Rabeprazole sodium tablet and rabeprazole sodium enteric-coated tablet
A technology of Rabeprazole Sodium Tablets and Beprazole Sodium Tablets, which is applied in the directions of medical preparations of non-active ingredients, pill delivery, digestive system, etc., and can solve the problem of low bioavailability of Rabeprazole Sodium and tablet Solve problems such as poor core dissolution rate, achieve good dissolution rate and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 Compatibility experiment of auxiliary materials
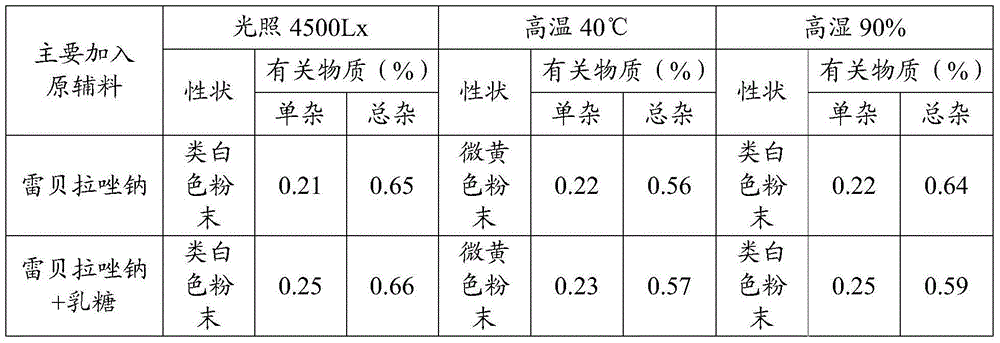
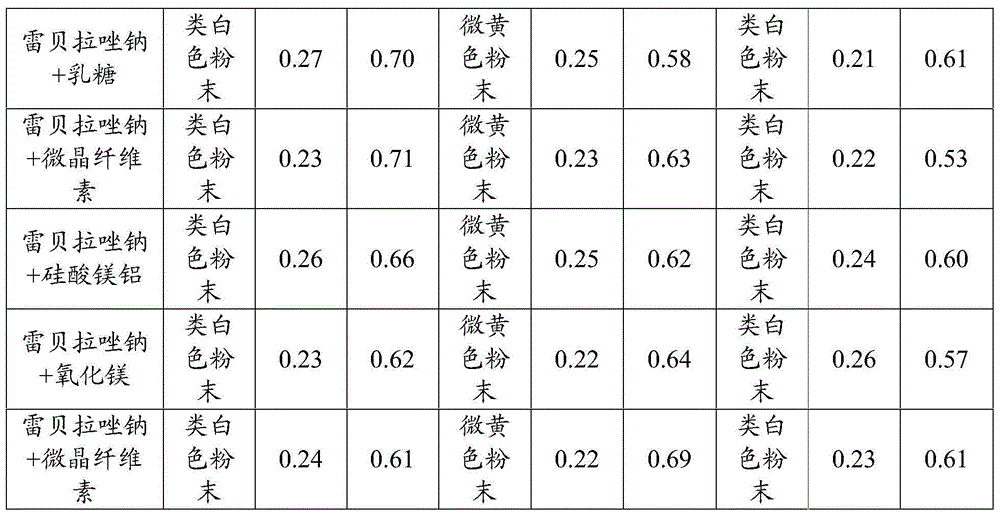
[0063] Mix rabeprazole sodium with lactose, microcrystalline cellulose, magnesium aluminum silicate, and magnesium oxide in a mass ratio of 1:5; rabeprazole sodium and microcrystalline cellulose are mixed in a mass ratio of 20:1. Mix, after mixing evenly; According to the method of influencing factors respectively, under the conditions of high temperature 40 ℃, high humidity 90%, light (4500Lx), place the sample after 10 days, investigate its properties and related substances, the results are shown in Table 1, table 1 is the experimental result of excipient compatibility provided by the embodiment of the present invention.
[0064] The excipient compatibility experiment result that table 1 the embodiment of the present invention provides
[0065]
[0066]
[0067] It can be seen from Table 1 that after rabeprazole sodium is mixed with various auxiliary materials, there is no obvious change in the dete...
