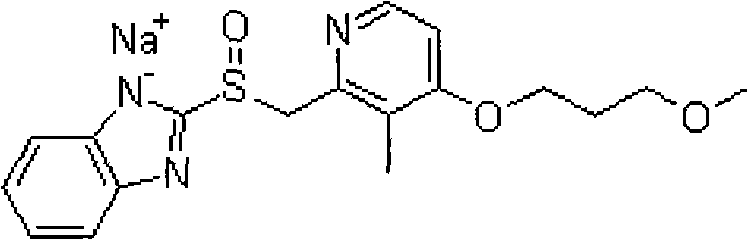
Stable rabeprazole sodium freeze-dried preparation and preparation method thereof
A technology of rabeprazole sodium and freeze-dried preparations is applied in the field of rabeprazole sodium freeze-dried preparations and preparations thereof, and can solve the problems of instability of rabeprazole sodium, slow absorption, low bioavailability and the like, and achieves the Effects of improved stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] prescription:
[0051] Rabeprazole Sodium 21.24g
[0052] Mannitol 40g
[0053] Appropriate amount of sodium hydroxide
[0054] Add water for injection to 2000ml
[0055]
[0056] Makes 1000 bottles
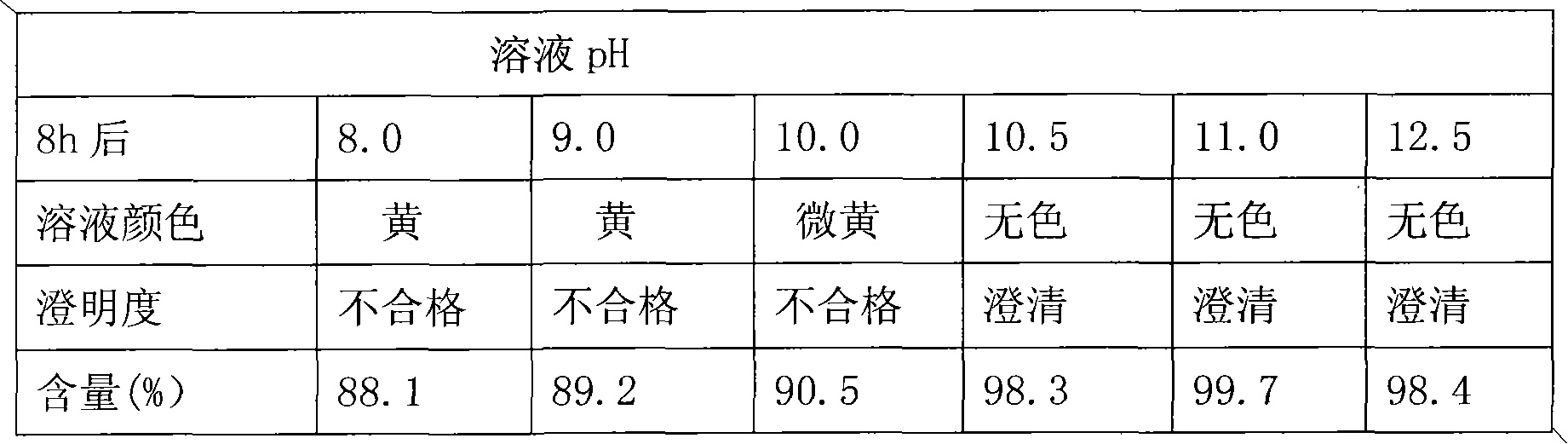
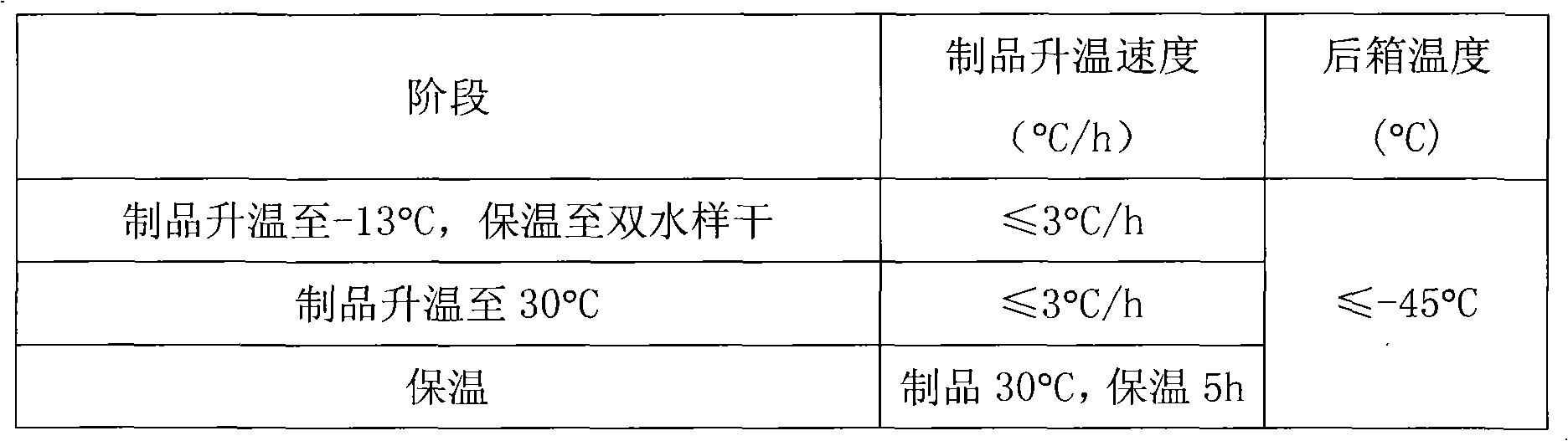
[0057] Take the prescribed amount of mannitol and rabeprazole sodium, put them in a sterile container, add water for injection, stir to dissolve, and mix well. Sodium hydroxide, a pH regulator, is added to adjust the medicinal solution within the range of 10.5 to 12.5, and the water for injection is supplemented to the prescribed amount. After complete dissolution, add 0.05% activated carbon to stir and adsorb for 20 minutes, decarbonize, sterilize and filter to measure the content of intermediates. After passing the test, the filtrate can be filled in sterile vials, half stoppered, and freeze-dried.
Embodiment 2
[0059] prescription:
[0060] Rabeprazole Sodium 21.24g
[0061] Mannitol 60g
