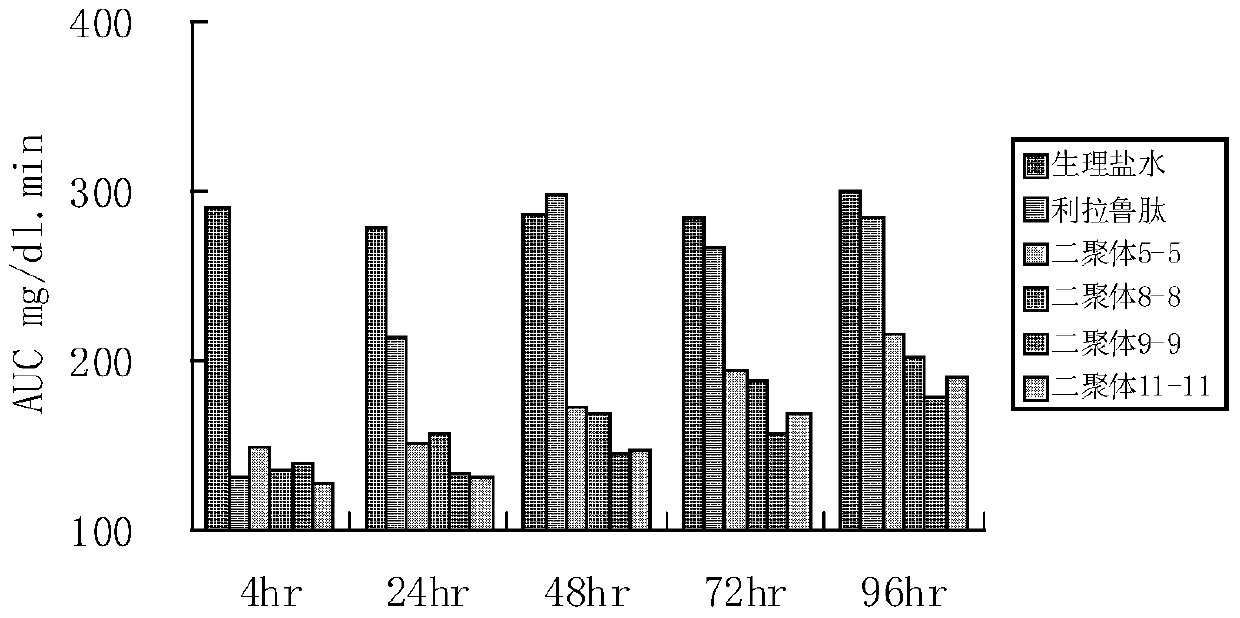
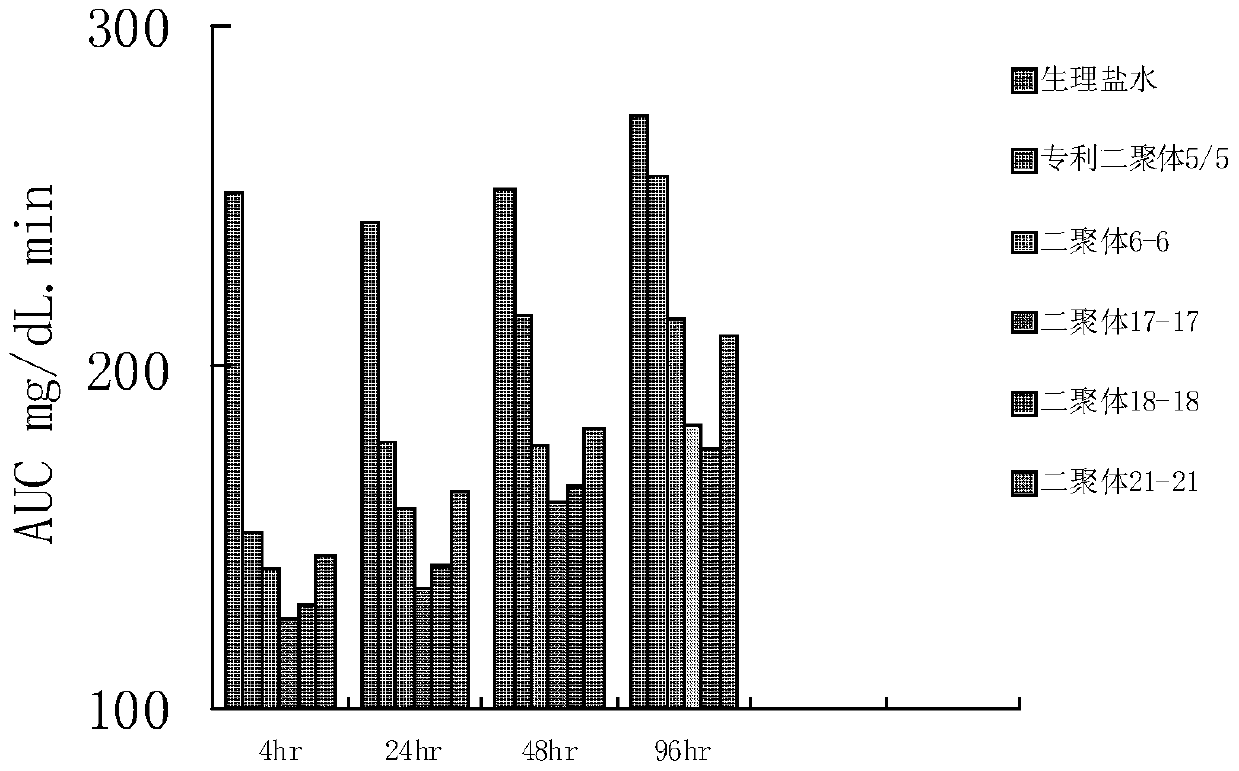
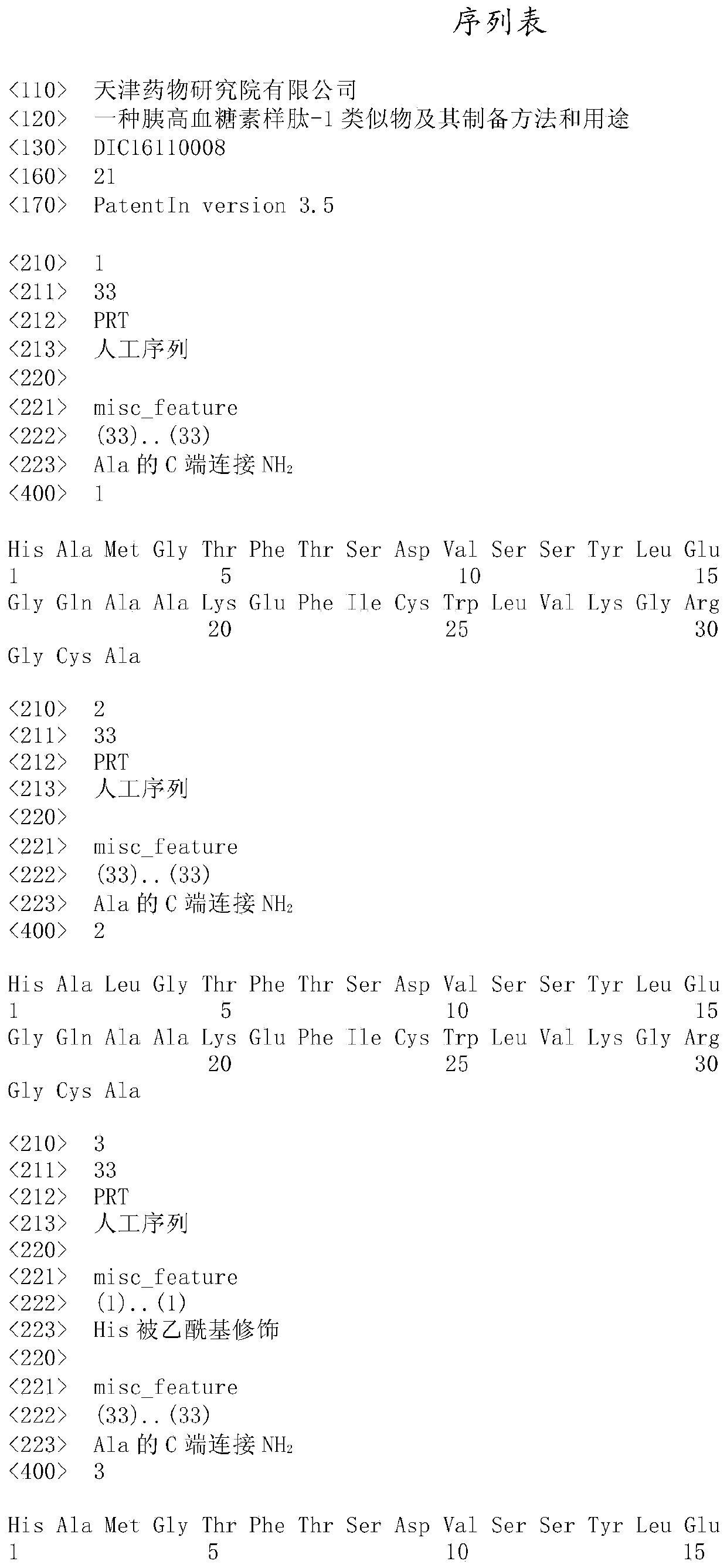
A kind of glucagon-like peptide-1 analogue and its preparation method and application
A technology for glucagon and analogues, which is applied in the field of application of glucagon-like peptide-1 (GLP-1) analogues and dimers, drugs, and can solve the problems of drug compliance that needs to be improved , to achieve the effects of improving clinical application compliance, avoiding safety risks, and overcoming short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of GLP-1 analogue monomer and dimer
[0051] A. Preparation of GLP-1 analogue monomer:
[0052] 1) Synthesis: The Fmoc method is used to implement the synthesis method step by step according to the following steps:
[0053] a) Fmoc-Arg-resin is obtained by coupling amino resin solid phase carrier and Fmoc protected arginine in the presence of activator system;
[0054] b) Connect amino acids in the amino acid sequence of the peptide sequence by solid-phase synthesis to obtain N-terminal Fmoc-protected and side chain protected peptide-resin conjugates; the amino acids with side chains take the following protective