Novel crystal form of rabeprazole sodium aquo-complex and preparation method of rabeprazole sodium aquo-complex
A technology of dex-rabeprazole sodium and rabeprazole sodium, which is applied in the field of new crystal forms of proton pump inhibitor drugs such as prazoles, can solve the problems of easy degradation, discoloration, poor stability, etc., and achieve high product purity, Low hygroscopicity, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Dissolve 2.25 g of NaOH in 100 mL of methanol, add 20 g of dex-rabeprazole under stirring, and react at room temperature for 1 h. After the reaction was completed, suction filtered, the filtrate was taken, and concentrated to dryness under reduced pressure to obtain dex-rabeprazole sodium as a solid. Add the obtained solid into 50 mL of acetone, stir at 40~50°C to dissolve, filter to remove insoluble matter. Stir and crystallize at about 10°C for 8 h. Suction filtration, washing with acetone, and drying gave 16.2 g of a white solid with a chemical purity of 99.98% and an ee value of 99.99%.
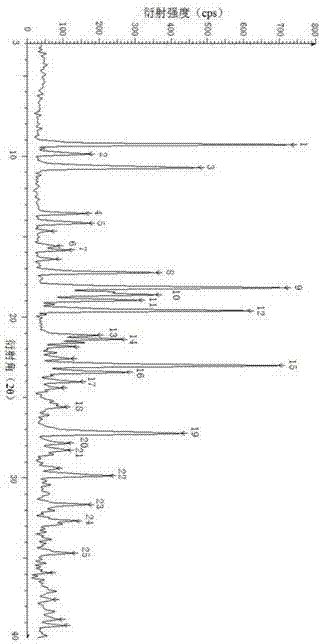
[0057] This crystalline compound is analyzed by X-ray powder diffraction, and its X-ray diffraction pattern is shown in figure 1 , hereinafter referred to as the crystal form Z crystal, the data are listed in the following table 1, omitted in table 1 figure 1 For some other weaker peaks given in , the error of the 2θ diffraction angle is ±0.2°.
[0058] Table 1 X-ray powder dif...
Embodiment 2
[0064] Dissolve 0.56 g of NaOH in 20 mL of methanol, add 5 g of dex-rabeprazole under stirring, and react at room temperature for 1 h. After the reaction was completed, suction filtered, the filtrate was taken, and concentrated to dryness under reduced pressure to obtain dex-rabeprazole sodium as a solid. Add the obtained solid into 15 mL butanone, stir at 40~50°C to dissolve, filter to remove insoluble matter. Add a small amount of seed crystals, stir and crystallize at about 5°C for 8 h. Suction filtration, washing with butanone, and drying gave 4.5 g of a white solid with a chemical purity of 99.97% and an ee value of 99.98%.
[0065] The crystal form compound was analyzed by X-ray powder diffraction, and the result showed that the compound had the same crystal form as the compound in Example 1. See the specific X-ray powder diffraction pattern Figure 5 , and the data are listed in Table 2 below. Omitted in Table 2 Figure 5 For some other weaker peaks given in , the ...
Embodiment 3
[0068] Embodiment 3 Stability and hygroscopicity experiment
[0069] Take an appropriate amount of Z crystal form compound obtained in Example 1 of the present invention and amorphous dex-rabeprazole sodium, put it in a glass plate, and place it at 40°C and 75% humidity for 10 days, and take samples on the 0th, 5th, and 10th day respectively , to investigate the stability of two different forms of compounds, the inspection indicators are appearance, weight, and purity, and the results are shown in Table 3.
[0070] Table 3 Stability investigation results
[0071]
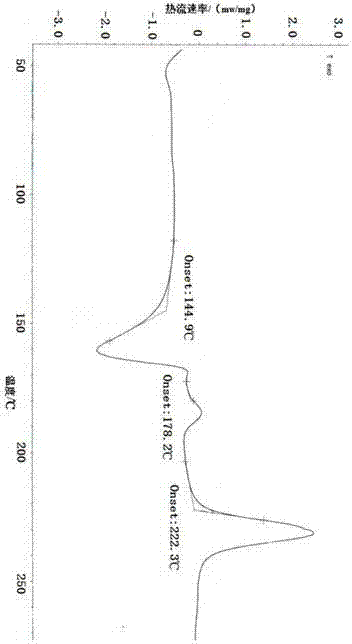
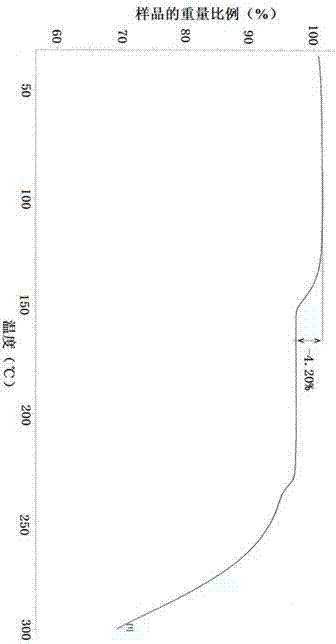
[0072] From the above experimental results, it can be found that amorphous dex-rabeprazole sodium has strong hygroscopicity, poor stability, and easy degradation and discoloration. The Z-type crystal of dex-rabeprazole sodium hydrate prepared in Example 1 of the present invention has good stability and low hygroscopicity, which is beneficial for long-term storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com