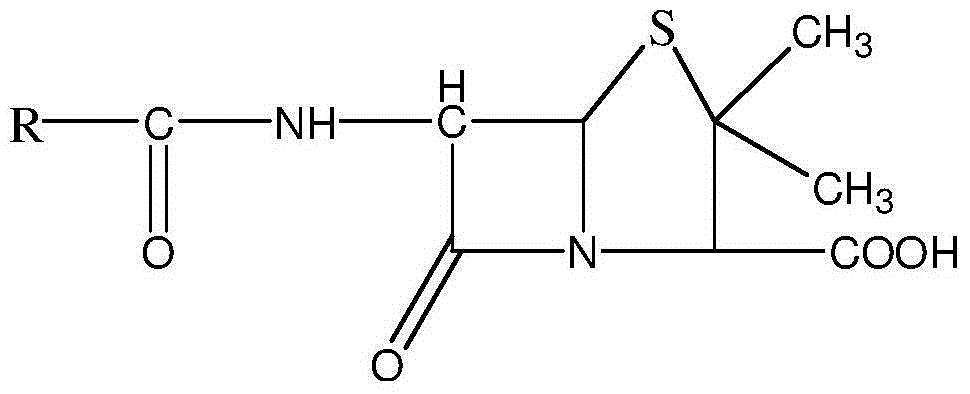
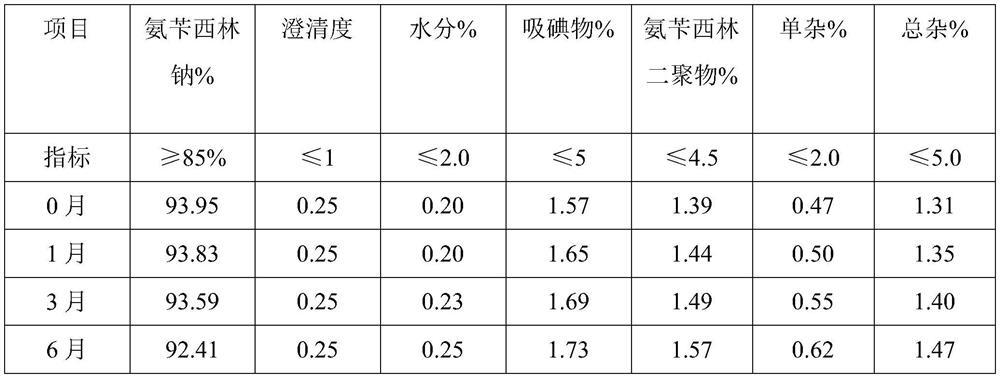
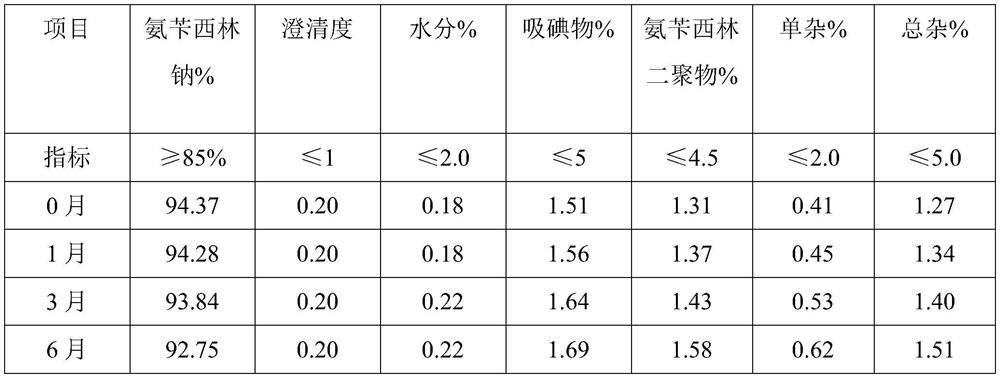
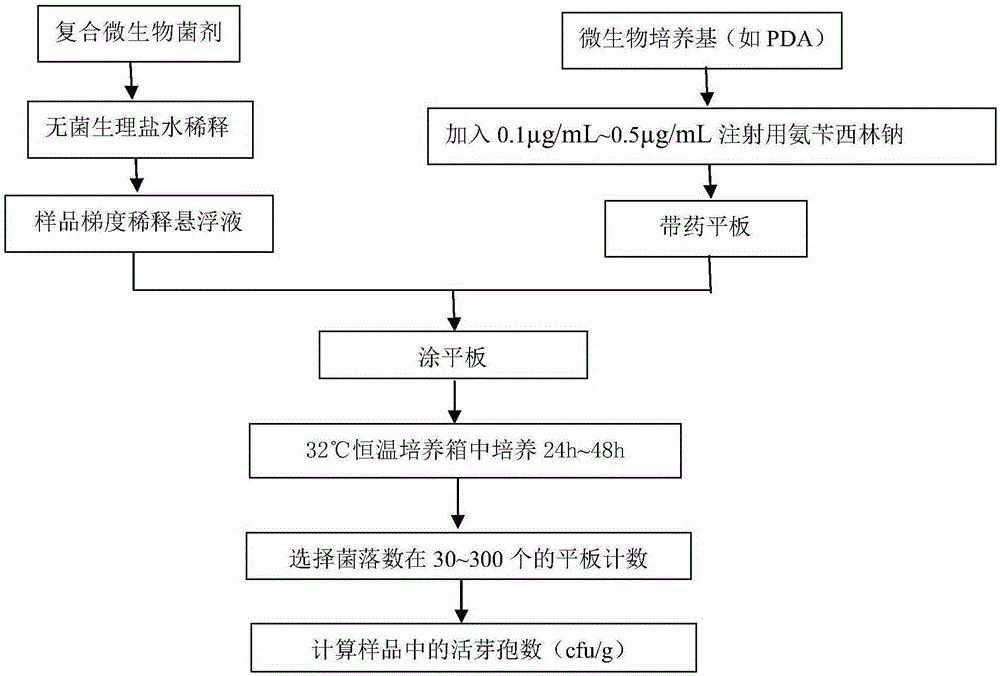
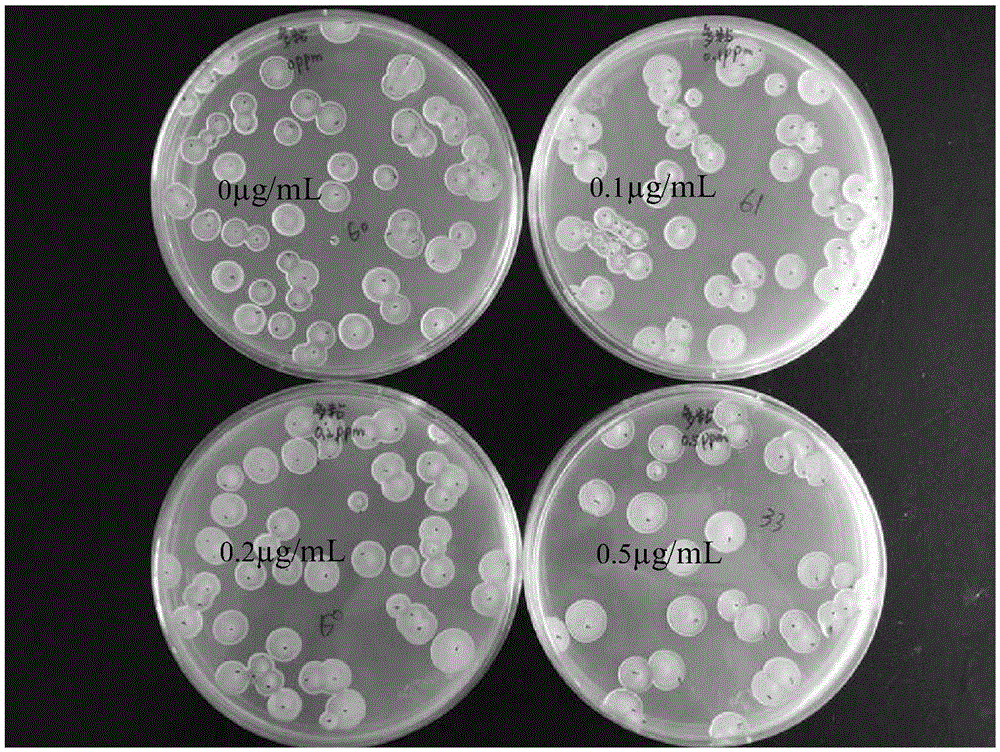
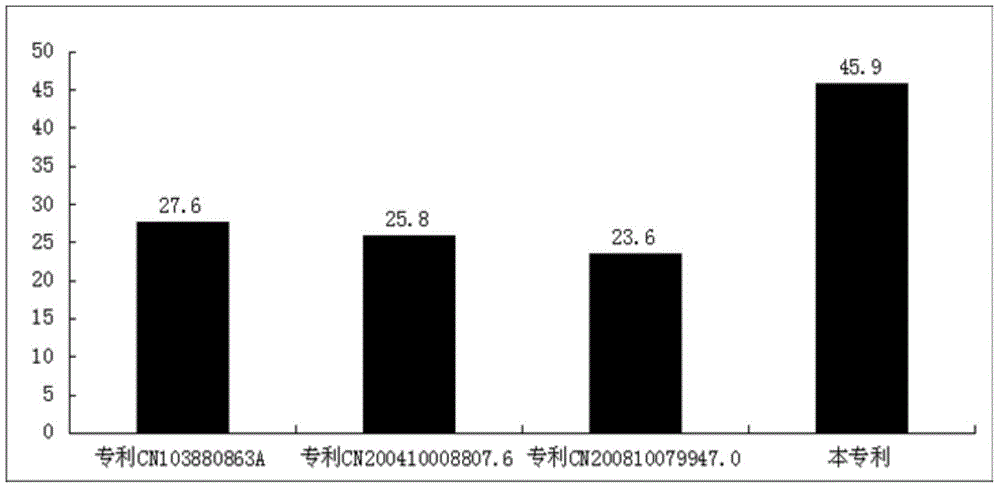
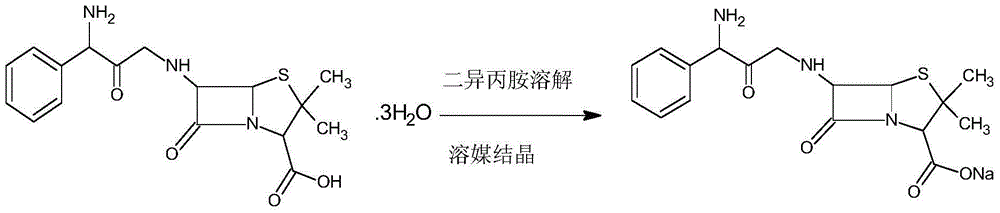
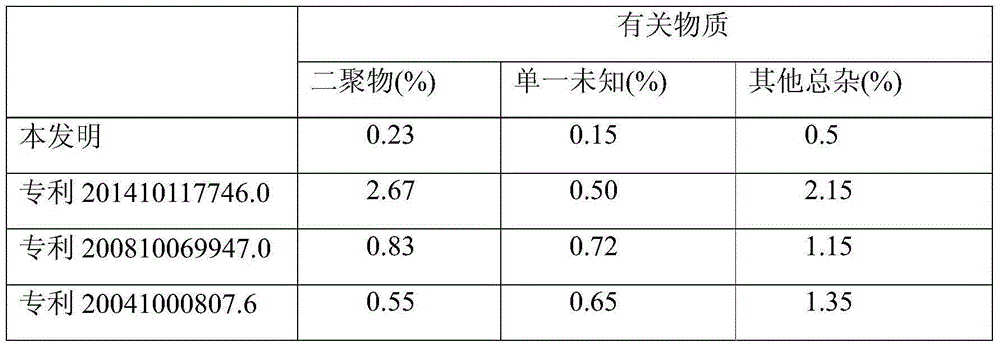
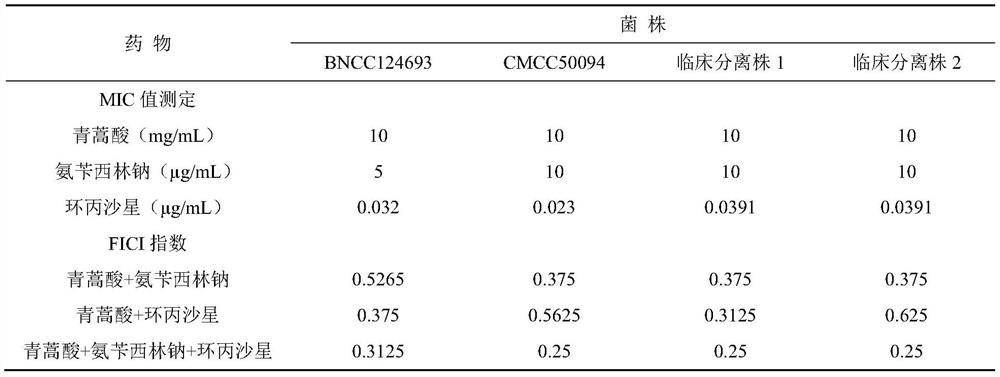
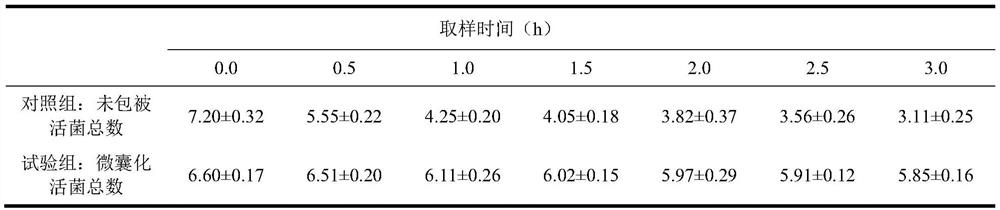
