A kind of high-purity mezlocillin sodium preparation and preparation method thereof
A technology for mezlocillin sodium and mezlocillin dry powder, which is applied in the directions of organic chemistry methods, active ingredients of heterocyclic compounds, organic chemistry, etc. Slow speed, product safety risks and other problems, to achieve the effect of good fluidity and dissolution speed, process optimization, and low insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This example provides a kind of preparation method of high-purity mezlocillin dry powder, the steps include the preparation of ampicillin sodium solution, the preparation of mezlocillin sodium condensation liquid, the preparation of mezlocillin dry powder three steps. Specifically:
[0033] 1-1) Preparation of ampicillin sodium solution.
[0034] According to the molar ratio of ampicillin and sodium hydroxide being 1:1 to 1:1.25, 20 g of the reaction raw materials ampicillin and 1.98 to 2.48 g of sodium hydroxide were weighed. Wherein, the purity of ampicillin is 98%, and the purity of sodium hydroxide is 99%. Sodium hydroxide is dissolved in water to prepare a sodium hydroxide solution with a mass concentration of 3.9-4.0%, which is ready for use.
[0035] Add 200g of water to the reaction kettle, dissolve the weighed ampicillin in the water, then add sodium hydroxide solution to the reaction kettle, control the adding speed of the sodium hydroxide solution, and carr...
Embodiment 2
[0049] This embodiment provides a kind of preparation method of high-purity mezlocillin sodium preparation, with the mezlocillin dry powder prepared in Example 1 as raw material, and then through the preparation of ampicillin sodium solution and the preparation of mezlocillin sodium condensation liquid 1. Three steps of preparing the mezlocillin sodium preparation to obtain the mezlocillin sodium preparation. Specifically:
[0050] 2-1) Preparation of mezlocillin sodium solution.
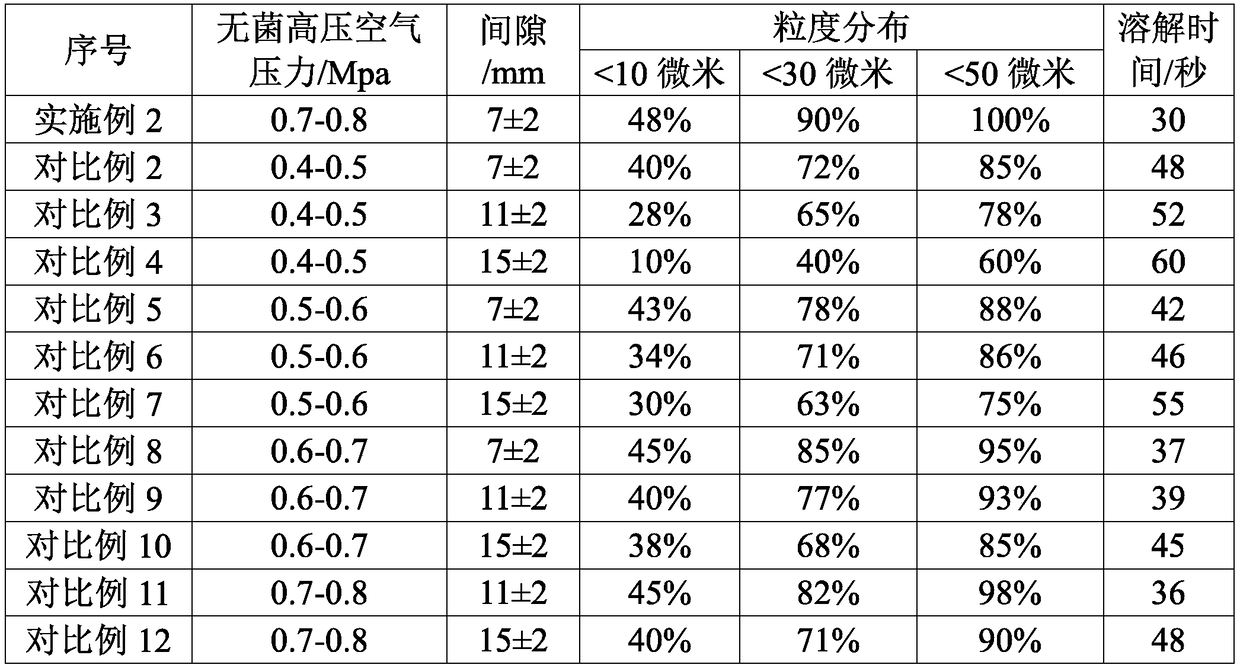
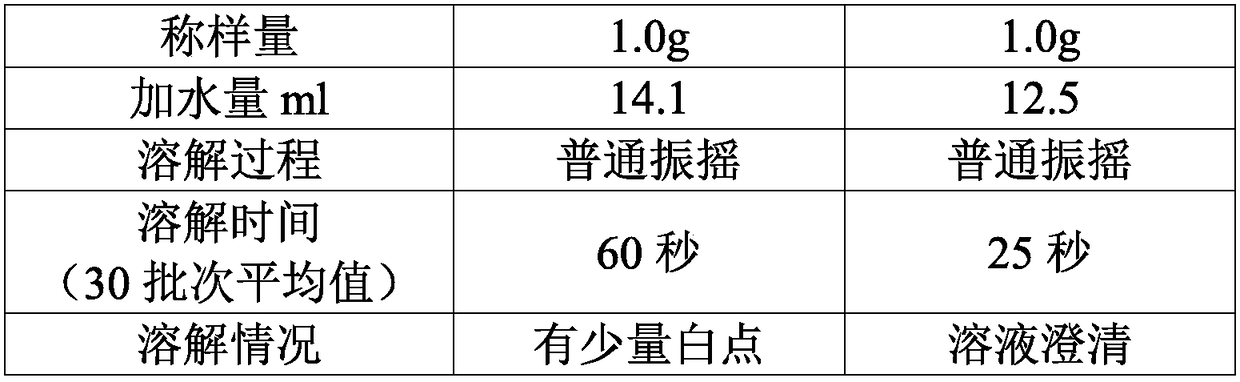
[0051] According to the mol ratio of mezlocillin and sodium bicarbonate is 1: 1~1: 2, take by weighing the mezzlocillin 100g that embodiment 1 makes, sodium bicarbonate 15g, sodium bicarbonate is dissolved in water, configures mass concentration 10% sodium bicarbonate solution, ready to use.
[0052] Slowly add the dry powder of mezlocillin into the sodium bicarbonate solution, control the pH at the end point of the feed solution to be 5.5-7.0, control the reaction temperature at 20-25°C, and cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com