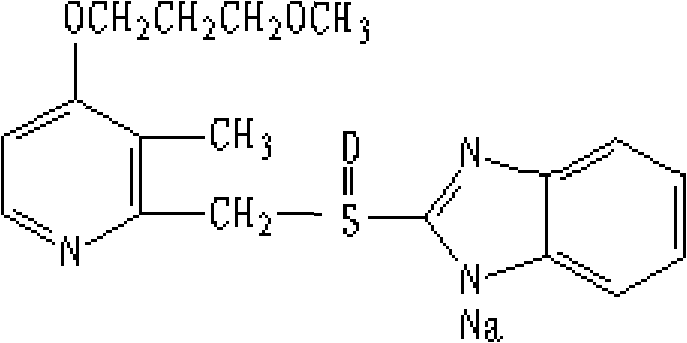Rabeprazole sodium composition and preparation method thereof
A technology of rabeprazole sodium and composition, applied in the field of rabeprazole sodium composition and preparation thereof, can solve the problems of large number of insoluble particles, slow dissolution, inconvenient injection and use, etc., and achieves excellent appearance and reduction of insoluble particles. , composed of simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
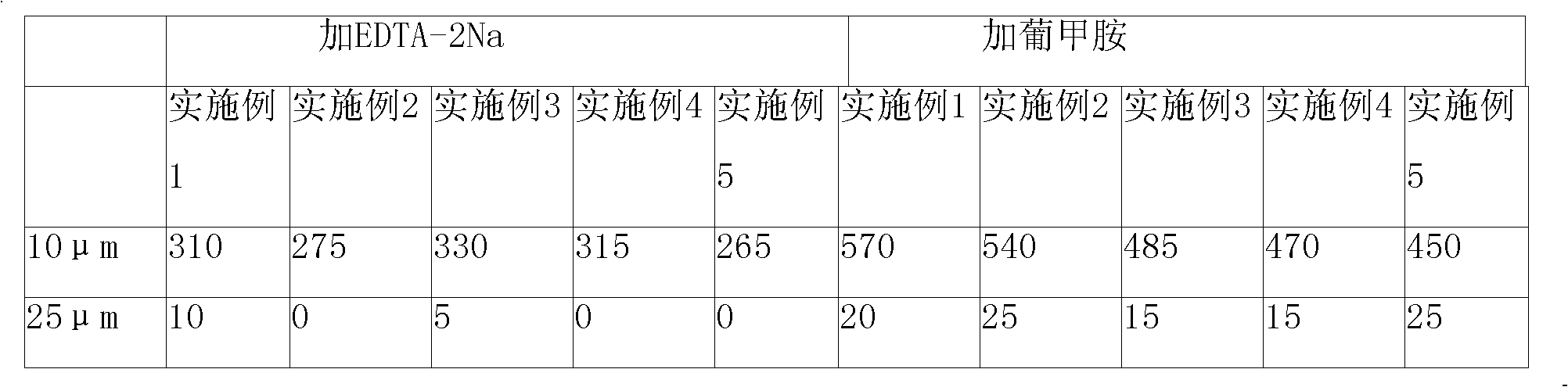
[0059] Rabeprazole sodium composition prescription:
[0060] Rabeprazole Sodium 20g
[0061] Disodium diethylamine tetraacetate 5.0g
[0062] Mannitol 150g
[0063] 1000 bottles
[0064] Rabeprazole sodium composition is prepared into the preparation method of rabeprazole sodium powder injection:
[0065] Dissolve 5.0g of disodium diethylamine tetraacetate in 4000ml of water for injection, then add 20g of rabeprazole sodium at a temperature of 30°C, stir to dissolve, then add 150g of mannitol, stir to dissolve Cool to room temperature, adjust the pH of the solution to 11.5; add 0.1% g / ml activated carbon to the above-prepared solution, stir for 15 minutes, filter and decarbonize; rapidly cool the filtrate from room temperature to -22°C, and maintain -22°C for a period of time for 2.5 hours, then lowered to -40°C, pre-frozen for 3 hours, vacuumized, raised the temperature of the frozen rabeprazole sodium to 5°C within 6 hours, maintained at 5°C for 1...
