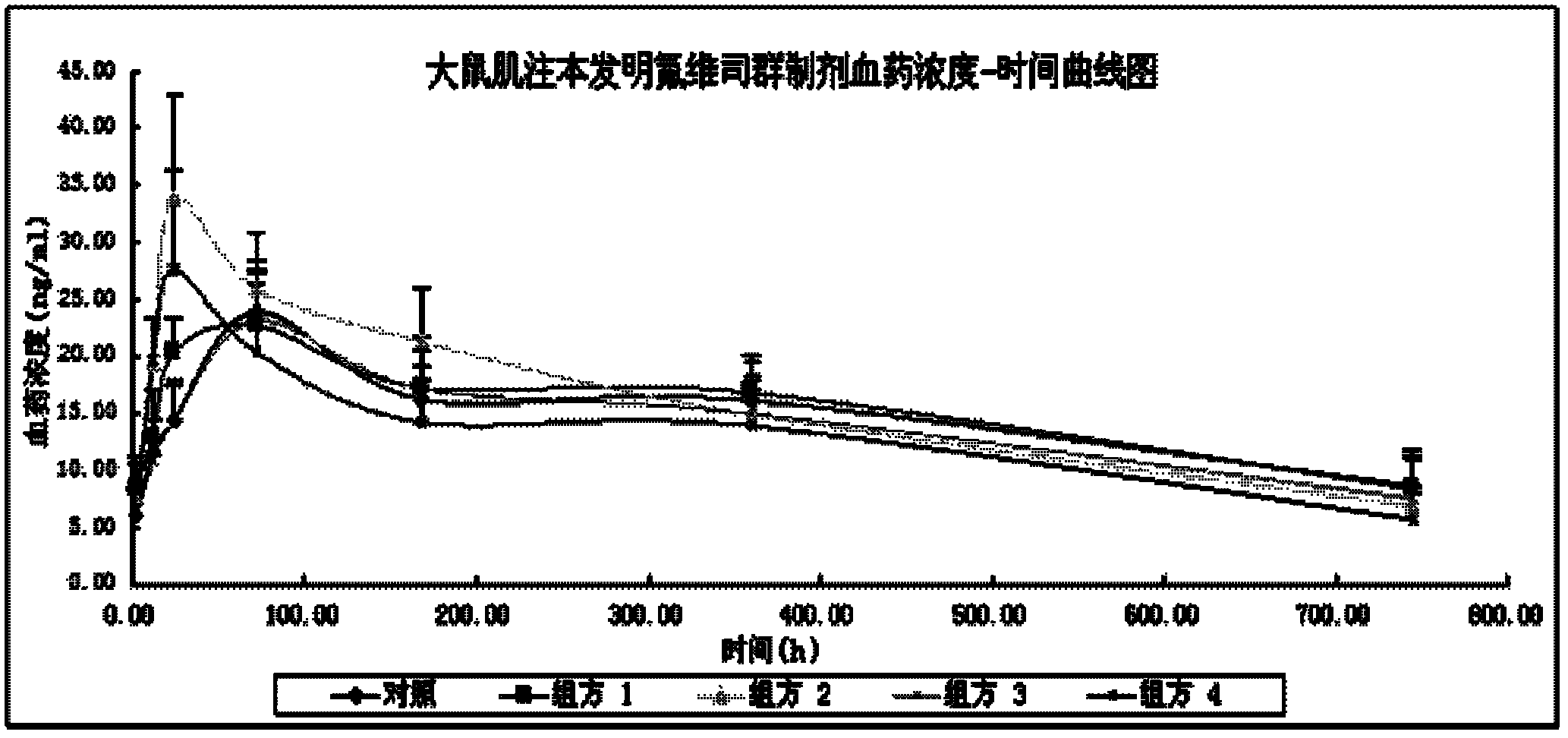
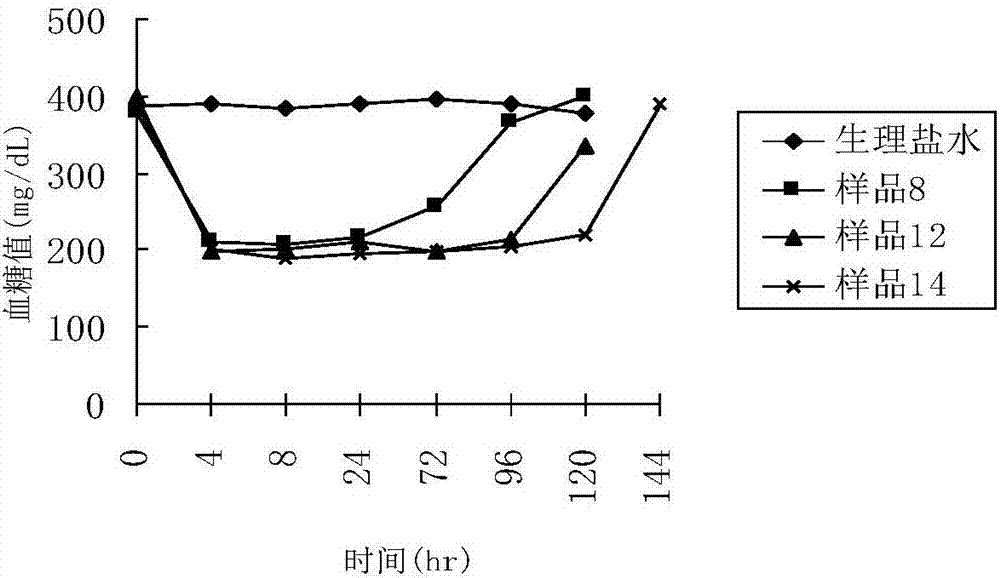
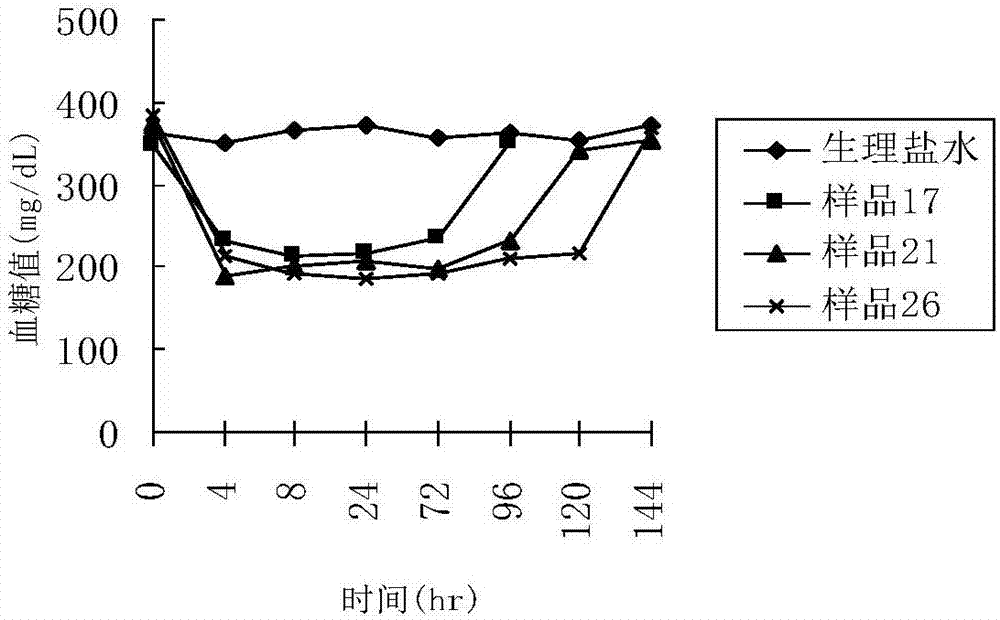
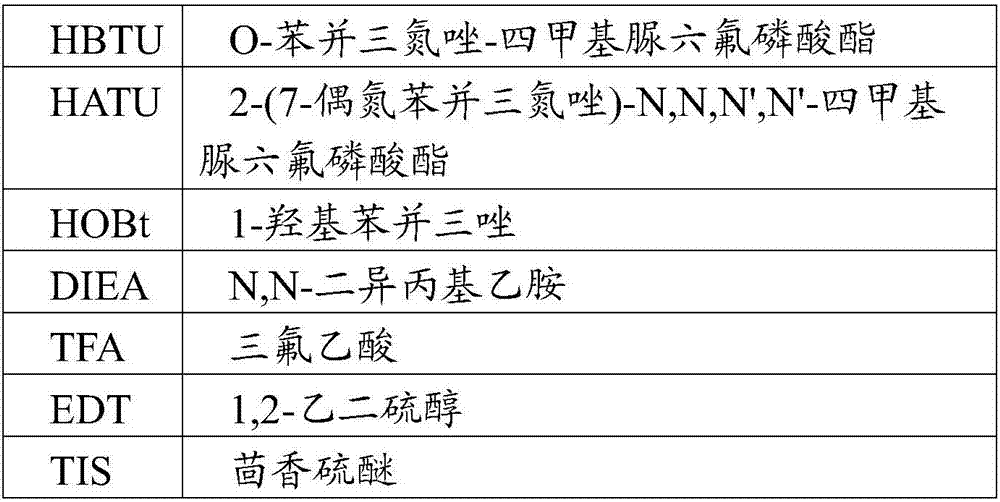
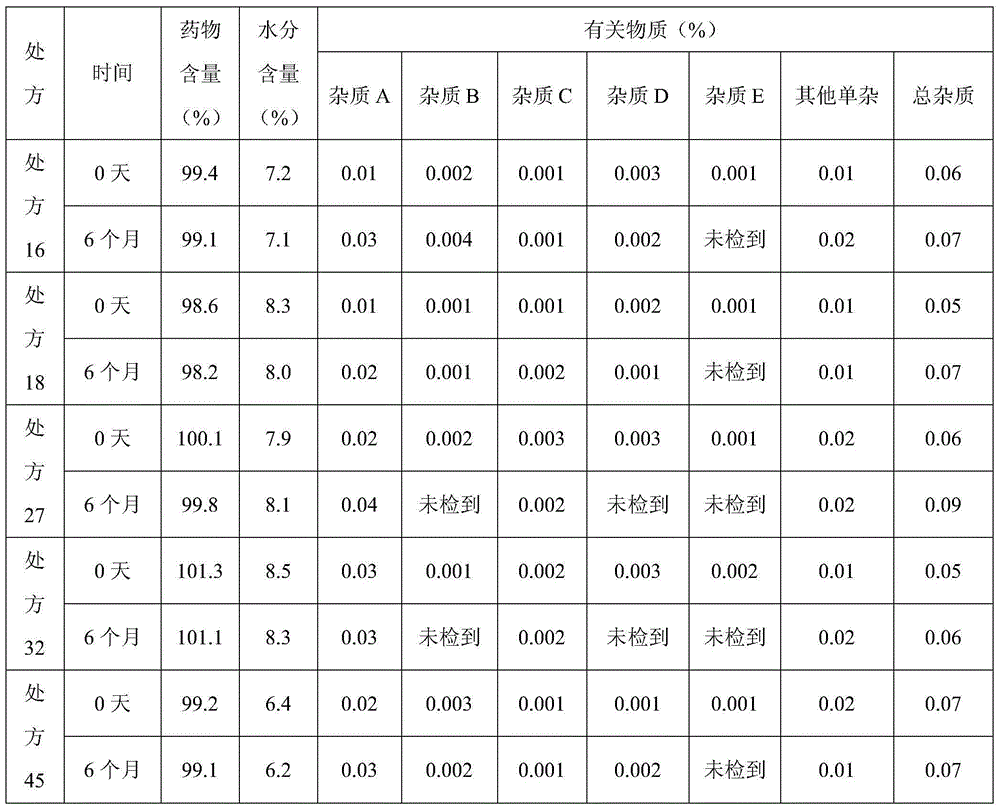
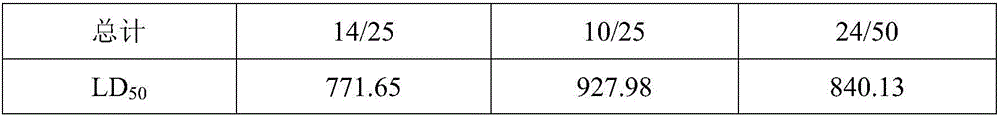
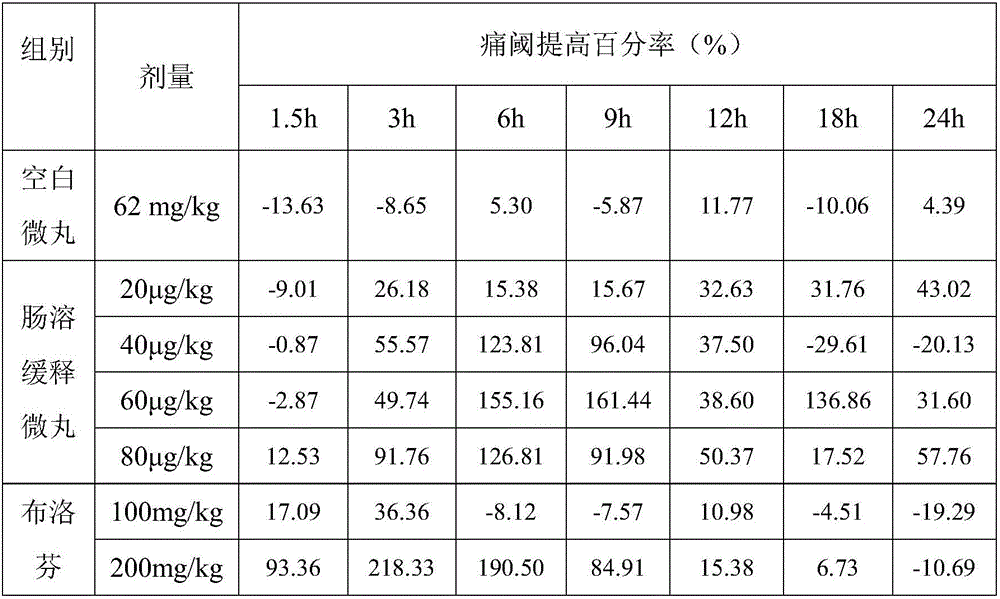
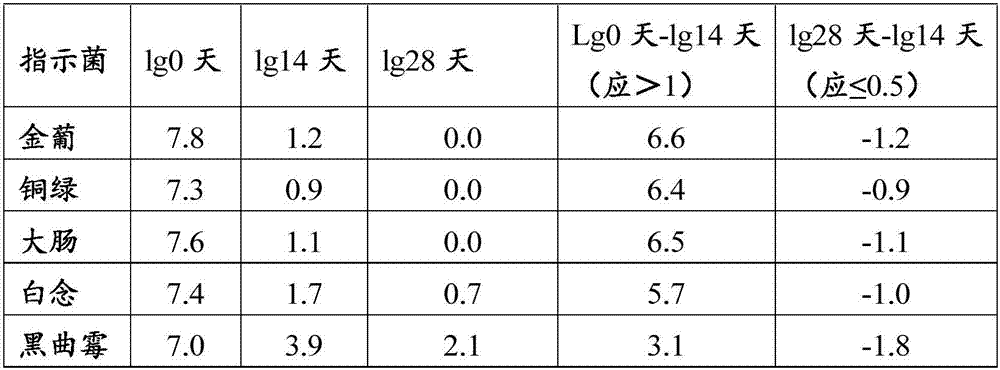
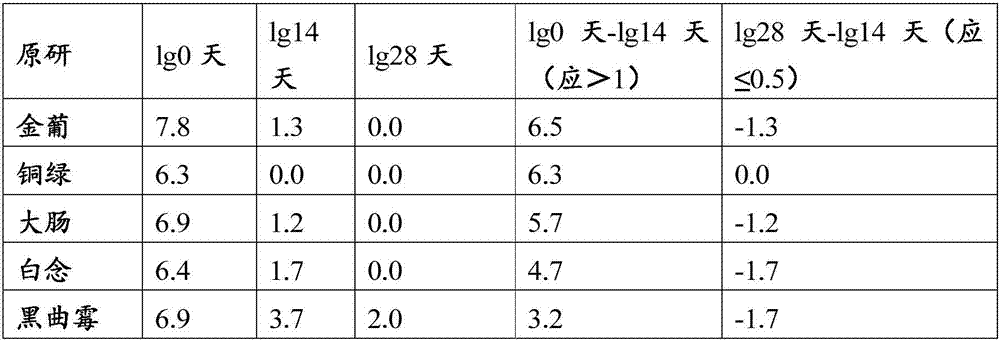
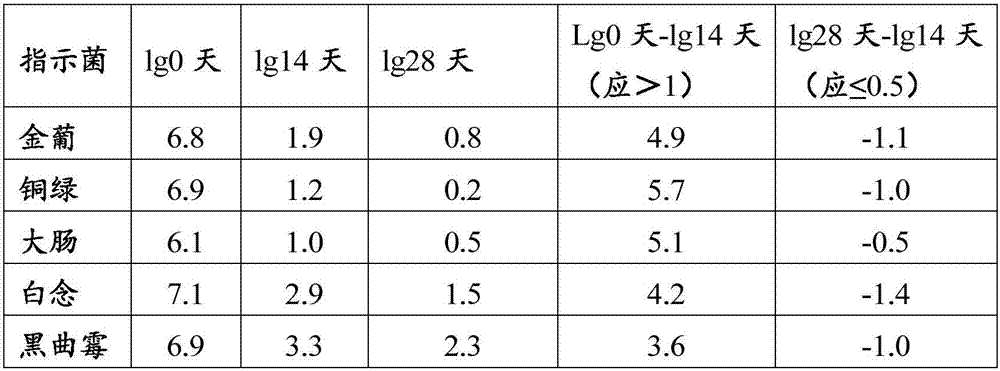
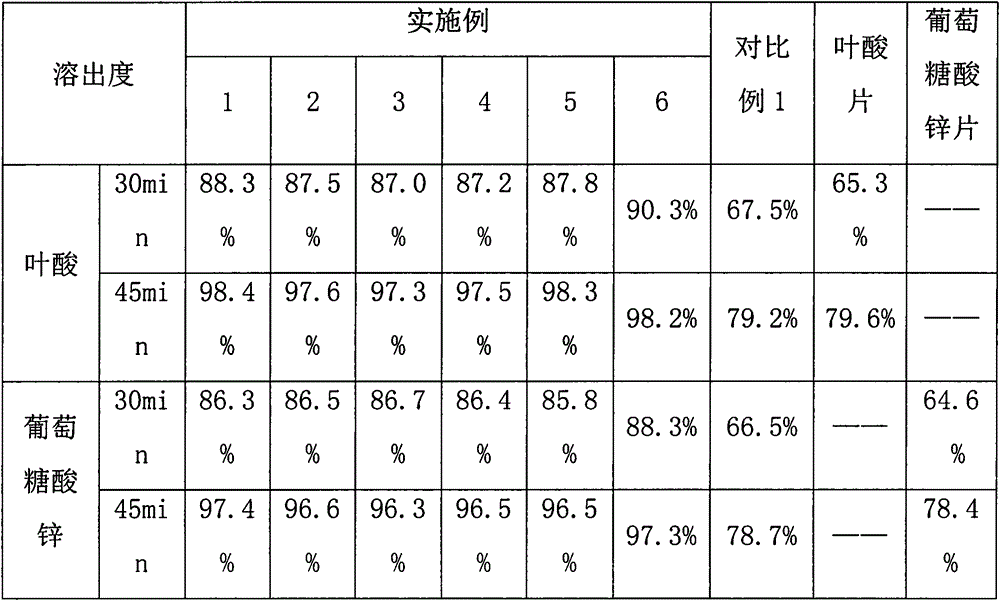
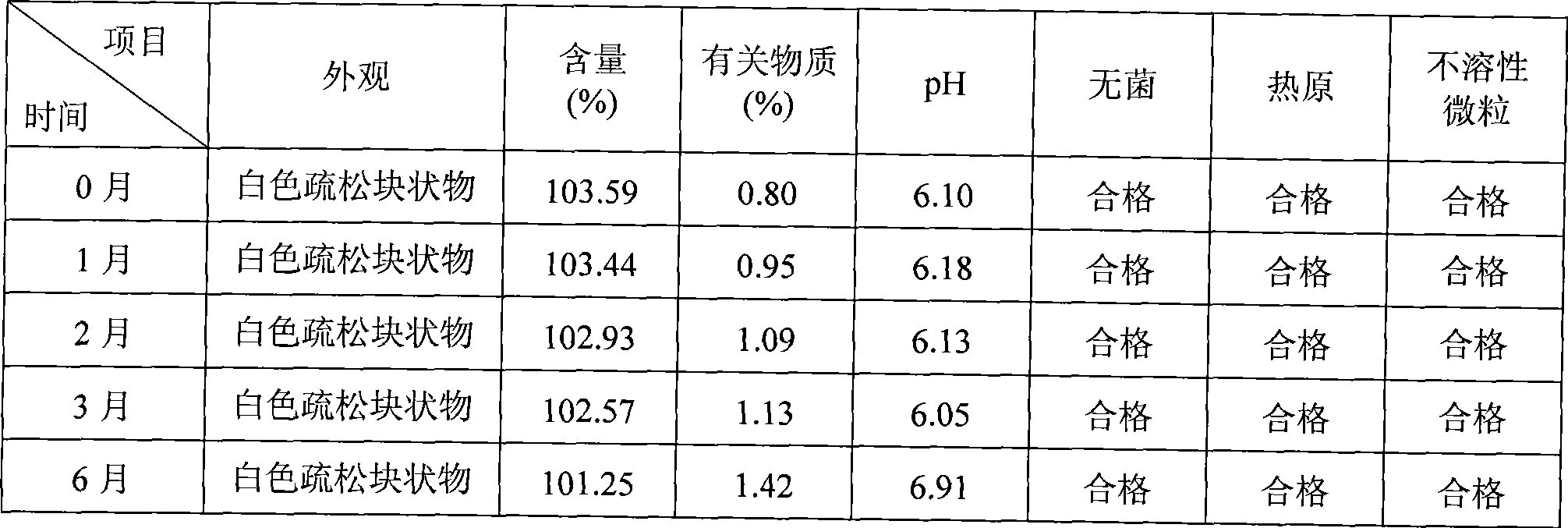
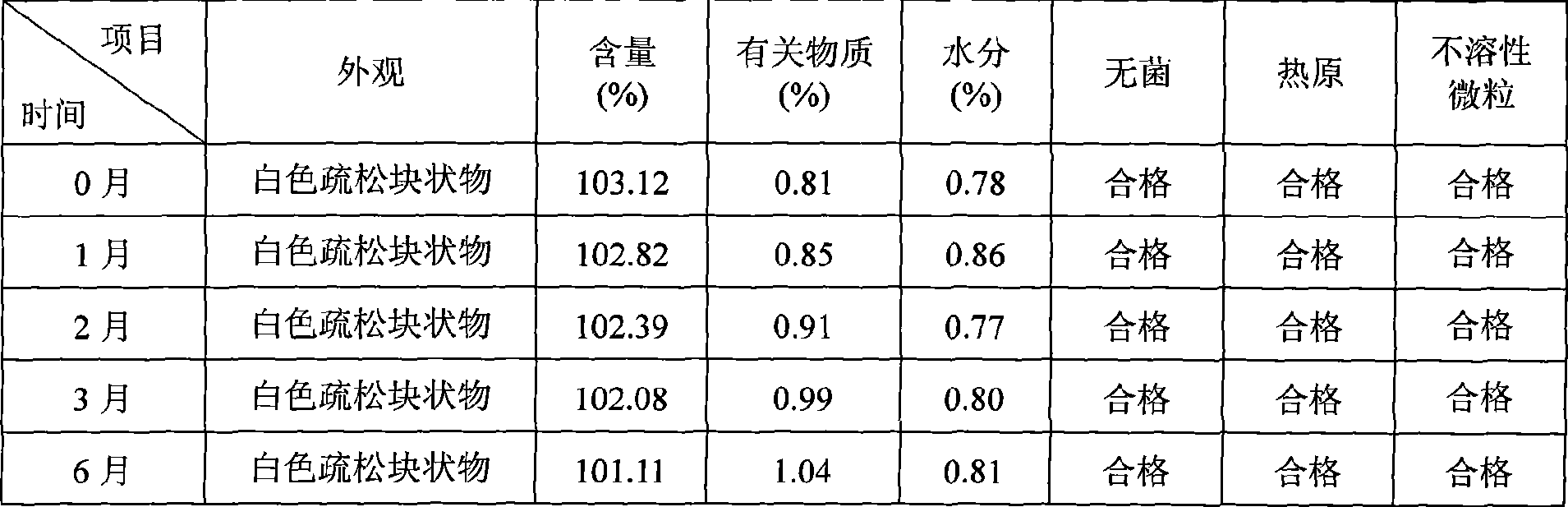
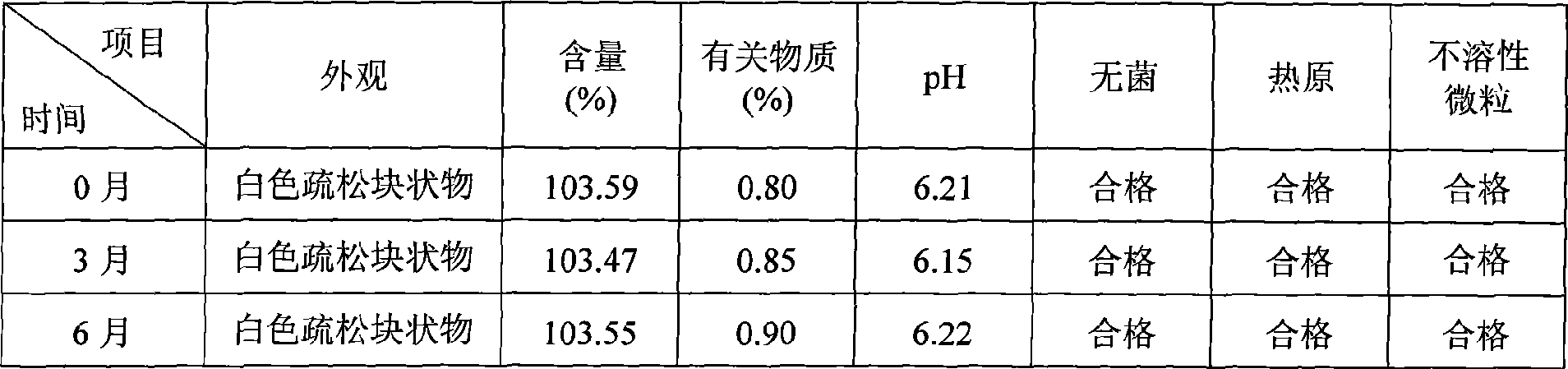
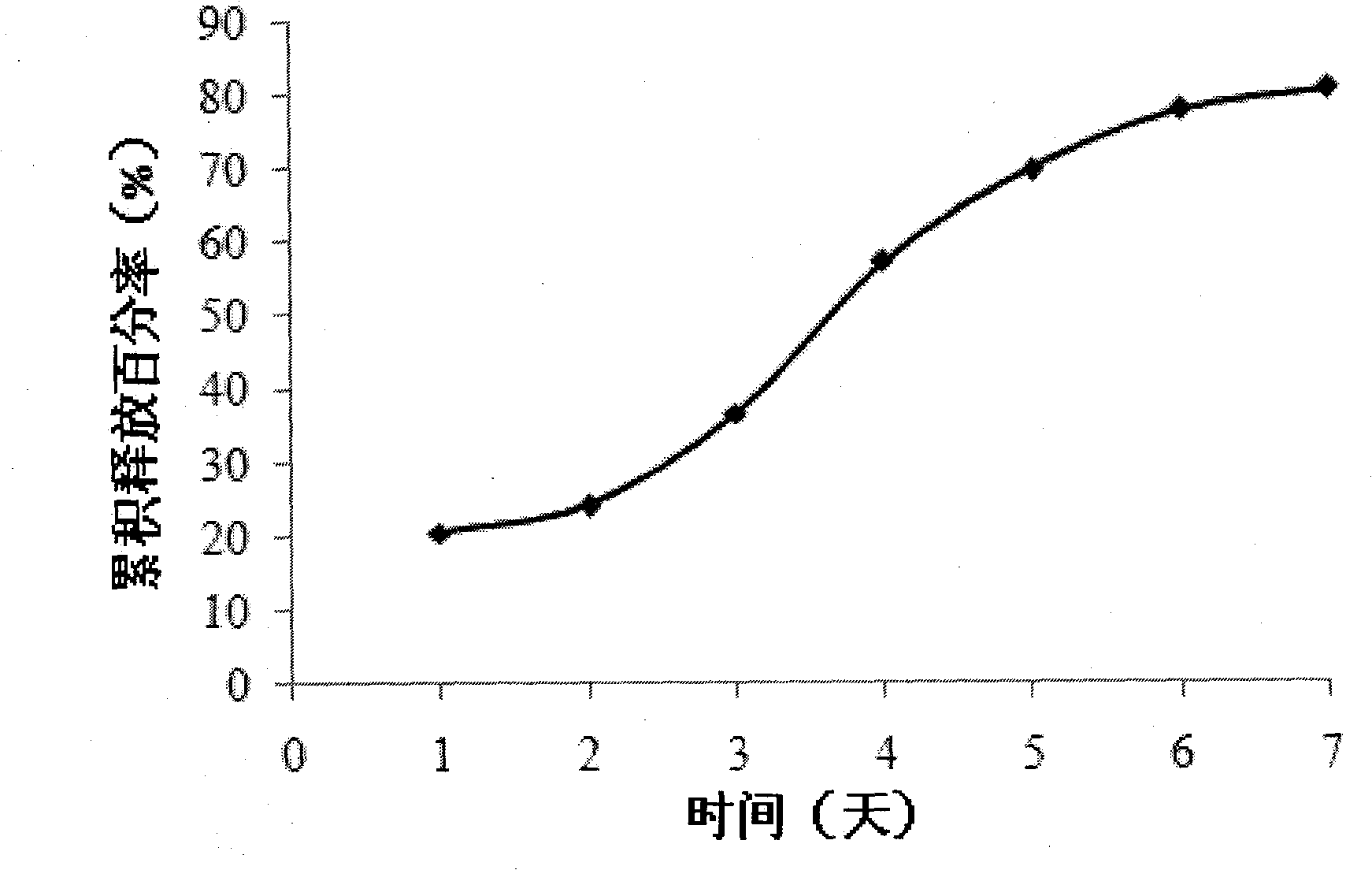
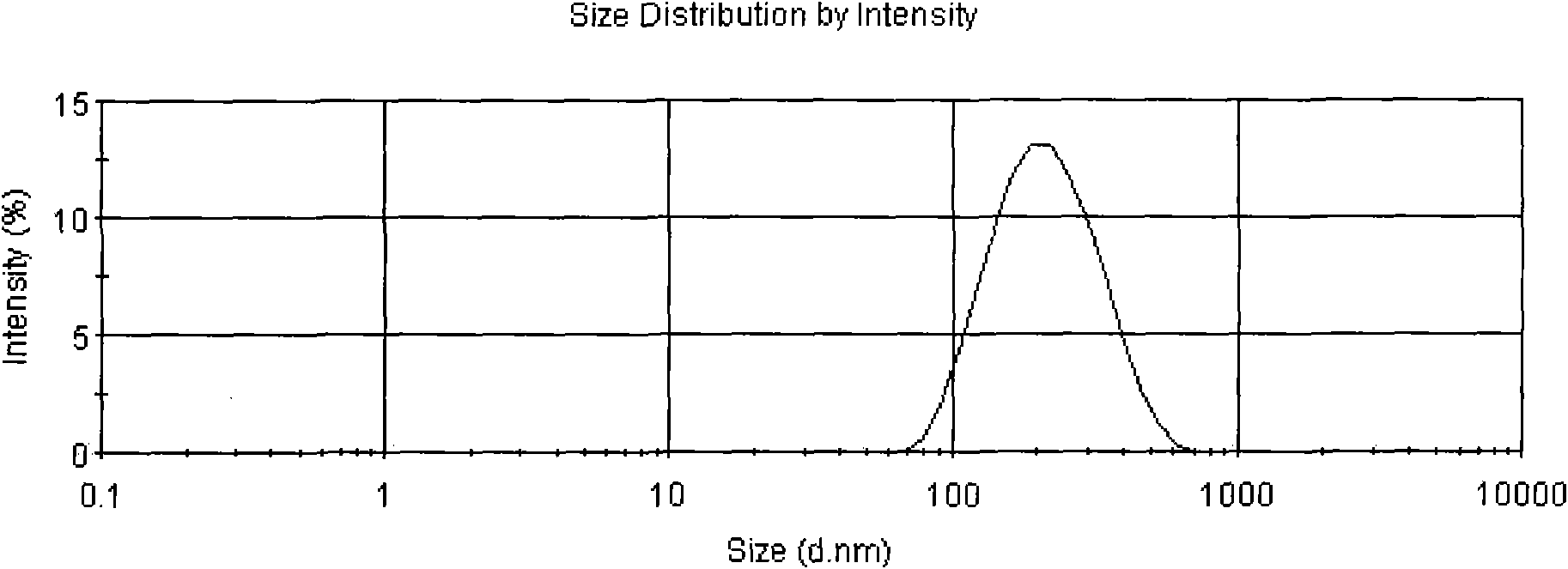
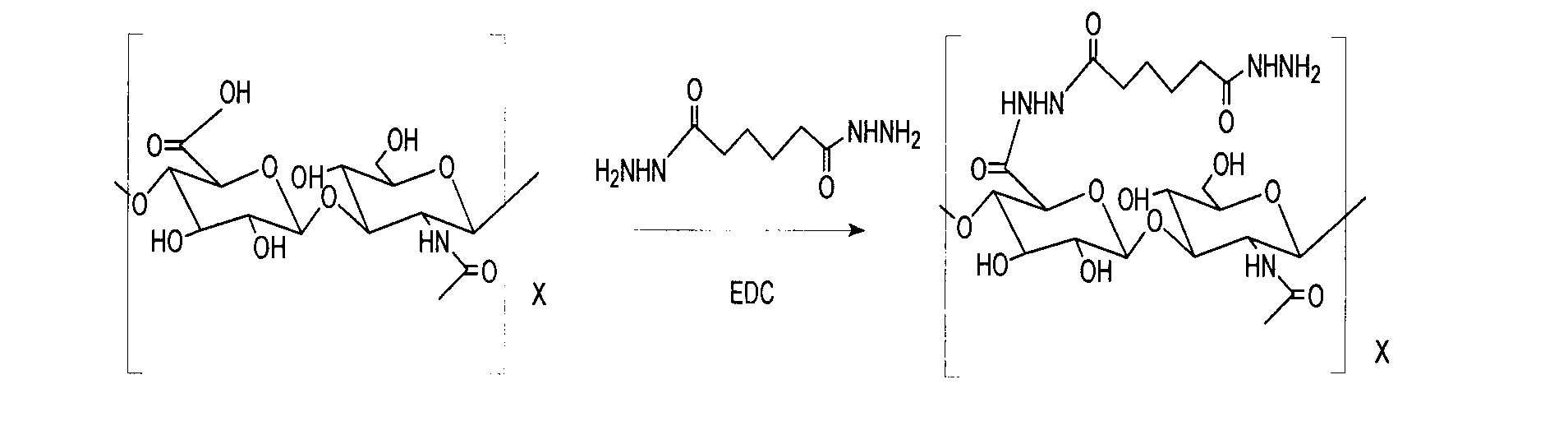
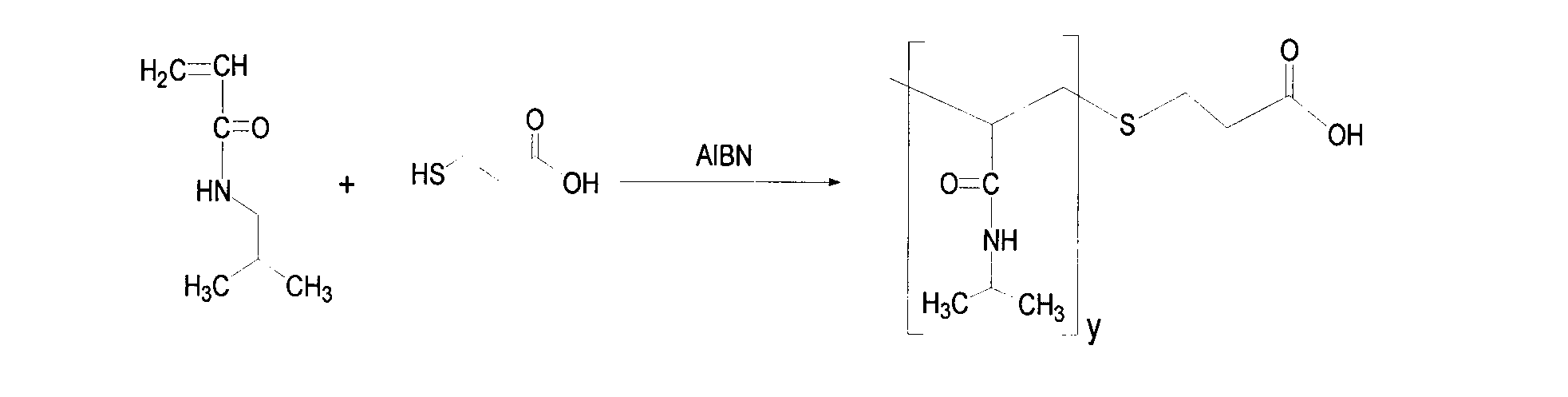
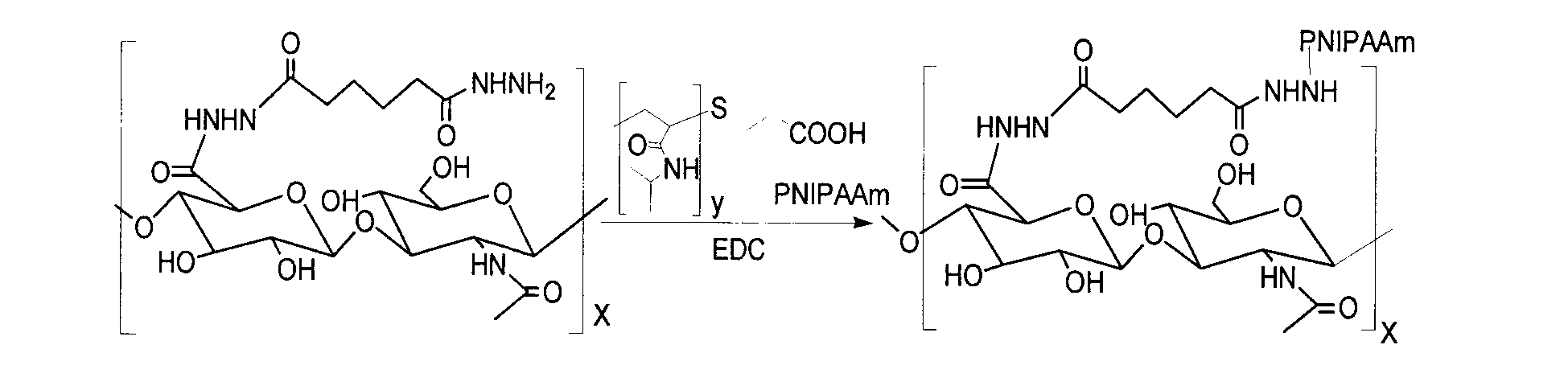
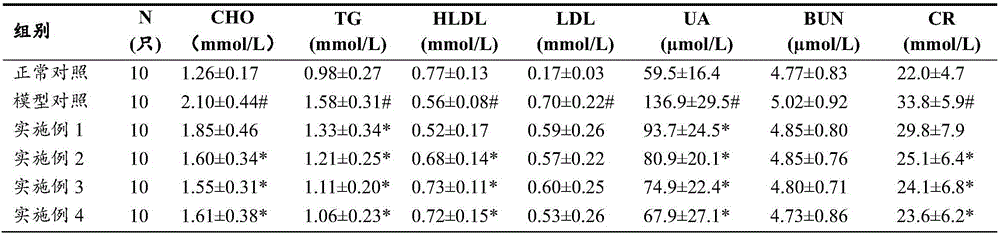
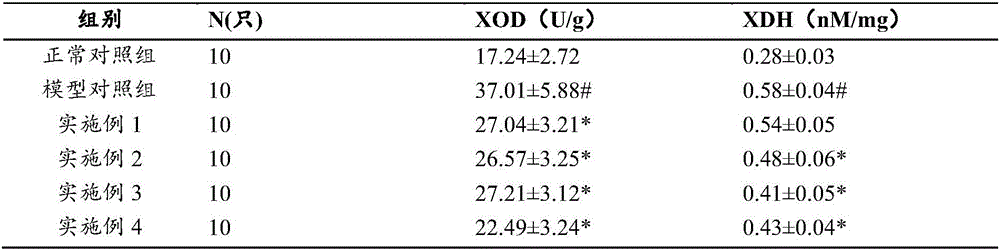
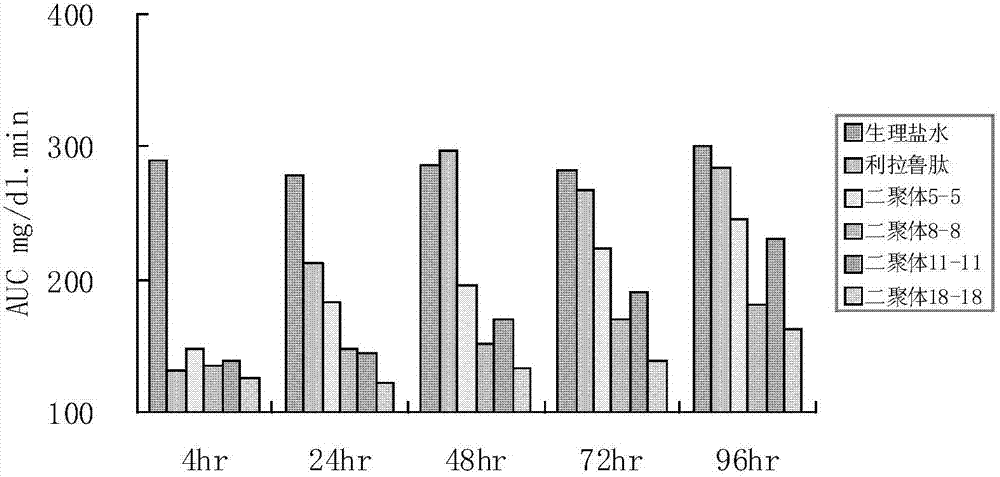
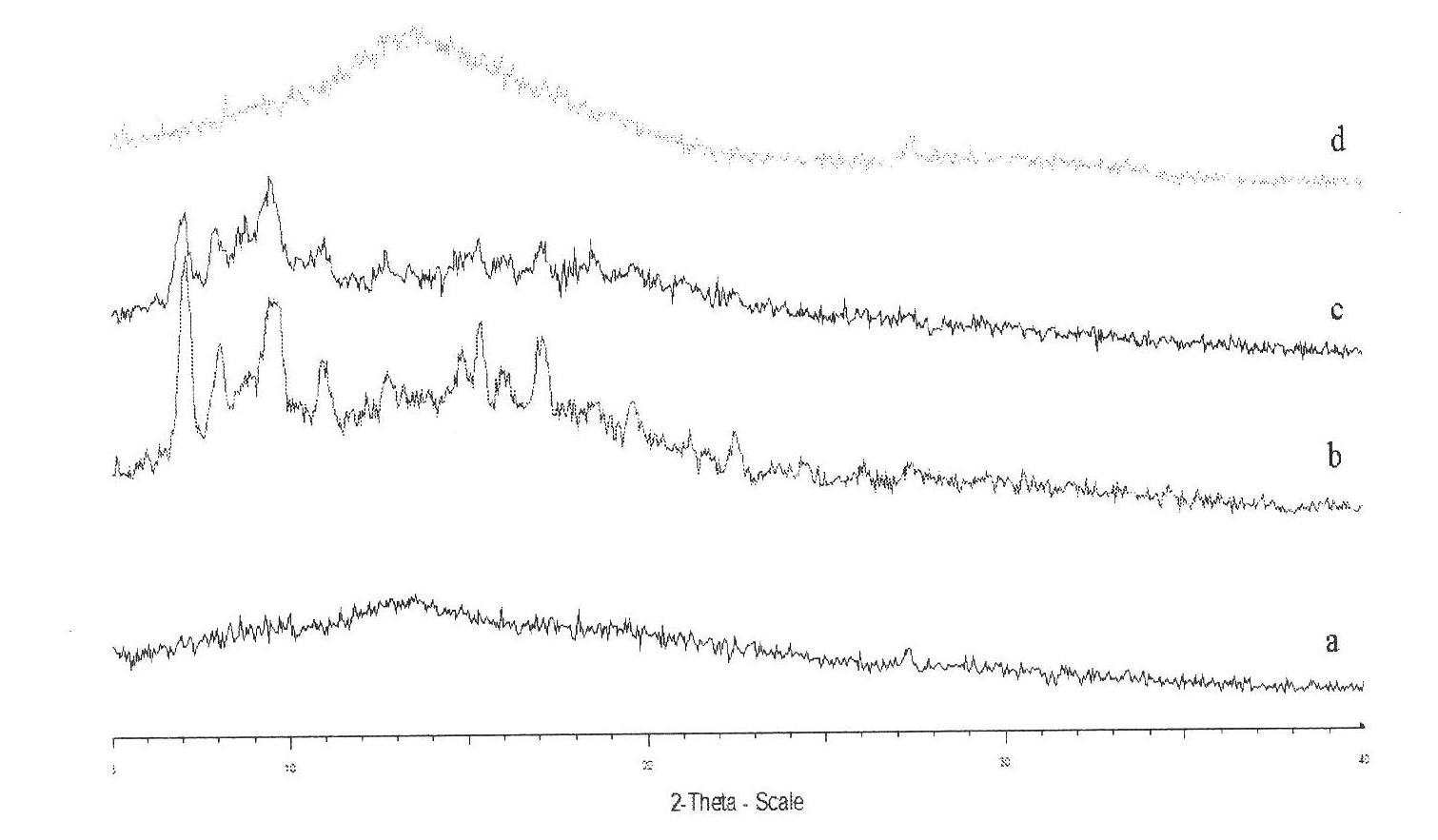
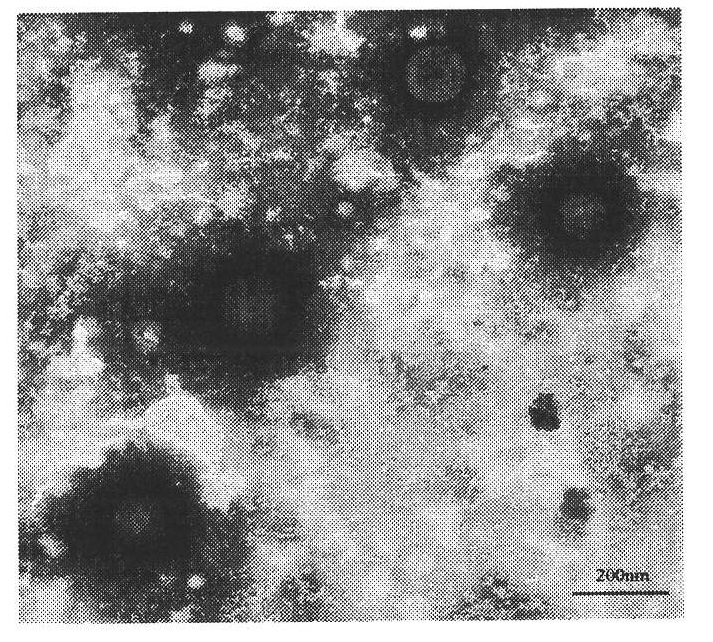
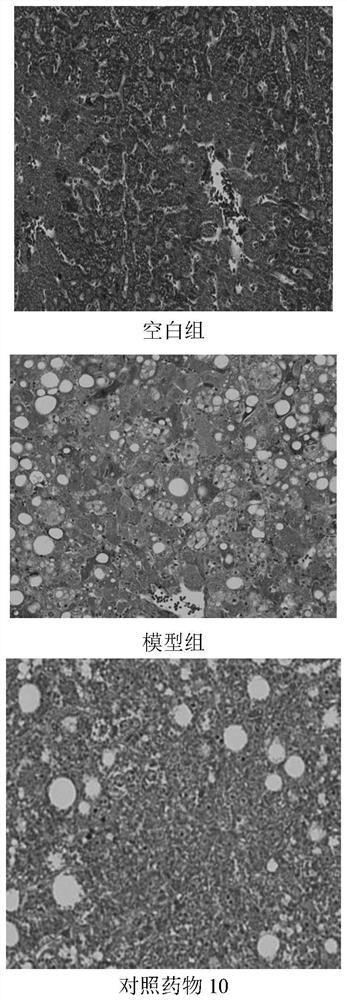