measures: tryptophan with tert-butoxy Carbonyl (Boc), oxy-tert-butyl (OtBu) for glutamic acid, tert-butoxycarbonyl (Boc) for lysine, trityl (Trt) for glutamine, tert-butyl for tyrosine (tBu) ), Serine uses trityl (Trt) or tert-butyl (tBu), aspartic acid uses oxy-tert-butyl (OtBu), threonine uses tert-butyl (tBu), histidine uses trityl Group (T...
Embodiment 2
[0070] Example 2 Preparation of N-terminal acylated GLP-1 analog dimer
[0071] 1) Synthesize N-terminal Fmoc-protected and side chain protected peptide-resin conjugates according to the monomer synthesis method in Example 1;
[0072] 2) The N-terminal Fmoc protective group is removed by conventional methods, the resin-peptide conjugate is suspended in an appropriate amount of pyridine, and an appropriate molar ratio of acetic anhydride or trifluoroacetic anhydride is added, mixed and placed to obtain N-terminal histidine Chemical peptide-resin conjugate;
[0073] 3) Cleavage according to the method in Example 1 to obtain the crude peptide, purify and freeze-dry to obtain the monomer target peptide;
[0074] 4) Prepare the dimer according to the method in Example 1.
[0075] The GLP-1 analog dimer formed by the monomers of the following sequence was prepared according to the above method:
[0076] The GLP-1 analog dimer 3-3 formed by SEQ ID NO 3 and SEQ ID NO 3,
[0077] GLP-1 analog d...
Embodiment 3
[0081] Example 3 Preparation of GLP-1 analogue monomer and dimer
[0082] A. Preparation of GLP-1 analogue monomer:
[0083] 1) Synthesis: The Fmoc method is used to implement the synthesis method step by step according to the following steps:
[0084] a) Fmoc-Ala-resin is obtained by coupling amino resin solid phase carrier and Fmoc protected glycine in the presence of activator system;
[0085] b) Connect amino acids in the amino acid sequence of the peptide sequence by solid-phase synthesis to obtain N-terminal Fmoc-protected and side chain protected peptide-resin conjugates; the amino acids with side chains take the following protective measures: tryptophan with tert-butoxy Carbonyl (Boc), oxy-tert-butyl (OtBu) for glutamic acid, tert-butoxycarbonyl (Boc) for lysine, trityl (Trt) for glutamine, tert-butyl for tyrosine (tBu) ), Serine uses trityl (Trt) or tert-butyl (tBu), aspartic acid uses oxy-tert-butyl (OtBu), threonine uses tert-butyl (tBu), histidine uses trityl Group (Tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com