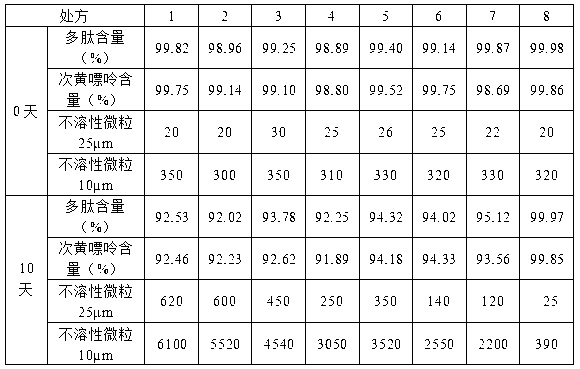
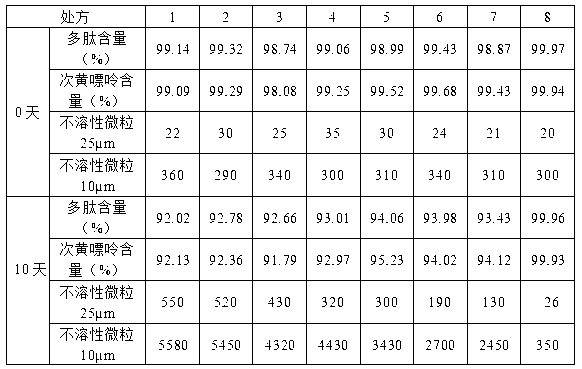
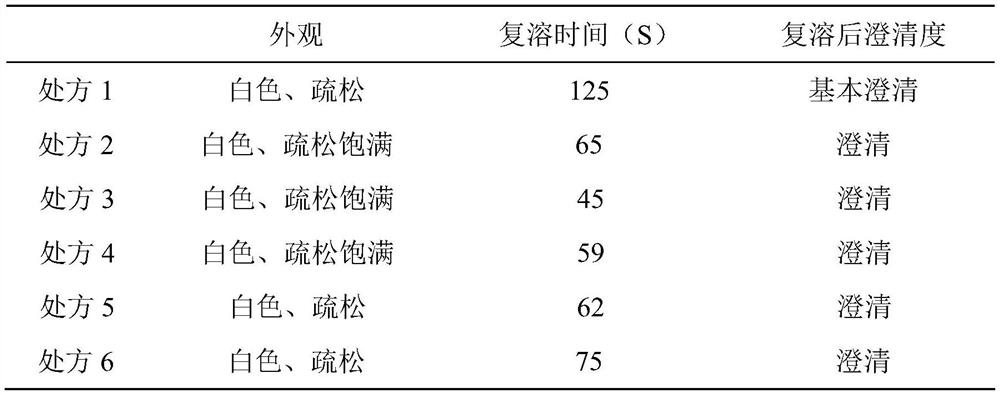
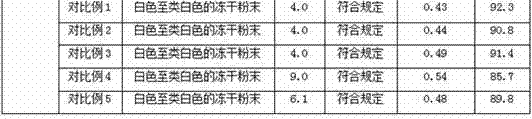
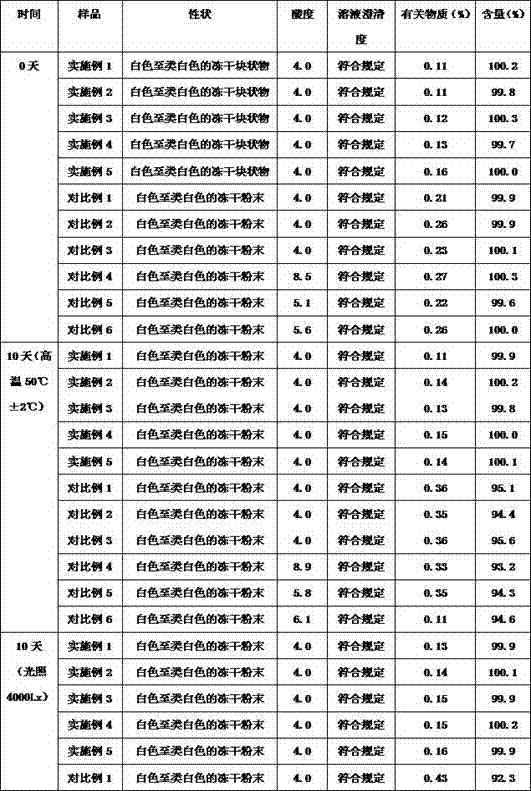
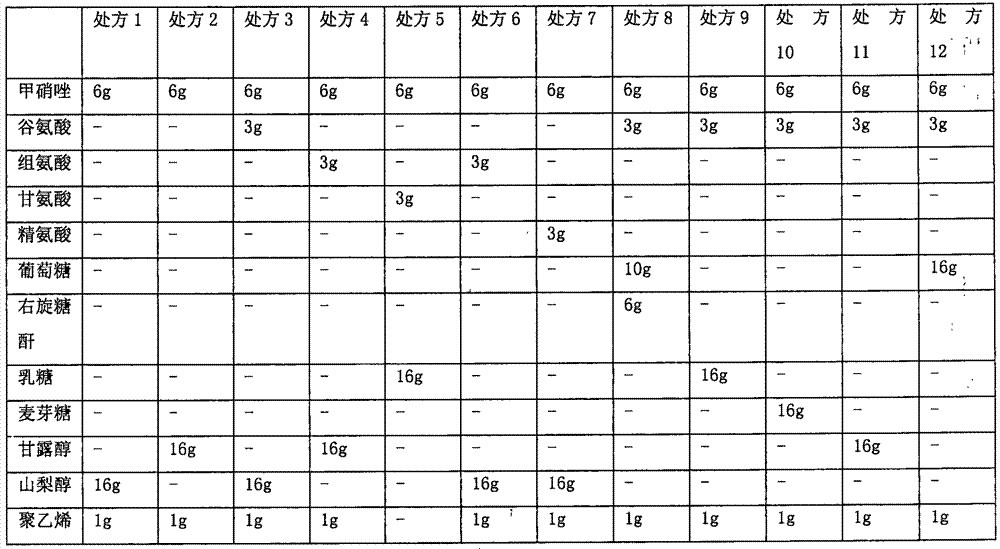
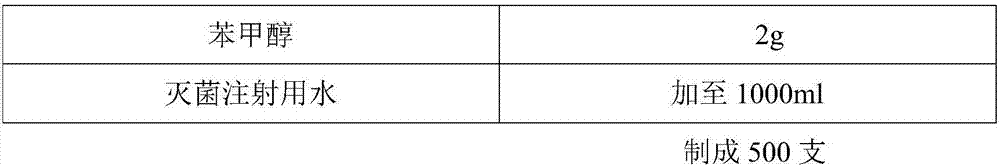
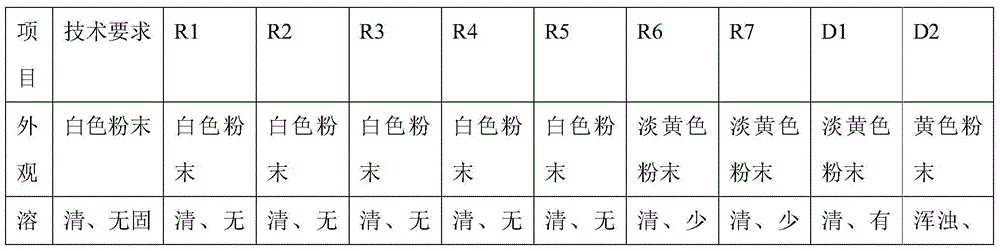
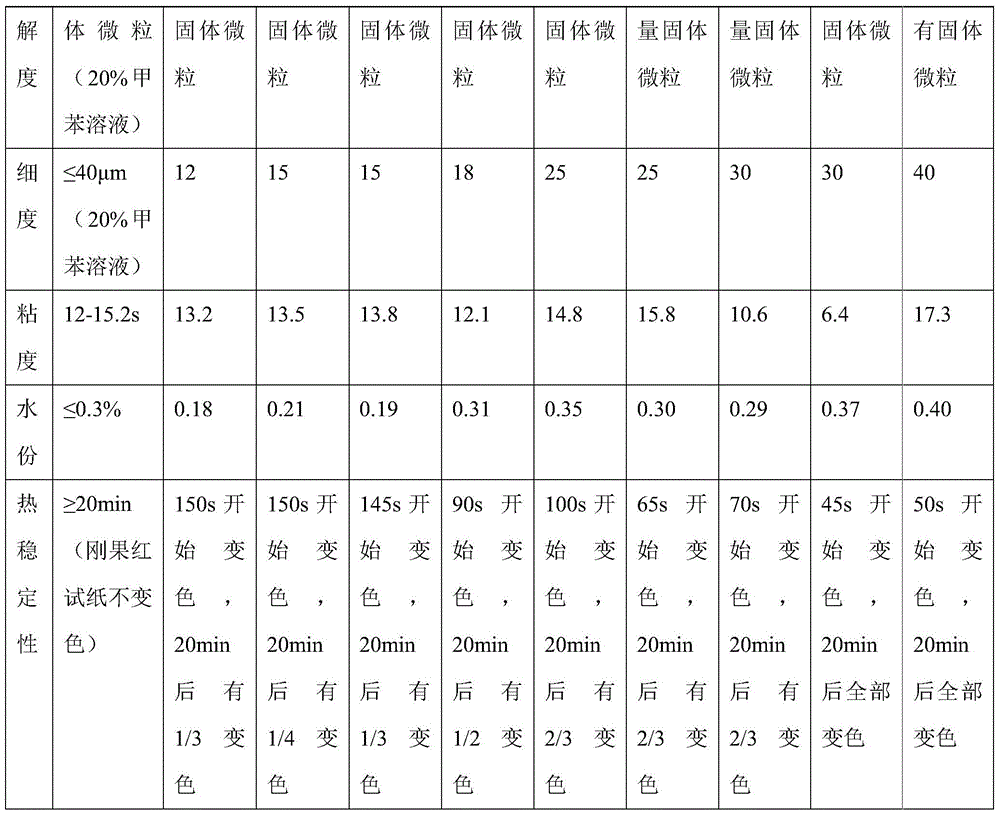
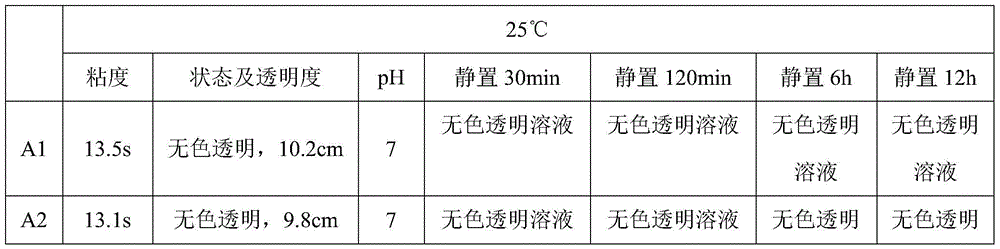
Patents
Literature
34results about How to "Less insoluble particles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
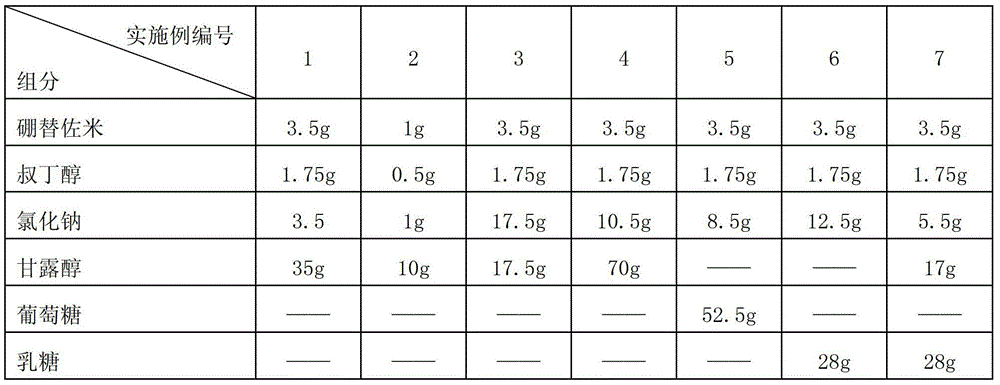
Drug composition of bortezomib and preparation method thereof
InactiveCN103212055AAccelerate the dissolution rateLess insoluble particlesPowder deliveryDipeptide ingredientsMannitolExcipient
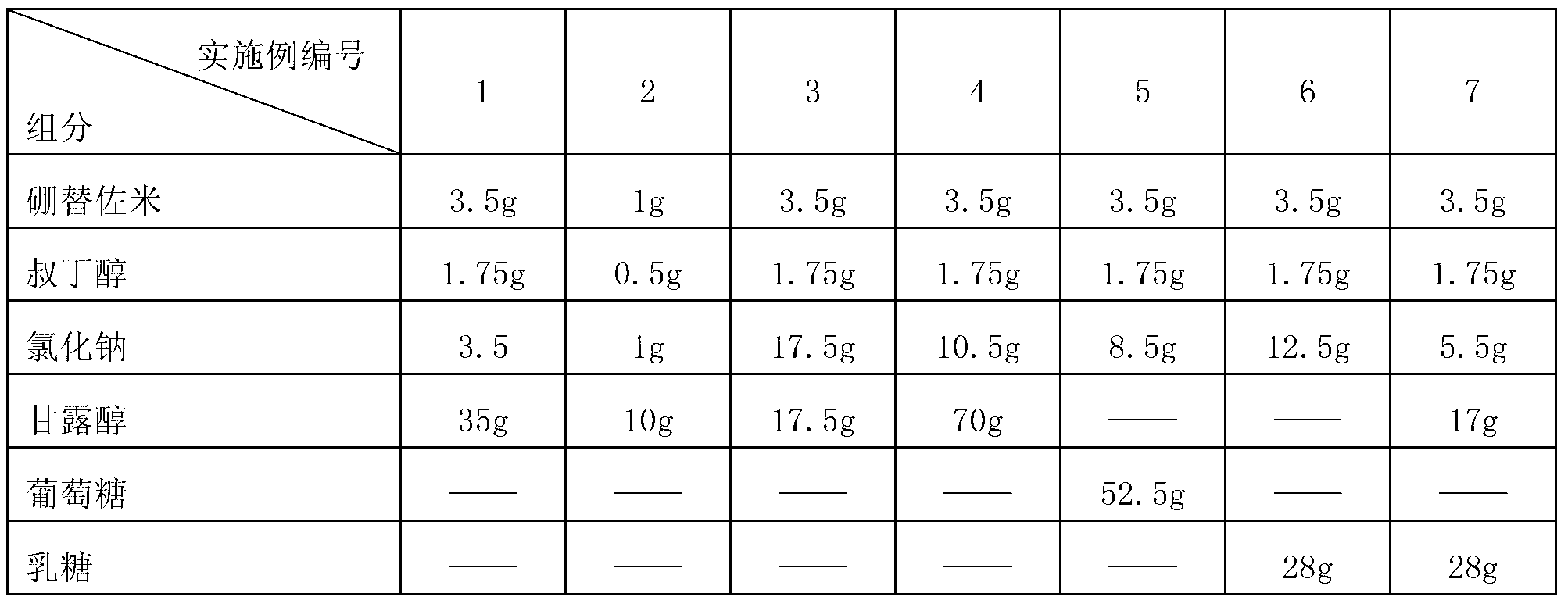
The invention belongs to the technical field of medicines and particularly relates to a drug composition of bortezomib and a preparation method thereof. The drug composition disclosed by the invention contains bortezomib, tert butyl alcohol, sodium chloride and an excipient in the mass ratio of 1:0.5:(1-5):(5-20). The drug composition adopts freeze-dried powder injection or water injection, preferably the freeze-dried powder injection. The drug composition contains tert butyl alcohol and sodium chloride besides the conventional excipient; through adding tert butyl alcohol, bortezomib can be rapidly dissolved and rapidly reacted with polyalcohol excipients such as mannitol to form more stable borate, so that the stability problem of the bortezomib freeze-dried powder injection per se is solved; and through adding the drug active constituent i.e. sodium chloride, the normal physiological and biochemical activities and functions in vivo are fully guaranteed.
