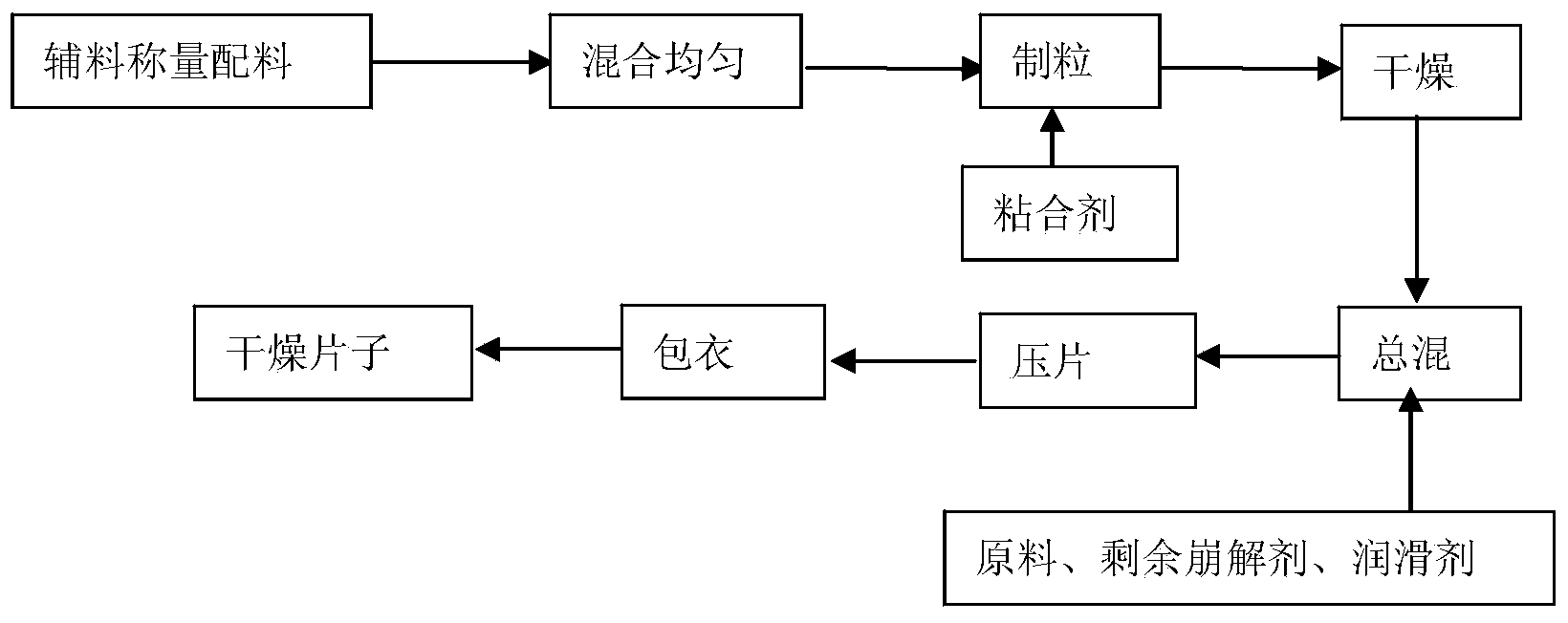
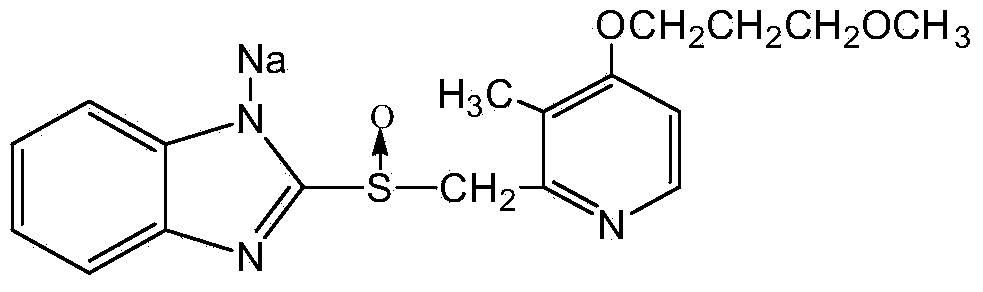
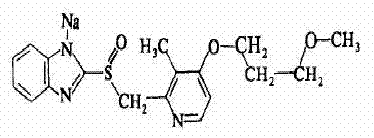
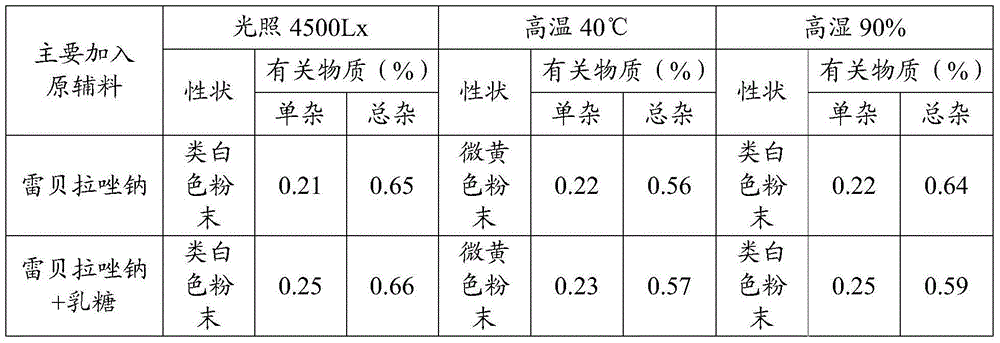
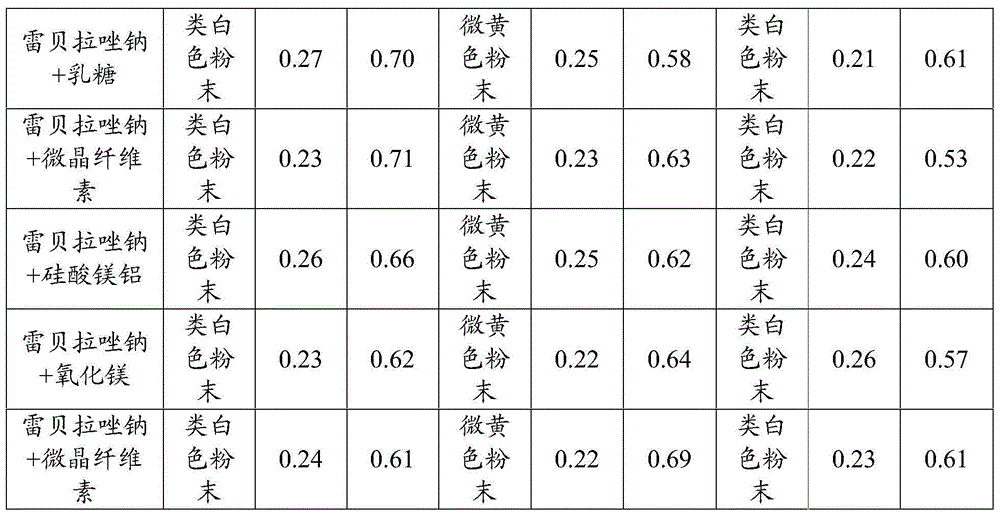
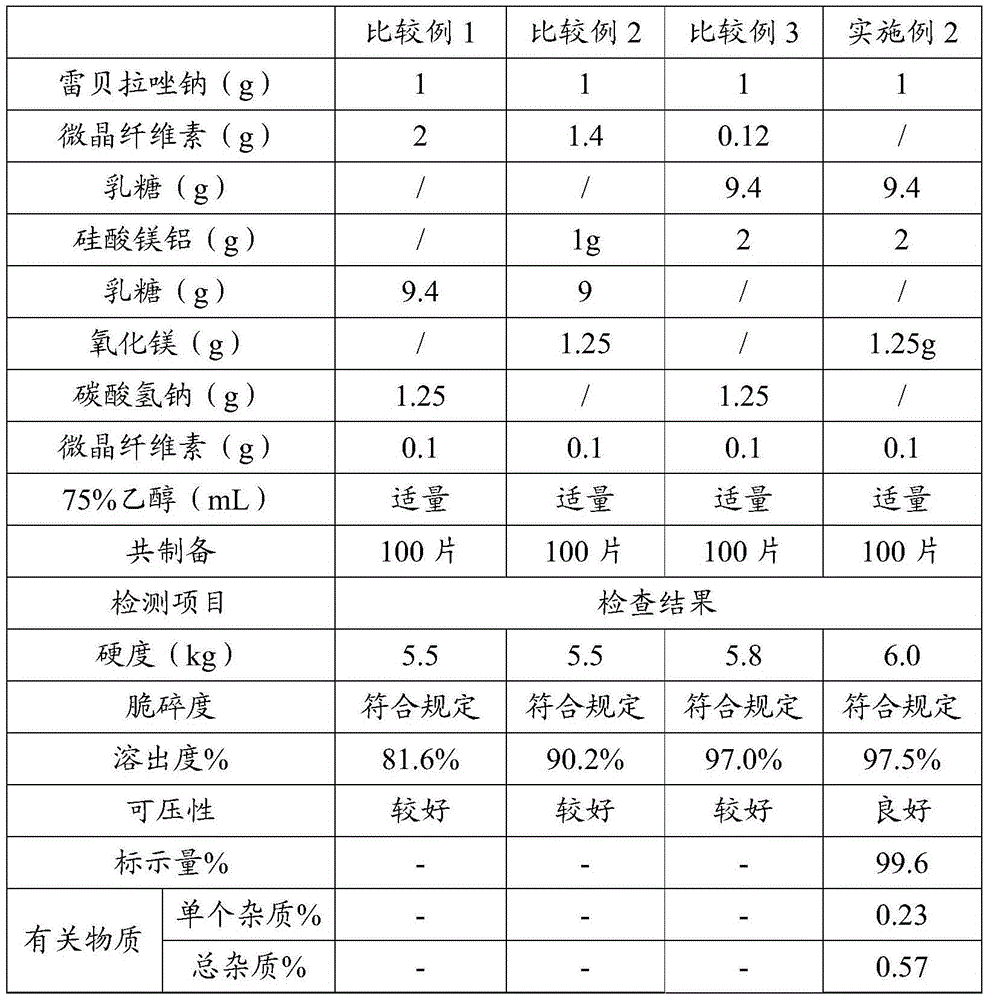
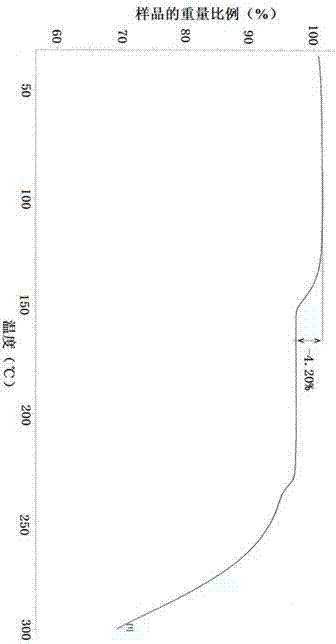
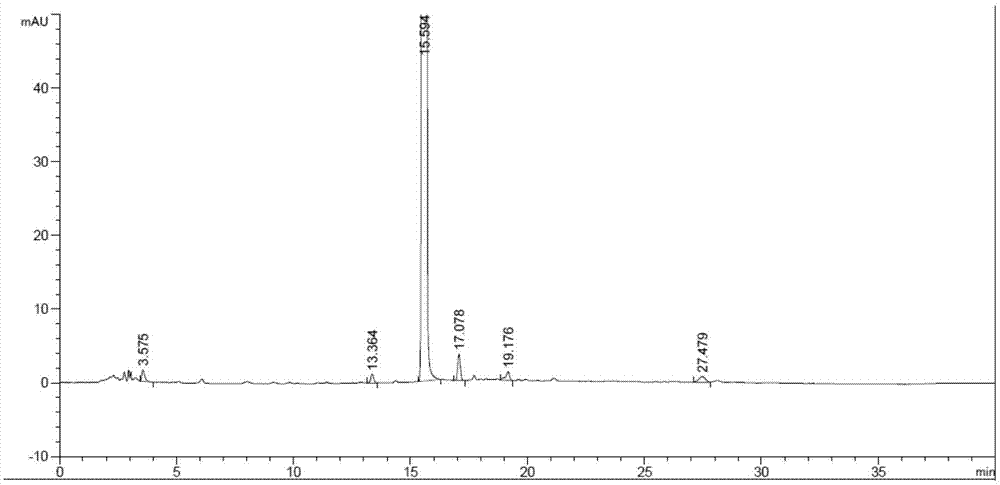
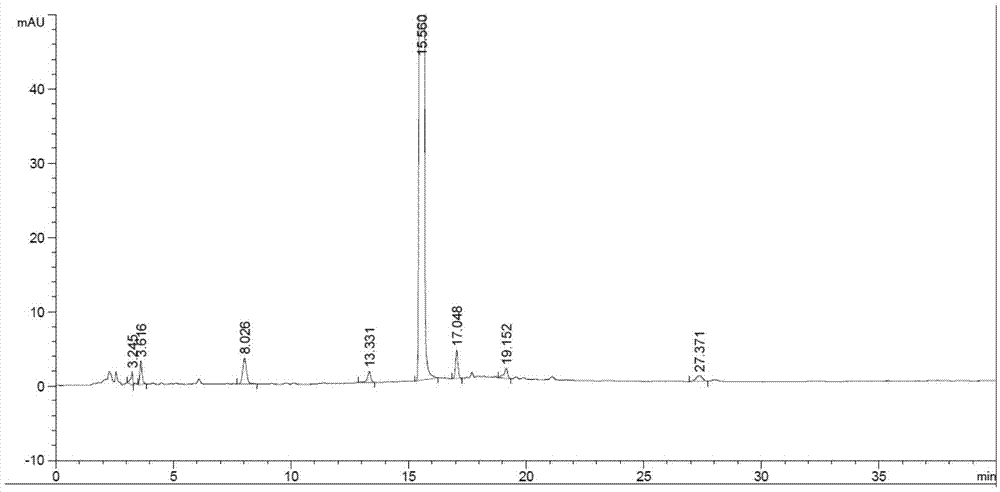
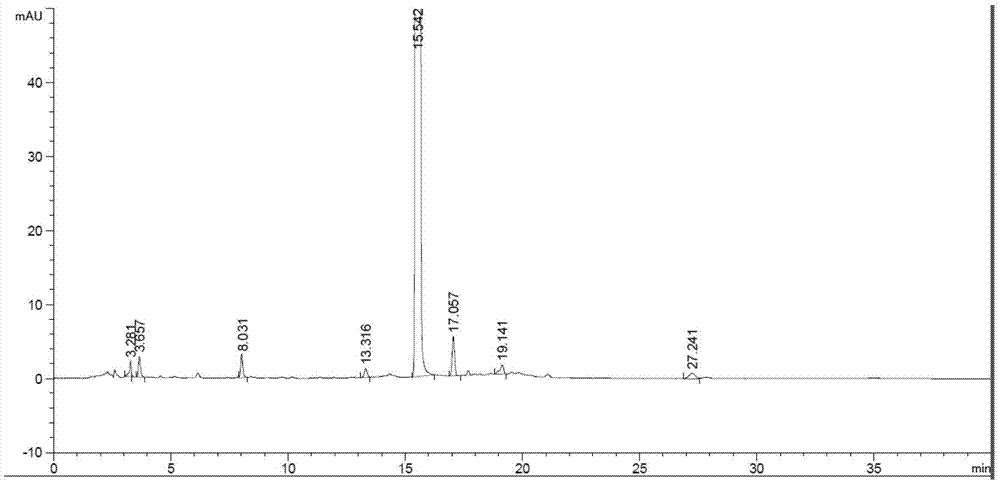
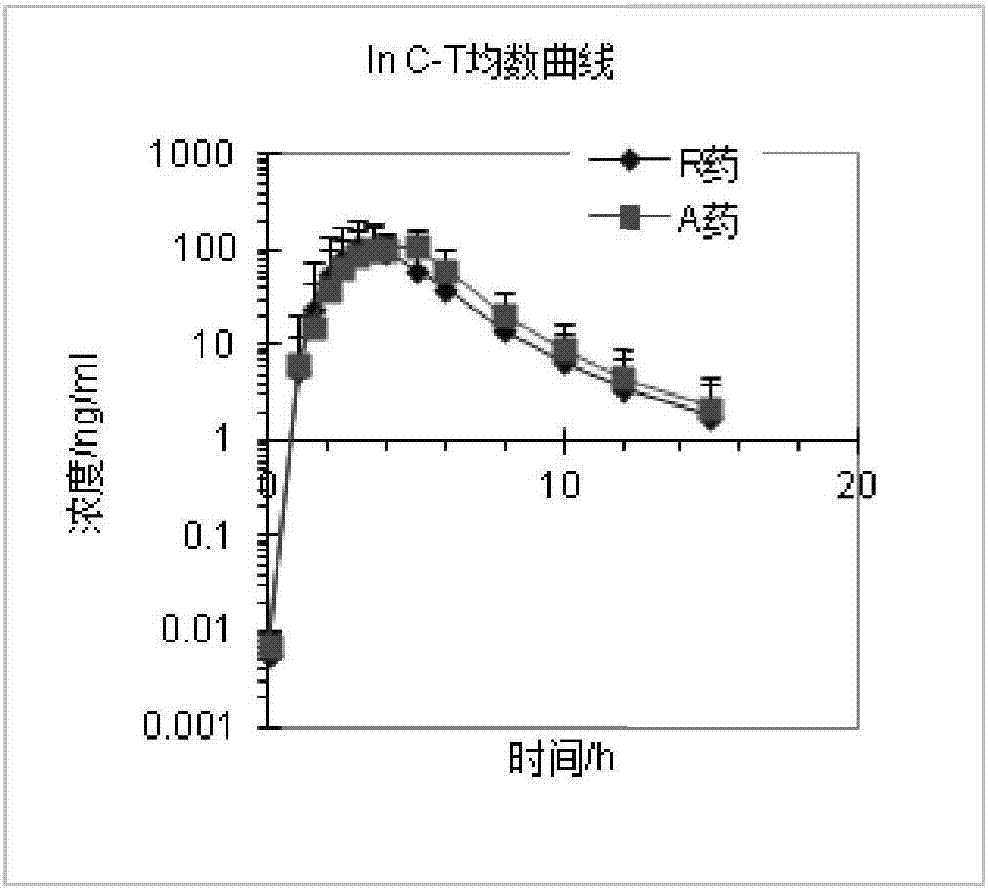
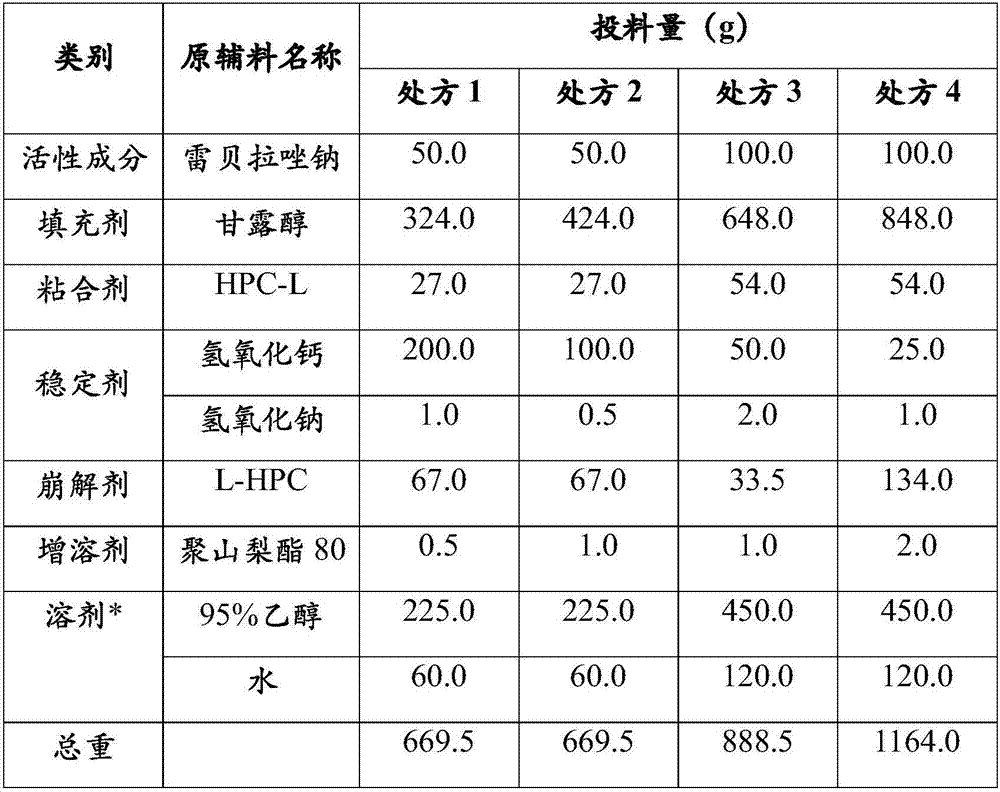
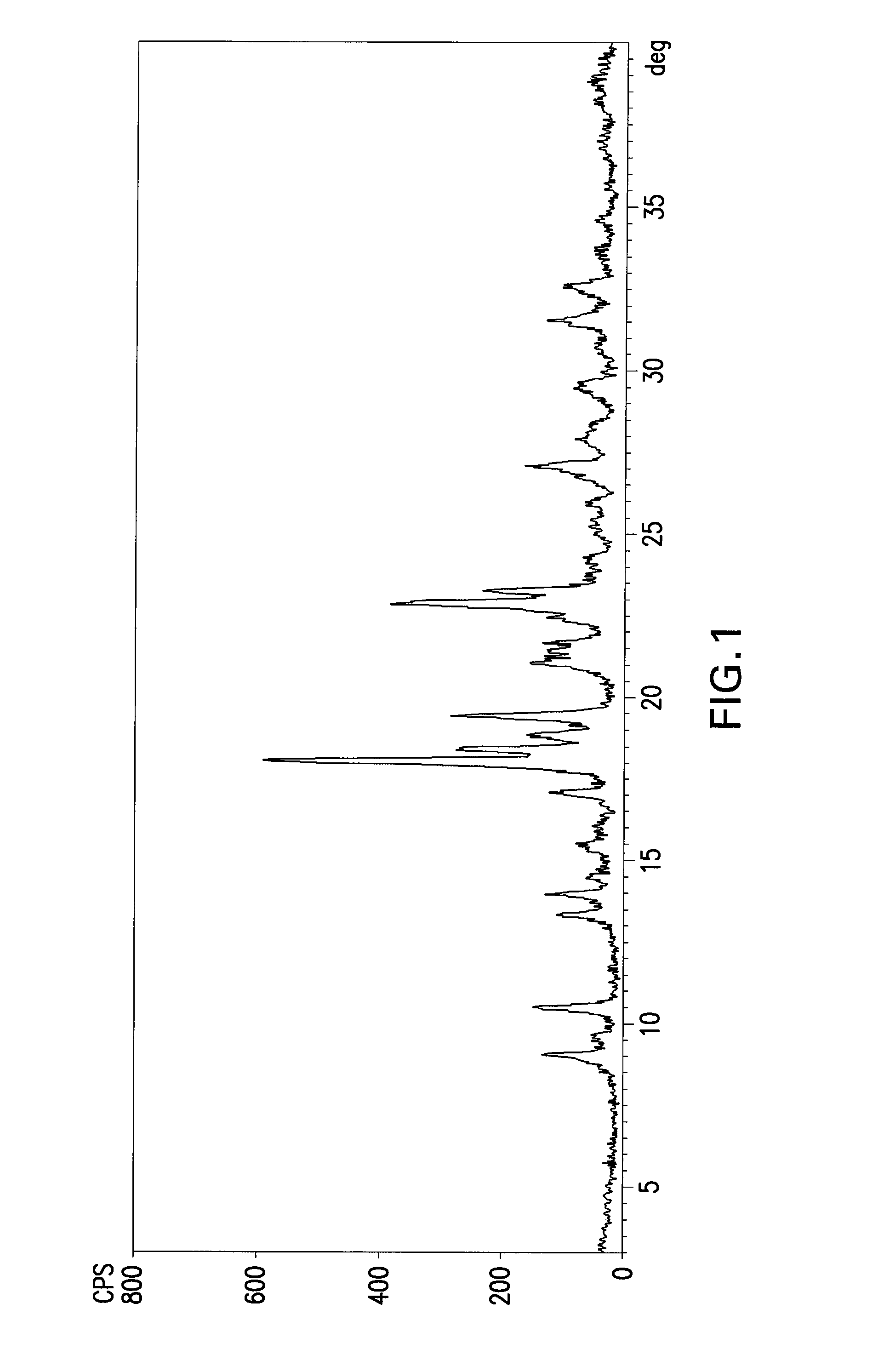
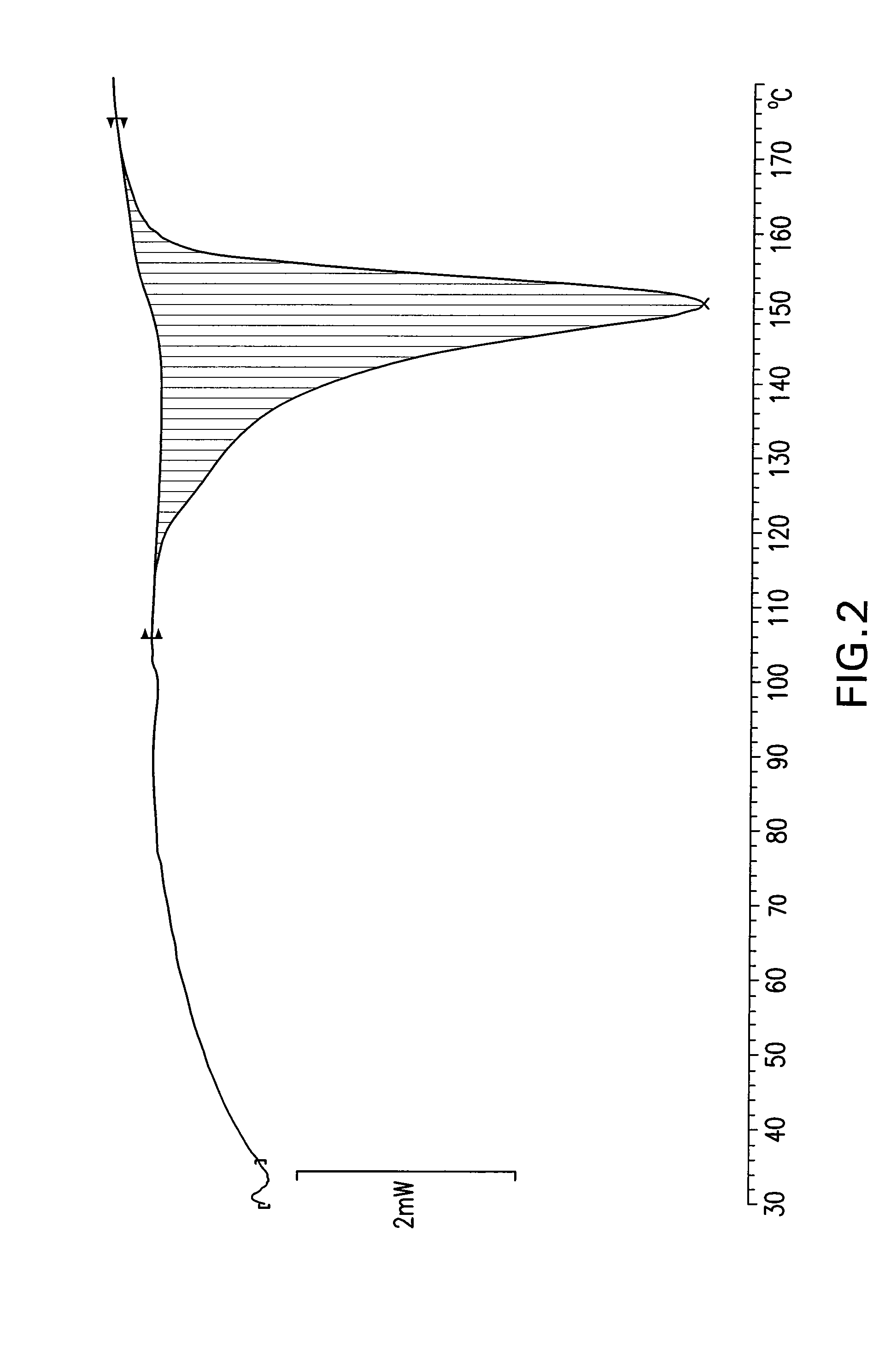
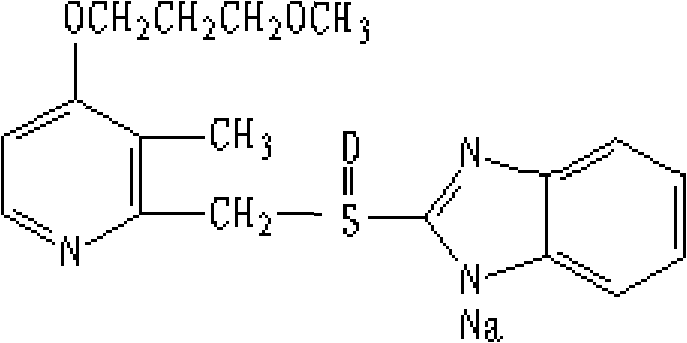Patents
Literature
113 results about "Rabeprazole Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
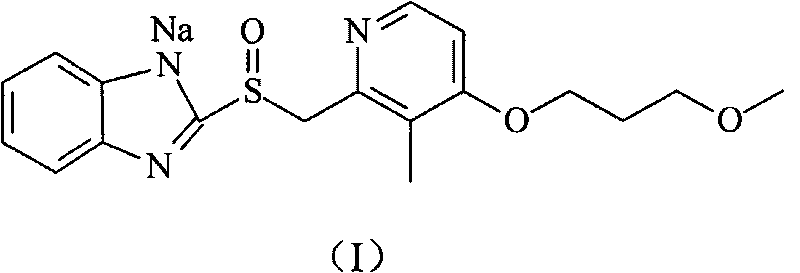
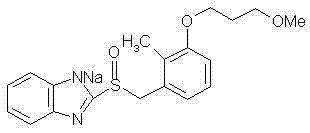
