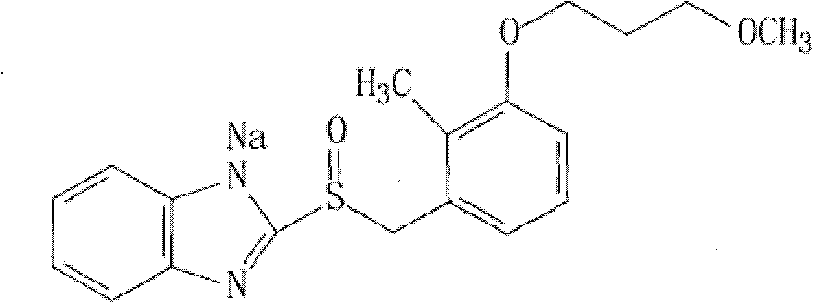
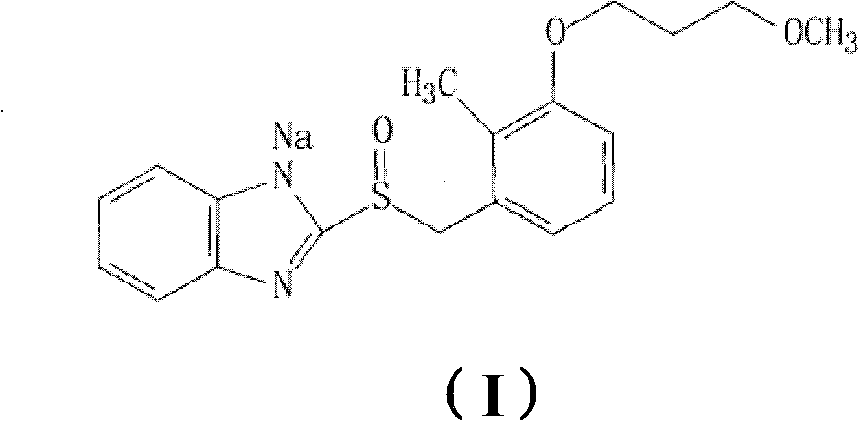
Sodium rabeprazole medicinal composite and method for preparing same
A technology of rabeprazole sodium and its composition, which is applied in the field of pharmaceutical preparations, can solve problems such as difficulty in preparing rabeprazole sodium solid preparations, large amount of alkaline stabilizer, and large total weight of dosage forms, etc., and achieve clinical compliance Good sex, low cost, suitable for swallowing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
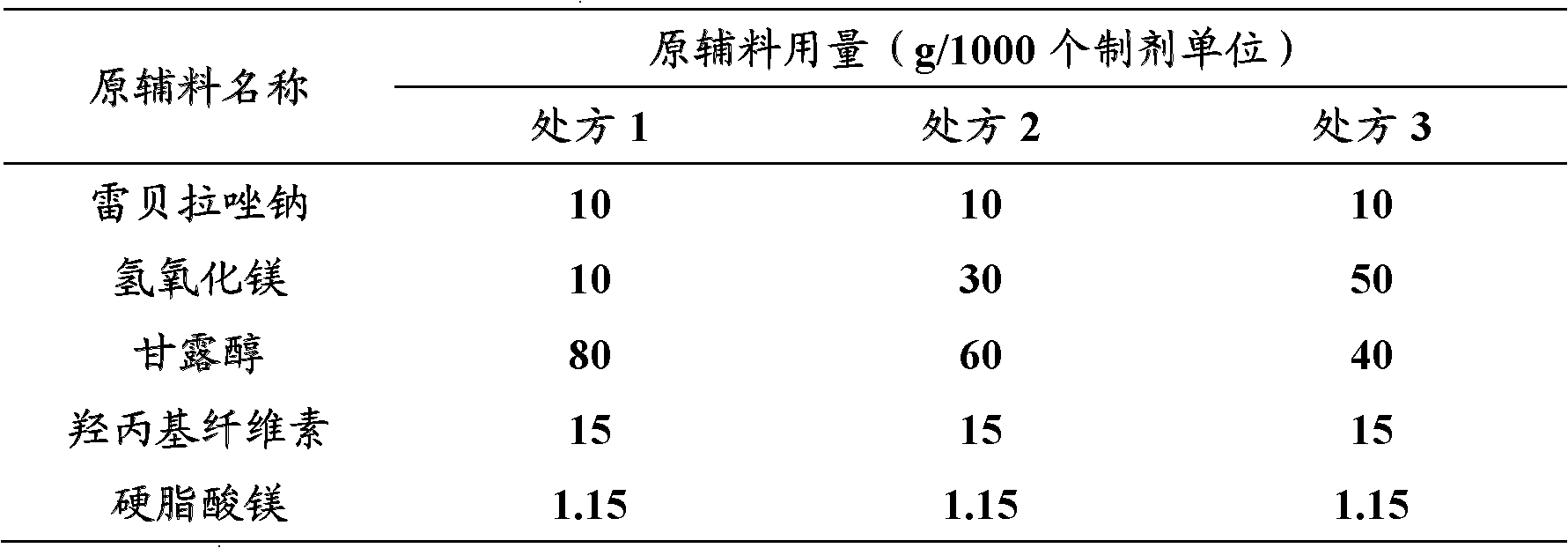
[0044] Table 1: Prescription
[0045]
[0046] Preparation Process:
[0047] Weigh the raw and auxiliary materials according to the prescription ratio in Table 1, first mix magnesium hydroxide, mannitol, and hydroxypropyl cellulose and pass through a 60-mesh sieve to mix evenly, then mix with the main ingredient of the prescription amount and pass through a 60-mesh sieve to Mix evenly, finally mix with magnesium stearate, powder direct compression preparation specification is 10mg, sheet weight is 116.15mg, tablet diameter is the rabeprazole sodium tablet of 7mm (can also directly fill No. 3 capsules and preparation specification is 10mg rabeprazole sodium capsules).
[0048] Those skilled in the art can also predict that the weight ratio of rabeprazole sodium: magnesium hydroxide: mannitol: hydroxypropyl cellulose: magnesium stearate is 10: 10: 80: 15: 1.15, 10: 30: 60 :15:1.15 or 10:50:40:15:1.15 formulations are also included in the present invention.
Embodiment 2
[0050] Table 2: Prescription
[0051]
[0052] Preparation Process:
