Propranolol hydrochloride gel preparation for external use and preparation method and application thereof
A technology of propranolol hydrochloride and gel preparation, which is applied in the field of medicine, can solve problems such as bradycardia, hypoglycemia, and adverse effects, and achieve the effects of improving stability, high drug loading, and good skin penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
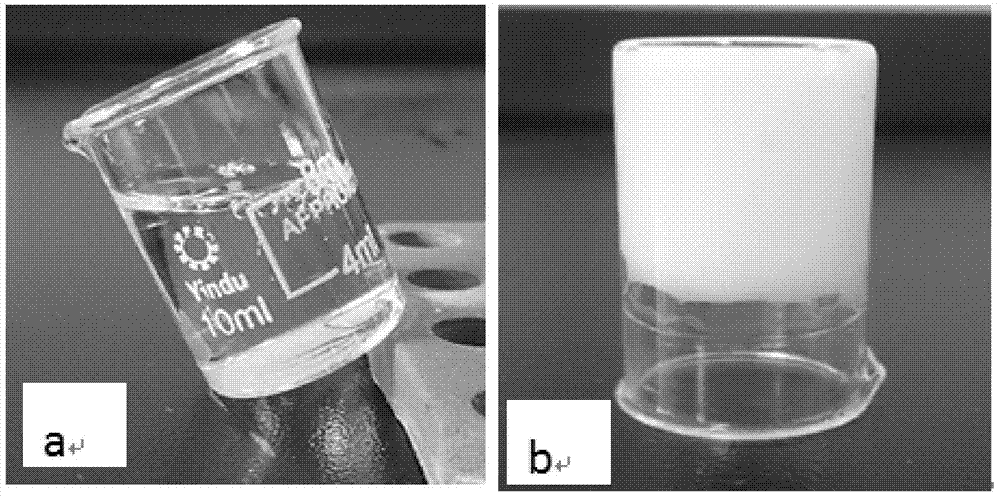
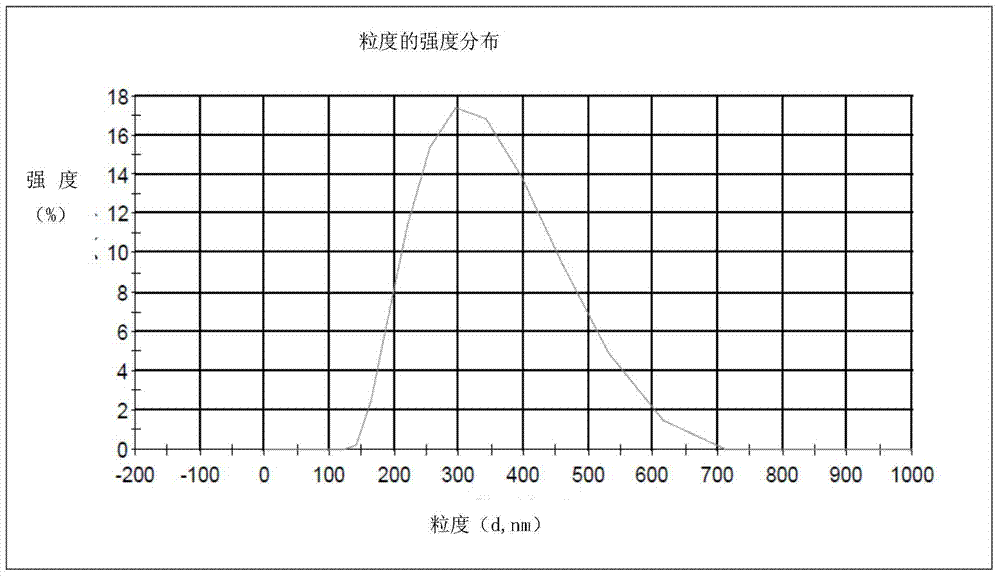
[0035] Weigh 4g of propranolol hydrochloride and 0.05g of sorbic acid, add an appropriate amount of water, heat the resulting mixture with a water bath to dissolve it completely, then add 15g of poloxamer 407, stir mechanically to make it swell completely; Ketone and 8g propylene glycol, stir to make it completely miscible, the resulting solution is added to the gel matrix that is dissolved in propranolol hydrochloride, under the mechanical stirring condition of 500 revs / min, stir 10 minutes, make propranolol hydrochloride Napolol gel preparation for external use (the total amount of the prescription is based on 100g). The gel preparation is uniform milky white, semi-solid gel, with a pH value of 5.24, and the average droplet diameter after being diluted 100 times is 306.6nm. After the sample is placed in a sealed container at 25°C±2°C for one month: there is no significant change in the appearance and properties, pH value, and particle size of the sample.
Embodiment 2
[0037] Weigh 10g of propranolol hydrochloride and 0.05g of sorbic acid, add an appropriate amount of water, heat the resulting mixture in a water bath to dissolve it completely, then add 15g of poloxamer 407, and stir mechanically to completely swell; Ketone and 8g propylene glycol, stir to make it completely miscible, the resulting solution is added to the gel matrix that is dissolved in propranolol hydrochloride, under the mechanical stirring condition of 500 revs / min, stir 10 minutes, make propranolol hydrochloride Napolol gel preparation for external use (the total amount of the prescription is based on 100g). The gel preparation is uniform milky white, semi-solid gel, with a pH value of 5.17, and the average droplet diameter after being diluted 100 times is 301.9nm. After the sample is placed in a sealed container at 25°C±2°C for one month: there is no significant change in the appearance and properties, pH value, and particle size of the sample.
Embodiment 3
[0039] Weigh 16g of propranolol hydrochloride and 0.05g of sorbic acid, add appropriate amount of water, heat the resulting mixture in a water bath to make it completely dissolved, then add 15g of poloxamer 407, stir mechanically to make it swell completely; Ketone and 8g propylene glycol, stir to make it completely miscible, the resulting solution is added to the gel matrix that is dissolved in propranolol hydrochloride, under the mechanical stirring condition of 500 revs / min, stir 10 minutes, make propranolol hydrochloride Napolol gel preparation for external use (the total amount of the prescription is based on 100g). The gel preparation is in the form of a uniform milky white semi-solid gel, with a pH value of 4.89, and the average droplet diameter after being diluted 100 times is 307.5nm. After the sample is placed in a sealed container at 25°C±2°C for one month: there is no significant change in the appearance and properties, pH value, and particle size of the sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com