Owner:HAINAN JINRUI PHARMA
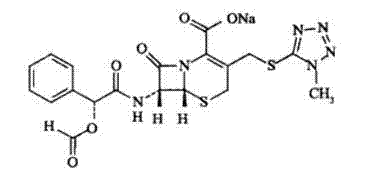
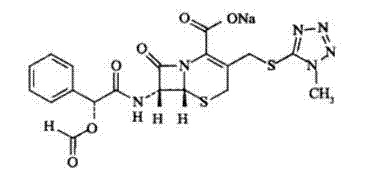
Cefamandole nafate compound and pharmaceutical composition thereof
ActiveCN103044453AImprove stabilityLess insoluble particlesAntibacterial agentsOrganic active ingredientsChemical compoundPharmaceutical Substances
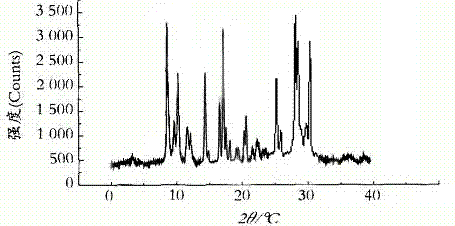
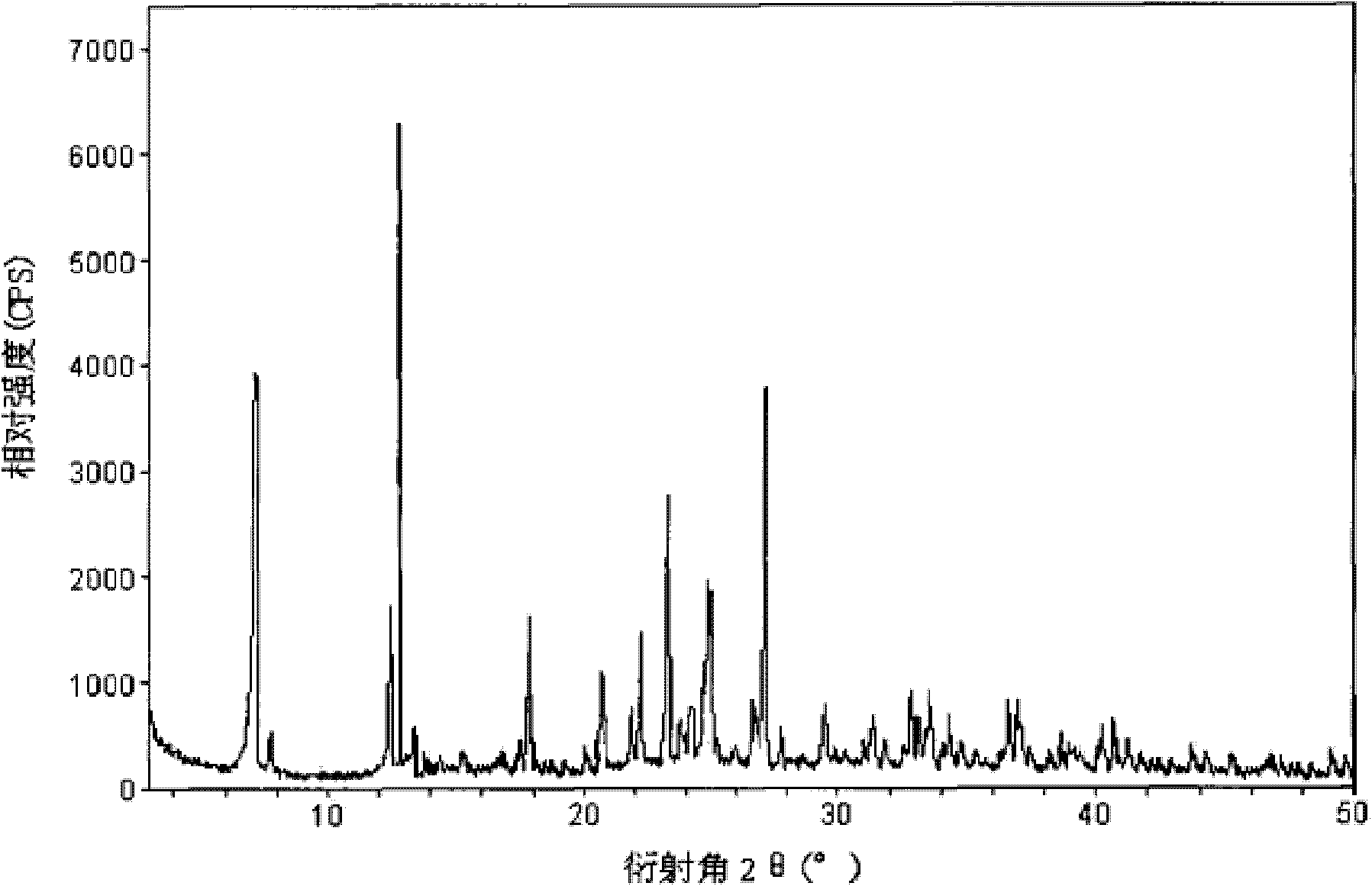
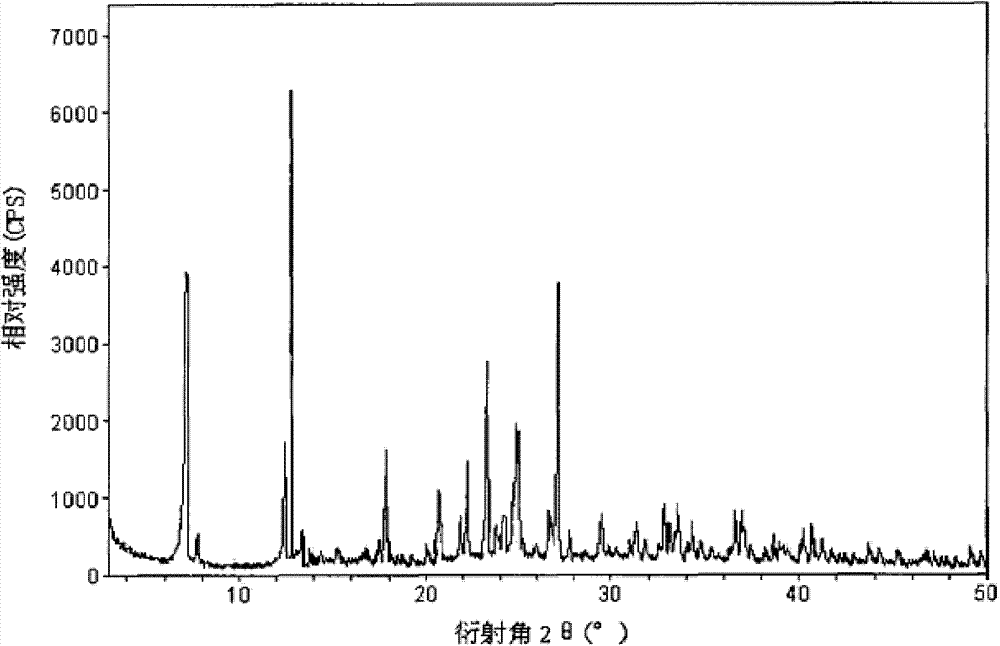
The invention relates to a cefamandole nafate compound and a pharmaceutical composition of the cefamandole nafate compound. The cefamandole nafate compound is crystal, and is determined by X-ray powder diffraction, and the characteristic peak in an atlas is shown as 8.8 degrees, 9.5 degrees, 10.2 degrees, 11.3 degrees, 12.0 degrees, 14.4 degrees, 16.8 degrees, 17.3 degrees, 20.6 degrees, 25.0 degrees, 27.8 degrees, 28.1 degrees and 30.2 degrees in a range of 20+ / 0.2 degrees. The pharmaceutical composition of the cefamandole nafate compound is a preparation and a pharmaceutical composition preparation containing the above cefamandole nafate compound and the preparation is powder-injection. The cefamandole nafate crystalline compound is high in stability and few in insoluble particles; a cosolvent is not needed, so that the compound can be directly and aseptically sub-packaged to obtain cefamandole nafate powder injection for injection. The cefamandole nafate crystalline compound is also fully mixed with aseptic sodium carbonate powder to be aseptically sub-packaged. The number of insoluble particles added with the powder injection of sodium carbonate is few on the basis of reaching standard request.