[0053] Weigh the raw and auxiliary materials according to the prescription ratio in Table 2. First, mix anhydrous sodium carbonate, mannitol, and hydroxypropyl cellulose and pass through a 60-mesh sieve to mix evenly, then mix with the main ingredient of the prescription amount and pass through a 60-mesh sieve. To mix evenly, finally mix with magnesium stearate, powder direct compression preparation specification is 10mg, sheet weight is 116.15mg, tablet diameter is the rabeprazole sodium tablet of 7mm (can also directly fill No. 3 capsule preparation specification 10 mg rabeprazole sodium capsules).
[0054] Those skilled in the art can also predict that the weight ratio of rabeprazole sodium: anhydrous sodium carbonate: mannitol: hydroxypropyl cellulose: magnesium stearate is 10: 10: 80: 15: 1.15, 10: 30: Formulations of 60:15:1.15 or 10:50:40:15:1.15 are also encompassed by the invention.
Embodiment 3
[0056] Table 3: Prescription
[0057]
[0058]
[0059] Preparation Process:
[0060] Weigh the raw and auxiliary materials according to the prescription ratio in Table 3, first mix anhydrous sodium bicarbonate, mannitol, and hydroxypropyl cellulose and pass through a 60-mesh sieve to mix evenly, then mix with the main drug of the prescription amount and pass through a 60-mesh sieve Sieve until mixed evenly, and finally mix with magnesium stearate, and the powder is directly compressed into tablets to prepare rabeprazole sodium tablets whose specification is 10 mg, tablet weight is 116.15 mg, and tablet diameter is 7 mm (it can also be prepared by directly filling No. 3 capsules) The specification is 10mg rabeprazole sodium capsules).
[0061] Those skilled in the art can also predict that the weight ratio of rabeprazole sodium: anhydrous sodium bicarbonate: mannitol: hydroxypropyl cellulose: magnesium stearate is 10: 10: 80: 15: 1.15, 10: 30 :60:15:1.15 or 10:50:40:15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com