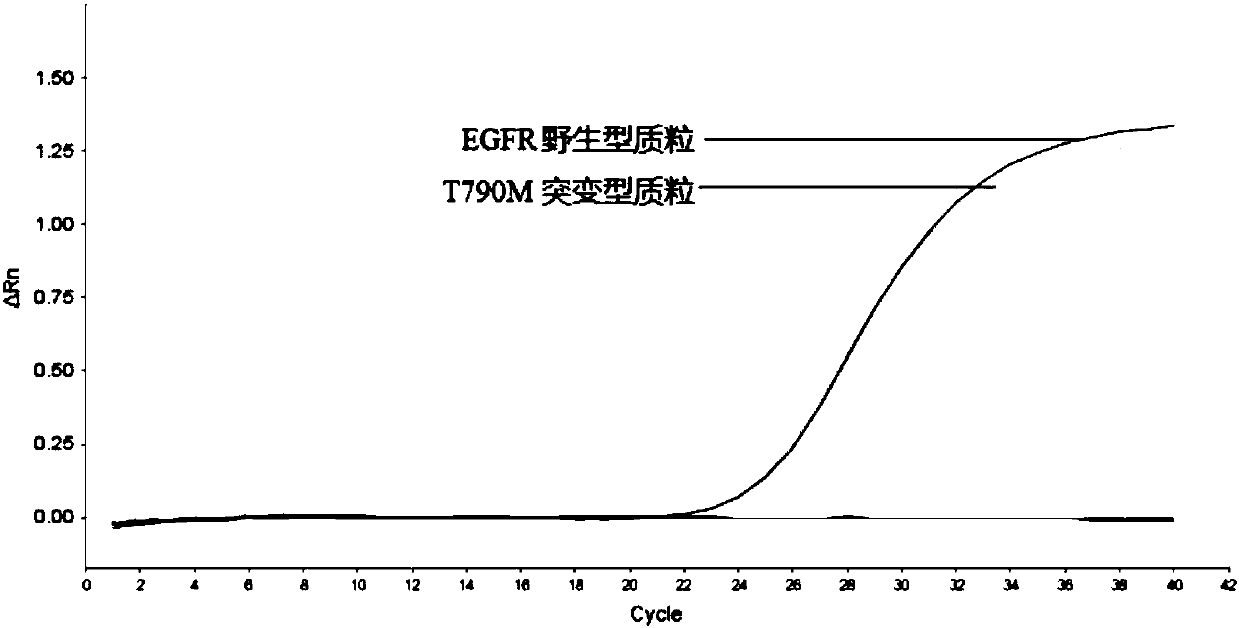
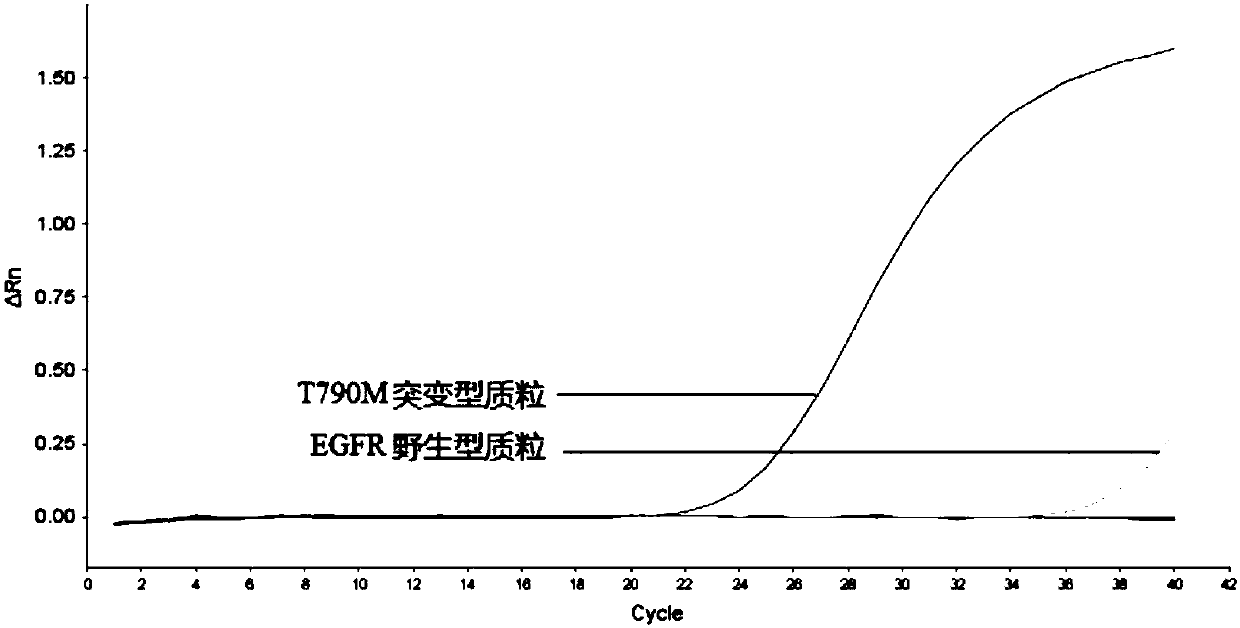
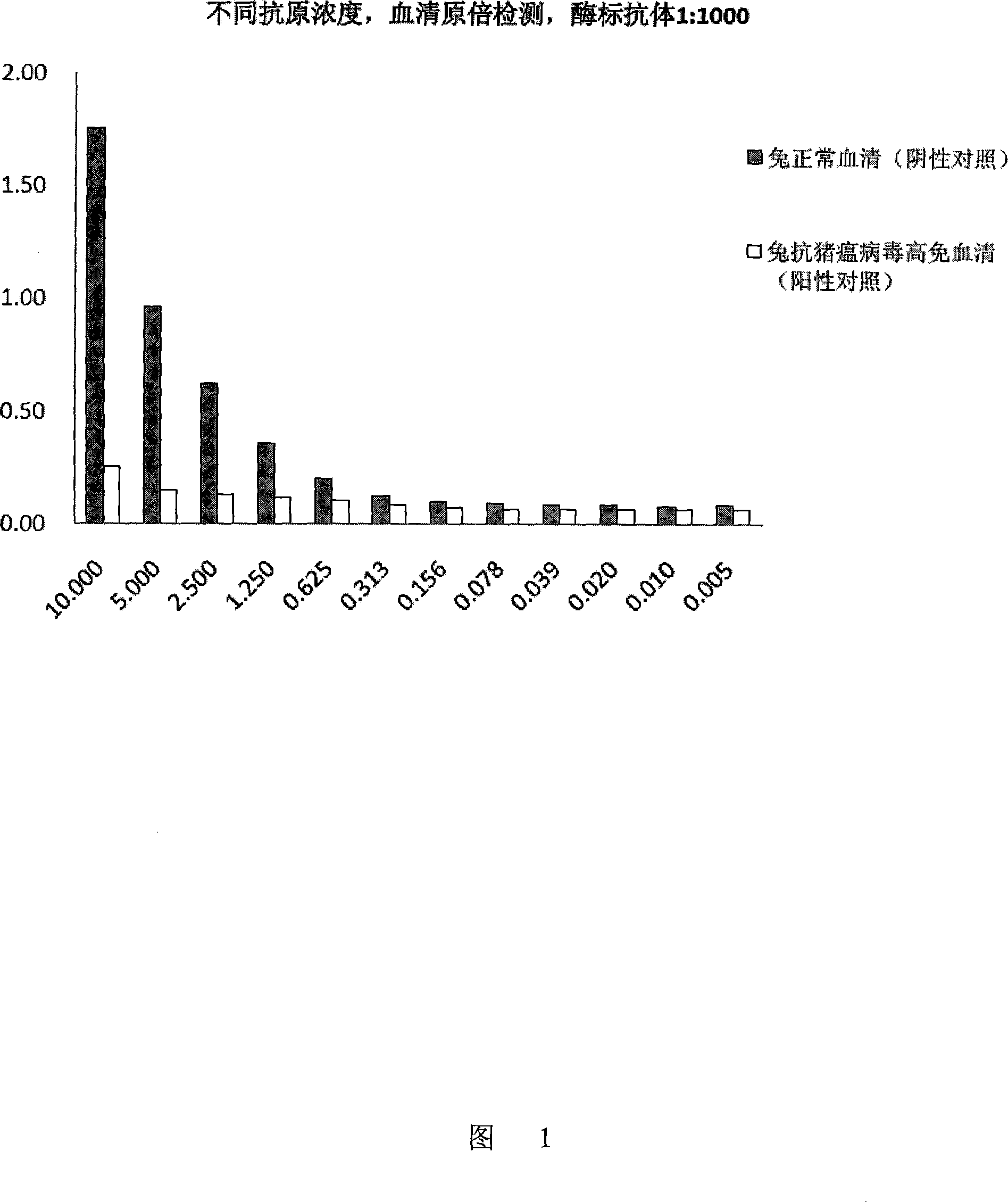
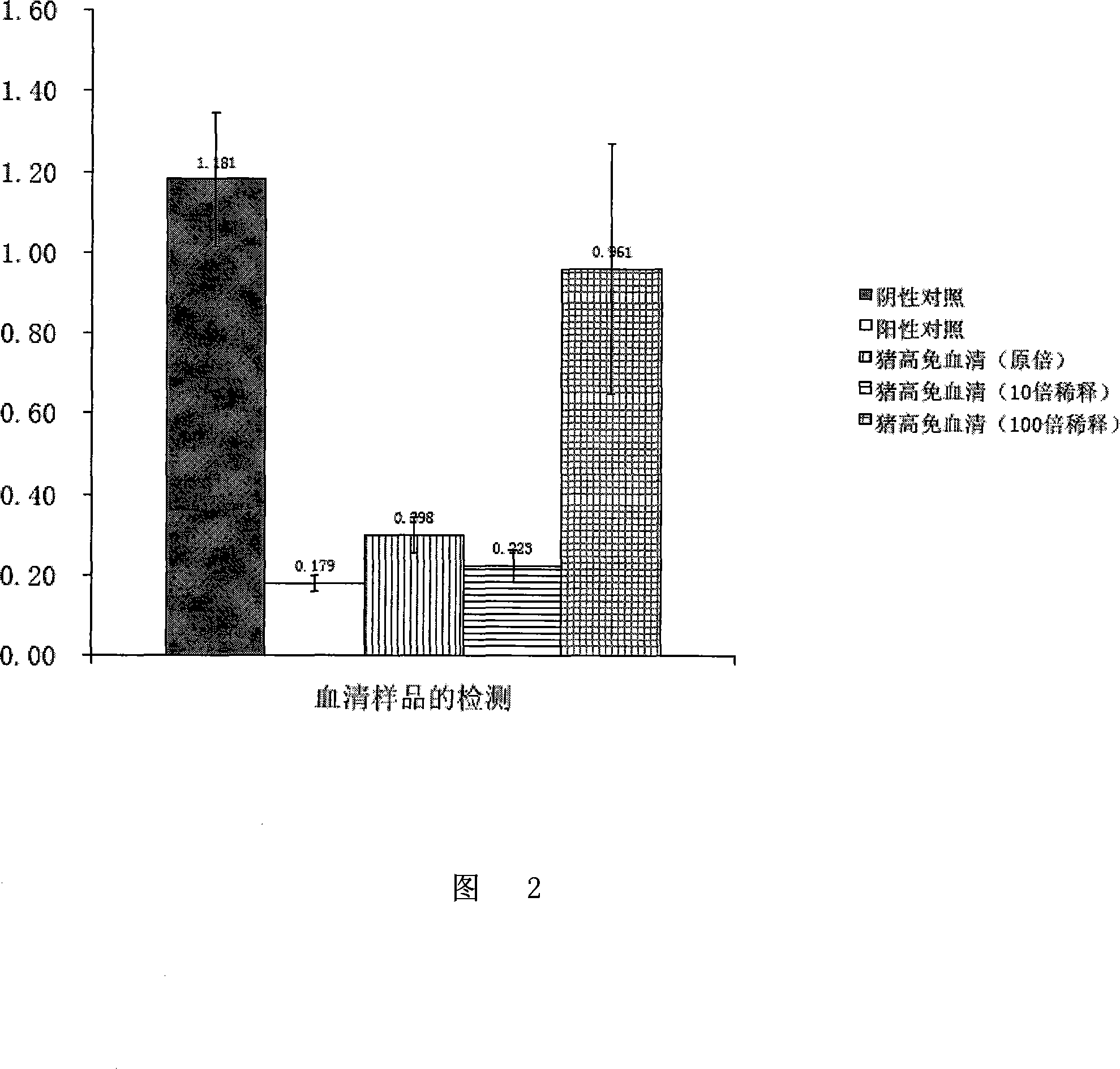
Patents
Literature
561results about How to "Guaranteed specificity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and HLA gene sequencing and typing method
InactiveCN101962676AWide detection coverageResolving Ambiguous Results IssuesMicrobiological testing/measurementDNA/RNA fragmentationTyping methodsHLA-B gene
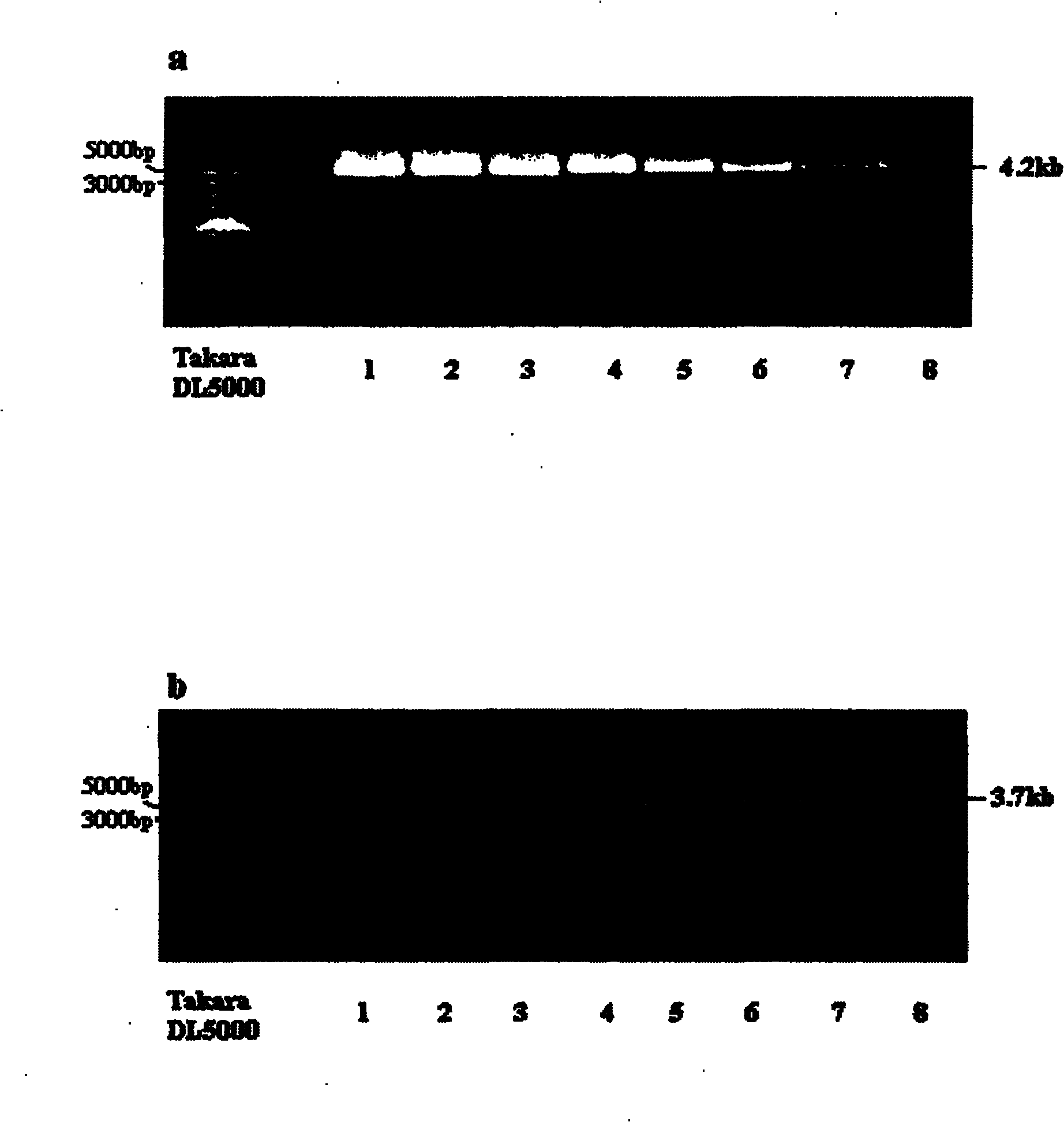
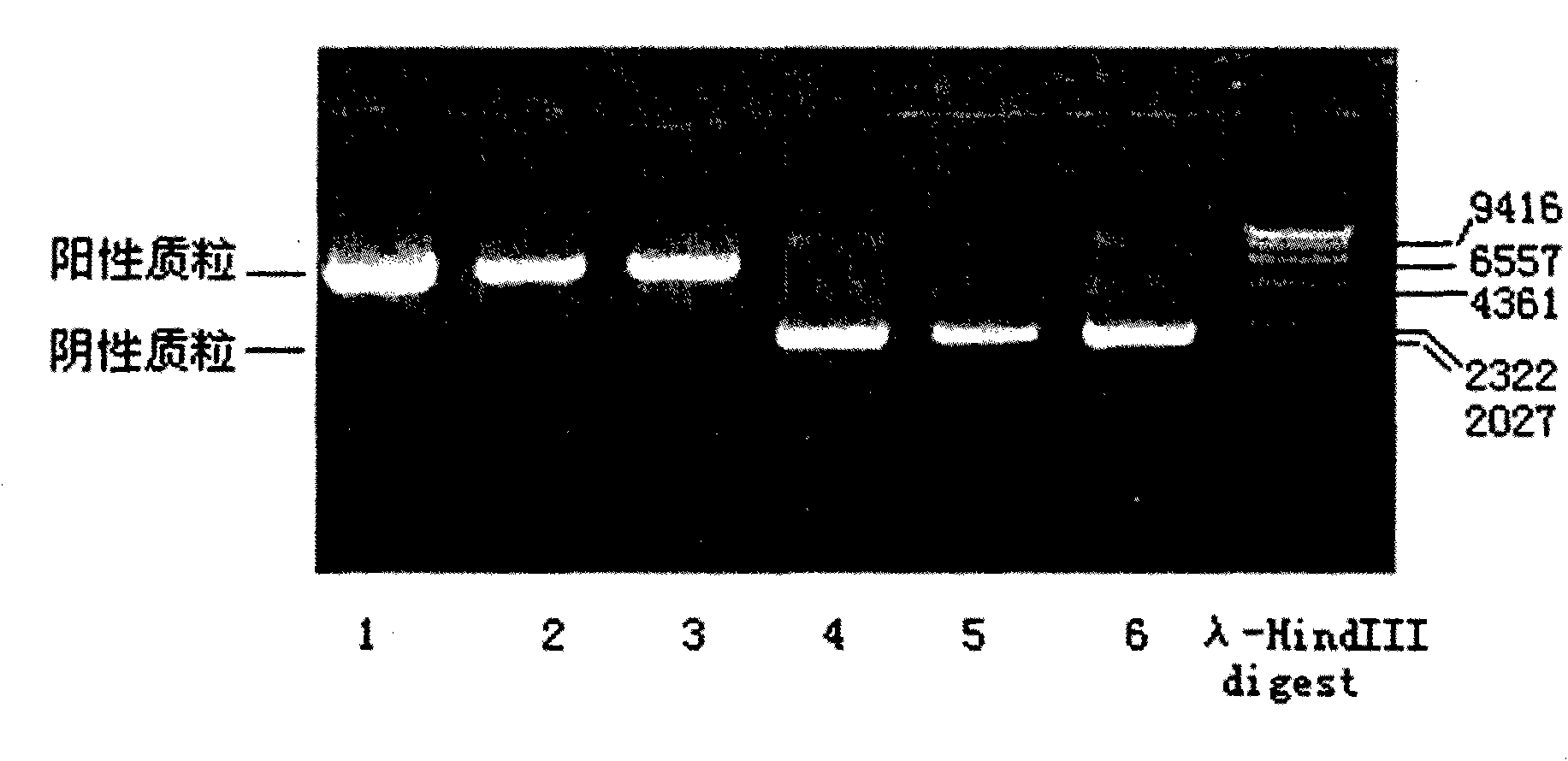
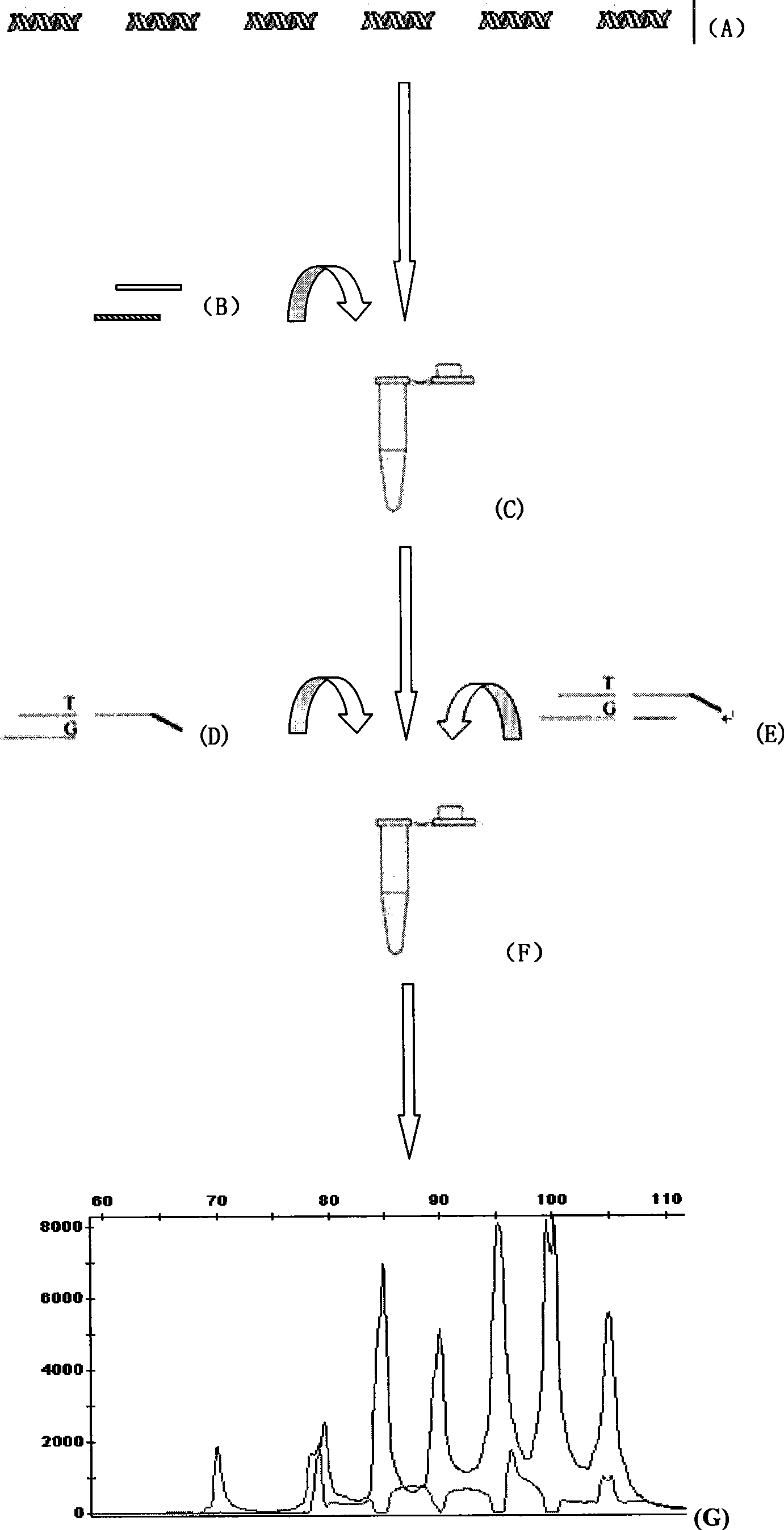
The invention discloses a human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and an HLA gene sequencing and typing method. The HLA-A and HLA-B gene full-length sequencing method comprises the following steps of: a, performing PCR amplification on about 4kb full-length sequences of HLA-A and HLA-B genes by using a pair of primers respectively; and b, cloning the amplification products to a pGEM-Tea sy vector, sequencing the full-length sequences by using ten walking sequencing primers in positive and negative directions respectively, and totally obtaining 38 allele 4.3kb full-length sequences of the HLA-A and 30 allele 3.7kb full-length sequences of the HLA-B. The HLA-A and HLA-B sequencing and typing method comprises the following steps of: performing PCR amplification on typing target areas of the HLA-A and HLA-B by using two pairs of primers respectively; and performing two directional sequencing on products by using fourteen sequencing primers respectively, wherein the HLA-DRB1 and HLA-DQB1 sequencing and typing method comprises the following steps of: amplifying sequences of second and third exons of DRB1 and DQB1 by adopting group specificity primers respectively; performing two directional sequencing on the second and third exons of the DRB1 by adopting eight group specificity primers and three sequencing primers; and performing two directional sequencing on the second and third exons of the DQB1 by adopting four sequencing primers respectively.
Owner:SHENZHEN BLOOD CENT