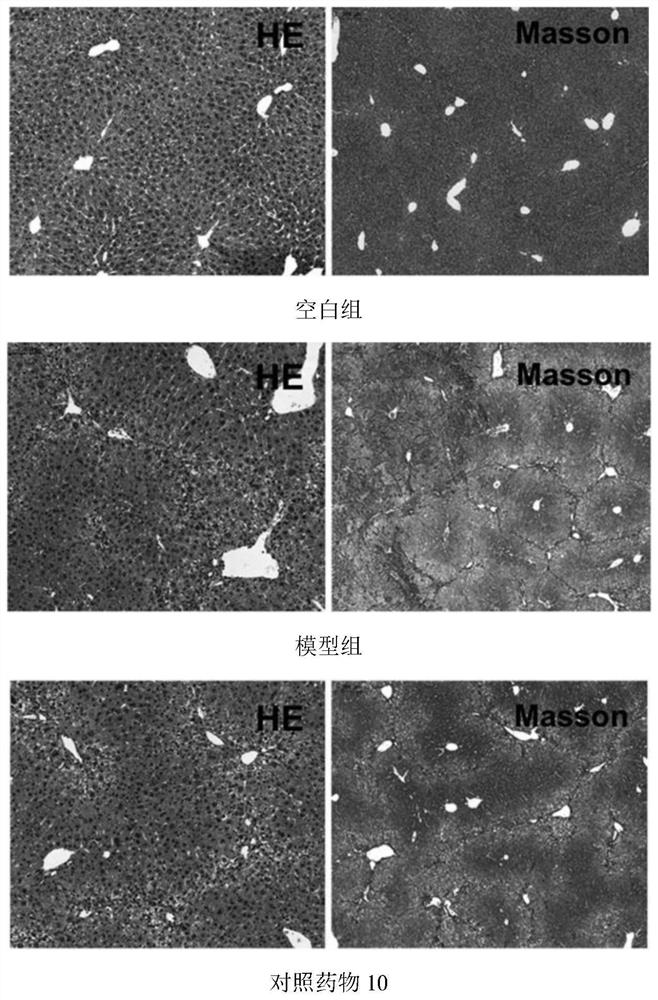
Patents
Literature
64results about How to "Improve clinical compliance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
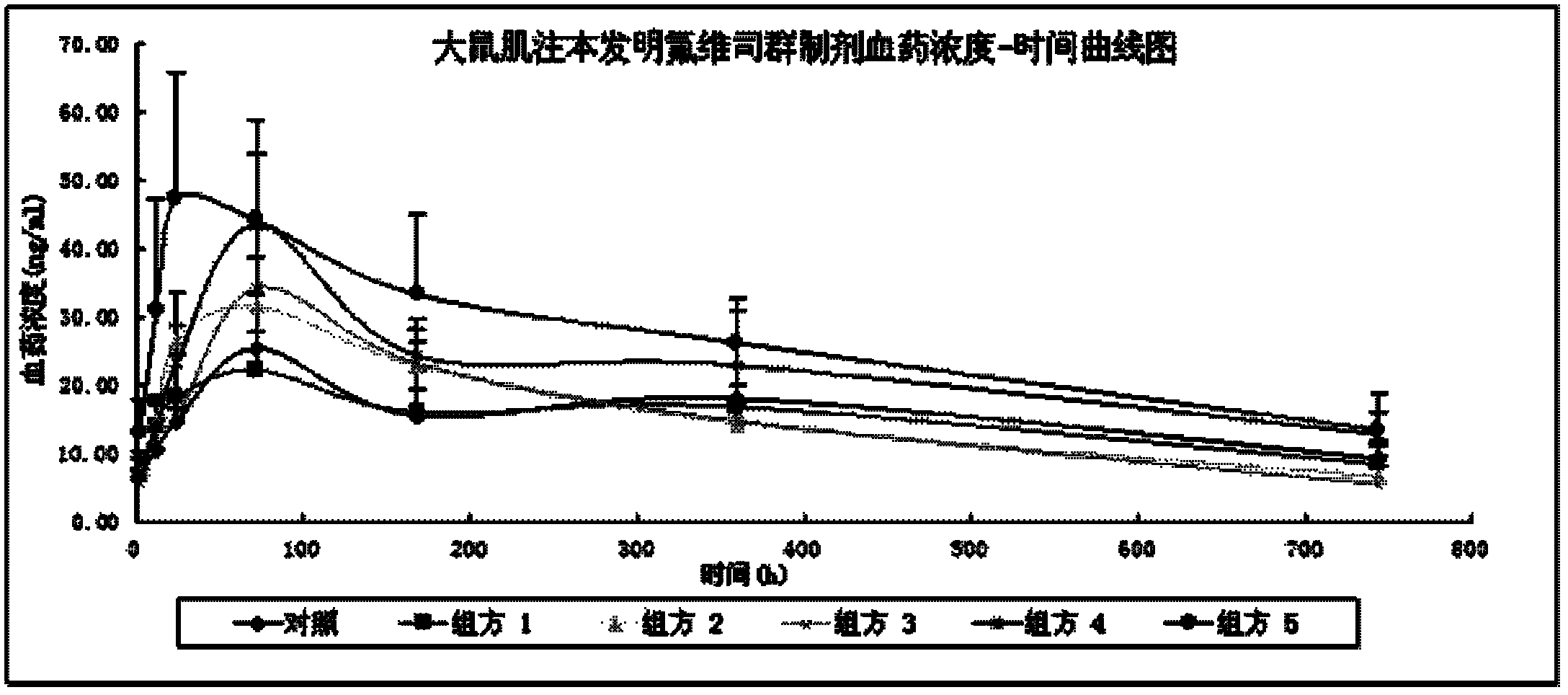
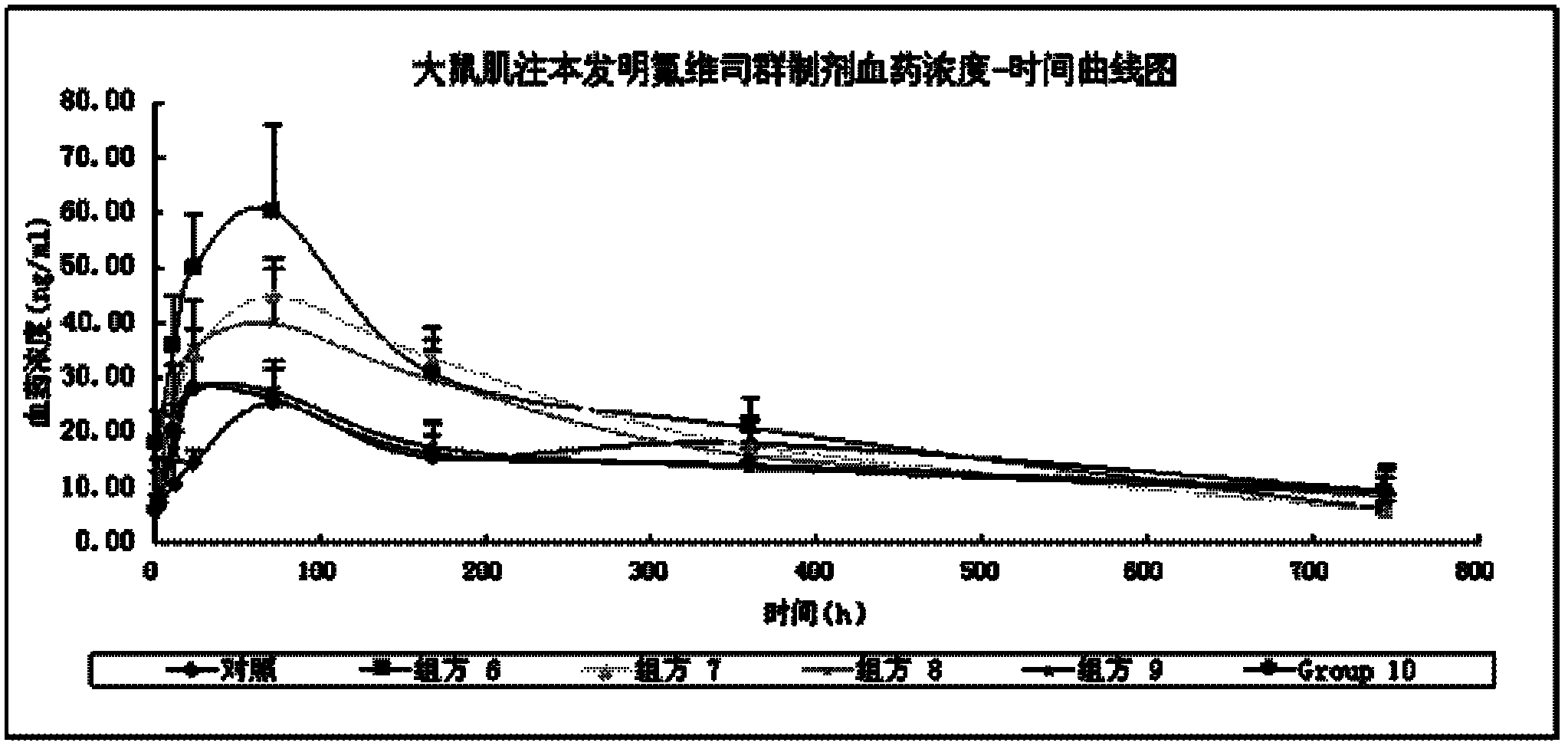
Long acting drug delivery system for treating breast cancer and preparation method and applications thereof
InactiveCN108159055AEnsure safetyIncrease concentrationOrganic active ingredientsPharmaceutical delivery mechanismProcainePhospholipid
The invention provides a long acting drug delivery system, which comprises fulvestrant or derivatives thereof and takes phospholipid as a sustained release material. The invention mainly discloses a high concentration formula of fulvestrant or derivatives thereof. The main component is fulvestrant or derivatives thereof. The sustained release material is different phospholipids or a mixture of phospholipids and different kinds of plant oil. The solvent is at least one of ethanol, ethyl