Glucagon-like peptide-1 (GLP-1) analogue monomer and dimer, preparation method therefor and application thereof
A technology of glucagon and analogues, applied in GLP-1 dimer, the application field of medicine, can solve the problems of short half-life of liraglutide, short half-life of GLP-1, inconvenient clinical use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the solid-phase synthesis of polypeptide
[0069] Using the solid-phase peptide synthesis method of the Fmoc strategy, the CS 336X instrument produced by CSBio Company was used to synthesize the cysteine-containing GLP-1 monomer of the present invention. The method of synthesis was carried out according to the manufacturer's instruction manual.
[0070] The prepared GLP-1 monomer containing cysteine was purified using an HPLC C18 semi-preparative column, and the mobile phase was acetonitrile. The dry powder of GLP-1 monomer containing cysteine is obtained by desalting and freeze-drying. The disulfide bonds in this example are formed by ammonium bicarbonate or other reducing agents.
Embodiment 2
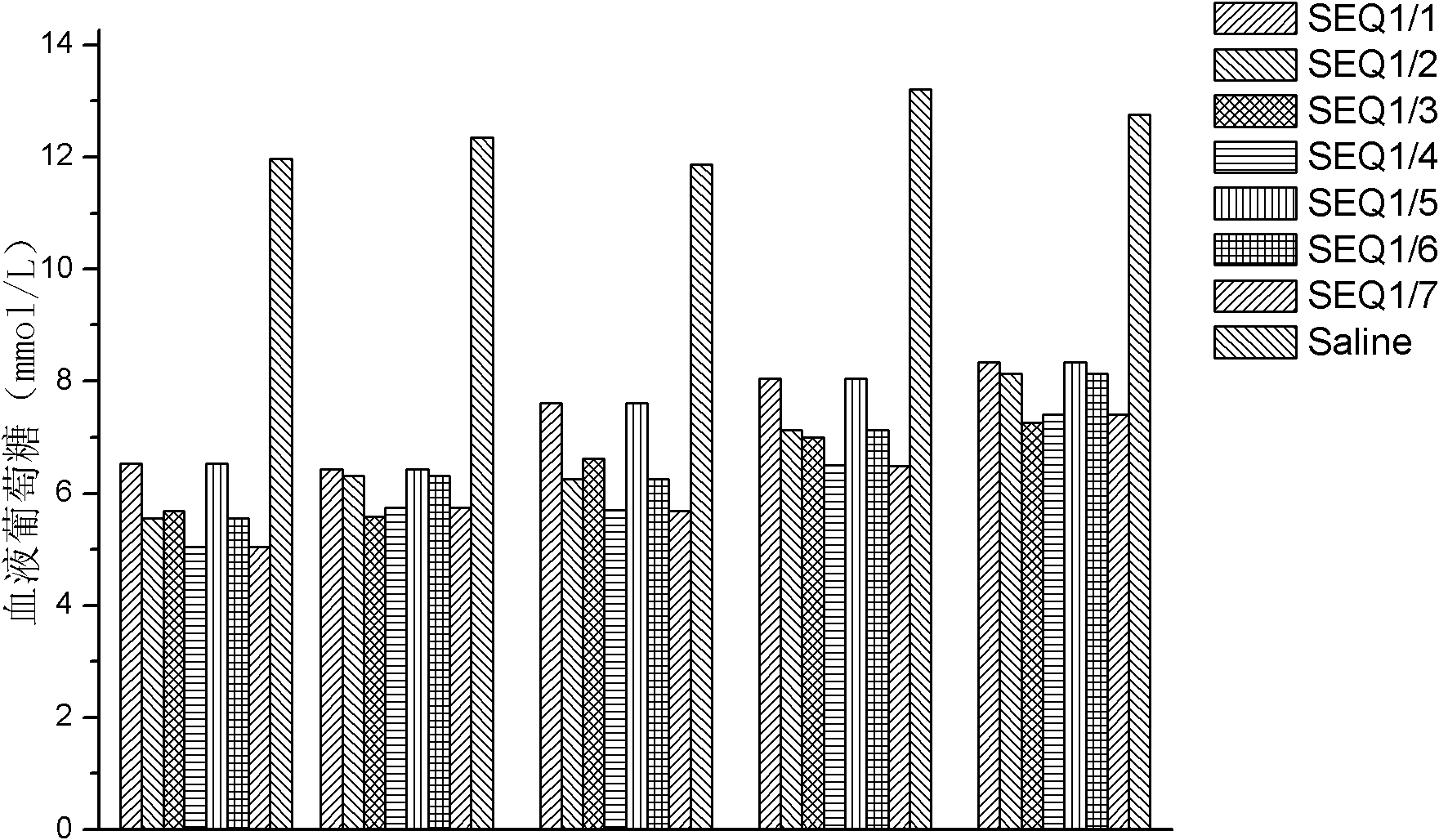
[0071] Example 2: GLP-1 dimer (formed from SEQ ID NO 1 and other GLP-1 monomers) related hypoglycemic function
[0072] In this embodiment, the GLP-1 dimer used is as follows:
[0073] The GLP-1 monomers containing cysteine are SEQ ID NO 1 and the GLP-1 dimer of SEQ ID NO 1, respectively;
[0074] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 1 and SEQ ID NO 2, respectively;
[0075] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 1 and SEQ ID NO 3;
[0076] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 1 and SEQ ID NO 4;
[0077] GLP-1 monomers containing cysteine are dimers of SEQ ID NO 1 and SEQ ID NO 5;
[0078] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 1 and SEQ ID NO 6;
[0079] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 1 and SEQ ID NO 7, respectively.