Preparing method for tr-gene products detecting oligonucleotides chip and use thereof
InactiveCN1584049AGuaranteed specificityTo achieve the purpose of mutual verificationMicrobiological testing/measurementHeterologousOligonucleotide chip
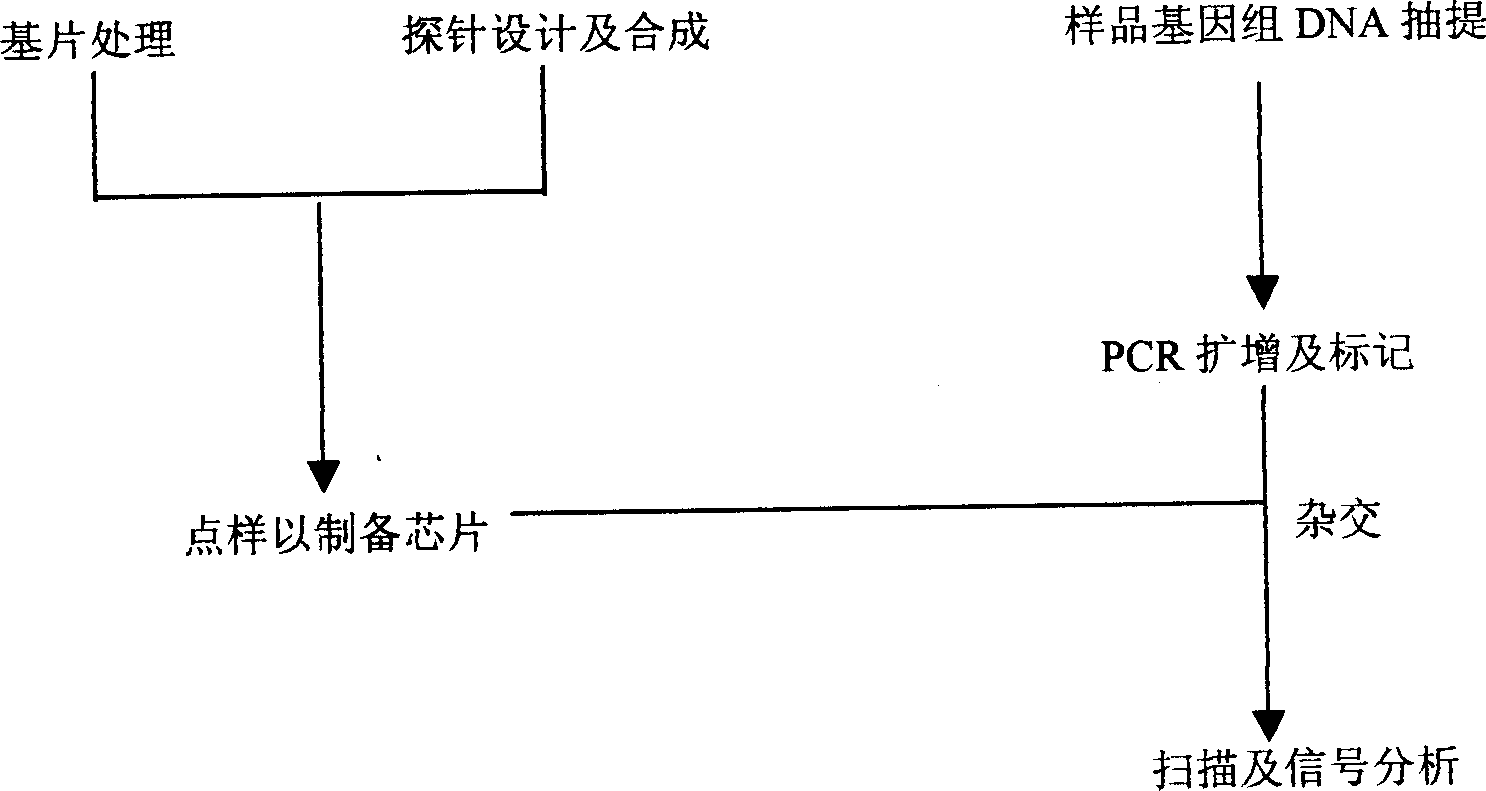
A method for preparing oligonucleotide chip for transgene product detection and use are disclosed. It includes: designing specific oligonucleotide probe and related primer by heterogeneric inserting gene, species internal standard gene, specific boundary sequence in transgene product gene set, fixing probe on glass slide to form transgene product detecting chip, amplifying DNA of plant to be tested by related primmer, marking by fluorescence, and hybridizing with chip. It can be use to acquire variable information.
Owner:国家质量监督检验检疫总局动植物检疫实验所 +2
Formulation of small RNA/DNA whitening product and preparation method thereof
InactiveCN101601635ASatisfied with the whitening effectProtect the skinCosmetic preparationsToilet preparationsAdditive ingredientSingle strand
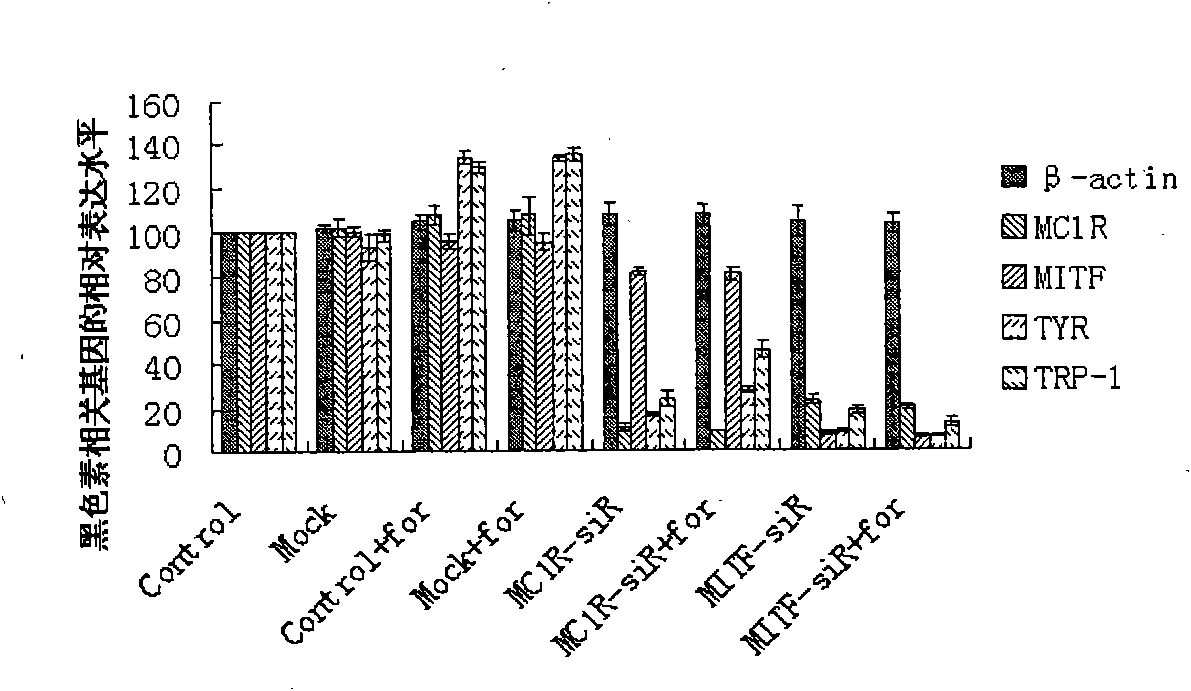
The invention relates to a small RNA / DNA whitening product series, which mainly consists of a small RNA skin whitening lotion and a small RNA skin beauty cream. The small RNA skin whitening lotion mainly comprises efficient active ingredients for removing skin pigmentation and whitening skin, such as nano single-strand or double-strand oligonucleotides for specially and efficiency restraining genes (Asip, Mclr, Mitf, Dct, Silv, Oca2, Myo5a, Loc51151, Rab27a, Pomc, OA1,F2rl1, Kinesin, Slc24a5, Tyr and Tyrp1) relevant to melanin generation and a transdermal agent for stimulating oligonucleotides to enter skin melanocytes; the small RNA skin beauty cream comprises a plurality of highly active materials for maintaining pure and white skin and small RNA segments to prevent the sunshine from damaging the protective components of the skin and remove free radicals, and anti-aging and anti-inflammatory effective molecules. In addition, the invention further provides a formulation, a method and a procedure for preparing the series of whitening products.