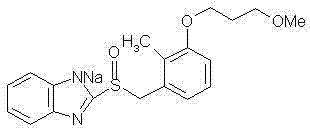
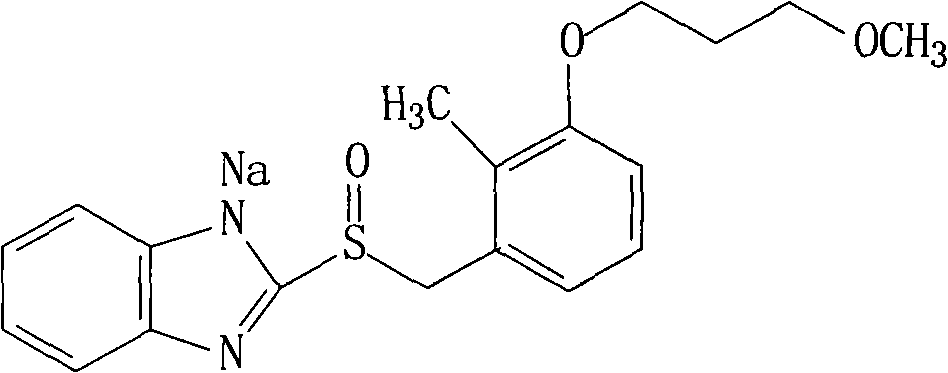
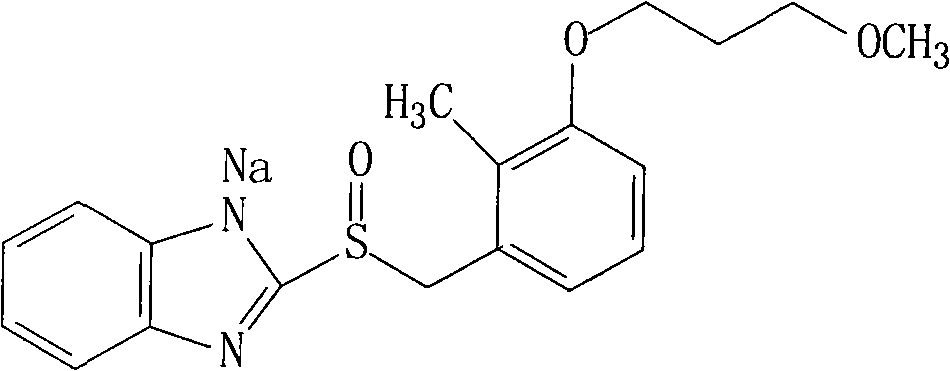
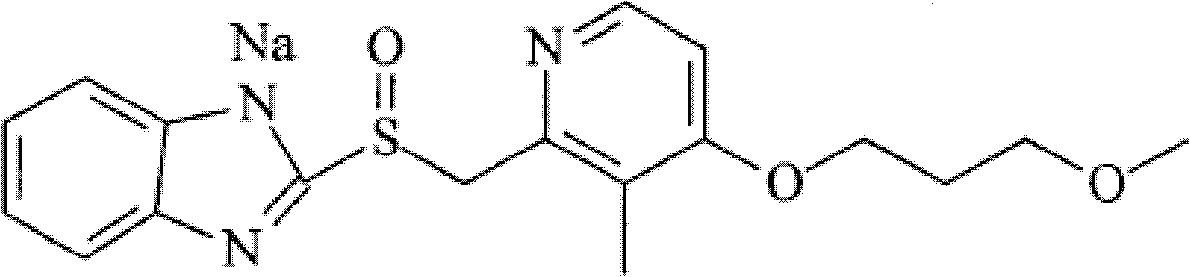
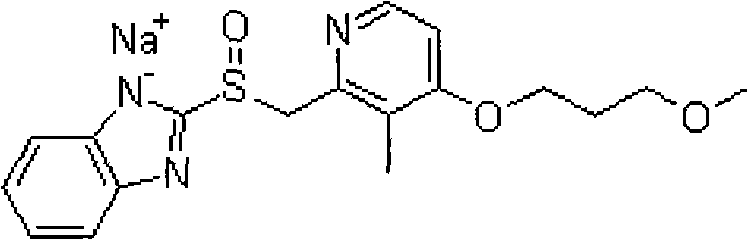
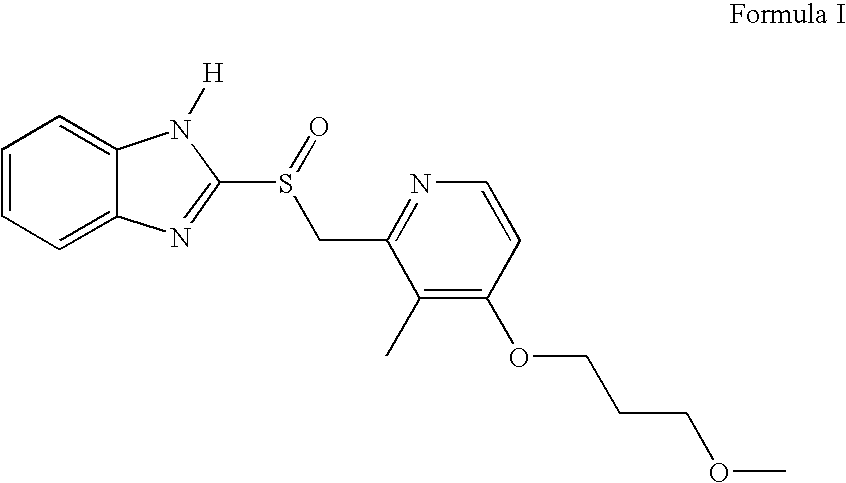
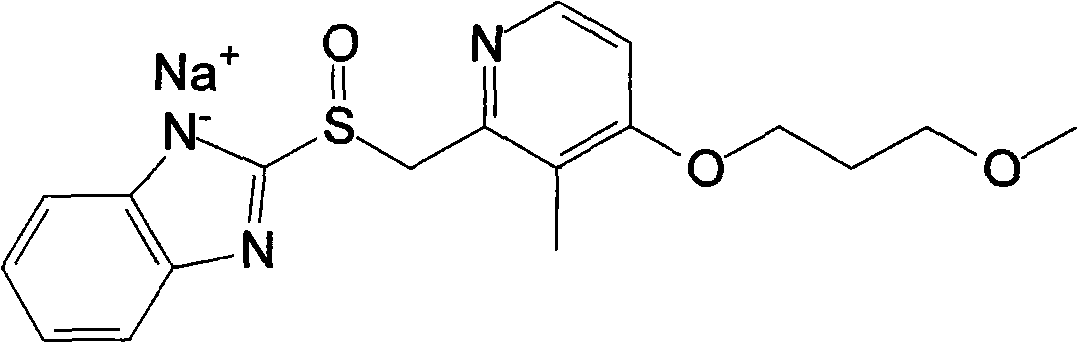
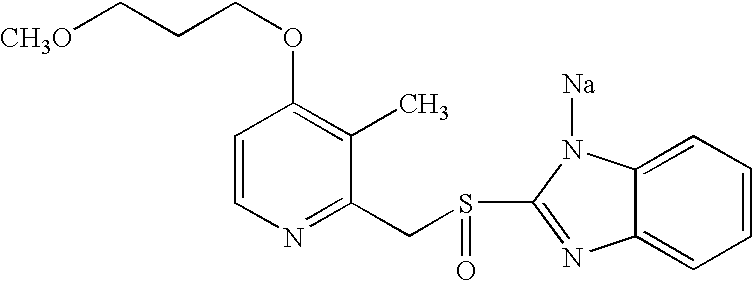
The sodium salt of the prodrug rabeprazole, a substituted benzimidazole proton pump inhibitor, with potential anti-ulcer activity. After protonation, accumulation, and transformation to the active sulfenamide within the acidic environment of gastric parietal cells, rabeprazole selectively and irreversibly binds to and inhibits the H+, K+ATPase (hydrogen-potassium adenosine triphosphatase) enzyme system located on the parietal cell secretory surface, inhibiting gastric acid secretion.