[0062] Potassium hydroxide appropriate amount
[0063] Add water for injection to 2000ml
[0064]
[0065] Makes 1000 bottles
[0066] Take the prescribed amount of mannitol and rabeprazole sodium, put them in a sterile container, add water for injection, stir to dissolve, and mix well. Add a pH regulator potassium hydroxide to adjust the medicinal solution within the range of 11.0±0.5, and make up the water for injection to the prescribed amount. After complete dissolution, add 0.05% activated carbon to stir and adsorb for 20 minutes, decarbonize, sterilize and filter to measure the content of intermediates. After passing the test, the filtrate can be filled in sterile vials, half stoppered, and freeze-dried.
Embodiment 3
[0068] prescription:
[0069] Rabeprazole Sodium 21.24g
[0070] Dextran 40 40g
[0071] Appropriate amount of sodium hydroxide
[0072] Add water for injection to 2000ml
[0073]
[0074] Makes 1000 bottles
[0075] Dissolve dextran 40 in hot water (above 80°C), cool to room temperature, add the main ingredient and stir to dissolve, add pH regulator sodium hydroxide to adjust the pH to 11.0±0.5 after it is completely dissolved, and make up enough water for injection. Add 0.05% activated carbon, stir and adsorb for 20 minutes, decarburize, sterilize and filter, measure the content of intermediates, and after passing the test, the filtrate can be filled in sterile vials, half stoppered, and freeze-dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com