Owner:JIANGSU GENECON BIOLOGICAL TECH
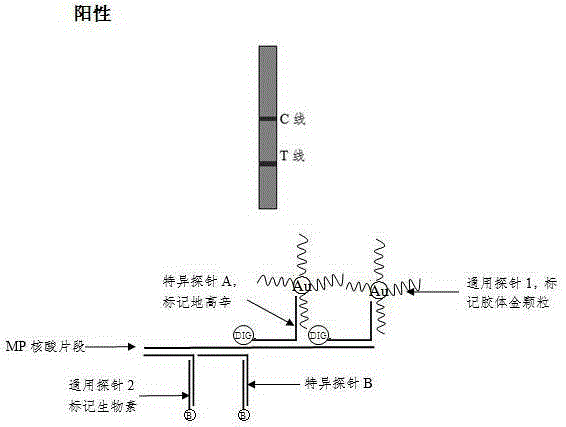
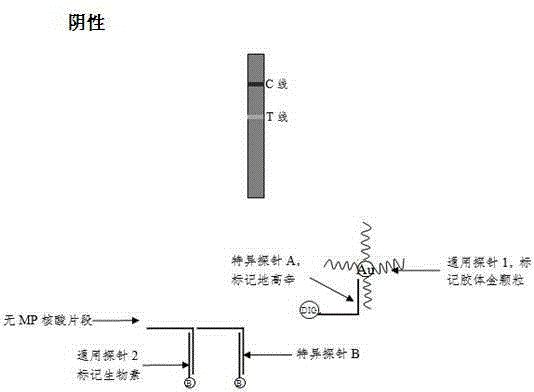
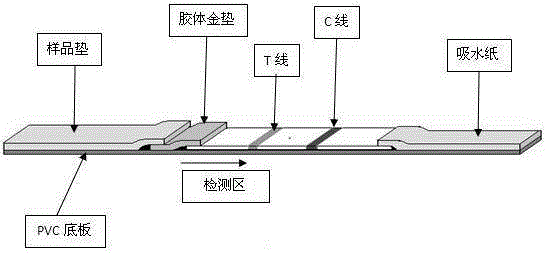
Method and kit for adopting colloidal gold chromatographic technique for detecting mycoplasma pneumoniae nucleic acid
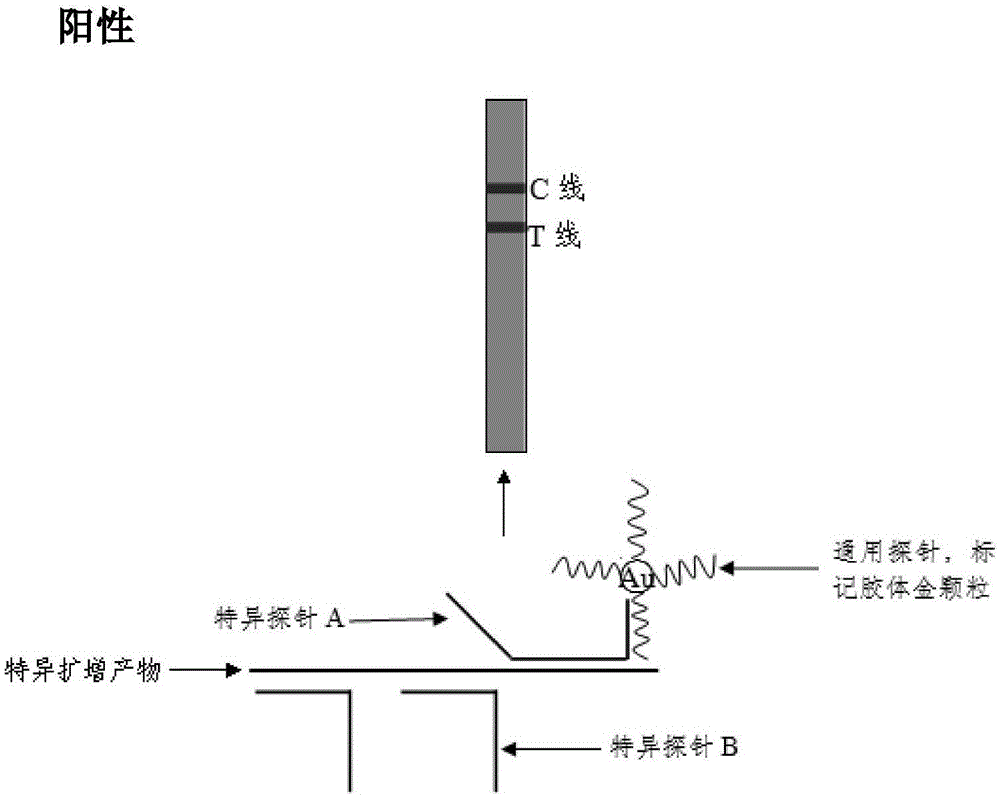
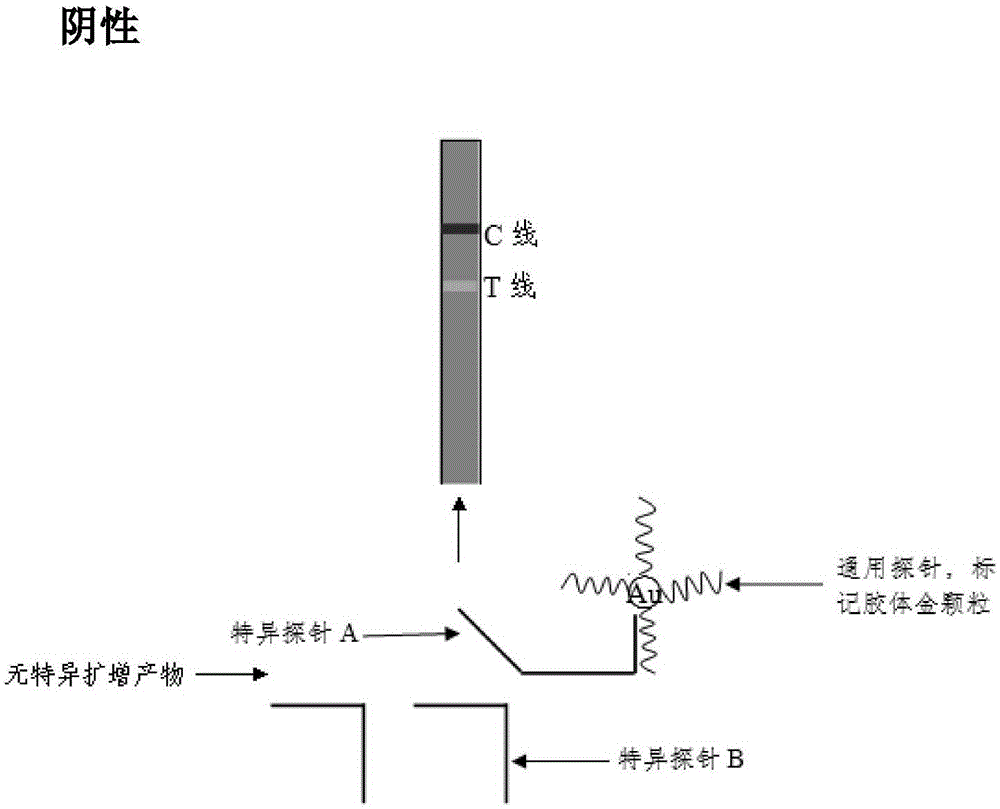
The invention discloses a method and a kit for adopting a colloidal gold chromatographic technique for detecting mycoplasma pneumoniae nucleic acid and belongs to the technical field of medical biochemistry. According to the method, a colloidal gold grain is directly marked on a nucleic acid probe; the sequence for the marked nucleic acid probe is designed as a universal sequence; the nucleic acid probe also can be used for detecting other pathogens. During the design process for the kit provided by the invention, the introduced special probe A and special probe B have the functions of bridge molecular components and a gold marked probe and an MP (Mycoplasma Pneumoniae) nucleic acid amplified fragment are successively combined with each other in series by the two probes, so that the special detection for the MP nucleic acid fragment is realized. More than two probes can be designed in each set of probes; such a design is beneficial to the increasing of the sensitivity of the test strip; the advantages of the amplification technique for the depending nucleic acid sequence of MP and the colloidal gold marked detection for the products after amplification are integrated; the technical demand on the experimenter is low; no special instrument is required; the popularization of the MP nucleic acid detection in basic and faraway rural medical institutions is easily realized.
Owner:武汉中帜生物科技股份有限公司
Kit and method for detecting mycobacterium tuberculosis infection and application
ActiveCN102004155ALow costQuality assuranceBiological testingMycobacterium InfectionsLatent tuberculosis
The invention belongs to the field of biomedicine examination, and particularly relates to a kit and a method for detecting mycobacterium tuberculosis infection and application. The invention discloses a novel mycobacterium tuberculosis detection reagent by screening specific T cell epitope of mycobacterium tuberculosis, wherein the reagent contains polypeptide or analog thereof represented by SEQ ID No.1-10. The method detects cell factors released from T cells by using single or more SEQ ID No.1-10 polypeptides to contact the T cells of mycobacterium tuberculosis infected individuals. The method can effectively detect active tuberculosis or latent tuberculosis infection, and is free from disturbance of Bacilli Calmette Guerin (BCG) inoculation vaccines. The invention also discloses a diagnostic kit and other application based on the polypeptide and the method. Compared with the gamma interferon release experiments in the prior art, the method can obviously improve the detection rate without reducing the specificity and has high clinical application value.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Nucleic acids of liquid-phase gene chip for synchronously detecting five porcine viruses and detection method thereof
ActiveCN104328218AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVMultiplex
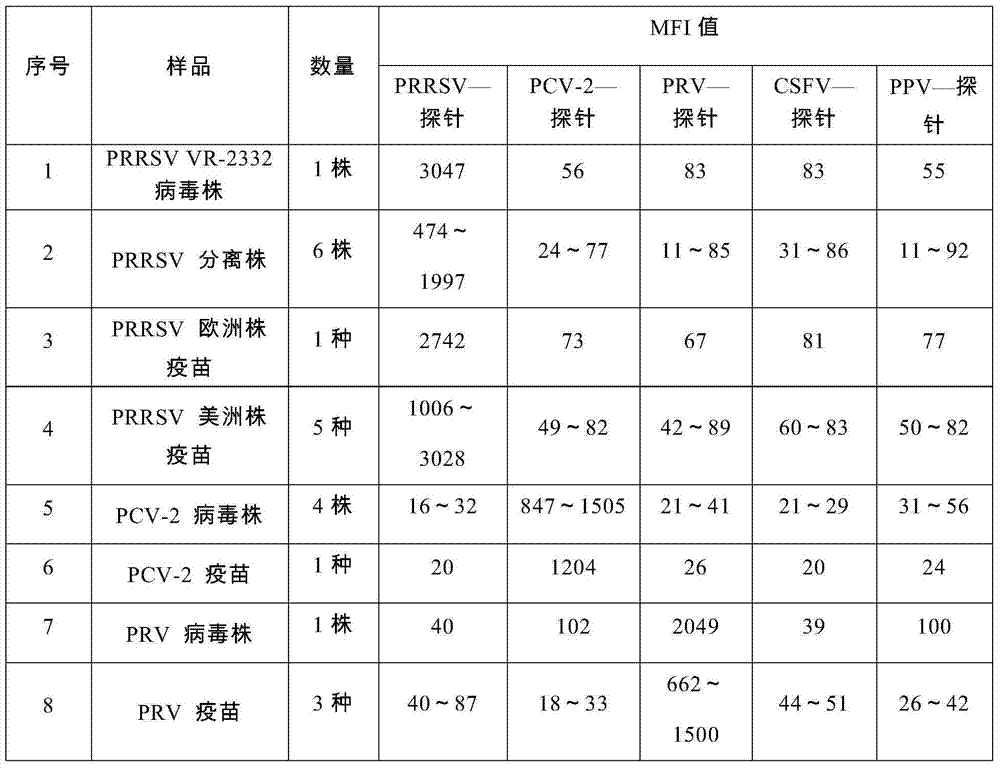
The invention provides a set of nucleic acids of a liquid-phase gene chip for synchronously detecting five porcine viruses, which comprise forward and reverse primers and hybrid probes for porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV2), porcine pseudorabies virus (PRV), classical swine fever virus (CSFV) and porcine parvovirus (PPV). The invention also provides a multiplex liquid-phase chip high-flux molecular biology detection method of the five porcine viruses. According to the method, porcine virus nucleic acids in the sample to be detected are extracted to perform multiplex unsymmetric nucleic acid amplification / multiplex liquid-phase gene chip (suspension chip) combined detection, thereby synchronously and accurately detecting and identifying the five porcine viruses in the sample to be detected. The method has the advantages of high specificity, high sensitivity, high stability, high flux and high detection speed, and is simple to operate.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Preparation method of electrochemical sensor for detection of heavy metal lead contaminants
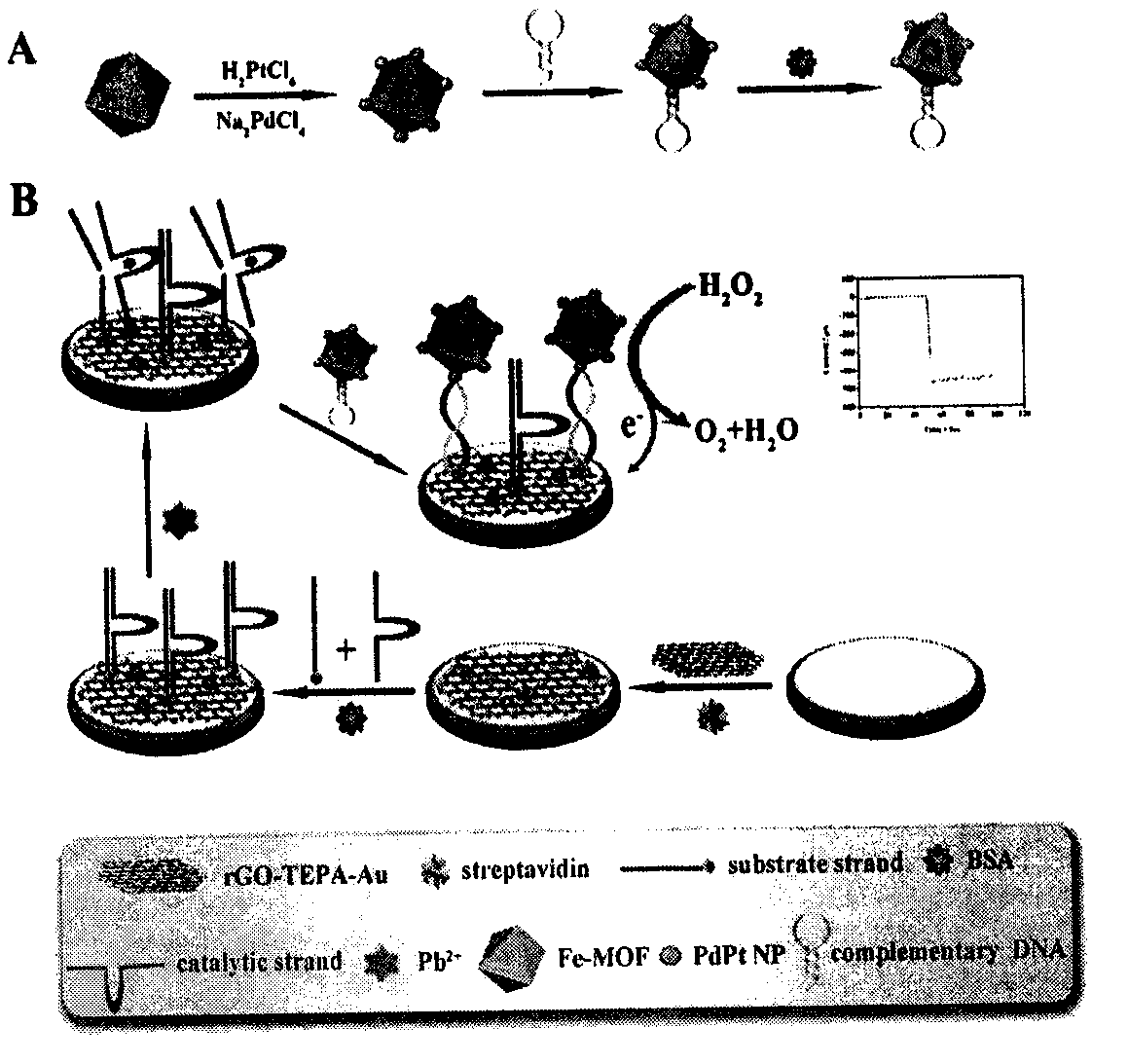
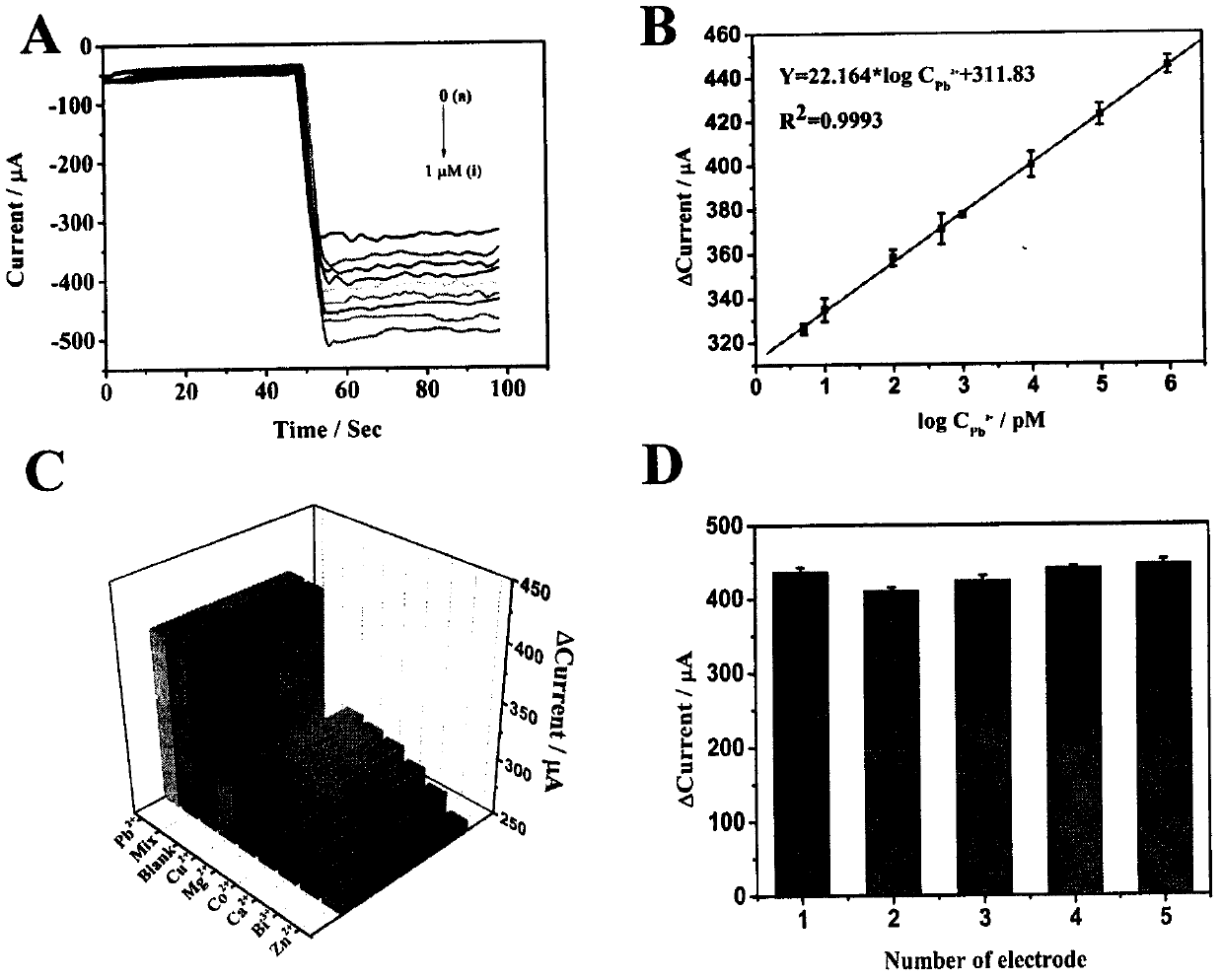
ActiveCN107621493AImprove catalytic performanceHigh sensitivityMaterial analysis by electric/magnetic meansElectrochemical gas sensorBimetallic nanoparticle
The invention relates to a preparation method and application of an electrochemical sensor for detection of heavy metal lead contaminants and belongs to the technical field of electrochemical detection. The preparation method is characterized by including; performing synthesis to obtain a Fe-MOFs nano material, reducing palladium-platinum bimetallic nanoparticles onto the Fe-MOFs nano material, and mixing a hairpin type DNA signal probe with the composite material to obtain a biological signal probe; performing layer-by-layer self-assembly through rGO-TEPA and avidin for fixing of '8-17'DNAzyme to obtain the electrochemical sensor for detection of the heavy metal lead contaminants. The sensor is successfully applied to detection of lead contaminants in the environment. The preparation method and application has the advantages of high flexibility, high specificity and rapid and convenient detection; experimental evidence is provided for research of lead contamination detection technology, and new concepts and new technology platforms are provided for monitoring the lead contaminants in the environment are provided.