Owner:HUNAN KELUN PHARMA
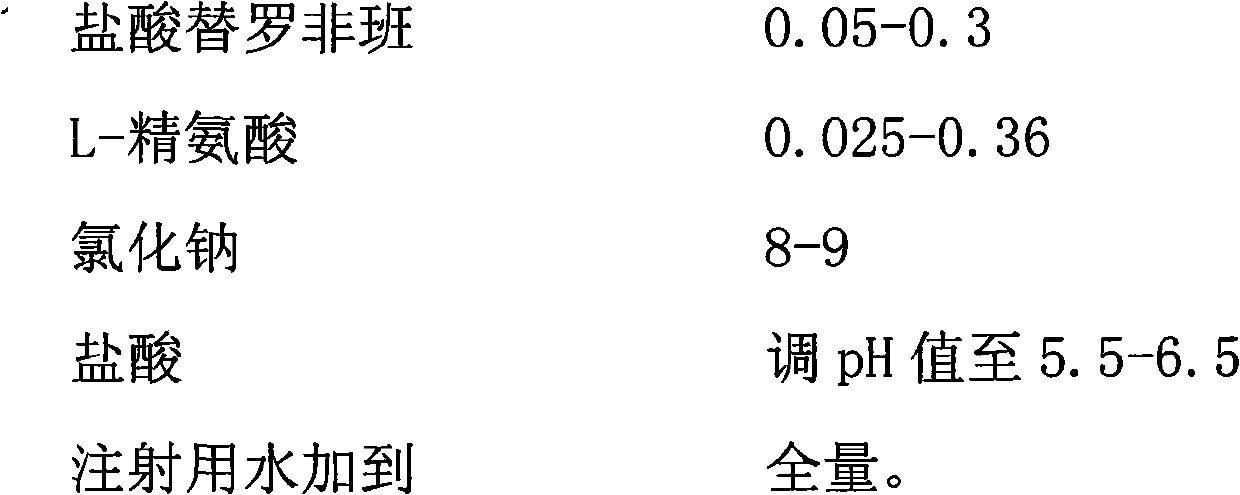
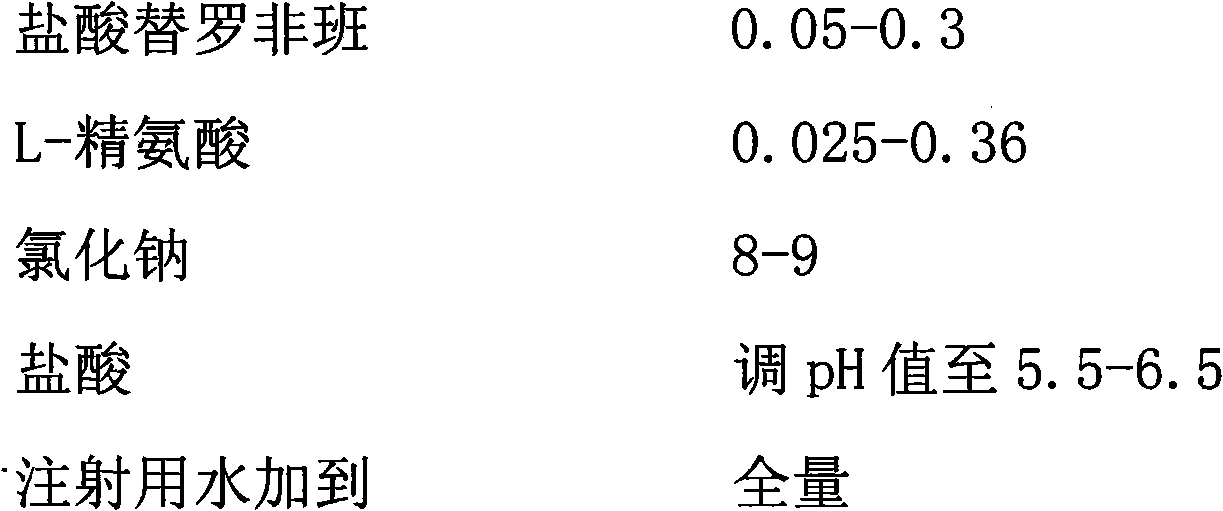
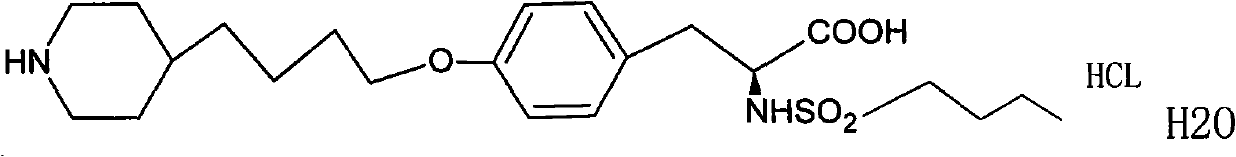
Tirofiban hydrochloride injecta and preparation method thereof
InactiveCN102600072ALess insoluble particlesOrganic active ingredientsPharmaceutical delivery mechanismAspirinTirofiban Hydrochloride
The invention provides a tirofiban hydrochloride injecta and a preparation method thereof. L-arginine is added into an injecta formula, and compared with a formula in which a pH (Potential Of Hydrogen) buffer solution is used as an accessory and a formula provided with arginine aspirin, insoluble particles of the tirofiban hydrochloride injecta are reduced in the formula with the L-arginine, and particularly, after the standing of the solution, the quantity of the insoluble particles is basically not increased as time goes on, so that the tirofiban hydrochloride injecta is ensured to be safe and effective.
Owner:武汉同源药业有限公司
Dantrolene sodium freeze-dried powder injection for injection and preparation method thereof
ActiveCN102302461AImprove toleranceDosing time is shortPowder deliveryOrganic active ingredientsForeign matterCLARITY
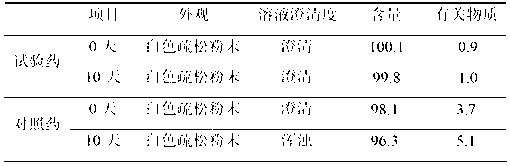
The invention belongs to the technical field of medicines, and particularly relates to a dantrolene sodium freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is prepared by freeze drying dantrolene sodium, lactobiose, a pH regulator and water for injection, wherein a mass ratio of the dantrolene sodium to the lactobiose is (1:0.5) to (1:2.5). The preparation method comprises the following steps of: adding the dantrolene sodium to the lactobiose in a prescription amount into the water for the injection, stirring, regulating the pH value to 9.0 to 10.5 by using the pH regulator, adding active carbon for injection, removing a heat source, decolorizing, filtering for decarburizing, performing fine filtering by using a filter membrane, packaging in separate bags, freezing and drying. The dantrolene sodium freeze-dried powder injection is studied on the basis of a freeze-dried process, namely the dantrolene sodium freeze-dried powder injection is cooled, heated by a small margin and is cooled again, so that the moisture of the freeze-dried product is reduced, and the freeze-dried product is high in redissolution and visible foreign matters and does not have insoluble granules. The dantrolene sodium freeze-dried powder injection for the injection is high in resolubility, clarity and stability, and low impurity content.
Owner:HAINAN JINRUI PHARMA
Medical freeze-dried sterile polyethylene film and preparation method thereof
The invention belongs to the technical field of high molecular materials and in particular relates to a medical freeze-dried sterile polyethylene film and a preparation method thereof. The medical freeze-dried sterile polyethylene film is prepared from the following raw materials in parts by weight: 6 to 8 parts of ultrahigh molecular weight polyethylene resin and 2 to 4 parts of metallocene polyethylene. The preparation method of the medical freeze-dried sterile polyethylene film comprises the steps of mixing, preparing the film, cutting the film, packaging and sterilizing. The polyethylene film provided by the invention has the characteristics of low temperature resistance, sterile performance, low insoluble particles, strong mechanical properties and the like, and can meet the freeze-dried production requirements of medicines, crude drugs, biological products, medical intermediates, chemical products and the like.
Owner:ZIBO HUA ZHI LIN PACKING PROD CO LTD
Drug composition of bortezomib and preparation method thereof
InactiveCN103212055BAccelerate the dissolution rateLess insoluble particlesPowder deliveryDipeptide ingredientsMANNITOL/SORBITOLFreeze-drying
The invention belongs to the technical field of medicines and particularly relates to a drug composition of bortezomib and a preparation method thereof. The drug composition disclosed by the invention contains bortezomib, tert butyl alcohol, sodium chloride and an excipient in the mass ratio of 1:0.5:(1-5):(5-20). The drug composition adopts freeze-dried powder injection or water injection, preferably the freeze-dried powder injection. The drug composition contains tert butyl alcohol and sodium chloride besides the conventional excipient; through adding tert butyl alcohol, bortezomib can be rapidly dissolved and rapidly reacted with polyalcohol excipients such as mannitol to form more stable borate, so that the stability problem of the bortezomib freeze-dried powder injection per se is solved; and through adding the drug active constituent i.e. sodium chloride, the normal physiological and biochemical activities and functions in vivo are fully guaranteed.
Owner:HAINAN JINRUI PHARMA CO LTD
Rabeprazole sodium composite lyophilized injectable powder and preparation method thereof
ActiveCN102552179AImprove stabilityLess insoluble particlesOrganic active ingredientsPowder deliverySodium sulfiteMeglumine
The invention provides a rabeprazole sodium composite, which comprises the following components: rabeprazole sodium parts by weight, meglumine 7.5-8.5 parts by weight, mannitol 100-170 parts by weight, sodium sulfite 9-10 parts by weight and ethylene diamine tetraacetic acid 4.5-5.5 parts by weight. The preparation process of the rabeprazole sodium composite lyophilized injectable powder preparedby the composites is greatly simplified, the lyophilized time of the lyophilized injectable powder is also shortened, production cost is reduced, appearance is full, stability and re-dissolution are good, and insoluble particles of injection solution are few.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Preparation method for freeze-dried powder injection of posaconazole
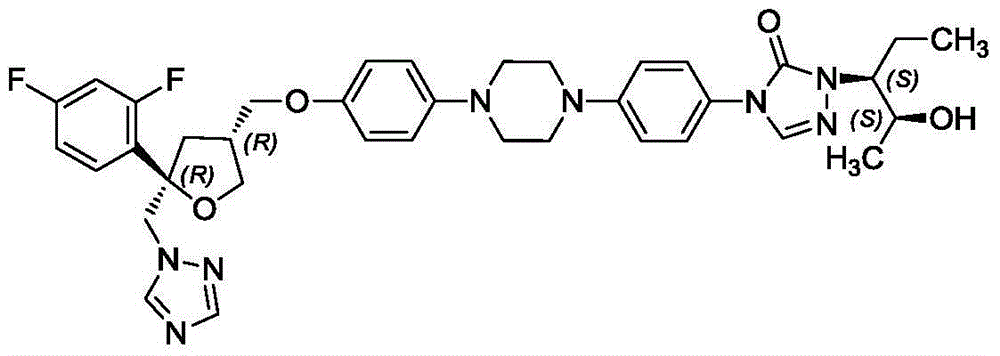
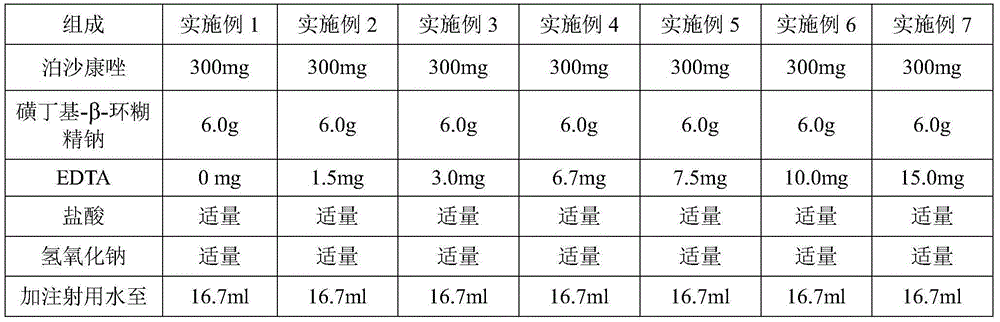
InactiveCN106265534ASolve the insolubleSolve problems that require the addition of organic solventsPowder deliveryOrganic active ingredientsFreeze-dryingAdditive ingredient
The invention relates to a freeze-dried powder injection of posaconazole and a preparation method thereof. The powder injection comprises active treatment ingredients consisting of posaconazole, cyclodextrin, a metal ion chelating agent and a pH conditioning agent. The preparation method comprises the following steps: dissolving cyclodextrin and disodium edetate under stirring; adjusting the pH value of a solution obtained in the previous step to 1 to 2; adding posaconazole and dissolving posaconazole under stirring so as to obtain a clear solution; adjusting the pH value of the clear solution to 2.0 to 3.5; allowing the volume of the solution to reach a fixed value through addition of water; and successively carrying out aseptic filtering, separation and freeze drying. According to the invention, through effective control of the proportions of posaconazole, the metal ion chelating agent and the like in a recipe, insoluble particles before and after combination of medicinal liquid is reduced, and the prepared freeze-dried powder injection of posaconazole has the advantages of good stability of active ingredients and convenience in transport and storage.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
(S)-Pantoprazole sodium lyophilized powder preparation for injection
ActiveCN106474074ALess irritatingReduce the introductionPowder deliveryOrganic active ingredientsEtherMicroparticle
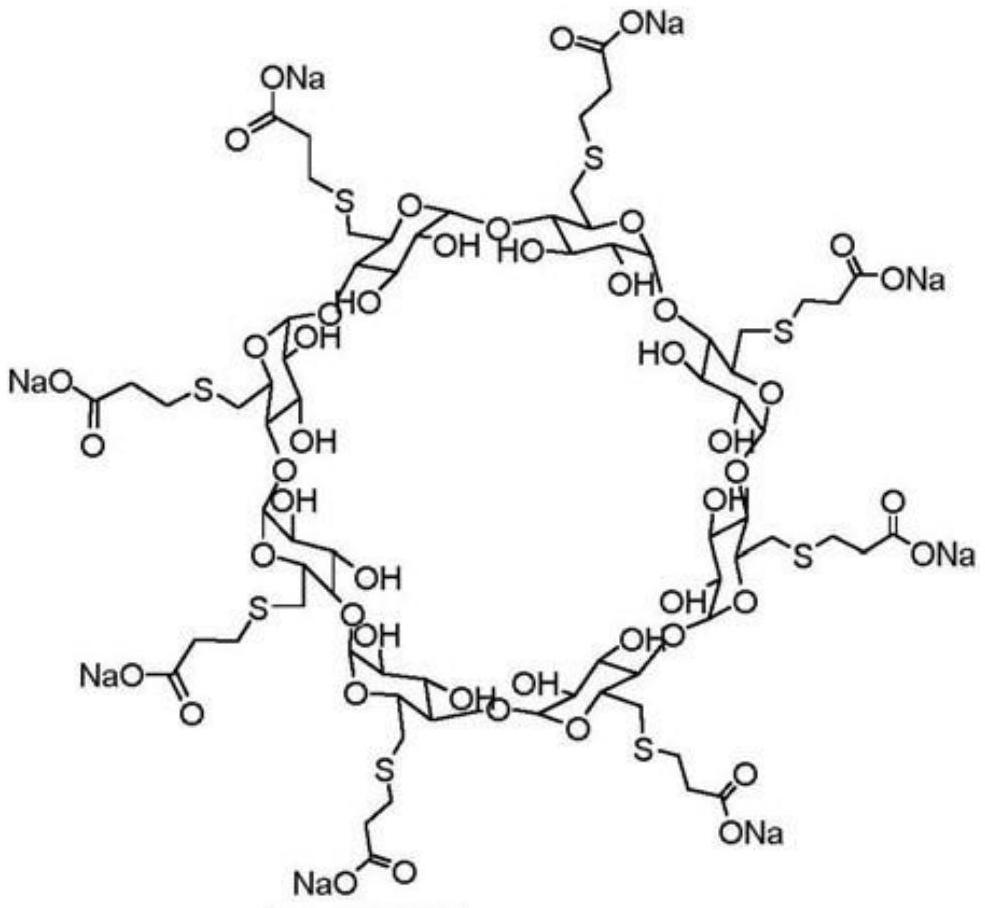
The invention relates to (S)-Pantoprazole sodium lyophilized powder preparation for injection, made from an active ingredient (S)-pantoprazole sodium, a stabilizer and a lyophilizing protectant, wherein the stabilizer is one of sulfobutyl ether-Beta-cyclodextrin, hydroxypropyl- Beta-cyclodextrin, and glucosyl-Beta-cyclodextrin. After the (S)-pantoprazole sodium lyophilized powder preparation for injection is placed for 24 months, R-configuration isomers are less than 0.3%; the number of insoluble microparticles is significantly decreased through optimization of a preparation process; the preparation process is simple and feasible and is suitable for industrial large-scale production.