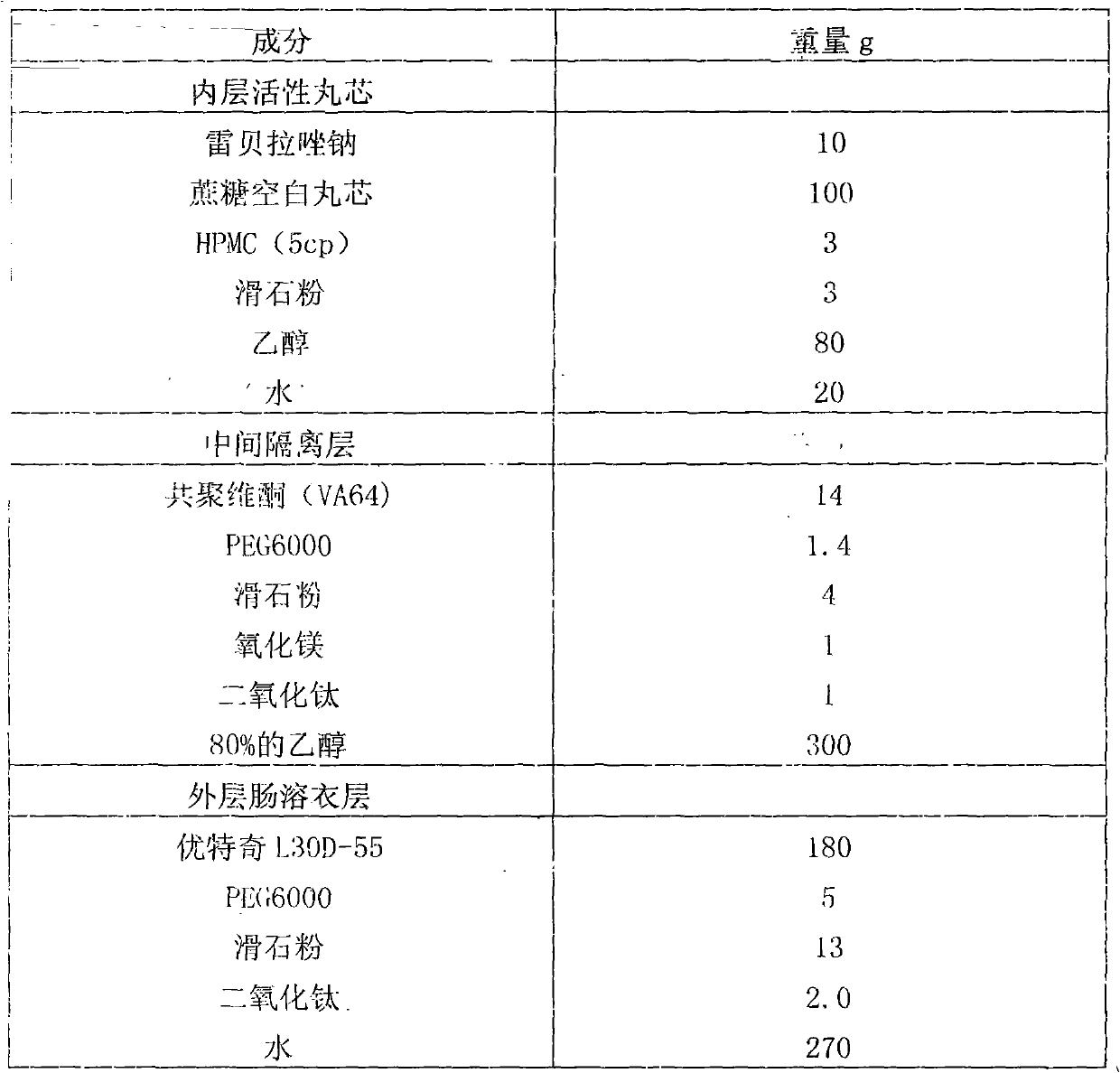
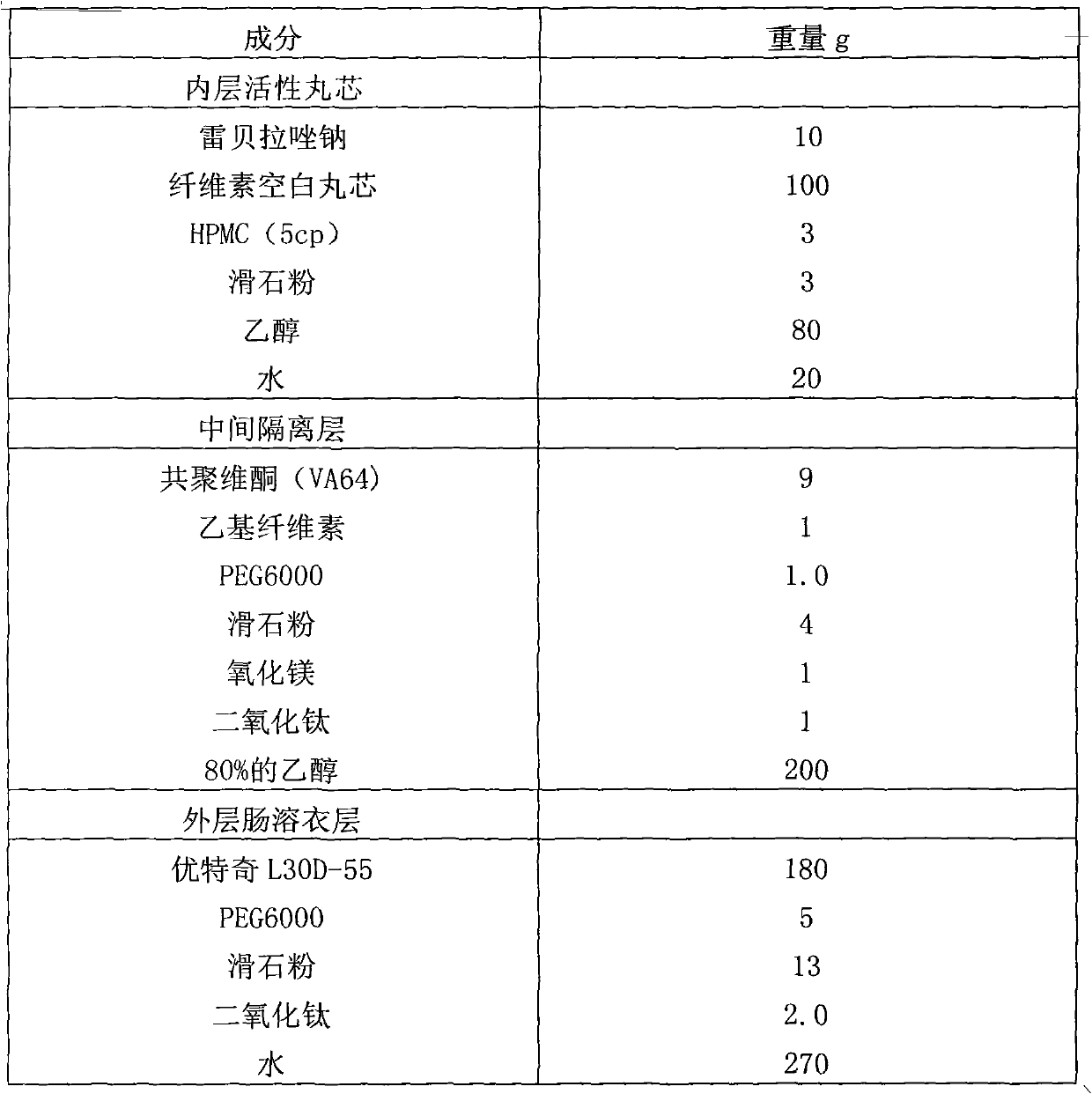
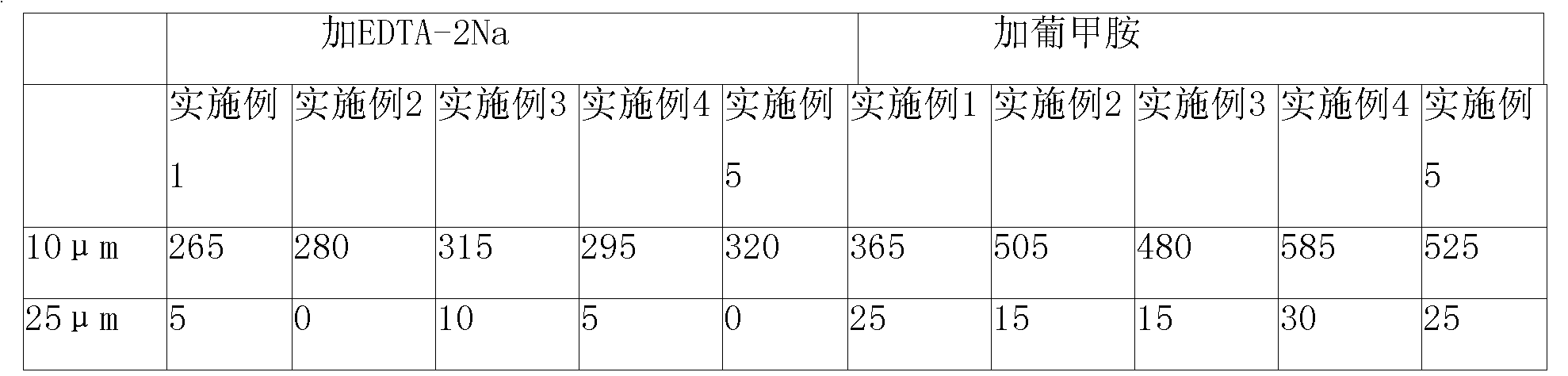
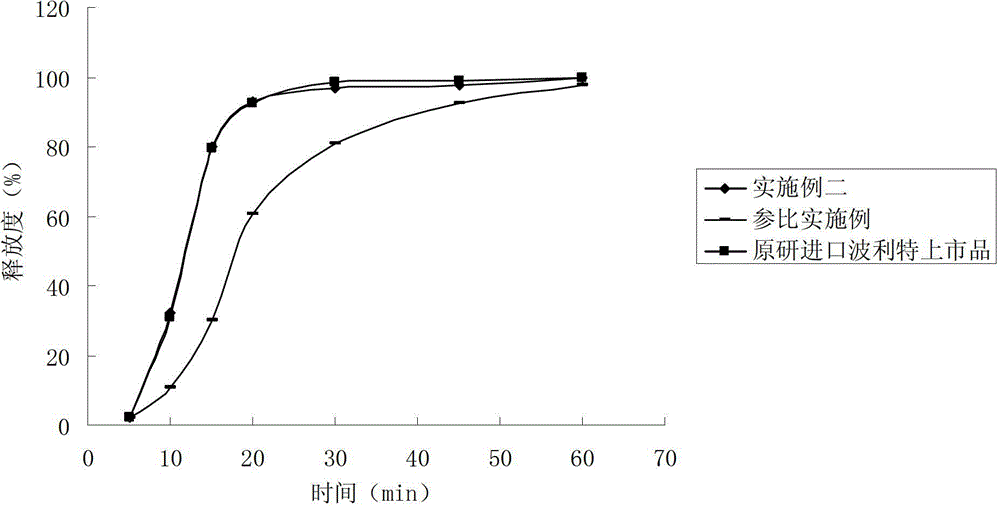
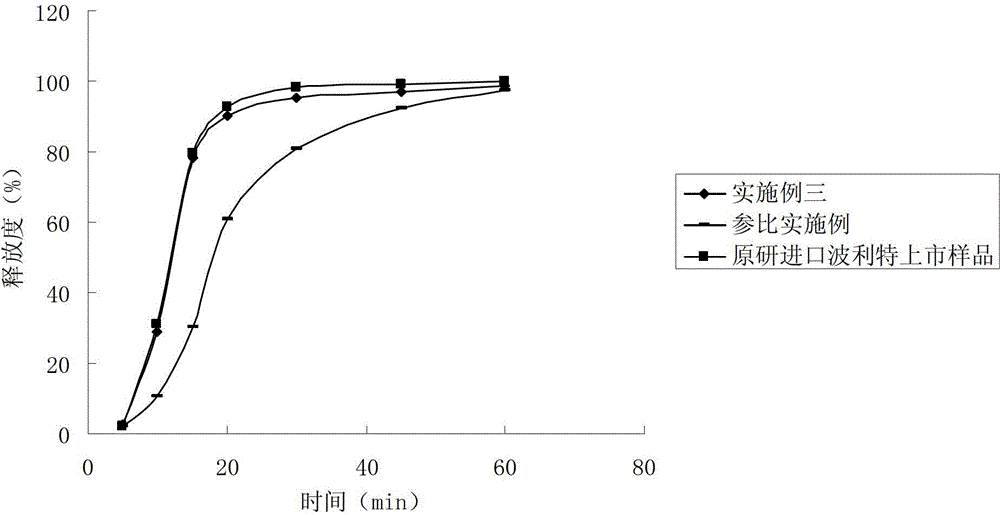
Rabeprazole sodium enteric-coated micro-pellet and preparation method thereof
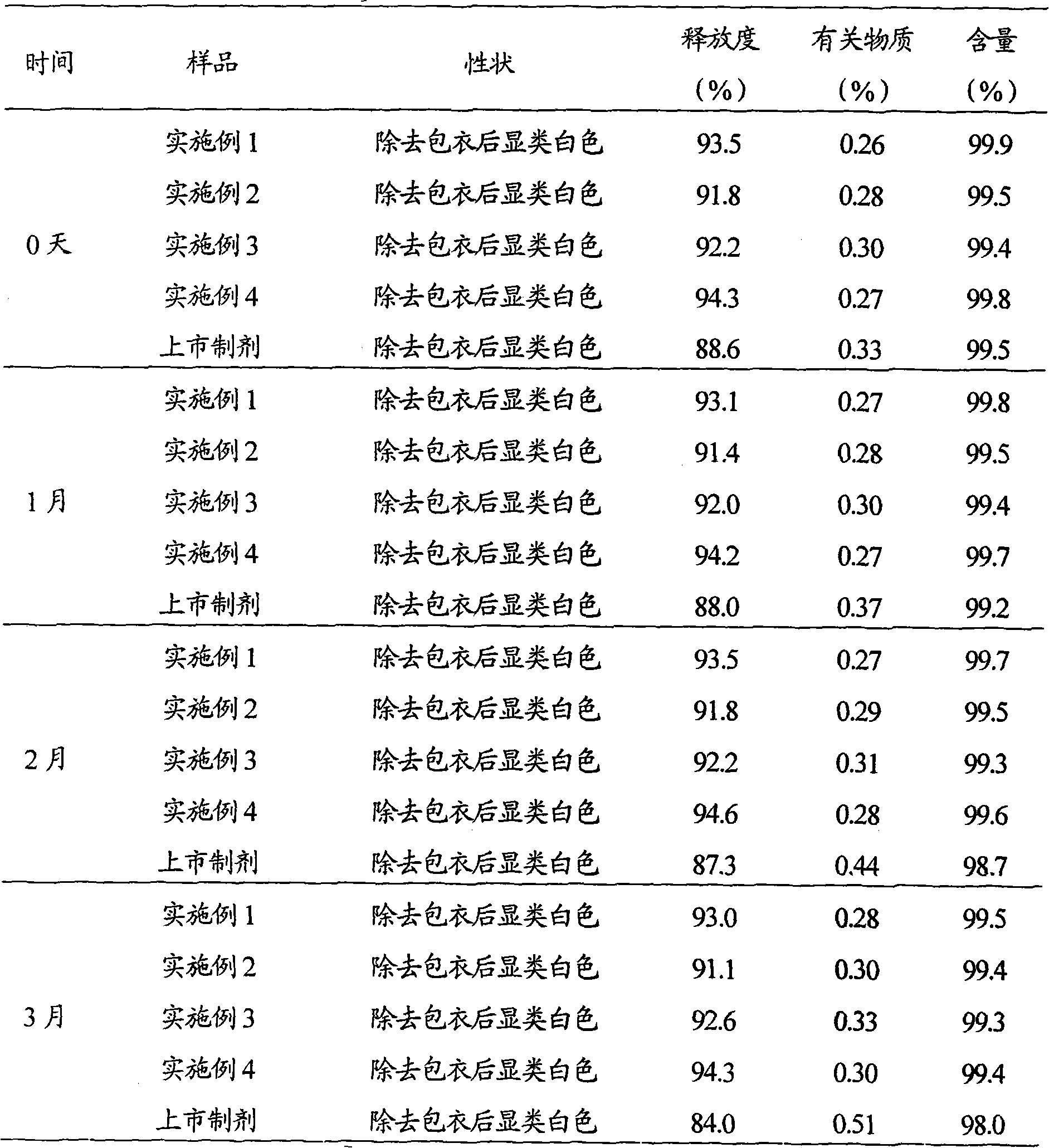
ActiveCN102552159AImprove stabilityQuick releaseOrganic active ingredientsDigestive systemSodium bicarbonateSolubility
The invention belongs to the field of medicament preparations, and relates to a rabeprazole sodium enteric-coated preparation and a preparation method thereof. A rabeprazole sodium enteric-coated micro-pellet disclosed by the invention comprises a blank pellet core, a medicament carrying layer, an isolating layer and an enteric-coated layer from inside to outside, wherein the weight increment of the medicament carrying layer is 10-20 percent; the weight increment of the isolating layer is 5-10 percent; the weight increment of the enteric-coated layer is 30-50 percent; the medicament carrying layer consists of rabeprazole sodium, hydroxypropyl methyl cellulose and talc powder; the isolating layer consists of copovidone, a plasticizing agent, talc powder, titanium dioxide and a stabilizing agent; the stabilizing agent is one or more of magnesium oxide, calcium oxide, sodium carbonate, sodium bicarbonate, potassium carbonate and potassium bicarbonate; and the enteric-coated layer consists of an enteric-coated material, a plasticizing agent, talc powder and titanium dioxide. The rabeprazole sodium enteric-coated micro-pellet disclosed by the invention has the effects of high solubility and high stability.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD +1
Rabeprazole sodium composition and preparation method thereof
ActiveCN101627996AReduce moistureImprove stabilityPowder deliveryOrganic active ingredientsSolubilityMicroparticle
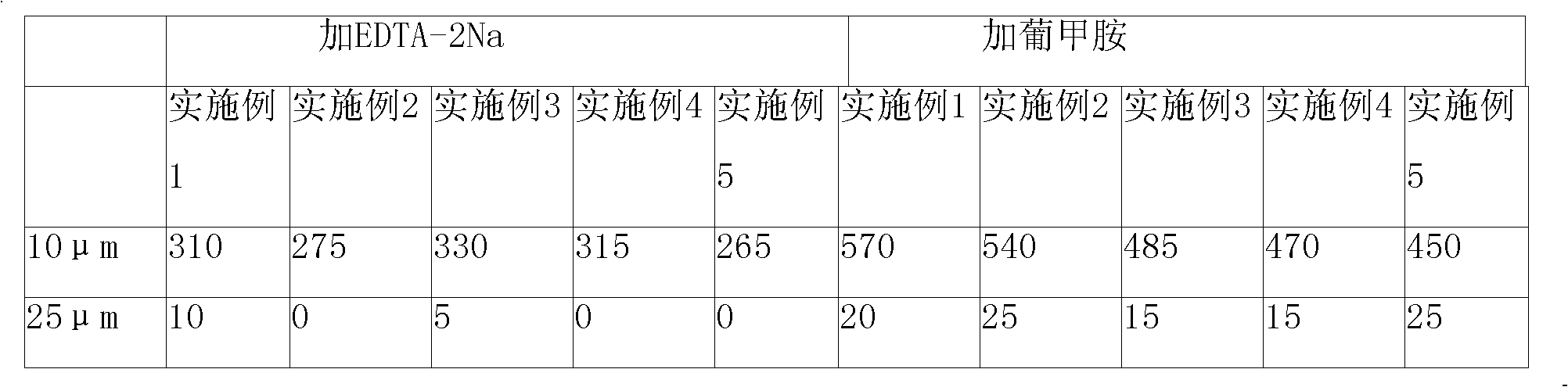
The invention provides a rabeprazole sodium composition which comprises the active ingredients of rabeprazole, mannitol and ethylene diamine tetraacetic acid, wherein the weight ratio of ethylene diamine tetraacetic acid to rabeprazole sodium is 0.05-0.5:1. The method for preparing the composition into solid powder-needle preparation comprises the following steps: dissolving the ethylene diamine tetraacetic acid solution with the prescription amount into injection water, adding and stirring the rabeprazole sodium with the prescription amount at a temperature of 25-35 DEG C for dissolving, then adding mannitol with the prescription amount, stirring the mannitol for dissolving, cooling to a room temperature, and regulating the pH value of the solution to 11.5-12.5; adding active carbon intothe confected solution, stirring and filtering for decarburization, and freezing and drying the obtained filtrate to obtain the rabeprazole frozen powder needle. The rabeprazole frozen powder needle has full appearance, favorable stability and solubility and little insoluble grains in the injection.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
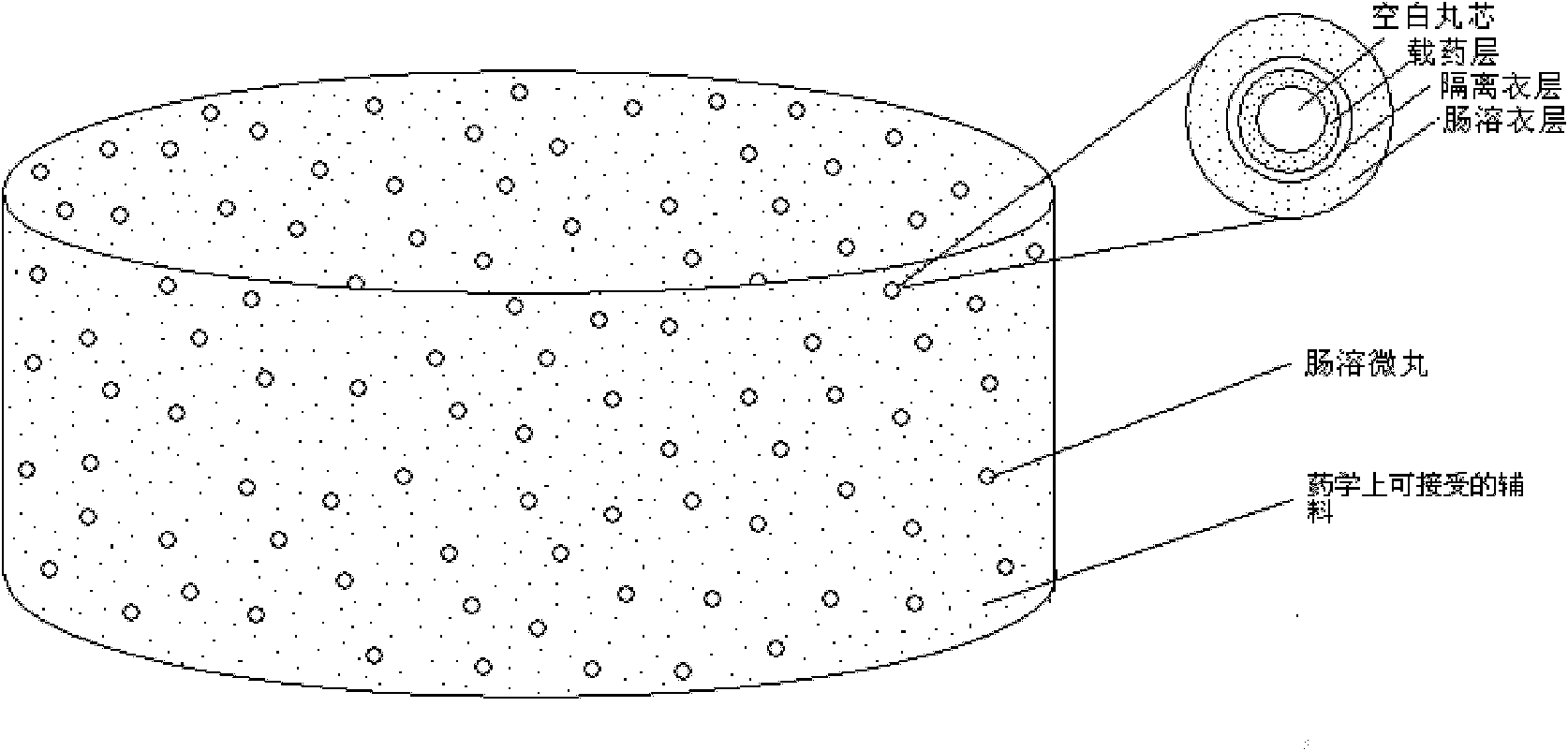
Rabeprazole sodium enterosoluble micro-particles and preparation method thereof
ActiveCN102652734AImprove complianceReduce adverse reactionsPowder deliveryOrganic active ingredientsRefluxMedicine
The invention belongs to the field of pharmaceutical preparations, and particularly relates to novel rabeprazole sodium enterosoluble micro-particles with high stability in an acid medium, and a preparation method thereof. Each rabeprazole sodium enterosoluble micro-particle comprises a blank pill core (1), a medicine-carrying layer (2), an isolation coating layer (3) and an enteric coating layer (4) in sequence from inside to outside. A pH value regulator is used in the medicine-carrying layer, the isolation coating layer is added between the medicine-carrying layer and the enteric coating layer, and particularly, a retarding agent is added into an enteric coating layer combination, so that the acid tolerance of a medicament active ingredient namely rabeprazole sodium which is extremely unstable in acid is well improved. The rabeprazole sodium enterosoluble micro-particles and a preparation thereof can be used for treating diseases such as active duodenal ulcer, benign active gastric ulcer, rodent or ulcerative gastroesophageal reflux accompanied by clinical symptoms and the like.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
High-purity sodium rabeprazole compound
InactiveCN101704811AOvercome purityOvercome the disadvantages of difficult purificationOrganic chemistryOrganic solventRabeprazole
