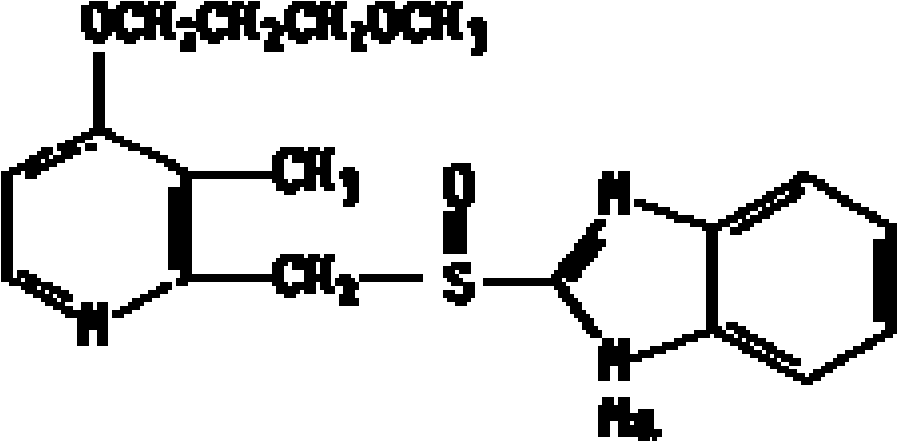
Rabeprazole sodium composite lyophilized injectable powder and preparation method thereof
A technology of rabeprazole sodium and freeze-dried powder injection, which is applied in the direction of freeze-dried delivery, drug combination, powder delivery, etc., and can solve the problem of many insoluble particles of freeze-dried powder injection, poor resolubility of freeze-dried powder injection, and complicated preparation process and other problems, to achieve the effect of less insoluble particles, good stability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Rabeprazole sodium composition prescription:
[0038]
[0039] Rabeprazole sodium composition is prepared into the preparation method of rabeprazole sodium powder injection:
[0040] Dissolve the rabeprazole sodium, meglumine, mannitol, sodium sulfite and disodium ethylenediaminetetraacetic acid in the prescribed amount first in water for injection, stir to make it dissolve, and adjust the pH of the solution to 11.5 with disodium hydrogen phosphate; Add 0.1% g / ml activated carbon to the solution prepared above, stir for 15 minutes, filter and decarbonize; cool the filtrate to -42°C, pre-freeze for 2 hours, vacuumize, heat up to 30°C at a rate of 5.5°C / h, and then After heat preservation for 1 hour, the freeze-dried powder of beprazole sodium is obtained.
Embodiment 2
[0042] Rabeprazole sodium composition prescription:
[0043]
[0044] Rabeprazole sodium composition is prepared into the preparation method of rabeprazole sodium powder injection:
[0045] Dissolve the prescribed amount of rabeprazole sodium, meglumine, mannitol, sodium sulfite, disodium ethylenediaminetetraacetic acid in water for injection, stir to dissolve, adjust the pH of the solution to 11.8 with sodium phosphate; Add 0.05% g / ml activated carbon to the solution, stir for 20 minutes, filter and decarbonize; cool the filtrate to -40°C, pre-freeze for 2 hours, vacuumize, heat up to 25°C at a speed of 5.8°C / h, and keep warm for 2 hours, the beprazole sodium freeze-dried powder was obtained.
Embodiment 3
[0047] Rabeprazole sodium composition prescription:
[0048]
[0049] Rabeprazole sodium composition is prepared into the preparation method of rabeprazole sodium powder injection:
[0050]First dissolve the prescribed amount of rabeprazole sodium, meglumine, mannitol, sodium sulfite, and disodium edetate in water for injection, stir to dissolve, and adjust the pH of the solution to 11.2; Add 0.5% g / ml activated carbon, stir for 15 minutes, filter and decarbonize; cool the filtrate to -45°C, pre-freeze for 1 hour, vacuumize, heat up to 27°C at a rate of 5.5°C / h, and keep it warm for 2 hours. The beprazole sodium freeze-dried powder was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com