Owner:SHANDONG NEWTIME PHARMA
Sugammadex sodium freeze-dried powder injection and preparation method thereof
PendingCN113456598ALess impuritiesSolve the problem of easy generation of large amounts of impuritiesPowder deliveryOrganic active ingredientsFreeze-dryingMedicine
The invention relates to the technical field of medicine technology, in particular to sugammadex sodium freeze-dried powder injection and a preparation method thereof. The preparation method of the sugammadex sodium freeze-dried powder injection comprises the following steps: adding sugammadex sodium into water for injection according to a prescription dosage, and stirring until sugammadex sodium is completely dissolved; adding a prescription amount of freeze-drying protection solution into the solution, stirring until the solution is completely dissolved, adjusting the pH value, stirring, filtering, filling and freeze-drying to obtain the target product. according to the method, the sugammadex sodium freeze-dried powder injection for injection is provided, and the problems that API serving as a cyclic molecular structure is unstable, and a large number of impurities are easily generated after high-temperature sterilization are solved; inert gas (nitrogen) is filled for protection after freeze-drying is finished, so that oxygen is effectively isolated, and the prepared sugammadex sodium freeze-dried powder injection has fewer impurities and is more stable; through optimization of a freeze-drying process, the prepared freeze-dried powder is shortest in redissolution time, the clarity and insoluble particles meet the requirements, the stability is higher, and the freeze-dried powder is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
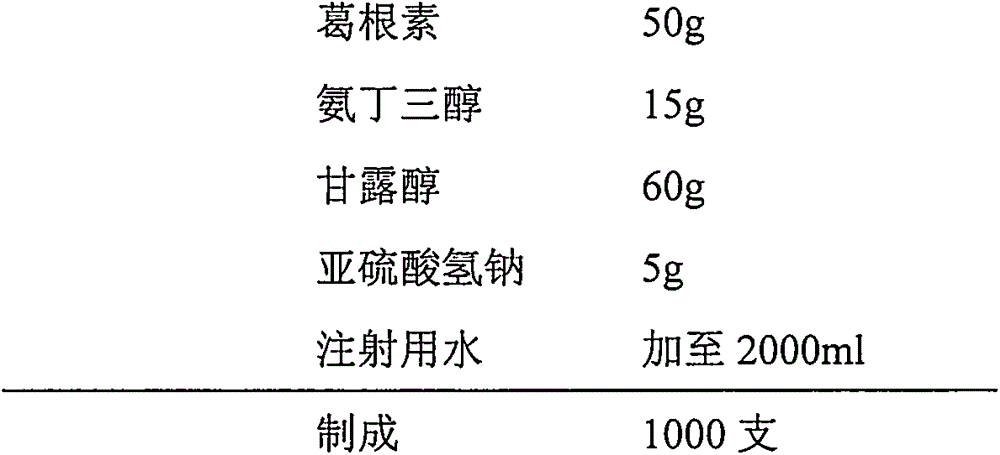
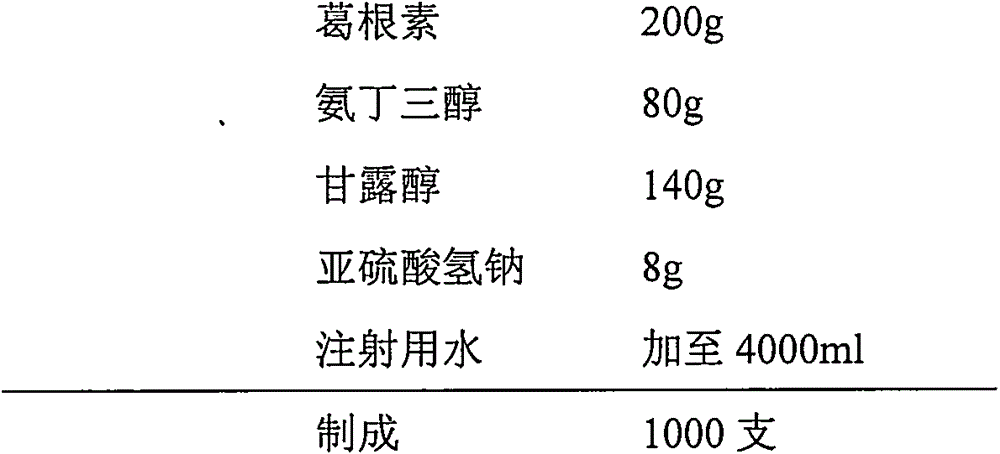
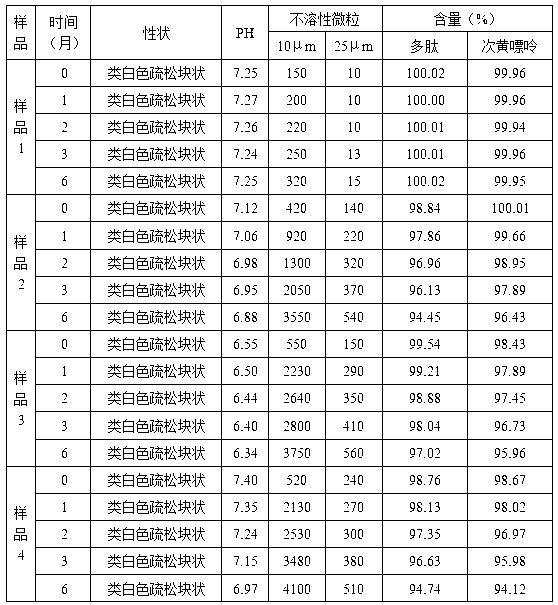
Injection puerarin containing tromethamine and preparation method of injection puerarin
InactiveCN105476964AGood resolubilityHigh clarityPowder deliveryOrganic active ingredientsFreeze-dryingMedicine
The invention relates to a puerarin freeze-dried powder injection and a preparation method thereof. The injection mainly comprises puerarin and tromethamine. Tromethamine used in the injection can obviously increase the redissolution speed of the puerarin freeze-dried powder injection; the amount of insoluble particles is reduced; and clinic security is improved.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD
Steam ejector for vinegar production
InactiveCN105543063AReduce dosageLess steam consumptionVinegar preparationAmylaseProcess engineering
The invention discloses a steam ejector for vinegar production. The steam ejector for the vinegar production comprises a steam nozzle, a suction chamber, a diffusion tube, an ejector pump, a mixing tank, an underflow pump, an ejection liquefier, a maintaining tank and a liquefaction tank which are sequentially connected. The steam ejector for the vinegar production has the superior effects as follows: liquefaction and gelatinization of a continuous ejection liquefaction method for the steam ejector for the vinegar production are performed in a closed container, so that the steam consumption is low, the heat loss is low, energy can be saved, the use amount of fuel coal is reduced, and the use amount of amylase can be reduced properly; moreover, the liquefaction quality of rice starch milk is not influenced, and the use amount of liquifying enzyme is reduced to 0.4L per ton of saccharide from 0.6L per ton of saccharide originally; with the adoption of the continuous ejection liquefaction method, continuous production of a saccharification process is realized, and the saccharification production environment is improved; due to the good effect of the continuous ejection liquefaction method, a saccharification liquid contains few insoluble particles, and the saccharification quality is improved.