Owner:CHONGQING MEDICAL UNIVERSITY
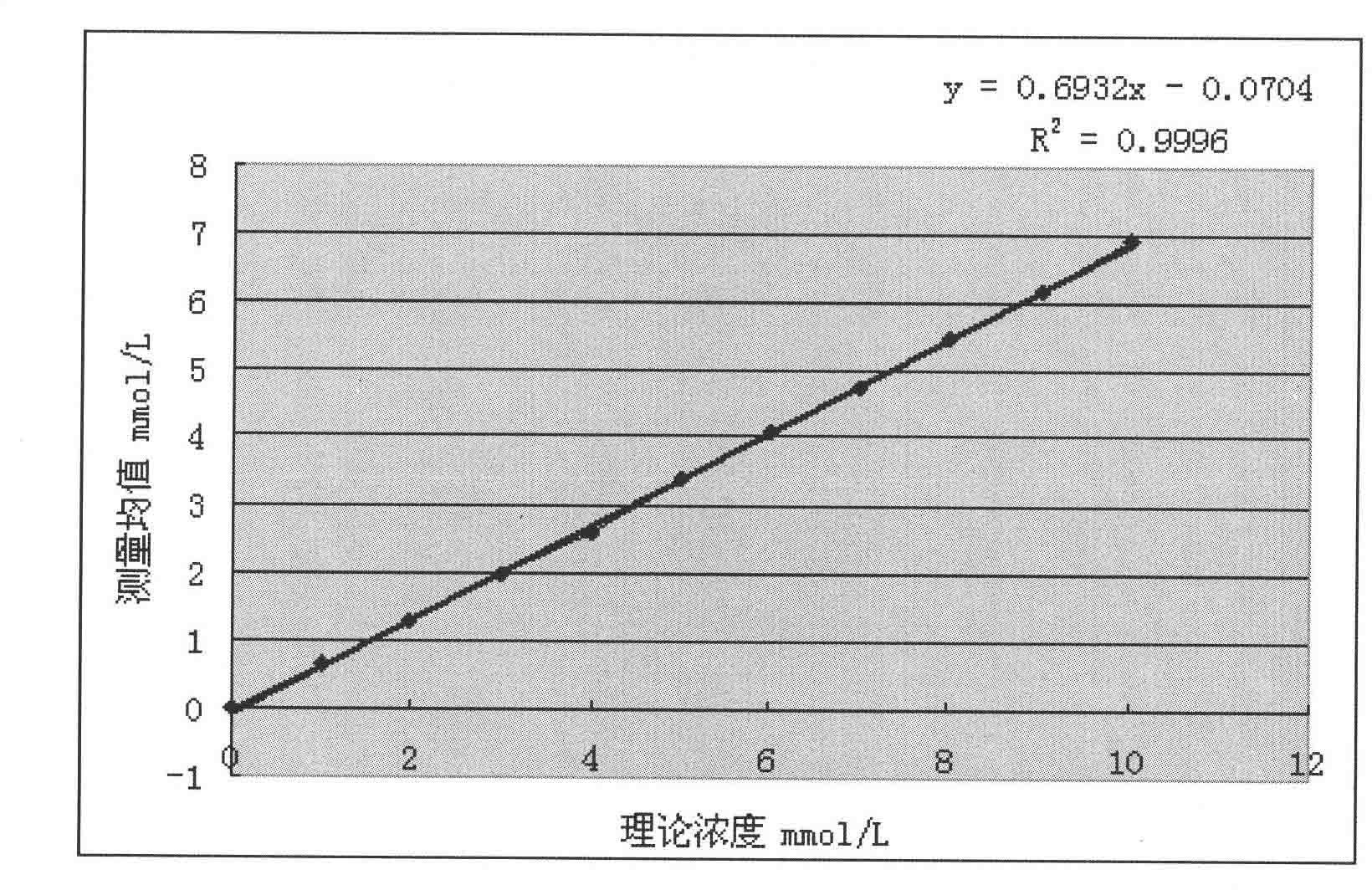
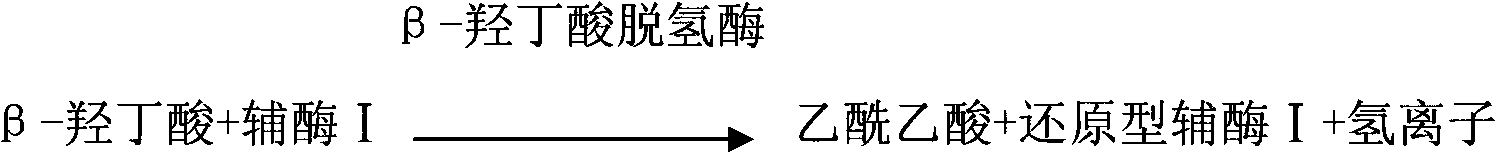
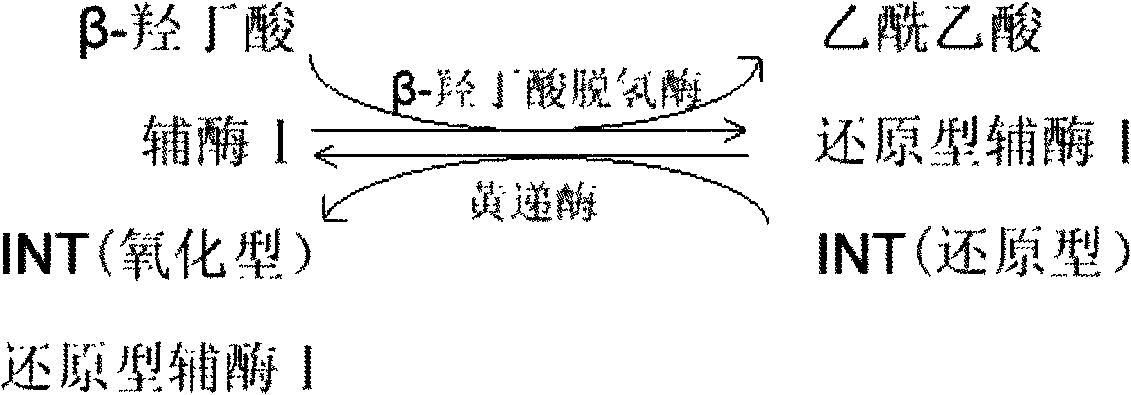
Liquid stable kit for measuring beta-hydroxybutyric acid by cyclic enzyme method
ActiveCN102435749AGuaranteed specificityStrong specificityColor/spectral properties measurementsBiological testingBeta-Hydroxybutyric acidPreservative
The invention discloses a liquid stable kit for measuring beta-hydroxybutyric acid by a cyclic enzyme method. The liquid stable kit consists of a reagent 1 and a reagent 2, wherein 1L of reagent 1 comprises 50 to 500mmol of buffer solution, 1 to 5KU of beta-hydroxybutyrate dehydrogenase, 1 to 5KU of diaphorase, 0.1 to 100g of surfactant, 1 to 100mmol of stabilizer, 0.1 to 100g of anti-interference agent I, 0.1 to 100g of anti-interference agent II, and 0.1 to 100ml of preservative; and 1L of reagent 2 comprises 50 to 500mmol of buffer solution, 1 to 20mmol of coenzyme I, 0.1 to 10mmol of nitrotetrazolium blue, 1 to 100mmol of stabilizer and 0.1 to 100ml of preservative. The liquid stable kit has the advantages of stability, wide linear range, high measurement accuracy, high antijamming capability and low cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Hyper-blocking fluorescence quantitative PCR method with high sensitivity for detecting rare mutation
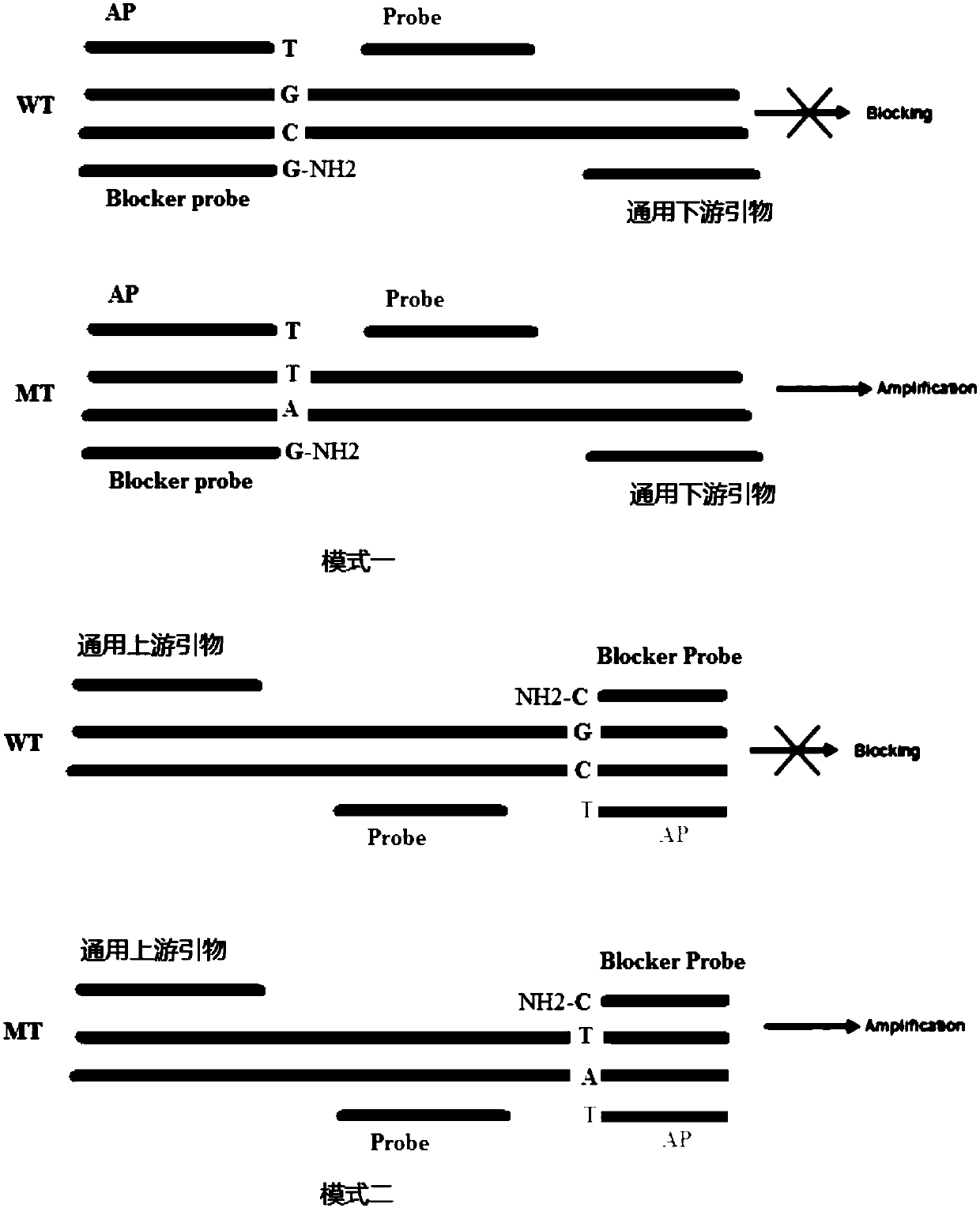
ActiveCN108048531ASpecific fastSpecificity does not affectMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMutation detection
The invention discloses a hyper-blocking fluorescence quantitative PCR method with high sensitivity for detecting rare mutation. The invention adopts specific primers and a probe technology and can rapidly detect gene point mutation in a blood sample. The method has the advantage that (1) the conventional ARMS primers are modified by LNA or mismatched bases are introduced into 3' tail 2th site totail 3th site so that the sensitivity of the mutation detection is not influenced and the specificity of the single base mutation based on the mutation specific primers is guaranteed, (2) the modifiedblocker probe completely complementary to the wild type gene is introduced so that the wild type background interference is further reduced with keeping the efficiency of the mutant amplification, (3) based on the high specificity modified blocker probe, the ARMS primers can detect different base mutation forms at the same site at the same time so that multiple sites in the same gene can be detected by only a few systems, (4) sensitivity is high and (5) a detection rate is fast.
Owner:SHANGHAI GENEPHARMA CO LTD
Method for detecting pig plague virus specific antibody and its ELISA reagent kit
InactiveCN101144818AGuaranteed specificityGuaranteed Differential DiagnosisMaterial analysis by observing effect on chemical indicatorElisa kitSwine Fever Virus
The invention discloses a method for detecting the specific antibody of classical swine fever virus and a special ELISA kit thereof. The kit includes a classical swine fever virus antigen and an enzyme-labeled classical swine fever virus single-epitope specific antibody; the said swine fever virus antigen is a polypeptide containing one or more than one amino acid residue sequence described in sequence 1. The detection reagent of the classical swine fever virus specific antibody of the present invention can carry out effective detection to the classical swine fever virus specific antibody by solid-phase antigen competition ELISA (blocking method); The B cell epitope ensures the differential diagnosis, and the high degree of conservation of the epitope among various strains ensures the specificity of detection.
Owner:TSINGHUA UNIV +2
Variant porcine reproductive and respiratory syndrome virus (PRRSV) TaqMan fluorescence quantitative RT-PCR detecting kit and application thereof
InactiveCN101736094AGuaranteed specificityStrong specificityMicrobiological testing/measurementMicroorganism based processesHighly pathogenicFluorescence
The invention discloses variant porcine reproductive and respiratory syndrome virus (PRRSV) TaqMan fluorescence quantitative RT-PCR detecting kit and application thereof. A primer and a TaqMan probe are designed and synthesized by referring to an NSP2 fragment gene sequence of the variant PRRSV and common PRRSV of a GenBank. By optimizing the reaction condition and constructing a standard plasmid product, a method for diagnosing the variant PRRSV by TaqMan fluorescence quantitative RT-PCR is established. A result indicates that the method has the advantages of strong specificity, high sensitivity, and the like and can detect the standard plasmid product with 264 copy numbers, and the virus quantity of 0.5623TICD50 is 10 times more sensitive than RT-PCR. By detecting 22 disease samples, 8 disease samples are positive, and the positive rate is 36.4 percent. Because the method has the advantages of quantification, high speed, accuracy, sensitivity, and the like, the invention is suitable for the diagnosis on the swinery infected variant PRRSV in the early stage, the medium stage and the later stage and plays an important role in effectively diagnosing, preventing and treating the highly pathogenic PRRSV.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +6
Method for quantitative determination of specific analyte with single trapping agent and reagent kit therefor
InactiveCN1700009AFacilitating the application of omics researchFacilitate disease diagnosisMaterial analysisAnalyteQuantitative determination
The invention relates to a method and its medium box for quantity measuring peculiar analysis substance by single capture medium. The method comprises the following steps: combining the tested analysis substance with solid phase capture medium, labeling analysis substance which has been captured by capture medium with report molecule, cleaning-up analysis substance from compound, recombining tested analysis substance with new solid phase capture medium, ascertaining content of analysis substance by report molecule's label signal. The medium box comprises capture device, testing device report molecule used as label and analysis substance eluent.
Owner:孙东旭
On-site detection immuno-chip and preparation method thereof and application
The invention discloses an on-site detection immuno-chip, comprising a chip carrier and an agarose gel layer which is paved on the chip carrier, wherein, the agarose gel layer is fixed with a plurality of antibody microarrays of 4*4, and chip-dedicated fences or Super PAP Pen scribings are used for separating the microarrays from each other; the double antibody sandwich principle is adopted to detect the antigen to carry out the dot matrix of corresponding capture antibody, positive control and negative control on the solid phase carrier simultaneously; the antibody protein is connected with the solid phase carrier through the covalent bond and physical adsorption; the sample liquid to be detected and the chip are directly incubated; the antigen to be detected in the samples combine with the corresponding antibody which is fixed on the chip; a specific monoclonal antibody probe marked by horse radish peroxidase is added and the macroscopic detection results can be obtained after the coloration of substrates. The invention can detect viruses in multiple aquatic animal samples and the results are macroscopic, thus being applicable to rapid and accurate detection of viruses of aquatic animals in breeding production.
