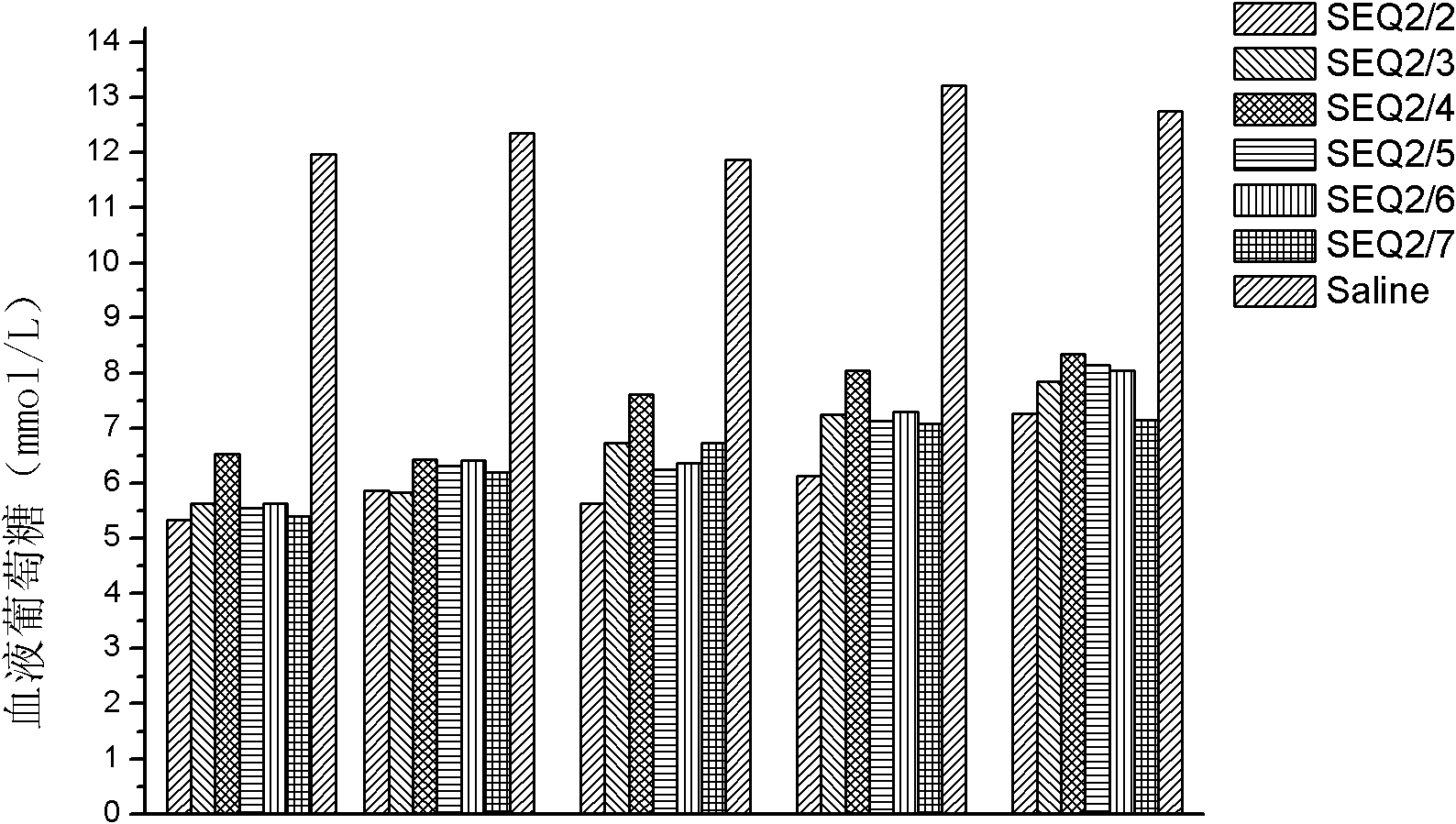
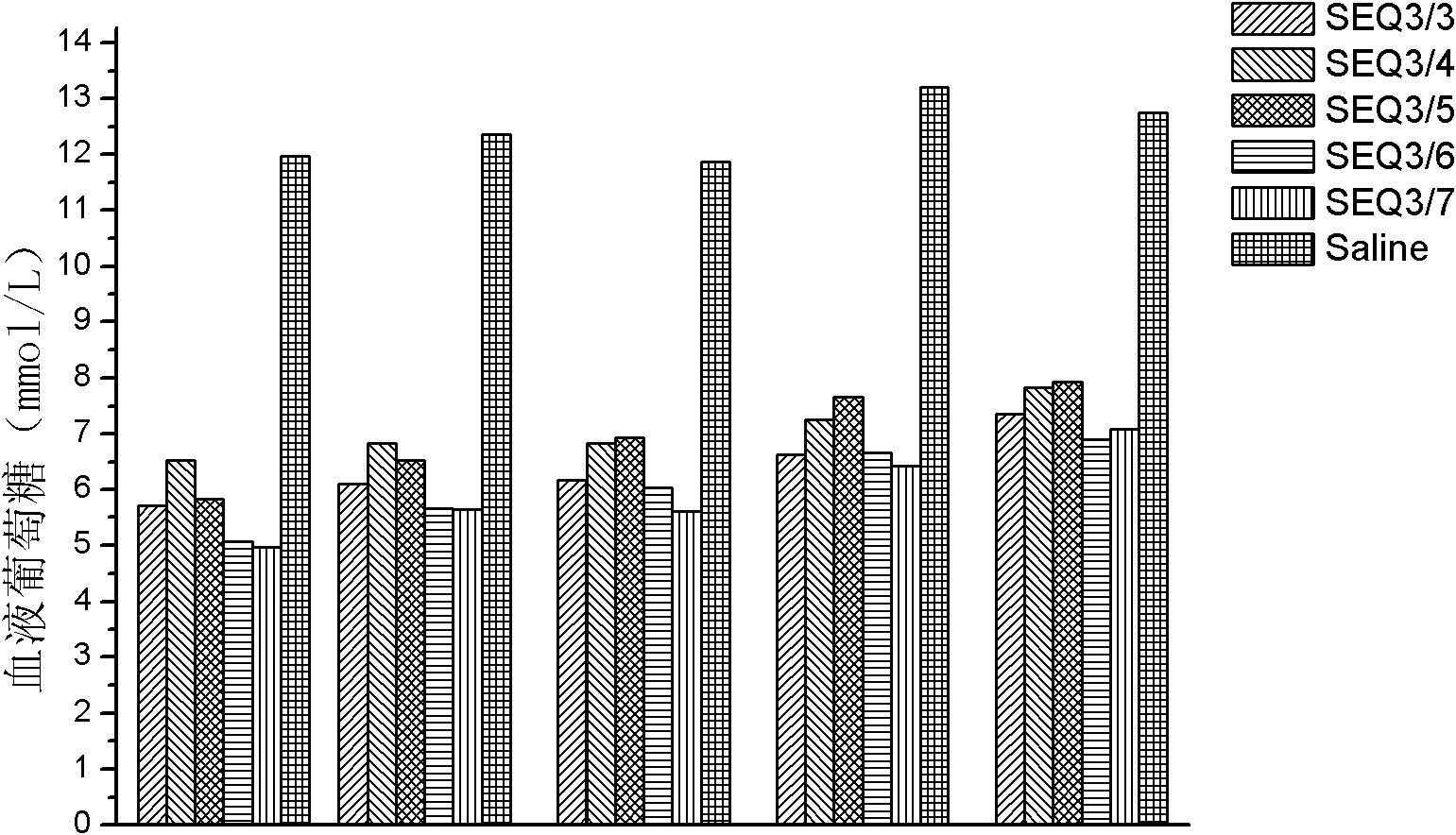
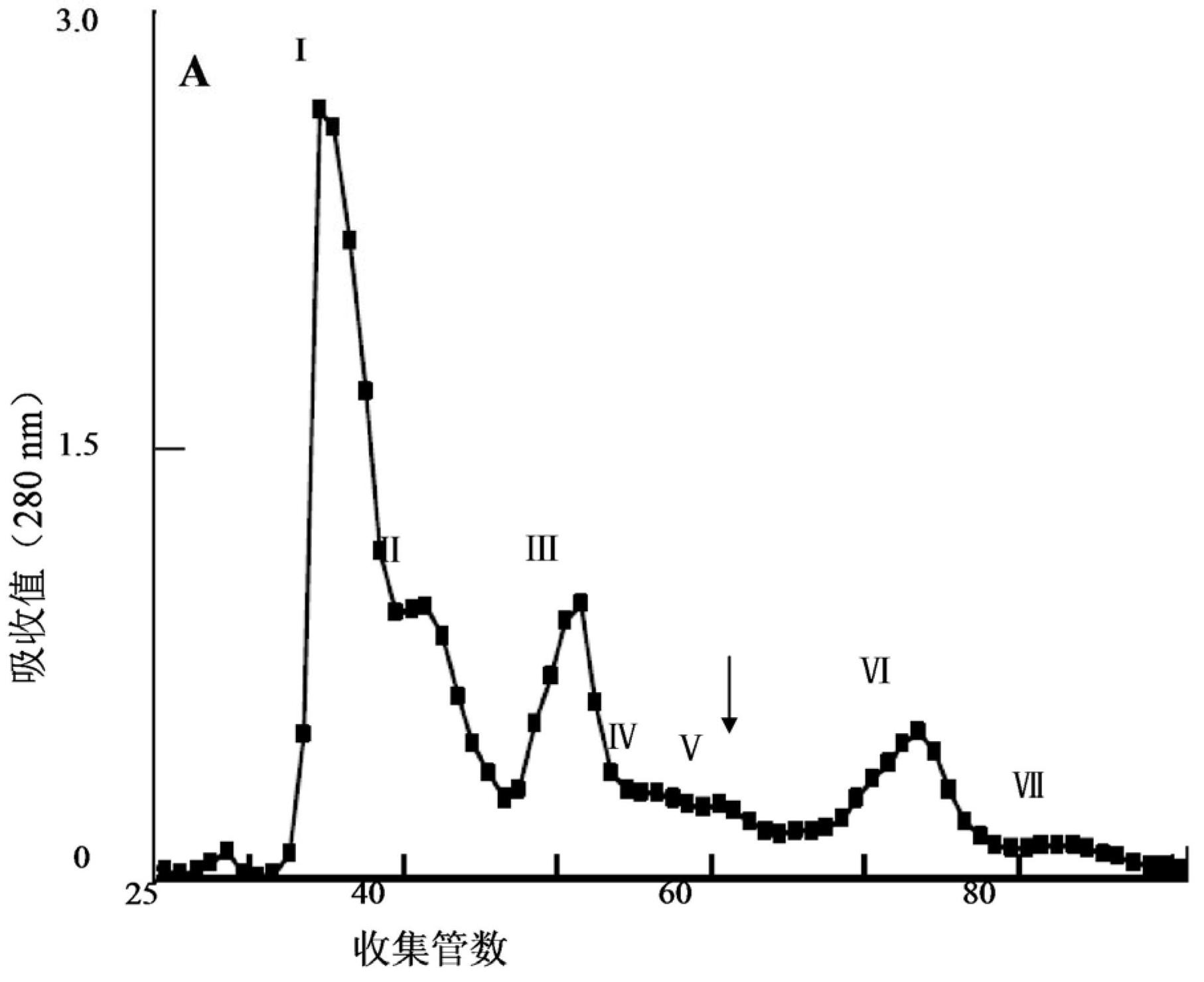
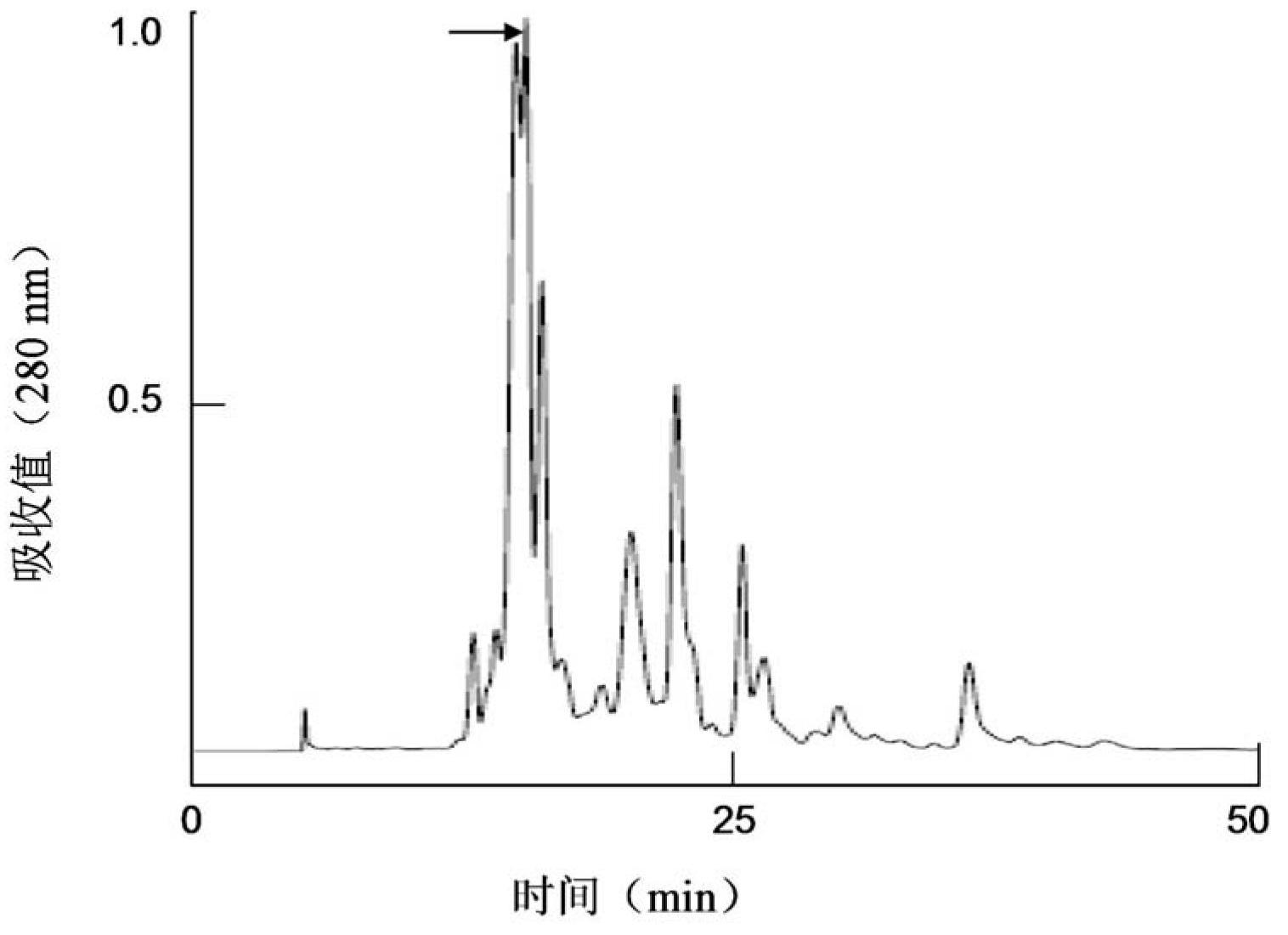
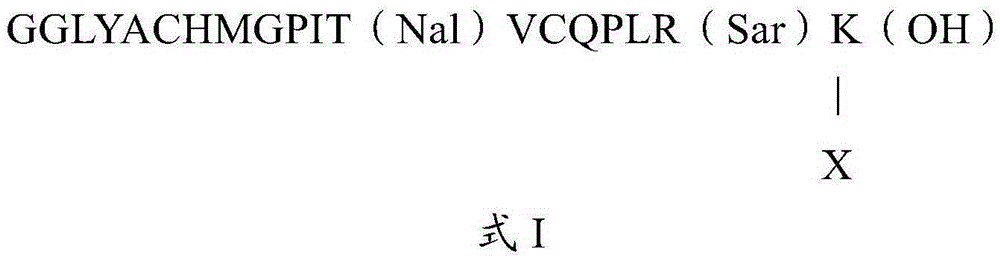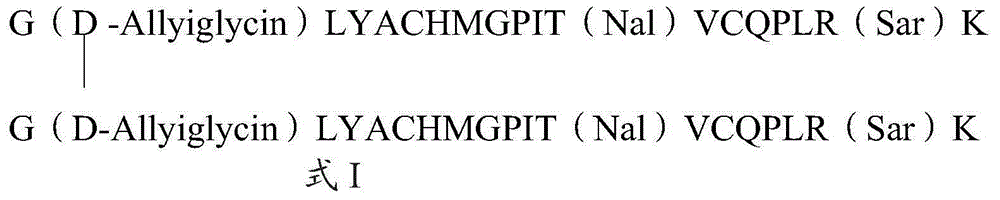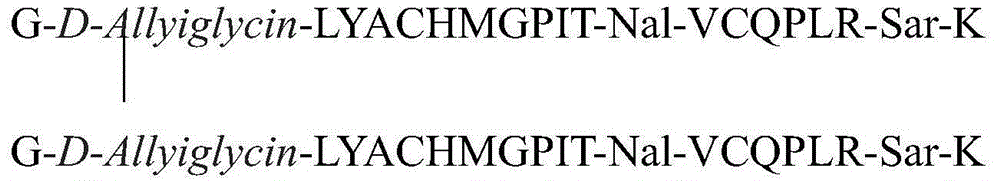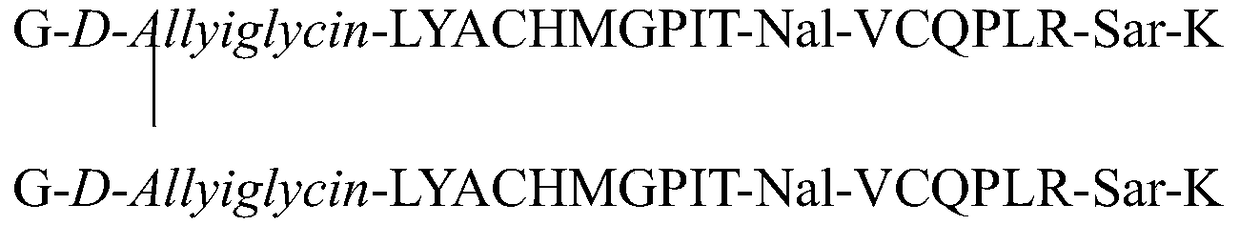Patents
Literature
56 results about "Cysteine formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
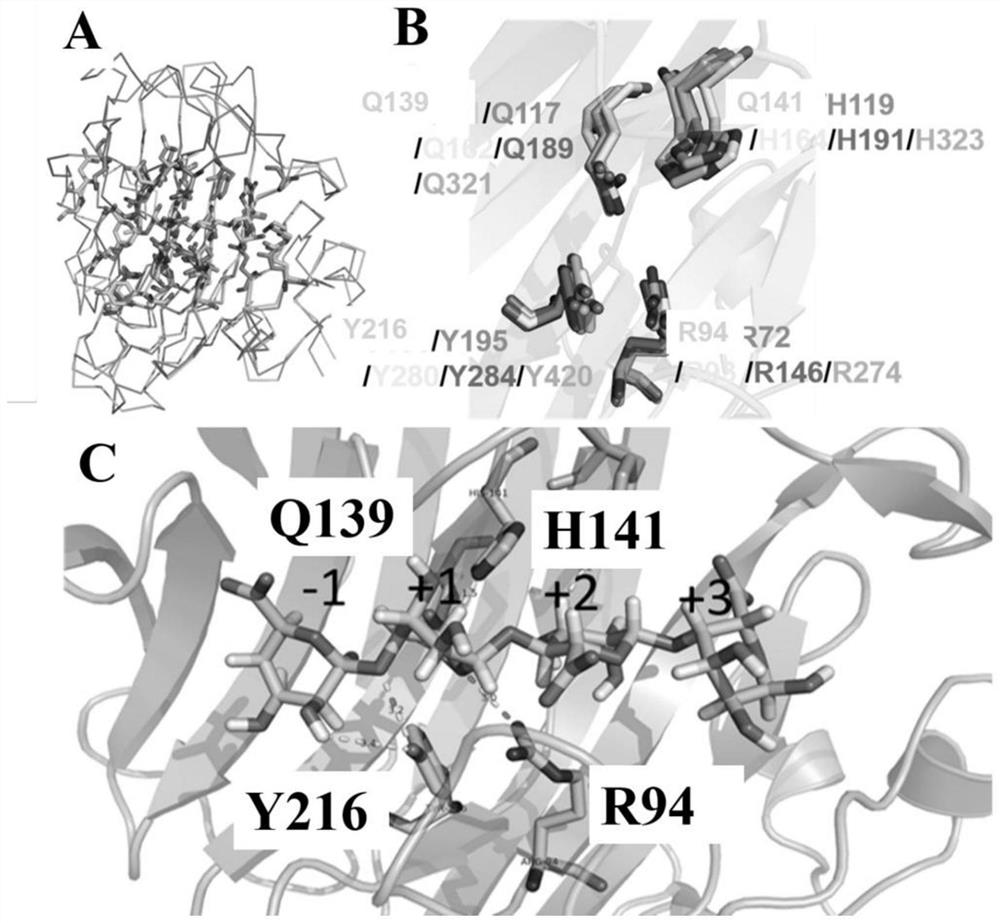
Glucagon-like peptide-1 analogue monomer and dimer, preparation method therefor and application thereof
InactiveUS20150232527A1Conducive to clinical promotion and applicationPeptide/protein ingredientsMetabolism disorderHalf-lifeGlucagon-like peptide-1
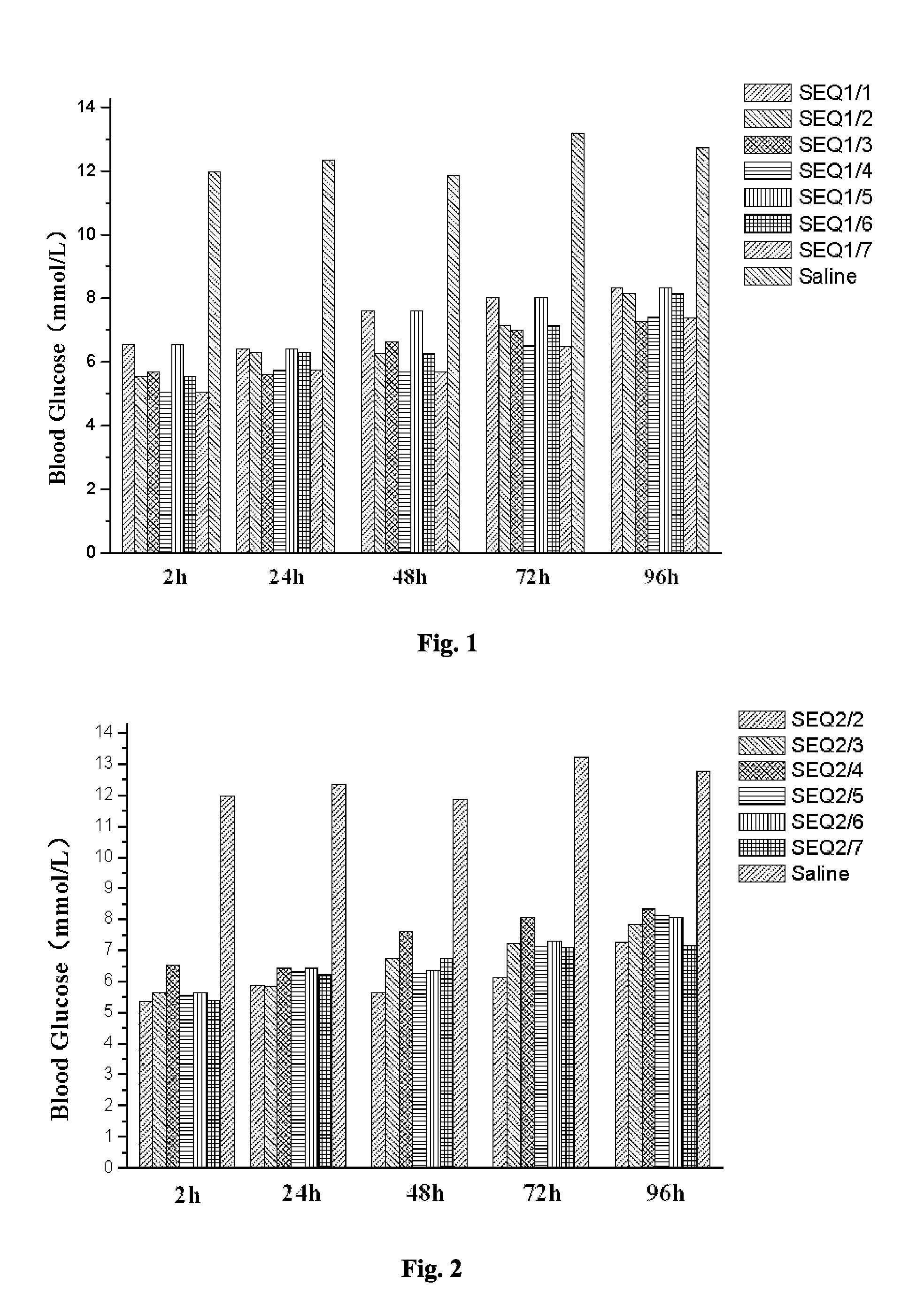
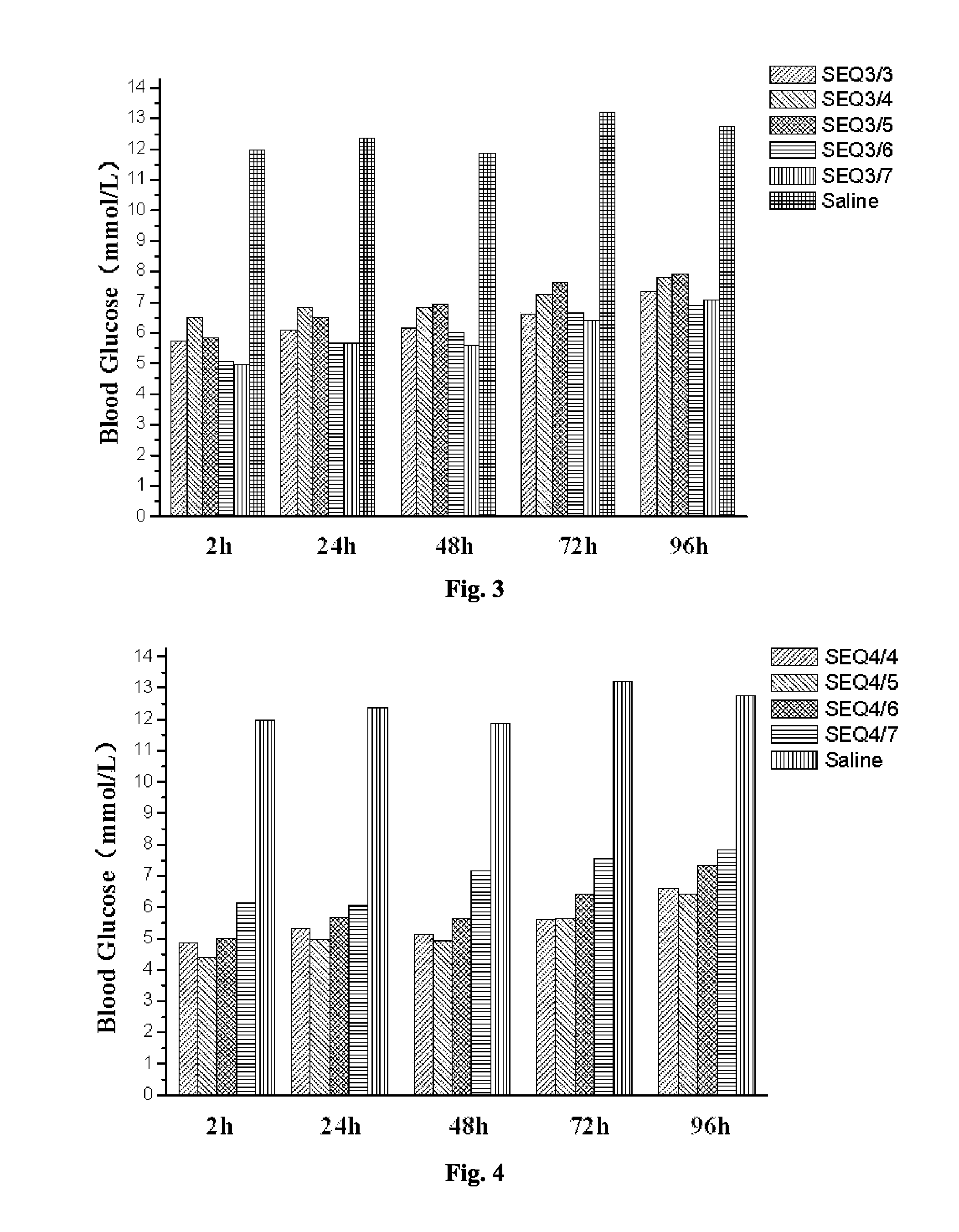
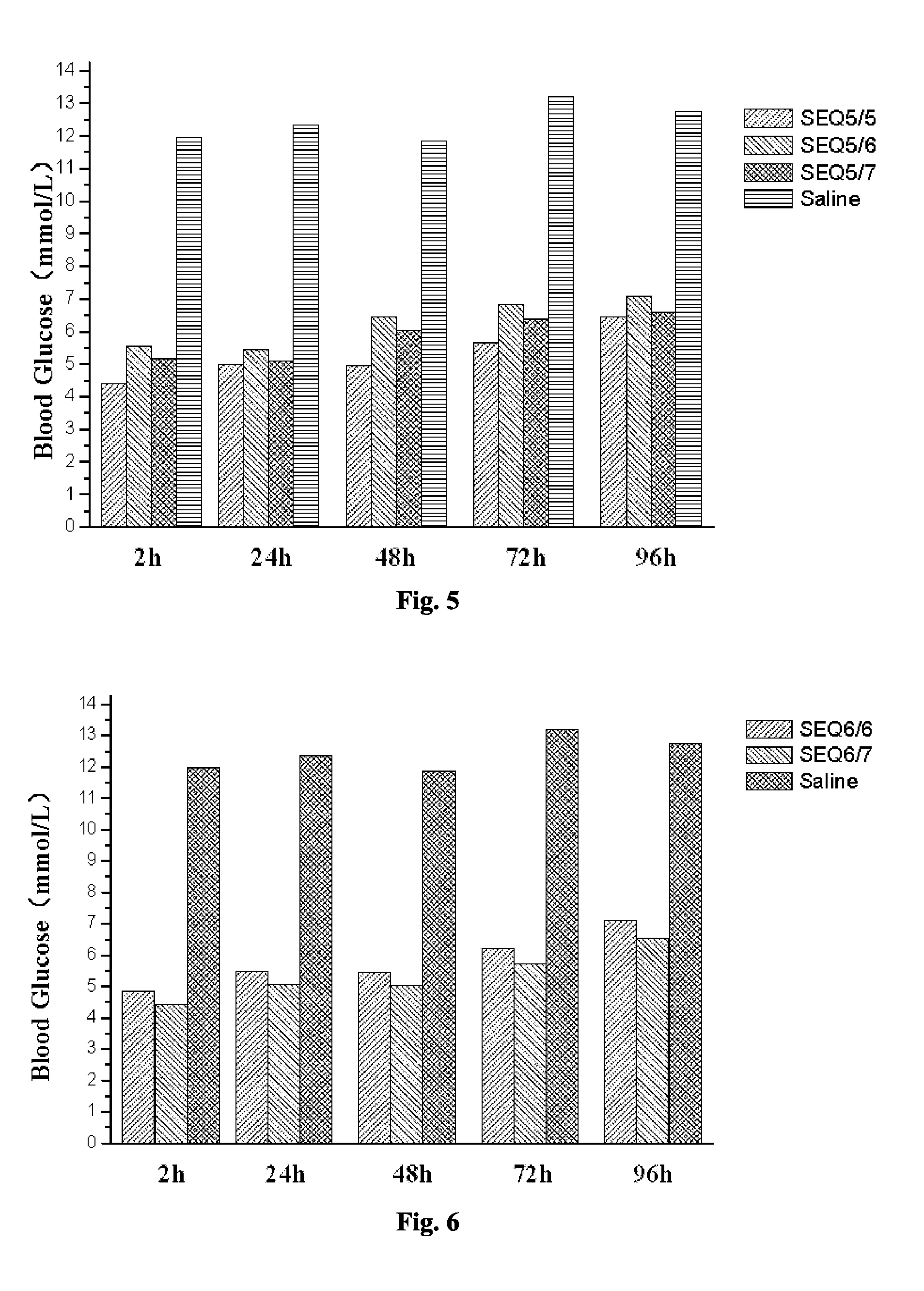
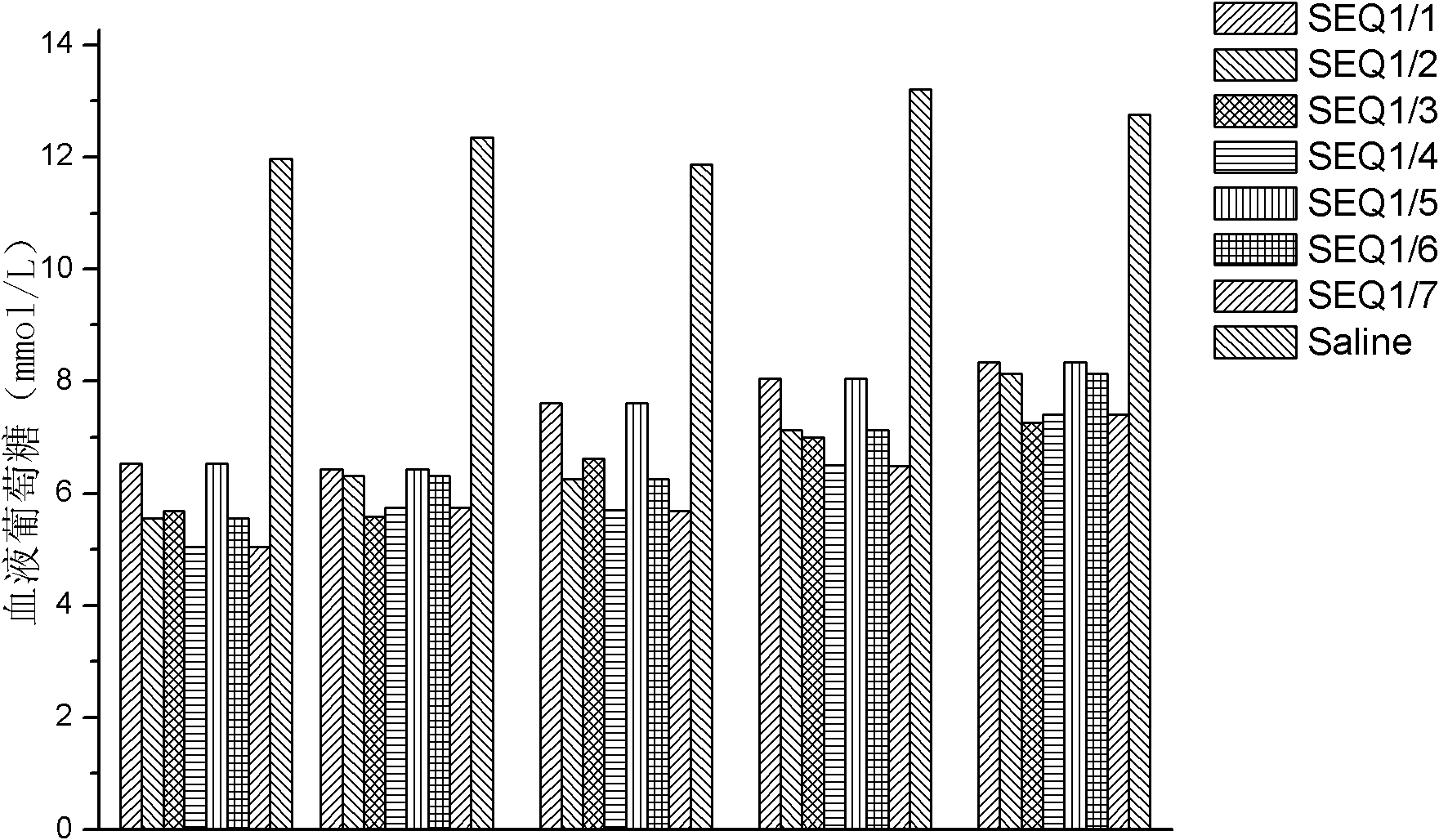
Provided are a glucagon-like peptide-1 (GLP-1) analogue monomer and dimmer, a preparation method thereof, and an application thereof. The GLP-1 analogue monomer comprises one cysteine; and the dimer is formed by two monomer molecules connected via an intermolecular disulfide bond formed by the cysteine. The GLP-1 monomer comprising cysteine has the following general formula: 7HAEX10TFTSX15VSSYLEX22X23AAKEFIX30WLX33KGRG37, wherein X10 is glycine or cysteine, X15 is aspartate or cysteine, X22 is glycine or cysteine, X23 is glutamine or cysteine, X30 is alanine or cysteine, and X33 is valine or cysteine; and only one of X10, X15, X22, X23, X30, and X33 is cysteine. The glucagon-like peptide-1 analogue dimer of the present invention has an in vivo half-life of more than 8 to 96 hours, thus facilitating clinical promotion and application.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Glucagon-like peptide-1 (GLP-1) analogue monomer and dimer, preparation method therefor and application thereof
ActiveCN102718858AOvercoming the problem of short half-lifeExtended half-lifePeptide/protein ingredientsMetabolism disorderHalf-lifeGlucagon-like peptide-1