Owner:XINJIANG XIAOCHU FOOD
Pharmacetutical for treating cardiovascular and cerebrovascular disease and its preparing process
InactiveCN1839868AGood curative effectLess insoluble particlesOrganic active ingredientsPowder deliveryDiseaseMedicine
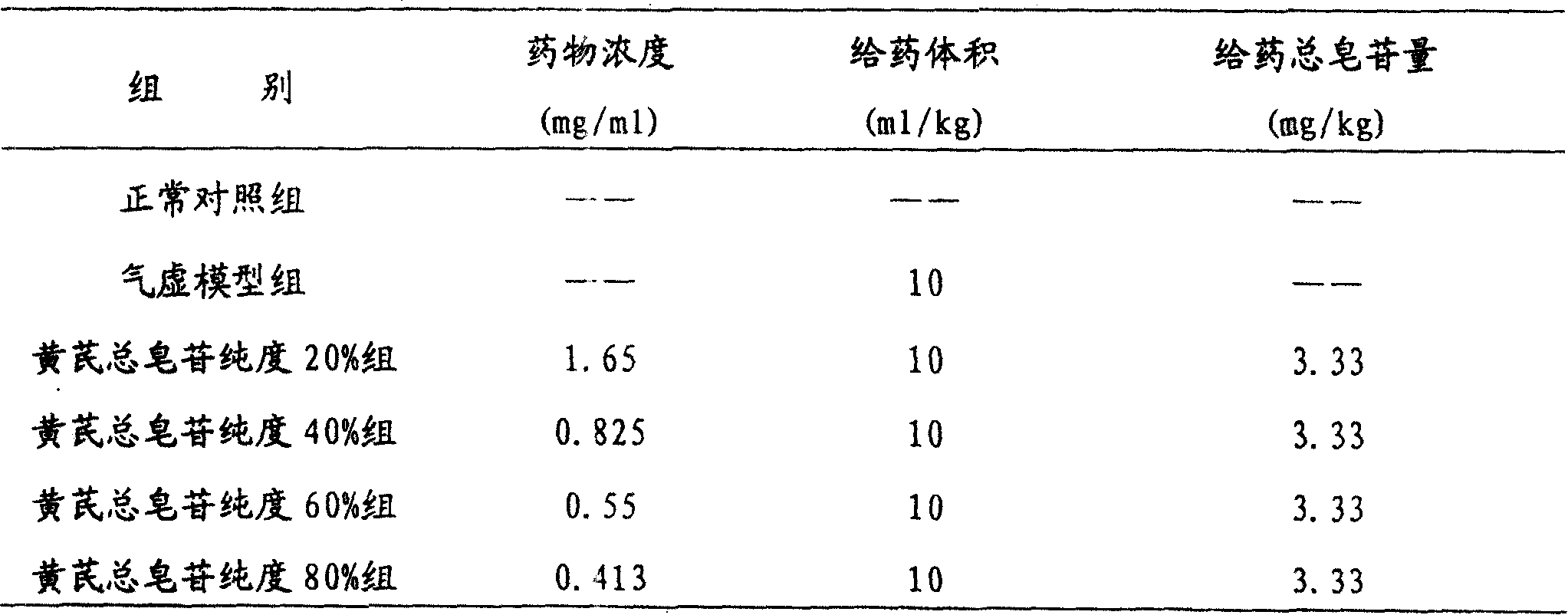
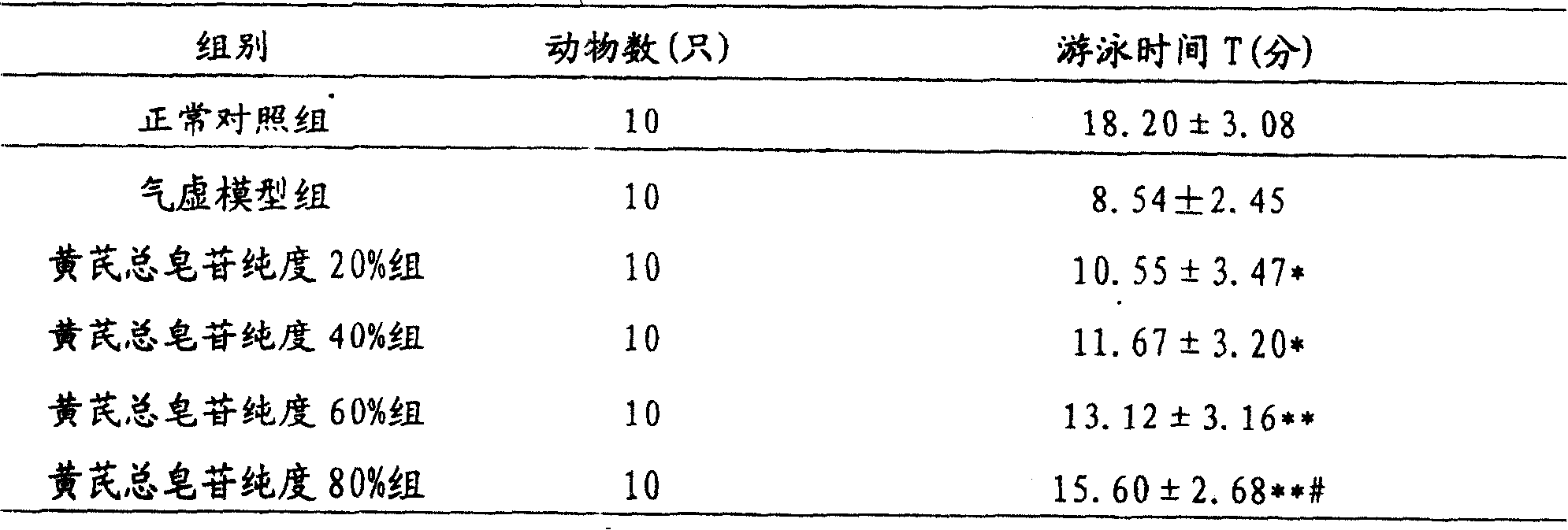
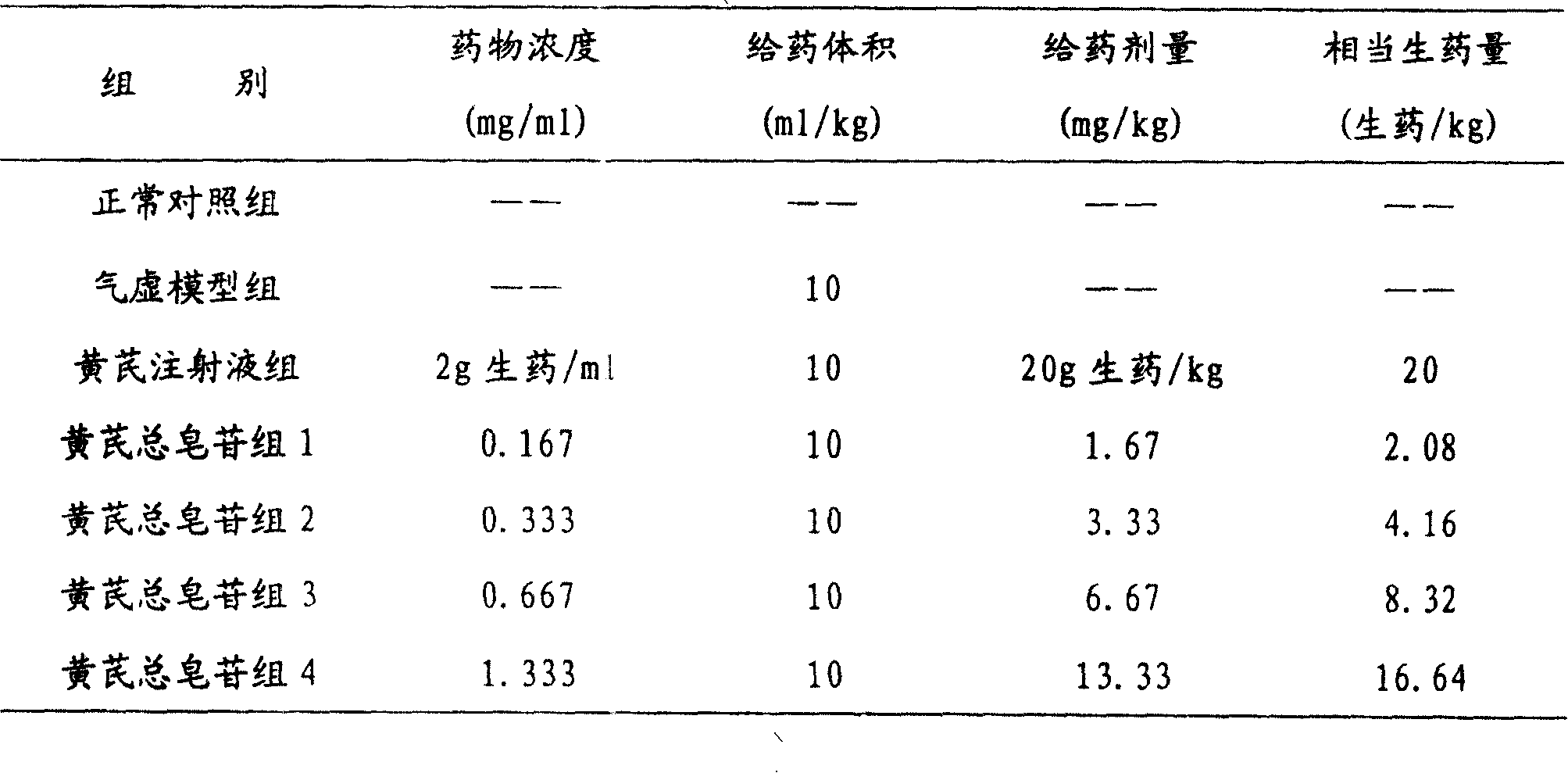
The invention relates to a pharmaceutical composition for treating cardiovascular and cerebrovascular diseases and its preparing process, wherein the composition comprises breviscapine and Radix Astragali saponin with a purity of over 80% extracted from astragalus root, the content of the Radix Astragali saponin being 20-30%.
Owner:成都和康药业有限责任公司
Muscular amino acid and nucleoside extract and pharmaceutical composition thereof
ActiveCN103191153AImprove stabilityLess insoluble particlesPowder deliveryNervous disorderNucleotideThio-
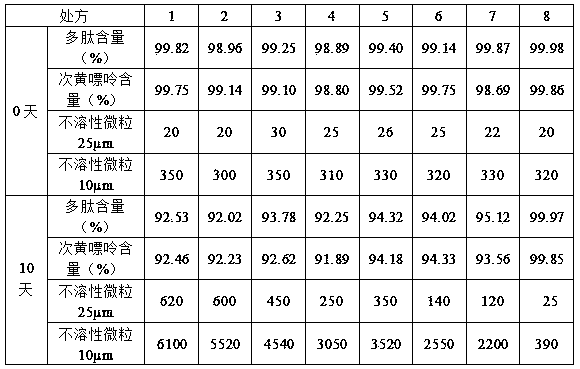
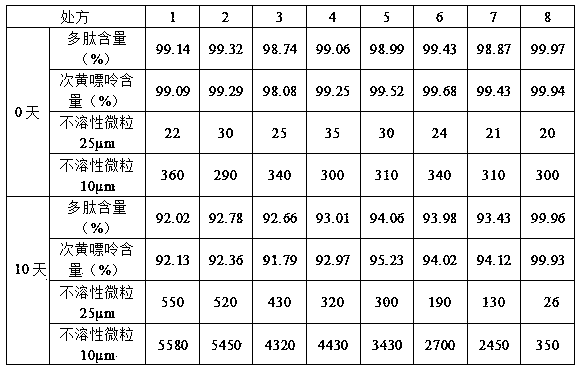
The invention relates to a muscular amino acid and nucleoside extract containing polypeptide, amino acid, nucleoside, nucleotide and other medicinal components. The extract is prepared by crushing cardiac muscles and muscles of a rabbit of which the connective tissue is removed to be mixed with water, homogenizing, freeing and performing ultrasonic treatment; adding potassium chloride into the homogenate, and performing ultrasonic agitation; and centrifuging to obtain supernate, filtering with a filter membrane, and performing ultrafiltration. The invention also provides a muscular amino acid and nucleoside medicinal composition injection and lyophilized powder, wherein the muscular amino acid and nucleoside medicinal composition consists of the following components: 1000ml of muscular amino acid and nucleoside solution, 1.5g of sorbitol, 0.2g of thioglycerol, and 1.0g of poloxamer 188; and the concentration of the polypeptide in the muscular amino acid and nucleoside solution is between 1.58 and 1.92mg / ml, and the concentration of hypoxanthine is between 0.22 and 0.28mg / ml. The muscular amino acid and nucleoside pharmaceutical composition lyophilized powder is prepared by lyophilizing 1,000ml of muscular amino acid and nucleoside solution, 40g of dextran 40, 0.2g of thioglycerol, 1.0g of poloxamer 188, and 0.5g of hydroxypropyl betadex. The muscular amino acid and nucleoside extract and the pharmaceutical composition thereof have a good effect of improving the stability of muscular amino acid and nucleoside and maintaining low insoluble particles.
Owner:HUNAN WUZHOUTONG PHARMA
Special intravenous injection drug transfer box for intravenous drug use configuration center
PendingCN108557226ACleanliness (biosafety assuranceLess insoluble particlesClosure with auxillary devicesRigid containersPediatricsHandover
The invention belongs to the technical field of transfer boxes, and discloses a special intravenous injection drug transfer box for an intravenous drug use configuration center. The special intravenous injection drug transfer box comprises a multi-layer box body and a box cover, the upper ends and the lower ends of both sides of the box body integrally form mutually matched buckles, and a handle is connected to the middle of the box cover in a bolted mode. Two parallel detachable soft clamps are connected into the box body in a clamped mode, and buckles matched with the upper side of the box body are separately and integrally formed on both sides of the box cover. According to the special intravenous injection drug transfer box for the intravenous drug use configuration center, liquid leakage of drugs caused by collision of a piston can be avoided and mutual contamination of syringes due to moving contact can be avoided, the number of drug groups can be directly counted without openingthe cover during transportation and handover, all components are detachable, and thorough cleaning and disinfection are facilitated.
Owner:SHENZHEN CITY BAOAN DISTRICT MATERNAL & CHILD HEALTH HOSPITAL
Dantrolene sodium freeze-dried powder injection for injection and preparation method thereof
ActiveCN102302461BGood formabilityHigh clarityOrganic active ingredientsPowder deliveryForeign matterCLARITY
The invention belongs to the technical field of medicines, and particularly relates to a dantrolene sodium freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is prepared by freeze drying dantrolene sodium, lactobiose, a pH regulator and water for injection, wherein a mass ratio of the dantrolene sodium to the lactobiose is (1:0.5) to (1:2.5). The preparation method comprises the following steps of: adding the dantrolene sodium to the lactobiose in a prescription amount into the water for the injection, stirring, regulating the pH value to 9.0 to 10.5 by using the pH regulator, adding active carbon for injection, removing a heat source, decolorizing, filtering for decarburizing, performing fine filtering by using a filter membrane, packaging in separate bags, freezing and drying. The dantrolene sodium freeze-dried powder injection is studied on the basis of a freeze-dried process, namely the dantrolene sodium freeze-dried powder injection is cooled, heated by a small margin and is cooled again, so that the moisture of the freeze-dried product is reduced, and the freeze-dried product is high in redissolution and visible foreign matters and does not have insoluble granules. The dantrolene sodium freeze-dried powder injection for the injection is high in resolubility, clarity and stability, and low impurity content.