lactate, 1,2-propylene glycol, and ethyl acetate. The analgesic is benzyl alcohol, lidocaine, procaine, or ropivacaine. The viscosity of the formula is 20-45 mPa.s, and the concentration of the formula is 60-300 mg / mL. The antioxidant of the formula is Ve, lipoic acid, and the like. The phospholipids can increase mutual solubility and has a sustained release function. The preparation is prepared in an aseptic filtration mode.
Owner:XIAN LIBANG PHARMA TECH
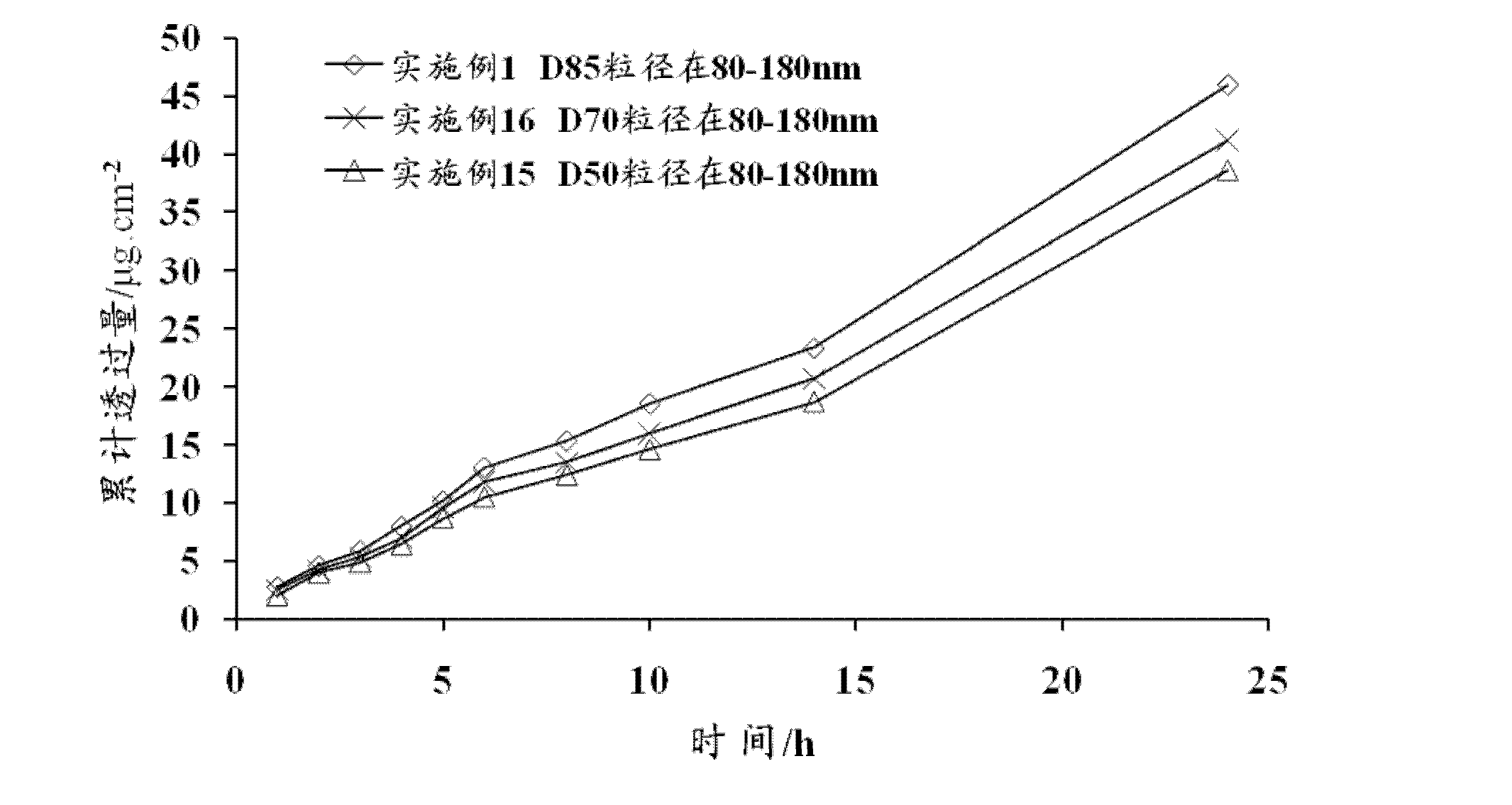
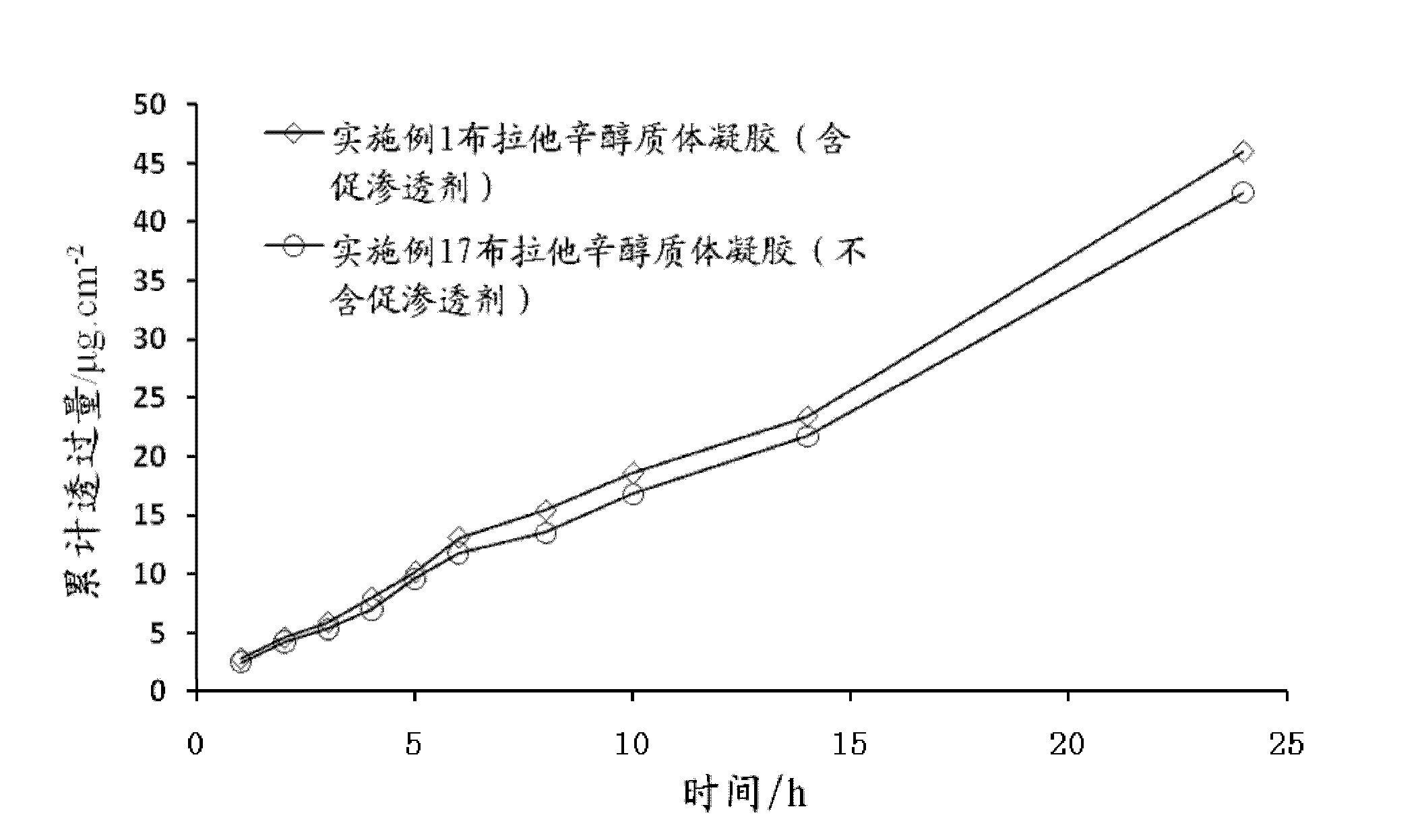
Bullatacin ethosome gel and preparation method thereof
InactiveCN102552147AImproves transdermal penetrationGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholAntioxidant
The invention discloses a bullatacin ethosome gel and a preparation method thereof. The bullatacin ethosome gel comprises ethosomes and a gel, wherein the ethosomes and the gel exit in a uniformly mixed state; the ethosomes are particles containing bullatacin, phospholipids, low-molecular weight alcohols, cholesterol, stabilizers, antioxidants and water; the particle size of the ethosomes ranges from 30 nm to 400 nm; the gel contains a gel matrix, penetration enhancers, humectant, antiseptics, pH regulators and water; and the ethosomes and the gel are combined at a certain weight ratio, and the ethosomes and the gel have specific compositions respectively. The bullatacin ethosome gel provided by the invention can reduce irritation to the skin and has good percutaneous penetration effects.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD
Small golden pellets and preparation method
ActiveCN1726969AEasy to swallowReduce lossesMammal material medical ingredientsGranular deliveryMyrrhCurative effect
A Chinese medicine ''Xiaojin micropill'' is prepared from 10 Chinese-medicinal materials including musk, myrrh, Chinese angelica root, earthworm, etc. Its preparing process is also disclosed. Its advantages are short dissolving time and quickly taking its high curative effect.
