp 26-BL and preparation method and application thereof. According to the invention, a cattle Brucella S19 genome DNA is used as a template, an upstream homologous arm of a bp26 genome is obtained, and a downstream homologous arm of a BLS gene, a L7 / L12 gene and a bp 26 gene is obtained; the gene fragments are connected in sequence, and a fragment delta-bp 26-BL is obtained; a pRE112 and the fragment delta-bp 26-BL are separately subjected to a double digestion and are connected, and a suicide homologous recombinant plasmid is obtained; the plasmid is introduced into a competent cell of a S19-delta bp 26 bacterial strain, in the selection pressure of chloramphenicol, and a homologous recombinant single recon is obtained, and then the homologous recombinant single recon is coated on a selection medium for culture, and the cattle Brucella recombination strain S19-delta bp 26-BL is obtained. The strain has the advantages of good heredity stability, growth characteristic and appropriate virulence, and can be used as a mark vaccine strain for clinic.
Owner:SOUTH CHINA AGRI UNIV
ELISA detection kit for bovine Brucella
ActiveCN105445473AEasy to operateReduce use costBiological material analysisBiological testingSerum igeElisa kit
The invention provides an ELISA detection kit for bovine Brucella, belonging to the technical field of protein engineering. In the kit provided by the invention, the mixed antigen of the outer membrane proteins OMP19, OMP22 and OMP28 of bovine Brucella is used as an envelope antigen of the ELISA kit; the concentration of each antigen is 1mu g / ml, and the antigens are mixed in a volume ratio of 1:1:1 to obtain the mixed antigen; the serum dilution is 1:200, and the dilution of the enzyme-labeled secondary antibody is 1:20,000; and the bovine Brucella is detected through an indirect ELISA method. The kit provided by the invention has the characteristics of high specificity, high sensitivity, high accuracy, easiness in operation and good reproducibility while the market application prospect is good.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Dual-gene deletion rough type bovine brucellosis and production method for vaccine thereof
InactiveCN104031874AAntibacterial agentsBacterial antigen ingredientsResistant genesBrucella Vaccine
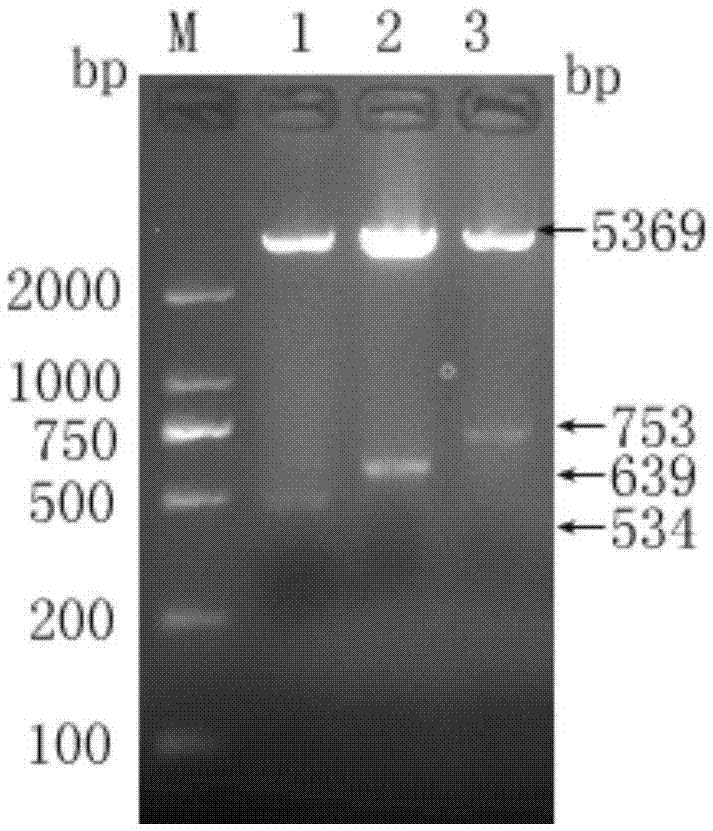