Owner:HAINAN JINRUI PHARMA CO LTD
Lansoprazole for injection
PendingCN113069421ALess clarityLess insoluble particlesOrganic active ingredientsPowder deliveryNaCl - Sodium chlorideNanofiltration
The invention discloses lansoprazole for injection, and particularly relates to lansoprazole for injection. The lansoprazole preparation for injection is a freeze-dried powder injection and is prepared from the following raw materials in parts by weight: 10-50 parts of lansoprazole, 40-80 parts of mannitol, 5-15 parts of meglumine and 1600-2400 ml of water for injection. The preparation process of the lansoprazole preparation for injection comprises the steps of liquid preparation, sterilization and filtration, filling and freeze drying, and the preparation process of mannitol comprises the steps of nanofiltration, decoloration and concentration, crystallization and recrystallization. The prepared lansoprazole for injection has less clarity and insoluble particles, can avoid the problems that the clarity of a solution is reduced and the insoluble particles are increased after the lansoprazole is stored for a period of time, can be matched with common diluting solvents such as 0.9% sodium chloride injection and 5% glucose injection, and has a wider clinical application range.
Owner:HAINAN JINRUI PHARMA
Muscular amino acid and nucleoside extract and pharmaceutical composition thereof
ActiveCN103191153BImprove stabilityLess insoluble particlesPowder deliveryNervous disorderNucleotideThio-
Owner:HUNAN WUZHOUTONG PHARMA
A kind of high-purity mezlocillin sodium preparation and preparation method thereof
ActiveCN105902543BFast dissolutionHigh purityOrganic chemistry methodsHeterocyclic compound active ingredientsMicroparticleAmpicillin Sodium
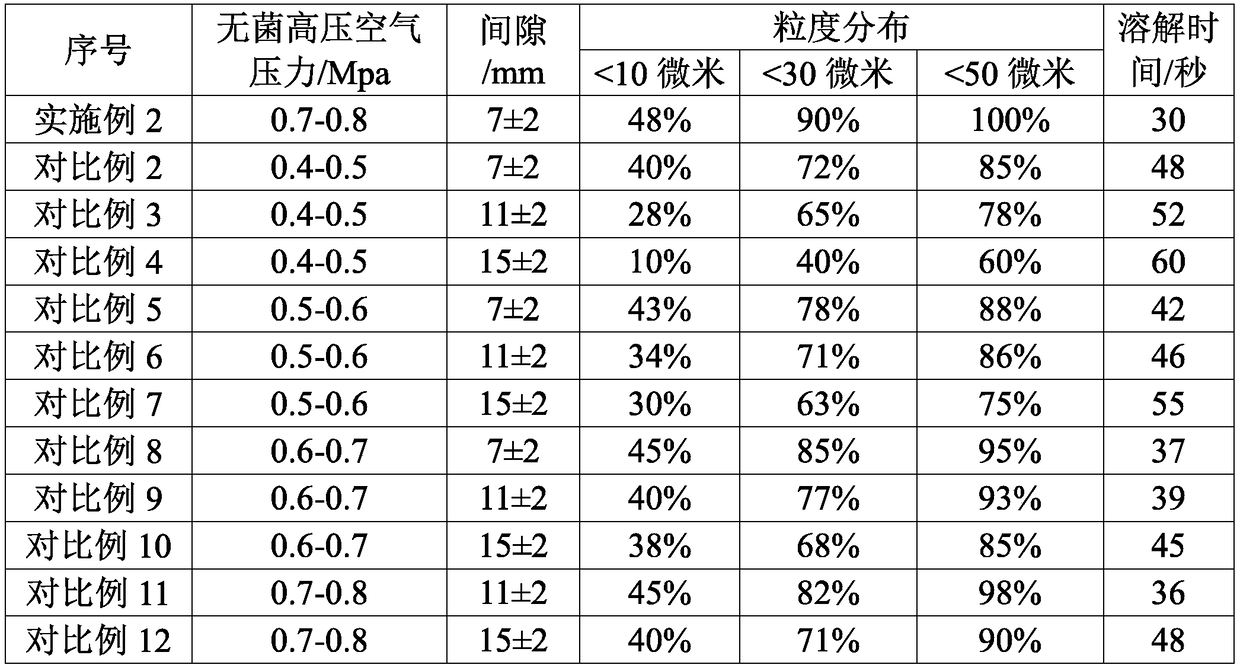
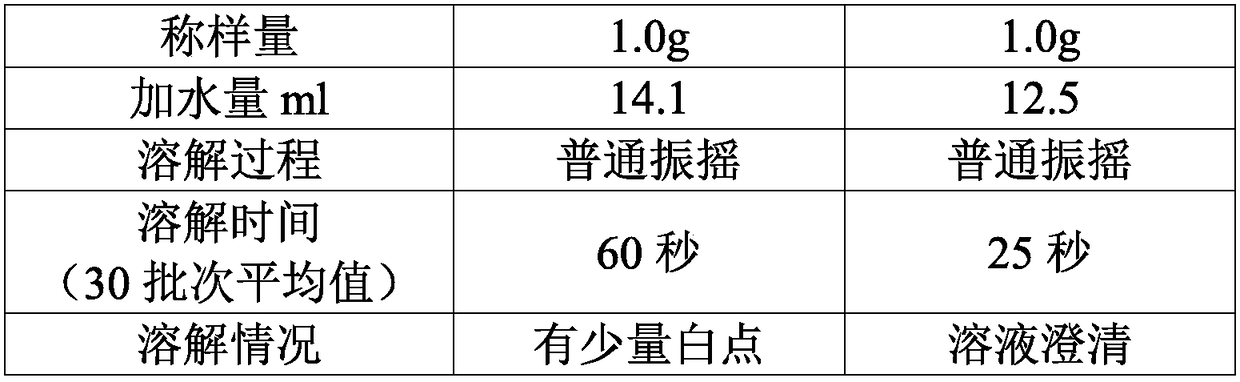
The invention discloses a high-purity mezlocillin sodium preparation. The high-purity mezlocillin sodium preparation comprises uniform granules with the grain sizes smaller than 50 micrometers. The content of total impurities in the high-purity mezlocillin sodium preparation is lower than or equal to 0.68%, and the content of polymers in the high-purity mezlocillin sodium preparation is lower than or equal to 0.06%. The invention further discloses a method for preparing the high-purity mezlocillin sodium preparation. The method includes six steps of preparing ampicillin sodium solution; preparing mezlocillin sodium condensation liquid; preparing mezlocillin dry powder; preparing mezlocillin sodium solution; preparing mezlocillin sodium active compounds; preparing the high-purity mezlocillin sodium preparation. The high-purity mezlocillin sodium preparation and the method have the advantages that existing problems can be solved by the aid of the high-purity mezlocillin sodium preparation prepared by the aid of novel crystallization technologies, and the quality of the high-purity mezlocillin sodium preparation can be greatly improved; the high-purity mezlocillin sodium preparation is high in dissolving rate and purity, low in impurity content and insoluble particle content, good in flowability and easy to separately package, and the like.
Owner:NORTH CHINA PHARMA COMPANY
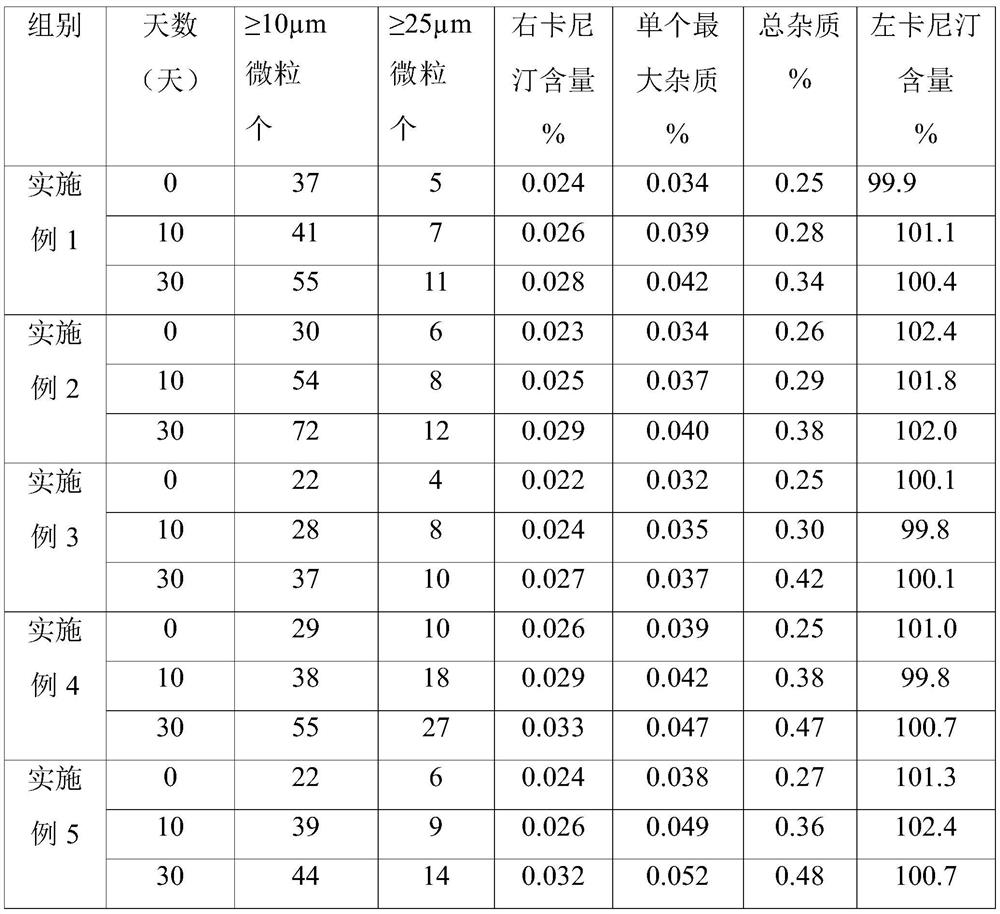
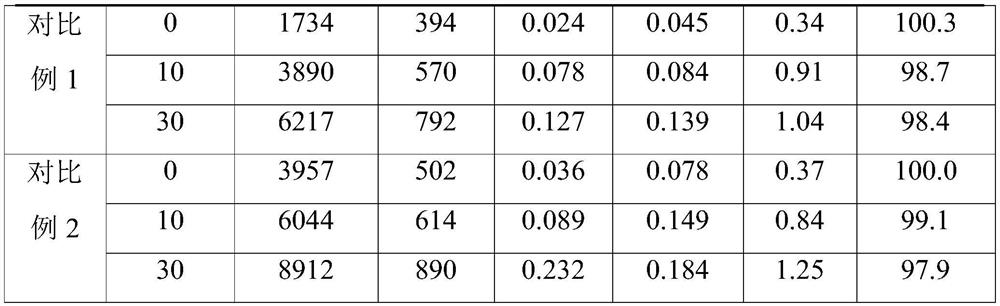
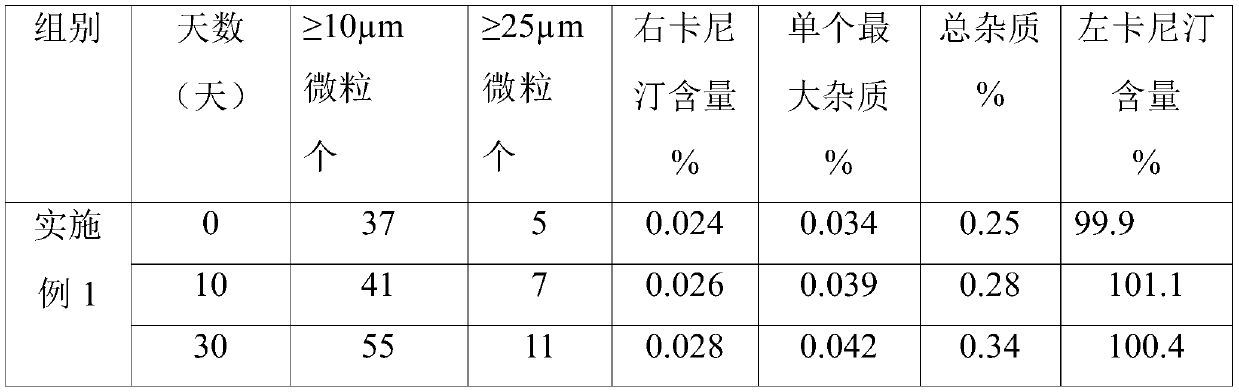
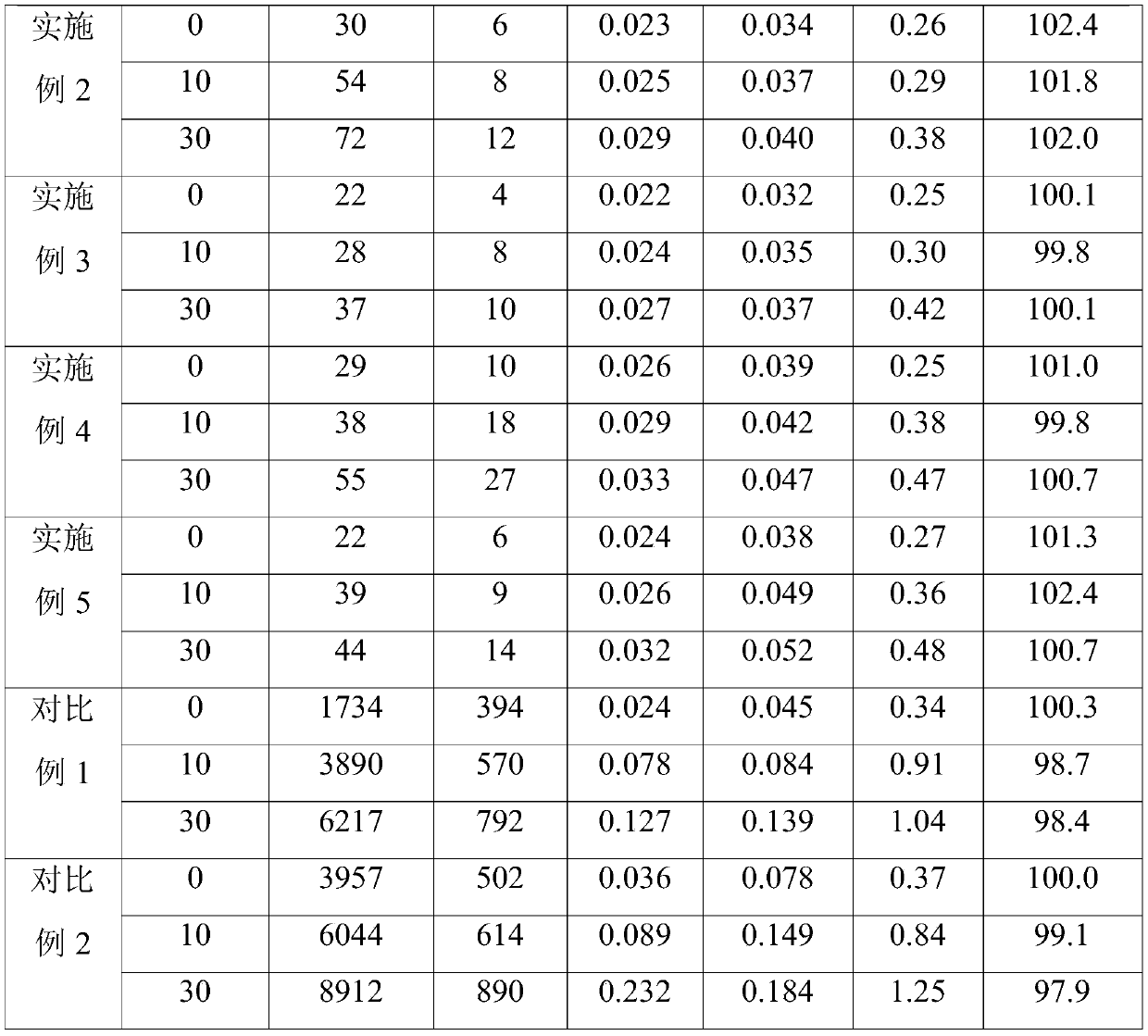
A kind of preparation method of L-carnitine injection preparation
ActiveCN109985011BSimple processSuitable for industrial productionOrganic active ingredientsPowder deliveryPhysical chemistryOrganic chemistry
Owner:哈尔滨誉衡制药有限公司 +1
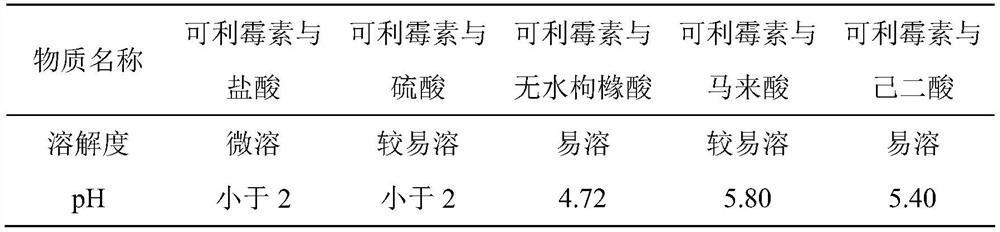
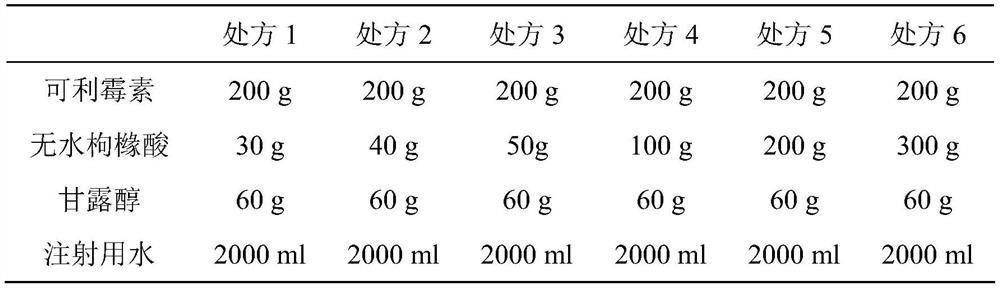
A kind of carrimycin freeze-dried powder preparation and preparation method thereof
ActiveCN111450066BEnhanced inhibitory effectImprove securityAntibacterial agentsOrganic active ingredientsO-Phosphoric AcidEthylic acid