The invention relates to a high-purity sodium rabeprazole compound, belonging to the technical field of medicine. The method includes the following steps: dissolving crude sodium rabeprazole synthesized by the reaction of rabeprazole and sodium hydroxide in water, adjusting pH value to be faintly acid to neutral by using solid acid salt, and collecting precipitated solid; after dissolving the solid with organic solvent, conducting elution and purification by using eluting agent through macroporous adsorption resin, and collecting eluent; and adjusting the pH value of the eluent to be alkaline, and collecting the precipitated solid to obtain the pure sodium rabeprazole.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Enteric-coated preparation of rabeprazole sodium and preparation method thereof
ActiveCN104337789APollution has little effectShorten the production cycleAntibacterial agentsOrganic active ingredientsSolventStabilizing Agents
The invention belongs to the field of medicines and in particular relates to an enteric-coated preparation of rabeprazole sodium, a proton pump inhibitor and a preparation method thereof. The properties of rabeprazole sodium are very unstable and rabeprazole sodium can be quickly degraded and discolored if rabeprazole sodium is prepared or stored improperly, thus directly affecting the curative effects and medication safety of the medicine. In order to solve the problems, the enteric-coated preparation is prepared by using rabeprazole sodium as the main medicine and adopting three layers of coatings, wherein the three coating layers are respectively an alkaline protective layer, an isolation layer and an enteric-coated layer from inside to outside; the alkaline protective layer comprises film forming agents, lubricating agents, light-screening agents, alkaline stabilizing agents and solvents and the pH value of the coating solution is 8.0-13.0; the isolation layer comprises film forming agents, lubricating agents, plasticizers, light-screening agents and solvents and the pH value of the coating solution is 7.0-8.0; the enteric-coated layer comprises film forming agents, plasticizers, lubricating agents, light-screening agents, stabilizing agents and solvents and the pH value of the coating solution is 5.0-6.0. The enteric-coated preparation has the characteristics of short product production cycle and less influence caused by environments, temperatures, humidity and dust pollution.
Owner:成都迪康药业股份有限公司
Dexrabeprazole sodium monohydrate crystal form and preparation method thereof
ActiveCN102924434AImprove stabilityImprove solubilityOrganic active ingredientsOrganic chemistrySODIUM MONOHYDRATEPhysical chemistry
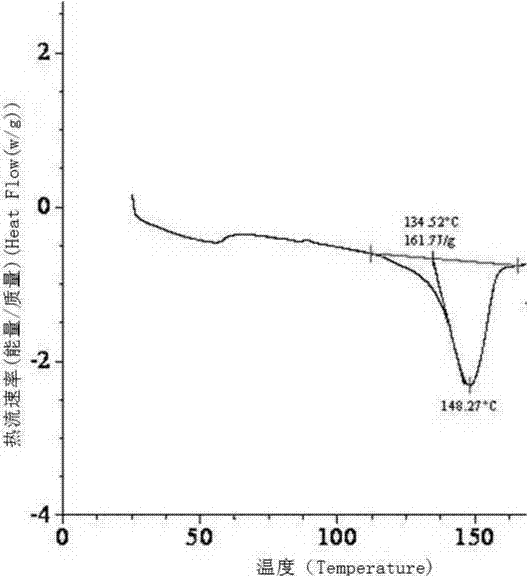
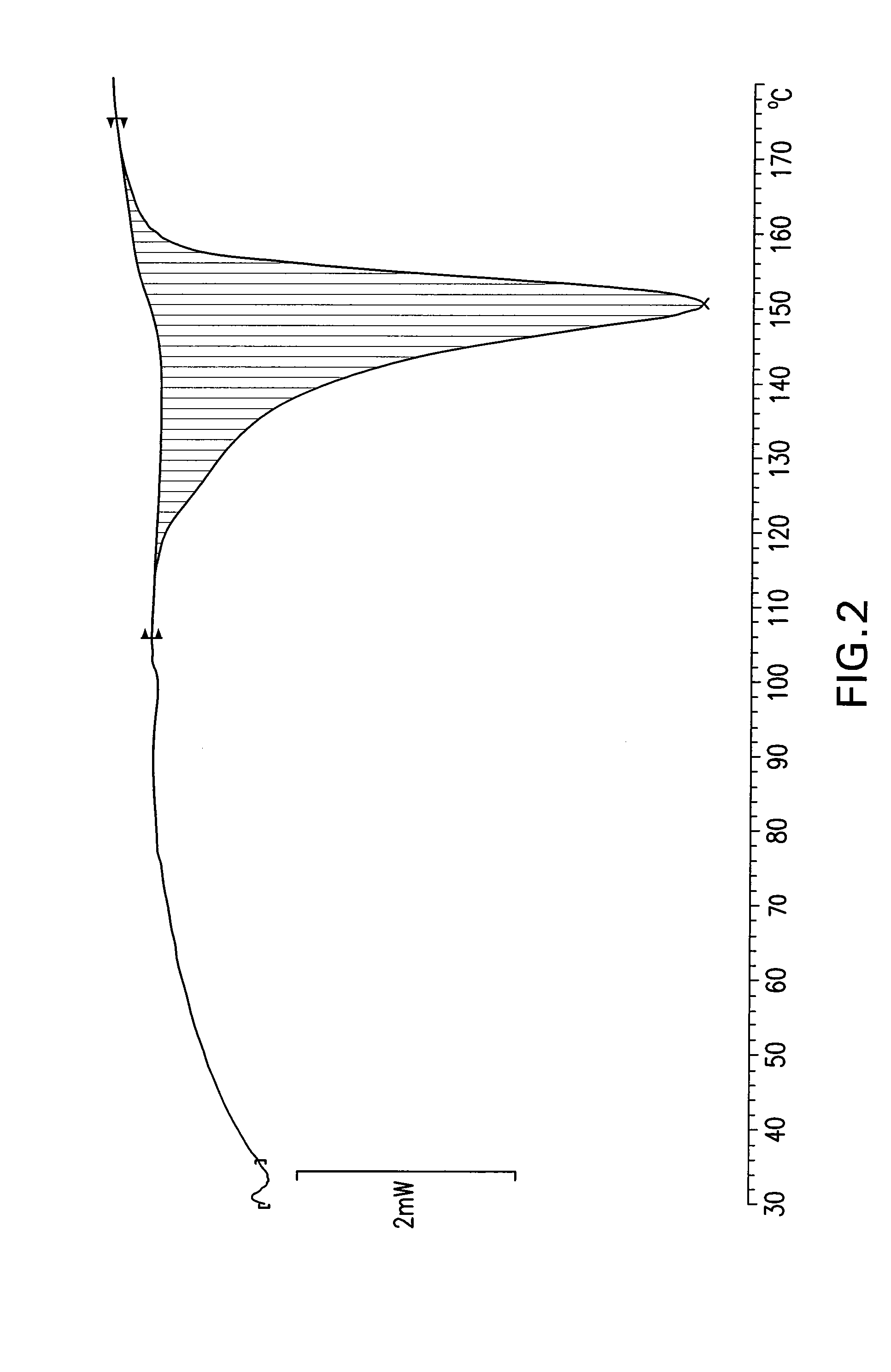
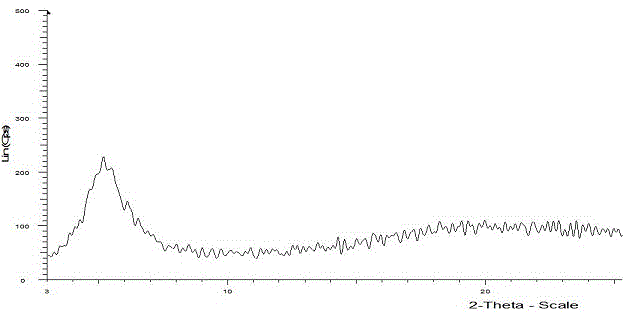
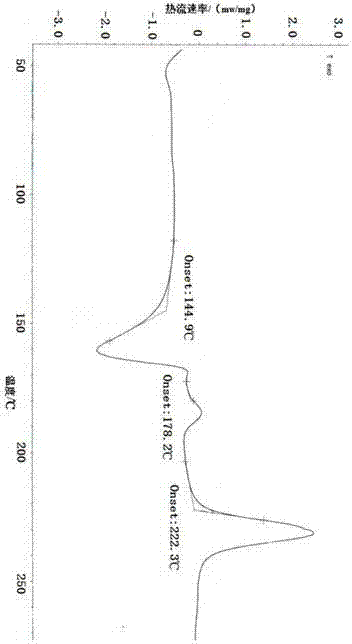
The invention provides a dexrabeprazole sodium monohydrate crystal form. The invention is characterized in that the dexrabeprazole sodium monohydrate crystal form has characteristic diffraction peaks at 10.5, 13.4, 17.1, 18.0, 18.5, 18.9, 19.5, 23.0, 23.3, 27.1 and 31.6 degrees in an X-ray powder diffraction spectrum represented by Cu-Kalpha radiation and 2theta; and an absorption peak exists at about 148.27 DEG C in a differential thermal analysis atlas of the dexrabeprazole sodium monohydrate crystal form. The crystal form has relatively high stability, solubility and dissolution, and relatively low hygroscopicity. The invention also provides a preparation method for the dexrabeprazole sodium monohydrate crystal form. The preparation method is simple, high in yield and low in preparation cost, and the quality of products is high.
Owner:JIANGSU CHENGXIN PHARMA
Sodium rabeprazole enteric-coated orally disintegrating tablets and preparation method thereof
ActiveCN101507718AImprove utilizationSmall particle sizeOrganic active ingredientsDigestive systemFiller ExcipientOrally disintegrating tablet
The invention relates to a Rabeprazole sodium enteric-coated orally disintegrating tablet and a method for preparing the same. The Rabeprazole sodium enteric-coated orally disintegrating tablet comprises the following components in percentage by weight: 1 to 5 percent of Rabeprazole sodium, 8 to 10 percent of blank pill core, 2 to 5 percent of stabilizer, 10 to 15 percent of isolation layer, 15 to 20 percent of enteric coating, 35 to 70 percent of filling agent and 10 to 20 percent of disintegrating agent. The invention provides the Rabeprazole sodium enteric-coated orally disintegrating tablet having the advantages of small grain size, quick absorption, high bioavailability, good taste, easy swallowing, good disintegrated effect, strong compressibility, and convenient use for patients and the method for preparing the same.