The invention provides a GLP-1 dimer, a preparation method therefore and an application thereof. The GLP-1 dimer is formed by two GLP-1 monomers containing cysteine connected via a disulfide bond formed by cysteine. The general formula of the GLP-1 monomer containing cysteine is 7HAEX10T FTSX15V SSYLE X22X23AAKEFIX30W LX33KGR G37; wherein X10 is aglycine or cysteine, X15 is aspartic acid or cysteine, X22 is glycine or cysteine, X23 is leucine or cysteine, X30 is alanine or cysteine, X33 is valine or cysteine, and only one among X10, X15, X22, X23, X30 and X33 is cysteine. The GLP-1 dimer in the invention has an in vivo half-life of more than 8 to 96 hours, thus greatly facilitating clinical promotion and application.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Application of scolopendra mutilans neurotoxin peptide omega-SLPTX-Ssmla
InactiveCN102671185AEnhancement effect is goodNervous disorderPeptide/protein ingredientsDiseaseSequence signal
The invention relates to application of scolopendra mutilans neurotoxin peptide omega-SLPTX-Ssmla, belonging to the field of biomedicine. A primary sequence of the omega-SLPTX-Ssmla comprises 83 amino acid residues, wherein three pairs of disulfide bonds are formed by six aminothiopropionic acids, and the molecular weight is 8811.3 Da. The code of the neurotoxin gene comprises 426 nucleotides of which include signal peptides, premise peptides and mature peptides. The scolopendra mutilans neurotoxin omega-SLPTX-Ssml has a quite obvious enhancement effect on calcium ion channel current on a dorsal ganglion root and is a novel calcium channel agonist. The scolopendra mutilans neurotoxin omega-SLPTX-Ssm provided by the invention can be used for increasing the openness of the calcium channel to enable calcium ions to flow internally and preparing medicaments for treating calcium channel diseases and can also be used as a new tool reagent for calcium channel researches.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Peptides as well as pharmaceutical composition and application thereof
ActiveCN106632603AAnalgesic effect hasPeptide/protein ingredientsAntipyreticCystine knotPharmacophore
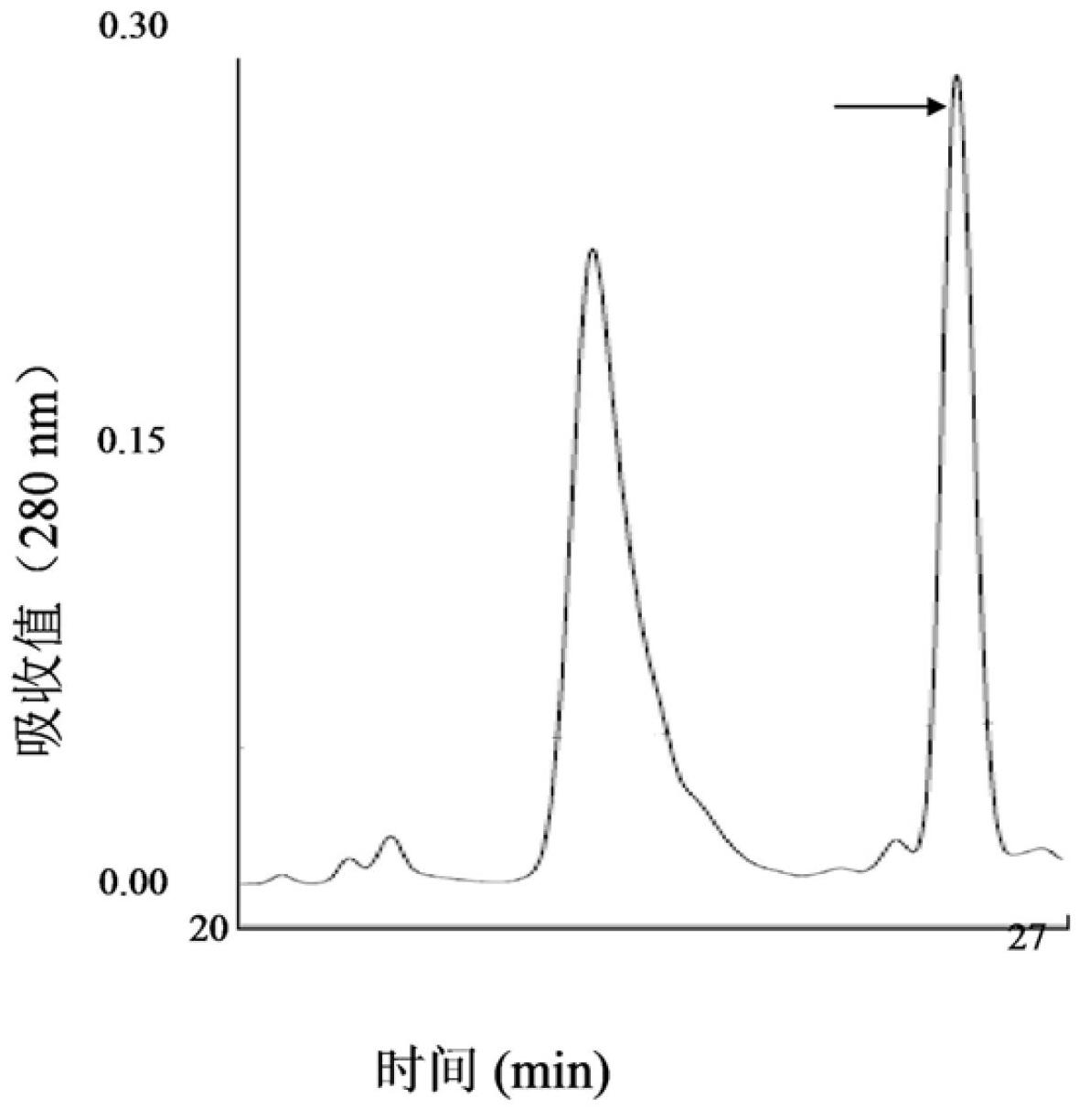
