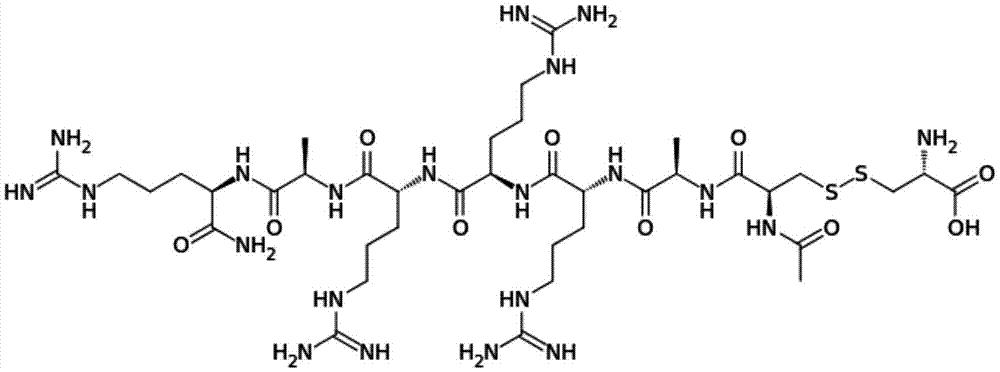
Method for synthesizing Etelcalcetide
A liquid-phase synthesis and l-cys technology, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problem of easy removal of guanidinium groups, difficult purification, and inability to obtain ideal yield and purity of final products and other problems, to avoid low conversion rate, less impurities, and facilitate large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Fmoc-D-Arg(Pbf)-NH 2 Synthesis
[0044] 1. The inventive method
[0045] Fmoc-D-Arg(Pbf)-OH (130g, 200mmol) was added to 1000ml of dichloromethane, cooled in an ice-water bath to below 10°C, added isopropyl chloroformate (27g, 220mmol, 1.1equ.), stirred After 5 minutes, DIPEA (31 g, 242 mmol, 1.21 equ.) was slowly added dropwise, and the resulting mixture was stirred while keeping the system temperature below 10° C., and the reaction was monitored by TLC until the raw material point disappeared.
[0046] 200 milliliters of ammonia water was slowly added to the above reaction solution, keeping the reaction temperature below 20° C., continued to stir for 2 hours, separated the liquid, washed the DCM layer twice, dried over anhydrous sodium sulfate, and obtained 115 g of a white solid after removing the solvent (yield 88.5%), and the HPLC purity was 95.2%.
[0047] 2. Comparison method
[0048] Fmoc-D-Arg(Pbf)-OH (130g, 200mmol) was added to 1000ml of dichlo...
Embodiment 2
[0051] Example 2: Fmoc-D-Ala-D-Arg(Pbf)-NH 2 Synthesis
[0052] Fmoc-D-Ala-OH (4.67g, 15mmol) was added to 100ml of dichloromethane, stirred for 10 minutes, cooled to below 10°C in an ice-water bath, and isopropyl chloroformate (2.0g, 16.5mmol, 1.1 equ.), after stirring for 5 minutes, slowly added DIPEA (2.33g, 18.1mmol, 1.21equ.), the resulting mixture kept the temperature of the system below 10°C, stirred, and TLC monitored the reaction until the raw material point disappeared.
[0053] Fmoc-D-Arg(Pbf)-NH 2 Remove Fmoc protecting group, H-D-Arg(Pbf)-NH 2 (7.0g, 16.5mmol) was dissolved by adding dichloromethane (50ml), and then added to the above reaction solution, keeping the temperature of the system below 10°C and continuing to stir for 10 hours until the starting point disappeared (TLC).
[0054] The reaction mixture solution was washed once with saturated sodium bicarbonate solution, washed twice with water, dried over anhydrous sodium sulfate, and 11.1 g of a white s...
Embodiment 3
[0061] Example 3: Fmoc-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-NH 2 Synthesis
[0062] Fmoc-D-Ala-R-Arg(Pbf)-NH 2 (8.9g 12mmol) was added in dichloromethane (200 milliliters), after stirring for 5 minutes, piperidine (5.1g, 60mmol) was added dropwise, and the reaction solution continued to stir until Fmoc-D-Ala-R-Arg(Pbf)- NH 2 Completely gone (TLC). Wash with dilute hydrochloric acid until the dichloromethane solution is neutral, separate the layers, dry the dichloromethane solution, and remove the solvent to obtain the oil H-D-Ala-D-Arg(Pbf)-NH 2 (5.3g, yield 85.8%), the oil was treated with ethyl acetate / n-hexane to obtain pure product.
[0063] Fmoc-D-Arg(Pbf)-OH (6.5g, 10mmol) was added to 100ml of N,N-dimethylformamide, cooled in an ice-water bath to below 10°C, and isopropyl chloroformate (1.34g, 11mmol, 1.1equ.), after stirring for 5 minutes, slowly added DIPEA (1.56g, 12.1mmol, 1.21equ.), the resulting mixture kept the system temperature below 10°C, stirred, and TLC monitore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com