Owner:OCEAN UNIV OF CHINA
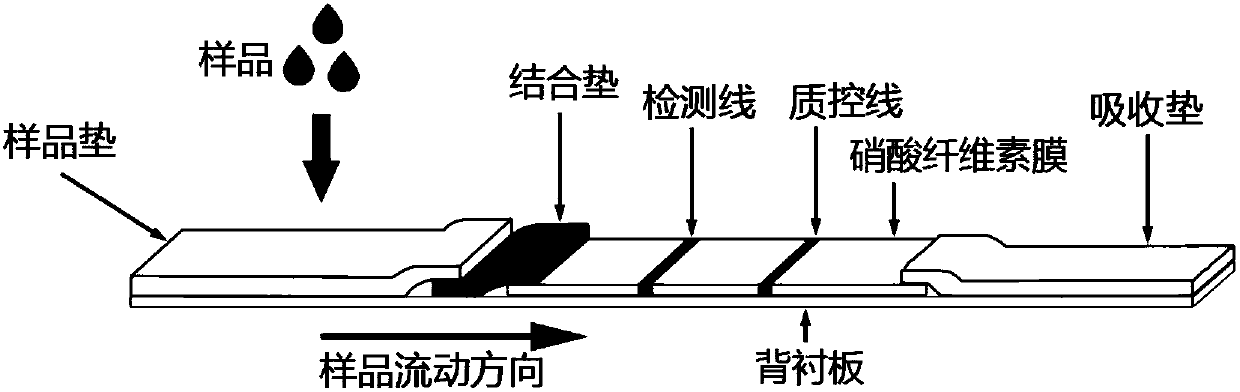
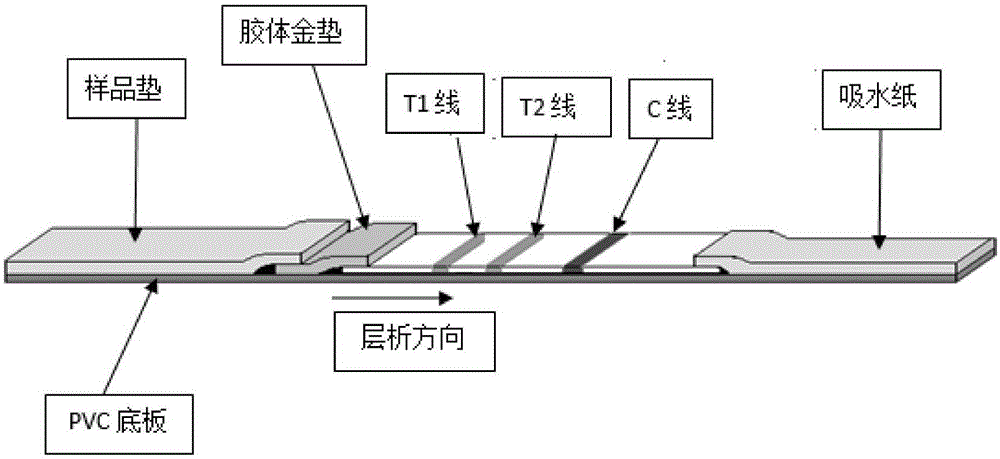
Immunochromatographic test strip, and production method and application thereof
InactiveCN107907679AGuaranteed specificityIndicating validityMaterial analysisQuality control systemMonoclonal antibody
The invention discloses an immunochromatographic test strip, and a production method and an application thereof. The test strip is characterized in that a labeling compound carries an inert protein connected with a special label and used for quality control, and a quality control line contains a monoclonal antibody specifically binding to the special label carried by the inert protein. The test strip has an independent quality control system, so the obtained test result has a high specificity and an anti-interference property, non-specific reactions brought by the self-bearing antibodies in human blood samples are effectively avoided, and the effectiveness of the test strip is effectively indicated.
Owner:NANJING VAZYME MEDICAL TECH CO LTD +1
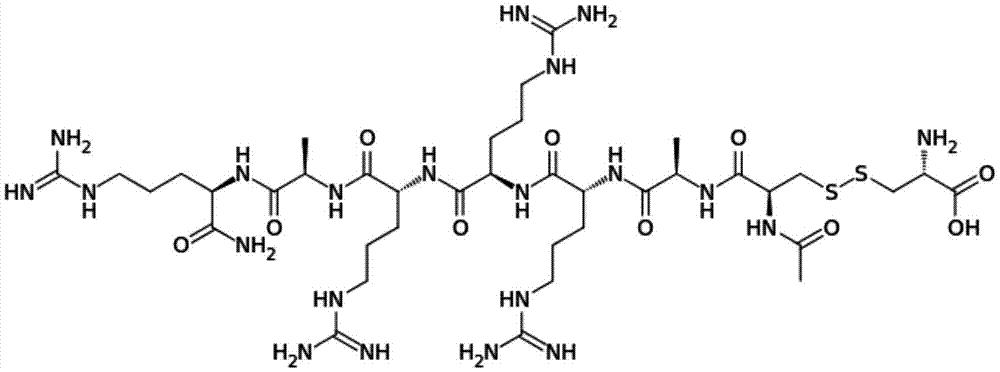
Method for synthesizing Etelcalcetide
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing Etelcalcetide. The method comprises the following steps: performing liquid-phase synthesis so as to obtain straight-chain heptapeptide which is subjected to acetylization at an N end and is subjected to amidation at a C end on the amino acid sequence of SEQ ID NO:1; performing NCS (Chlorosuccinimide) chlorination on L-Cys, and substituting chlorine on the sulfydryl, and generating L-Cys (SCl); performing coupling reaction on the straight-chain heptapeptide and L-Cys (SCl) to generate disulfide, thereby obtaining Etelcalcetide. As the Etelcalcetide is synthesized by using a whole liquid-phase method, relatively small amounts of reagents and solvents are used when being compared with those of a solid-phase method, the synthesis is green and environmental-friendly, no expensive resin is used, and thus the cost is lowered. Meanwhile, as NCS which is cheap and easy to obtain and cysteine are adopted to form an active intermediate to construct the disulfide bond, the defects that a conventional method is low in conversion rate and low in purity are avoided, and large-scale production of the Etelcalcetide can be facilitated.
Owner:HYBIO PHARMA
Reagent for detecting brucella and complex probe fluorescence quantitative PCR (polymerase chain reaction) brucella detection method
InactiveCN102146466ASimple and efficient operationGuaranteed specificityMicrobiological testing/measurementFluorescence/phosphorescenceQuenchingFluorescence
The invention provides a reagent for detecting brucella. The reagent comprises an upstream primer, a downstream primer, a fluorescence probe and a quenching probe; the gene sequence of the upstream primer is 5'-caagggcaaggtggaagatt-3'; the gene sequence of the downstream primer is 5'-ctgcgaccgatttgatgttt-3'; the gene sequence of the fluorescence probe is 5'-fam-atcgtttccgggtaaagcgtcgcca-P-3'; and the gene sequence of the quenching probe is 5'-cgctttacccggaaacga-Dabcyl-3'. The invention also provides a complex probe fluorescence quantitative polymerase chain reaction (PCR) brucella detection method using the reagent. The method is simple and convenient in operation, efficient, quick and specific; the detection time of the brucella is greatly shortened; the quantitative detection of a sample can be completed in about 2 hours; and the reagent and the method have significance for early diagnosis of brucella disease.
Owner:浙江国际旅行卫生保健中心
Detection method of invasive fungus infection, detection kit and application
ActiveCN109055502ASimplify detectionSimplify operabilityMicrobiological testing/measurementDNA/RNA fragmentationCell freeFluorescence
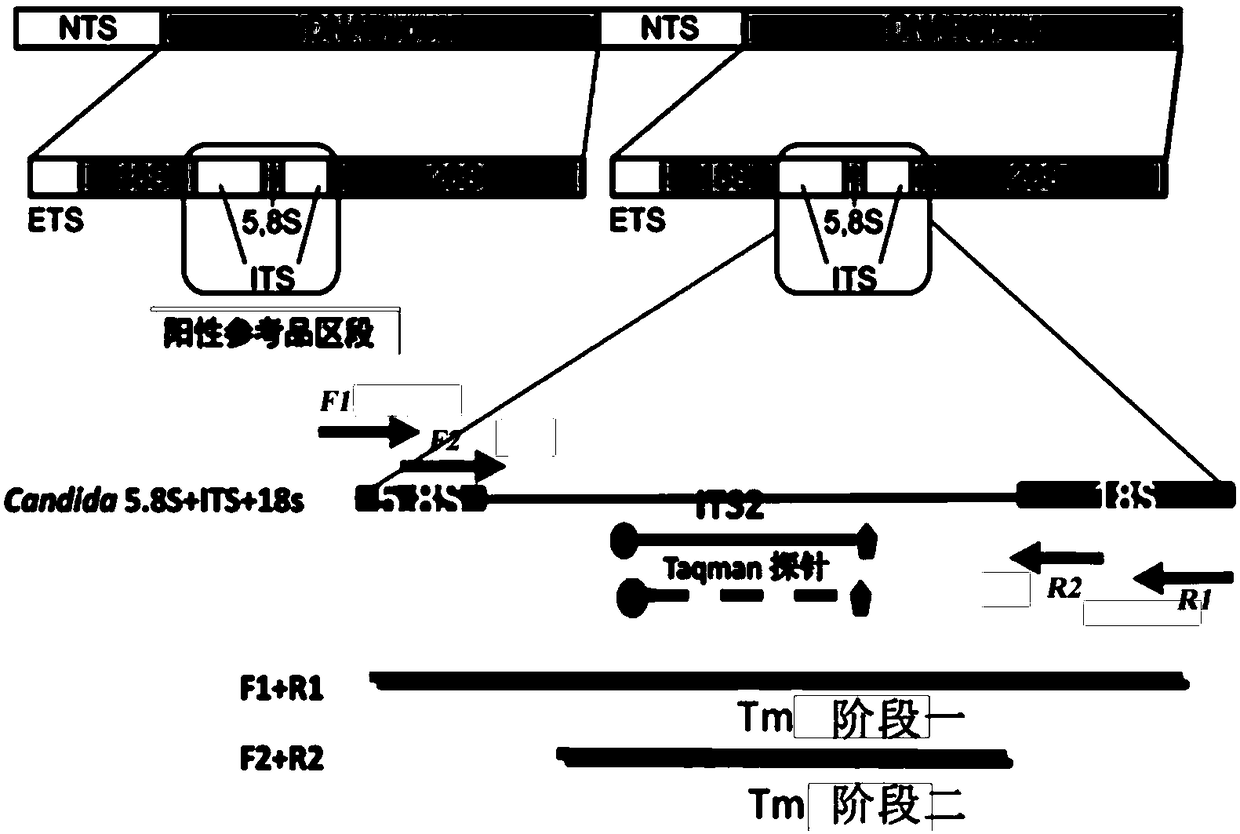
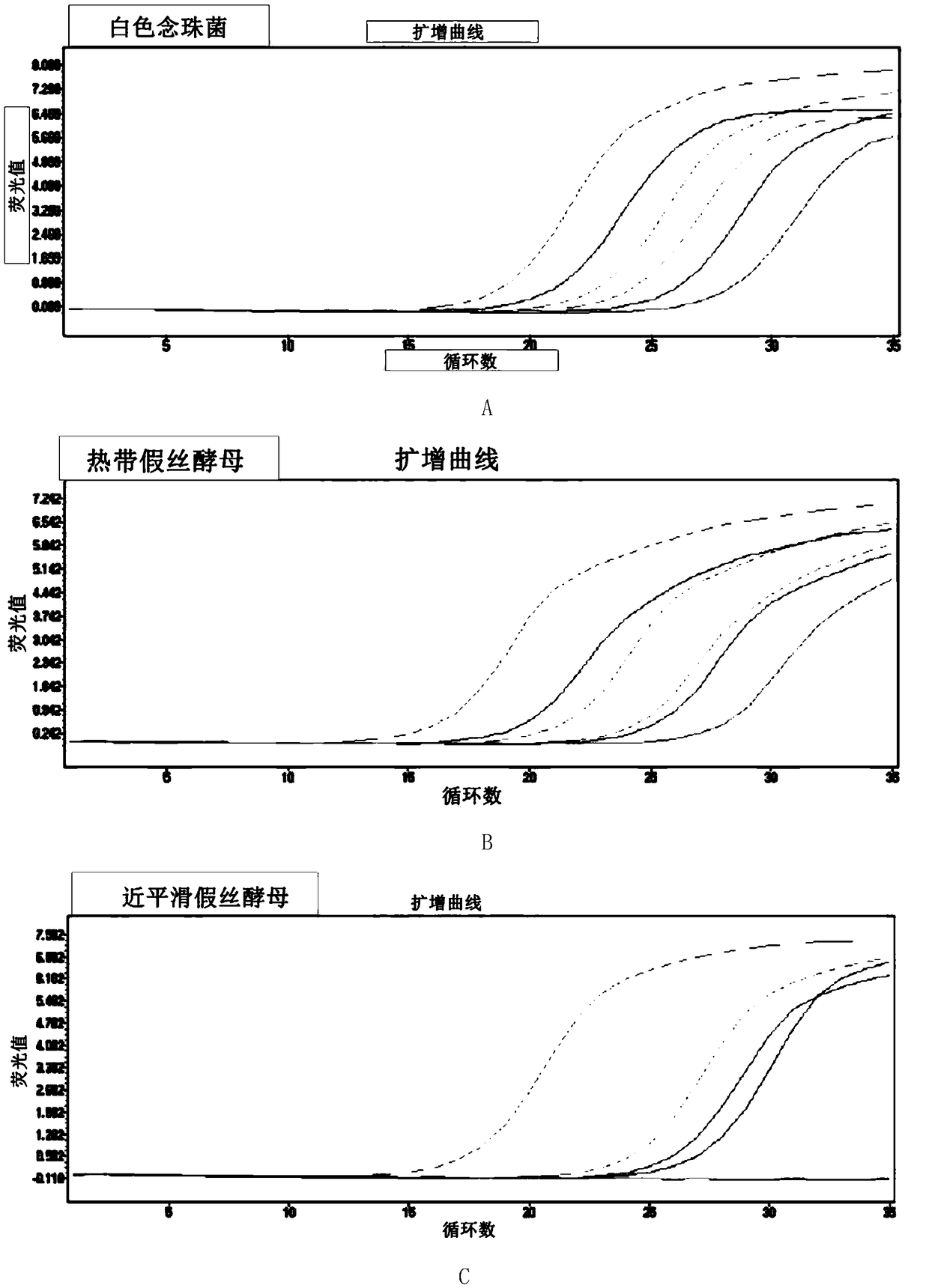
The invention provides a fast multiplex PCR identification diagnosis detection method of invasive fungus infection based on cfDNA (cell free DNA). The method can be used for identifying the invasive infection caused by clinic common and high-incidence Candida albicans, Candida tropicalis, Candida parapsilosis, Candida krusei, Candida glabrata and Aspergillus fumigtus. An amplification primer is designed according to characteristic genome segments of each fungus category and species; a detection fluorescence probe of a strain can be distinguished according to the amplification segment design; the real time PCR can be performed on a sample to be tested; high sensitivity of nested PCR and high specificity and multi-target performance advantages of multiple fluorescent hybrid probe PCR are integrated for identifying the fungus strain. The invention also provides a PCR diagnosis kit for the invasive fungus infection and application thereof.
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
Method and kit for realizing multiple detection of nucleic acid through colloidal gold chromatographic technology
PendingCN105154565ARapid multiplexingSimple multiplex detectionMicrobiological testing/measurementCelluloseComplementary pair
The invention discloses a method and kit for realizing multiple detection of nucleic acid through a colloidal gold chromatographic technology, and belongs to the field of medical biotechnology. The invention provides a nucleic acid detection test strip, a universal probe is marked with colloidal gold particles to be fixed on a glass cellulose film, the universal probe is designed into a universal sequence, an NC film on the test strip have one or more detection lines and a quality control line, each detection line is coated with a section of nucleic acid sequence which can be in specific hybridization combination with a corresponding specific probe B series; the quality control line is coated with a section of nucleic acid sequence which can in specific hybridization combination with a specific probe A series; the specific probe A series is in complementary pairing hybridization with the universal probe; the probe marked with gold and a nucleic acid amplified fragment are combined in series by virtue of the specific probe A series and the specific probe B series, so that the specific detection of the nucleic acid fragment can be realized. The method has the advantages that the technical requirements of experimenters are low, the required detection time is short, special instruments are not required, and the method is easy to popularize towards grass-roots and remote countryside medical institutions.
