Lyophilized powder injection of rabeprazole sodium medicinal composition and preparation method of lyophilized powder injection
A technology of rabeprazole sodium and freeze-dried powder injection, applied in the field of freeze-dried powder injection of rabeprazole sodium pharmaceutical composition and its preparation, can solve the problems of slow dissolution, large number of insoluble particles, and inconvenient injection, etc. Achieve the effect of stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
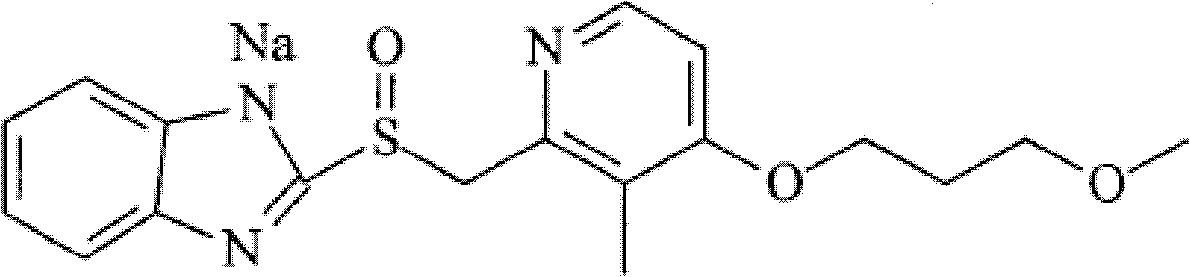
[0079] [Compound Example 1] Preparation of rabeprazole sodium crystalline compound
[0080] 1) Add 60 g of the raw drug rabeprazole sodium into 300 ml of water, stir and dissolve to obtain an aqueous solution of rabeprazole sodium;
[0081] 2) Add a mixed solution of 480ml tetrahydrofuran and methanol (the volume ratio of tetrahydrofuran and methanol is 3:1) to the above-mentioned rabeprazole sodium aqueous solution under stirring at a speed of 300r / min, decolorize with activated carbon at 25°C, filter, get the filtrate;
[0082] 3) The filtrate was dropped into 1440ml of acetonitrile, cooled to 5°C, crystals were precipitated, filtered, and vacuum-dried to obtain 54.1g of white rabeprazole sodium crystalline compound.