The invention belongs to the technical field of medicine, and relates to a corimycin freeze-dried powder preparation and a preparation method thereof. The freeze-dried powder preparation is prepared by corimycin, an organic acid or an inorganic acid, a proppant and water for injection. to make. Wherein, every 1000ml of water for injection contains 40-115g of corimycin, 20-100g of organic acid or inorganic acid, and 15-30g of proppant. The weight ratio of carrimycin, organic acid or inorganic acid is: 1:1-5:1, preferably 2:1-4:1. Inorganic acid includes hydrochloric acid, sulfuric acid or phosphoric acid; described organic acid includes citric acid, anhydrous citric acid, maleic acid, adipic acid, acetic acid, methanesulfonic acid, ethanesulfonic acid, fumaric acid, tartaric acid, apple Acid, pyroglutamic acid, lactic acid, succinic acid or C1-C4 straight chain or branched chain alkane sulfonate unsubstituted or substituted by any position of 1-3 hydroxyl groups. The freeze-dried powder injection of the invention has the characteristics of short reconstitution time, few insoluble particles, easy preparation and relatively stable characteristics.
Owner:沈阳信达泰康医药科技有限公司
A kind of stephanine hydrochloride freeze-dried powder, preparation method and application thereof
ActiveCN104922081BImprove stabilityImprove bioavailabilityOrganic active ingredientsPowder deliveryFreeze-dryingSodium Chloride Injection
Owner:广州少伯控股集团有限公司
Freeze-dried metronidazole powder injection and preparation method thereof
InactiveCN106937944AShort reconstitution timeLess insoluble particlesAntibacterial agentsOrganic active ingredientsIonMetroNIDAZOLE Injection
The invention relates to a metronidazole freeze-dried powder preparation for injection and a preparation method thereof, which comprises metronidazole, glutamic acid, sorbitol and polyvinylpyrrolidone. It can effectively reduce nitrite and iron ions in metronidazole injection, avoid infusion pain, phlebitis, and side effects such as dizziness, chest tightness, and diarrhea caused by impurities such as nitrite. Dry powder injection has the advantages of short reconstitution time, less insoluble particles, easy reconstitution, and improved stability.
Owner:北京圣传创世科技发展有限公司
A kind of L-pantoprazole sodium freeze-dried powder preparation for injection
ActiveCN106474074BLess irritatingReduce the introductionPowder deliveryOrganic active ingredientsCyclodextrinBULK ACTIVE INGREDIENT
The invention relates to a sterile freeze-dried powder of S-pantoprazole sodium for injection, comprising active ingredient S-pantoprazole sodium, a stabilizer, and a freeze-drying protective agent; wherein the stabilizer is sulfobutyl-β One of ‑cyclodextrin, hydroxypropyl‑β‑cyclodextrin, glucosyl‑β‑cyclodextrin. The R-configuration isomer of the product of the present invention is less than 0.3% after 24 months of storage; the number of insoluble particles is significantly reduced through the optimization of the preparation process; the preparation process of the present invention is simple and easy, and is suitable for industrial scale production.
Owner:SHANDONG NEWTIME PHARMA
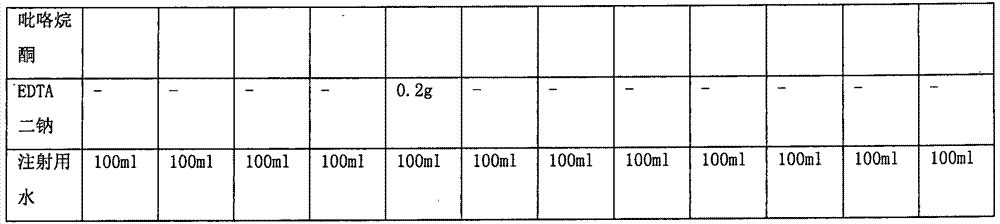
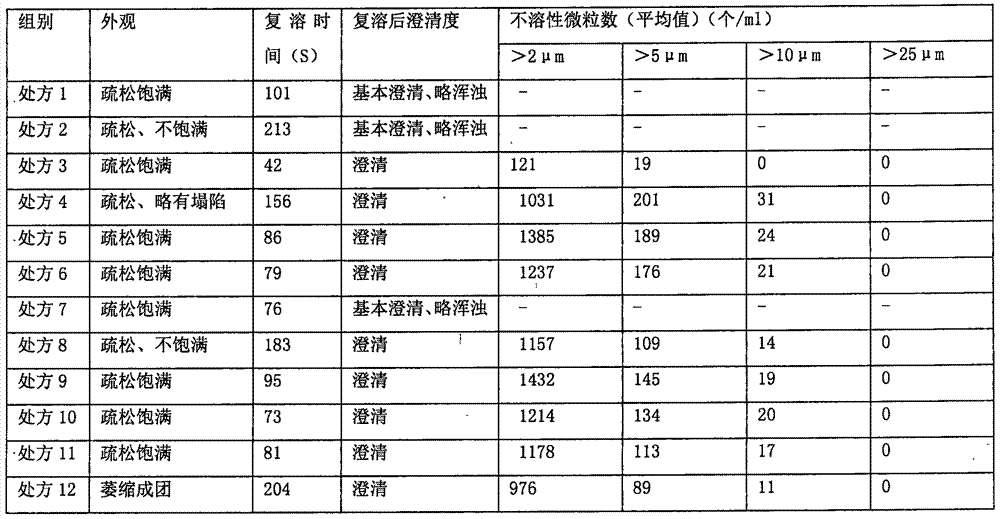
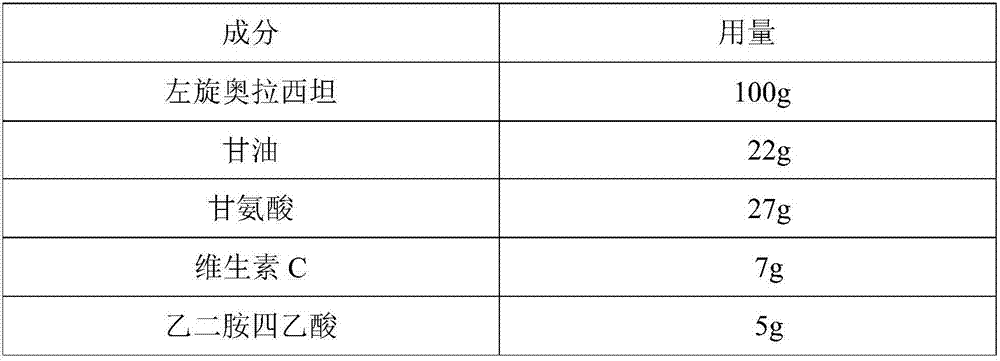
L-oxiracetam hydro-acupuncture for injection and preparation method thereof
InactiveCN107115276ANo changeNot easily oxidizedOrganic active ingredientsNervous disorderSolubilityAcetic acid
An L-oxiracetam hydro-acupuncture for injection is disclosed. According to the invention, 58-72% of L-oxiracetam, 8-22% of glycerin, 13-25% of glycine, 3-8% of vitamin C, 2-5% of ethylene diamine tetraacetic acid and 1-3% of benzyl alcohol which are used as raw materials and auxiliary materials undergo steps of concentrated mixing, diluted mixing, encapsulation, sterilization, inspection and packaging to prepare the L-oxiracetam hydro-acupuncture for injection. During the sterilization process of the L-oxiracetam hydro-acupuncture for injection, pH value of the solution is barely changed, the product is not easy to oxidize, and impurity increasing amount is only 0.02% during the sterilization process. Dissolvability of main drug of the product is good. Insoluble particles are lower than 25 microns. Stability is good. The period of validity is as long as 18 months and above. The product has less impurity during the period of validity, and the total impurity content is lower than 0.28%. Pains of patients are obviously reduced during the injection process, and patients' compliance is good. The product is worthy of market promotion.