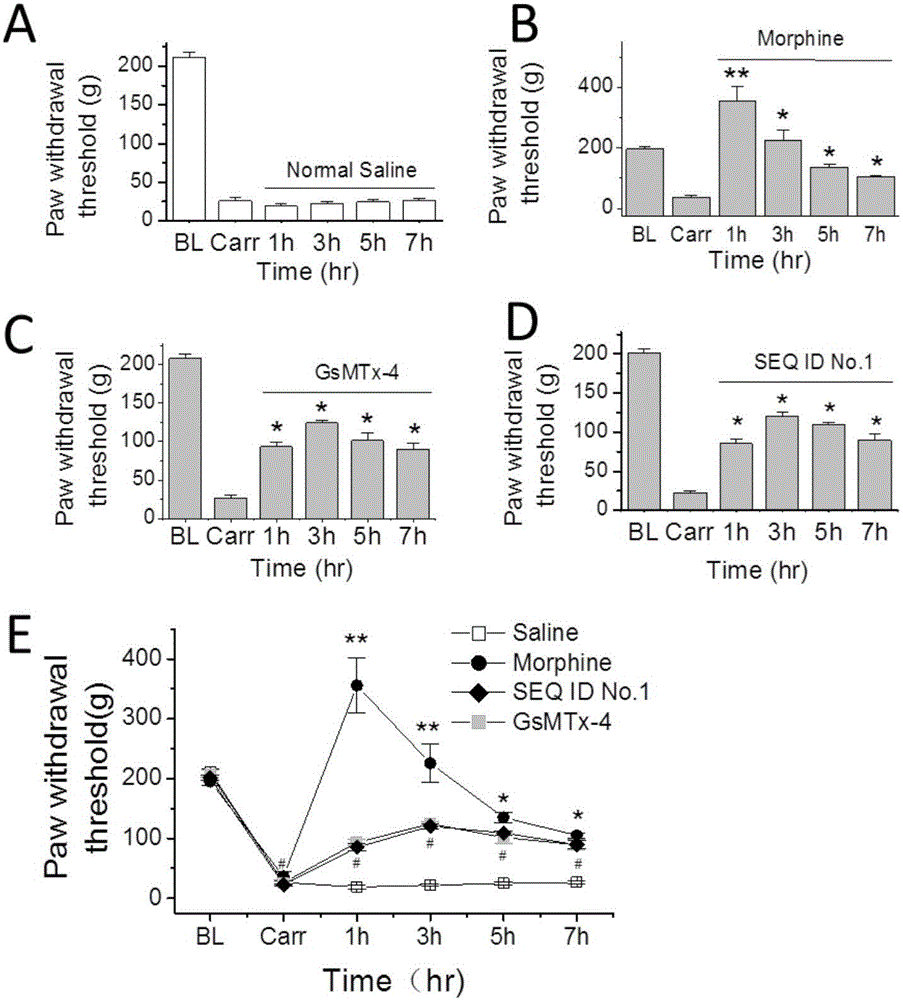
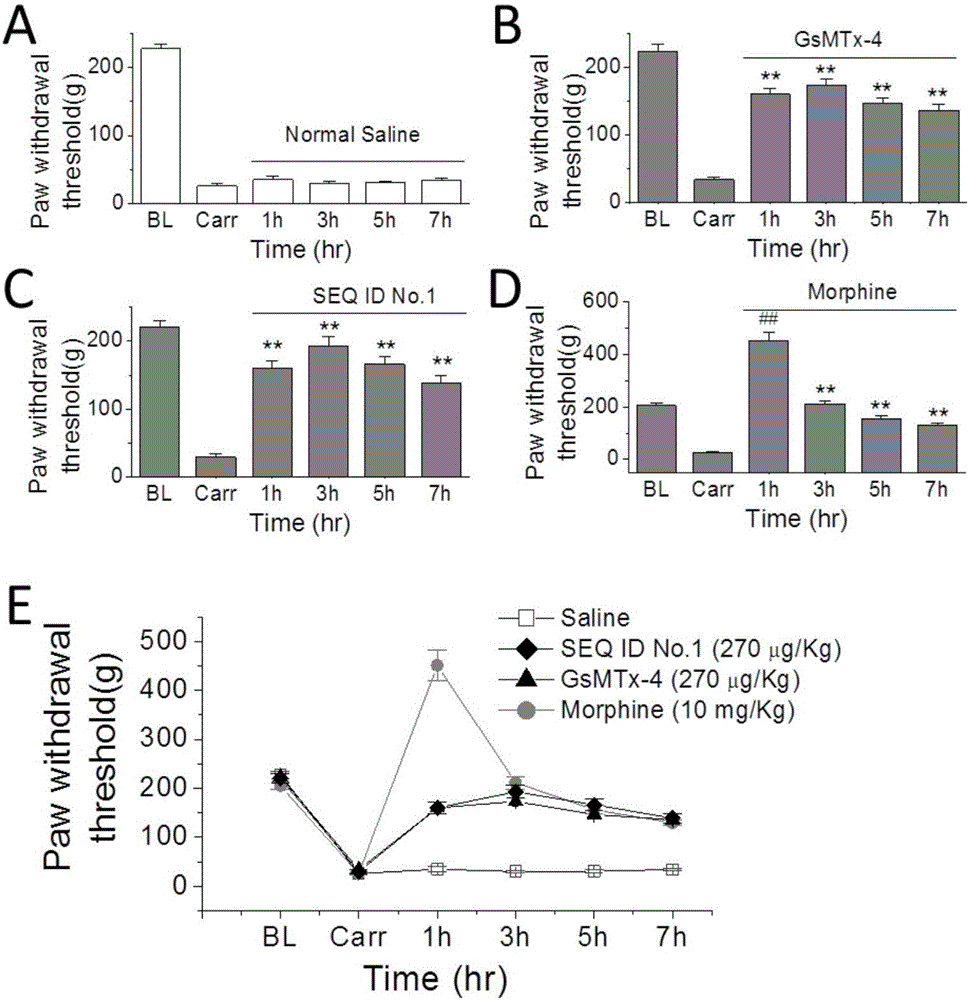
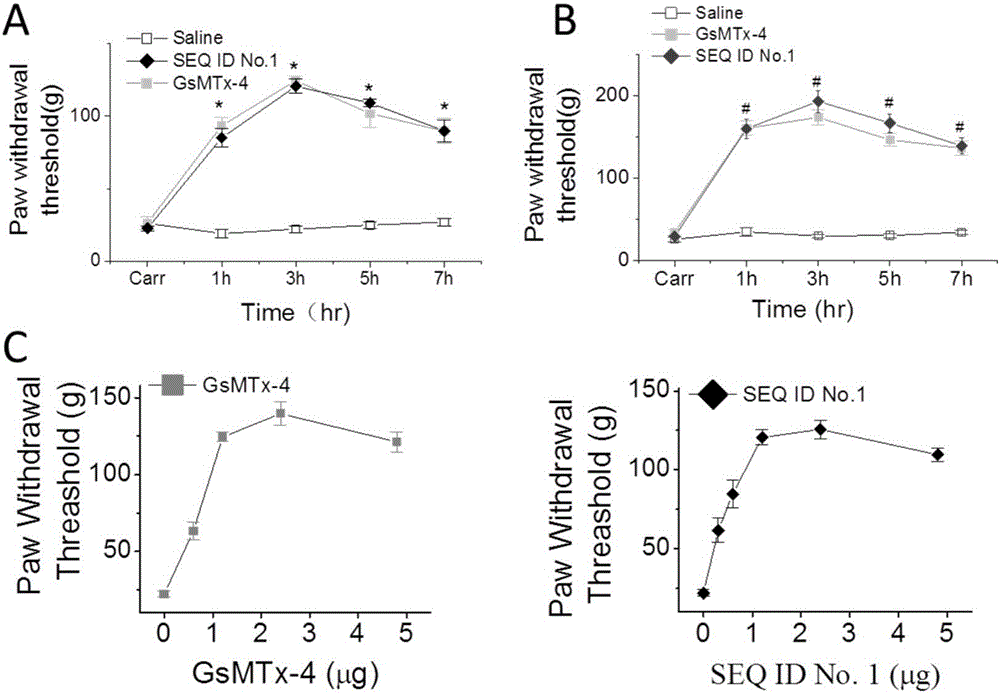
The invention provides a group of peptides as well as a pharmaceutical composition and application thereof. The peptides are as shown in sequence tables; the peptides have amino acid sequences with an analgesic effect, or are pharmacologically acceptable salts of the peptides. A ring is formed by two cysteines at the head end and the tail end of each peptide. A pharmacophore (a structural domain) which has abirritation in the GsMTx-4 peptide is determined by comparing multiple inhibitor cystein knot (ICK)-containing peptide sequences such as GsMTx-4 and GsMTX-2 and carrying out behavior test on a rat pain model.
Owner:唐琼瑶 +1
Anabaena PCC7120 fat oxygenase gene
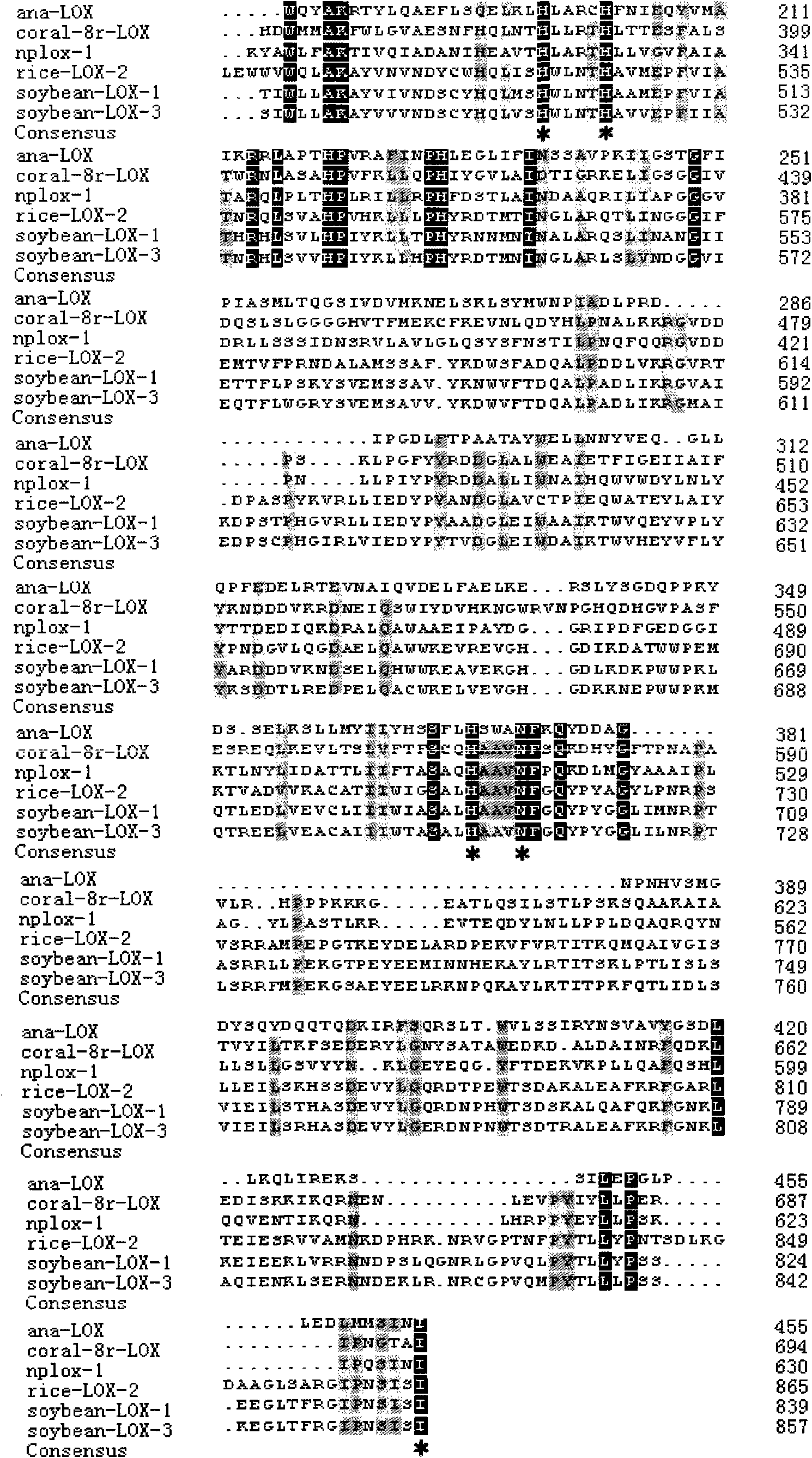
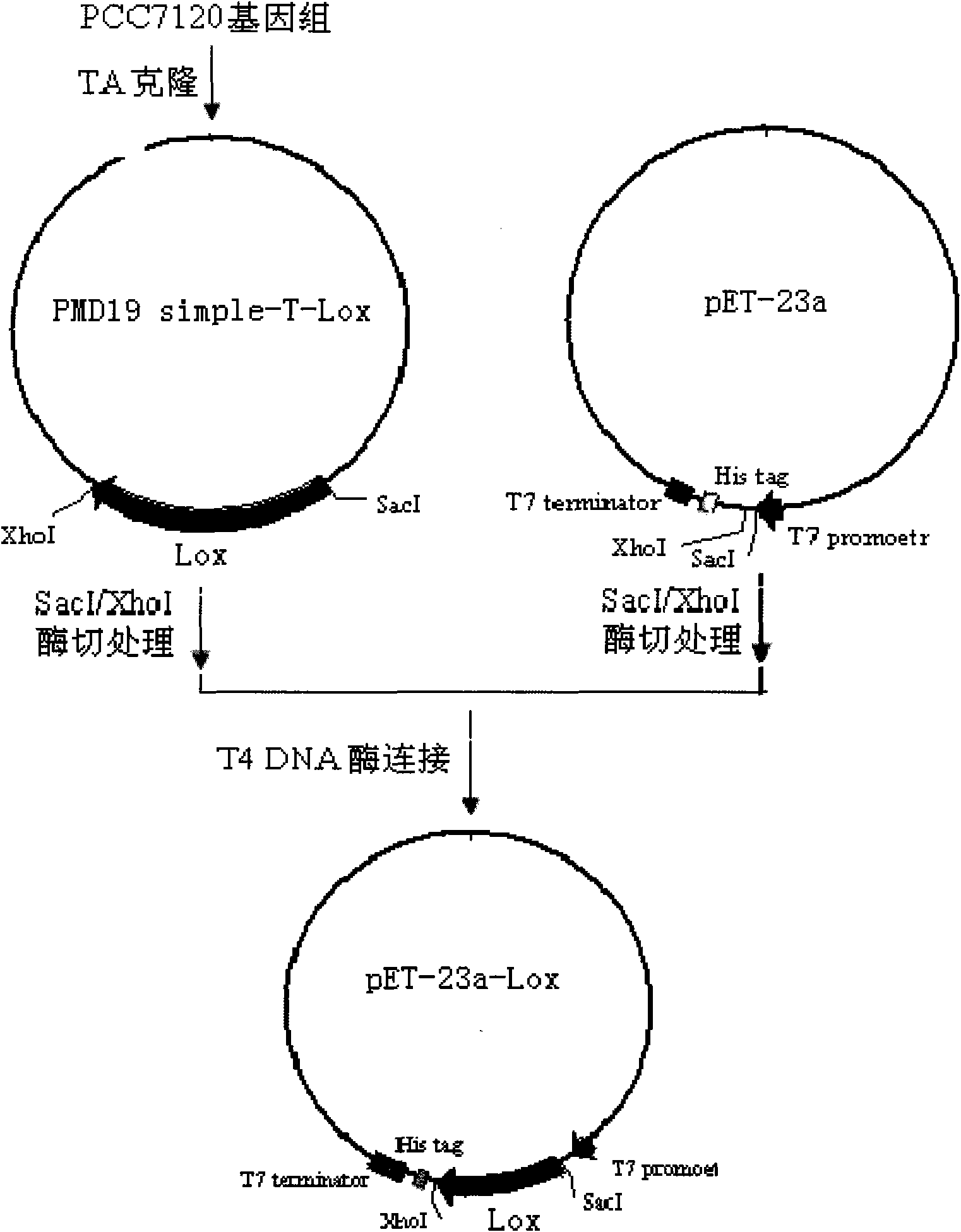
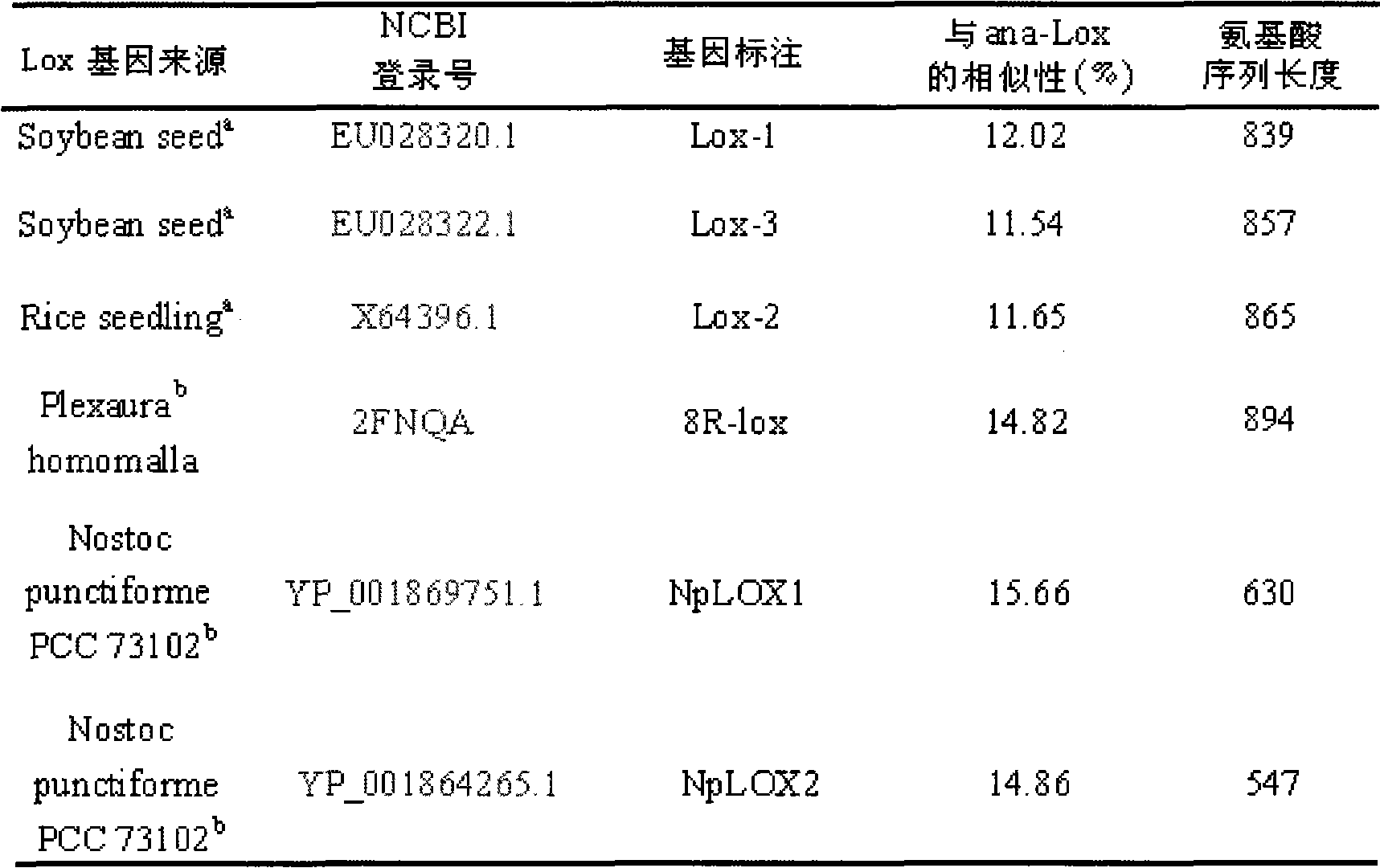
InactiveCN101643740AEfficient expressionHas LOX activityMicroorganism based processesOxidoreductasesDouble bondDibenzoyl Peroxide
The invention relates to an anabaena PCC7120 fat oxygenase gene which belongs to the biologic technical field of the food industry. The gene is derived from anabaena sp. PCC7120 and is multiplied andobtained from genome DNA by PCR, colibacillus is converted after an expression vector is inserted and is induced to express, and the expressed recombinant enzyme catalyzes the oxidation of unsaturatedfatty acid to generate a fatty acid hydrogen overoxidation derivative with conjugated double bonds. The hydroperoxide is used as an unstable primary enzyme promoting product and can further generatea series of oxidation secondary reactions. In the improvement of the quality of flour, the hydroperoxide has an important application value in the following two aspects: (1) oxidizing cysteine with reducibility, forming a disulfide bond, applying to the improvement of the quality of the flour, enhancing the strength of gluten and replacing potassium bromate with a carcinogenic action; and (2) oxidizing pigments, applying to the bleaching of the flour and replacing benzoyl peroxide.