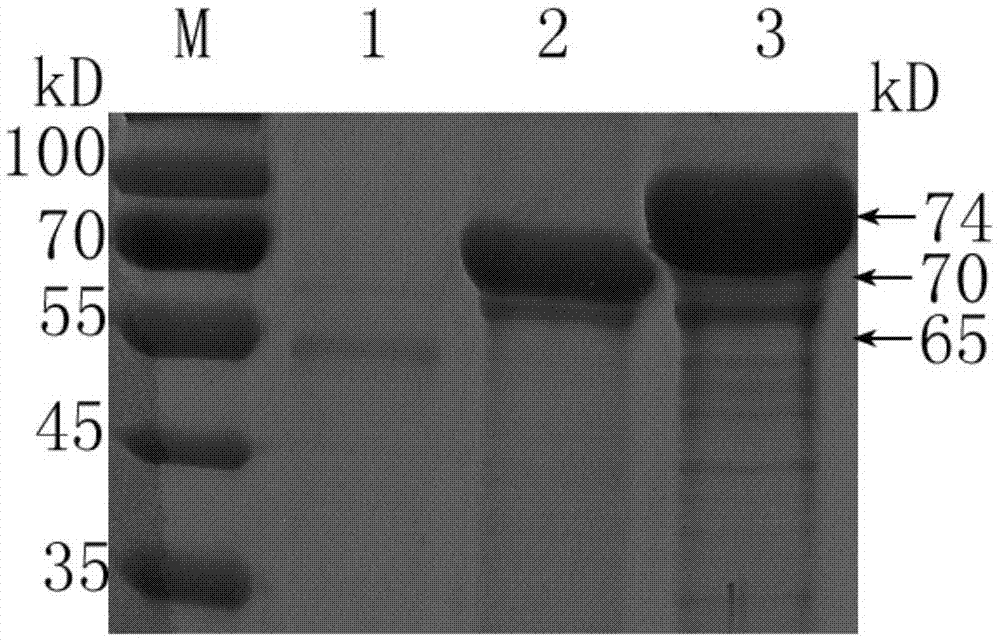
The invention relates to dual-gene deletion rough type bovine brucellosis and a production method for a vaccine thereof. A recombinant bacterial strain deletes a WboA gene and a vjbR gene of a bovine brucellosis 2308 strain by virtue of a non-resistance gene screening technology. Due to deletion of two genes, on the one hand, a condition (cased by WboA gene deletion) for forming an O chain in a smooth type bovine brucellosis cell wall LPS (lipopolysaccharide) structure is lost, and colonial morphology is changed into a rough type from a smooth type; and on the other hand, due to deletion of vjbR gene, toxicity of recombinant bacteria and viability in a cell are descended, so that infection ability of bovine brucellosis on a target animal is lowered, and therefore, safety of the vaccine is further improved. By using the bacterial strain to produce the vaccine, a current situation that a bovine brucellosis vaccine immune animal and a wild strain-infected animal are difficult to distinguish is changed, and safety of the existing vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Brucella detection kit and Brucella detection method
ActiveCN112322764AThe detection process is fastQuick checkMicrobiological testing/measurementMicroorganism based processesBrucella IgGBrucella
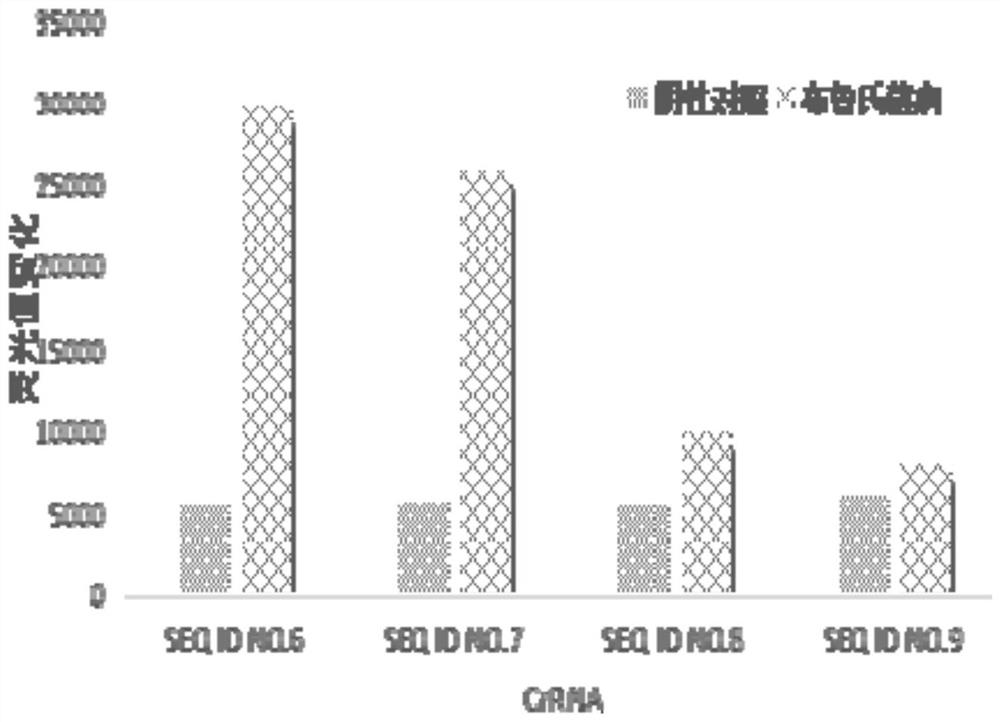
The invention discloses crRNA, a kit and a detection method for detecting brucella. The crRNA is selected from the following sequences: crRNA1, the sequence of which is shown as SEQ ID NO. 6; crRNA2,the sequence of which is as shown in SEQ ID NO. 7; crRNA3, the sequence of which is as shown in SEQ ID NO. 8; and crRNA4, the sequence of which is as shown in SEQ ID NO. 9. According to the present invention, crRNA is designed and screened based on the Brucella Omp25 gene, meanwhile, a CRISPR-Cas12a system and an RPA isothermal amplification technology are combined, whether Brucella exists in a to-be-detected sample or not can be detected within a short time, the operation is easy, the detection speed is high, the cost is low, repeated detection can be achieved, at the same time, the detectionsensitivity and the detection specificity are remarkably improved, wherein the lowest detection limit can reach 10 copies per microliter.