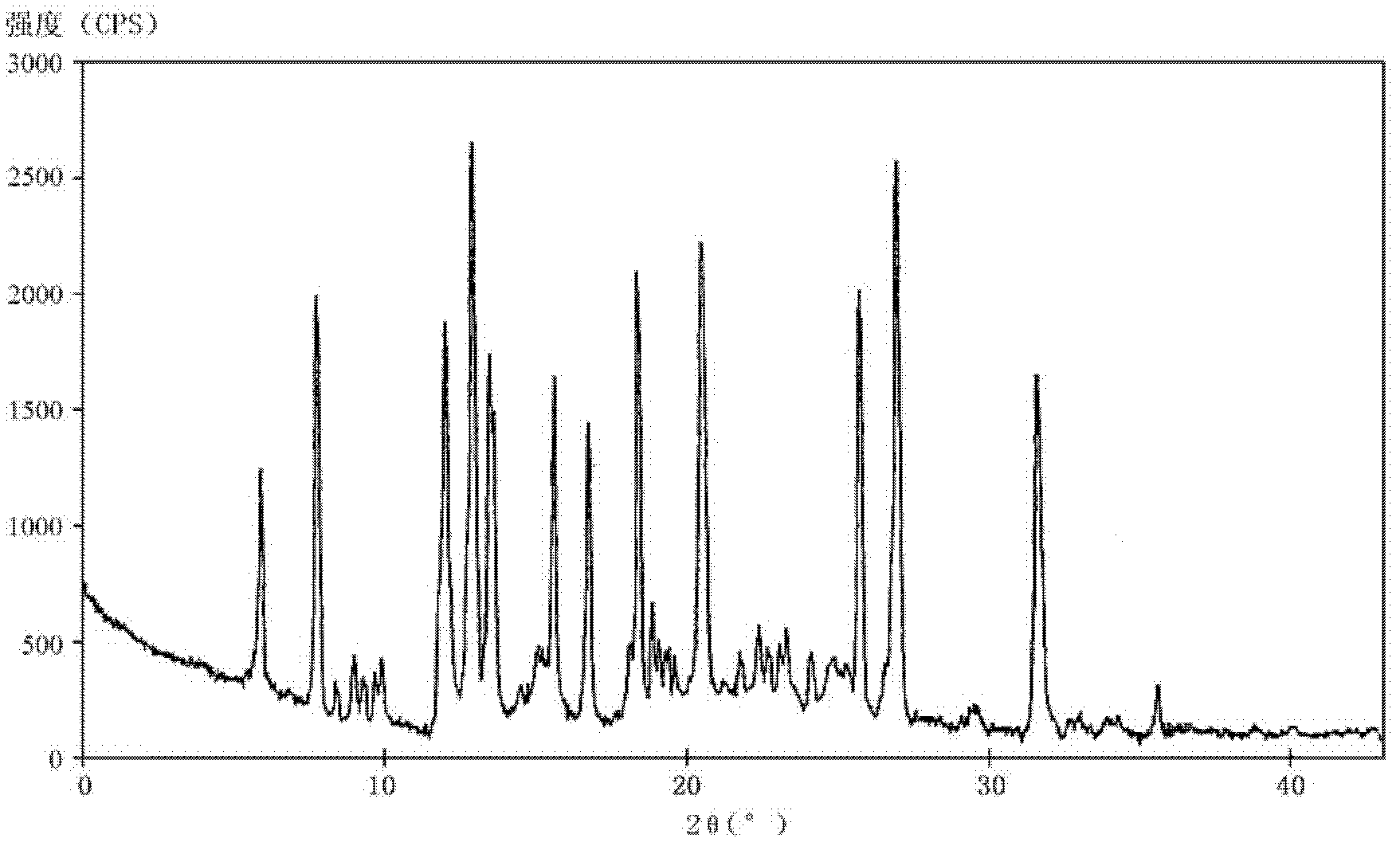
[0083] The melting point of the crystalline compound detected by capillary method is 162-163°C.
[0084] Adopt U.S. Perkin-Elmer company PE2400II elemental analyzer, elemental analysis (%) is: measured value (calculated value), C: 56.68 (56.68), H: 5.29 (5.3...
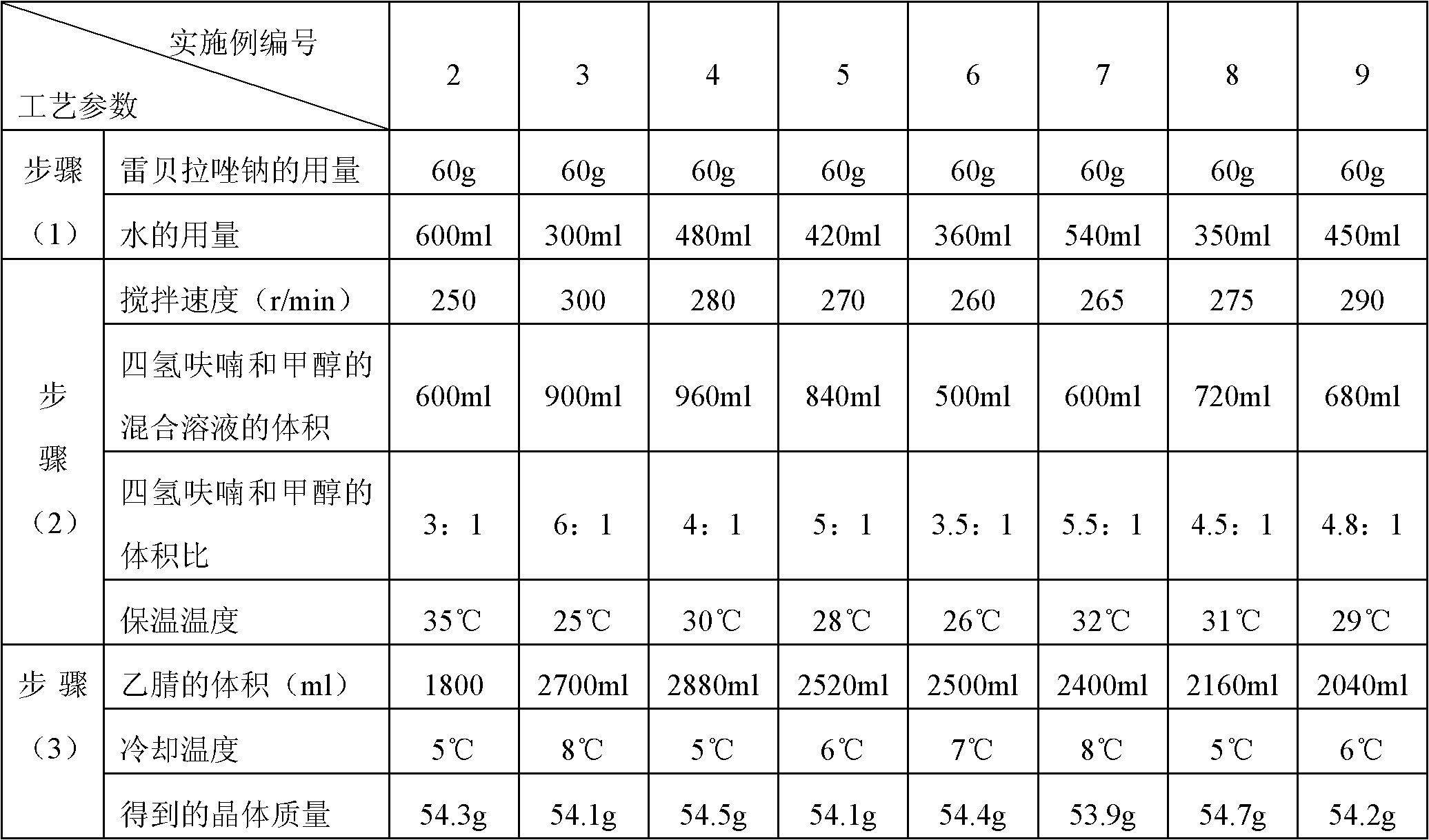
Embodiment 2-9
[0088]
[0089] The melting point of the crystalline compound obtained in Example 2-9 is detected by capillary method to be 162~163° C., and the crystalline compound obtained in Compound Example 2-9 is analyzed by the PE2400II elemental analyzer of American Perkin-Elmer Company, and the results and implementation Example 1 is similar.
[0090] Simultaneously, the crystalline compounds of compound examples 2-9 were measured by powder X-ray diffraction method, and the X-ray powder diffraction patterns represented by 2θ±0.2° diffraction angle were similar to those of Example 1.
[0091] [composition embodiment 1] rabeprazole sodium pharmaceutical composition
[0092] composition:
[0093]
[0094] Preparation method: Mix 20 g of rabeprazole sodium crystalline compound prepared in compound example 1, 5.0 g of disodium diethylamine tetraacetate and 150 g of mannitol, and divide into 1000 bottles to obtain the product.
[0095] The following are composition examples 2-9, the...
preparation Embodiment 1
[0099] [Formulation Example 1] Rabeprazole Sodium Freeze-dried Powder Injection
[0100] Prescription: 20mg / bottle (based on rabeprazole sodium)
[0101]
[0102] Preparation:
[0103] 1) Dissolve the disodium diethylamine tetraacetate of prescription quantity in 2500ml water for injection first, then add the rabeprazole sodium crystalline compound prepared by the compound embodiment 1 of prescription quantity under the condition of 30 ℃ of temperature, stir Make it dissolve, then add 150g of mannitol, stir and dissolve, then cool to room temperature, then add the prescribed amount of meglumine and sodium sulfite, adjust the pH of the solution to 11.5, and add water for injection to the full amount;
[0104] 2) Add 0.1% g / ml activated carbon to the above-prepared solution, stir for 15 minutes, and filter to decarbonize;
[0105] 3) Rapidly cool the filtrate obtained in step 2) from room temperature to -22°C, maintain -22°C for 2.5 hours, then drop to -40°C, pre-freeze for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com