Cyclosporine A and amphiphilic hyaluronic acid derivative composition and preparation method thereof
A technology of hyaluronic acid and cyclosporine, which is applied in the field of medicine, can solve the problems of low bioavailability, and achieve the effects of simple production process, high drug loading, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of Amphiphilic Hyaluronic Acid Derivatives (HA-g-PNIPAAm)
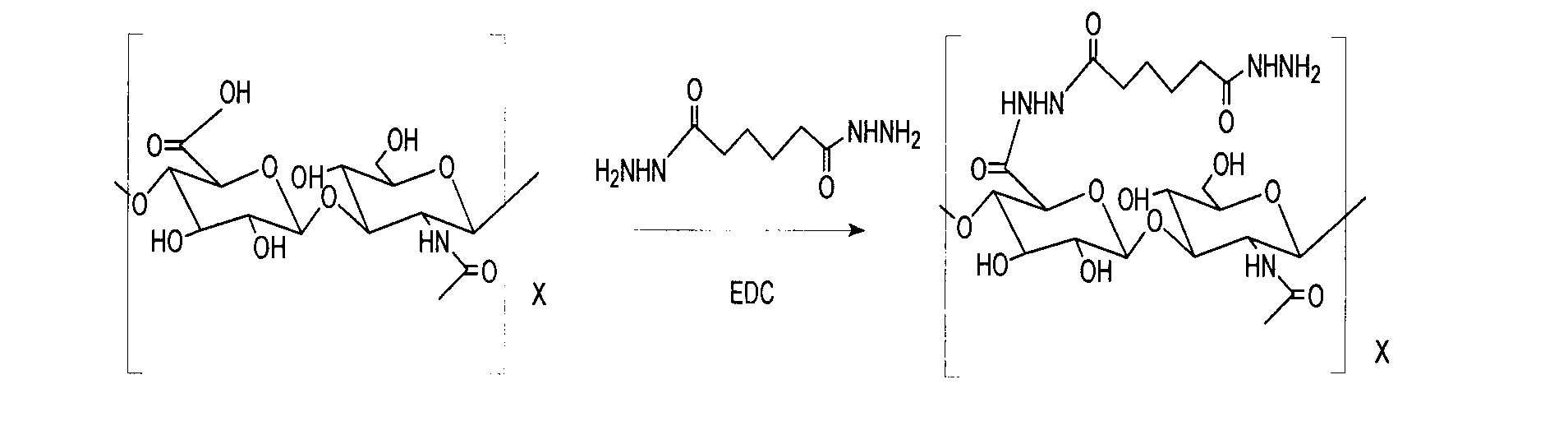
[0029] a. Preparation of hyaluronic acid intermediate AHA
[0030] Accurately weigh 100 mg of hyaluronic acid HA, and add 200 ml of distilled water to fully dissolve the HA. Then add 1.736 g of adipic acid dihydrazide ADH to the above solution, mix thoroughly and stir for 20 minutes, and adjust the pH to 4.8 with 0.1 mol HCl solution. Add 20ml of ethanol and stir for 30 minutes. Add 0.191 g of EDC·HCl, adjust the pH value with 0.1 mol HCl solution, and keep the reaction pH at 4.8. After 2 hours, the reaction ends, and adjust the pH value to 7.0 with 0.1 mol NaOH solution. The reaction solution was transferred into a pre-washed dialysis bag (molecular weight cut off 7KDa). 0.1mol NaCl was used as the dialysate. After 24 hours of dialysis, deionized water was used as the dialysate. After 24 hours of dialysis, it was centrifuged for 15 minutes at 3500 rpm. The supernatant was taken, passe...