Owner:武汉中帜生物科技股份有限公司
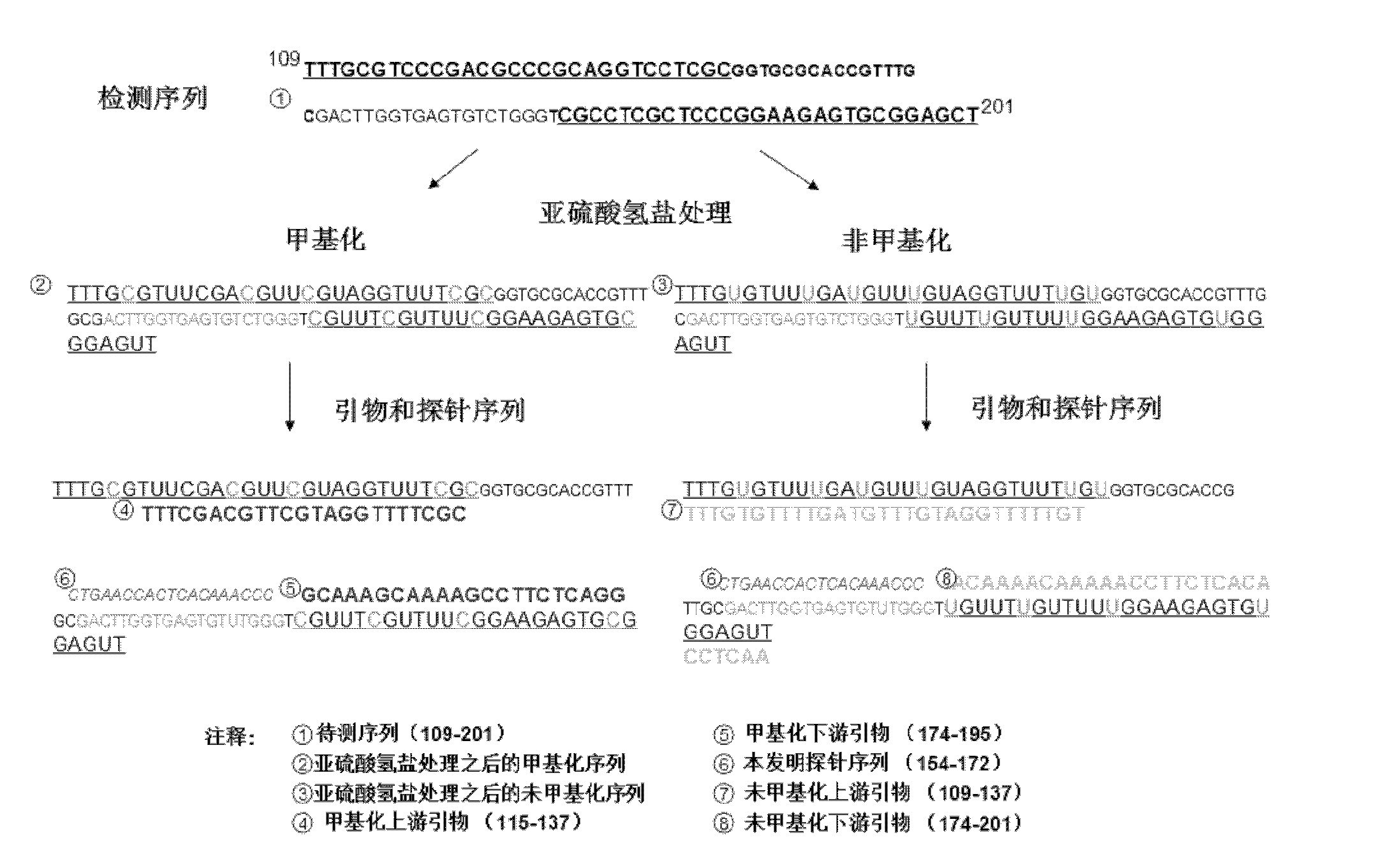
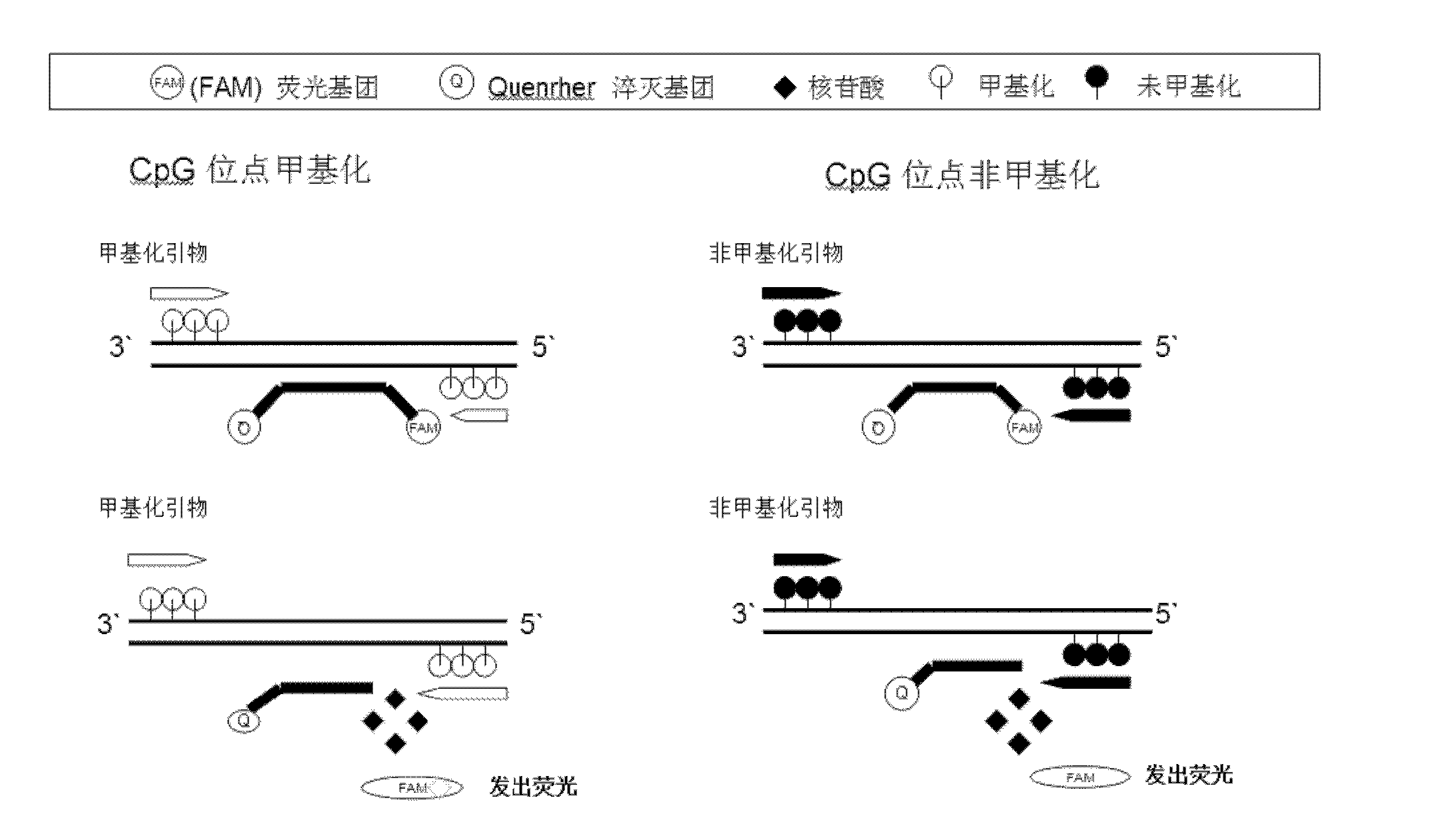
Taqman hydrolysis probe and method for quantitatively detecting methylation level of MGMT (O6-methylguanine-DNA-methyltransferase)
ActiveCN102424857AStrong specificityGuaranteed specificityMicrobiological testing/measurementDNA/RNA fragmentationO6-methylguanineFluorescence
The invention provides a Taqman fluorescent hydrolysis probe and a method for quantitatively detecting the methylation level of MGMT (O6-methylguanine-DNA-methyltransferase) by using the Taqman hydrolysis probe. The fundamental sequence of the Taqman fluorescent hydrolysis probe is GATTTGGTGAGTGTUTGGG, and the complementary region of the fundamental sequence and a genomic DNA (deoxyribonucleic acid) sequence extends upstream no more than 5nt and extends downstream no more than 5nt. According to the invention, the specificity of PCR (polymerase chain reaction) is doubly guaranteed; the percent of a methylated DNA template in a mixed sample can be quantitatively calculated; and the method has all the advantages (such as speediness, sensitiveness, no pollution, and the like) of the real-time quantitative PCR. The application of detection results of the invention can provide clinical guide for medicaments for carrying out chemotherapy on malignant tumors such as cerebral gliomas and the like; and the method has the advantages of high specificity, high sensitivity, accurate quantification, and the like.
Owner:陕西佰美医学检验有限公司
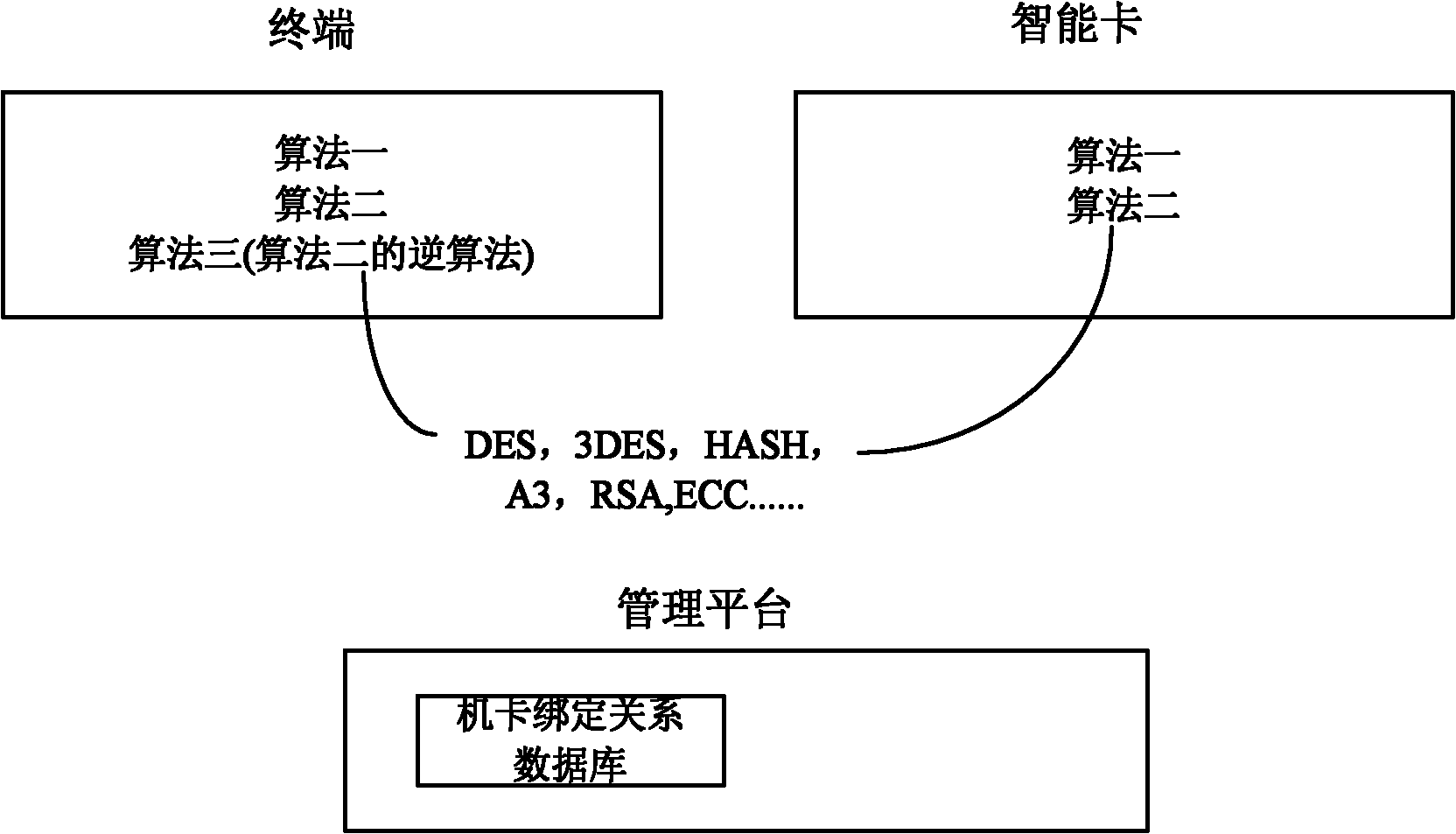
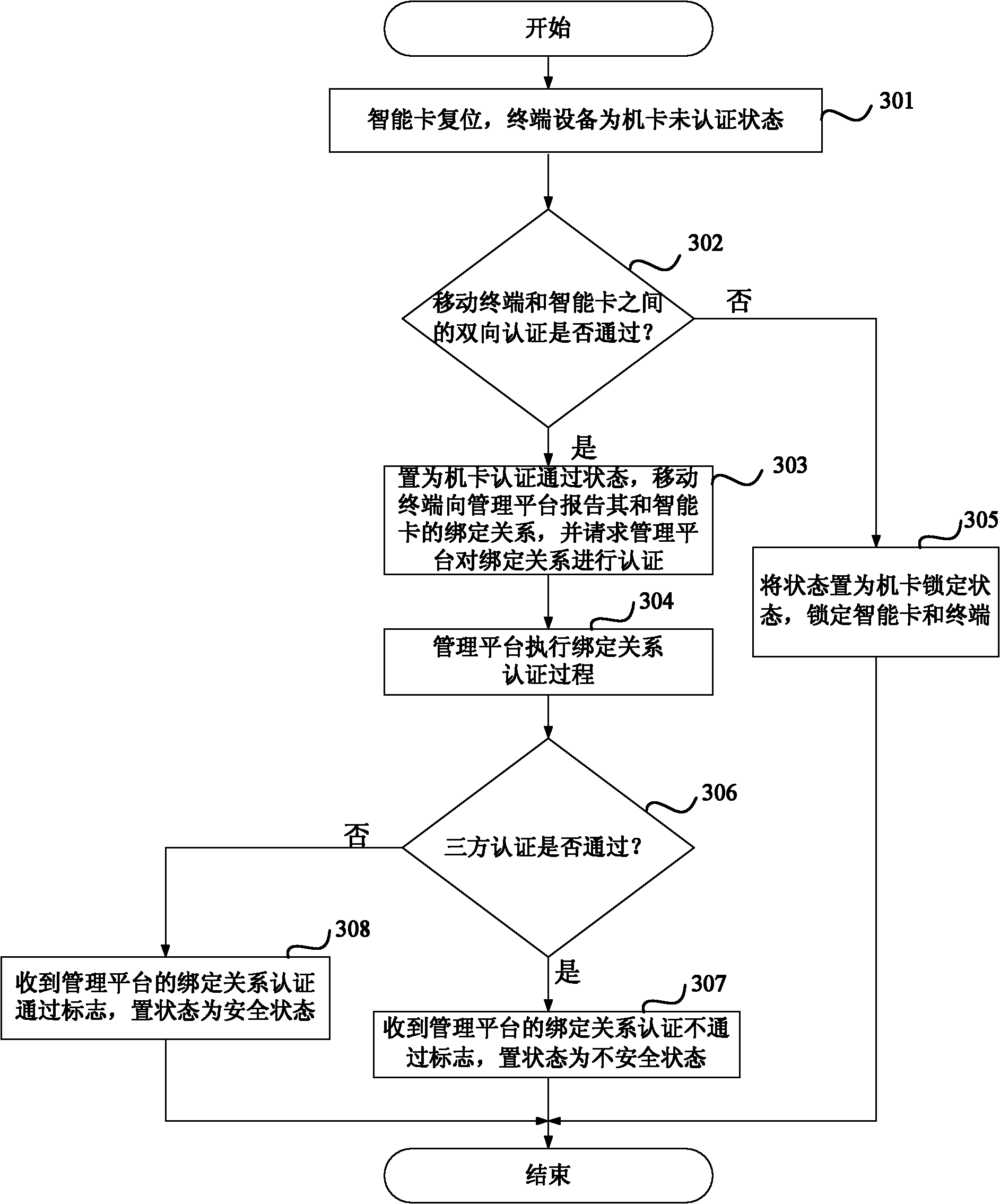
Three-party authentication method and device as well as intelligent card supporting two-way authentication
ActiveCN102833066AEnsure safetyGuaranteed specificityUser identity/authority verificationSmart cardAuthentication
The invention discloses three-party authentication method and device as well as an intelligent card supporting two-way authentication. The three-party authentication method comprises the following steps: authentication is performed between a terminal and the intelligent card; after the authentication between the terminal and the intelligent card is passed, the terminal reports a binding relationship between the terminal and the intelligent card to a management platform, and requests authentication of the binding relationship to the management platform; the management platform performs authentication of the binding relationship between the terminal and the intelligent card, if the authentication of the binding relationship is passed, the three-party authentication is judged to be passed, or else, the three-party authentication is judged not to be passed. By adopting the three-party authentication method and device as well as the intelligent card supporting two-way authentication, the safety of the terminal and the intelligent card are both ensured, and meanwhile, the binding relationship between the terminal and the intelligent card can be dynamically authenticated. The management platform side has a control and management right for the terminal and card equipment so that an operator can conveniently develop own business, and the specificity and safety of the terminal and the intelligent card during development of business of Internet of things are ensured.