Owner:江苏博嘉生物医学科技有限公司
Method for quickly identifying Brucella based on mass spectrum technology and application thereof
ActiveCN102851362AAccurate identificationMicrobiological testing/measurementMicroorganism based processesQuarantineBrucella
The invention relates to a method for quickly identifying Brucella based on a mass spectrum technology and application thereof. The invention discloses a method for quickly identifying Brucella, which comprises the steps of PCR (polymerase chain reaction) amplification, SAP (serum alkaline phosphatase) digestion, transcription, nucleic acid enzyme cutting, purification, detection with a mass spectrometer and the like. Based on the method, a common Brucella nucleic acid fingerprint database is established. According to a peak mass spectrogram generated in experiments, Brucella in a sample to be detected can be classified and identified, and the results can be widely used in the fields of Brucella typing and classification, environmental health and public safety quarantine and the like.
Owner:BIOYONG TECH
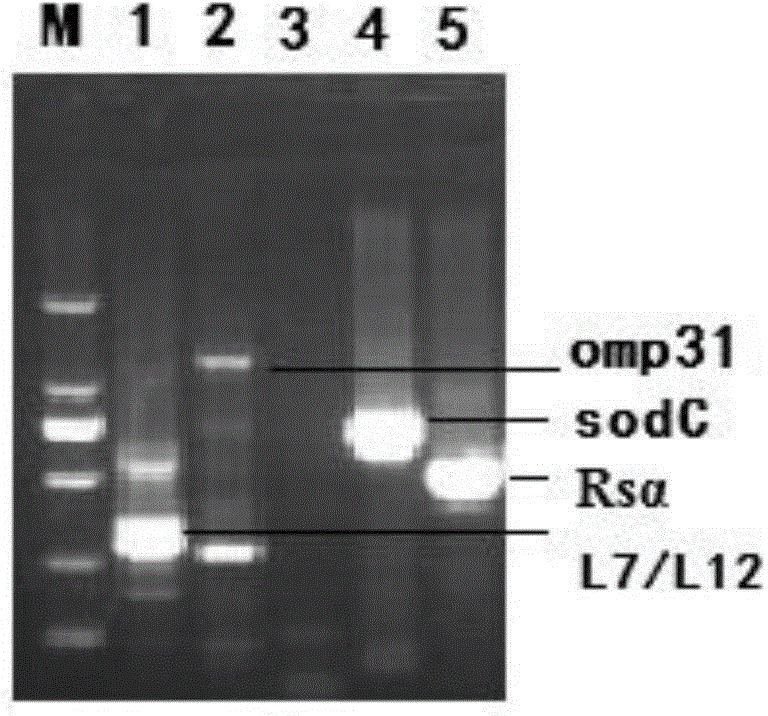
Construction method and expression method of coexpression vector of L7/L12, Omp31, Rs alpha and sodC Brucella immune proteins
InactiveCN104152480AAvoid interactionGuaranteed stable expressionAntibacterial agentsBacterial antigen ingredientsGene engineeringBiology
The invention discloses a construction method and an expression method of a coexpression vector of L7 / L12, Omp31, Rs alpha and sodC Brucella immune proteins. The disclosed coexpression vector includes a double expression vector pETDuet-1 and a double expression vector pRSFDuet-1, which are both expressed in the same host bacteria, wherein the two double expression vectors are respectively connected with encoding genes of two Brucella immune proteins. On this basis, the invention also discloses the construction method and the expression method of the coexpression vector. L7 / L12, Omp31, Rs alpha and sodC Brucella immune proteins all with good immunity functions can be expressed simultaneously by the coexpression vector disclosed by the invention and are effectively used for preparing subunit vaccine of the Brucella gene engineering and preventing and controlling various brucellosis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Cattle and sheep brucellosis indirect enzyme-linked immunosorbent assay antibody detection kit and preparation method thereof
InactiveCN103543261AIncreased sensitivityStrong specificityBiological material analysisEscherichia coliBiology
The invention relates to a cattle and sheep brucellosis indirect enzyme-linked immunosorbent assay antibody detection kit and a preparation method thereof, belongs to the field of detection of pathogens of zoonotic infections and aims at solving the problems of low sensitivity, poor specificity and the like in detection of Brucella by adopting other antigens. The kit is assembled by taking a recombinant Brucella BCSP31 protein with expression induced by SUMO as an expression vector in Escherichia coli to serve as an