Owner:成都迪康药业股份有限公司
Rabeprazole sodium crystal compound and preparing method thereof
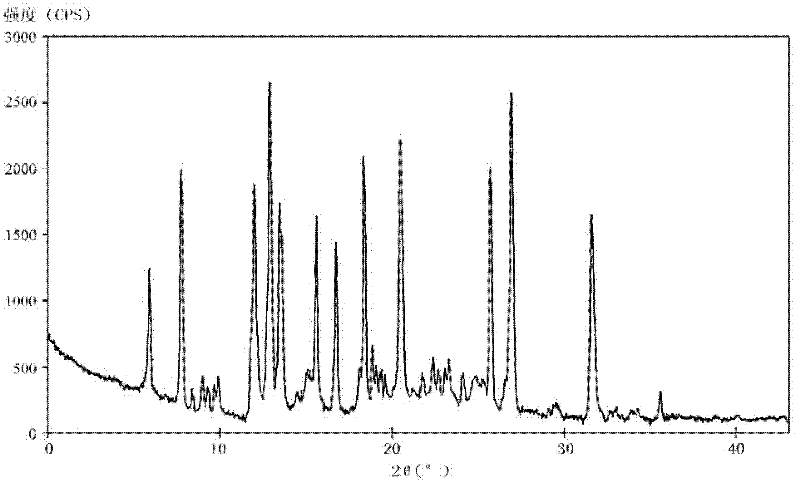
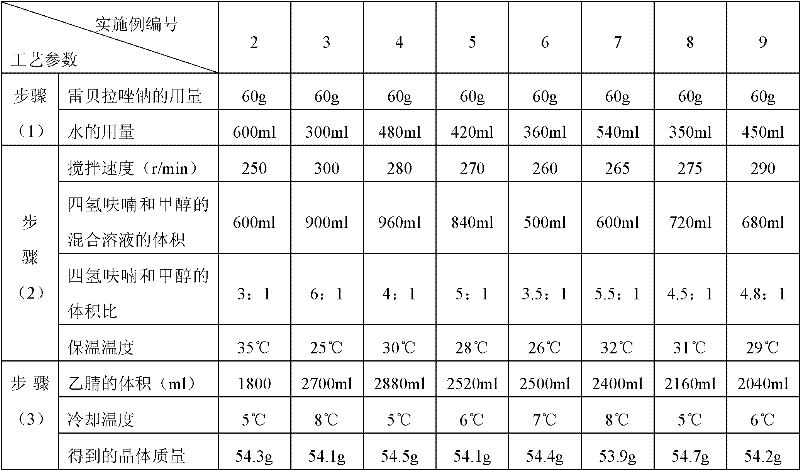
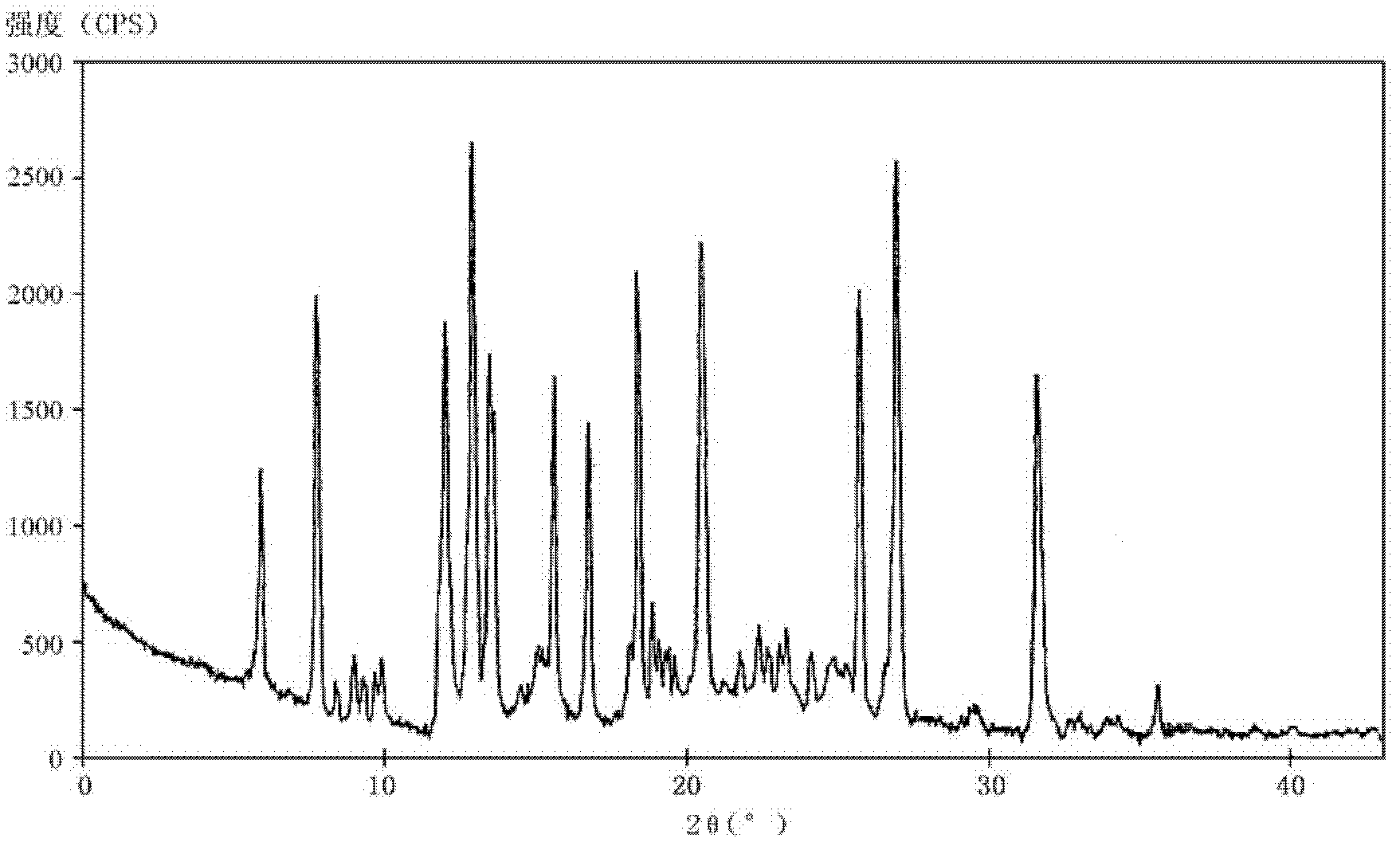
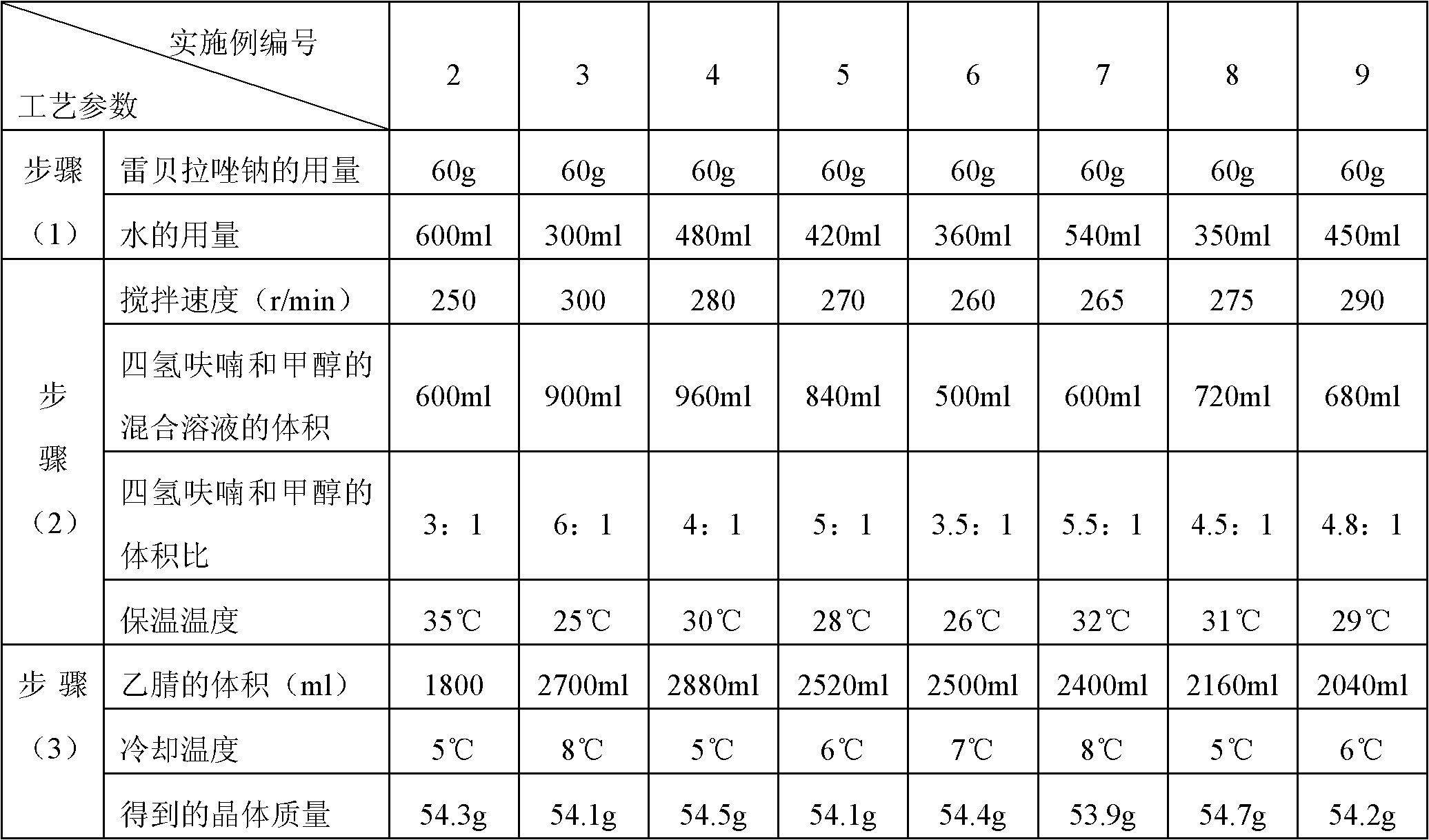
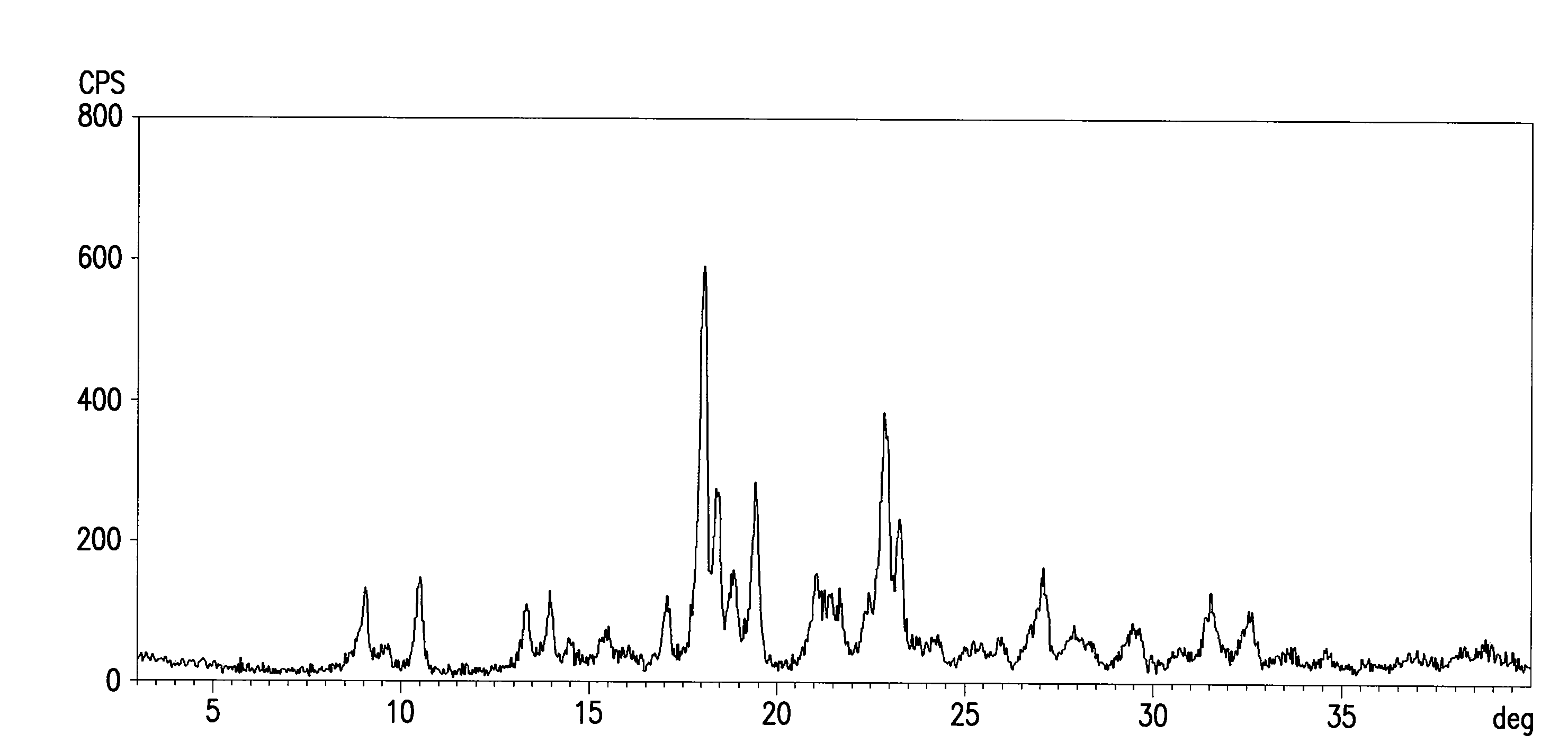
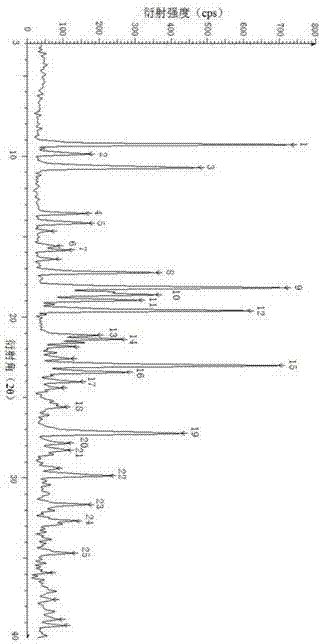
The invention relates to a rabeprazole sodium crystal compound and a preparing method thereof. The crystal compound is determined by a powder X-ray diffraction determining method, and an X-ray powder diffraction spectrum represented by 2 theta + / - 0.2 degree diffraction angles shows characteristic diffraction peaks at the positions of 5.8 degrees, 7.5 degrees, 12.1 degrees, 12.8 degrees, 13.3 degrees, 15.6 degrees, 16.7 degrees, 18.3 degrees, 20.4 degrees, 25.7 degrees, 26.8 degrees and 31.5 degrees. The preparing method comprises the following steps of: 1) adding rabeprazole sodium serving as a crude drug in water, stirring and dissolving to obtain a rabeprazole sodium water solution; 2) adding a mixed solution of tetrahydrofuran and methanol into the rabeprazole sodium water solution, conducting heat-insulation decoloration at the temperature of 25-35 DEG C by active carbon and filtering to obtain filtrate; and 3) dropping the filtrate into acetonitrile, cooling, crystallizing, filtering and drying in vacuum, thus obtaining the rabeprazole sodium crystal compound. The crystal compound has good stability and strong inhibitory action on Helicobacter pylori.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Rabeprazole sodium enteric-coated pellet and preparation method thereof
ActiveCN103599087AGood compatibilityImprove securityOrganic active ingredientsDigestive systemInsulation layerAdhesive
A rabeprazole sodium enteric-coated pellet is composed of a pellet core, an insulation layer and an enteric-coated layer from the inside to the outside, wherein by the total weight of the enteric-coated pellet, the content of the pellet core is 50 to 70 wt%, the content of the insulation layer is 10 to 20 wt%, and the content of the enteric-coated layer is 20 to 40 wt%; the pellet core comprises rabeprazole, a filling agent, an adhesive, a disintegrating agent, and an alkalizer, by the total weight of the pellet core, the content of the rabeprazole is 7 to 9 wt%, the content of the filling agent is 60 to 75 wt%, the content of the adhesive is 5 to 10 wt%, the disintegrating agent is 4 to 8 wt%, and the content of the alkalizer is 5 to 12 wt%. The rabeprazole sodium enteric-coated pellet has the advantages of low cost, stable quality, and good safety.
Owner:HAINAN HAILI PHARMACEUTICAL CO LTD
A stable rabeprazole sodium compound
The invention belongs to the technical field of medicines and particularly relates to a rabeprazole sodium compound and a preparation method thereof. Rabeprazole sodium provided by the invention contains less water and has the advantages of chemical purity up to 99.9%, maximum impurity content less than 0.1%, optical purity up to 99.96%ee and good stability. The compound provided by the inventionhas low production cost and stable quality, and is suitable for industrial production.