Owner:CHENGDU YONGKANG PHARM CO LTD
Fulvestrant or fulvestrant derivative sustained release preparation and preparation method thereof
InactiveCN102600064ALow incidence of adverse reactionsGrowth inhibitionOrganic active ingredientsOrganic non-active ingredientsVegetable oilAntioxidant
The invention relates to a long-acting fulvestrant or fulvestrant derivative sustained release preparation and preparation method of the long-acting fulvestrant or fulvestrant derivative sustained release preparation. The formula of the preparation provided by the invention comprises (1) 10mg / ml to 500mg / ml fulvestrant or fulvestrant derivative served as the active compound based on the content of preparation, (2) 3% to 80% of ketone compound or dimethyl sulfoxide served as the cosolvent based on the total weight of the preparation, (3) dispersing agent using vegetable oil or synthetic grease (ester), (4) analgesic, and (5) optional antioxidant.
Owner:XIAN LIBANG PHARMA
GLP-1 (glucagon-like peptide-1) analogue modified with PEG (polyethylene glycol)
ActiveCN107266557AGood metabolic stabilityProlong half-life in vivoNervous disorderPeptide/protein ingredientsDiseaseMetabolic stability
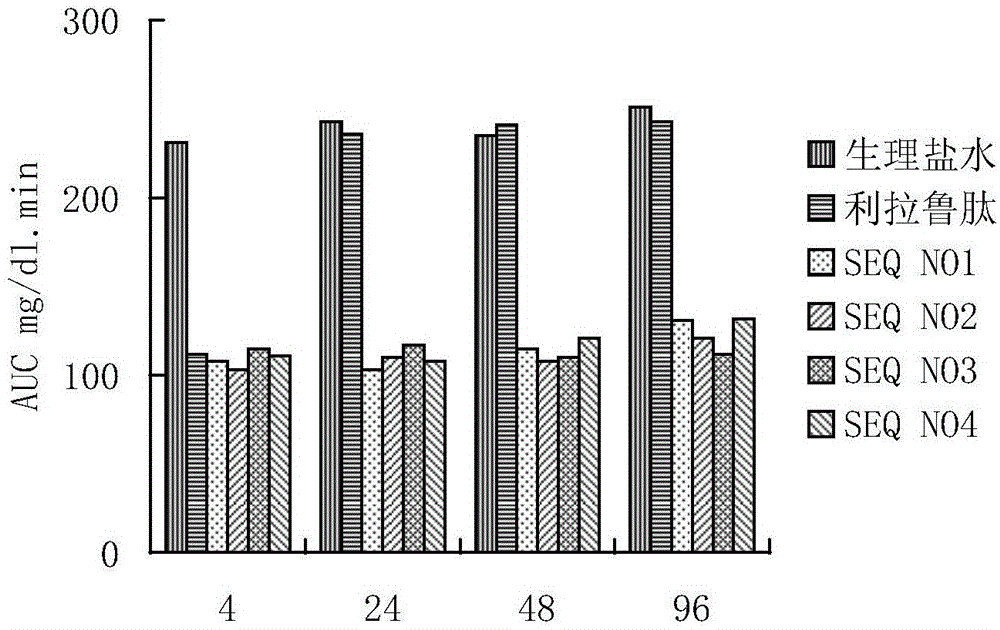
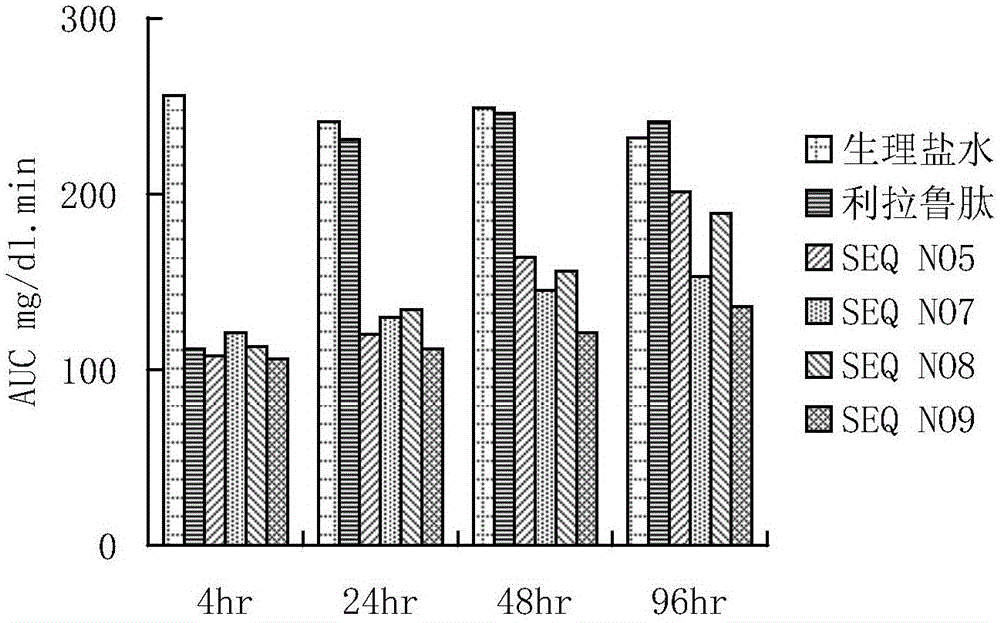
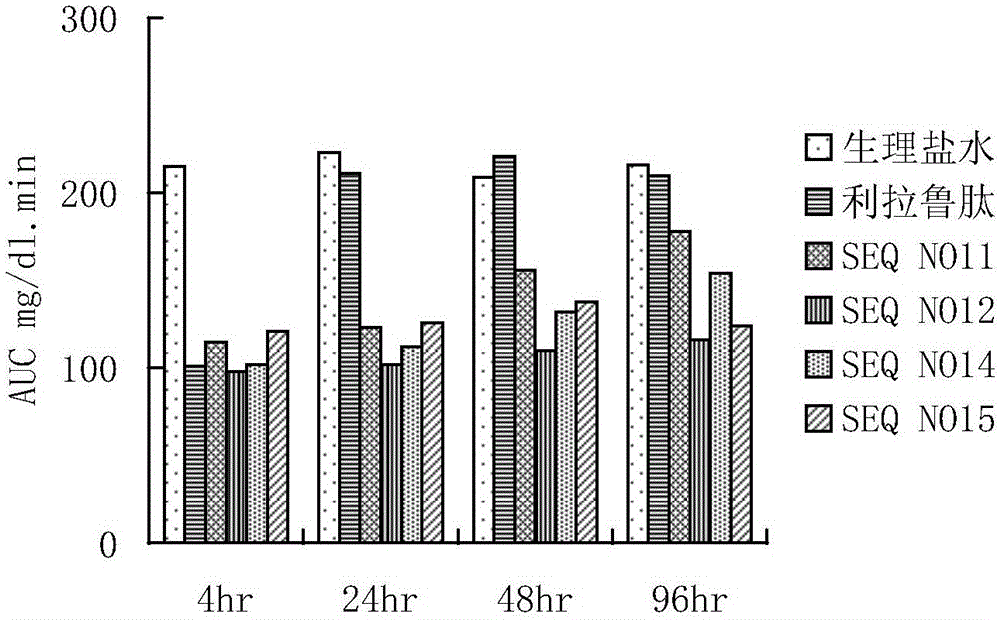
The invention provides a GLP-1 (glucagon-like peptide-1) analogue modified with PEG (polyethylene glycol). The GLP-1analogue has an amino acid sequence shown in SEQ ID NO: 1-19, and the cysteine residue of the amino acid sequence is modified with a PEG group. The invention further provides an application of the analogue or analogue composition in preparation of drugs for treating diabetes, obesity and Alzheimer's disease. The polypeptide has better metabolism stability, has remarkably prolonged in-vivo half-life period, solves the problem that the half-life period of natural GLP-1 is short, can substantially improve the clinical application compliance and has higher application value.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Sildenafil rapidly disintegrating film composition with high drug loading capacity
ActiveCN104586820AHigh drug loadingSmall sizeOrganic active ingredientsMacromolecular non-active ingredientsDiseaseAdditive ingredient
The invention belongs to the field of pharmaceutical preparation and especially relates to a sildenafil film composition. The invention provides a sildenafil rapidly disintegrating film composition with high drug loading capacity. The sildenafil rapidly disintegrating film composition with high drug loading capacity comprises (a) an active pharmaceutical ingredient which is sildenafil or pharmaceutically acceptable salt, and (b) a film forming material which accounts for 5-50%(w / w) of the composition and comprises gelatin which accounts for 10%-100% of the film forming material, (c) a plasticizer and (d) a taste regulator, wherein the biological availability of the active ingredient sildenafil in the film composition is about 60%. The composition disclosed by the invention takes gelatin as a main film forming material, and the obtained sildenafil film agent has the advantages of high drug loading capacity, good physical performance, perfect stability, nice taste and high biological availability, and is capable of rapidly disintegrating in oral cavity. The composition disclosed by the invention is used for treating male erectile dysfunction disease.
Owner:QILU PHARMA CO LTD
Glucagon-like peptide-1 analog and uses thereof
ActiveCN109384839AGood hypoglycemic activityReduce dosePeptide/protein ingredientsMetabolism disorderHalf-lifePolyethylene glycol
The present invention provides a precursor polypeptide or a pharmaceutically acceptable salt of a glucagon-like peptide-1 analogue, wherein the precursor polypeptide has an amino acid sequence represented by the following general formula I HX1X2GTFTSDVSSYLEEX3AAX4EFIX5WLVKX6X7X8X9, and X1, X2, X3, X4, X5, X6, X7, X8 and X9 represent arbitrary amino acids. The present invention further provides a glucagon-like peptide-1 analog or a pharmaceutically acceptable salt thereof. According to the present invention, the glucagon-like peptide-1 analog is obtained by conjugating a polypeptide optimized based on an endogenous GLP-1 (7-36 / 37) sequence and polyethylene glycol having a specific structure, has strong blood glucose lowering activity so as to reduce the dosage, and has significantly-prolonged half-life in vivo so as to be expected to improve clinical compliance; and the precursor polypeptide and the endogenous GLP-1 (7-36 / 37) are highly homologous in the sequence so as to reduce the immunogenicity, such that the precursor polypeptide has good potential in the drug development application.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Long-acting glucagon-like peptide-1 analogue and application thereof
ActiveCN106554409AGood hypoglycemic effectGood metabolic stabilityNervous disorderPeptide/protein ingredientsHalf-lifeGlucagon-like peptide-1
The invention provides a long-acting glucagon-like peptide-1 analogue. The long-acting glucagon-like peptide-1 analogue is characterized in that the polypeptide has a structure shown as a general formula I: Z1-HX8EX10T FTSDV SSYLE X22QAAK EFIX30W LVKX35RG-Z2, wherein, any two residues of X8, X10, X22, X30, X35 are cysteine, Z2 is -H or -HN2; any one residue of X8, X10, X22, X30, X35 is cysteine, and Z2 is a -CG sequence. The glucagon-like peptide-1 analogue expressed by the general formula I always contains two cysteine residues to form an intramolecular disulfide bond, so that rapid degradation of the analogue in vivo is avoided, and the in-vivo half life is obviously prolonged.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Tetrodotoxin enteric-coated and sustained-release pellet, and preparation method and application thereof
ActiveCN105919979AControlled drug releaseHas long-term analgesic effectOrganic active ingredientsNervous disorderSustained release pelletsPharmaceutical formulation
The invention provides a tetrodotoxin enteric-coated and sustained-release pellet and a preparation method thereof, and relates to the field of a medicine preparation technology and application. The tetrodotoxin enteric-coated and sustained-release pellet sequentially comprises a blank pellet core, an upper medicine layer, a sustained-release layer, an isolation layer and an enteric layer from inside to outside, wherein the upper medicine layer is tetrodotoxin containing a cosolvent and a bonding agent; according to a weight ratio, the weight of the sustained-release layer accounts for 20 percent to 40 percent of the weight of the medicine containing pellet; the weight of the isolation layer accounts for 2 percent to 8 percent of the weight of the sustained-release pellet; the weight of the enteric layer accounts for 10 percent to 30 percent of the weight of the isolation pellet. The blank pellet core is used as a medicine carrier; a fluidized bed medication method is used for preparing the tetrodotoxin medicine-carried pellet; the sustained-release layer, the isolation layer and the enteric layer are sequentially sprayed to obtain the tetrodotoxin enteric-coated and sustained-release pellet. The advantages of medicine release controllability, a long-period pain relieving effect, good clinic use complaisance, high safety and the like are realized. In addition, the blank pellet core fluidized bed medication and coating method is applicable to the preparation of the ultra-low-specification tetrodotoxin medicine oral sustained-release preparation; the medication rate is high; the content uniformity is good.
Owner:XIAMEN ZHAOYANG BIOLOGICAL ENG
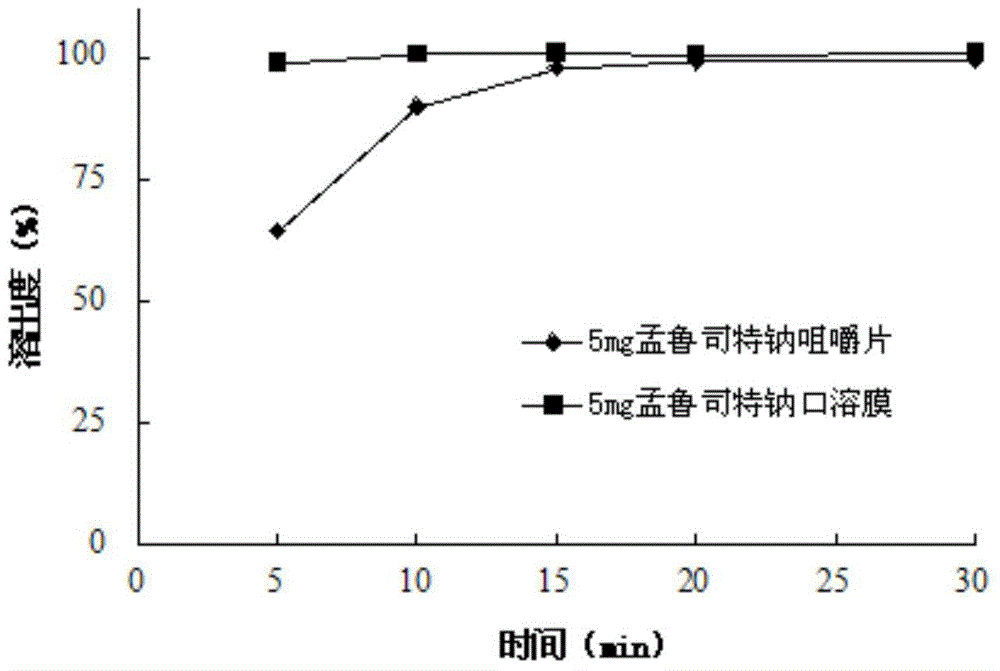
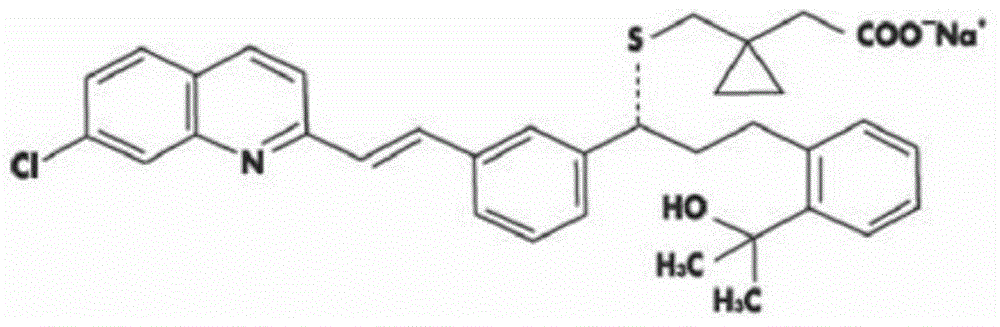
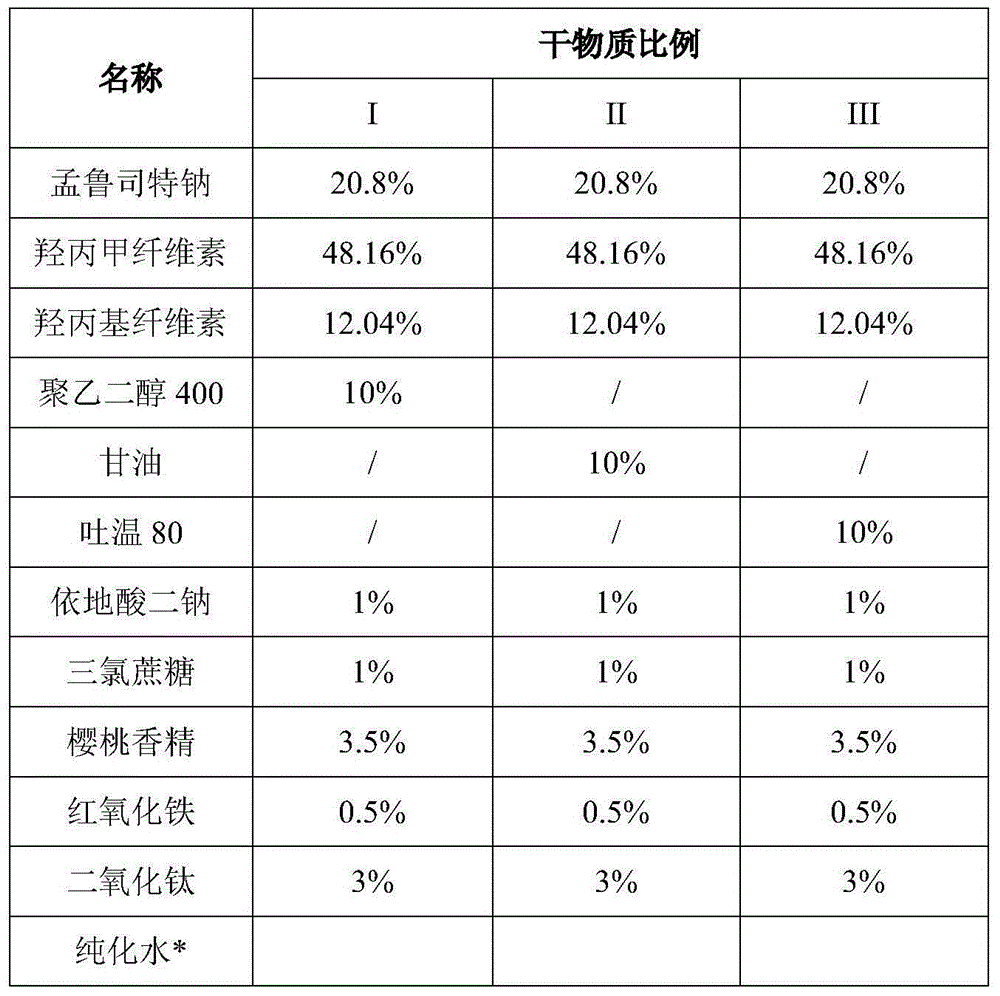
Stable Montelukast oral film preparation
The invention relates to a stable Montelukast oral film preparation. The oral film preparation contains an effective amount of Montelukast or pharmaceutically acceptable salts of the Montelukast, a drug stabilizer at least comprising edetic acid and / or editate as well as pharmaceutically acceptable auxiliary materials. The Montelukast oral film preparation obtained according to the formula is accurate in dose, stable in quality and capable of effectively preventing oxidization and degradation of effective components, has the advantages of bright color, good taste, capability of being quickly dissolved in an oral cavity without water, improves the administration compliance of a patient and is particularly suitable for infants and patients suffering from dysphagia.