antigen-coated ELISA (enzyme-linked immunosorbent assay) plate, adding serum to be detected and rabbit anti-bovine HRP-IgG or rabbit anti-goat HRP-IgG and adding a washing buffer solution, a blocking solution, a TMB (3,3',5,5'-Tetramethylbenzidine) primer solution and a stop solution for matching. The prokaryotic expression vector, namely the SUMO, is utilized to express the Brucella BCSP31 protein in an efficient and soluble manner, the kit is used for establishing a Brucella ELISA detection method, the detection has better repeatability and specificity, and cattle and sheep Brucella can be fast and efficiently detected by utilizing the method.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
Kit for simultaneously detecting four pathogenic bacteria and non-diagnostic detection method thereof
ActiveCN105950759AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesTime efficientYersinia pestis
The invention discloses a kit for simultaneously detecting four pathogenic bacteria Yersinia pestis, Frencisella tularensis, Burkholderia pseudomallei and Brucella by using fluorescent quantitative PCR (polymerase chain reaction) and a non-diagnostic detection method. The kit comprises specific primers and probes corresponding to the four pathogenic bacteria. The kit disclosed by the invention is convenient to use, and has the advantages of low reagent consumption, low cost, high detection specificity and high sensitivity. The detection method can detect four pathogenic bacteria for one sample, thereby greatly simplifying the operational process, reducing the repetitive operation steps, saving the time, reducing the labor consumed by repetitive operation, effectively saving the cost and implementing quick screening.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Therapeutic vaccine for malignant tumors and composition thereof
The invention relates to a therapeutic vaccine for malignant tumors and a composition thereof. The therapeutic vaccine for malignant tumors is a tumor cell line which contains plasmid of antisense nucleic acid of human transforming growth factor beta (TGF-beta2); the therapeutic vaccine composition for malignant tumors comprises the therapeutic vaccine for malignant tumors and an immunopotentiator, and the immunopotentiator is one selected from the group consisting of a Corynebacterium parvum preparation, a non-cell Corynebacterium parvum preparation, a BCG polysaccharide, a nucleic acid preparation, a Nocardia rubra-cell wall skeleton preparation, a group A Streptococcus preparation, a non-cell group A Streptococcus preparation, a Pseudomonas aeruginosa preparation, a non-cell Pseudomonas aeruginosa preparation, a Brucella preparation, a non-cell Brucella preparation, a non-cell Mycobacterium vaccae preparation and a non-cell Mycobacterium smegmatis preparation, preferably the non-cell Corynebacterium parvum preparation; and the malignant tumors include a lung cancer, a liver cancer, a pancreatic cancer, leukemia, lymphoma, an ovarian cancer, a colon cancer, a stomach cancer and a breast cancer.