Owner:NANJING AGRICULTURAL UNIVERSITY
R. grahami rnmunoregulating polypeptide, gene and variant and its application in medicine production
InactiveCN101037473AEasy to synthesizeSimple structurePeptidesCyclic peptide ingredientsImmunomodulationsIsoelectric point
The invention relates to an Odorrana grahami immunomodulation peptide, gene and variant and the application in the pharmacy belonging to the biomedicine field. The Odorrana grahami immunomodulation peptide is a circular peptide with a molecular weight of 1791.12 D and a isoelectric point of 9.84. The whole sequence of the Odorrana grahami immunomodulation peptide is Thr Ser Arg Cys Tyr Ile Gly Tyr Arg Arg Lys Val Val Cys Ser (TSRCYIGYRRKVVCS), wherein the fourth cysteine and 14th cysteine form intramolecular disulfide bond. The code gene is composed of 307 nucleotides, wherein the code mature parts are from 141th to 186th nucleotide. The produced variant is produced by substituting the fourth amino acid in the original sequence of the Odorrana grahami immunomodulation peptide. The artificial synthetic Odorrana grahami immunomodulation peptide and variant thereof has a strong immunomodulation activity and tumor resistance activity and can be used for immunomodulation, tumor treatment and chemotherapeutics. The invention has the advantages such as simple sequence and convenient synthesis.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Odorranagrahami antimicrobialpeptides and application thereof
InactiveCN100522993CNo hemolytic activityPeptide/protein ingredientsPeptidesChromatographic separationHigh pressure
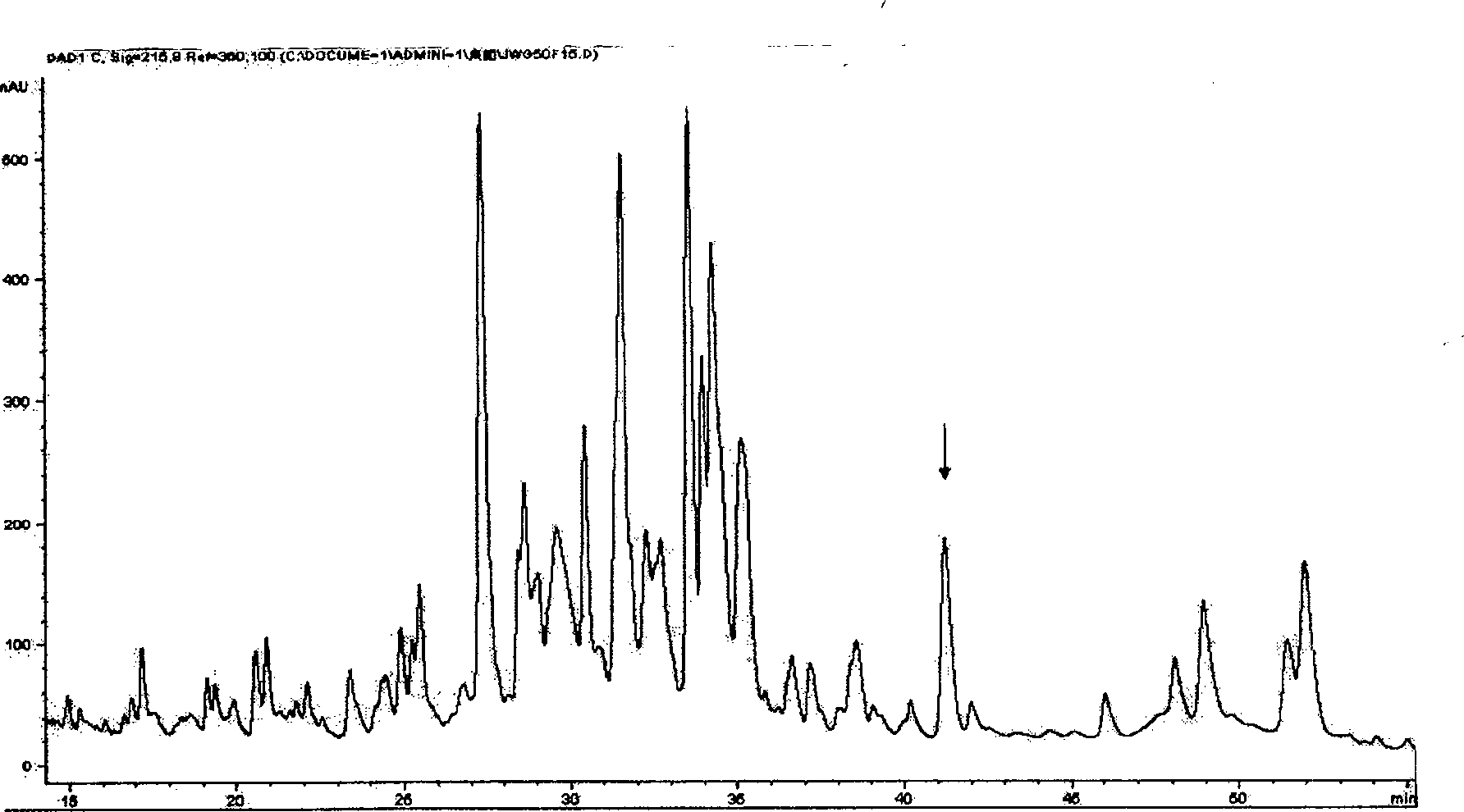
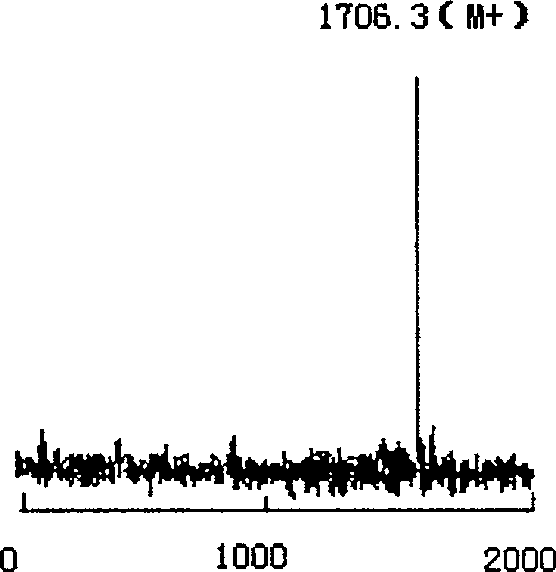
The present invention relates to rana grahami antibiotic peptide and its application, and belongs to the field of biomedicine. Rana grahami antibiotic peptide is one kind of cyclic polypeptide separated from the secretion of amphibian rana grahami, and has molecular weight of 1705.3 dalton, isoelectric point of 9.39, and primary structure of polypeptide sequence Leu Lys Gly Cys Trp The Lys Ser Ile Pro Pro Lys Pro Cys Phe(LKGCWTKSIPPKPCF) with cysteines in the 4-th and 14-th forming intramolecular disulfide bond. Its preparation process includes collecting secretion of ether stimulated rana grahami, centrifuging to eliminate precipitate, freeze drying, chromatographic separation in filtering gel column and separating purification in reverse high pressure liquid chromatogram. The rana grahami antibiotic peptide has powerful effect of inhibiting bacteria, fungi and viruses and no hemolytic activity, and may be used in preparing medicine for treating pathogenic microbe infection diseases.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Sequence symmetric modified IGG4 bispecific antibodies
ActiveUS10221251B2Enhanced antibodyEfficient preparationHybrid immunoglobulinsAntipyreticAntiendomysial antibodiesAmino acid substitution
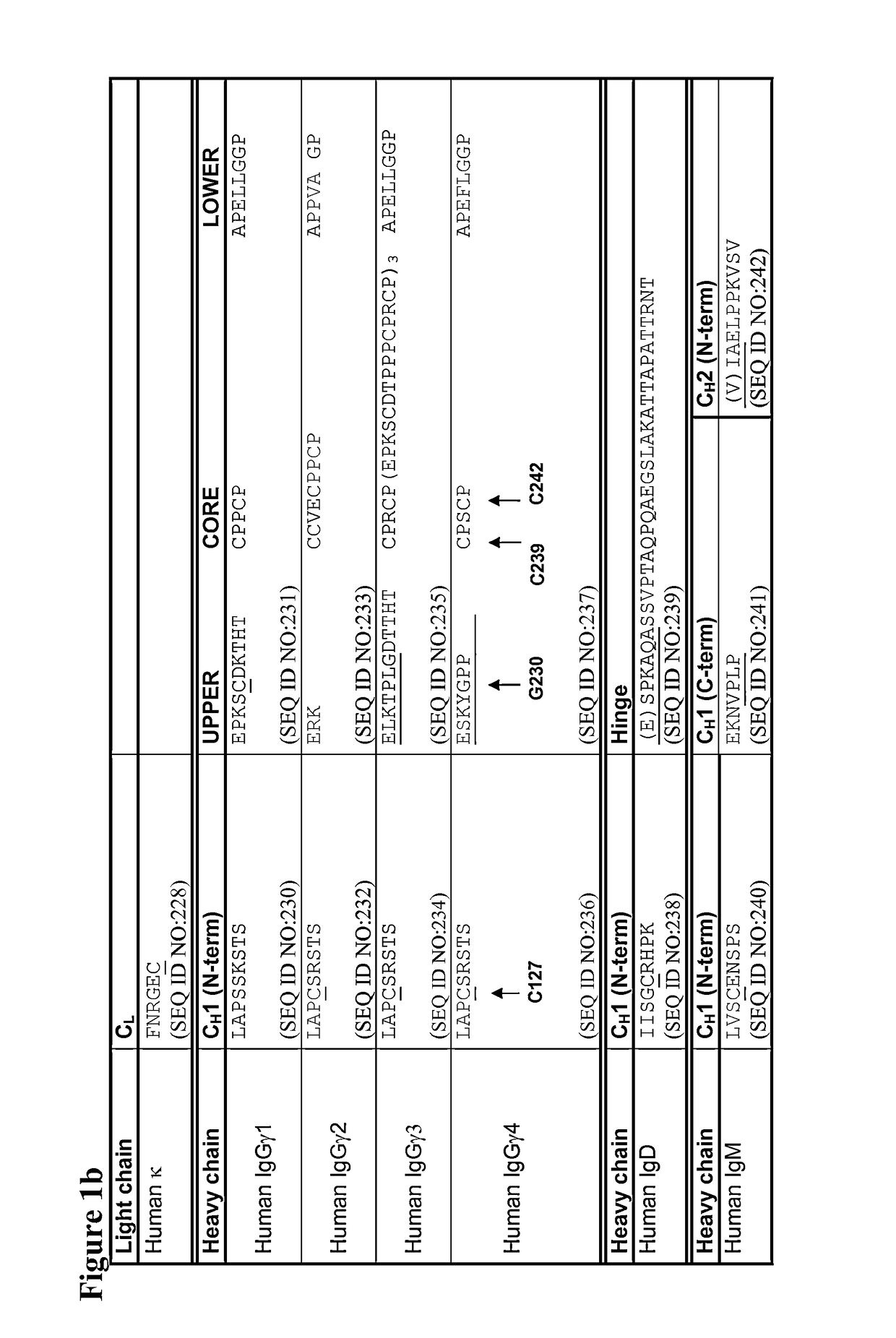
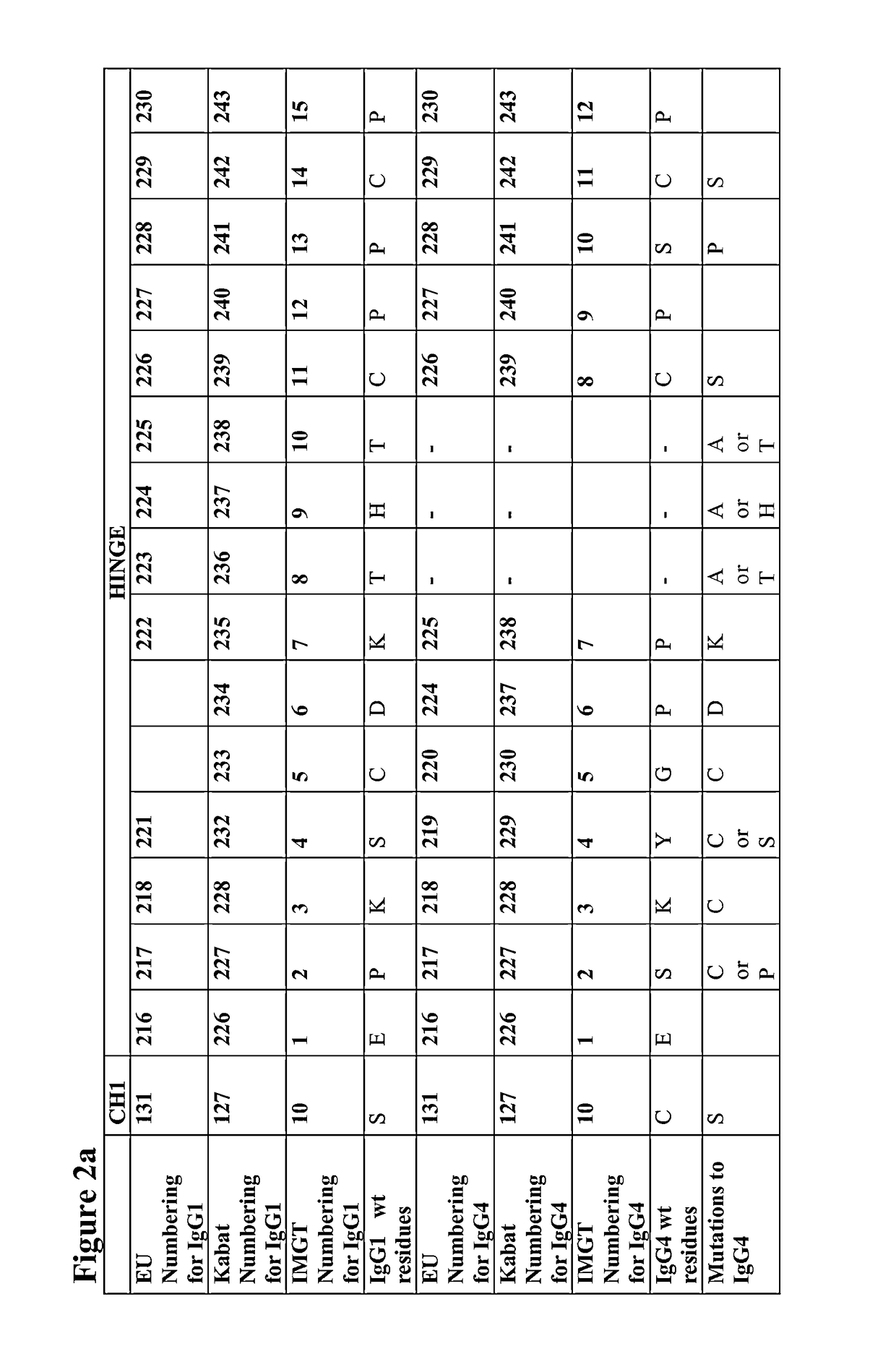
The present disclosure relates to a symmetric bispecific antibody of the class IgG4 comprising two heavy chains which each comprise a variable domain, CH1 domain and a hinge region, wherein in each heavy chain: the cysteine in the CH1 domain which forms an inter-chain disulphide bond with a cysteine in a light chain is substituted with another amino acid; and optionally one or more of the amino acids positioned in the upper hinge region is substituted with cysteine, wherein the constant region sequence of each heavy chain is similar or identical and the variable region in each heavy chain is different, formulations comprising the same, the use of each of the above in treatment and processes for preparing said antibodies and formulations.
