Dexrabeprazole sodium monohydrate crystal form and preparation method thereof
A technology of dex-rabeprazole sodium and rabeprazole sodium, which is applied in the field of dex-rabeprazole sodium monohydrate crystal form and its preparation, can solve the problems of poor stability and solubility, unsatisfactory dissolution rate, Problems such as strong hygroscopicity, to achieve good stability, good product quality, and low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of dex-rabeprazole sodium monohydrate crystals
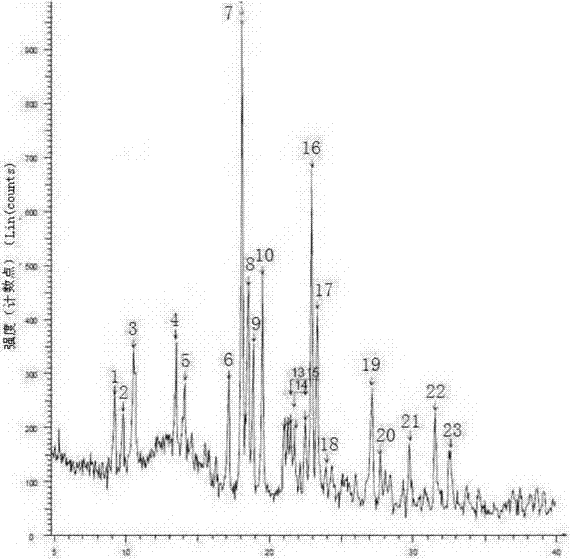
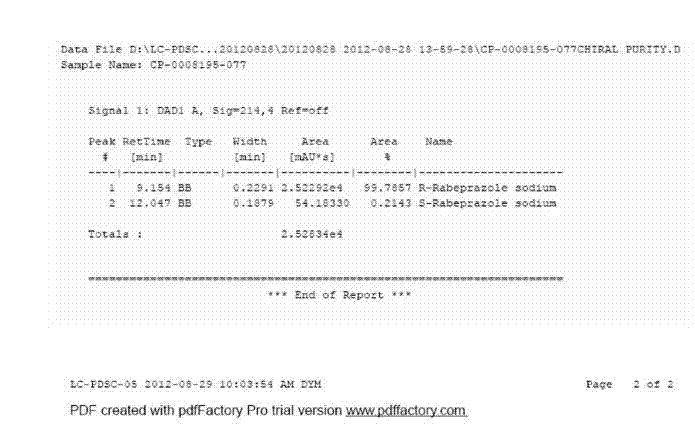
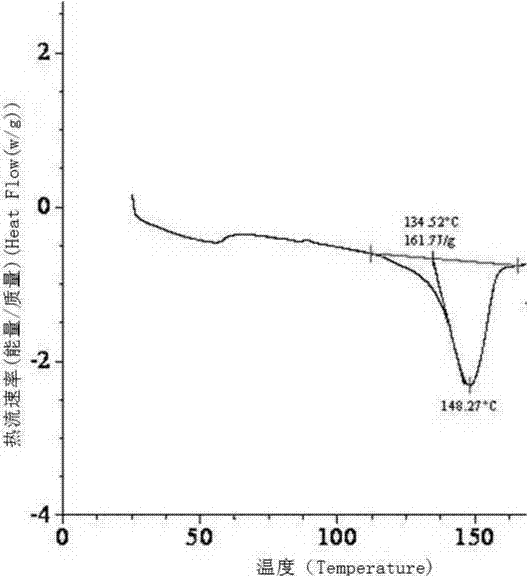
[0050] Add 3.9g of sodium hydroxide into 80ml of ethanol, stir to dissolve it, add 35g of dex-rabeprazole and 3.5g of activated carbon into the solution, stir at room temperature for 1h, filter with suction, rinse the filter cake twice with 30ml of ethanol, The obtained filtrates were combined, and the solvent in the filtrate was evaporated with a rotary evaporator to obtain a white solid. The obtained solid was vacuum-dried at 40°C for 24 hours, added to 80ml of ethyl acetate, stirred to dissolve, added 240ml of methyl tert-butyl ether and a small amount of Seed crystals, stir at room temperature for 8 h, filter, and rinse the obtained white solid with 30 ml of methyl tert-butyl ether twice, and dry the obtained solid in vacuum at 45° C. for 24 h to obtain the crystalline form of dex-rabeprazole sodium monohydrate. The X-ray powder diffraction pattern of the solid obtained is as follows figure 1 , and the dif...
Embodiment 2
[0052] Synthesis of dex-rabeprazole sodium monohydrate crystals
[0053] Add 3.9g of sodium hydroxide into 80ml of ethanol, stir to dissolve it, add 35g of dex-rabeprazole and 3.5g of activated carbon into the solution, stir at room temperature for 1h, filter with suction, rinse the filter cake twice with 30ml of ethanol, The obtained filtrates were combined, and the solvent in the filtrate was evaporated with a rotary evaporator to obtain a white solid. The obtained solid was vacuum-dried at 40 ° C for 24 h, added to 80 ml of ethyl acetate, stirred to dissolve, and 240 ml of methyl tert-butyl ether was added. Stir for 16 h, filter, and rinse the obtained white solid twice with 30 ml of methyl tert-butyl ether. The obtained solid is vacuum-dried at 45° C. for 24 h to obtain the crystalline form of dex-rabeprazole sodium monohydrate. The resulting solid had the same physical properties as the product obtained in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com