Owner:熊慧
Rough type Brucella melitensis attenuated strain and vaccine thereof
ActiveCN104845916AImprove securityImprove immunityAntibacterial agentsBacterial antigen ingredientsImmune effectsBrucella Vaccine
The invention relates to a rough type Brucella melitensis attenuated strain and a vaccine thereof. The rough type Brucella melitensis attenuated strain RM57 is screened by using an antibiotics and M factor serum combined domesticating and screening technology. The safety of the rough type attenuated strain is remarkably improved, but the favorable immune effect for Brucella melitensis is stilled retained. If the attenuated strain is prepared into a Brucella melitensis vaccine, the situation that a Brucella melitensis vaccine immunized animal and a wild strain infected animal are difficult to distinguish is changed, and the safety of the existing vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Brucella live vaccine and production method thereof
The invention relates to brucella and a production method of a brucella vaccine. In the vaccine, a recombinant brucella rS2-deltaWboA strain is taken as a production strain. A smooth recombinant strain is transformed into a rough recombinant strain by deleting base sequences among WboA genes 1-897bp of a brucella S2 strain and eliminating the forming condition of an O chain in a smooth brucella cell wall LPS (Lipopolysaccharide) structure with a non-resistant gene screening technology. The safety of the rough recombinant strain is improved remarkably, and a good immune effect on brucelliasis is still kept. Due to the adoption of the strain to the production of the vaccine, the current situation that a brucella vaccine immune animal cannot be distinguished from a wild strain infected animal easily is changed, and the safety of the conventional vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
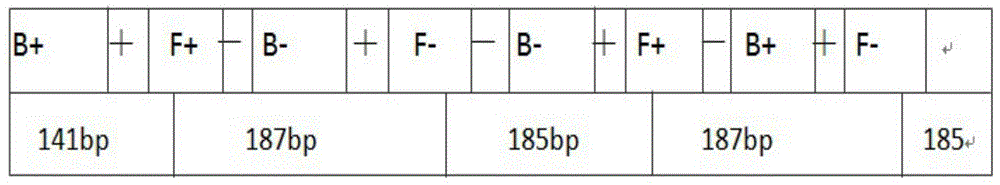
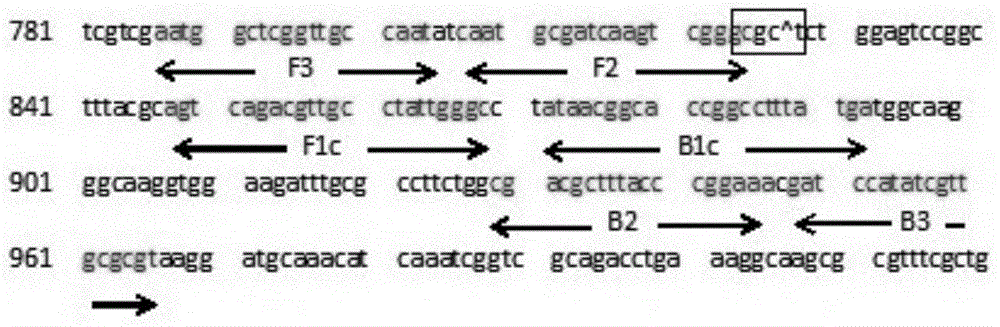
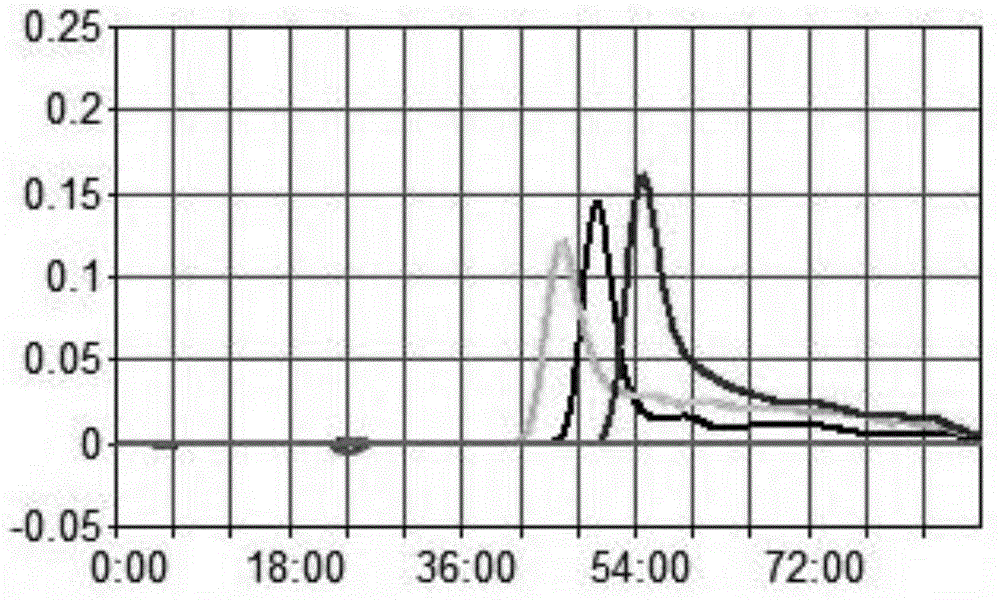
LAMP primer for detecting Brucella and kit containing same
ActiveCN103602721AStrong specificityAchieving Specific DetectionMicrobiological testing/measurementMicroorganism based processesBrucellaCell biology
The invention relates to an LAMP primer for detecting Brucella and a kit containing the same. The LAMP primer comprises inner primers, namely FIP and BIP, outer primers, namely F3 and B3, and two pairs of ring primers, namely LF1 and LB1 or LF2 and LB2; the kit comprises the LAMP primer, Bst DNA polymerase, and a reaction buffer solution containing dNTPs. The kit utilizes loop-mediated isothermal amplification reactions (LAMP), carries out a specific amplification on target gene BCSP31 by using Bst polymerase and the LAMP primer, and is capable of rapidly, accurately, and conveniently detecting the Brucella in a human blood sample or milk. The detection method has a high sensitivity, the lower limit of detection can reach 35 fg, the reaction time is short, expensive instruments are not needed, the operability is strong, moreover, the product detection is convenient, the product can be detected by naked eyes or through a dyeing method, so the kit has a very high practical value.