Owner:UCB PHARMA SRL
Serine protease inhibitor of Rana grahami, its gene and application
InactiveCN1883703ASimple structureEasy to synthesizePeptide/protein ingredientsDigestive systemComplete sequenceNucleotide composition
Disclosed is an pharmacy application of both a serine protease inhibitor of Rana grahami Graham's frog and a gene thereof, pertaining to biomedicine field. Serine protease inhibitor of Rana grahami Graham`s frog is a cyclic polypeptide encoded by a gene of Rana grahami Graham's frog, which is a Chinese amphibian. Said serine protease inhibitor has a molecular weight of 1951.38 dolton, an isoelectric point of 9.51, and no enzymatic activity. The complete sequence thereof is NH2-AVNIPFKVHFRCKAAFC-COOH,in which Cys at 12th site and Cys at 17th site form an intramolecularly disulfide bond. The coding gene is composed of 301 nucleotides, and the mature coding part is nucleotides from 160th site to 213th site. Artificial serine protease inhibitor of Rana grahami Graham's frog has strong suppression activity for serine protease, and can be used in pharmacy for preparing medicine for tumor, gastritis, and pancreatitis. Said serine protease inhibitor has a simple sequence and can be synthesized with ease.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Amino terminal brain natriuretic peptide precursor polypeptide, antibody, preparation method thereof, detection kit and detection method thereof
ActiveCN110092824AImproving immunogenicityHigh affinityAntibody mimetics/scaffoldsOvalbuminDisulfide bondAntibody
The present invention discloses an amino terminal brain natriuretic peptide precursor polypeptide. The amino terminal brain natriuretic peptide precursor polypeptide is formed by respectively adding first cysteine and second cysteine near an amino terminal end and a carboxy terminal end of a specific amino acid sequence of a native amino terminal brain natriuretic peptide precursor. The first cysteine and the second cysteine are capable of forming a disulfide bond, the specific amino acid sequence is selected from one or more of the polypeptide sequences shown as SEQ ID NO. 1, and / or the specific amino acid sequence is selected from one or more of the polypeptide sequences shown as SEQ ID NO. 2, the vicinity of the amino terminal is 1 to 3 amino acids at the amino terminal of the specificamino acid sequence, and the vicinity of the carboxyl terminal is 1 to 3 amino acids at the carboxy terminal of the specific amino acid sequence. The invention also discloses an antibody and a preparation method thereof. The invention also discloses a detection kit. The invention also discloses a method for detecting the amino terminal brain natriuretic peptide precursor.
Owner:SHENZHEN YHLO BIOTECH
Stable CD3-CD19 resisting mini-type difunctional antibody of disulfide bond and preparation method thereof
InactiveCN101899115AActivity unchangedRetain activityImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationPeriplasmic spaceDisulfide bond
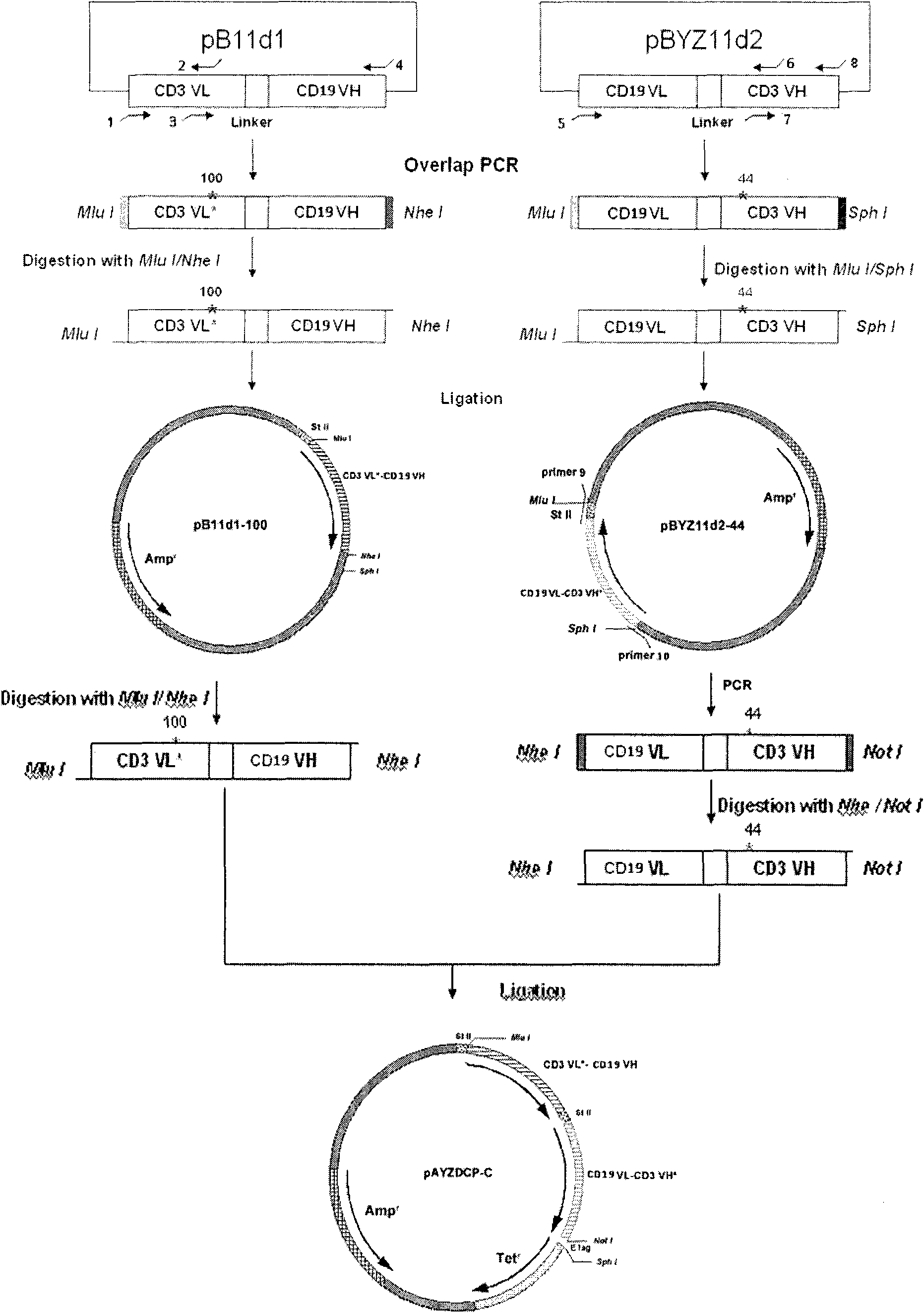
The invention relates to a stable CD3-CD19 resisting mini-type difunctional antibody of disulfide bond and a preparation method thereof. The preparation method comprises the steps of based on the CD3-CD19 resisting antibody, introducing amino acid Cys mutation in the CD3VL-resisting 100th amino acid Ser and CD3VH-resisting 44th amino acid Gly, wherein the expressed product has the structure of disulfide bond formed by the CD3VL-resisting 100th and the CD3VH-resisting 44th cysteines shown in the sequences 3 and 4. The stable CD3-CD19 resisting mini-type difunctional antibody of disulfide bond introduces cysteine mutation in the bits of VL-100 and VH-44 in the CD3-resisting variable region, and the disulfide bond is formed in a bacterium periplasm cavity between the variable region fragments of the two expressed single chains, i.e., between the CD3VL-CD19VH resisting segment and the CD19VL-CD3VH resisting segment, thereby enhancing the stability of the antibody; besides, a mutational site is located in a conservative framework region and two mutational sites in the space conformation are closer enough without generating tension so that the activity of the transformed difunctional antibody remains unchanged and the stability is enhanced.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
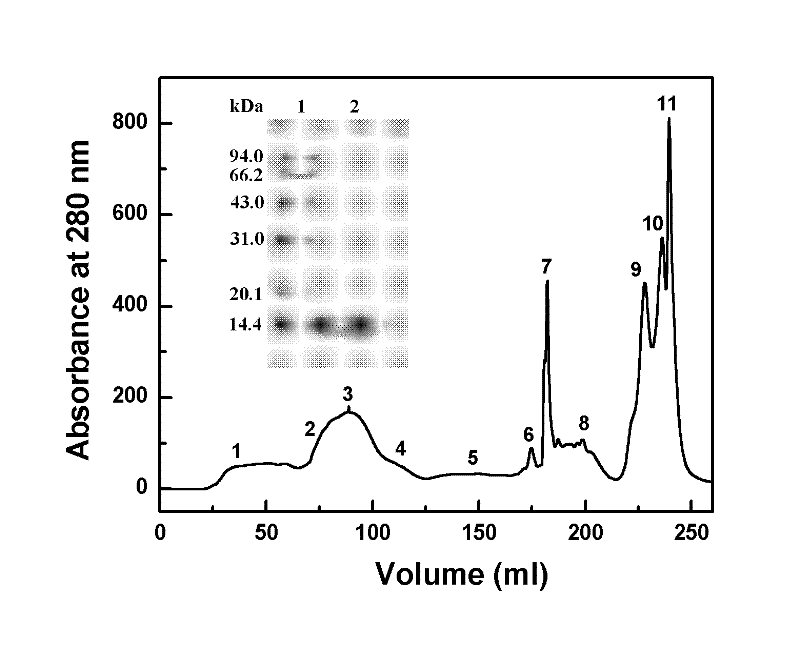
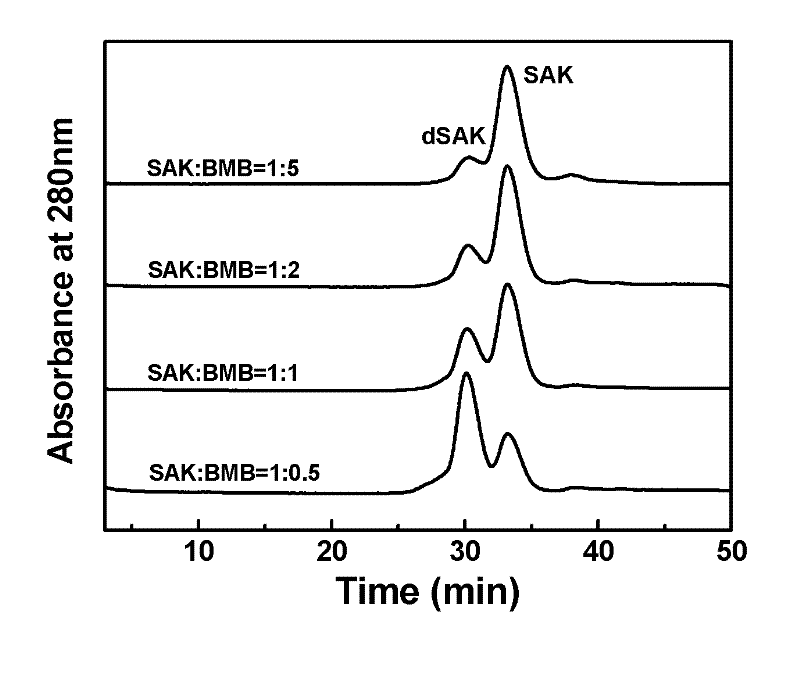
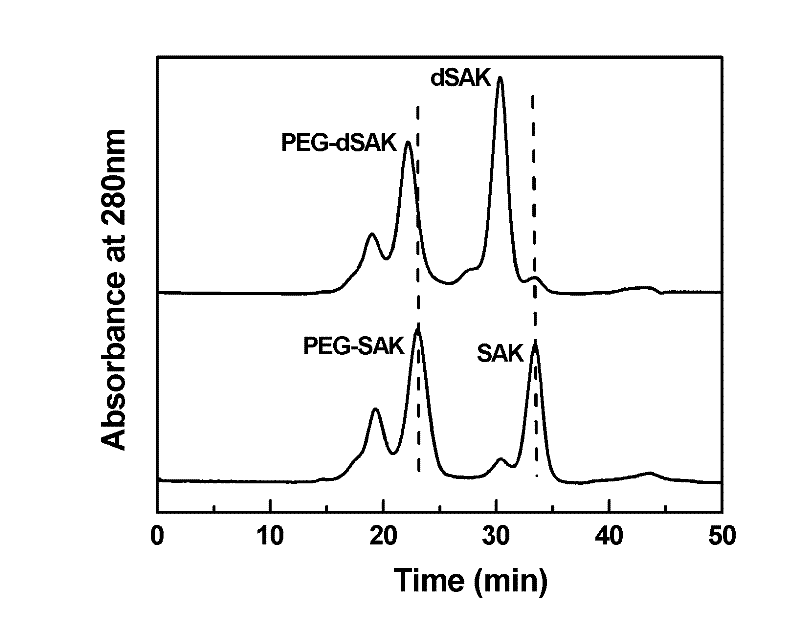
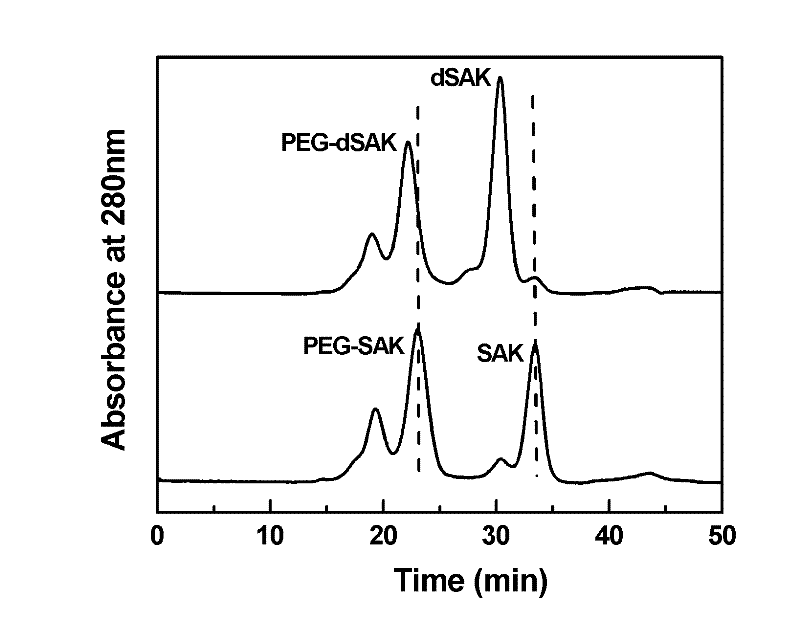
Preparation method of recombinant staphylokinase dimer and site-specific modificaton of polyethylene glycol
The invention discloses a staphylokinase mutant, wherein cysteine is introduced to the C-end of the staphylokinase mutant. Compared with the wild type staphylokinase, the C-end of the mutant has three more amino acids, namely glycine-glycine-cysteine (Gly-Gly-Cys). The invention also discloses a preparation method of the dimer of the staphylokinase mutant. The dimerization method of the staphylokinase is that the two maleimide groups of the homotype bifunctional binder 1,4-dimaleimide butane and the cysteine at the C-end of the staphylokinase are utilized to form a stable covalent bond. The invention also discloses a preparation method for realizing the N-end site-specific modificaton of polyethylene glycol by using the dimer of the staphylokinase mutant. Under special conditions, methoxy polyethylene glycol-propionaldehyde is connected with one N-end of the staphylokinase dimer molecule through the covalent bond. The biological activity of the polyethylene glycol modified product of the staphylokinase dimer is higher than that of the modified product of the staphylokinase monomer.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
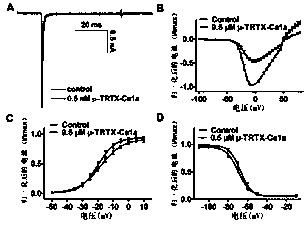
Application of sodium channel blocker mu-TRTX-Ca1a as analgesic drug
ActiveCN110713530AGood analgesic effectGood inhibitory effectPeptide/protein ingredientsAntipyreticDisulfide bondingIonic Channels
The invention relates to an amino acid sequence and a gene sequence of cyriopagopus albostriatus toxin mu-TRTX-Ca1a, and application, and belongs to the field of biomedicine. The mu-TRTX-Ca1a contains38 amino acid residues, wherein six cysteines form three pairs of disulfide bonds, and the molecular weight is 4289.31 Da. The cyriopagopus albostriatus toxin mu-TRTX-Ca1a can preferentially inhibitsodium channel subtype Nav1.7. The cyriopagopus albostriatus toxin mu-TRTX-Ca1a can remarkably alleviate the pain caused by a mice formalin model, an acetic acid writhing model and a hot plate model,and can be used as a drug for treating pain.
Owner:HUNAN NORMAL UNIVERSITY
Modification and dimerization preparation and application of novel growth hormone releasing hormone similar peptide
ActiveCN111533800AEnhance release activityHigh activityNervous disorderPeptide/protein ingredientsDisulfide bondingDimer
The invention provides application of a molecular allosteric body and dimer of a human growth hormone releasing hormone (hGHRH) similar peptide in treating GH deficiency, infertility, senile dementiaand diabetes and enhancing immunity. The dimer is formed by connecting two identical hGHRH similar peptide monomers containing a single cysteine molecule allosteric structure through a disulfide bondformed by cysteine, and an H-shaped structure (intramolecular single Ser-to-Cys substitution) is formed. A side chain epsilon-amino of one lysine of the GHRH similar peptide is also subjected to fattyacid chain modification. Through fatty acid modification, the GH sustained release time of the GHRH similar peptide monomer or dimer is prolonged in vivo, and the longest release time reaches 17 days. The invention finds that the GHRH dimer with long-acting activity has an Aib-to-2Ala substitution structure, an 18C-to-18C disulfide bond formation structure, a C20 fatty acid chain modification structure and a C-terminal amidation structure.