Owner:TIANJIN HANRUI PHARMA
Rabeprazole sodium compound and novel preparation method thereof
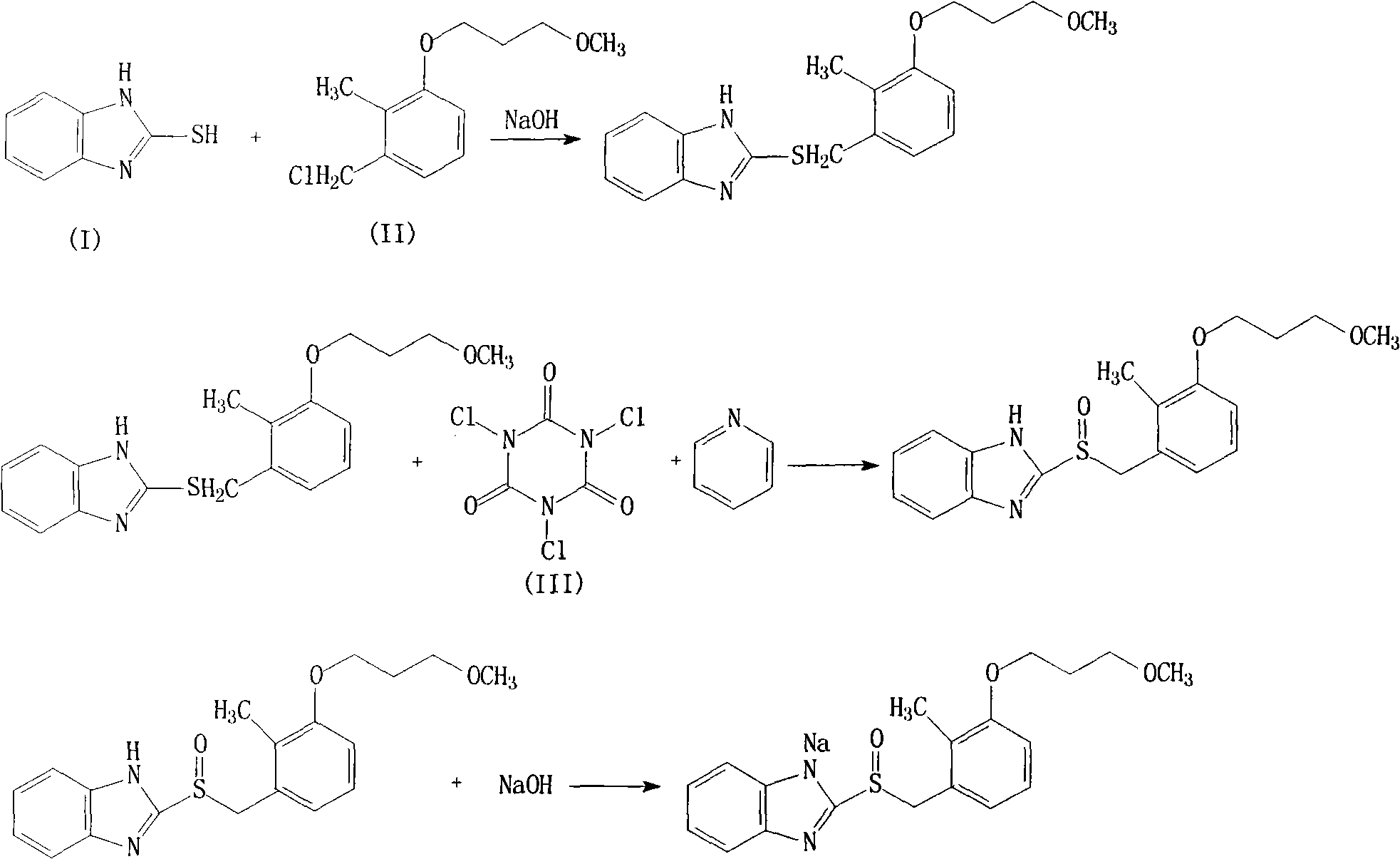
The invention aims at providing a novel rabeprazole sodium compound and a novel preparation method thereof. A novel oxidizing agent of trichloroisocyanuric acid is adopted for controlling the oxidization from thioether into sulfoxide, and the oxidization from the thioether into the sulfoxide is perfectly controlled. Because the invention shortens the reaction steps, and few other impurities are introduced in the process of synthesizing chiral rabeprazole sodium, so the purification process is easier, the purity and the yield of obtained finally products are high, and the invention is applicable to large-scale industrial production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Lyophilized powder injection of rabeprazole sodium medicinal composition and preparation method of lyophilized powder injection
ActiveCN102552178AStrong ability to suppress acidReduce the incidence of bleedingOrganic active ingredientsPowder deliverySulfite saltStrong acids
The invention relates to a lyophilized powder injection of a rabeprazole sodium medicinal composition and a preparation method of the lyophilized powder injection. The medicinal composition is composed of rabeprazole sodium and pharmaceutically acceptable auxiliary materials, wherein the rabeprazole sodium is a rabeprazole sodium crystal compound. By utilizing a powder X-ray diffraction determination method to determine, characteristic diffraction peaks are displayed at parts of 5.8 degrees, 7.5 degrees, 12.1 degrees, 12.8 degrees, 13.3 degrees, 15.6 degrees, 16.7 degrees, 18.3 degrees, 20.4 degrees, 25.7 degrees, 26.8 degrees and 31.5 degrees by an X-ray powder diffraction pattern which is represented by a 2 theta+ / -0.2 degree diffraction angle; and the pharmaceutically acceptable auxiliary materials comprise mannitol and disodium ethylene diamine tetraacetate and further comprise meglumine and sodium sulfite. The prepared rabeprazole sodium powder injection is full in appearance andgood in redissolving property and has fewer insoluble particles; and a test shows that the lyophilized powder injection has a stronger acid-inhibiting capability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Process for Synthesis of Proton Pump Inhibitors
A process for preparation of rabeprazole sodium comprising oxidation of wet or dry rabeprazole sulphide with sodium hypohalite in water or a mixture of water and water miscible solvent using alkali metal hydroxide and catalyst is disclosed herein. The present invention also discloses process for preparation of rabeprazole sulphide.
Owner:CIPLA LTD
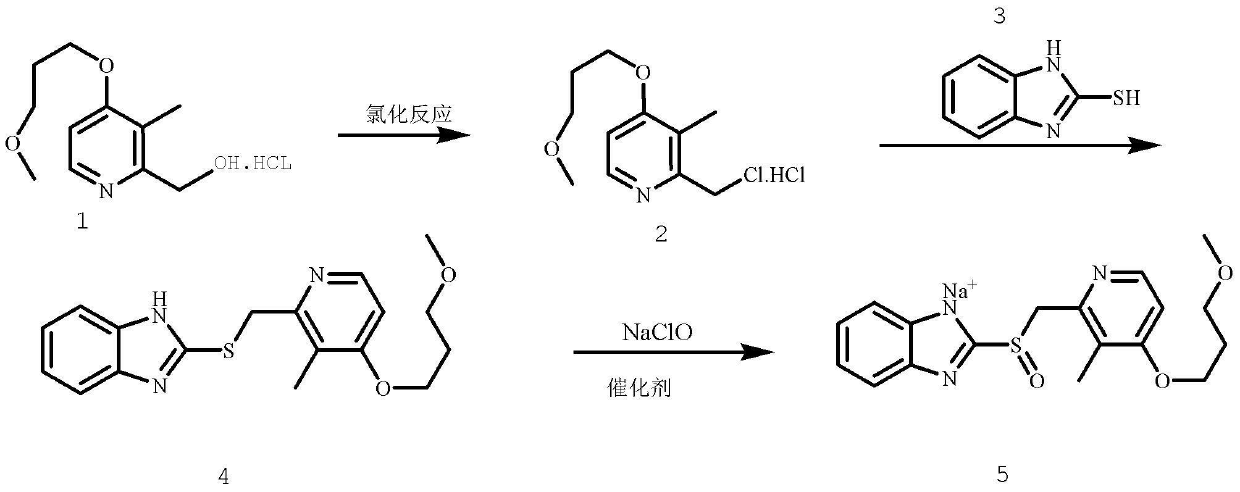
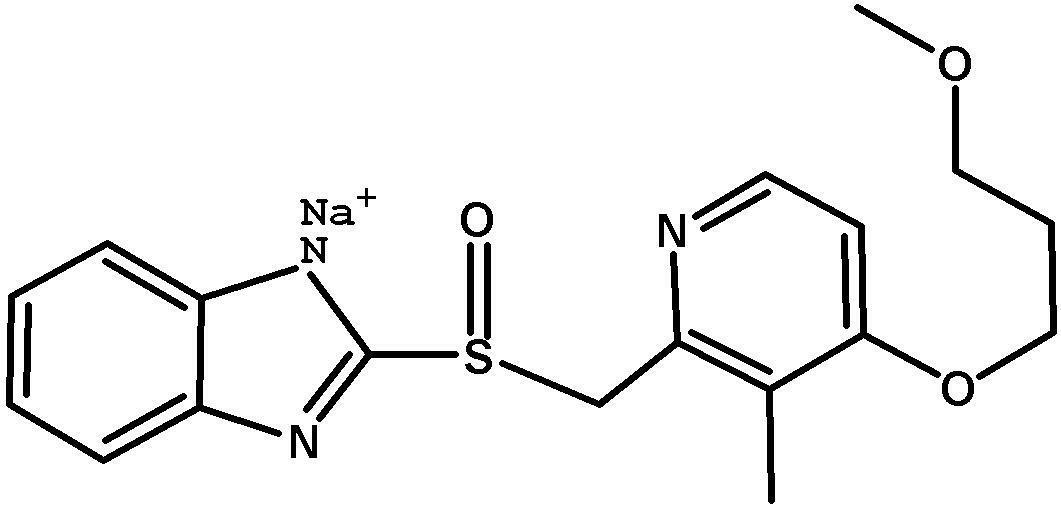
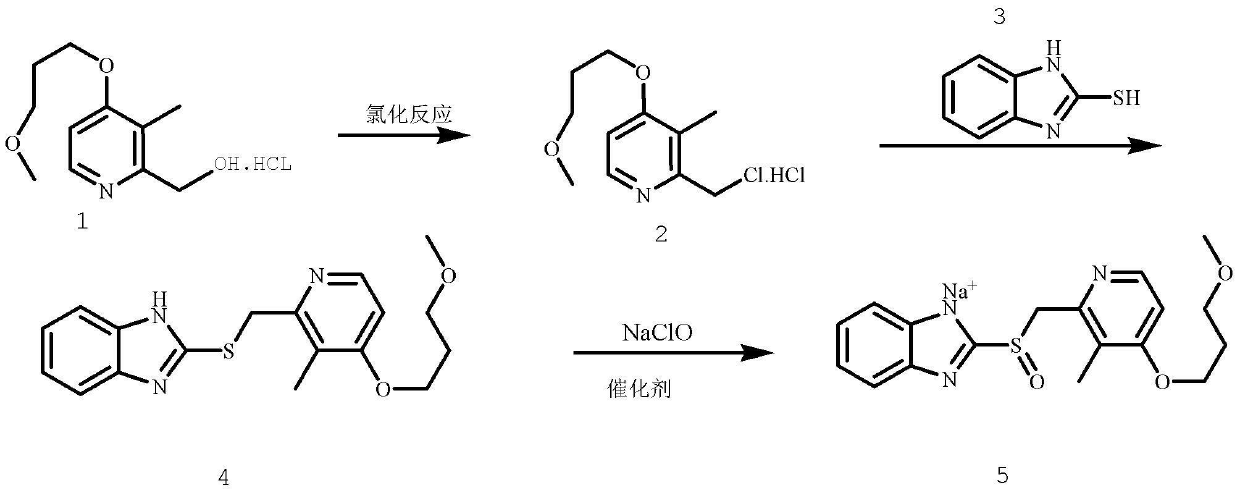
Method for pure water phase preparation of rabeprazole sodium
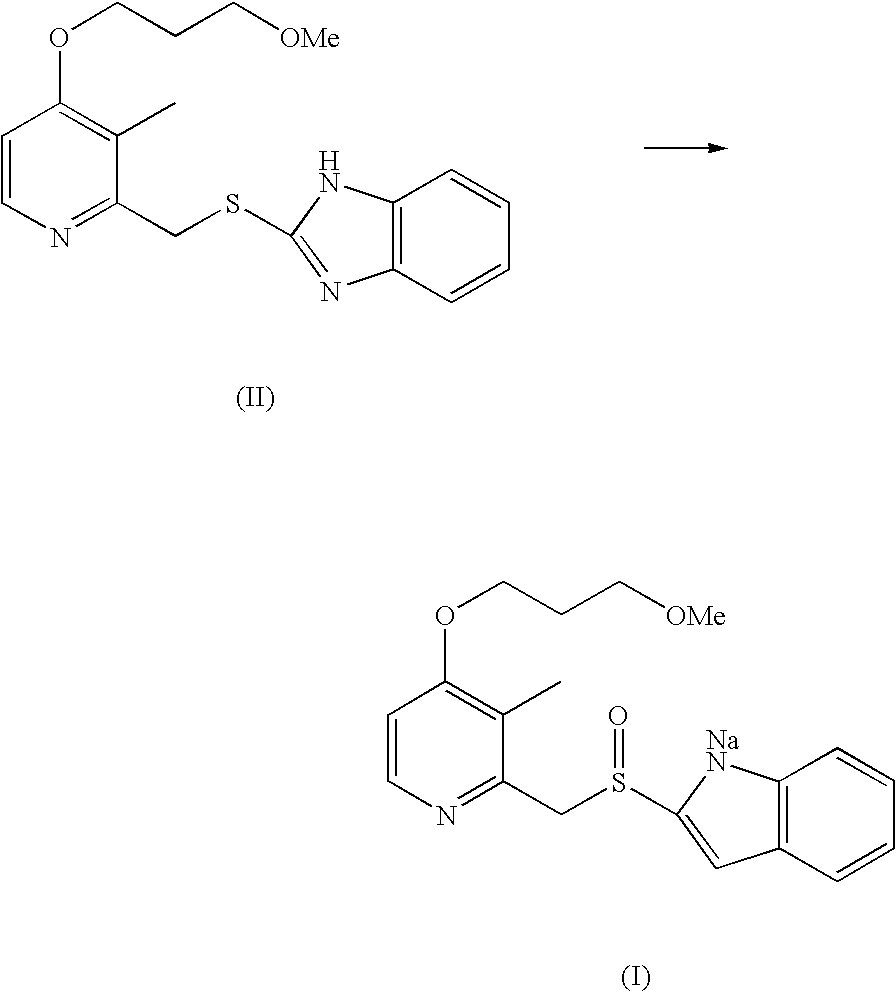
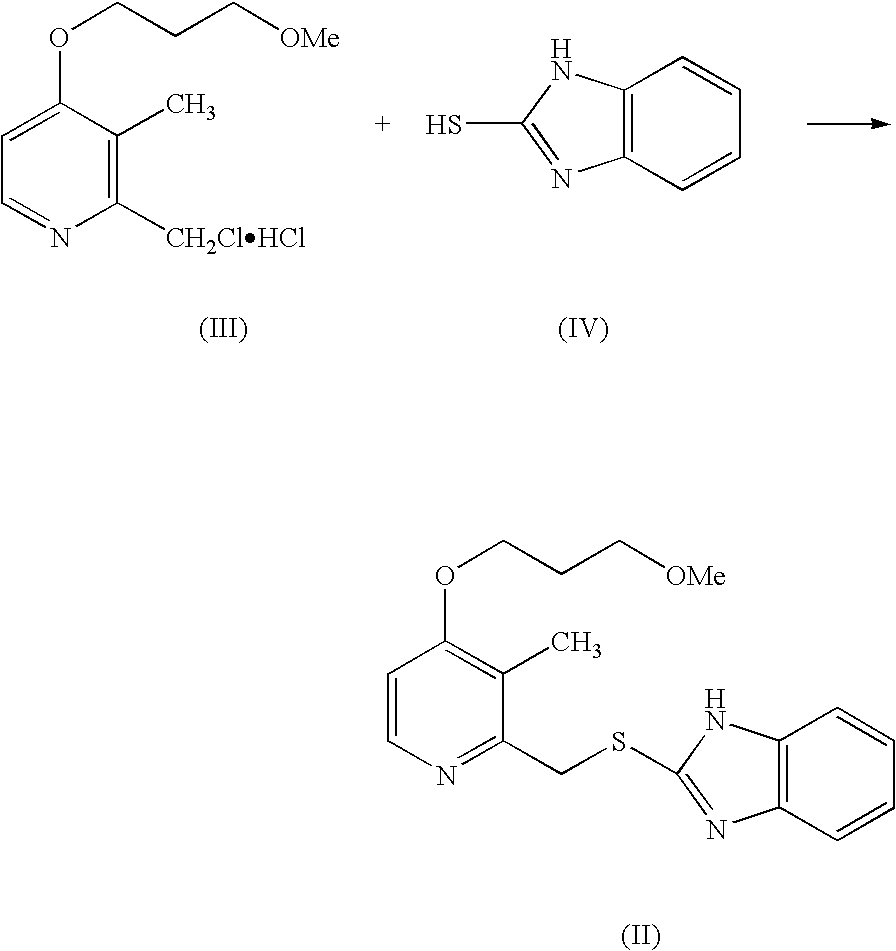
The invention provides a method for pure water phase preparation of rabeprazole sodium and belongs to the technical field of medicines. The method is characterized in that an intermediate, 2-hydroxymethyl-3-methyl-4-(3-methoxyl propoxy) pyridine hydrochloride, of the rabeprazole sodium is adopted as a raw material to be subjected to chlorination reaction, condensation reaction and oxidation reaction, so as to synthesize the rabeprazole sodium through three steps. The method adopts a novel chlorination reaction system and a novel sodium hypochlorite oxidation system, and can achieve a higher chloridization rate and excellently control a sulfur ether intermediate to be oxidized into sulfoxide; the reaction conditions are wild; both the reaction conversion rate and the reaction selectivity are very high; few reaction by-products are produced; the whole process can be carried out under the condition of no solvent; the content of residual solvent is lowered; time-consuming and toilsome vacuum rectification is not needed; the operation is simplified to a great extent; the process flow is short; and suitability for industrial application is achieved.
Owner:DALIAN UNIV OF TECH
Sodium rabeprazole freeze-dried powder injection for injection, preparation method thereof and detection method thereof
ActiveCN102106829AQuality improvementHigh precisionPowder deliveryOrganic active ingredientsSide effectMedicine
The invention relates to a sodium rabeprazole freeze-dried powder injection for injection, a preparation method thereof and a detection method thereof, and belongs to the technical field of medicines. In the sodium rabeprazole freeze-dried powder injection for the injection, medicinal content and the stability of relevant substances are improved by screening an additament in a prescription and the pH value of a preparation process and controlling the pH value of a finished product. The sodium rabeprazole freeze-dried powder injection for the injection has the advantages of low relevant substances, fewer side effects, high stability and safety, low production cost and the like, and is convenient to store and apply. In addition, by studying the detection method of the freeze-dried powder injection, the detection method has high accuracy, repeatability, sensitivity and specificity, and the quality of the injection can be better controlled.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Rabeprazole sodium powder injection and preparation method thereof
Rabeprazole sodium is developed by Eisai Co., Ltd. Japan, and is mainly used for treating active duodenal ulcer and optimum active gastric ulcer. The invention provides a rabeprazole sodium freeze-dried powder injection, which has the advantages of high stability, unique technology and few insoluble grains dissolved in transfusion liquid. The invention is superior to the existing technical schemein the aspects of impurities and relevant substances, so good curative effect and low adverse reaction rate are obtained. Excipients of the invention adopt mannite, and have the concrete weight ratio of rabeprazole sodium / mannite=1 / 2 to 2.5, and preferably, the weight ratio of rabeprazole sodium / mannite=1 / 2. PH regulating agents of the injection of the invention adopt sodium hydroxide, the PH value regulation range before the freeze drying is between 10.5 and 11.5, and preferably, the PH value is regulated to 11.
