Alginate Lyase Mutant with High Catalytic Activity and Its Application
An alginate lyase and a technology for catalyzing alginate, applied in the field of protein engineering, can solve the problems of long cycle, time-consuming and high blindness, and achieve the effects of reducing reaction time, improving catalytic efficiency, and accelerating industrial development and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
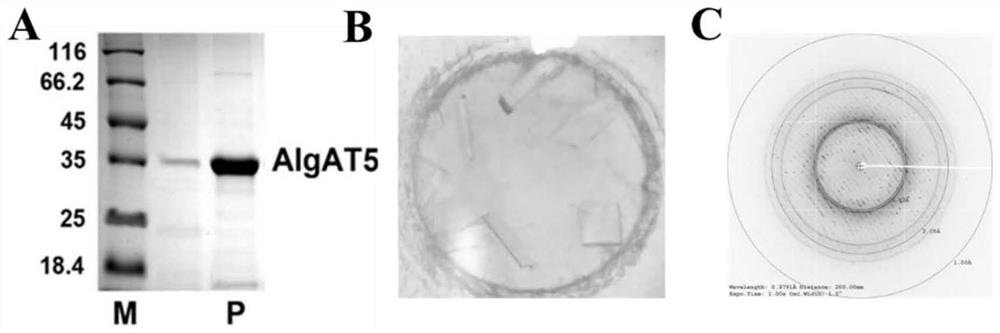
[0053] Example 1. Analysis of the crystal structure of the alginate lyase AlgAT5.
[0054] 1.1 Protein expression and purification
[0055] A novel thermophilic bacterium thatdegrades brown algae[J].Applied and environmental microbiology,2016,82(3 ): 868-877.] Source Alginate lyase AlgAT5 was used for protein expression using the pET-30a(+) vector. Pick a single clone of the BL21(DE3) expressing strain containing the AlgAT5 gene from the solid medium plate cultured overnight in LB with kanamycin resistance in a constant temperature incubator at 37°C, add kanamycin liquid to 5 mL of LB Seeds were grown overnight at 37°C at 200 rpm in the medium. The next day, the activated cells were transferred to 500 mL of LB liquid medium containing kanamycin, cultured at 200 rpm at 37 °C until the OD600 was 0.5-0.8, and IPTG with a final concentration of 1 mM was added, and placed at 22-25 °C. Incubate with shaking at 200 rpm in a constant temperature shaker for 16-18 h to induce protein...
Embodiment 2
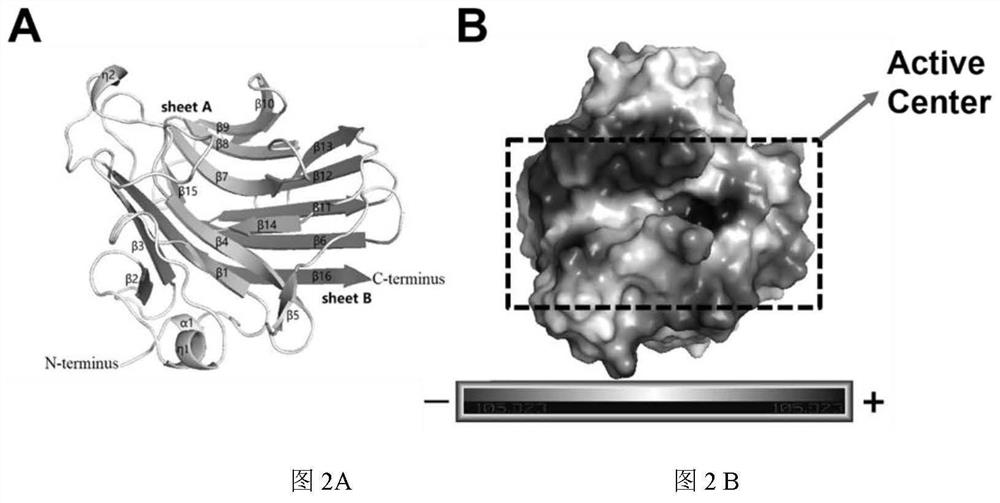
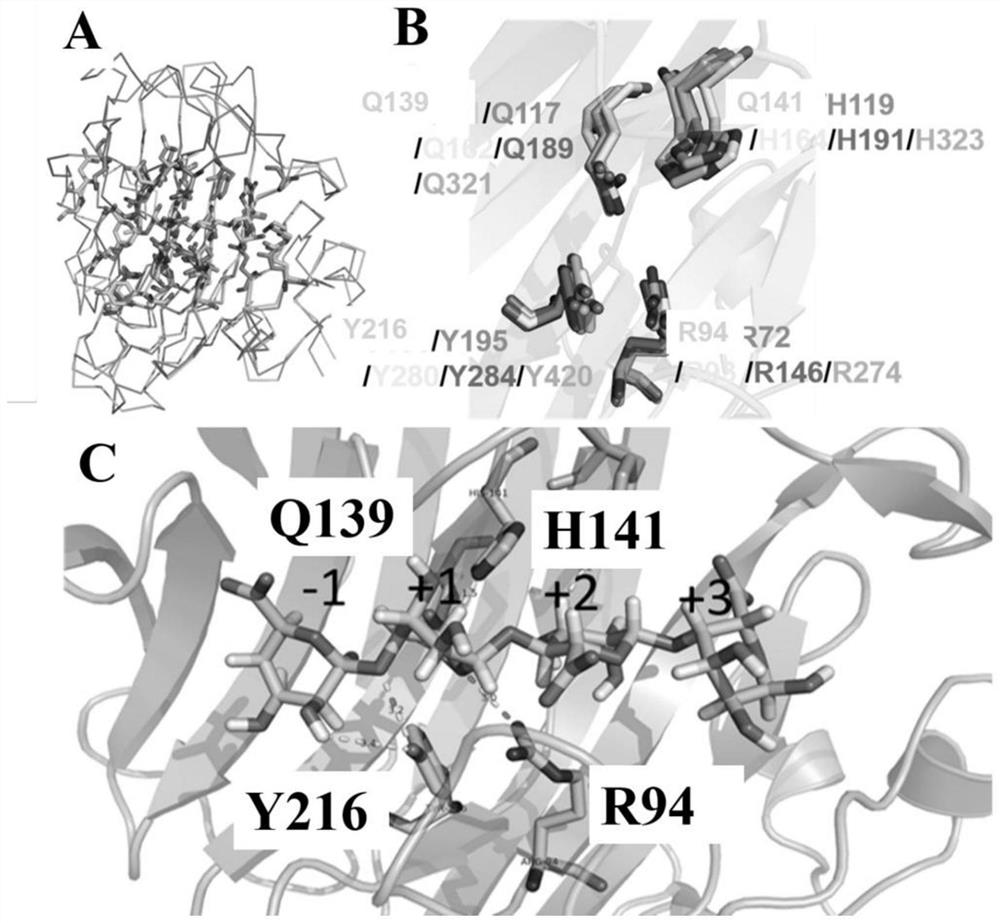
[0073] Example 2. Protein-based sequence and structural analysis of the catalytic amino acid mutation in the active center to study its effect on the enzymatic activity of AlgAT5