Owner:深圳纳福生物医药有限公司
Method for selectively protecting restriction enzyme cutting sites in trypsin digestion process
ActiveCN111607004ALow affinityReduce or even eliminate the enzyme cutting effectFusions for plasma life prolongingOxidoreductasesDisulfide bondingEnzyme digestion
A method for selectively protecting restriction enzyme cutting sites in a trypsin digestion process is characterized in that a polypeptide to be digested by trypsin is prepared, cysteines are arrangedon the two sides of arginine of the polypeptide, two cysteines form a disulfide bond, and the disulfide bond stretches across arginine. Appropriate sites are selected to insert cysteine or unimportant amino acids are mutated into cysteine on two sides of arginine needing to be protected in polypeptide, and two cysteines stretch over arginine to be protected to form a disulfide bond. Through the conformation change caused by the disulfide bond, steric hindrance is formed at the enzyme cutting sites, the affinity of trypsin and arginine carboxyl terminals is reduced, and the enzyme digestion effect is greatly weakened or even eliminated.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Alginate lyase mutant with high catalytic activity and application thereof
ActiveCN112980822AImprove lyase catalytic efficiencyHigh catalytic efficiencyBacteriaBiofuelsMicroorganismLyase
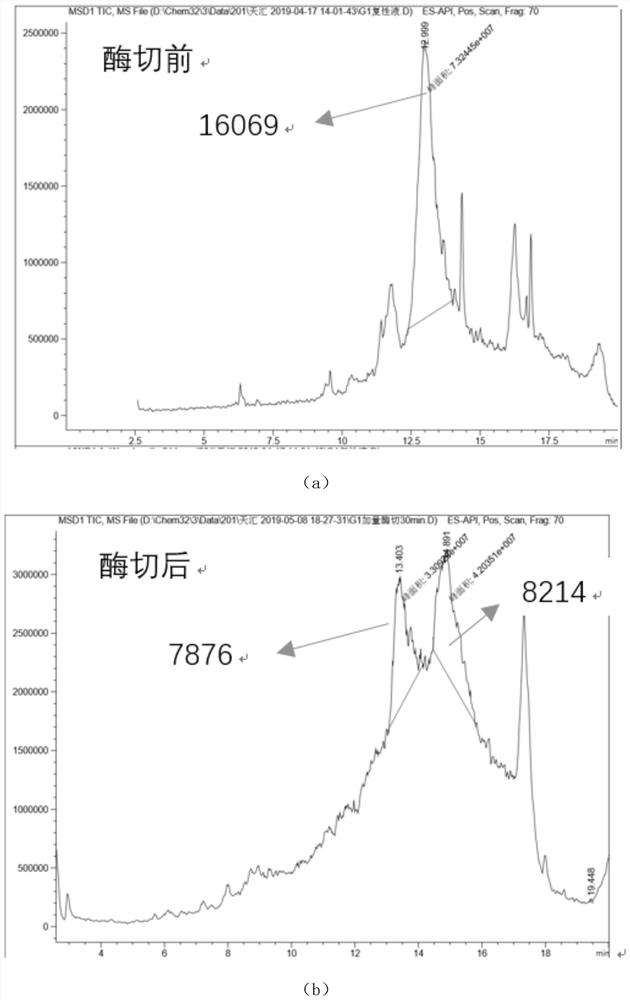
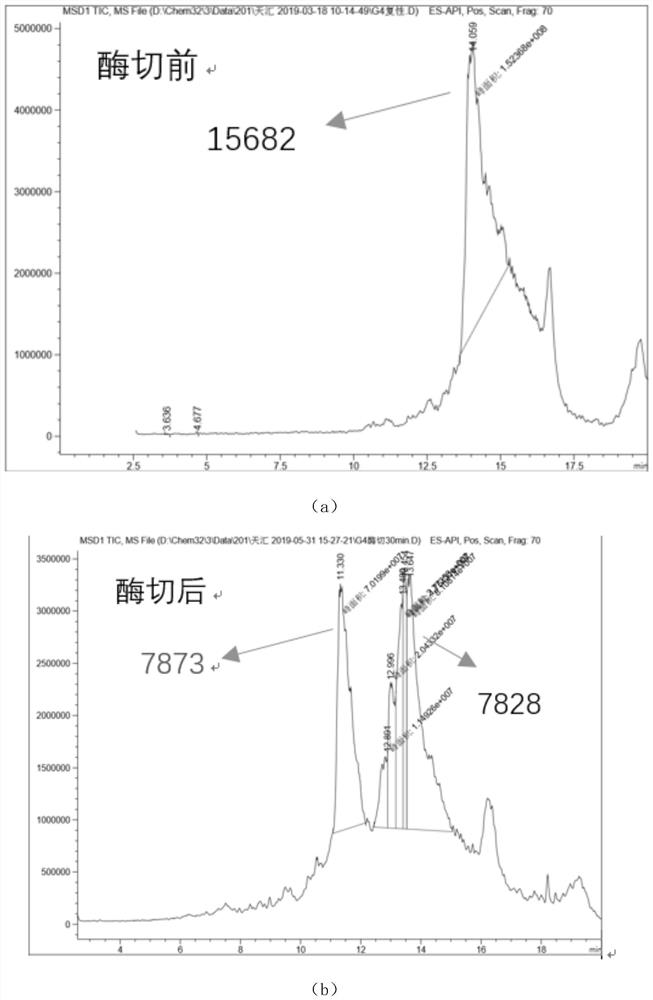
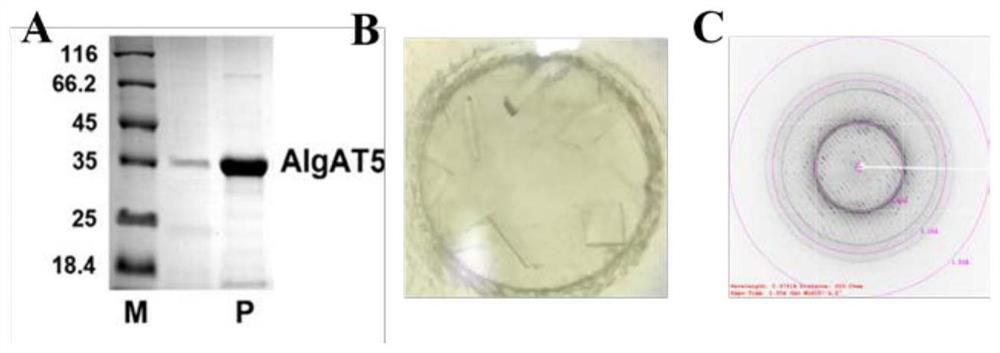
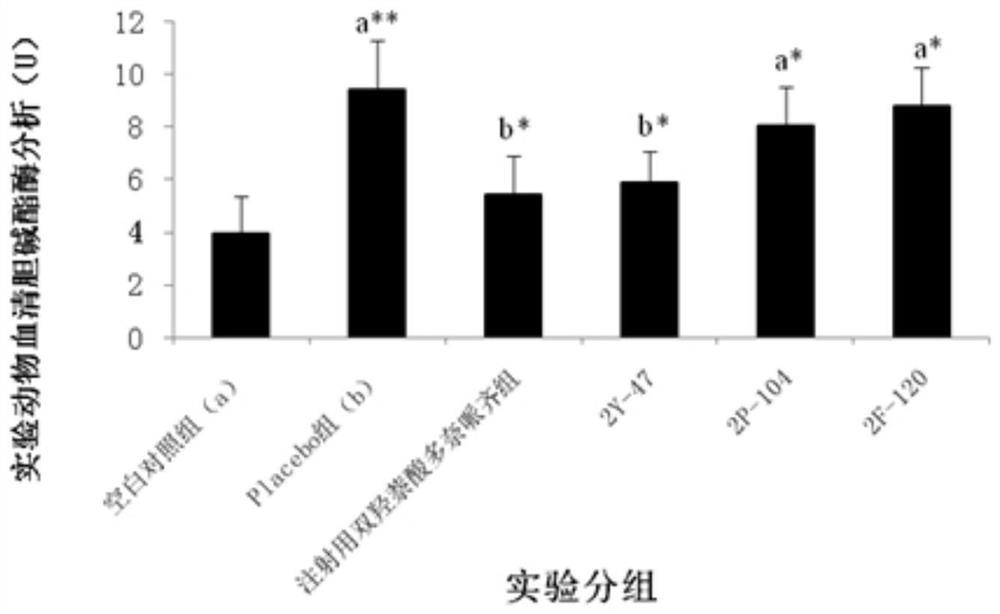
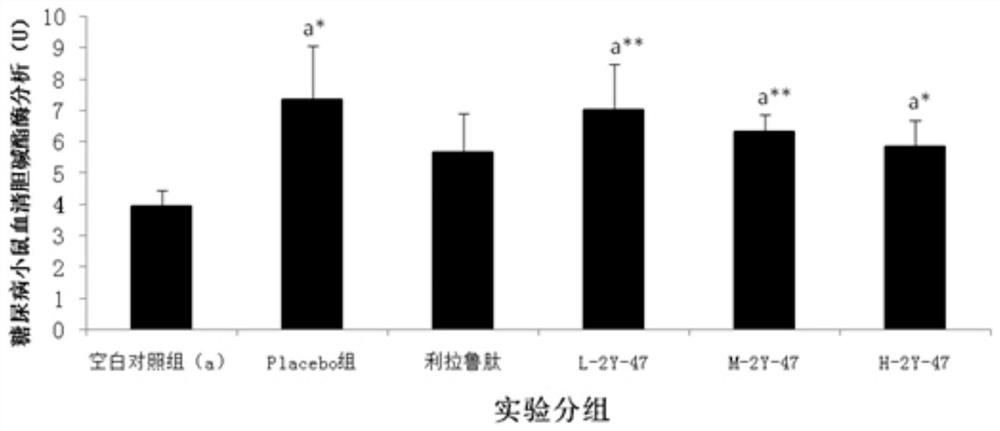
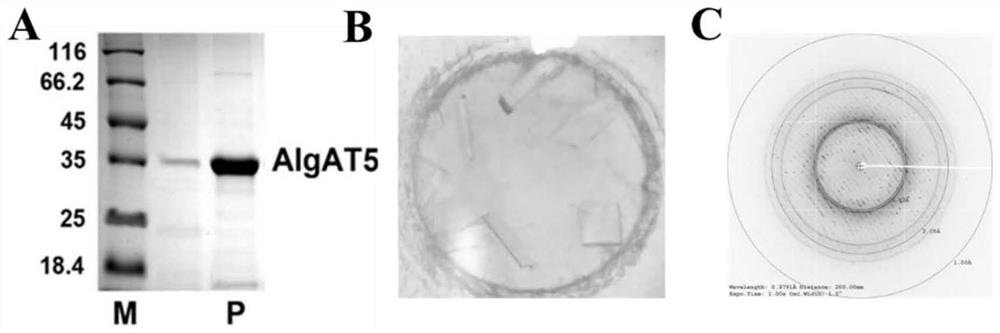
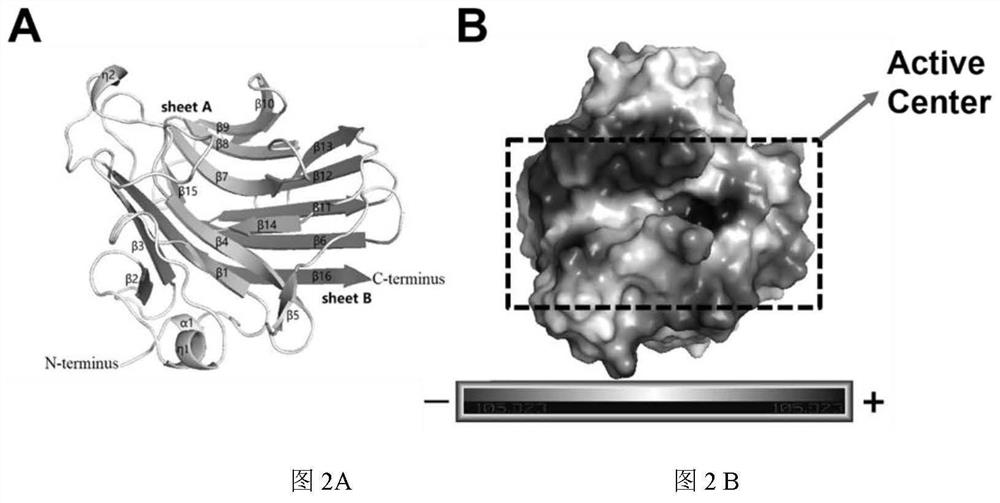
The invention relates to the technical field of protein engineering, in particular to an alginate lyase mutant with high catalytic activity and application thereof. The alginate lyase mutant is obtained by mutation of any one or more sites of cysteine at the 82<nd> site, cysteine at the 95 site, aspartic acid at the 146 site, cysteine at the 209 site and amino acids forming hydrogen bonds with the cysteine at the 82<nd> site, the 95 site or the 209 site of alginate lyase AlgAT5. The alginate lyase mutant or the expression microorganism of the alginate lyase mutant can catalyze algin to be subjected to beta elimination reaction to prepare alginate oligosaccharide, so that the catalysis efficiency of algin lyase is remarkably improved, the reaction time is shortened, and industrial development and application of the algin lyase are accelerated.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Hoplobatrachus rugulosus proteinase inhibition peptide and its gene and application of inhibition peptide in pharmacy
ActiveCN106478811ASimple structureInhibitory activityAntibacterial agentsCosmetic preparationsNucleotideAmino acid composition
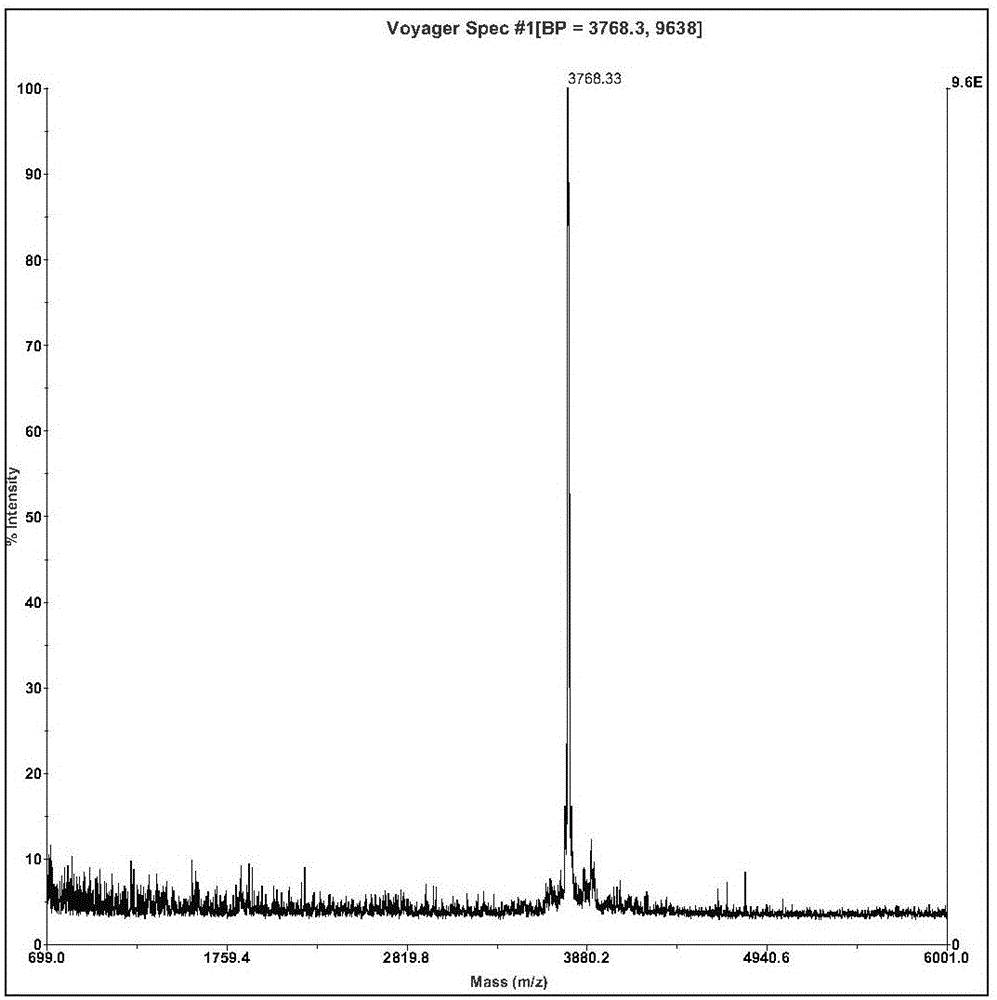
The invention relates to an active polypeptide and its gene and an application of the inhibition peptide in pharmacy. The hoplobatrachus rugulosus proteinase inhibition peptide is cyclopeptide composed of 33 amino acids, a molecular weight is 3768.33 dalton, the isoelectric point is 6.303, and an amino acid sequence is SEQ ID NO.1, and a third cysteine and an eighth cysteine of the polypeptide form intramolecular disulfide bond. A gene sequence of the hoplobatrachus rugulosus proteinase inhibition peptide is composed of SEQ ID NO.4, wherein, the matured hoplobatrachus rugulosus proteinase inhibition peptide is 379th-477th site nucleotide. The hoplobatrachus rugulosus proteinase inhibition peptide has the characteristics of simple structure, convenient artificial synthesis, wide antibacterial spectrum, and strong activity. and can be taken as a medicine for treating pathogenic microorganism infectious diseases and a medicine having functions of beauty treatment and skin caring.
Owner:SOUTHERN MEDICAL UNIVERSITY
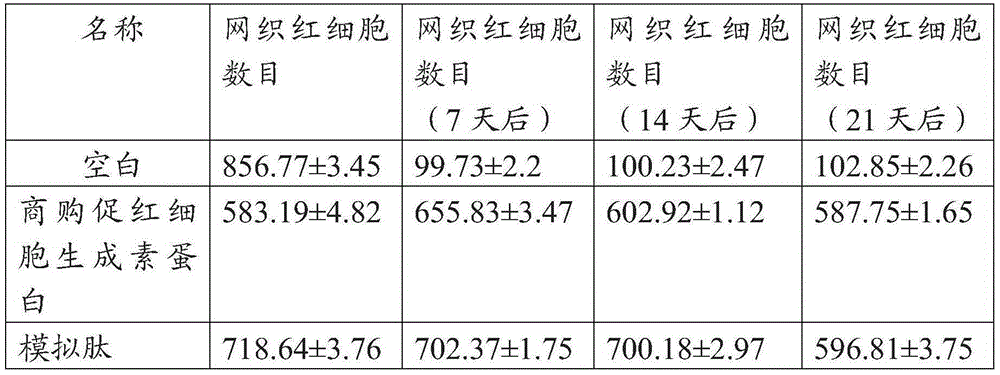
Hydroxyethyl starch modified hemopoietin mimic peptide and preparation thereof and application thereof
InactiveCN105418754ACounts have no appreciable effectCount impactPeptide/protein ingredientsAntipyreticDiseaseHydroxyethyl starch
The invention relates to a hydroxyethyl starch modified hemopoietin mimic peptide with a long-acting erythropoiesis promoting function and a medicinal salt thereof. The invention further provides a preparation method for the hydroxyethyl starch modified hemopoietin mimic peptide and use thereof in preparing drugs for treating diseases which are characterized by being short of erythrocyte and erythrogenin or being short of erythrocyte groups or being in defect of erythrocyte groups. The hydroxyethyl starch modified hemopoietin mimic peptide provided by the invention is represented by a formula I as shown in the description, wherein two cysteines form a disulfide bond, X represents succinic hydroxyethyl starch, C represents a lysine at a tail end, and (K) is condensed with carboxyl of X through free amino of a side chain.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Long-acting erythropoietin mimic peptide, and preparation method and application thereof
ActiveCN106554395APacking has no appreciable effect onCompaction effectNervous disorderPeptide/protein ingredientsDiseaseErythropoiesis
The invention relates to an erythropoietin mimetic peptide derivative having a long-acting erythropoiesis function and a medicinal salt thereof. The invention also provides a preparation method of the erythropoietin mimetic peptide derivative, and a use of the erythropoietin mimetic peptide derivative in the preparation of medicines for treating diseases characterized by lacking of erythropoietin or erythrocyte population deficiency or defect. The erythropoietin mimetic peptide derivative is a polypeptide represented by SEQ ID NO:1 GGLYACHMGPITNalVCQPLRSarKVPGPGVPGPGVPGPGVPGPG, wherein two cysteines of the polypeptide form a disulfide bond, and the N end is acetylated.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of recombinant staphylokinase dimer and site-specific modificaton of polyethylene glycol
The invention discloses a staphylokinase mutant, wherein cysteine is introduced to the C-end of the staphylokinase mutant. Compared with the wild type staphylokinase, the C-end of the mutant has three more amino acids, namely glycine-glycine-cysteine (Gly-Gly-Cys). The invention also discloses a preparation method of the dimer of the staphylokinase mutant. The dimerization method of the staphylokinase is that the two maleimide groups of the homotype bifunctional binder 1,4-dimaleimide butane and the cysteine at the C-end of the staphylokinase are utilized to form a stable covalent bond. The invention also discloses a preparation method for realizing the N-end site-specific modificaton of polyethylene glycol by using the dimer of the staphylokinase mutant. Under special conditions, methoxypolyethylene glycol-propionaldehyde is connected with one N-end of the staphylokinase dimer molecule through the covalent bond. The biological activity of the polyethylene glycol modified product of the staphylokinase dimer is higher than that of the modified product of the staphylokinase monomer.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Artificial synthetic antibacterial peptide with beta-hairpin structure
InactiveCN108084248ABroaden the formation processImprove stabilityAntibacterial agentsCosmetic preparationsEscherichia coliArginine
The invention discloses an artificial synthetic antibacterial peptide with a beta-hairpin structure. The amino acid sequence is as follows: Cys-Ile-Cys-Arg-Arg-Gly-Phe-Cys-Val-Cys. Two arginines existin the corner position of the antibacterial peptide to form active sites where positive charges are concentrated; at the same time, a glycine exist in the corner position, so that the formation and stability of the corner structure are improved; four cysteines exist in the two arm positions to form two pairs of disulfide bonds; at the same time, hydrophobic amino acids exist in the two arm positions, so that the hydrophobicity of two arms is enhanced, and the insertion of the two arms into a bacterial cell membrane is facilitated; and the amidation design exist at the sequence C-terminus, sothat the charge influences at two ends of the sequence are reduced. Experiments show that the above antibacterial peptide provided by the invention can effectively inhibit or even kill staphylococcusaureus, escherichia coli, and aerobic spore bacteria, and has a good inhibitory effect on most of gram-positive bacteria.