Owner:JUMPCAN PHARMA GRP
Crystalline form of rabeprazole sodium
Rabeprazole sodium in the monohydrate crystalline form, pharmaceutical compositions thereof, the use thereof in therapy, a process for its preparation, and the use thereof for the purification of rabeprazole sodium.
Owner:DIPHARMA FRANCIS
Stable rabeprazole sodium freeze-dried preparation and preparation method thereof
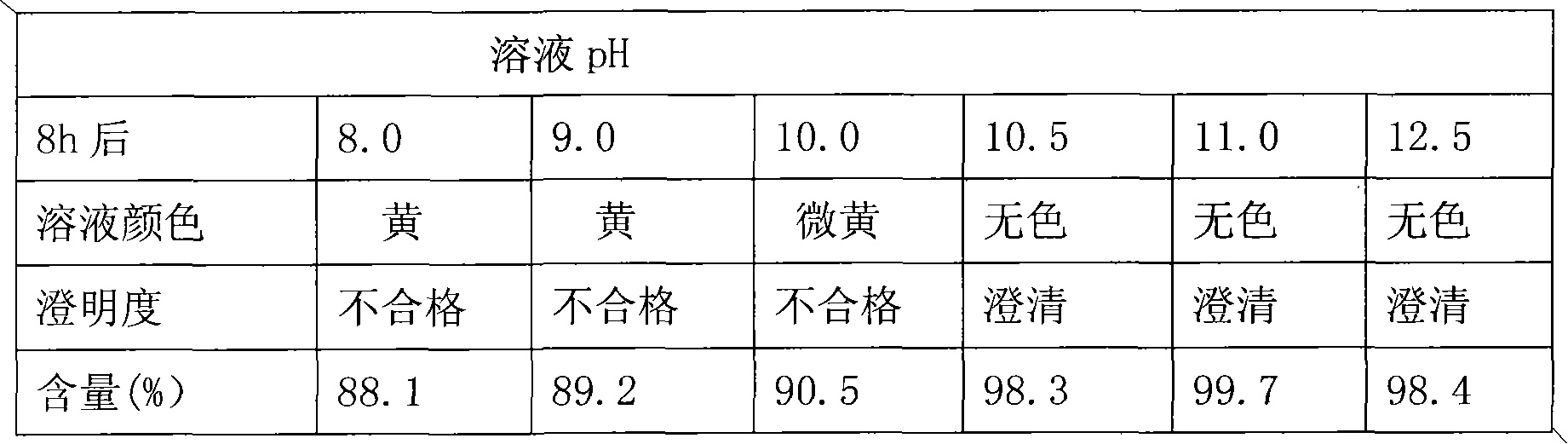
The invention relates to the field of medicament preparations, and particularly relates to a stable rabeprazole sodium freeze-dried preparation and a preparation method thereof. The freeze-dried powder injection consists of active component rabeprazole sodium, a pharmaceutically acceptable excipient and a pH regulator, wherein the pH regulator ensures that the pH of a solution is maintained in a range of between 10.5 and 12.5 when being dissolved once again before the solution is not freeze-dried and after the solution is freeze-dried. The rabeprazole sodium freeze-dried preparation has the characteristic of high stability.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD +1
Process for preparing rabeprazole sodium
InactiveUS20100121068A1Efficiently and more conveniently obtainedPreparing amorphous rabeprazole sodium more efficiently and more convenientlyOrganic chemistryOrganic solventRabeprazole Sodium
The present invention provides a process for preparing the amorphous rabeprazole sodium. The process comprises the following steps: (a) Contacting rabeprazole sodium compound with a solvent system to thereby obtain a clear solution under a first temperature, wherein said solvent system is a mixture of at least two categories of organic solvents; (b) Stirring said clear solution of step (a) under a second temperature for a certain time period to obtain a solution containing resultant separated solid, wherein said second temperature is equal to or lower than said first temperature; (c) Filtering said solution containing resultant separated solid obtained from step (b) to obtain a wet solid; and (d) Drying said wet solid to obtain an amorphous rabeprazole sodium compound.
Owner:SYN TECH CHEM & PHARM
Preparation method of new crystal form of dexrabeprazole sodium
InactiveCN104910135ANo changeGood crystal stabilityOrganic chemistry methodsAlkaline waterFiltration
Owner:NANJING KEFEI PINGSHENGHUI PHARMA CO LTD +2
Rabeprazole sodium for injection as well as preparation method and detection method thereof
InactiveCN102440967ANo apparent in vitro hemolysisNo obvious agglutinationAntibacterial agentsPowder deliveryDiseaseDuodenal ulcer
The invention relates to rabeprazole sodium for injection as well as a preparation method and a detection method thereof. The rabeprazole sodium for injection is a medical preparation for injection, which is prepared from rabeprazole sodium as an active ingredient, a stabilizing agent, a pH value regulator and pharmaceutically acceptable auxiliary materials. The rabeprazole sodium for injection, disclosed by the invention, is rapid in effect taking and high in bioavailability, is used for treating gastrohelcosis, duodenal ulcer, erosive gastroesophageal reflux disease, helicobacter pylori injection, Zollinger-Ellision syndrome and the like and is more suitable for being used as a substitutive medicament when oral preparations for treating the diseases have no effect.
Owner:SHANDONG DANHONG PHARMA
Rabeprazole sodium tablet and rabeprazole sodium enteric-coated tablet
The invention provides a rabeprazole sodium tablet which comprises the following components in part by weight: 20-40 parts of rabeprazole sodium, 260-300 parts of lactose, 50-80 parts of magnesium silicate, 35-45 parts of magnesium oxide and 1-5 parts of microcrystalline cellulose. According to the invention, the components of the rabeprazole sodium tablet and the dosage of each component are controlled, and the rabeprazole sodium tablet has high dissolution rate and bioavailability through the integrated action of all components and is reasonable in component design. Experimental results show that the dissolution rate of the rabeprazole sodium tablet is 97.5%. The invention further provides a rabeprazole sodium enteric-coated tablet which is prepared from the rabeprazole sodium tablet above; the rabeprazole sodium tablet is reasonable in component design, so that the prepared rabeprazole sodium enteric-coated tablet has good stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Rabeprazole sodium liposome enteric-coated tablets
InactiveCN101966161AImprove stabilitySolve the problem of heat discolorationOrganic active ingredientsDigestive systemCure rateCholesterol
The invention discloses rabeprazole sodium liposome enteric-coated tablets and a preparation method and application thereof to treating gastroesophageal reflux disease (GERD). The rabeprazole sodium liposome enteric-coated tablets comprise rabeprazole sodium liposome solid, alkaline substances and other common auxiliary materials for a solid preparation, wherein the rabeprazole sodium liposome solid comprises the following components in part by weight: 10 to 20 parts of rabeprazole sodium, 40 to 90 parts of dioleoyl phosphatidylcholine, 15 to 50 parts of cholesterol and 8 to 30 parts of sodium glycocholate. The rabeprazole sodium liposome enteric-coated tablets have the advantages of improving stability, increasing dissolution, solving the problem of color change when being heated, and the like, and when the rabeprazole sodium liposome enteric-coated tablets are used for treating the GERD, the curative ratio is higher, the total effective rate is higher, and the clinical superiority is more obvious.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Novel crystal form of rabeprazole sodium aquo-complex and preparation method of rabeprazole sodium aquo-complex
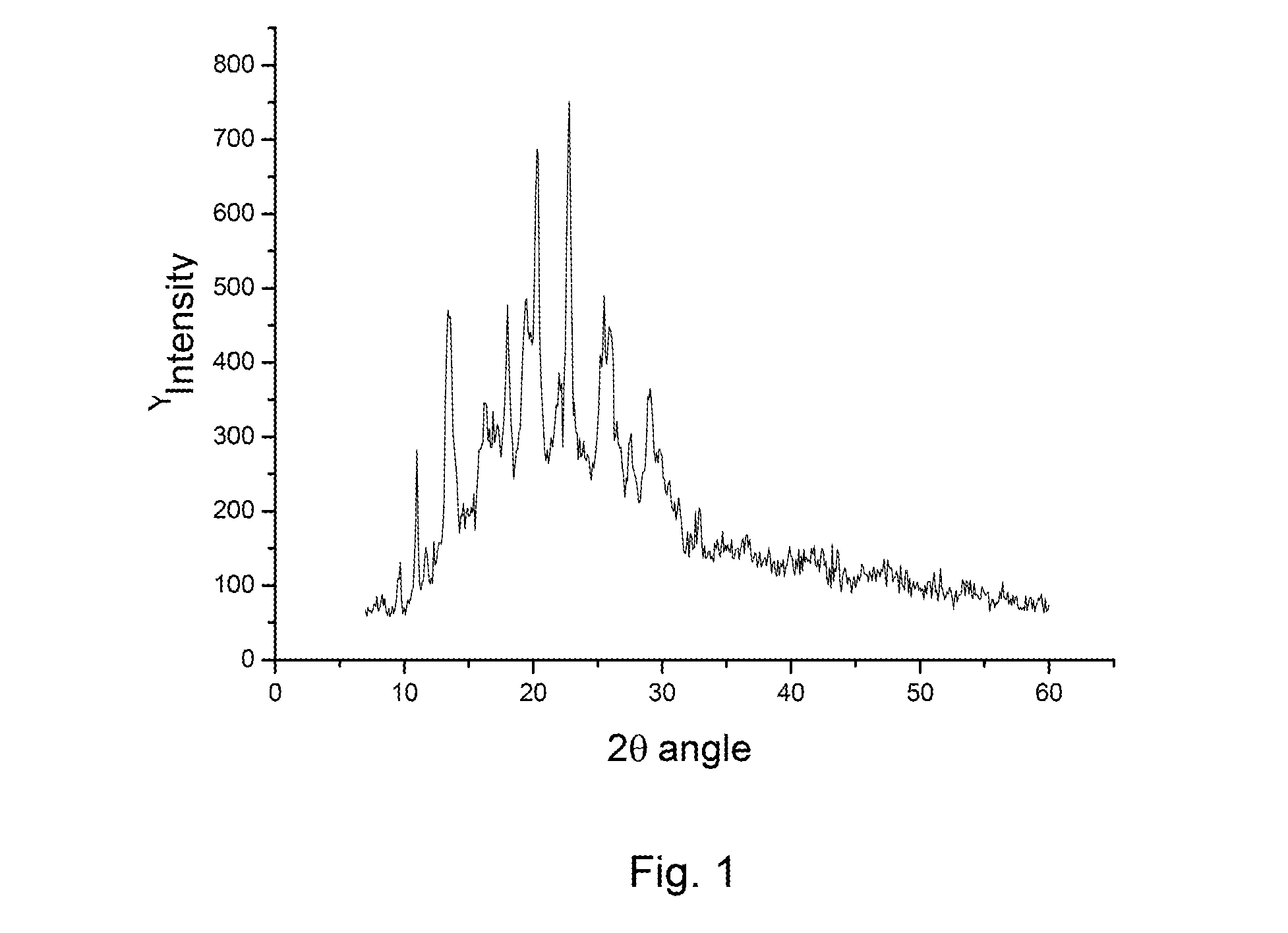
ActiveCN104725358AImprove stabilityLow hygroscopicityOrganic active ingredientsOrganic chemistry methodsRabeprazole SodiumK-alpha
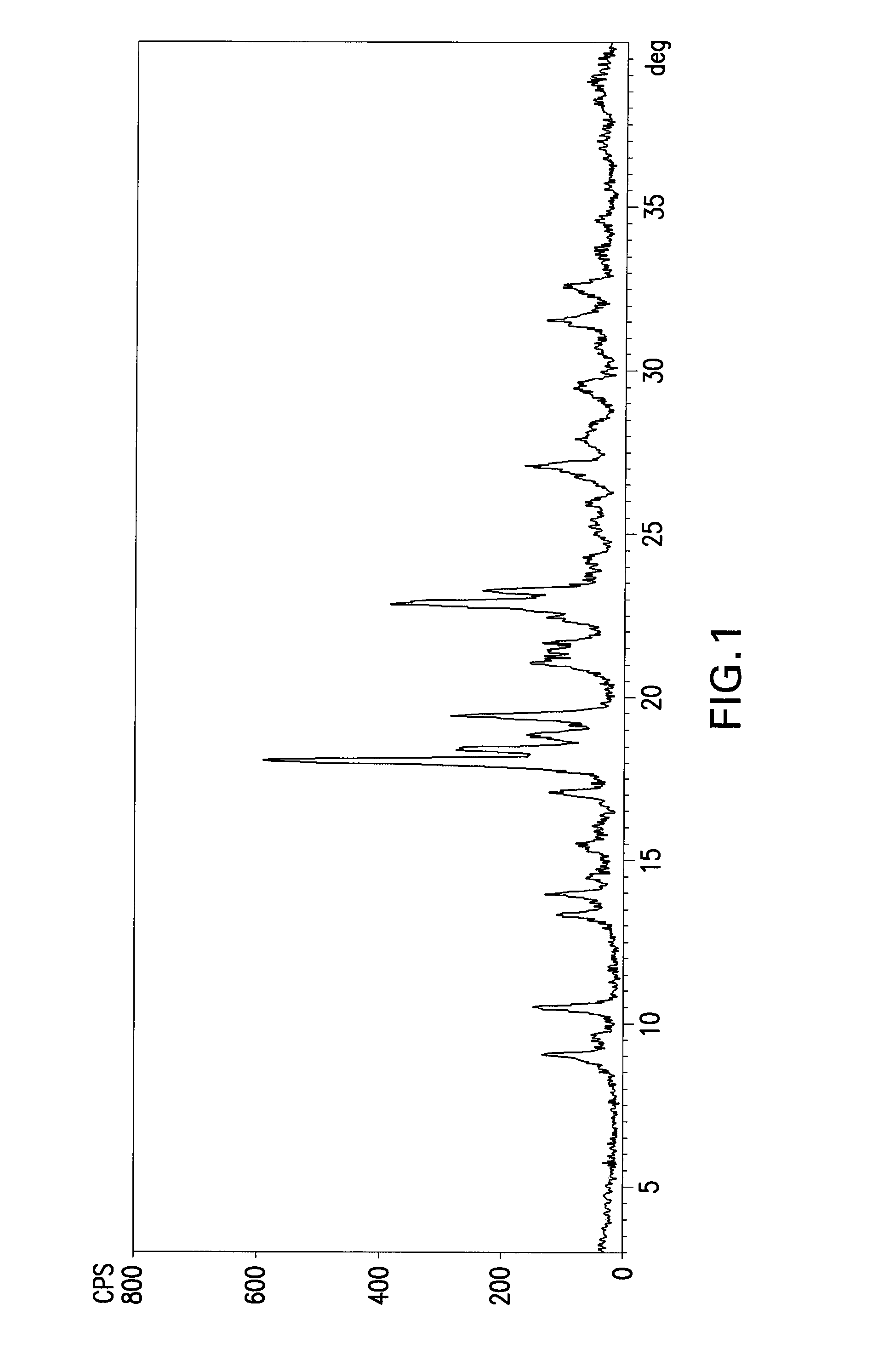
The invention relates to a novel crystal form of rabeprazole sodium aquo-complex and a preparation method of the novel crystal form. The novel crystal form is called the Z-type crystal. The Z-type crystal of the rabeprazole sodium aquo-complex is characterized in that in the X-ray powder diffraction pattern expressed by Cu-K alpha radiation and a 2theta+ / -0.2DEG diffraction angle, the Z-type crystal has characteristic diffraction peaks at 9.3, 10.7, 18.2, 19.6, 21.2, 23.0, 27.2 and 29.9.
Owner:燃点(南京)生物医药科技有限公司
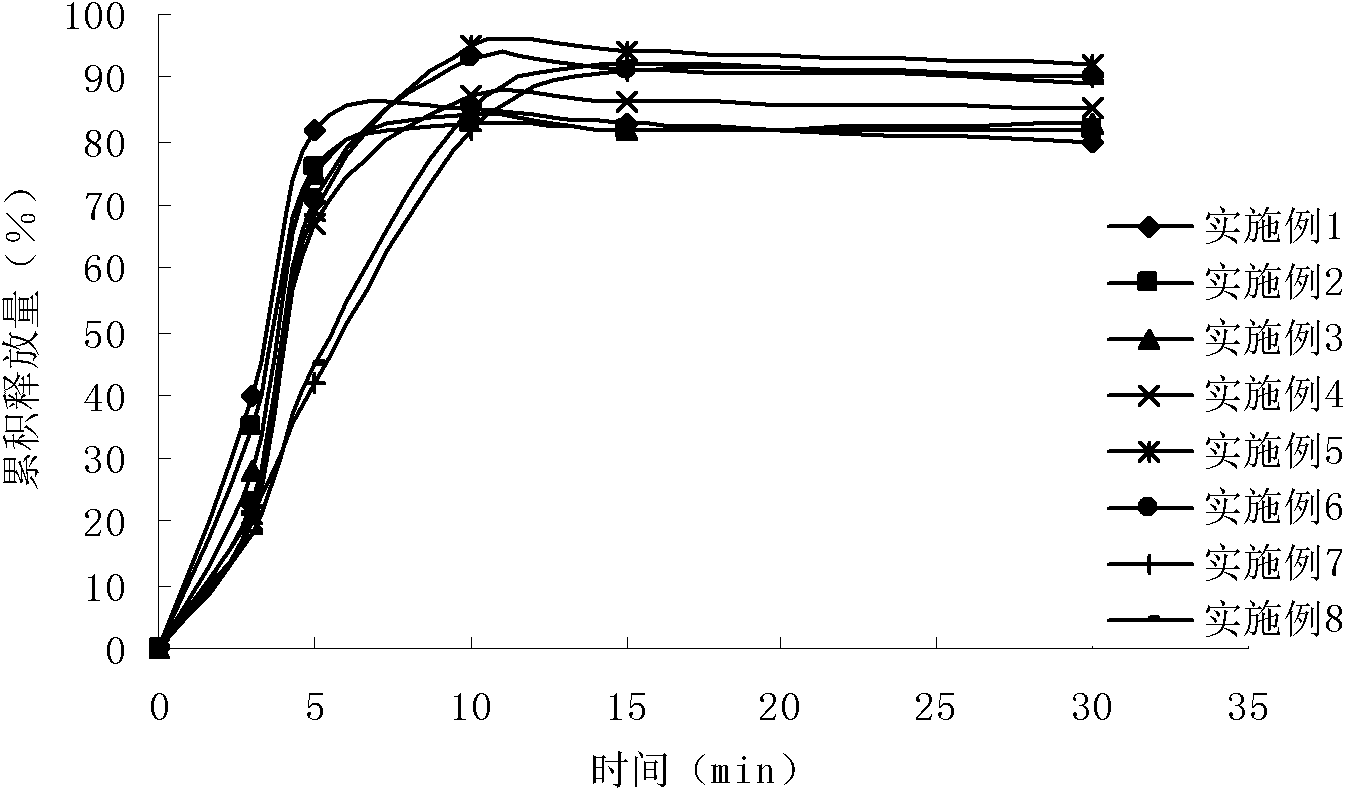
Rabeprazole enteric-coated micro pellet, and preparation method thereof
ActiveCN105434398AFeel comfortableShorten the timeOrganic active ingredientsDigestive systemSolubilityDrug release rate
The invention relates to a rabeprazole enteric-coated micro pellet, and a preparation method thereof. The rabeprazole enteric-coated micro pellet comprises a core pellet, a drug-carrying layer, an insulating coating layer, and an enteric coating layer from inside to outside; the weight of the drug-carrying layer accounts for 25 to 30%, the weight of the insolating coating layer accounts for 22 to 27%, and the weight of the enteric coating layer accounts for 13 to 17%; the drug-carrying layer is composed of rabeprazole or rabeprazole sodium, sucrose, hydroxypropyl methyl cellulose, sodium hydrogen sulfite, and lauryl sodium sulfate; the insulating coating layer is composed of hydroxypropyl methyl cellulose, polyethylene glycol 6000, titanium dioxide powder, and disodium hydrogen phosphate; and the enteric coating layer is composed of C-type acrylic resin, polyethylene glycol 6000, and talcum powder. A drug release curve and a drug intestinal absorption rate curve which are coincident with each other are obtained via appropriate controlling on enteric solubility and drug release rate of the rabeprazole enteric-coated micro pellet; existing time of drugs in intestinal tract in free states is shorter; less degradation is caused; bioavailability is high; and it is shown by clinical experiments that obvious effective rate is higher, stability is higher, and the rabeprazole enteric-coated micro pellet is convenient for patients to eat.