Owner:黄耀江
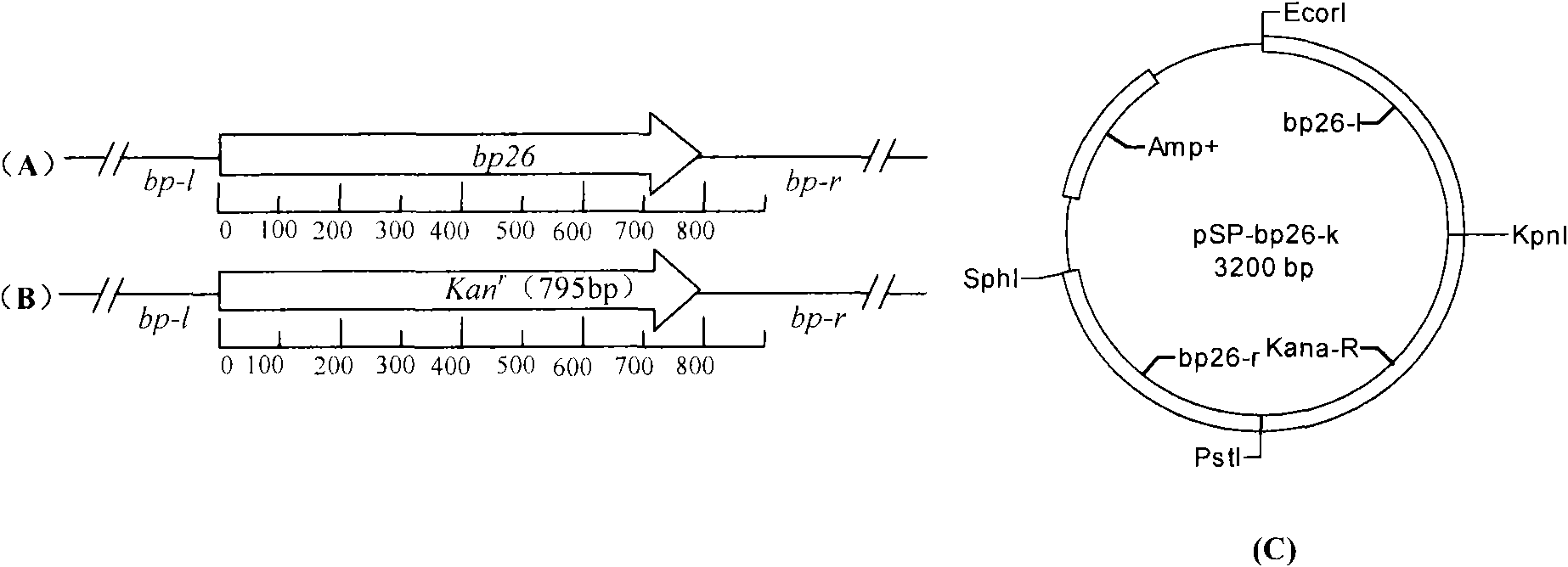
Brucella melilitensis bp26 gene-deleted M5-90 vaccine strain
The invention relates to a recombined brucella melilitensis, wherein the recombined brucella melilitensis completely deletes the bp26 gene. Safe tests prove that the brucella melilitensis is consistent with a parent vaccine strain. Virulent attack protection tests prove that a mutant strain has immune protection consistent with the parent vaccine strain. The mutant strain has significance for distinguishing vaccine immunity from wild type brucellosis infection from the standpoint of serology and controlling, monitoring and purifying brucellosis disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Brucella omp31 antigenic epitope and monoclonal antibody thereof, and application of brucella omp31 antigenic epitope and monoclonal antibody thereof
InactiveCN104086634AGood specificity recognition abilityAccurate identificationAntibacterial agentsBacterial antigen ingredientsBrucella antigenEpitope
The invention discloses a brucella omp31 antigenic epitope and a monoclonal antibody thereof, and an application of the brucella omp31 antigenic epitope and the monoclonal antibody thereof. The sequence of the epitope is GGYIGINAGYAGGKFK. The invention also discloses the monoclonal antibody identifying the epitope, and the application of the epitope and the monoclonal antibody in preparing reagents or medicines used for diagnosing, preventing or treating brucellosis.