Owner:ZHEJIANG OCEAN UNIV
Erythropoietin mimic peptide, dimer thereof, and preparation methods and application of erythropoietin mimic peptide and dimer
ActiveCN105153293ACounts have no appreciable effectCount impactPeptide/protein ingredientsUrinary disorderRed blood cellHalf-life
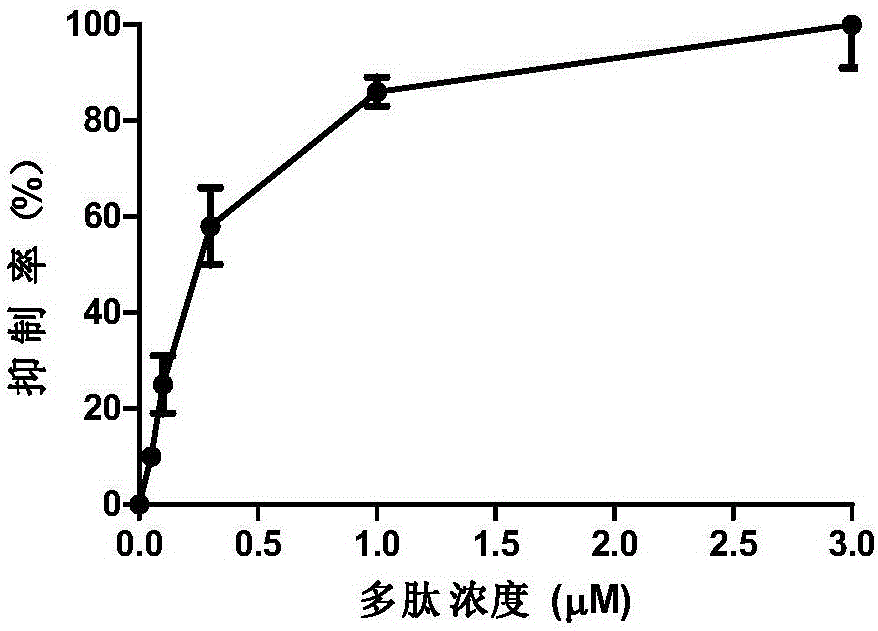
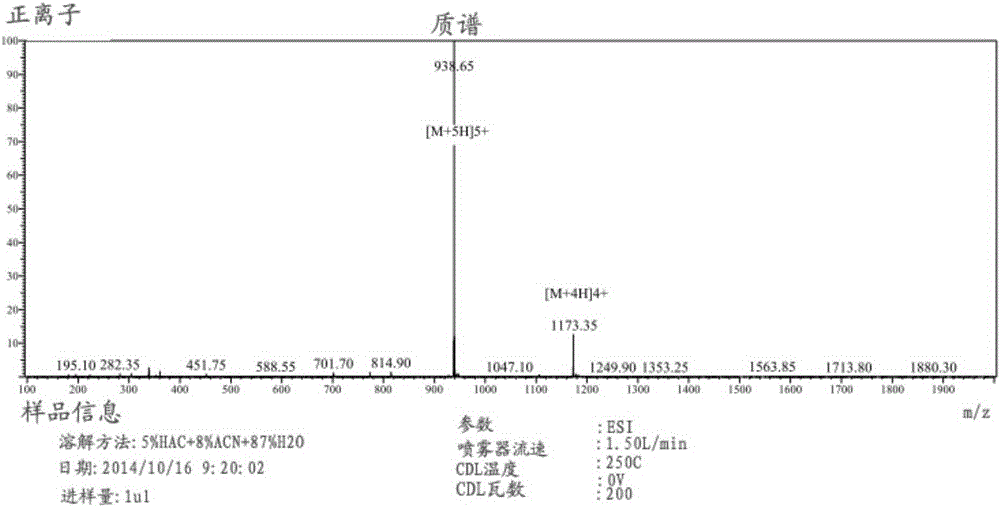
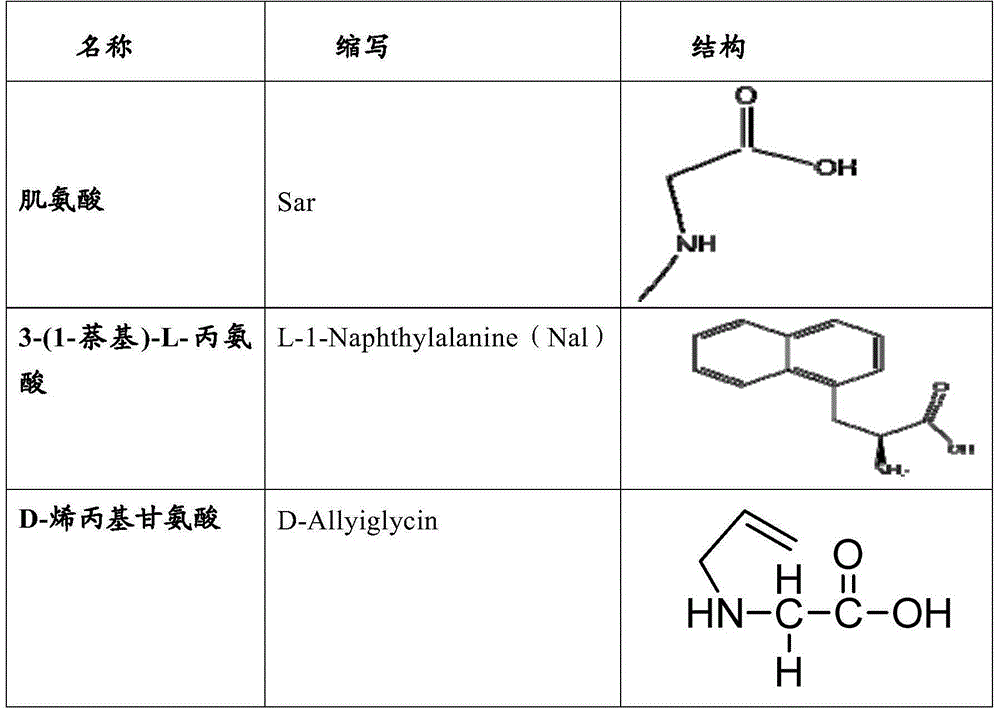
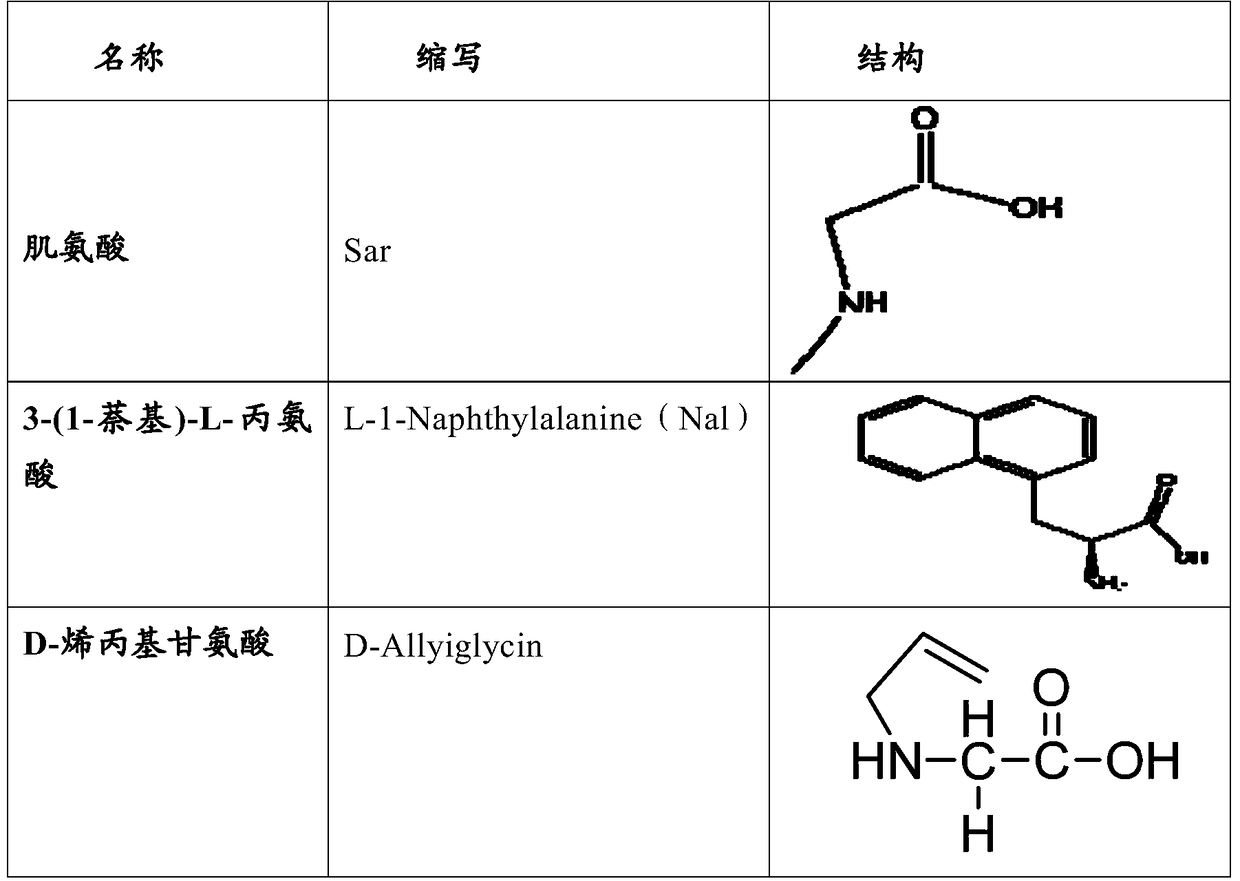
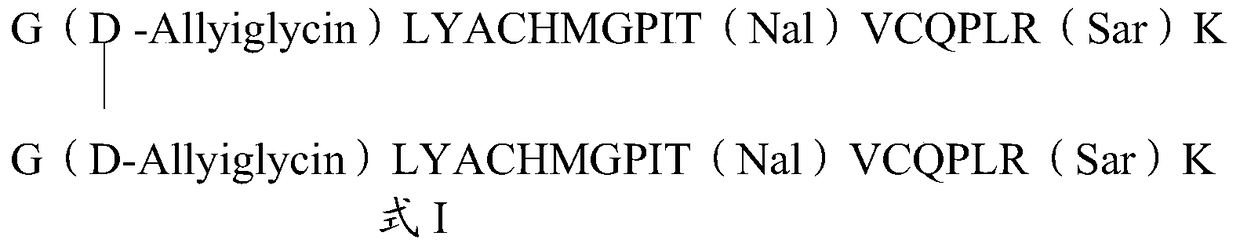
The invention relates to erythropoietin mimic peptide. The erythropoietin mimic peptide is characterized in that the amino acid sequence of monomer peptide of the mimic peptide is shown as GX1LYACHMGPITX2VCQPLRX3K in SEQ ID NO:1, wherein X1 is allylglycine (D-Allyiglycin), X2 is 3-(1-naphthyl)-L-alanine (Nal), X3 is sarcosine (Sar), 6-bit and 15-bit cysteine (C) forms an intramolecular disulfide bond, and N is acetylized in end. The invention further provides a preparation method of the mimic peptide, a preparation method of dimer and pharmaceutical salt of the mimic peptide. The invention further provides a pharmaceutical composition containing the mimic peptide or the dimer or the pharmaceutical salt of the mimic peptide. The erythropoietin mimic peptide, the dimer and the pharmaceutical salt of the mimic peptide can excite generation of erythrocyte and significantly prolong the half-life period of drugs in human bodies.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Hemopoietin mimic peptide, and preparation method and application thereof
ActiveCN105367629APacking has no appreciable effect onHemoglobin content had no appreciable effectNervous disorderPeptide/protein ingredientsDiseaseCeanothic acid
The invention relates to a hmopoietin mimic peptide with long-acting erythropoiesis-promoting function and pharmaceutical salts. The invention also provides a preparation method for the above mopoietin mimic peptide and application of the mopoietin mimic peptide to prepare medicines treating diseases with the character of erythrocyte lack, erythropoietin lack, or erythrocyte population lack or defect. The provided hemopoietin mimic peptide is an alkyl acid conjugate of a lysine (K) side-chain dissociated amino at the terminal of a peptide chain shown as SEQ ID NO:1, the structural general formula is shown as a formula I: GGLYACHMGPITNalVCQPLRSarK-X, wherein two cysteine form a disulfide bond, the N terminal is acylated, and X represents lauric acid or palmitic acid.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation and application of novel ghrelin-like peptide modification and dimerization
ActiveCN111533800BEnhance release activityHigh activityNervous disorderPeptide/protein ingredientsDisulfide bondingDimer
The invention provides the molecular degeneration of human growth hormone release hormone (HGHRH) peptides and its di clustering in the treatment of GH deficiency, infertility, dementia, diabetes, and enhanced immunity.The di agent of the present invention is two similar HGHRH -like peptide monomers that contain a single beurine molecular degeneration are connected by a sulfur (sulfurine formed by cysteine, and form an H -shaped structure (internal single ser → CYS inside the molecule → CYSreplace).The present invention also modifies the fatty acid chain of one of the lysine ε‑ amino groups of one of the lysine in the GHRH -like peptide.Extend the GHRH -like peptide monomer or di -polycatus in the body through fatty acid decoration, up to 17 days in the GH in the body.The present invention found that GHRH diodes with long -term activity have AIB → 2 ALA replacement, 18 C‑ 18 C formal sulfur formation, C20 fatty acid chain modification, and C -side amineization structure.
Owner:深圳纳福生物医药有限公司
Alginate Lyase Mutant with High Catalytic Activity and Its Application
ActiveCN112980822BImprove lyase catalytic efficiencyHigh catalytic efficiencyBacteriaBiofuelsMicroorganismFucoidan
The invention relates to the technical field of protein engineering, in particular to a mutant of alginate lyase with high catalytic activity and its application. The mutants are cysteine at position 82, cysteine at position 95, aspartic acid at position 146, cysteine at position 209, and cysteine at position 82, 95 or 209 of alginate lyase AlgAT5 Any one or several positions of amino acids that form hydrogen bonds with amino acids are mutated. The mutant of the present invention or the microorganism expressing it can catalyze the β-elimination reaction of alginate to prepare alginate oligosaccharides. This method significantly improves the catalytic efficiency of the alginate lyase, reduces the reaction time, and helps to accelerate the industrial development and application of the alginate lyase.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
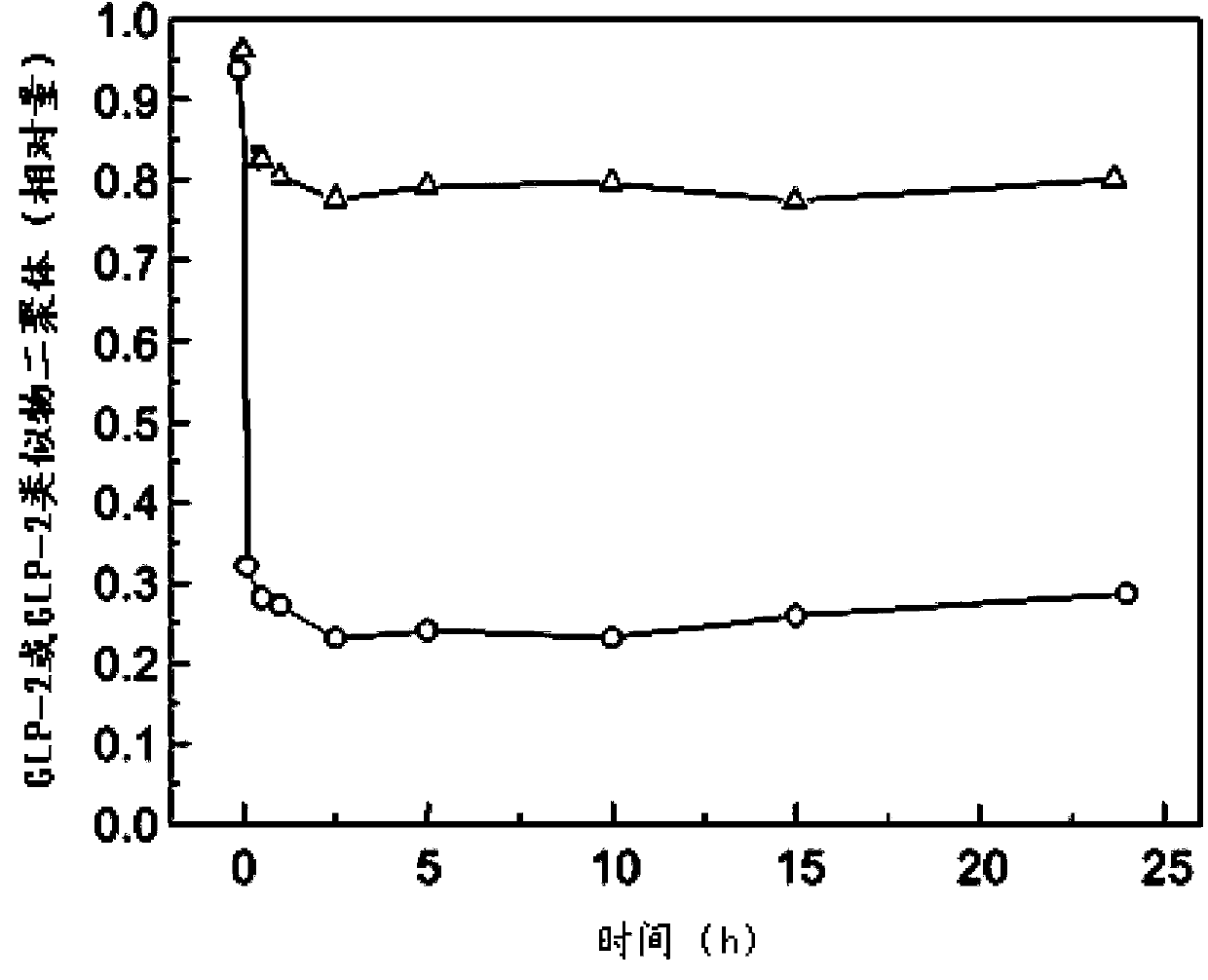
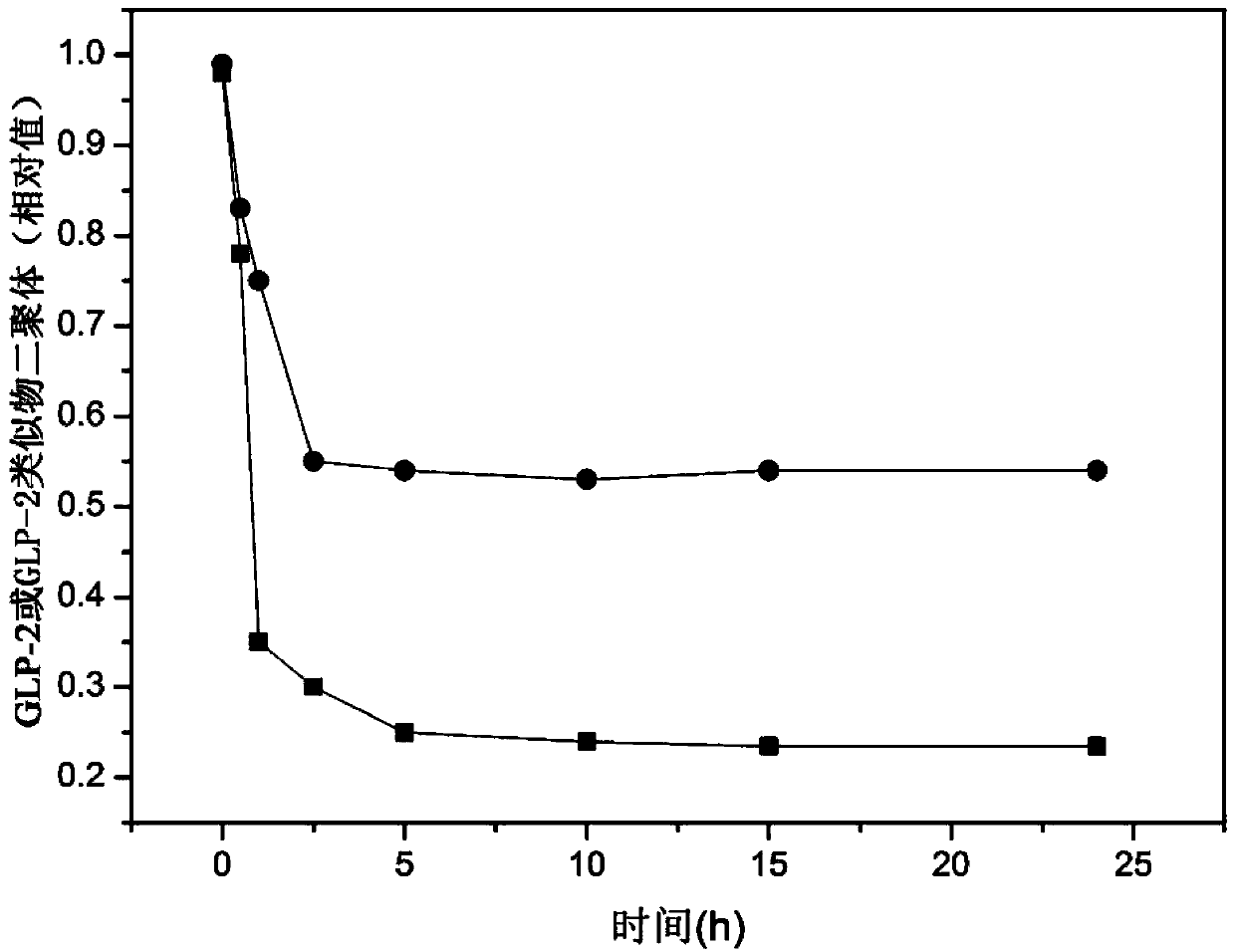
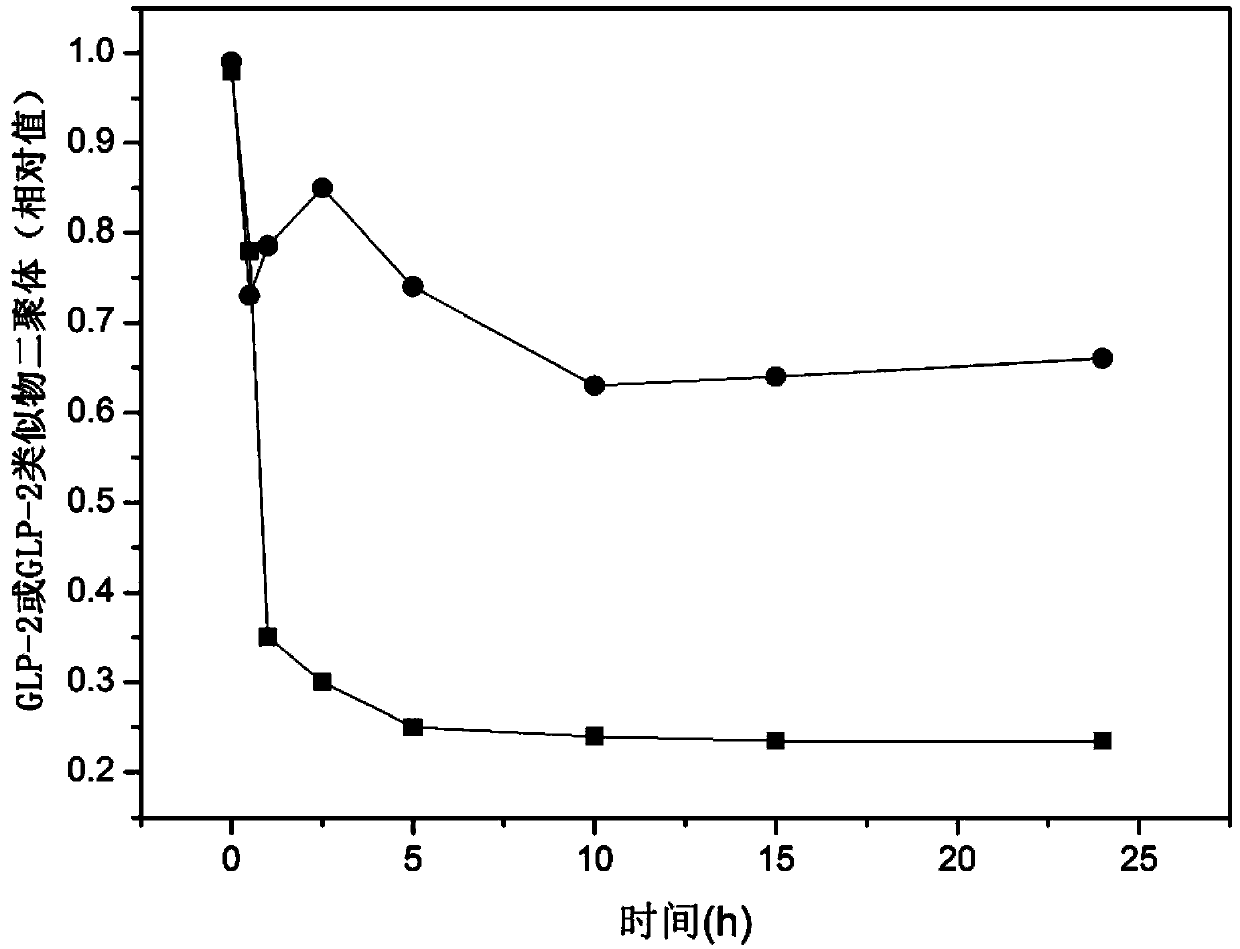
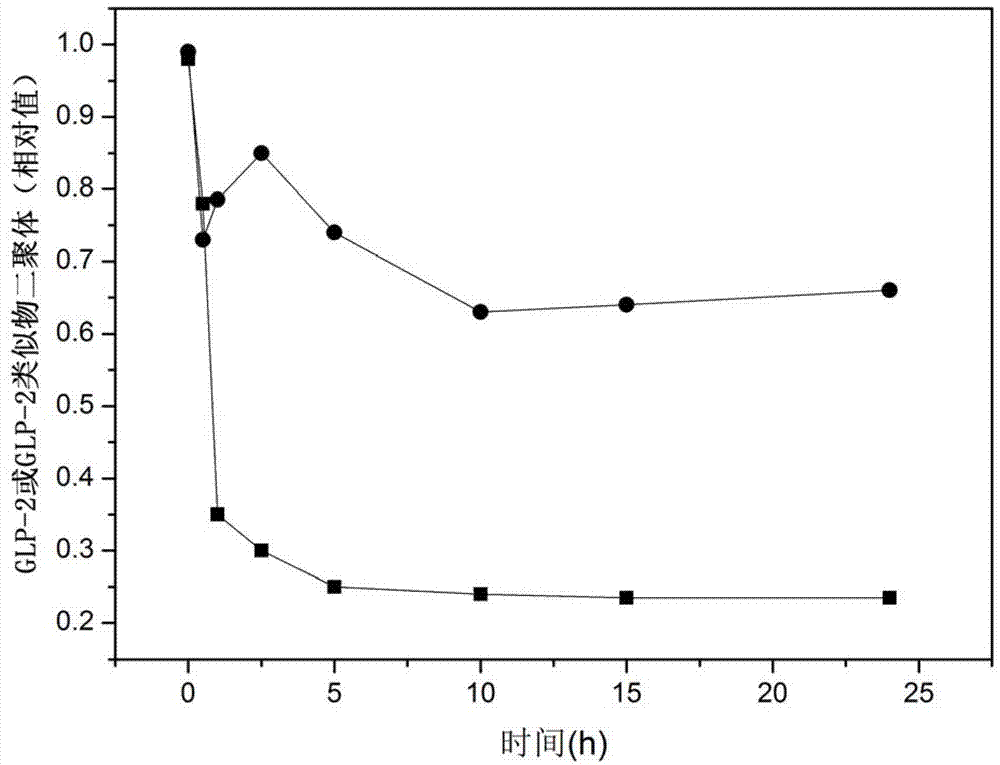
Glucagon-like peptide-2 analogue dimer, preparation method and application thereof
ActiveCN103864917AOvercoming the problem of short half-lifeExtended half-lifePeptide/protein ingredientsDigestive systemDiseaseHalf-life