Owner:QILU PHARMA CO LTD
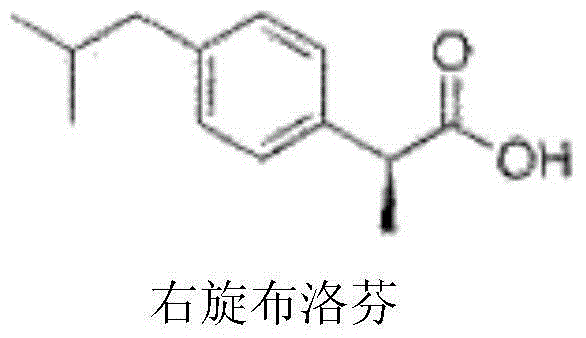
Dexibuprofen injection drug composition and preparation method thereof
ActiveCN104546697AImprove complianceGood analgesic effectOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a dexibuprofen injection and a preparation method thereof. The dexibuprofen injection per ml comprises 10-90 mg of dexibuprofen. The high temperature resistance and hard light tolerance of a dexibuprofen injection prepared according to the invention are obviously superior to those in the prior art, the product stability is better, the quality is better, the requirements of clinical intravenous drip can be satisfied, and the clinical compliance is better.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Colored medicinal charcoal film coated tablet preparation method
ActiveCN101103997AEasy to acceptImprove clinical complianceDigestive systemCarbon active ingredientsColoredExcipient
The invention discloses a preparation method of painted medicinal carbon film-coating tablet produced by the raw material of medicinal carbon and medicinal excipients. The invention is characterized in that painted medicinal carbon film coating tablets are produced in the preparation method, thus the visual defect of black medicinal carbon is overcome and the painted tablets are easy to be accepted by patients. The smooth and round form of the painted medicinal carbon film coating tablet can help effectively guarantee the tablet quality and improve the clinical compliance of patients.
Owner:河北长天药业有限公司
Esomeprazole magnesium suspension tablet and preparation method thereof
InactiveCN104027320APrecise split doseSuitable for childrenOrganic active ingredientsDigestive systemEsomeprazole PillSuspending Agents
The invention provides an esomeprazole magnesium suspension tablet which is prepared by mixing and tabletting an esomeprazole pill, a filler, corrigent, a disintegrating agent, a suspending agent, a lubricant and the like, wherein the esomeprazole pill is prepared by carrying the medicine in a pill core, wrapping an isolating layer and wrapping an enteric coating. Because of nicking, the esomeprazole magnesium suspension tablet can be torn apart for taking; a small pill tabletting process is adopted, the divided dose is accurate, the enteric effect of the pill is not affected after the tablet is torn apart, the tablet can be reasonably taken according to the age and the weight of children, and the clinical compliance is improved. The esomeprazole magnesium suspension tablet provided by the invention can be rapidly dispersed into suspension, is beneficial for distribution of the medicine in gastrointestinal tracts, can improve the bioavailability of the medicine, and is also applicable to patients with dysphagia.
Owner:杭州新诺华医药有限公司
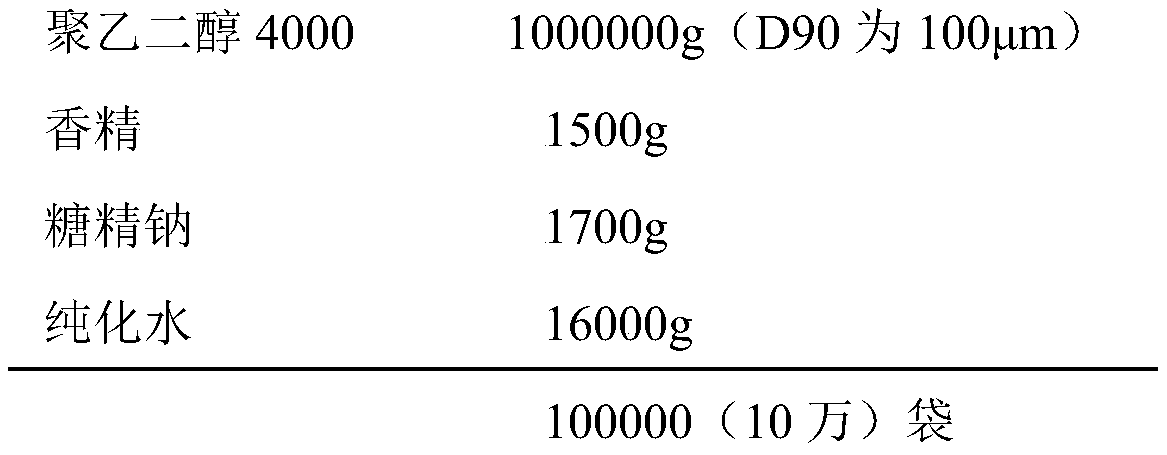
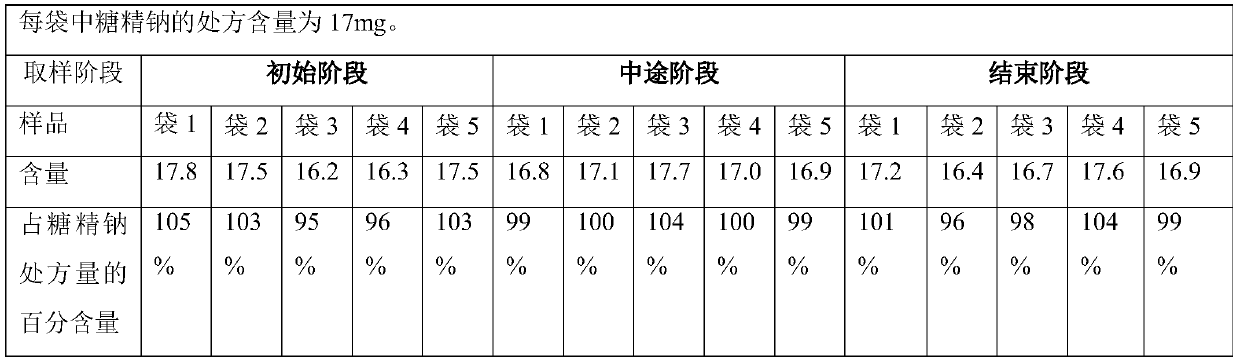
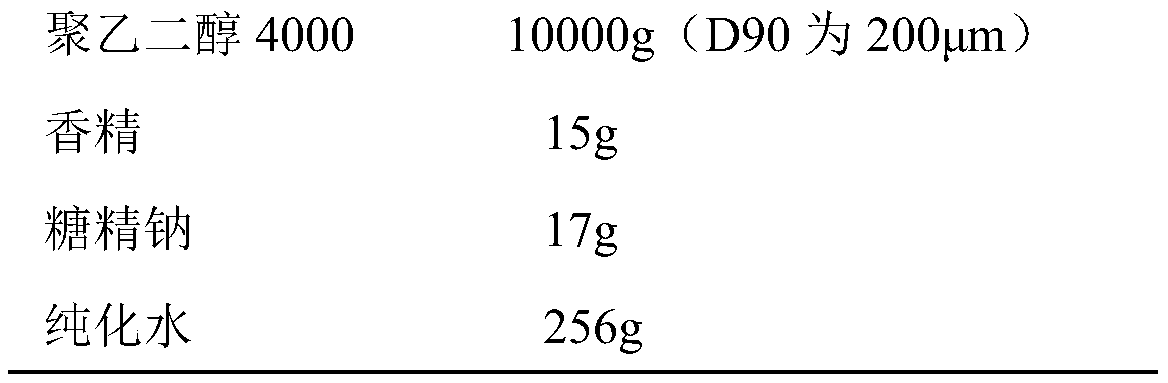
Method for preparing polyethylene glycol 4000 powder
InactiveCN110680805AImprove clinical complianceImprove high-volume production efficiencyPowder deliveryDigestive systemComposite materialPolythylene glycol
The invention discloses a method for preparing polyethylene glycol 4000 powder. The prescription of the polyethylene glycol 4000 powder consists of active ingredients of polyethylene glycol 4000, andinactive ingredients of saccharin sodium and essence. The method comprises the following steps: firstly, dissolving the saccharin sodium and the essence into a solution by using a proper solvent, thenuniformly mixing the solution with the polyethylene glycol 4000 in a spray feeding manner, and finally, completing packaging to obtain the polyethylene glycol 4000 powder. According to the method, the technical problem of non-uniform mixing of auxiliary materials in the preparation process of the polyethylene glycol 4000 powder is well solved, and the clinical use compliance of the product is improved.
Owner:CHONGQING PHARSCIN PHARM CO LTD
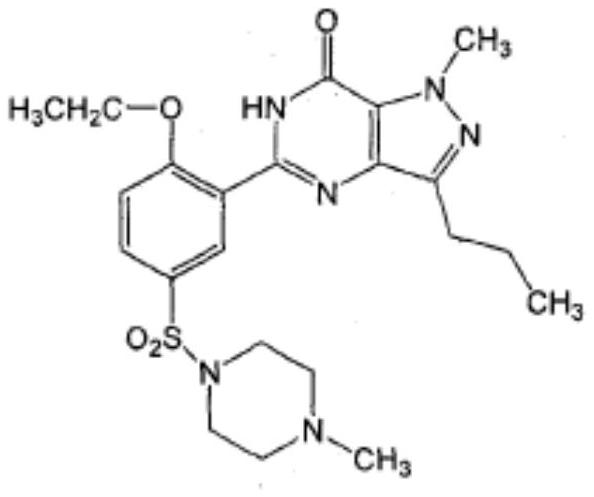
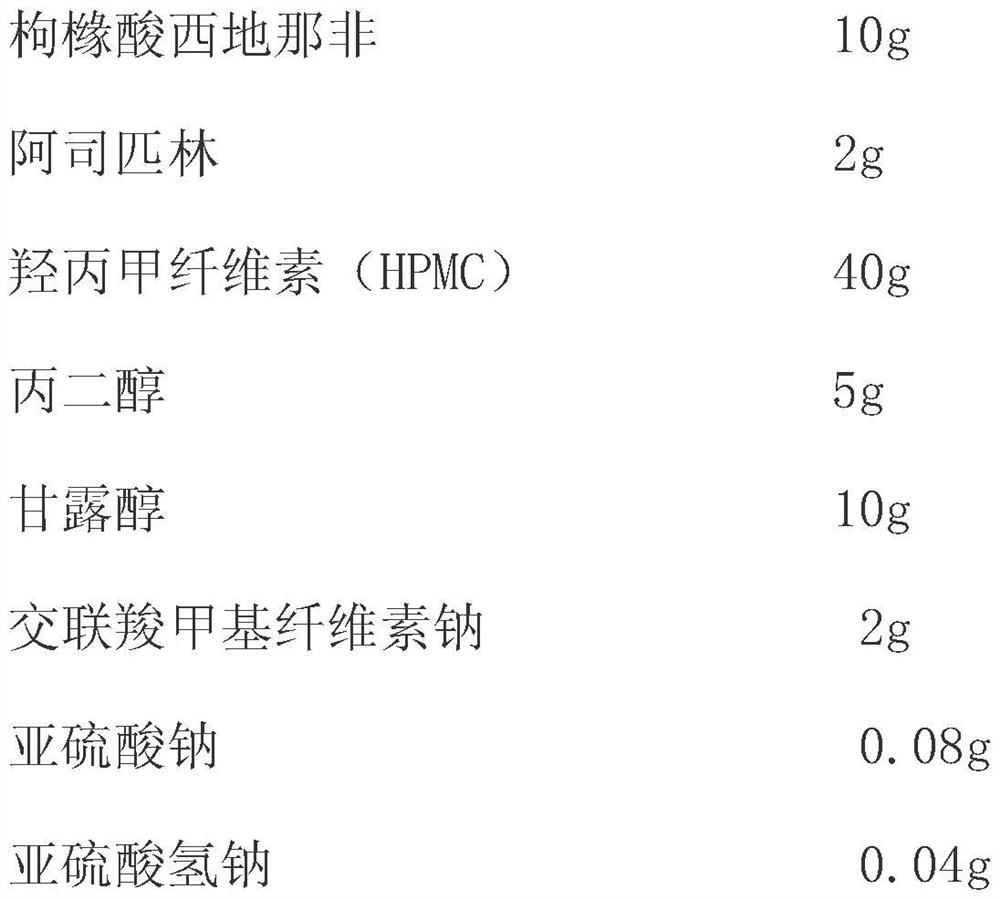
Sildenafil oral membrane pharmaceutical composition and preparation method thereof
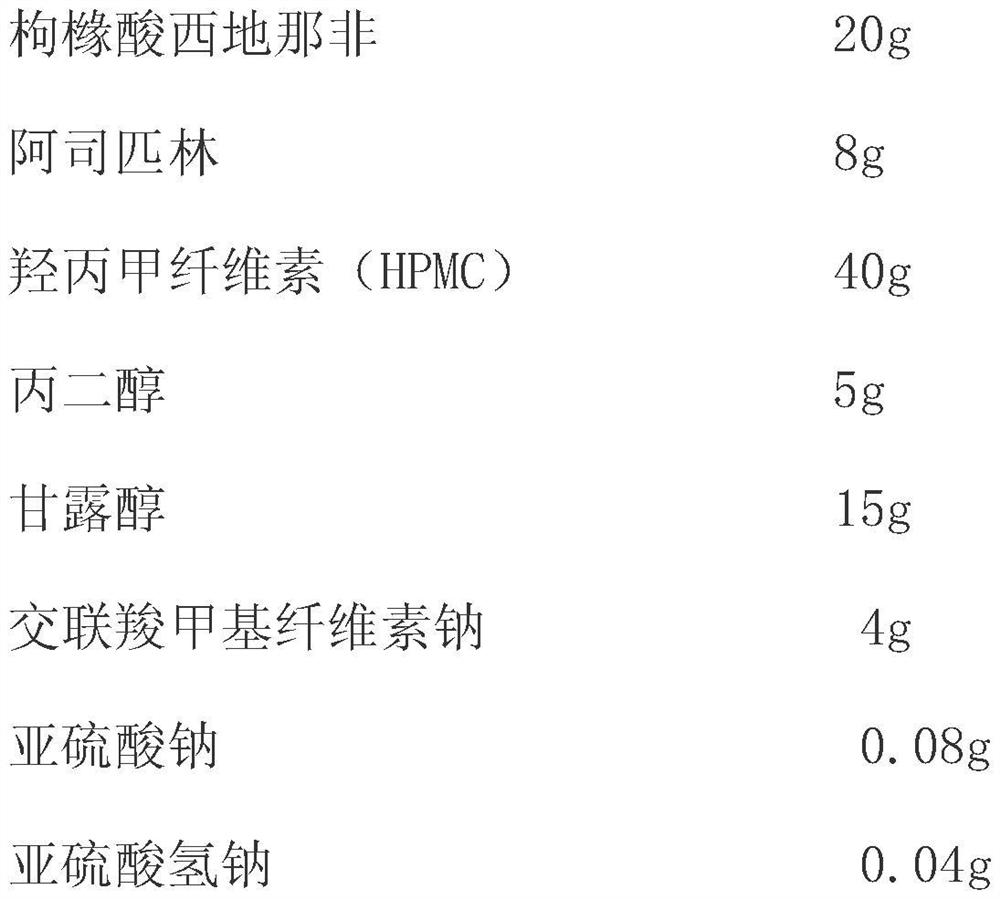
InactiveCN111956652ADisintegrates quicklyRapid dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsSexual impotenceSide effect
The invention belongs to the technical field of urinary science, and particularly relates to a compound preparation for treating erectile dysfunction, a compound preparation for treating erectile dysfunction and a solvent coating method for preparing the compound preparation. The effective medicine component of the compound preparation are a compound preparation sublingual membrane of sildenafil citrate and aspirin, and the compound preparation further comprises a disintegrating agent, a humectant, a stabilizer, a film forming material and a plasticizer, wherein the mass ratio of the sildenafil citrate to the aspirin is 1:(0.1-0.9). The sildenafil citrate in the compound preparation sublingual membrane is quick to disintegrate and high in bioavailability, gastrointestinal tract damage andliver first-pass effect can be avoided, toxic and side effects are reduced, sudden death caused by coronary heart disease and the like after sildenafil is taken is prevented, the side effects are greatly reduced, and a synergistic interaction effect is achieved. The compound preparation not only can improve the clinical use compliance of existing marketed dosage forms, but also can improve the clinical curative effect, and has particularly high clinical development and use values.