Owner:SOUTHERN MEDICAL UNIVERSITY
Pig breed brucella bax inhibitor-1 (BI-1) gene deletion strain and construction method and application thereof
ActiveCN110699312APromote growthHigh degree of polymerizationAntibacterial agentsBacterial antigen ingredientsBrucellaTGE VACCINE
The invention discloses a pig breed brucella bax inhibitor-1 (BI-1) gene deletion strain and a construction method and application thereof. The strain is obtained by replacing a pig breed brucella BI-1 gene with a kanamycin resistance gene, and the method includes the main steps that a pig breed brucella S2 vaccine strain genome is taken as a template, PCR amplification is conducted to obtain an upstream homologous arm and a downstream homologous arm of the BI-1 gene, the upstream homologous arm and the downstream homologous arm of the BI-1 gene and a kanamycin resistance gene fragment also obtained through PCR amplification are together cloned into a pUC18 plasmid, and a homologous recombination deletion plasmid pUC18-BI1-KanR is obtained. The plasmid is used for electrotransformation ofa pig breed brucella competent cell, and through PCR identification, an obtained positive transformant is the pig breed brucella BI-1 gene deletion strain. Compared with a wild strain, the deletion strain shows the characteristics of decreasing the growth rate, reducing colonies, increasing cell membrane permeability, enhancing cell polymerization and the like, and it is indicated that the deletion strain has the potential of serving as a brucella attenuated live vaccine.
Owner:NORTHWEST A & F UNIV
DNA loaded Brucella ghost composite vaccine
InactiveCN108690823ALittle side effectsImprove securityAntibacterial agentsBacterial antigen ingredientsSide effectA-DNA
The invention discloses a DNA loaded Brucella ghost composite vaccine. The preparation method comprises following steps: introducing a suicide plasmid that contains a nucleic acid molecule encoding atemperature sensitive regulatory protein cI857, a nucleic acid molecule encoding a bacteriophage splitting protein E, and a nucleic acid molecule encoding a bacterial nuclease protein A into Brucella;utilizing a homologous recombination technology to obtain recombinant Brucella; culturing the recombinant Brucella to obtain a bacterial solution, processing the bacterial solution at a high temperature, collecting bacterial cells, and adding target DNA to obtain the DNA loaded Brucella ghost composite vaccine. The composite vaccine has following advantages: (1) the vaccine has the characteristics of bacterial ghost, compared with a conventional killed vaccine or an attenuated live vaccine, the side effect of the composite vaccine is small, the safety is high, and the protection effect is good; and (2) the bacterial ghost is a safe and effective carrier for delivering DNA vaccines, can introduce nucleic acid vaccines into antigen presenting cells, and performs high efficient expression. The composite vaccine has an important meaning for controlling the epidemic spreading of brucellosis and has a wide application range.
Owner:INNER MONGOLIA HUAXI BIOTECH
Multiplex PCR primer probes and kit for detecting pet-derived zoonotic pathogens
ActiveCN109609665ASimplify detection workloadShorten detection timeMicrobiological testing/measurementDNA/RNA fragmentationSequence analysisCompanion animal
The present invention provides multiplex PCR primer probes and a kit for detecting pet-derived zoonotic pathogens. Bartonella henselae, toxoplasma gondii and brucella belonging to pet-derived zoonoticpathogens are subjected to gene sequence analysis and comparison, triple fluorescent PCR detection primers and probes suitable for single reaction and simultaneous detection of the three pathogens are designed, and nucleotide sequences of the primers and probes are shown in SEQ ID NO.1-9, respectively. The present invention also discloses a method and a detection kit for detecting the above 3 pathogens. The detection method simplifies detection workload of 2 / 3 or more of the original, and shortens detection time by about 1 hour, and detection sensitivities of the bartonella henselae, toxoplasma gondii and brucella are 5, 5 and 50 copies, respectively. The multiplex PCR primer probes have no non-specific amplification of 23 common pathogens and canine and cat chromosomes, and show good specificity.