[0080] Dissolve 1 mg of each of the above-mentioned dimers (7 species in total) in 1 ml of norma...
Embodiment 3
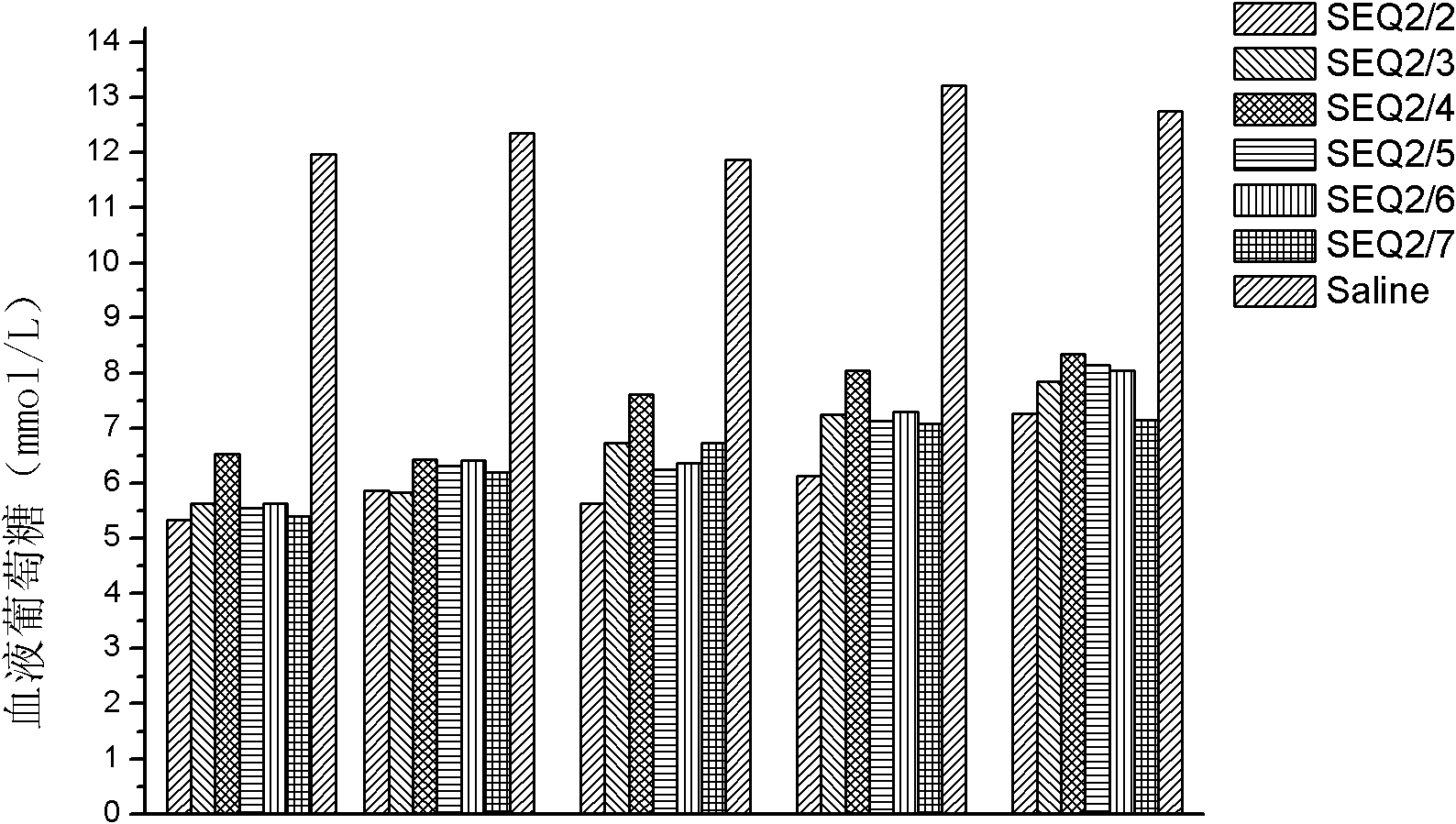
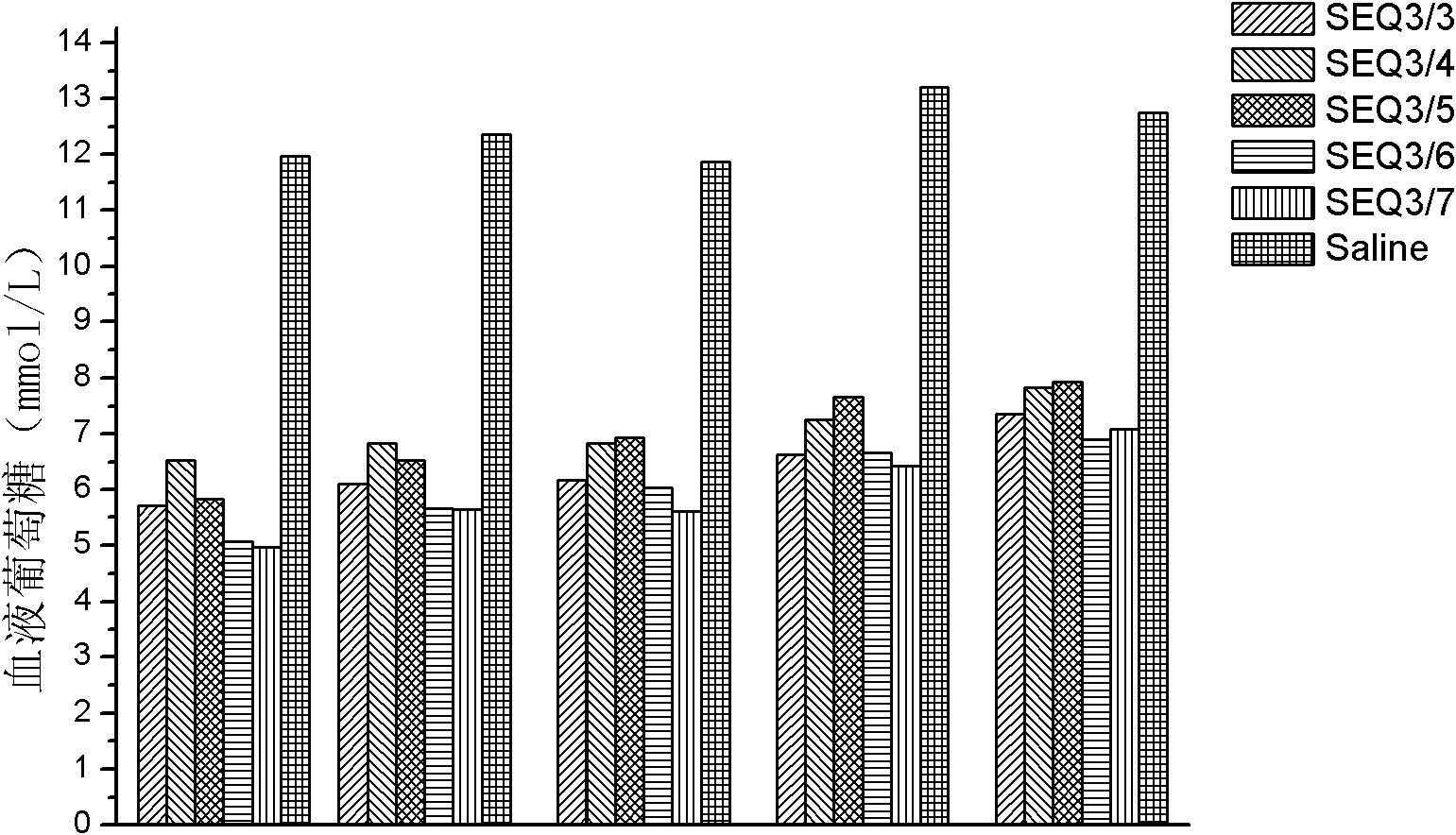
[0082] Example 3: GLP-1 dimer (formed by SEQ ID NO 2 and other GLP-1 monomers) related hypoglycemic function.
[0083] In this embodiment, the GLP-1 dimer used is as follows:
[0084] The GLP-1 monomers containing cysteine are SEQ ID NO 2 and the GLP-1 dimer of SEQ ID NO 2;
[0085] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 2 and SEQ ID NO 3;
[0086] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 2 and SEQ ID NO 4, respectively;
[0087] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 2 and SEQ ID NO 5;
[0088] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 2 and SEQ ID NO 6;
[0089] GLP-1 monomers containing cysteine are GLP-1 dimers of SEQ ID NO 2 and SEQ ID NO 7, respectively.
[0090] Dissolve 1 mg of each of the above-mentioned dimers (a total of 6 species) in 1 ml of normal saline, and inject them subcutaneously into mice (200 μl / mouse, 6 / group, purchased from Shanghai Expe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com