Owner:衣锦浣香电子商务有限公司
Multiplex ligation-dependent probe amplification detection kit for simultaneously detecting five swine disease viruses, primers and probes
ActiveCN102943129AGuaranteed sensitivityGuaranteed specificityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMultiplex ligation-dependent probe amplification
The invention discloses a multiplex ligation-dependent probe amplification detection kit for simultaneously detecting five swine disease viruses, primers and probes. The multiplex ligation-dependent probes are shown in sequence tables SEQ ID NO:1 to SEQ ID NO:10; and the primers are shown in sequence tables SEQ ID NO:11 to SEQ ID NO:12. By using the primers, the probes and / or the multiplex ligation-dependent probe amplification detection kit containing the primers and the probes, five important swine disease pathogens such as a swine influenza virus, a swine reproductive and respiratory syndrome virus, a pseudorabies virus, a swine transmissible gastroenteritis virus and a foot-and-mouth disease virus can be simultaneously detected, thereby saving the detection time and cost and being beneficial to accurately diagnosing the epidemic diseases in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
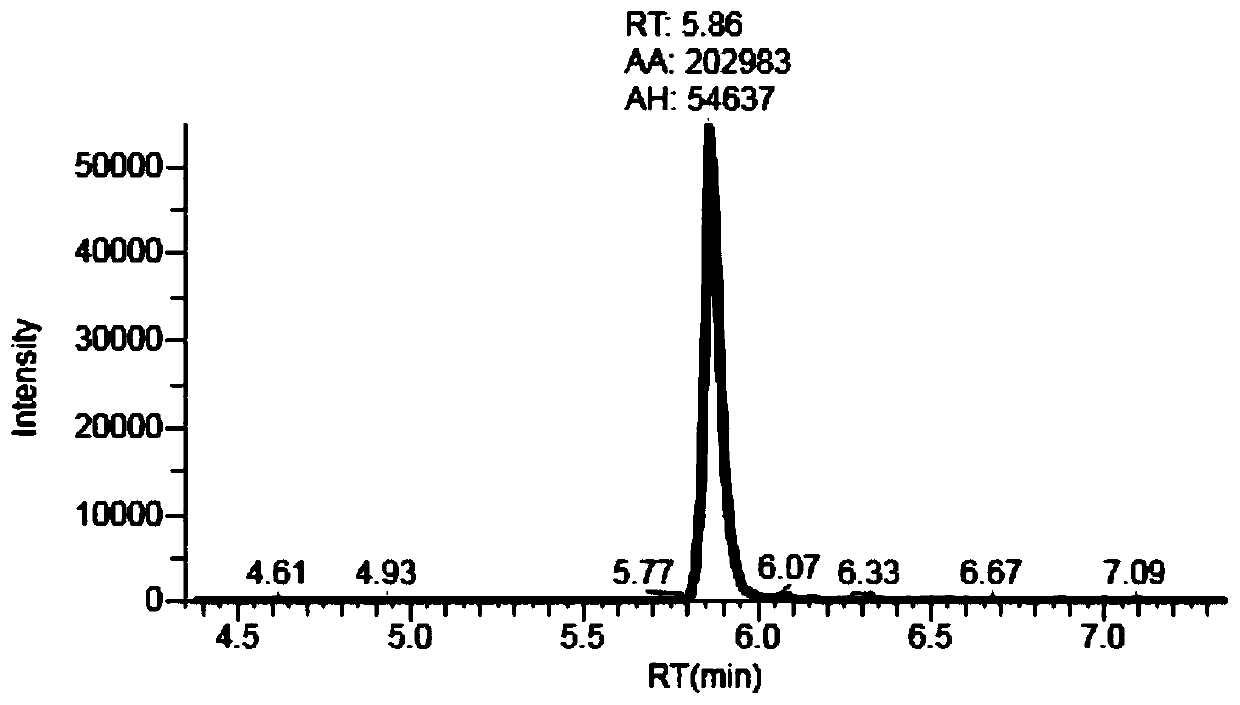
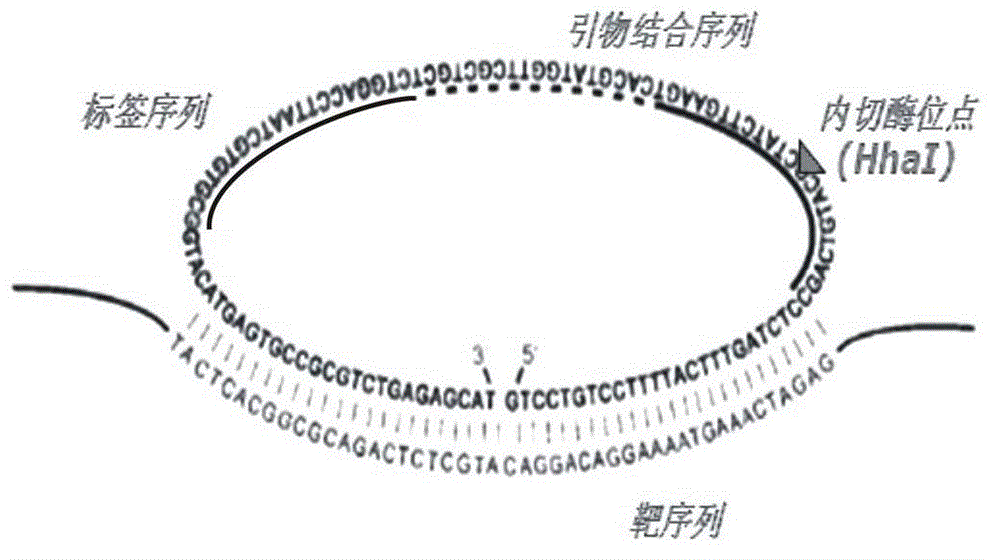
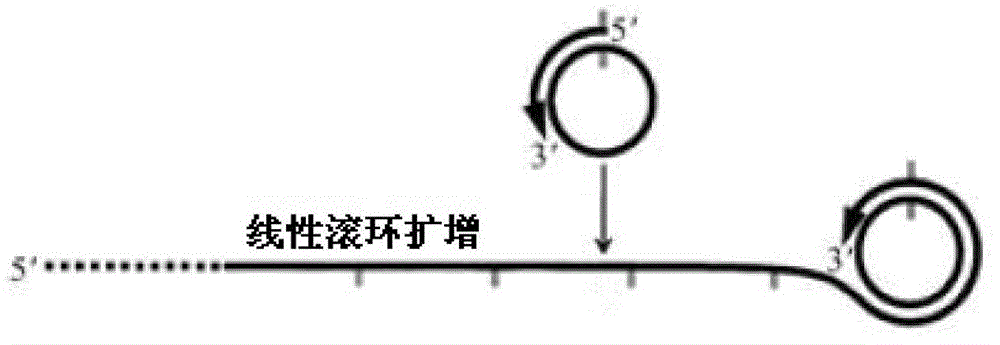
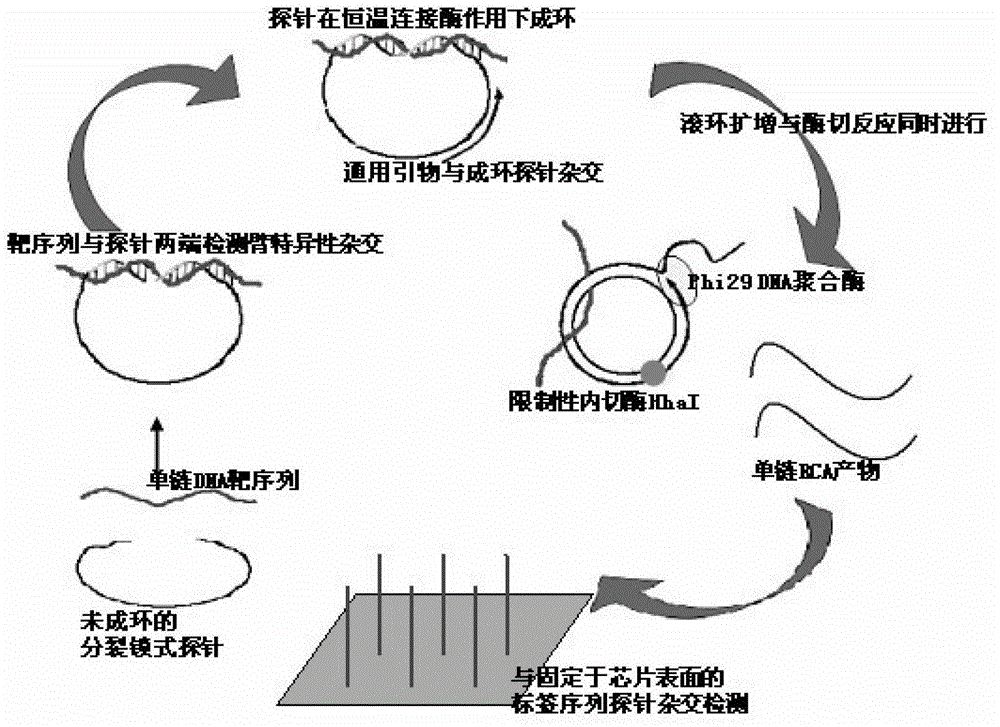
Multi-RCA (rolling circle amplification) method based on split padlock probes
InactiveCN102719550AEasy to operateStrong specificityMicrobiological testing/measurementPolymerase LExonuclease I
The invention discloses a multi-RCA (rolling circle amplification) method based on split padlock probes. Each novel split padlock probe is about 90bp in length and comprises four parts, namely a detection arm, a universal primer area, an HhaI endonuclease site and a tag sequence area, wherein an amplification system is composed of a connection system and an RCA system. The concrete detection method comprises the steps of: firstly, carrying out coupled reaction, mixing a target sequence DNA (deoxyribonucleic acid) segment and four split padlock probes of which the final concentration is 1mol / L, hybridizing for 15 minutes after boiling and degenerating, adding T4DNA ligase and T4DNA ligase buffer solution, supplying 10 muL of reaction system; carrying out exonuclease I and exonuclease III exterior contact for 45 minutes at 37 DEG C, preparing an annular template and then carrying out RCA reaction; mixing, boiling and degenerating 10 muL of connection product and universal primer, respectively adding dNTP, phi29DNA polymerase, HhaI restriction enzyme and buffer solution to form 20 muL of reaction system, stewing for 60 minutes at 37 DEG C; finally detecting single-chain DNA product of RCA with a specific tag sequence, thus obtaining a corresponding test conclusion according to the result.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Forward and reverse ABO typing and RhD blood type detecting card
ActiveCN104569444AGuaranteed specificityGuaranteed FeaturesBiological testingIonic strengthMicro column
The invention discloses a forward and reverse ABO typing and RhD blood type detecting card comprising a detecting card with six micro-columns, wherein the first three micro-columns in the six micro-columns are respectively filled with equivalent A-resistant gel, B-resistant gel and D-resistant gel, and the left three micro-columns are filled with equivalent blank gel. The forward and reverse ABO typing and RhD blood type detecting card is prepared by using the following method comprising the steps of swelling dextran gels; preparing all antibody diluents; preparing all antibody working solutions; washing the gels; and subpackaging to form the RhD blood type detecting card. According to the forward and reverse ABO typing and RhD blood type detecting card, crosslinked dextran gels with different sizes and sieving ranges are mixed and swelled by using purified water, so that the specificity is effectively ensured, meanwhile, the forward and reverse ABO typing and RhD blood type detecting card has very high sensitivity, and the ion strength of a system is effectively ensured.
Owner:合肥天一生物技术研究所有限责任公司
Human body intestinal canal flora detection parting and quantitative reagent kit
InactiveCN101509034AGuaranteed specificityEnabling multiple typing quantificationMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceBioinformatics
The invention relates to a method for detecting, genotyping and quantifying human intestinal flora, consisting of universal primer PCR amplification and specific probe LDR. In the PCR amplification, the 16sr DNA fragment of selected bacteria genus is amplified by the designed universal primer synchronously; the specific LDR probe of each bacteria genus is utilized to achieve the goal of genotyping through ligation reaction; and LDR signal is detected by ABI Prism 377 type sequencer, and the detected fluorescence value is taken as the quantifying basis. Aiming at the universal primer of intestinal microflora, the detection method of the invention effectively amplifies all target intestinal microflora 16sr DNA sequence, thereby ensuring that each target sequence and the template of low copy therein can be detected, further ensuring the specificity of the specific probe of each bacteria genus, completely identifying each target sequence and realizing multiplex genotyping quantification.
Owner:DONGHUA UNIV
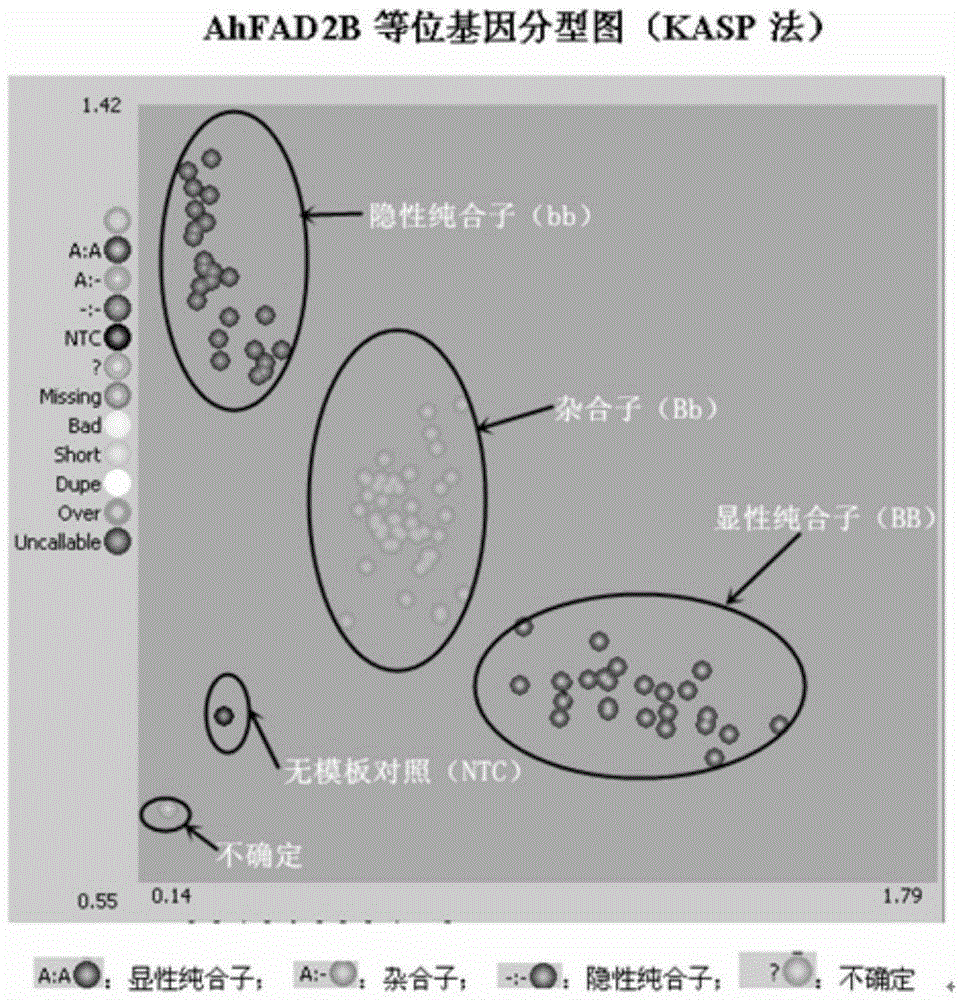
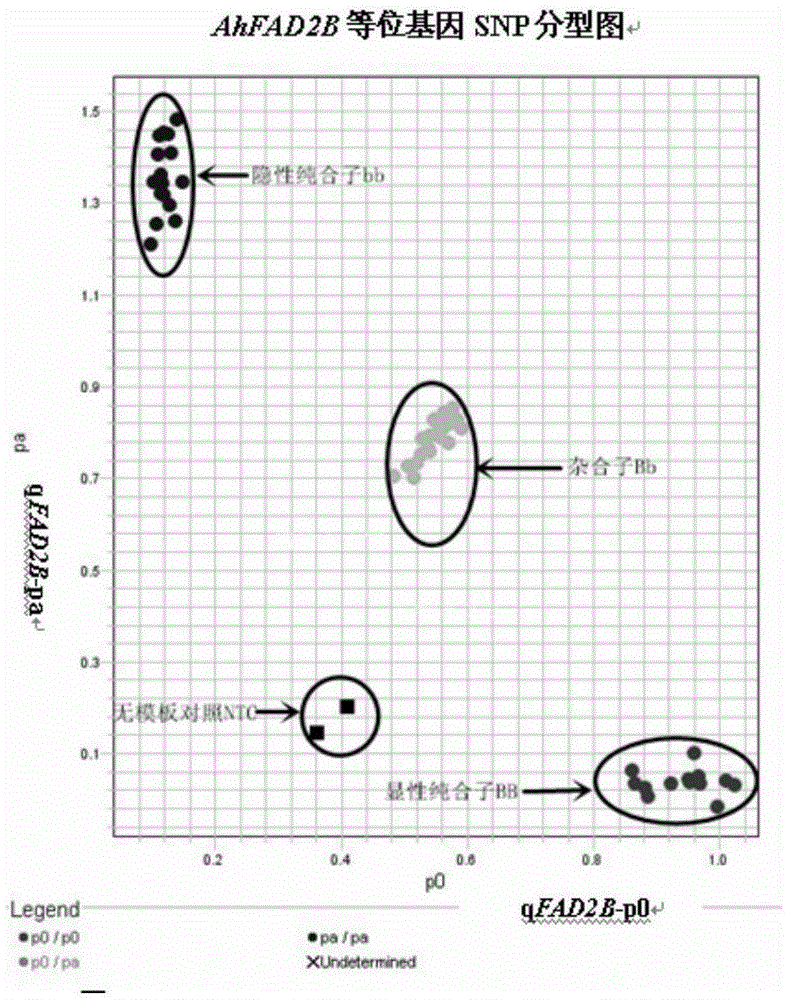
Primers and method for detecting SNP gene typing of AhFAD2B genes of peanuts in high throughput
InactiveCN105385778AGuaranteed accuracyGuaranteed specificityMicrobiological testing/measurementDNA/RNA fragmentationBiologySNP genotyping
The invention relates to primers and a method for detecting SNP gene typing of AhFAD2B genes of peanuts in high throughput. The primers comprise the specific primer of which the nucleotide sequence is shown as SEQ ID No.1 and SEQ ID No.2 and a universal prime of which the nucleotide sequence is shown as SEQ ID No.3. According to the primers and method, the allelic variation condition of the AhFAD2B genes can be detected and identified in high throughput, the maximum sample quantity of one-time detection can reach 1536, the detection cost is low, rapidness and accuracy are achieved, and the efficiency and accuracy of peanut breeding selection are greatly improved.