Owner:西安博睿康宁生物科技有限公司
Fluoroquinolone antibiotic degrading bacterium and application thereof in compost
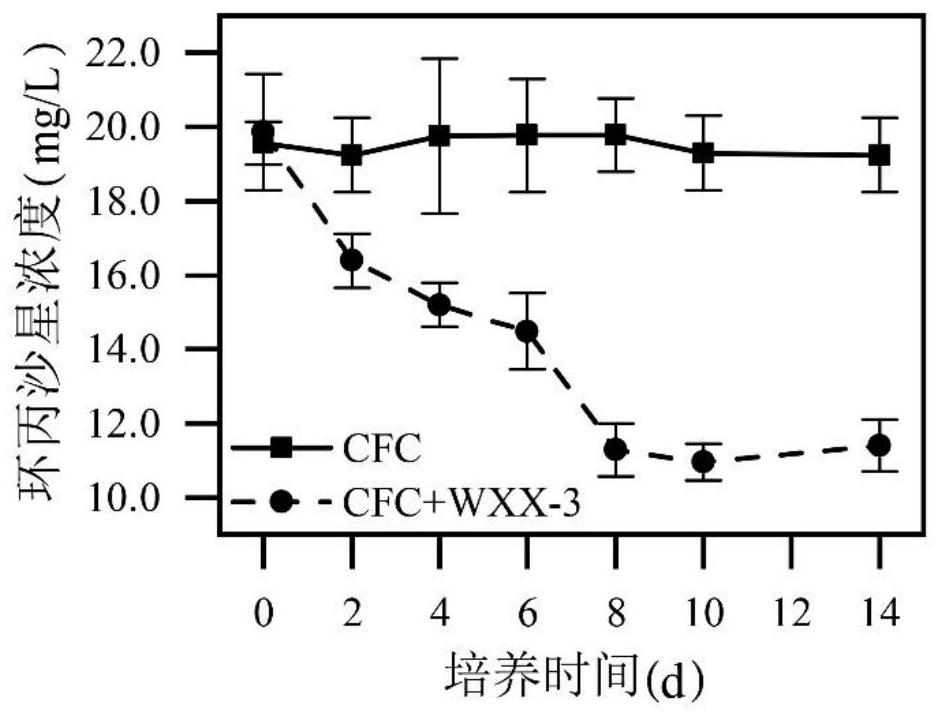
ActiveCN113308413AEfficient degradationImprove securityBio-organic fraction processingBacteriaBiotechnologyFeces
The invention relates to the technical field of microorganisms, in particular to a Brucella WXX-3 strain and application of the Brucella WXX-3 strain in degradation of fluoroquinolone antibiotics. The preservation number of the strain provided by the invention is CGMCC NO.22259. The WXX-3 strain provided by the invention can efficiently degrade fluoroquinolone antibiotics, and according to the embodiment, the highest degradation rate of ciprofloxacin reaches 44.7% after the strain is added into an LB liquid culture medium containing 20mg / L ciprofloxacin and is cultured in a dark place; besides, the strain is inoculated into livestock and poultry manure for propagation expanding for 8-10 days, then conditioning agents such as wood chips and straw are added for conventional composting for 30 days, and the degradation rate of ciprofloxacin can reach 85.7%; meanwhile, compost prepared from the strain can degrade antibiotic resistance genes, so that the safety of compost products is improved, and the strain is suitable for large-scale popularization and application.
Owner:INST OF SOIL SCI CHINESE ACAD OF SCI
Colloidal gold test paper bar for antibody detection of sheep Brucella
The invention relates to a colloidal gold test paper bar for antibody detection of sheep Brucella. In the invention, lipopolysaccharide (LPS) extracted from a Brucella vaccine strain (S2) is taken as an envelope antigen of the colloidal gold test paper bar and improves the sensitivity of detection. Monoclonal-antibody-labeled colloidal gold of a mouse anti-sheep IgGFc terminal is prepared into a gold colloid pad of the colloidal gold test paper bar for antibody detection, and nonspecific adsorption of antibody protein by SPA in the tradition is changed. The monoclonal-antibody-labeled colloidal gold is taken as a colloidal gold labeled protein, so that specificity of detection is further improved. A set of serum for controlling quality of the test paper bar is prepared, and specificity and sensitivity of the test paper bar is ensured. The test paper bar can be used for on-site sampling, is convenient and rapid to use, and is quite suitable for application to a basic farm or a basic veterinarian.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com