The present invention provides a glucagon-like peptide-2 analogue dimer, a preparation method and an application thereof, wherein two identical or different GLP-2 analogue monomers form a disulfide bond through cysteines on the monomers so as to prepare the dimer. The dimer preparation method comprises: adopting an Fmoc solid phase polypeptide synthesis method to synthesize a GLP-2 analogue monomer, and making the synthesized GLP-2 analogue monomers form a disulfide bond between the monomers. The application is an application of the GLP-2 analogue dimer in preparation of drugs for treatment of gastrointestinal related diseases. According to the present invention, with the GLP-2 analogue dimer formed from the GLP-2 analogue monomer, the problem of the short half-life of the GLP-2 is overcome, and the half-life of the GLP-2 analogue dimer can achieve more than 8-96 h in vivo, and is significantly prolonged compared with the half-life of the GLP-2 administered separately so as to substantially and easily achieve clinical promotion and application.
Owner:天津天诚新药评价有限公司
A kind of erythropoietin mimetic peptide and its dimer, as well as preparation method and application
ActiveCN105153293BCounts have no appreciable effectCount impactPeptide/protein ingredientsUrinary disorderHalf-lifeSarcosine
The invention relates to erythropoietin mimic peptide. The erythropoietin mimic peptide is characterized in that the amino acid sequence of monomer peptide of the mimic peptide is shown as GX1LYACHMGPITX2VCQPLRX3K in SEQ ID NO:1, wherein X1 is allylglycine (D-Allyiglycin), X2 is 3-(1-naphthyl)-L-alanine (Nal), X3 is sarcosine (Sar), 6-bit and 15-bit cysteine (C) forms an intramolecular disulfide bond, and N is acetylized in end. The invention further provides a preparation method of the mimic peptide, a preparation method of dimer and pharmaceutical salt of the mimic peptide. The invention further provides a pharmaceutical composition containing the mimic peptide or the dimer or the pharmaceutical salt of the mimic peptide. The erythropoietin mimic peptide, the dimer and the pharmaceutical salt of the mimic peptide can excite generation of erythrocyte and significantly prolong the half-life period of drugs in human bodies.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Black frame toad antioxidant peptide and its gene and its application in pharmaceuticals
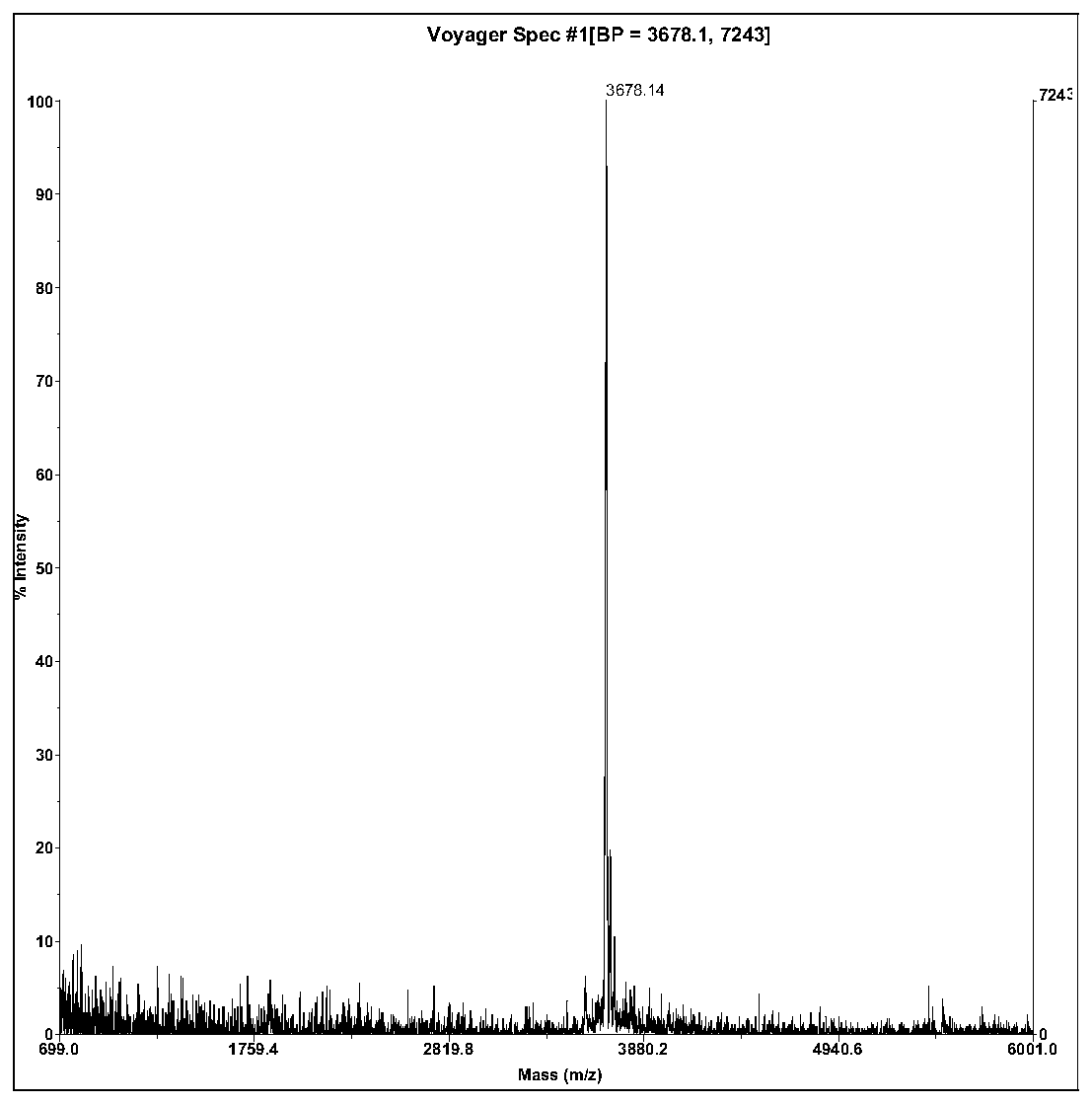
ActiveCN106397564BSimple structureSignificant lectinCosmetic preparationsNervous disorderCyclic peptideDisulfide bonding
The invention relates to a bufo melanostictus antioxidative peptide and its gene and an application in pharmacy. The bufo melanostictus antioxidative peptide is cyclic peptide consisting of 33 amino acids, the molecular weight is 3678.14 Dalton, and the isoelectric point is 10.33, and the amino acid sequence is shown as SEQ ID NO.1; the third cysteine and the eighth cysteine of the polypeptide are formed to be an intramolecular disulfide bond. The gene sequence of the bufo melanostictus antioxidative peptide consists of SEQ ID NO.4, wherein the matured bufo melanostictus antioxidative peptide encoded with functions is the 394-492 bit of nucleotide. The matured functional polypeptide amino acid sequence is deduced by gene of bufo melanostictus antioxidative peptide; the compounded bufo melanostictus antioxidative peptide has very strong agglutinin, serine protease inhibitor and antioxygenation.
Owner:SOUTHERN MEDICAL UNIVERSITY
Glucagon-like peptide-2 analog dimer and its preparation method and application
ActiveCN103864917BOvercoming the problem of short half-lifeExtended half-lifePeptide/protein ingredientsDigestive systemDisulfide bondingDimer
The present invention provides a glucagon-like peptide-2 analogue dimer, a preparation method and an application thereof, wherein two identical or different GLP-2 analogue monomers form a disulfide bond through cysteines on the monomers so as to prepare the dimer. The dimer preparation method comprises: adopting an Fmoc solid phase polypeptide synthesis method to synthesize a GLP-2 analogue monomer, and making the synthesized GLP-2 analogue monomers form a disulfide bond between the monomers. The application is an application of the GLP-2 analogue dimer in preparation of drugs for treatment of gastrointestinal related diseases. According to the present invention, with the GLP-2 analogue dimer formed from the GLP-2 analogue monomer, the problem of the short half-life of the GLP-2 is overcome, and the half-life of the GLP-2 analogue dimer can achieve more than 8-96 h in vivo, and is significantly prolonged compared with the half-life of the GLP-2 administered separately so as to substantially and easily achieve clinical promotion and application.
Owner:天津天诚新药评价有限公司
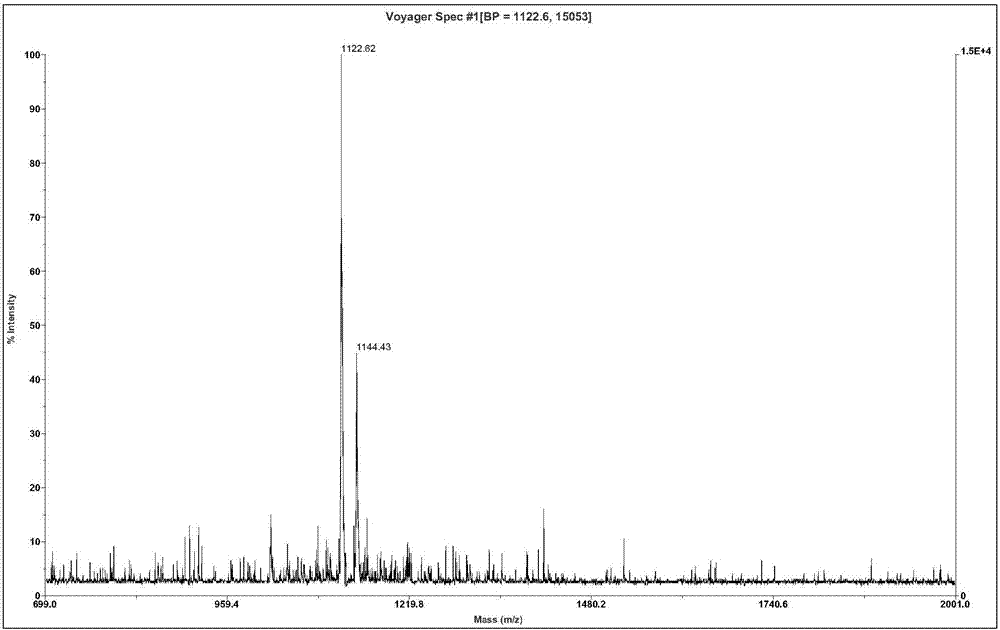
Fejervarya multistriata antioxidative peptide and gene and application thereof
InactiveCN107501395ASimple structureEasy to synthesizeCosmetic preparationsNervous disorderFood additiveNucleotide
The invention relates to active polypeptide, a gene thereof and an application of active polypeptide to pharmacy. The fejervarya multistriata antioxidative peptide is a cyclopeptide comprises nine amino acids, has the molecular weight of 1122.62 Dalton and the isoelectric point of 8.01, and has the amino acid sequence of Arg Cys Phe Tyr Phe Gly Gln Cys Thr (RCFYFGQCT) (SEQ ID NO.1). Cysteines in the second and eighth places of the polypeptide form an intramolecular disulfide bond, and threonine in the ninth place is amidated. The gene sequence of the fejervarya multistriata antioxidative peptide comprises SEQ ID NO.4, and nucleotides in the 196-222<nd> are coded with functional mature fejervarya multistriata antioxidative peptide. The fejervarya multistriata antioxidative peptide is simple in structure and convenient to synthesize, and is applied to prepare a medicine and food additive having an antioxidative effect.
Owner:SOUTHERN MEDICAL UNIVERSITY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com