[0074] Protein-based sequence and structural analysis of AlgAT5 found that the enzyme is the same as the typical three-dimensional structure of PL7 family alginate lyase, AlgAT5 is also a β-jelly roll fold, containing two anti-parallel β-sheets (sheet A and sheet B ). The structure includes 1 α-helix, 16 β-sheets, and 2 η-helices. Sheet A contains 9 β-strands(β1,residues 13-16;β4,residues66-73;β7,residues 106-113;β8,residues119-125;β9,residues 128-132;β10,residues 136-142; β14,residues 182-185;andβ15,residues 187-189), sheet B contains 7 β-strands(β3,residues 48-53;β6,residues 88-95;β11,residues 151-158;β12,residues 161 -166; β13, residues 169-175 and β16, residues 202-215). The remaining β-strands (β2, residues 39-41; andβ5, residues 77-79), 1 α-helix (α1, residues 4-7), 2 η-helices (η1, residue...
Embodiment 3
[0079] 1) Based on protein sequence and structural analysis, the acquisition of alginate lyase mutants:
[0080] The template used for point mutation was the plasmid template pET30a-AlgAT5. The PCR reaction system for point mutation is as follows:
[0081] Forward primer (10pM) 1μL Reverse primer (10pM) 1μL template DNA 1μL 2×KAPA PCR SuperMix 50μL H 2 O
43μL
[0082] PCR amplification conditions were as follows: pre-denaturation, denaturation, annealing, extension, 33 cycles, extension.
[0083] 1 94℃ 10min 2 94℃ 30sec 3 67℃ 30sec 4 72℃ 6min 5 72℃ 10min
[0084] To obtain different mutants, the primers used are shown in the table below:
[0085]
[0086] According to the above process, the different mutants obtained are:
[0087] The mutant R94A mutates the arginine R at the 94th position of the amino acid sequence of algin lyase AlgAT5 into alanine A, and the amino acid is show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com