Sodium rabeprazole freeze-dried powder injection for injection, preparation method thereof and detection method thereof
A technology of rabeprazole sodium and freeze-dried powder injection, which is applied in the field of medicine, can solve problems such as acute clinical consequences, and achieve the effects of strong specificity, high precision, and high efficiency and energy saving in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
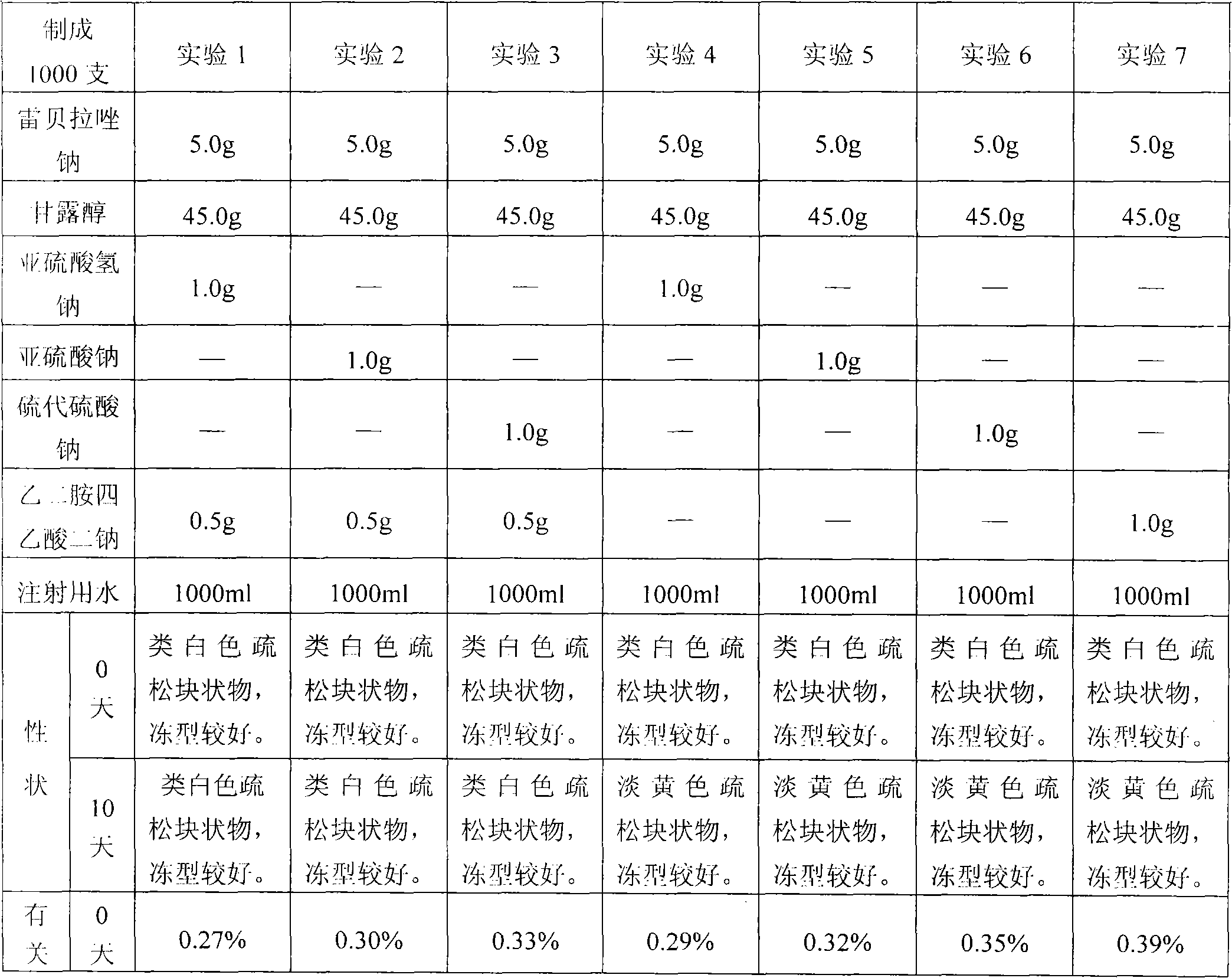
[0163] Prescription: Rabeprazole Sodium 5.0g
[0164] Mannitol 45.0g
[0166] Disodium edetate 0.5g
[0167] Add water for injection to 1000ml / 1000 sticks
[0168] Preparation method: Dissolve the prescribed amount of sodium sulfite, mannitol, and disodium edetate in about 60% of water for injection at 60±5°C, add 0.1% (g / ml) activated carbon, absorb for 10 minutes, decarbonize and filter Filtrate is cooled to room temperature, adjusts pH to 12.0 with the NaOH solution of 1mol / L, adds the rabeprazole sodium of recipe quantity, stirs to dissolving, adds water for injection to full amount; Measure intermediate solution pH value, content; Use 0.22 Fine filtration with μm microporous membrane, filling, semi-stoppering, freeze-drying, stoppering, capping, inspection, and packaging.