Owner:KAMP PHARMA
Application of polyphenols in resisting coronavirus
The invention discloses an application of various compounds such as polyphenols in resisting coronavirus. The invention relates to the application of one or more of a polyphenol substance, a proton pump inhibitor, p-benzoquinone and a derivative thereof, an active ingredient of sappan wood, TDZD-8, thiomersalate, alkannin, Tidelusib, protoflavin, rabeprazole sodium, PX-12, Dixanthophygen, methyl cholate, carbamofluorine, corilagin, dihydromyricetin, chloramine T, merbromin, gallocatechin, fraxetin, meisoindigo, a lactic acid ethacridine monohydrate and sousoprazole in preparation of drugs for treating and / or preventing diseases caused by coronavirus. In-vitro enzyme activity experiments show that various compounds such as 1, 4-naphthoquinone and the like can well inhibit the activity of main protease in the coronavirus, and the defect that diseases caused by the coronavirus cannot be treated in the prior art is overcome.
Owner:SHANGHAI TECH UNIV
Preparation process of rabeprazole sodium enteric capsules
ActiveCN107019680AIncrease productivityImprove bioavailabilityOrganic active ingredientsDigestive systemCelluloseHard Capsule
The invention discloses a preparation process capable of improving bioavailability of rabeprazole sodium enteric capsules. The rabeprazole sodium enteric capsules prepared by the invention are prepared from rabeprazole sodium enteric mini-pills and hard capsule shells, wherein each rabeprazole sodium enteric mini-pill comprises a drug-loaded pill core, an isolation layer and an enteric layer; each drug-loaded pill is prepared from rabeprazole sodium, mannitol, low substituted hydroxypropy cellulose, high substituted hydroxypropyl cellulose L, calcium hydroxide, sodium hydroxide, and tween 80. Each isolation layer is prepared from ethyl cellulose, high substituted hydroxypropyl cellulose L and magnesium stearate; the enteric layer is prepared from acrylic resin, triethyl citrate and talcum powder. According to the preparation process, the production efficiency of the rabeprazole sodium enteric capsules can be improved, the produced rabeprazole sodium enteric capsules are uniform in quality, good in stability and high in dissolution in vitro; moreover, the bioavailability of rabeprazole sodium enteric capsules can be obviously improved, and the rabeprazole sodium enteric capsules have good market prospect.
Owner:珠海润都制药股份有限公司
Crystalline form of rabeprazole sodium
Rabeprazole sodium in the monohydrate crystalline form, pharmaceutical compositions thereof, the use thereof in therapy, a process for its preparation, and the use thereof for the purification of rabeprazole sodium.
Owner:DIPHARMA FRANCIS
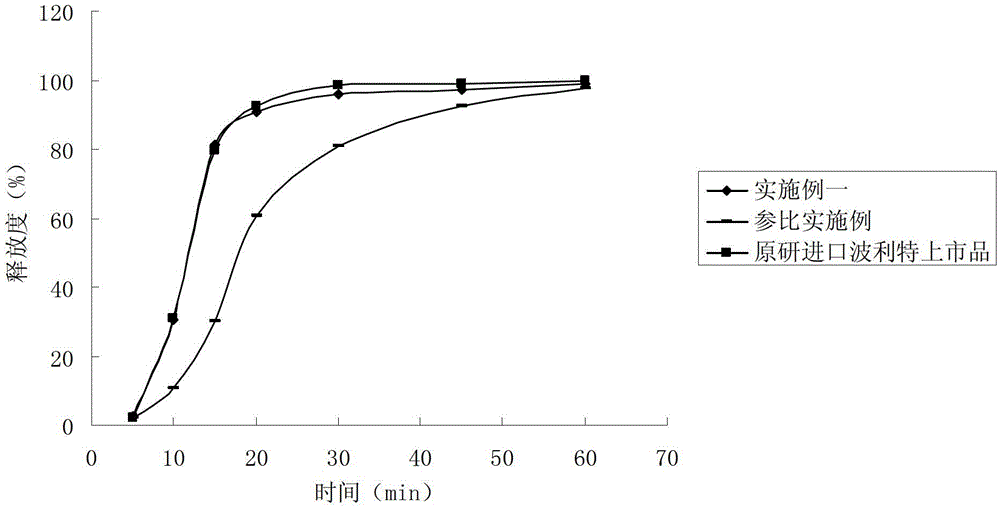
Establishment method of release curve of rabeprazole sodium enteric-coated micro-pill capsule in acidic medium
ActiveCN105021559AEasy to degradeOvercoming the disadvantage of heavy interferenceColor/spectral properties measurementsCentrifugationTest sample
The invention discloses an establishment method of a release curve of a rabeprazole sodium enteric-coated micro-pill capsule in an acidic medium. The method comprises the steps: taking the rabeprazole sodium enteric-coated micro-pill capsule, adopting an oar-method device, taking the acidic medium as a release medium, respectively taking solutions at different rotation speeds and time points, filtering, measuring to take the dissolution filtrate, carrying out appropriate treatment, and then taking the treated filtrate as a test sample solution; additionally taking a rabeprazole sodium reference substance, adding the acidic medium, carrying out appropriate treatment, and thus obtaining a reference substance solution; and measuring the absorbance by using an ultraviolet-visible spectrophotometry, calculating the release degree and drawing the release curve. The method overcomes the defects that rabeprazole sodium is easy to degrade in the acidic medium and is difficult to effectively detect by high performance liquid chromatography, and enteric-coated accessories seriously interfere determination results in ultraviolet-visible spectrophotometry, innovatively adopts simple acid addition precipitation centrifugation to remove accessory interference, detects degradation products of rabeprazole sodium, indirectly detects the rabeprazole sodium release degree, and has the advantages of being simple, quick and accurate.
Owner:广东彼迪药业有限公司
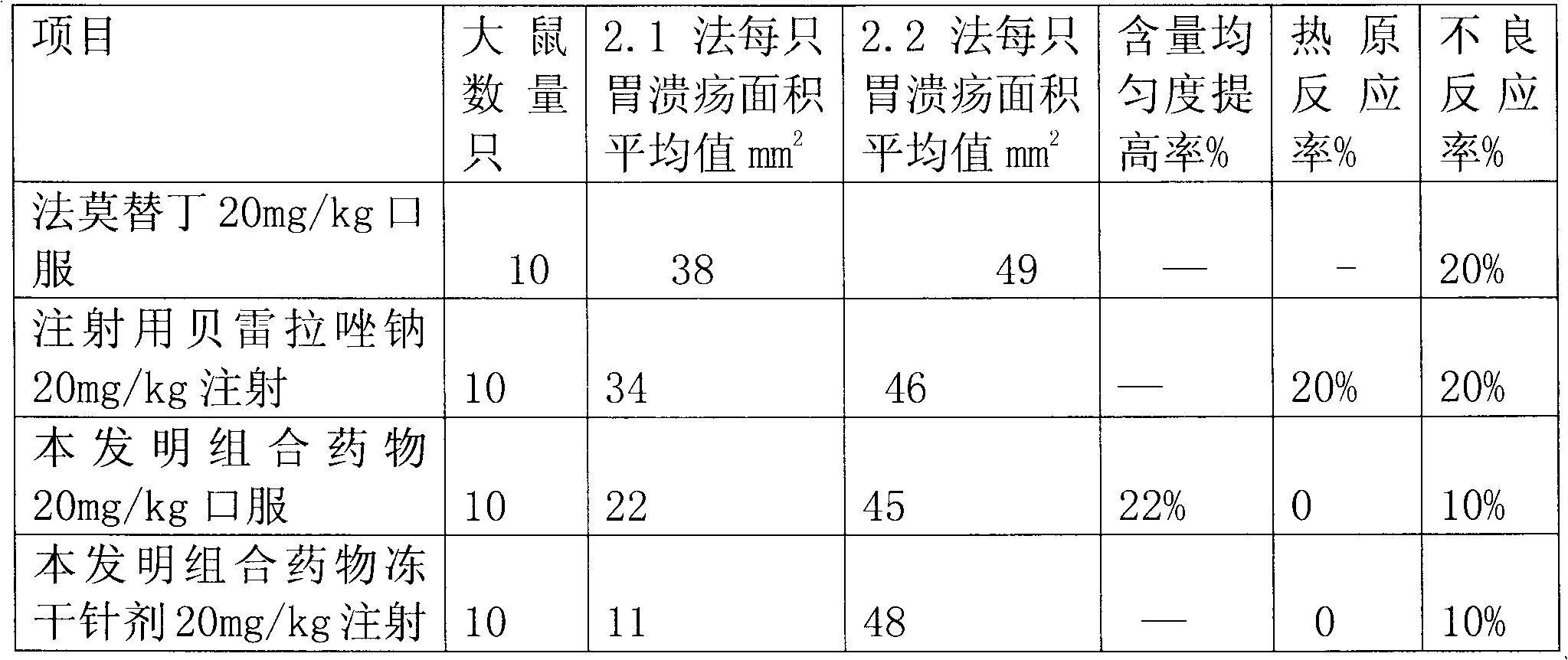
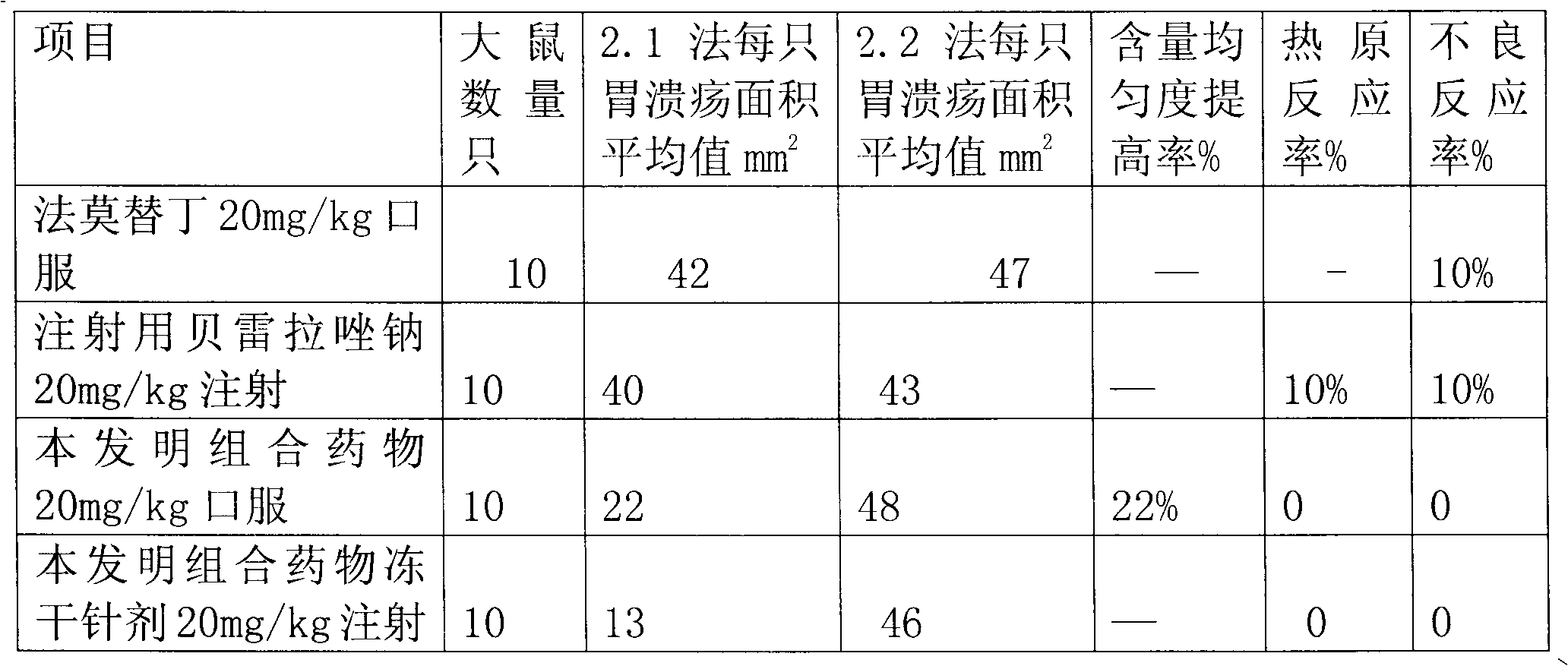
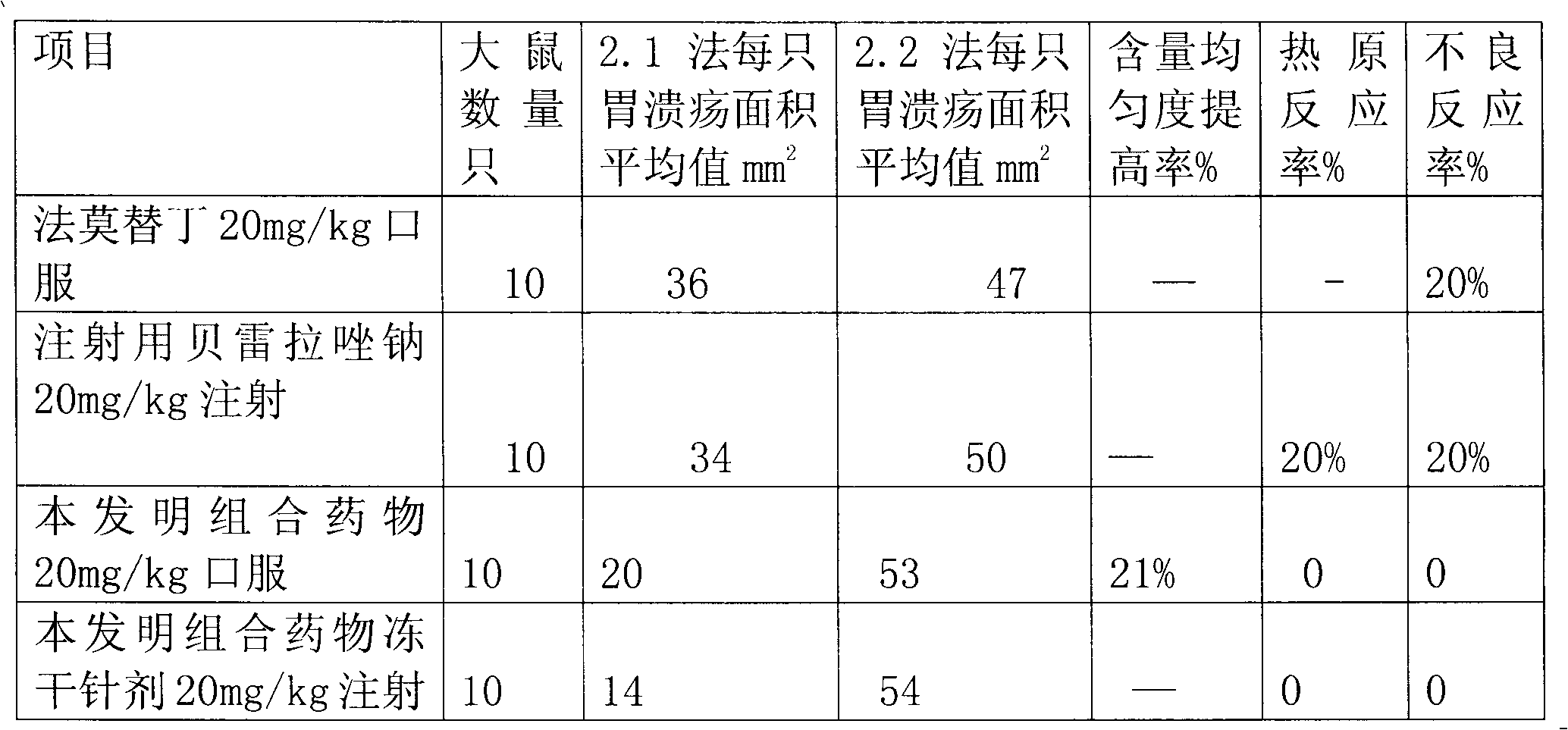
Rabeprazole sodium combined medicament and preparation process thereof
InactiveCN102091070AEliminate adverse reactionsGood regeneration performanceOrganic active ingredientsDigestive systemDrugs preparationsRabeprazole Sodium
The invention provides a rabeprazole sodium combined medicament and a preparation process thereof. The rabeprazole sodium combined medicament is characterized by comprising the following raw materials as components in proportion by weight: 5-10 of ilaprazole sodium, 20-30 of composition of reduced glutathione and hepatocyte growth-promoting factors in proportion by weight of 1:10, and 50-60 of diammonium glycyrrhizinate. According to a pharmaceutically allowable dose of rabeprazole sodium, pharmaceutical preparations in dosage forms of injection, lyophilized powder injection, enteric coated tablets, enteric coated capsules, spray and the like of the rabeprazole sodium combined medicament are respectively prepared for treating gastric ulcer.
Owner:吴赣英
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com