Muscular amino acid and nucleoside extract and pharmaceutical composition thereof
A technology of carnosine glycosides and extracts, which is applied in the field of medicine, and can solve problems such as easy oxidation, condensation, polymerization, instability of hydrolytic enzymes, and complicated process of adding hydrolytic enzymes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
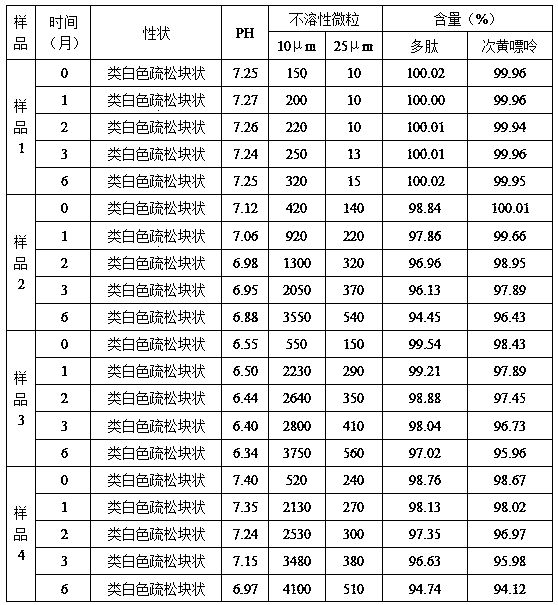
[0059] Example 1 Preparation of Carnosine Glycoside Extract
[0060] Take a total of 1000g of myocardium and muscle (weight ratio 1:1) of a healthy rabbit, remove the connective tissue, wash it with water for injection until it is bloodless, and grind it in a meat grinder. The weight ratio of the ground tissue and water for injection is 0.68 Mix at a ratio of :1, adjust the pH to 5.7 with acetic acid, homogenize with a high-speed tissue grinder, adjust the temperature of the homogenate to 24°C, and keep the temperature for 1 hour; freeze the homogenate at -30°C for 12 hours and then store it at 40°C Thaw and repeat this process 3 times; place the homogenate under an ultrasonic field with a power of 0.8KW and a frequency of 25KHz, add potassium chloride with a weight of 2.5% of the homogenate weight to the homogenate, keep the temperature at 20°C and start stirring. The stirring speed is 250 rev / min, and it is stirred under this ultrasonic field for 2 hours. After 2 hours, the ...
Embodiment 2
[0061] Example 2 Preparation of Carnosine Glycoside Extract
[0062] Take a total of 1000g of myocardium and muscle (weight ratio 1:1) of a healthy rabbit, remove the connective tissue, wash it with water for injection until it is bloodless, and grind it in a meat grinder. The weight ratio of the ground tissue and water for injection is 0.72 Mix at a ratio of :1, adjust the pH to 6.0 with acetic acid, homogenize with a high-speed tissue grinder, adjust the temperature of the homogenate to 27°C, and keep the temperature constant for 1 hour; freeze the homogenate at -32°C for 12 hours and then store it at 44°C Thaw and repeat this 3 times; place the homogenate under an ultrasonic field with a power of 0.8KW and a frequency of 25KHz, add potassium chloride with a weight of 2.5% of the homogenate weight to the homogenate, keep the temperature at 25°C and start stirring. Stirring speed is 250 rpm, stirring in this ultrasonic field for 2 hours, after 2 hours, place the homogenate in...
Embodiment 3
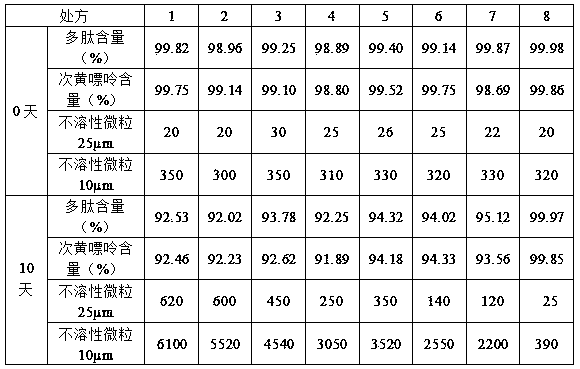
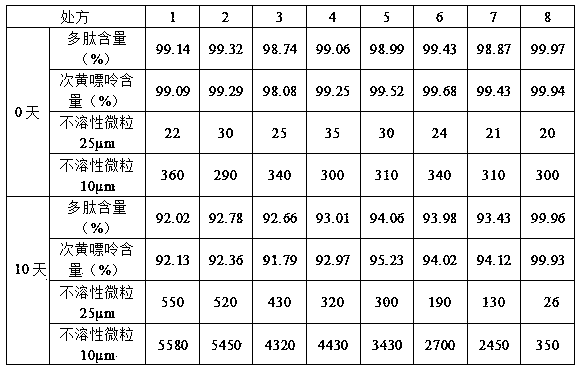
[0063] Example 3 Preparation of sarcosinoside pharmaceutical composition injection (5ml)
[0064] Take the carnosine glycoside extract prepared in Example 1, measure the content of polypeptide and hypoxanthine in the solution, adjust the concentration of the solution, add water for injection to 1000ml to make the concentration of polypeptide: 1.74mg / ml, and the concentration of hypoxanthine: 0.25mg / ml; add 1.5g of sorbitol, 0.2g of thioglycerol, and 1.0g of poloxamer 188 to the sarcosaminoglycan glycoside solution in turn, stir and dissolve at 32°C, filter with a 0.22μm filter membrane, subpackage after passing the test, and heat at 121°C Sterilize for 20 minutes to obtain the carnosine glycoside pharmaceutical composition injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com