Owner:BIOTECH RES CENT SHANDONG ACADEMY OF AGRI SCI
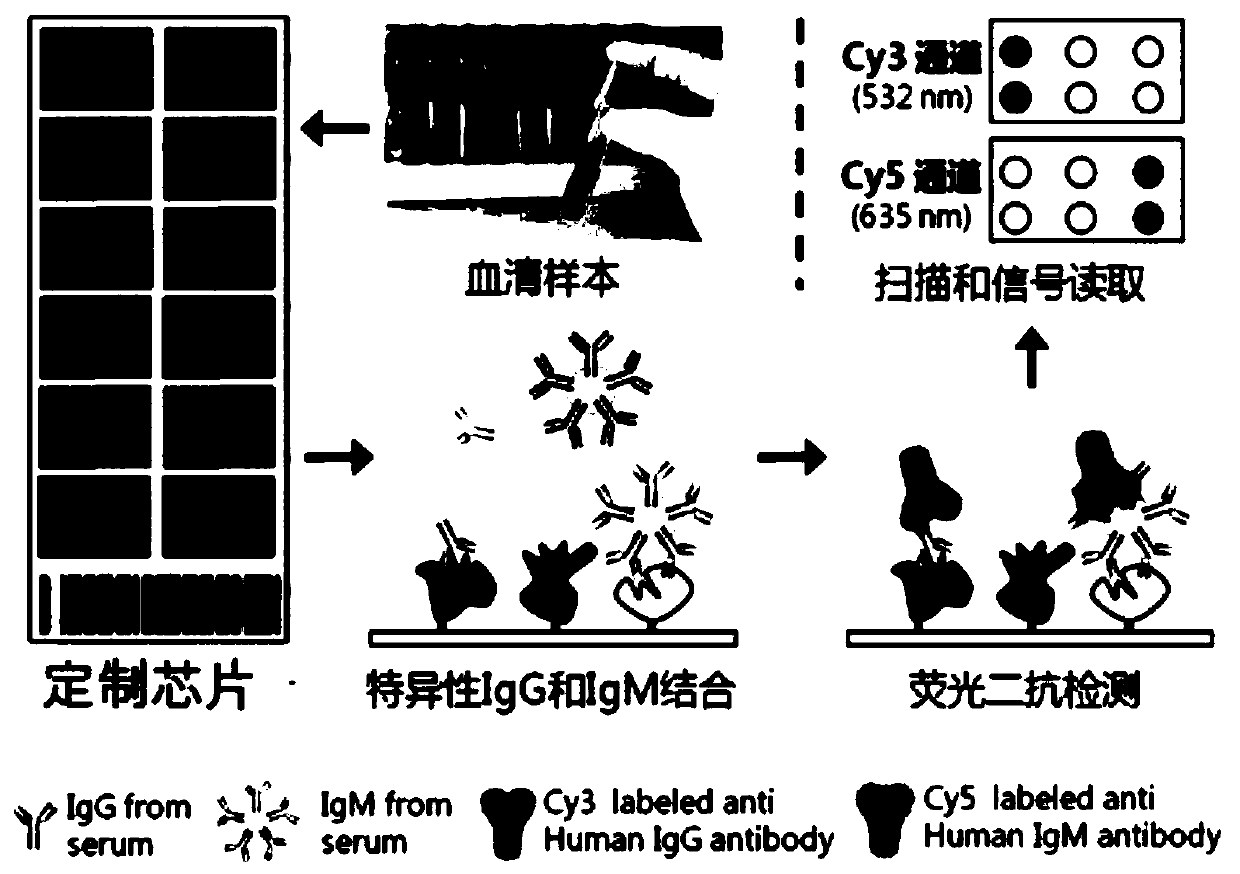
Serum protein marker for early screening and diagnosis of esophageal squamous carcinoma, kit and detection method
ActiveCN110716044AGuaranteed specificityGood reference valueColor/spectral properties measurementsOncologyBiomedicine
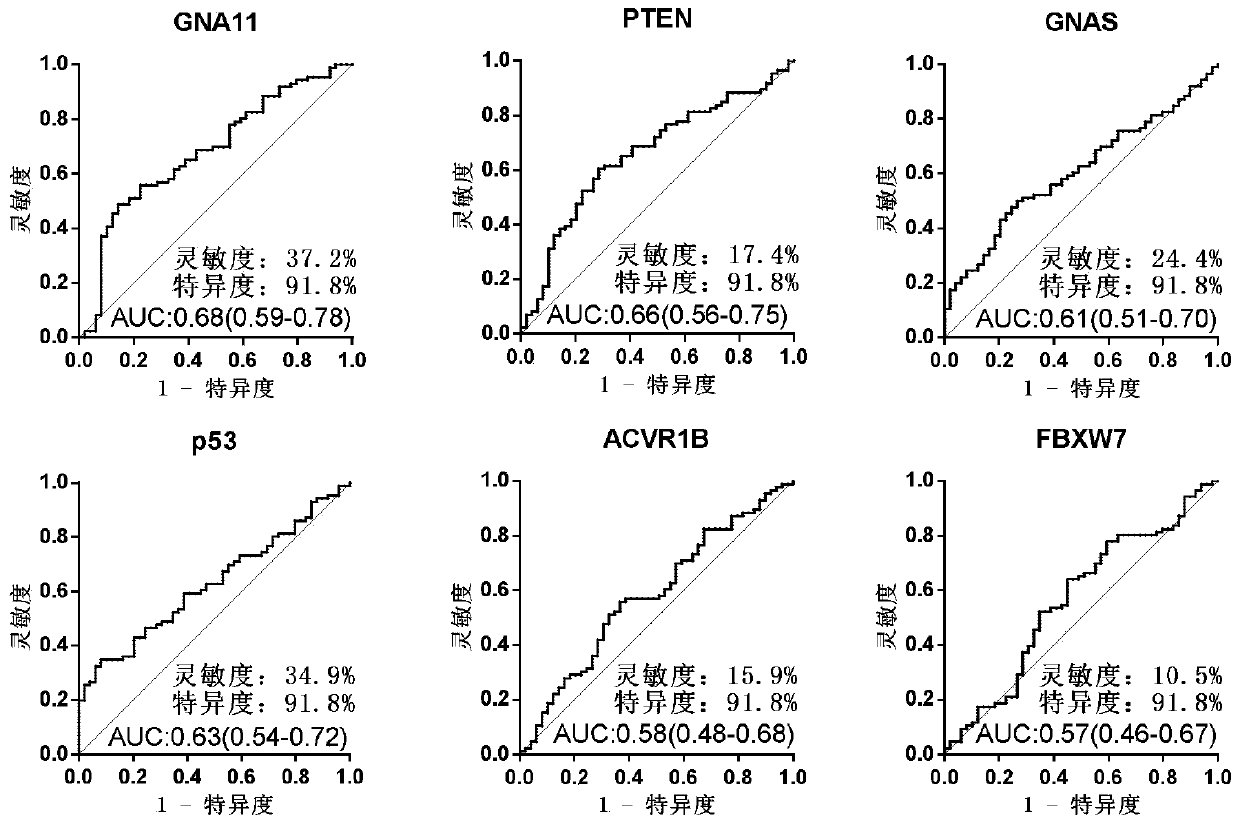
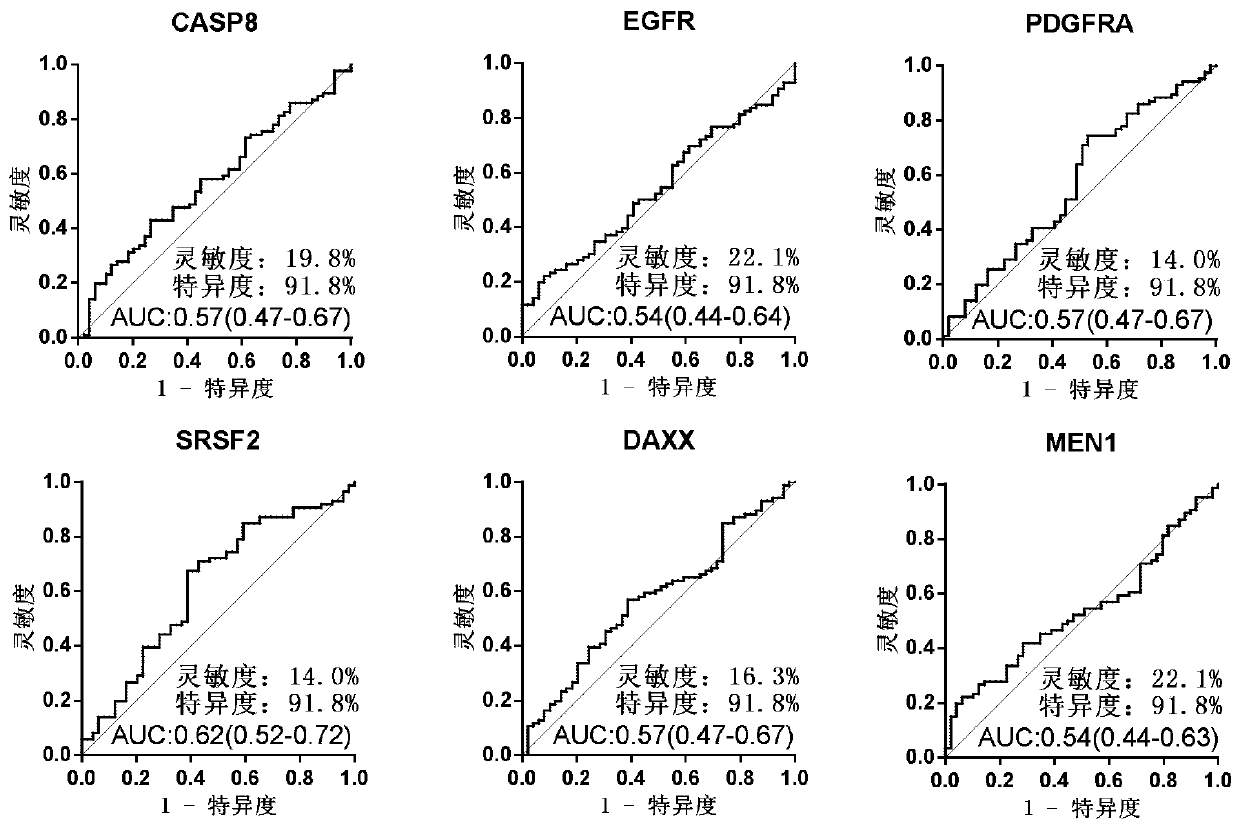
The invention discloses a serum protein marker for early screening and diagnosis of esophageal squamous carcinoma, and belongs to the field of biomedical technology. The serum protein marker is any one or a combination of two or more of proteins encoded by P53, GNA11, GNAS, PTEN, ACVR1B, FBXW7, EGFR, PDGFRA, SRSF2, MEN1, DAXX or CASP8 genes. Based on the role played by cancer driving genes in tumorigenesis and development, a human protein chip encoded by 138 cancer driving genes is customized, the human protein chip contains 180 human-derived recombinant proteins for screening out potential markers capable of diagnosing caners or other markers capable of characterizing cancers, early detection serum markers of the esophageal squamous carcinoma are primarily screened out by the protein chip, and then are verified by an ELISA indirect method experiment, a group of joint serum protein markers of esophageal squamous carcinoma for the early screening and diagnosis of the esophageal squamouscarcinoma is screened out at last for assisting the clinical diagnosis of the esophageal squamous carcinoma, so that the serum protein marker has better reference value.
Owner:ZHENGZHOU UNIV
Detection primer for EML4-ALK (anaplastic lymphoma kinase) fused gene mutation, probe and detection kit
InactiveCN106978497AImprove design requirementsLess componentsMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceQuality control system
The invention relates to the fields of biotechnologies and medicines, particularly discloses a detection primer for EML4-ALK (anaplastic lymphoma kinase) fused gene mutation, a probe and a detection kit, and aims to detect the EML4-ALK fused gene mutation. The detection primer comprises sequences as shown in SEQ ID NO: 1 to SEQ ID NO: 14. The sequence of the probe is as shown in SEQ ID NO: 15, of which the end 5' is modified with FAM or VIC (vasoactive intestinal contractor), and the end 3' is modified with NFQ-MGB (non-fluorescent quencher-myohemoglobin). The kit comprises the detection primer, the probe and a quality control system. The detection primer can specifically identify 13 EML4-ALK fusion mutation types, is high in sensitivity and can detect 5-copied mutations; the whole reverse transcription PCR (polymerase chain reaction) and fluorescence PCR detection process can be completed only by 80 minutes.
Owner:WUHAN YZY MEDICAL SCI & TECH
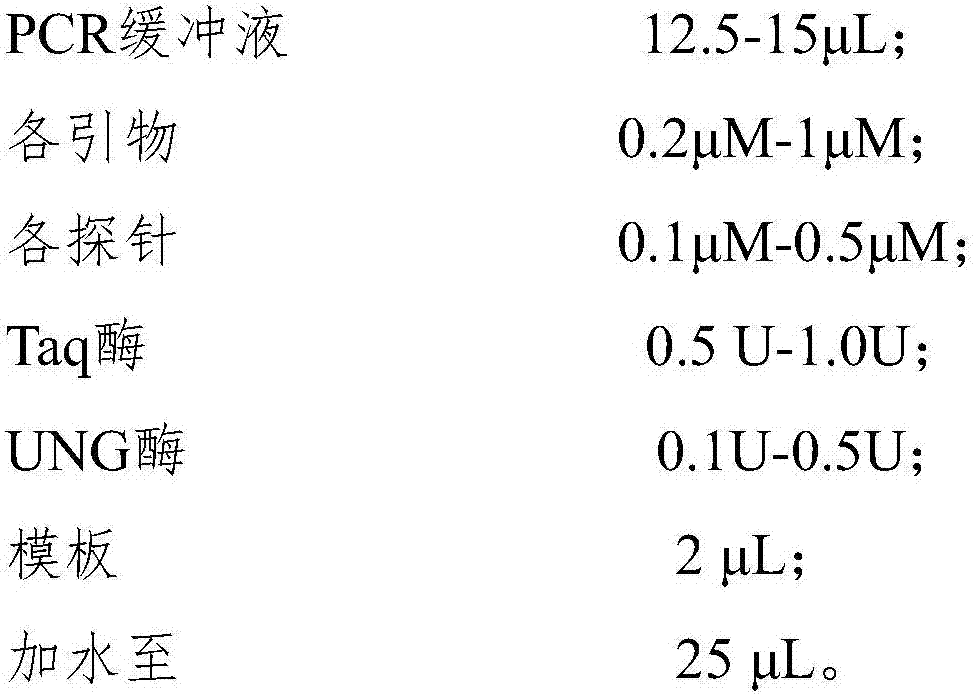
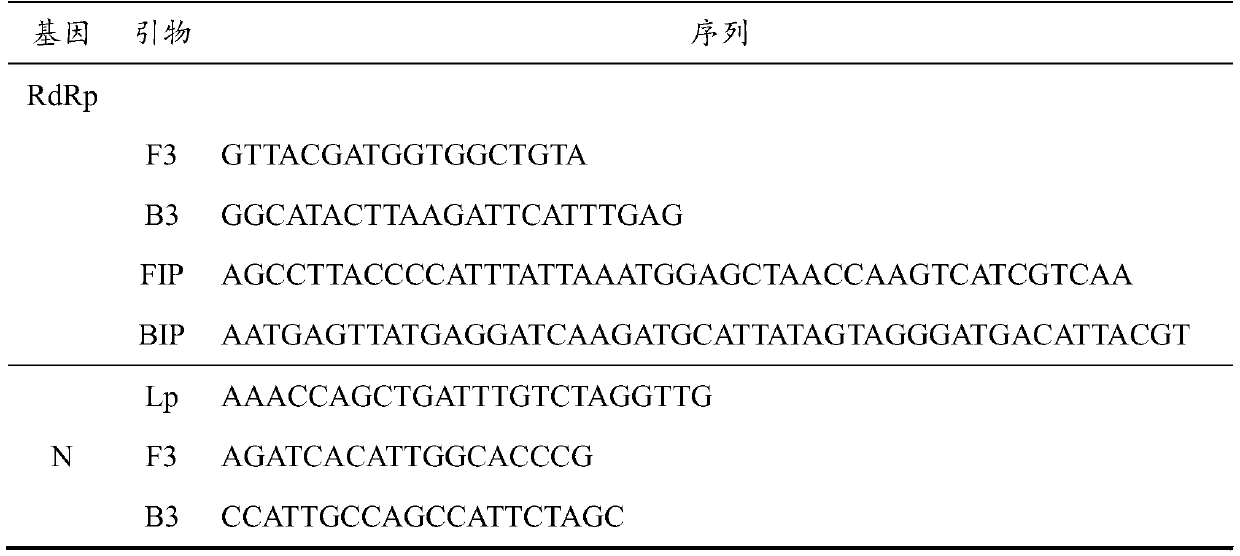
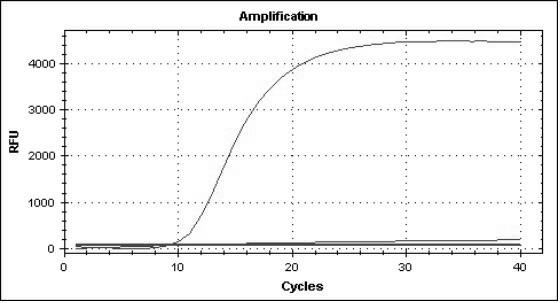
Loop-mediated isothermal amplification detection primer set, reagent kit and method for SARS-CoV-2
PendingCN111321249AStrong specificityRapid responseMicrobiological testing/measurementAgainst vector-borne diseasesVirologyBioinformatics
The invention provides a loop-mediated isothermal amplification detection primer set, reagent kit and method for SARS-CoV-2, and belongs to the technical field of medical science and molecular biologydiagnosis. Based on stability and uniqueness of RdRp, E and N genes in the SARS-COV-2, three primer sets are designed to perform amplification detection on the gene, the primer concentration is reduced, the reaction specificity is improved, and the false positive is reduced. Therefore, the detection primer set, reagent kit and method, provided by the invention are high in detection speed, high insensitivity and high in specificity, and have favorable practical application value.
Owner:JINAN CENTER HOSPITAL
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com