Owner:CHONGQING RUNZE PHARM CO LTD
A kind of preparation method of chlorinated rubber
The invention discloses a preparation method of chlorinated rubber. The preparation method includes the steps that firstly, natural rubber is added into a reaction kettle containing reaction media and auxiliaries, stirring is performed for 10-20 min, the weight ratio of natural rubber to the reaction media and to the auxiliaries is 1: (10-15): (0.02-0.15), and the reaction media are a hydrochloric acid solution with the mass fraction being 10-15%; secondly, chlorine is added into the reaction kettle, a chlorination reaction is performed, a coarse chlorinated rubber product is obtained, and a chlorination reaction condition includes that firstly reaction is performed at 90-110 DEG C for 2-3 h, and then reaction is performed at 50-70 DEG C for 2-3 h; thirdly, the coarse chlorinated rubber product obtained in the second step is subjected to deacidifying treatment, and chlorinated rubber is obtained through drying. The chlorinated evenness degree is high, the chlorination degree is high, product transparency is high, and solubility is good.
Owner:LINYI AOXING CHEM CO LTD
Levocarnitine injection preparation and preparation method thereof
ActiveCN109953949ASimple processGuaranteed stabilityOrganic active ingredientsMetabolism disorderTechnical supportCombinatorial chemistry
Owner:HARBIN GLORIA PHARMA +1
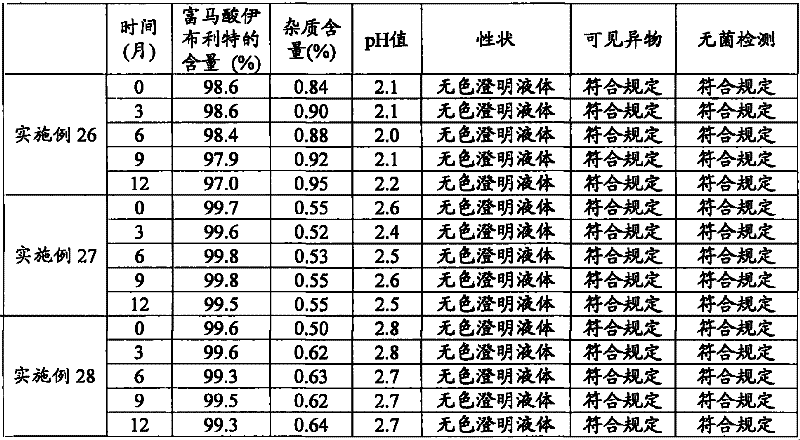
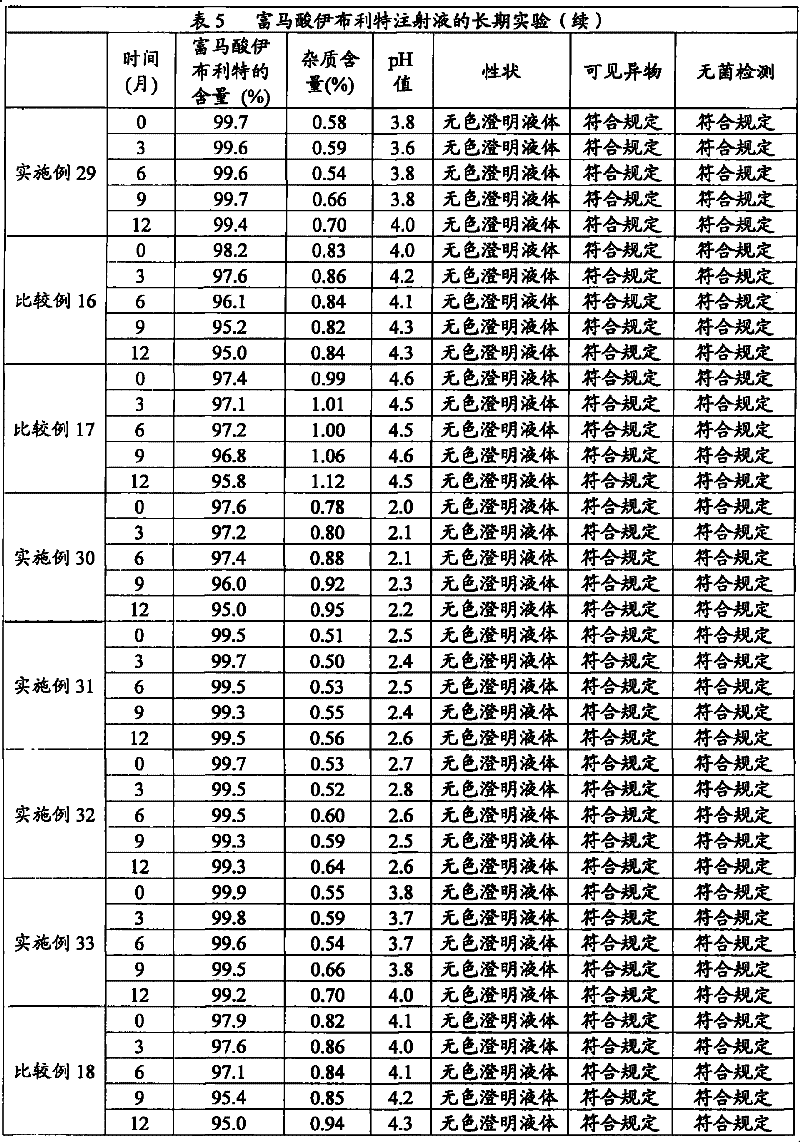
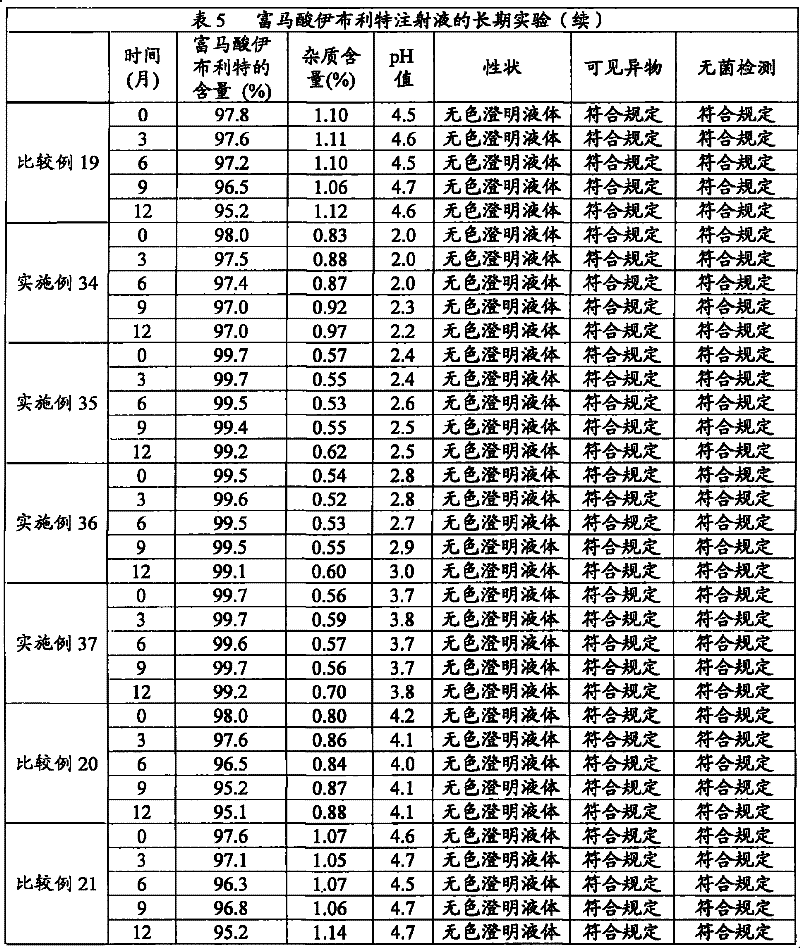
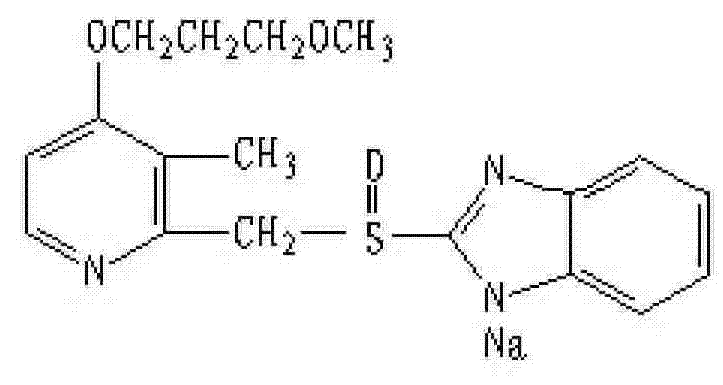
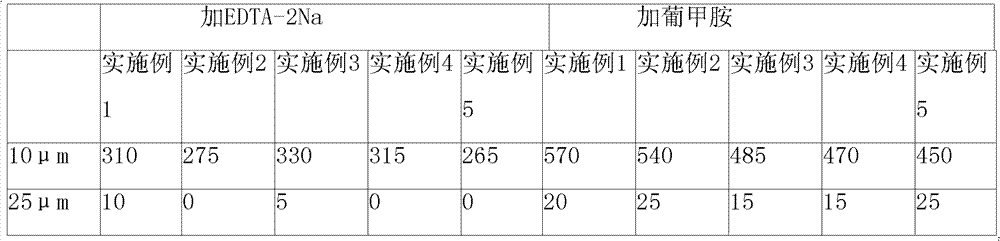
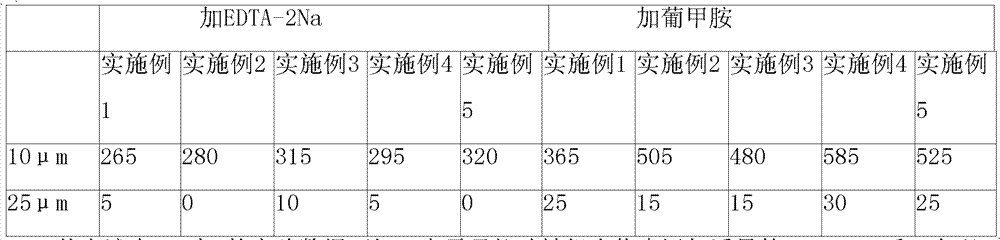
Composition containing ibutilide fumarate and preparation method and application thereof
ActiveCN101843607BImprove thermal stabilityLess impuritiesInorganic non-active ingredientsPharmaceutical delivery mechanismMedicineIbutilide Fumarate
The invention discloses a composition containing ibutilide fumarate and a preparation method and application thereof. The pH value of the composition containing ibutilide fumarate is smaller than 4.0. The preparation method comprises the step of adjusting the pH value of the composition to a set pH value by utilizing a pH regulating agent. The application refers to the application of the composition containing ibutililde fumarate in preparing antiarrhythmic drugs. The composition containing ibutilide fumarate has good thermal stability and has little change on the content of the ibutilide fumarate and reduction of impurities after sterilized.
Owner:JIANGSU JIUXU PHARMA
Rabeprazole sodium composition and preparation method thereof
ActiveCN101627996BLoose textureControllable texturePowder deliveryOrganic active ingredientsSolubilityMicroparticle
The invention provides a rabeprazole sodium composition which comprises the active ingredients of rabeprazole, mannitol and ethylene diamine tetraacetic acid, wherein the weight ratio of ethylene diamine tetraacetic acid to rabeprazole sodium is 0.05-0.5:1. The method for preparing the composition into solid powder-needle preparation comprises the following steps: dissolving the ethylene diamine tetraacetic acid solution with the prescription amount into injection water, adding and stirring the rabeprazole sodium with the prescription amount at a temperature of 25-35 DEG C for dissolving, then adding mannitol with the prescription amount, stirring the mannitol for dissolving, cooling to a room temperature, and regulating the pH value of the solution to 11.5-12.5; adding active carbon into the confected solution, stirring and filtering for decarburization, and freezing and drying the obtained filtrate to obtain the rabeprazole frozen powder needle. The rabeprazole frozen powder needle has full appearance, favorable stability and solubility and little insoluble grains in the injection.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Pharmacetutical for treating cardiovascular and cerebrovascular disease and its preparing process
InactiveCN100387236CGood curative effectLess insoluble particlesPowder deliveryOrganic active ingredientsDiseaseMedicine
The invention relates to a pharmaceutical composition for treating cardiovascular and cerebrovascular diseases and its preparing process, wherein the composition comprises breviscapine and Radix Astragali saponin with a purity of over 80% extracted from astragalus root, the content of the Radix Astragali saponin being 20-30%.
Owner:成都和康药业有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com