Embodiment 2 and comparative example 1~3
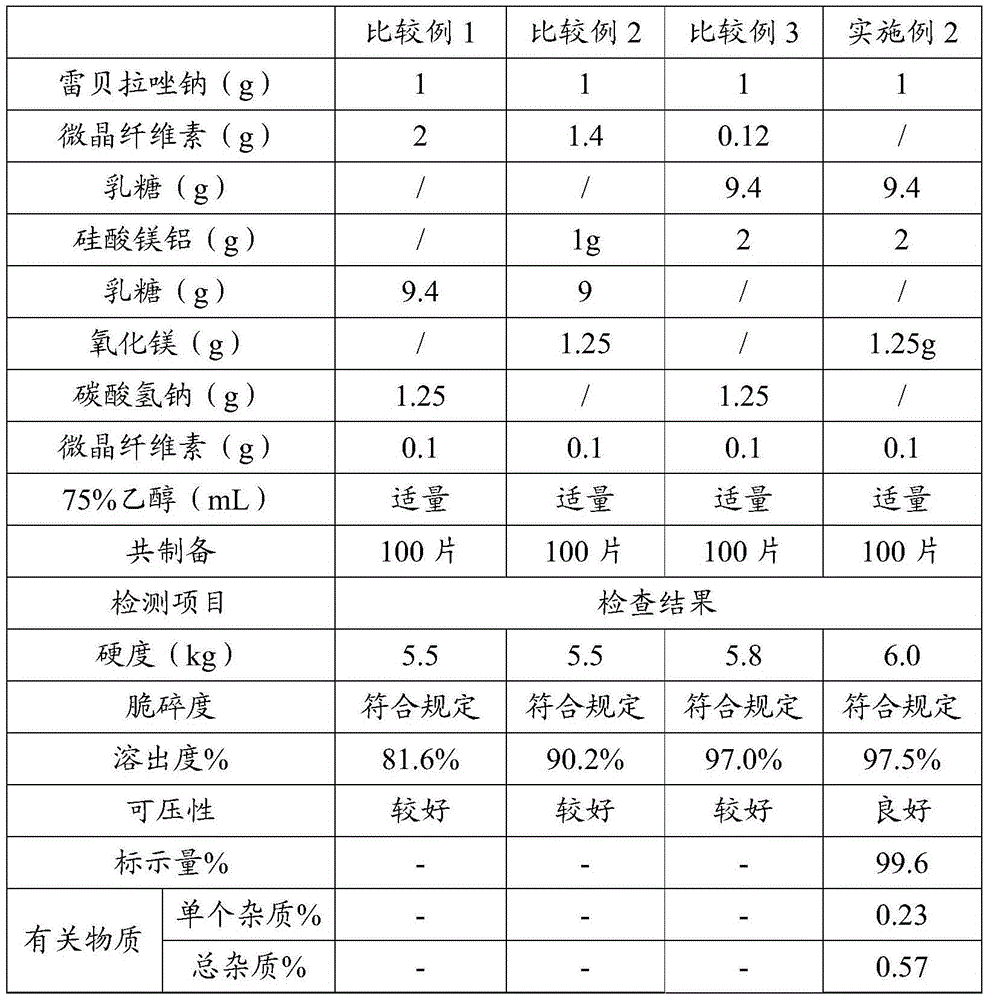
[0068] Embodiment 2 and comparative example 1~3 prescription screening
[0069] Rabeprazole sodium tablets were prepared according to the prescription in Table 2. Table 2 shows the prescription and performance test results of the rabeprazole sodium tablets provided in Example 2 and Comparative Examples 1-3.
[0070] After mixing the various substances in the prescription in Table 2 evenly, add ethanol with a mass concentration of 75% to prepare a soft material, dry it at 50°C and compress it into tablets to obtain rabeprazole sodium plain tablets.
[0071] Test the dissolution rate of rabeprazole sodium tablet according to the following method:
[0072] Get Rabeprazole Sodium Tablets, adopt dissolution assay (Chinese Pharmacopoeia 2010 edition two appendix XC first method) device, with phosphate buffer (pH6.8) (get 0.1mol / L hydrochloric acid solution and 0.2mol / L sodium phosphate solution, mix uniformly according to 3:1, if necessary, use 2mol / L hydrochloric acid solution or...
Embodiment 3
[0076] Example 3 screening of spacer fluid dosage
[0077] The spacer solution includes a medicinal gastric-soluble film coating premix with a mass ratio of 27:309 and an ethanol solution with a mass concentration of 85%.
[0078] Select the amount of spacer liquid relative to the Rabeprazole Sodium Tablets prepared in Example 2 to increase the weight by 3-4%, 6-7%, 7-8%, and detect whether it is effective for the Rabeprazole Sodium Tablets prepared after coating treatment. The quality of the sodium prazole product is affected, and the weight gain of the rabeprazole sodium plain tablet prepared by the intestinal solution relative to Example 2 is fixed at 10%. The test results are shown in Table 3, and Table 3 is the screening of the amount of spacer solution provided by the embodiment of the present invention result.
[0079] Table 3 Screening results of spacer fluid dosage provided by the embodiments of the present invention
[0080]
[0081]
[0082] It can be seen f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com