Owner:CHONGQING CONQUER PHARML
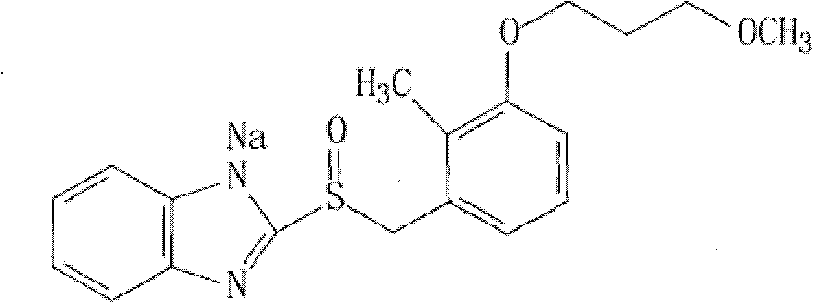
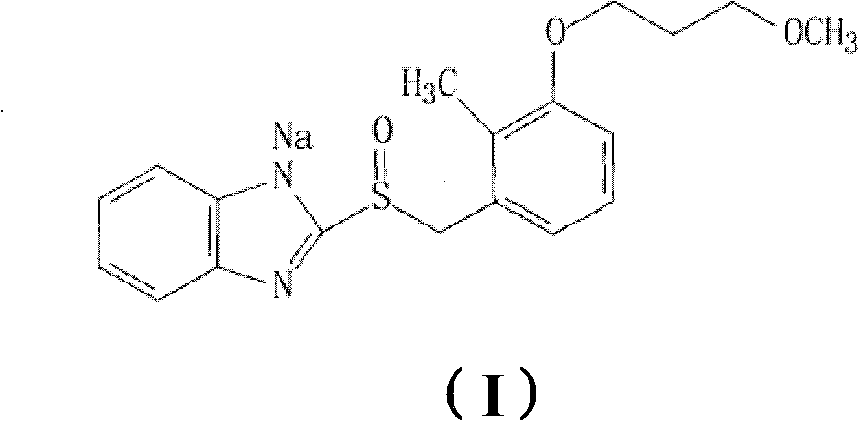
Sodium rabeprazole medicinal composite and method for preparing same
InactiveCN102614144AImprove stabilitySolve technical problems that are easy to degradeOrganic active ingredientsDigestive systemMedicineBULK ACTIVE INGREDIENT
The invention discloses a sodium rabeprazole medicinal composite, which contains the active substances of sodium rabeprazole and stabilizing agent, wherein the weight ratio of the stabilizing agent and the sodium rabeprazole is 0.05-10 / 1, and the sodium rabeprazole medicinal composite also contains other optional pharmaceutically acceptable additives. The composite uses less amount of the stabilizing agent, has good stability and a simple preparing technology.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Polyethylene glycol electrolyte oral liquid and preparing method thereof
ActiveCN107028876AOverall small sizeImprove complianceOrganic active ingredientsDigestive systemSodium bicarbonatePatient compliance
The invention relates to polyethylene glycol electrolyte oral liquid and belongs to the field of medicine. The polyethylene glycol electrolyte oral liquid comprises polyethylene glycol, sodium bicarbonate, sodium chloride and potassium chloride. The polyethylene glycol electrolyte oral liquid has the advantages that the oral liquid is a solution and does not contain any preservative, patients can take the oral liquid directly without preparation, and the oral liquid is convenient to take and safe; the oral liquid is a concentrated preparation, required dosage is small, only 25 mL of the oral liquid is required for treating constipation, 50 mL is required for gut purge, adverse reaction like nausea and abdominal distension are avoided, and patient compliance is high.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Male health composition containing folic acid and zinc gluconate, and preparation method of preparations thereof
ActiveCN106177964AReduce adverse reactionsIdeal generationOrganic active ingredientsInorganic non-active ingredientsSodium bicarbonateMedicine
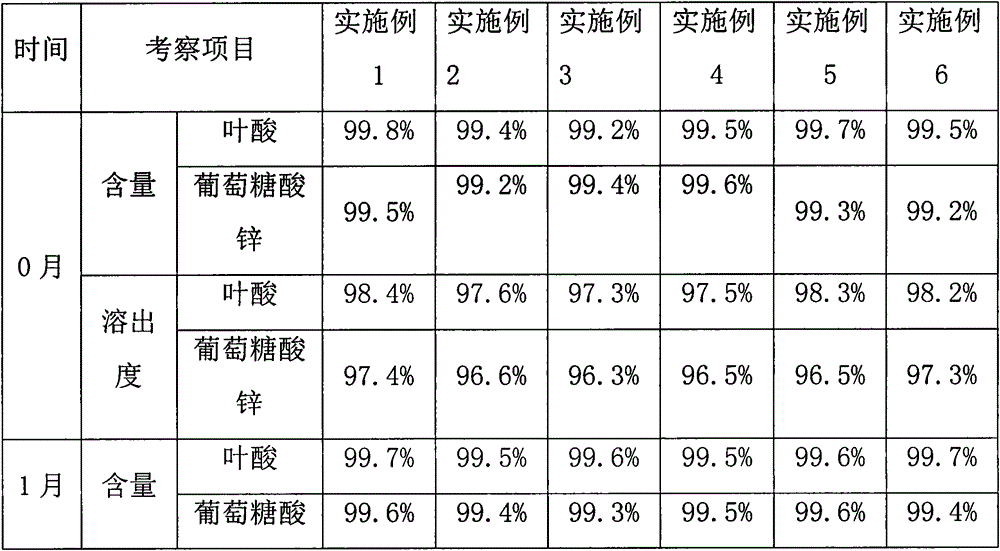
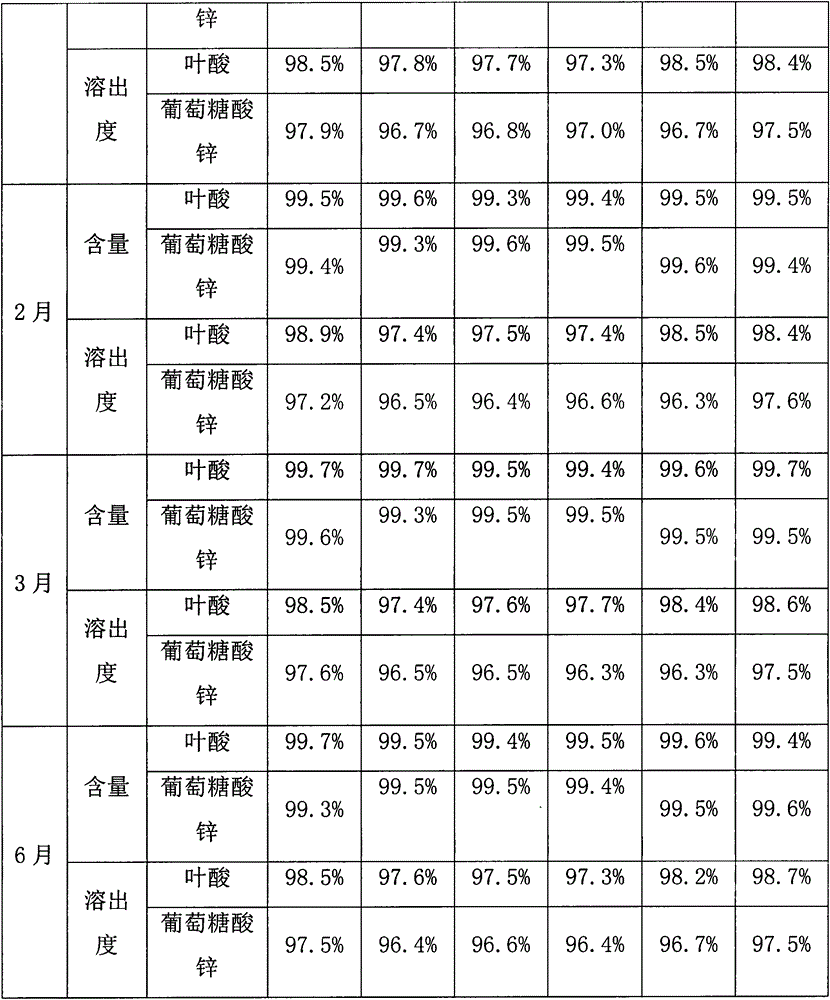
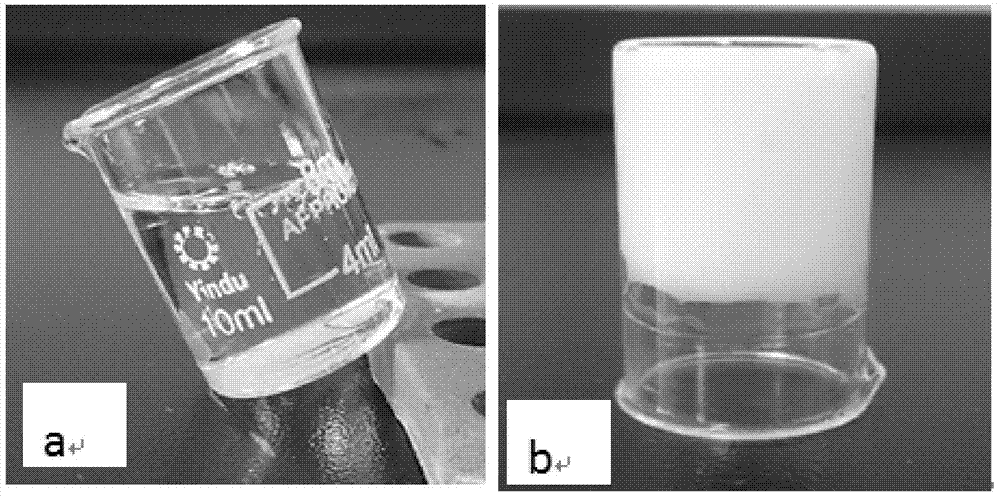
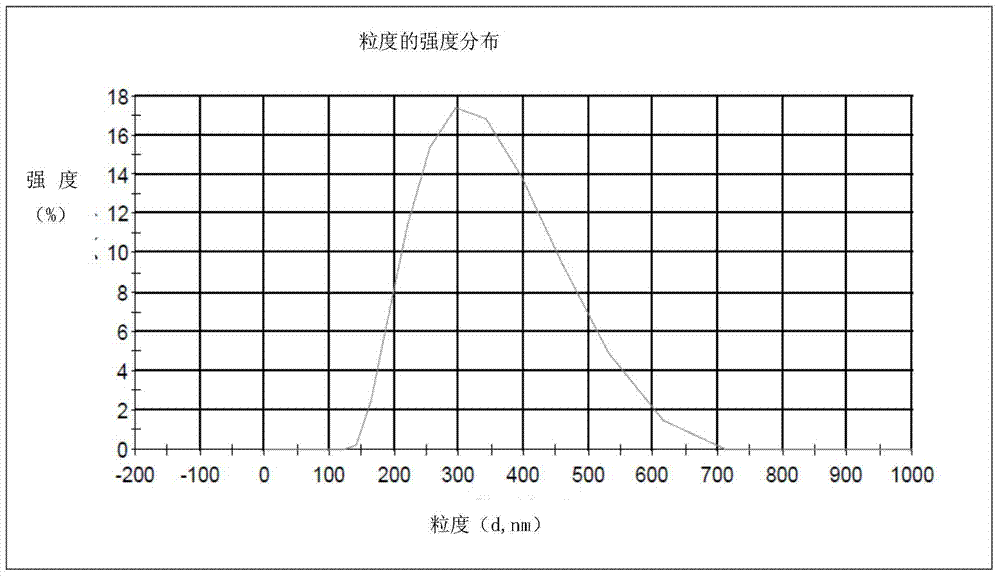
The invention provides a male health composition containing folic acid and zinc gluconate, and a preparation method of preparations thereof. The health composition contains folic acid and zinc gluconate, and also contains sodium bicarbonate and a pharmaceutically acceptable auxiliary material; and a weight part ratio of folic acid to zinc gluconate to sodium bicarbonate is 0.2-0.8:35-70:100-500. The health composition can be used for preparing oral solid preparations not limited to a tablet, a capsule and a powder, improves the dissolution rate and the dissolution degree of folic acid and zinc gluconate, substantially reduces zinc gluconate induced gastrointestinal bad reaction, and improves the bioavailability of zinc gluconate.
Owner:北京斯利安药业有限公司
Propranolol hydrochloride gel preparation for external use and preparation method and application thereof
ActiveCN105434336AImprove stabilityImprove treatment efficiencyOrganic active ingredientsAerosol deliveryGel preparationSkin permeability
The invention provides a propranolol hydrochloride gel preparation for external use and a preparation method and application thereof. The propranolol hydrochloride gel preparation for the external use contains at least 3% by weight of propranolol hydrochloride. The propranolol hydrochloride gel preparation for the external use has good stability and skin permeability and has a significant curative effect on treatment and prevention of infantile hemangioma located in different subcutaneous depths.
Owner:WUHAN CONFORM PHARMA CO LTD
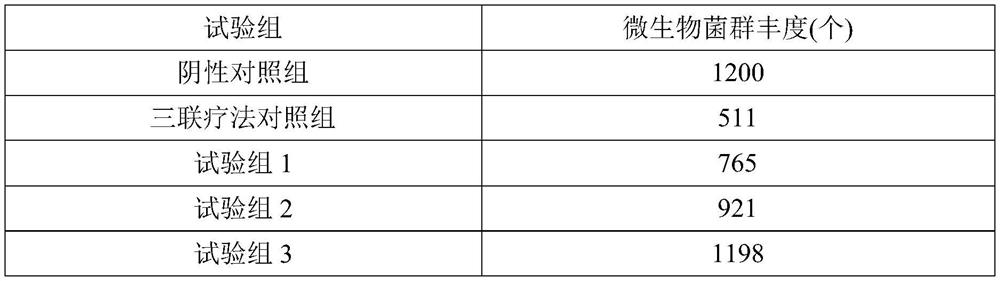
traditional Chinese medicine composition capable of resisting helicobacter pylori and application
InactiveCN111905017AImprove securityRepair Toxic Side EffectsAntibacterial agentsDigestive systemGut floraIntestino-intestinal
The invention belongs to the technical field of traditional Chinese medicine preparation, and particularly relates to a traditional Chinese medicine composition capable of resisting helicobacter pylori and an application. The traditional Chinese medicine composition is improved on the basis of famous Zuojin pills and Xianglian pills and comprises a rhizoma coptidis extract, a fructus evodiae and coptis chinensis extract, and a cortex phellodendri extract, wherein the content of berberine is greater than 50%, the effect of resisting the helicobacter pylori is good, besides, through the combination with the fructus evodiae and costus roots, the side effects of the product are effectively eliminated, and the safety of the medicine is improved; the traditional Chinese medicine composition is used in combination with a clinical standard therapy (triple therapy) for treating HP, so that the curative effect can be further improved, and intestinal flora disorder caused by the triple therapy and toxic and side effects of the triple therapy can be repaired.
Owner:SOUTHWEST UNIVERSITY
Ciclosporin medicament composition for injection administration
InactiveCN101502641AFix stability issuesSolve the technical problems of resolubilityCyclic peptide ingredientsImmunological disordersSolubilityIrritation
The invention relates to ciclosporin medicine composition of injection drug administration, comprising ciclosporin which serves as an active ingredient, Carmowax15-hydroxystearate (solutol HS 15) which serves as a solubilizer, alcohols or esters which serve as latent solvent and water for injection which serves as solvent. The medicine composition can be prepared into injecta or lyophilized preparation. The medicine composition of the invention not only solves the problem that severe anaphylactic reaction exists as the existing ciclosporin injecta contains much Cremophor EL, but also prevents severe anaphylactic reaction from occurring and causes little acrimony; the medicine composition also solves the problem that the previous technology can not solve the stability and solubility of the ciclosporin injecta and avoids the insufficiencies of complex preparation technique and low cost caused by the adoption of technologies such as nanometer technology and lipidosome technology; the medicine composition further solves the technical problem of the stability and solubility of lyophilized powder injecta, which can not be solved by the previous technology, as a result, the medicine composition features good stability and solubility; the preparation technique of the injecta is simple, the quality control is simple and convenient, the production cost is relatively low, thus greatly reducing economic burden of the patients in terms of medicine use.