[0169] Detection method:
[0170] Related substances are measured according to high performance liquid chromatography (two appendix V D of Chinese Pharmacopoeia versio...
Embodiment 2
[0176] Prescription: Rabeprazole Sodium 5.0g
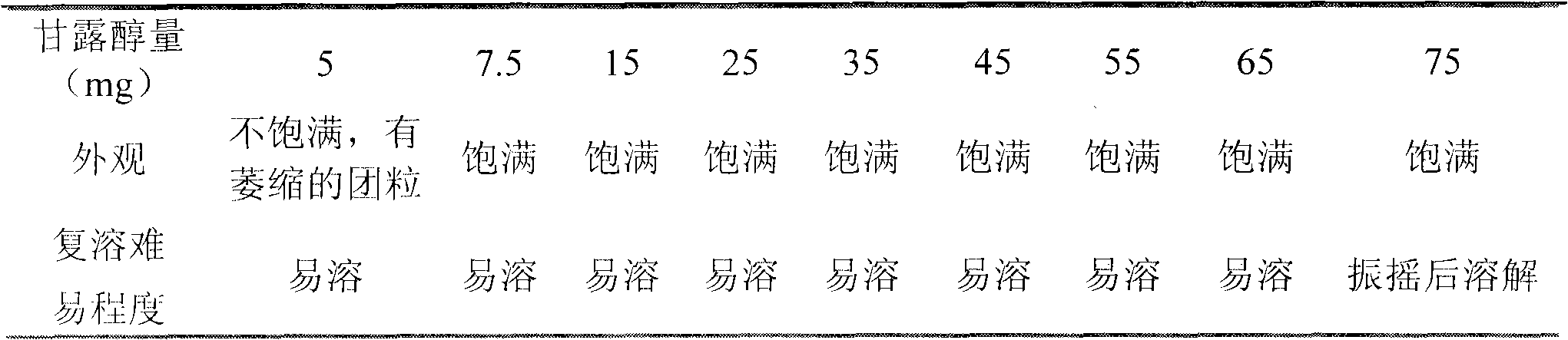
[0177] Mannitol 7.5g
[0178] Sodium sulfite 1.0g
[0179] Disodium edetate 0.5g
[0180] Add water for injection to 1000ml / 1000 sticks
[0181] Preparation method: Dissolve the prescribed amount of sodium sulfite, mannitol, and disodium edetate in about 60% of water for injection at 60±5°C, add 0.1% (g / ml) activated carbon, absorb for 10 minutes, decarbonize and filter Filtrate is cooled to room temperature, adjusts pH to 12.5 with the NaOH solution of 1mol / L, adds the rabeprazole sodium of recipe quantity, stirs to dissolving, adds water for injection to full amount; Measure intermediate solution pH value, content; Use 0.22 Fine filtration with μm microporous membrane, filling, semi-stoppering, freeze-drying, stoppering, capping, inspection, and packaging.
[0182] The relevant substances and contents were detected to be 0.33% and 99.8% respectively.
Embodiment 3
[0184] Prescription: Rabeprazole Sodium 1.0g
[0185] Mannitol 10.0g
[0186] Sodium sulfite 1.0g
[0187] Disodium edetate 0.5g
[0188] Add water for injection to 1000ml / 1000 sticks
[0189] Preparation method: Dissolve the prescribed amount of sodium sulfite, mannitol, and disodium edetate in about 60% of water for injection at 60±5°C, add 0.1% (g / ml) activated carbon, absorb for 10 minutes, decarbonize and filter Filtrate is cooled to room temperature, adjusts pH to 12.3 with the NaOH solution of 1mol / L, adds the rabeprazole sodium of recipe quantity, stirs to dissolve, adds water for injection to full amount; Measure intermediate solution pH value, content; Use 0.22 Fine filtration with μm microporous membrane, filling, semi-stoppering, freeze-drying, stoppering, capping, inspection, and packaging.
[0190] Detection method:
[0191] Related substances are measured according to high performance liquid chromatography (two appendix V D of Chinese Pharmacopoeia version ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com