Embodiment 2
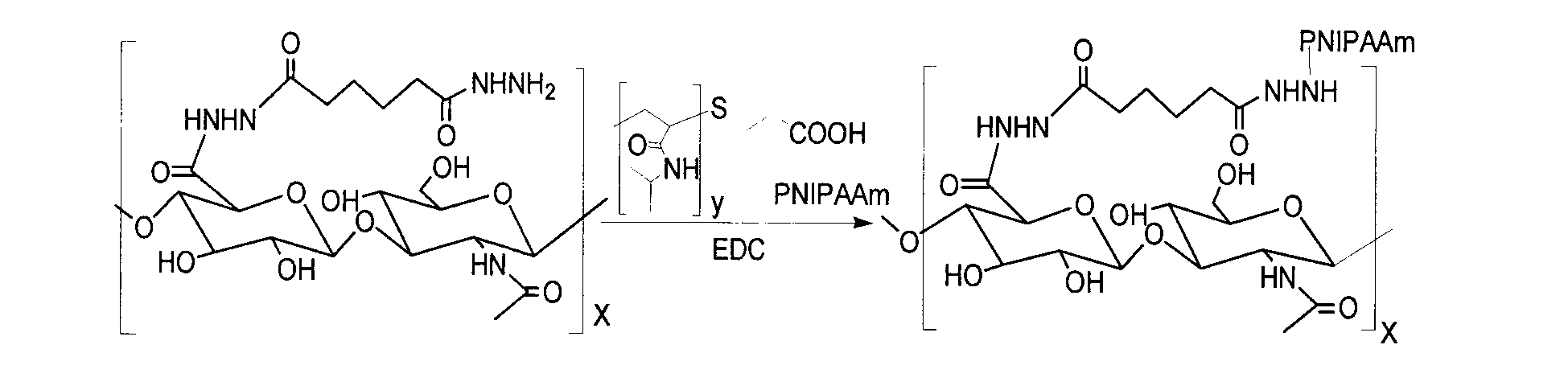
[0035] Example 2 Preparation of cyclosporine A and amphiphilic hyaluronic acid derivatives (HA-g-PNIPAAm)
[0036] The drug was loaded by dialysis, and the drug was cyclosporine CsA. Weigh 20mg of HA-g-PNIPAAm, add 2ml of double distilled water, and fully swell at room temperature for 30min. Dissolve 6 mg of cyclosporine A in a pharmaceutically acceptable organic solvent and add dropwise to the above solution, and ultrasonically pass through the probe for 30 minutes, and the drug and HA-g-PNIPAAm form a nanogel. Transfer the above nanogel into a pre-washed dialysis bag (molecular weight cut-off 3500Da), dialyze with deionized water for 1 day, freeze-dry for 36 hours after pre-freezing for 24 hours, and obtain cyclosporine A and amphiphilic hyaluronic acid derivatives Composition freeze-dried product. In the above preparation method, acceptable organic solvents for dissolving cyclosporine A include methanol, ethanol, chloroform, ethyl acetate, acetone, ether, preferably ethan...
Embodiment 3
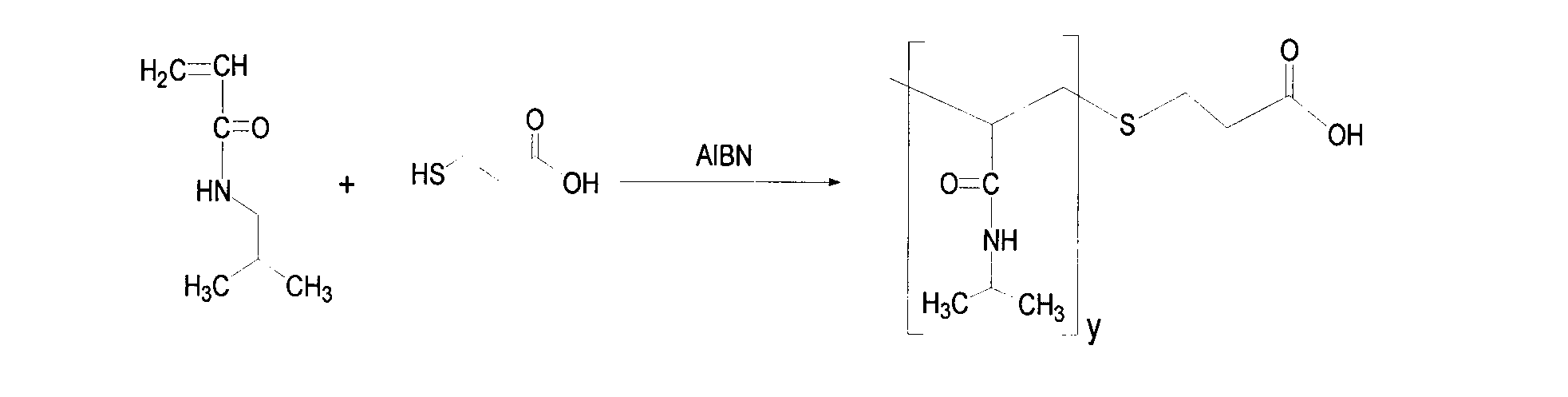
[0037] Example 3 Characterization of Substitution Degree of Amphiphilic Hyaluronic Acid Derivatives
[0038] Dissolve 0.10g of hyaluronic acid intermediate AHA in 40ml of water; respectively take 0.10g, 0.20g, and 0.30g of poly-N isopropylacrylamide PNIPAAm-COOH with carboxyl groups at the end and dissolve them in deionized water, and then add 0.10g to the solution. gEDC·HCl, incubated at 4°C for 48h. The two solutions were thoroughly mixed, the pH was adjusted to 5.6, and the mixture was stirred at room temperature for 24 h. The reaction solution was transferred into a pre-washed dialysis bag (molecular weight cut-off 3500Da), dialyzed with deionized water for 3 days, pre-frozen for 24 hours and then freeze-dried for 36 hours to obtain the frozen amphiphilic hyaluronic acid derivative HA-g-PNIPAAm. Dried product, refrigerated at -4°C for later use. Calculate the degree of substitution (DS) of the sample with formula (1), W is the mass, and MW is the molecular weight. The re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com