Owner:姚定全
Leurocristine sulfate liposome compositions, and its prepn. method
ActiveCN1559408ASmall toxicityGood curative effectOrganic active ingredientsAntineoplastic agentsVincristine SulfateLipid composition
A lipid composition of vincristine sulfate in the form freeze dried powder for intravenous injection or drip is prepared from the vincristine sulfate, phosphatide and cholesterin.
Owner:诺桥制药(成都)有限责任公司
Externally applied traditional Chinese medicine composition for treating gynecological diseases, transdermal medicine delivery patch and application
PendingCN112315945AGreat psychologyHuge physical burdenPharmaceutical non-active ingredientsSexual disorderGYNECOLOGIC DISORDERSHomalomena
The invention discloses an externally applied traditional Chinese medicine composition for treating gynecological diseases. The composition comprises the following traditional Chinese medicine components: cortex acanthopanacis, obscured homalomena rhizome, garden balsam stem, notopterygium root, radix angelicae pubescentis, daemonorops draco, frankincense, myrrh, safflower, folium artemisiae argyi, parasitic loranthus, teasel root, rhizoma sparganii, curcuma zedoary, radix paeoniae rubra, pericarpium zanthoxyli, dandelions, radix angelicae dahuricae and radix saposhnikoviae. A preparation method of a transdermal medicine delivery patch prepared from the traditional Chinese medicine components comprises the steps of preparing superfine medicine powder and preparing the patch. Besides, the invention further discloses application steps of the transdermal medicine delivery patch to treatment of gynecological diseases. The traditional Chinese medicine composition provided by the invention has a good curative effect and few side effects; According to the transdermal medicine delivery patch prepared from the traditional Chinese medicine composition, by means of an ultrasonic introductiontechnology, effective components in medicines permeate into deep tissues through local skin, so that relatively high medicine concentration is rapidly achieved, and the purpose of treatment is furtherachieved. A transdermal medicine delivery technology is good in directionality, accurate and controllable in action range, high in treatment efficiency, small in local irritation and good in clinicalcompliance.
Owner:FIRST PEOPLES HOSPITAL OF YUNNAN PROVINCE +1
Recombinant human endostatin nanoparticle composition for injection and preparation method thereof
InactiveCN101954065AFacilitated releaseSimple process routePowder deliveryPeptide/protein ingredientsCarrying capacityFreeze-drying
The invention relates to a recombinant human endostatin nanoparticle composition for injection and a preparation method thereof. The recombinant human endostatin nanoparticle composition can be prepared by methods such as a covalent bond linking method and an ionic induction method and by using biologically degradable polymer as a carrier. The recombinant human endostatin nanoparticle composition can be prepared into injection and freeze-dried powder injection, has high medicament carrying capacity and uniform particle size distribution, is suitable for intravenous injection and can delay the release of recombinant human endostatin.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD
Cyclosporine A and amphiphilic hyaluronic acid derivative composition and preparation method thereof
InactiveCN103191411AImprove securityHigh drug loading efficiencySenses disorderCyclic peptide ingredientsSide effectMedicine
The invention belongs to the technical field of medicines and discloses a cyclosporine A and amphiphilic hyaluronic acid derivative composition and a preparation method thereof. The composition is prepared from cyclosporine A and the amphiphilic hyaluronic acid derivative. The composition has the characteristics of high medicine loading capacity, good stability and good biocompatibility and can overcome such defects of the ophthalmic cyclosporine preparations as lower bioavailability and toxic and side effects. The preparation method is simple and is convenient to use.
Owner:CHINA PHARM UNIV
Chinese medicine composition for preventing and treating hyperuricemia and hyperlipemia and preparation method thereof
ActiveCN105749072AMeet the characteristics of long-term medicationWith dehumidification and seepageMetabolism disorderSkeletal disorderCannabisSide effect
The invention discloses a Chinese medicine composition for preventing and treating hyperuricemia and hyperlipemia and a preparation method thereof, and relates to medicine or health-care food for preventing and treating hyperuricemia and hyperlipemia.The Chinese medicine composition for preventing and treating hyperuricemia and hyperlipemia contains extract of a Chinese medicine formula, and according to the formula, the Chinese medicine composition is prepared from, by weight, 3-20 parts of coix seeds, 5-40 parts of glabrous greenbrier rhizome, 3-20 parts of plantain herbs, 1-9 parts of Chinese atractylode rhizome, 1-12 parts of the root of bidentate achyranthes, 1-9 parts of papaw and 1-9 parts of fructus cannabis.The Chinese medicine composition has the functions of clearing away dampness, eliminating water seepage and inducing diuresis for treating stranguria, can obviously regulate and treat the hyperuricemia and hyperlipemia and is free of toxic and side effects after being taken for a long time; the preparation technology is stable, and production feasibility is good.The medicine or health-care food for preventing and treating the hyperuricemia and hyperlipemia contains an effective dose of the Chinese medicine composition for preventing and treating the hyperuricemia and hyperlipemia.
Owner:SHENZHEN ELDERLY MEDICAL RES INST +2
Glucagon-like peptide-1 (GLP-1) analogs, and preparation method and application thereof
ActiveCN107236034AGood hypoglycemic effectOvercoming the problem of short half-lifeNervous disorderPeptide/protein ingredientsHydrogenHalf-life
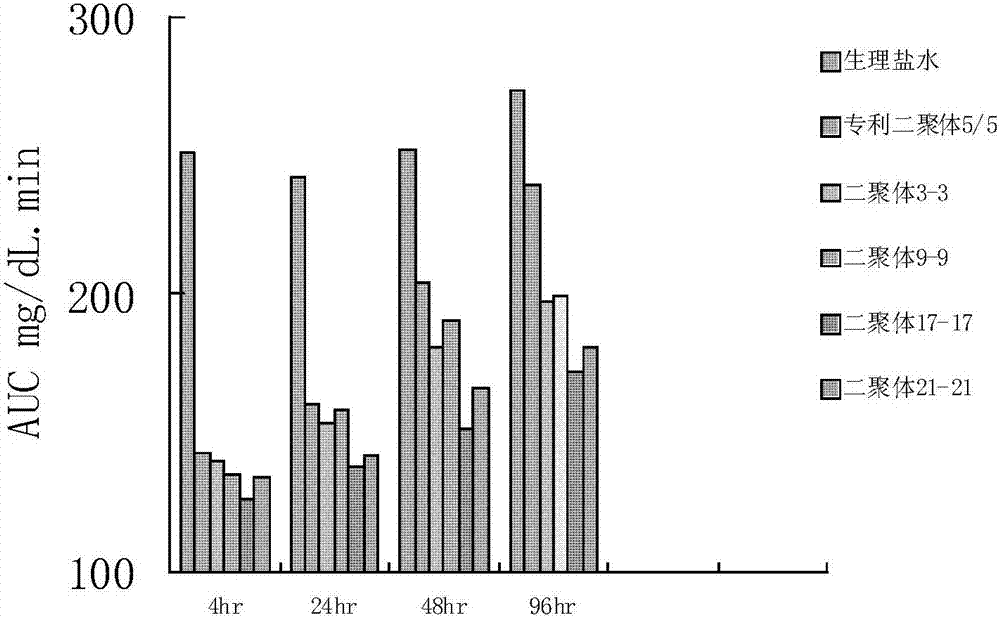
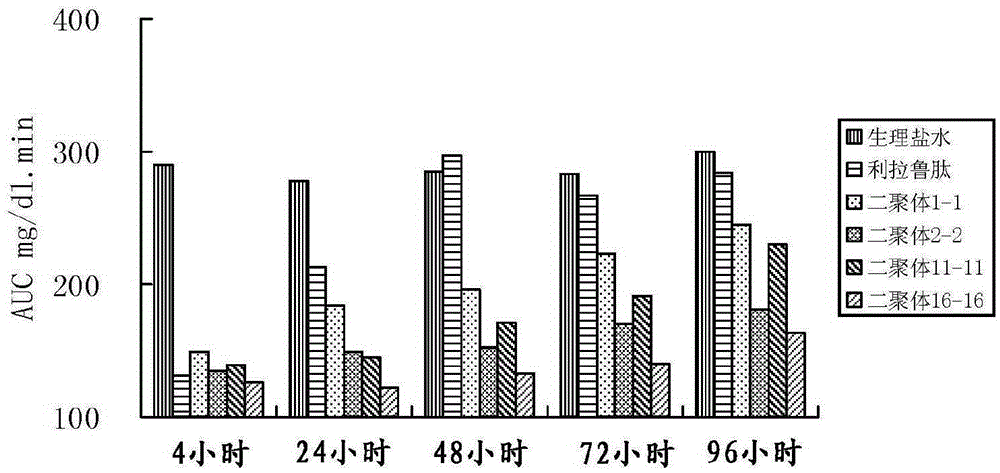
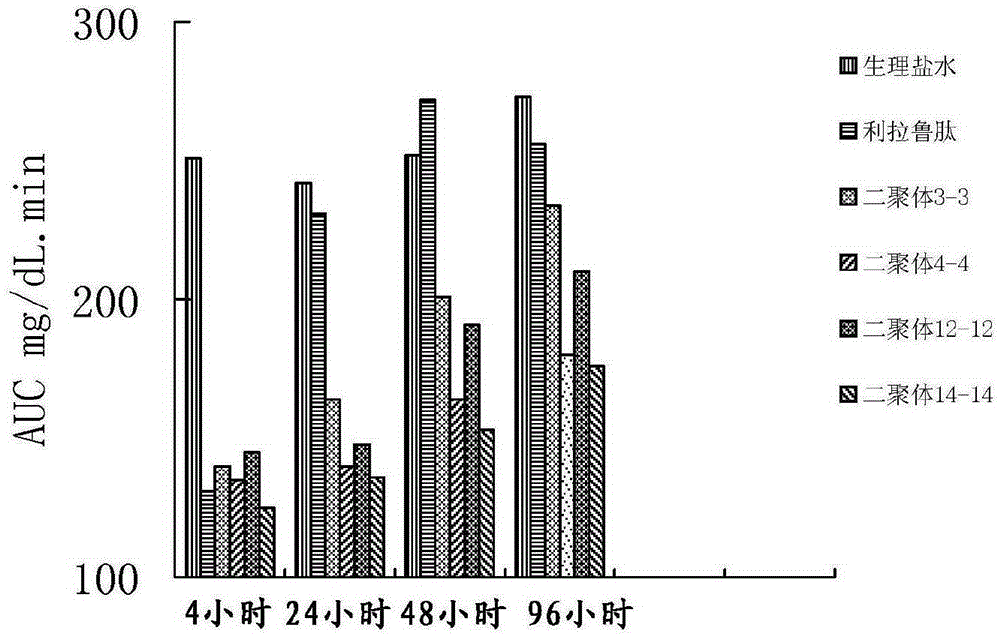
The invention provides glucagon-like peptide-1 (GLP-1) analog monomer peptides. The monomer peptides have the general formula 1: Z1HAX1GTFTSDVSSYLE X2QAAKEFICWLVKGRX3, wherein Z1 is hydrogen, acetyl or trifluoroacetyl; X1 is Met, Leu, Pro, Phe or Tyr; and X2 is Gly, Glu or Aib (2-aminoisobutyric acid). The invention also provides a dimer formed by the monomer peptides, a preparation method of the dimer, and application of the dimer in preparing drugs. The dimer of the GLP-1 analog monomers provided by the invention has the obvious effect of lowering the blood sugar, and the in vivo half life can reach 12-72 hours or above, thereby solving the problem of short half life in the natural GLP-1, and greatly enhancing the clinical application compliance.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Long-acting glucagon-like peptide-1 analogue dimer and application thereof
InactiveCN106554408AGood hypoglycemic effectOvercoming the problem of short half-lifeNervous disorderPeptide/protein ingredientsMonomerPeptide
The invention provides a long-acting glucagon-like peptide-1 analogue dimer. The long-acting glucagon-like peptide-1 analogue dimer is characterized in that the dimer is formed by two glucagon-like peptide-1 analogue monomers, the glucagon-like peptide analogue-1 monomer is shown as a general formula Z1HX1EX2TFTSDVSSYLEGQAAKEFIX3WLVKX4RG, wherein, Z1 is H, acetyl or trifluoroacetyl; X1 is Ala, Leu, Val, Gly, Ile; X2 is Gly, Cys; X3 is Ala, Cys; and X4 is Gly, Aib. The long-acting glucagon-like peptide-1 analogue dimer has long-acting hpyerglycemic effect, can increase the clinical medication compliance, is highly homologous with endogenous GLP-1(7-37), and can avoid the security risk.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Combination of cyclosporine A and alkylation serum albumin and preparation method thereof
InactiveCN102038933AHigh drug loadingHigh encapsulation efficiencyCyclic peptide ingredientsImmunological disordersAlkyl transferSerum protein albumin
The invention relates to the field of pharmaceutical preparation, and discloses a combination of cyclosporine A and alkylation serum albumin and a preparation method thereof. The combination has the characteristics of high drug-loading rate and stability, and can overcome the defects of serious side effects in traditional cyclosporine A preparation and the like. The preparation method has mature process, is simple and suitable for industrialized mass production.
Owner:CHINA PHARM UNIV
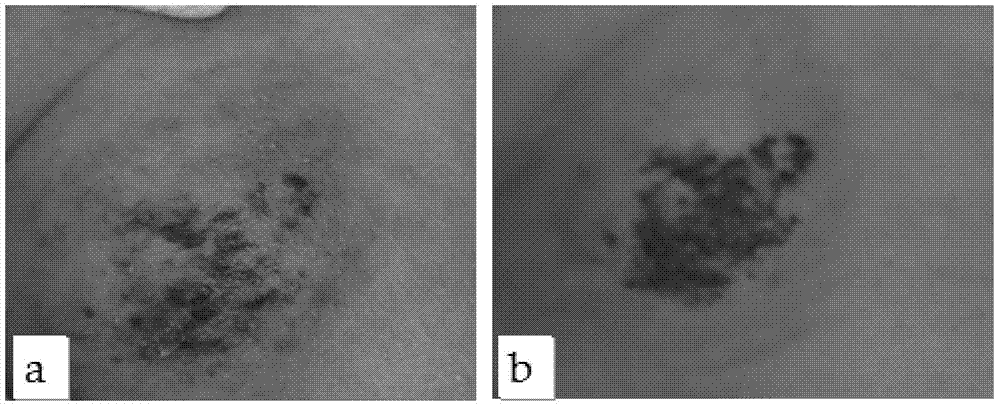
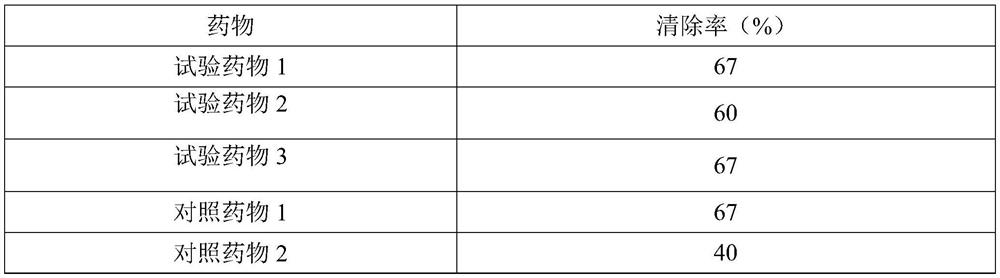
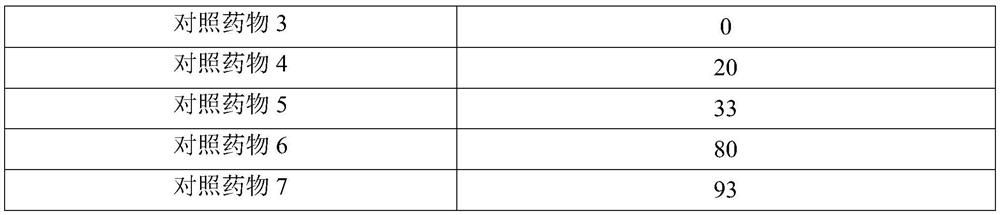
Application of Xianglian product or combination of Xianglian product and antibiotics in anti-helicobacter pylori drugs
InactiveCN113144015AGood anti-Hp effectEnhanced anti-Hp effectAntibacterial agentsTetracycline active ingredientsFatty liverHelicobacter pylori
The invention provides an application of an Xianglian product or a combination of the Xianglian product and antibiotics in anti-helicobacter pylori drugs aiming at the characteristics of poor safety, poor clinical compliance and the like of the existing Xianglian product in resisting Hp or treating or preventing fatty liver, hepatic fibrosis, cirrhosis and liver cancer, and also relates to a preparation method of a super-concentrated Xianglian product. The Xianglian product has a good anti-Hp effect, and when the Xianglian product is combined with one antibiotic or two antibiotics, the safety of the drugs is improved. The Xianglian product also has the effects of treating or preventing fatty liver, hepatic fibrosis, liver cirrhosis and liver cancer.
Owner:重庆伊士腾生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com