Embodiment 2
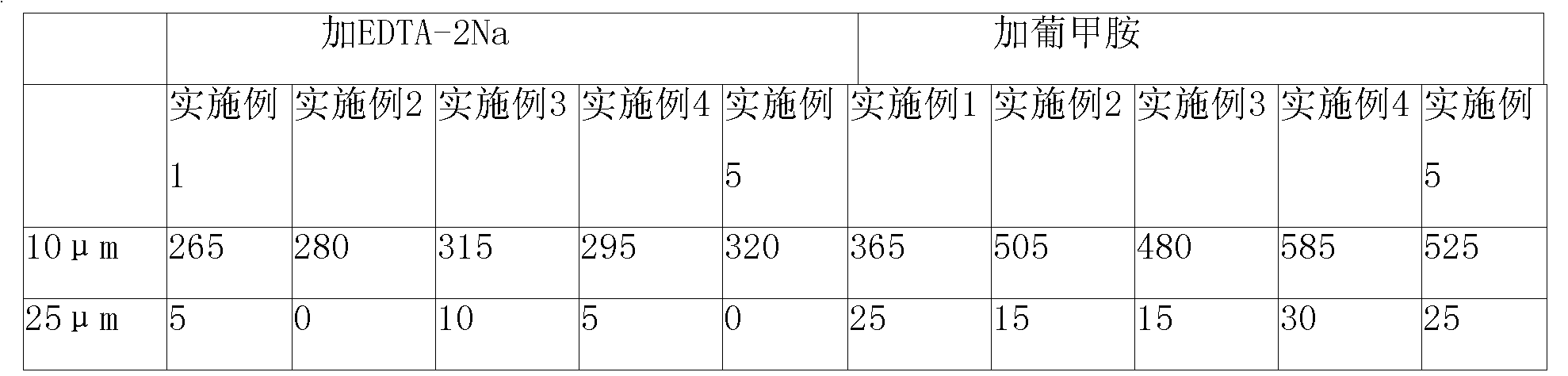
[0067] Rabeprazole sodium composition prescription:
[0068] Rabeprazole Sodium 20g
[0069] Disodium diethylamine tetraacetate 3.0g
[0070] Mannitol 200g
[0071] 1000 bottles
[0072] Rabeprazole sodium composition is prepared into the preparation method of rabeprazole sodium powder injection:
[0073] Dissolve 3.0g of disodium diethylamine tetraacetate in 4000ml of water for injection, then add 20g of rabeprazole sodium at a temperature of 25°C, stir to dissolve, then add 200g of mannitol, stir to dissolve Cool to room temperature, adjust the pH of the solution to 12.0; add 0.15% g / ml activated carbon to the above-prepared solution, stir for 15 minutes, filter and decarbonize; rapidly cool the filtrate from room temperature to -20°C, and maintain -20°C for a period of time for 2.5 hours, then lowered to -45°C, pre-frozen for 3 hours, vacuumed, raised the temperature of the frozen rabeprazole sodium to 8°C within 5 hours, maintained at 8°C for 18 hour...
Embodiment 3
[0075] Rabeprazole sodium composition prescription:
[0076] Rabeprazole Sodium 20g
[0077] Disodium diethylamine tetraacetate 6.0g
[0078] Mannitol 100g
[0079] 1000 bottles
[0080] Rabeprazole sodium composition is prepared into the preparation method of rabeprazole sodium powder injection:
[0081] Dissolve 6.0g of disodium diethylamine tetraacetate in 4000ml of water for injection, then add 20g of rabeprazole sodium at a temperature of 35°C, stir to dissolve, then add 100g of mannitol, stir to dissolve Cool to room temperature, adjust the pH of the solution to 12.5; add 0.05% g / ml activated carbon to the above-prepared solution, stir for 15 minutes, filter and decarbonize; quickly cool the filtrate from room temperature to -25°C, and maintain -25°C for a period of time 3.0 hours, then lower to -43°C, pre-freeze for 4 hours, vacuumize, raise the temperature of the frozen rabeprazole sodium to -5°C within 5 hours, continue to heat up to, mainta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com