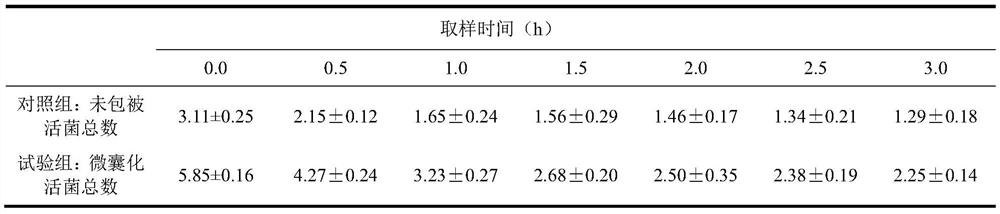
Patents
Literature
52 results about "Ampicillin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
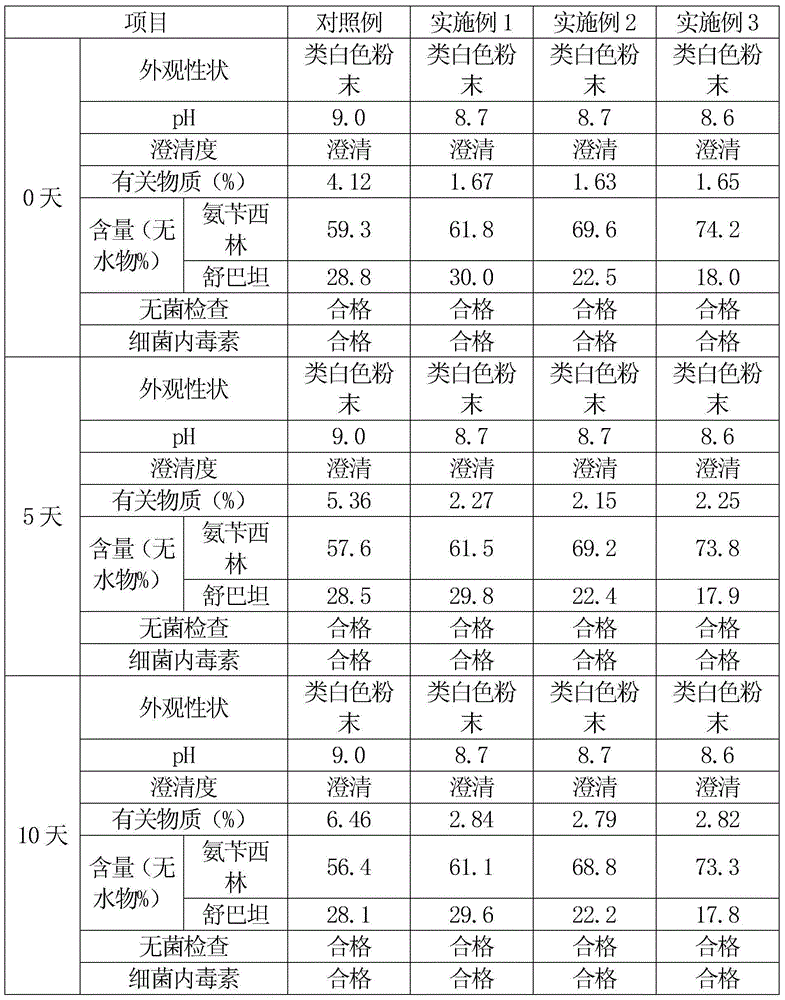
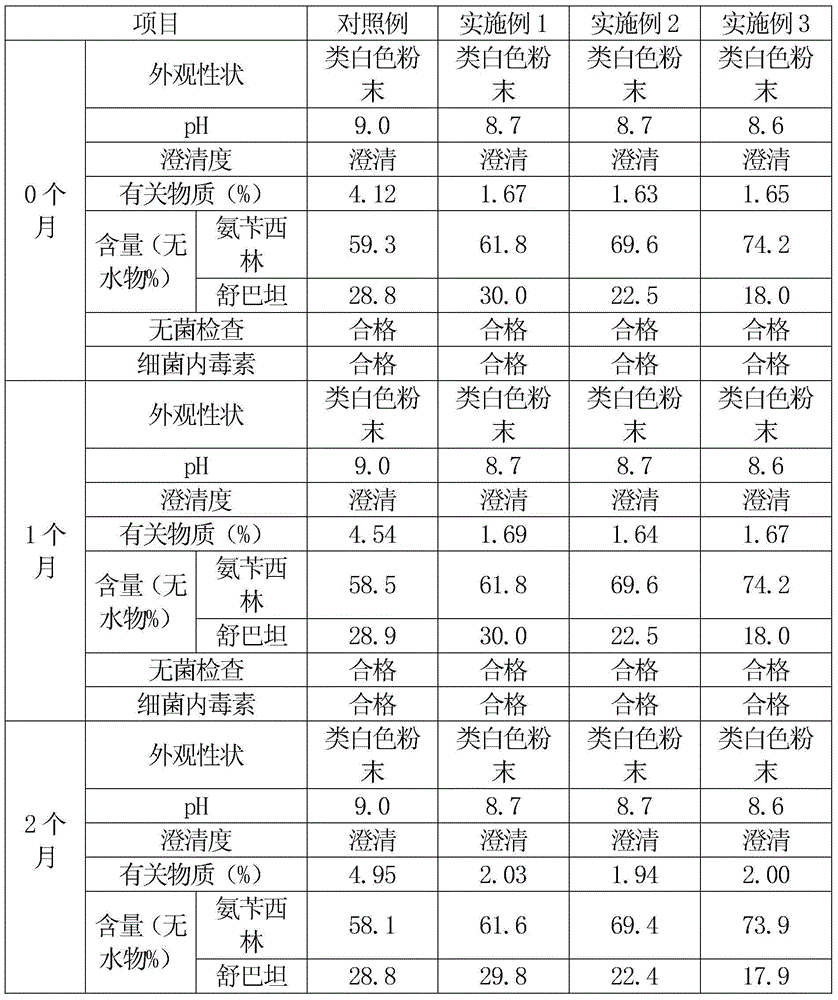
The sodium salt form of ampicillin, a broad-spectrum semisynthetic derivative of aminopenicillin. Ampicillin sodium inhibits bacterial cell wall synthesis by binding to penicillin binding proteins, thereby inhibiting peptidoglycan synthesis, a critical component of the bacterial cell wall.
Making process of Chinese cypress paper with wide-spectrum antiseptic function for cygarette
InactiveCN1417413ABroad spectrum antibacterialAvoid infectionPaper/cardboardPolyvinyl alcoholAntiseptic solutions
In the present invention, China cypress paper is painted with the ink and rolled, so that wide-spectrum antiseptic ink with inorganic antiseptic, organic antiseptic and Chinese medicinal antiseptic is painted on the surface to produce wide-spectrum antiseptic China cypress paper. The present invention features the ink, which consists of modified rosin, PVA, dibutyl phthalate, butyl aldehyde condensate, anatase type titania, medical alcohol, ferrite yellow calcium carbonate and talcum powder; has silver ion, copper ion, Ampicillin sodium and Eight Health Restoring Medicine mixture; and is produced through reaction, grinding, and dispersion.
Owner:YUNNAN YUXI TIPPING PAPER FACTORY +1
Kit for detecting beta-lactam antibiotic ligand in milk by receptor method and detection method thereof
ActiveCN101813698AShort detection timeHigh detection throughputDepsipeptidesTesting dairy productsPenicillin bindingDiluent
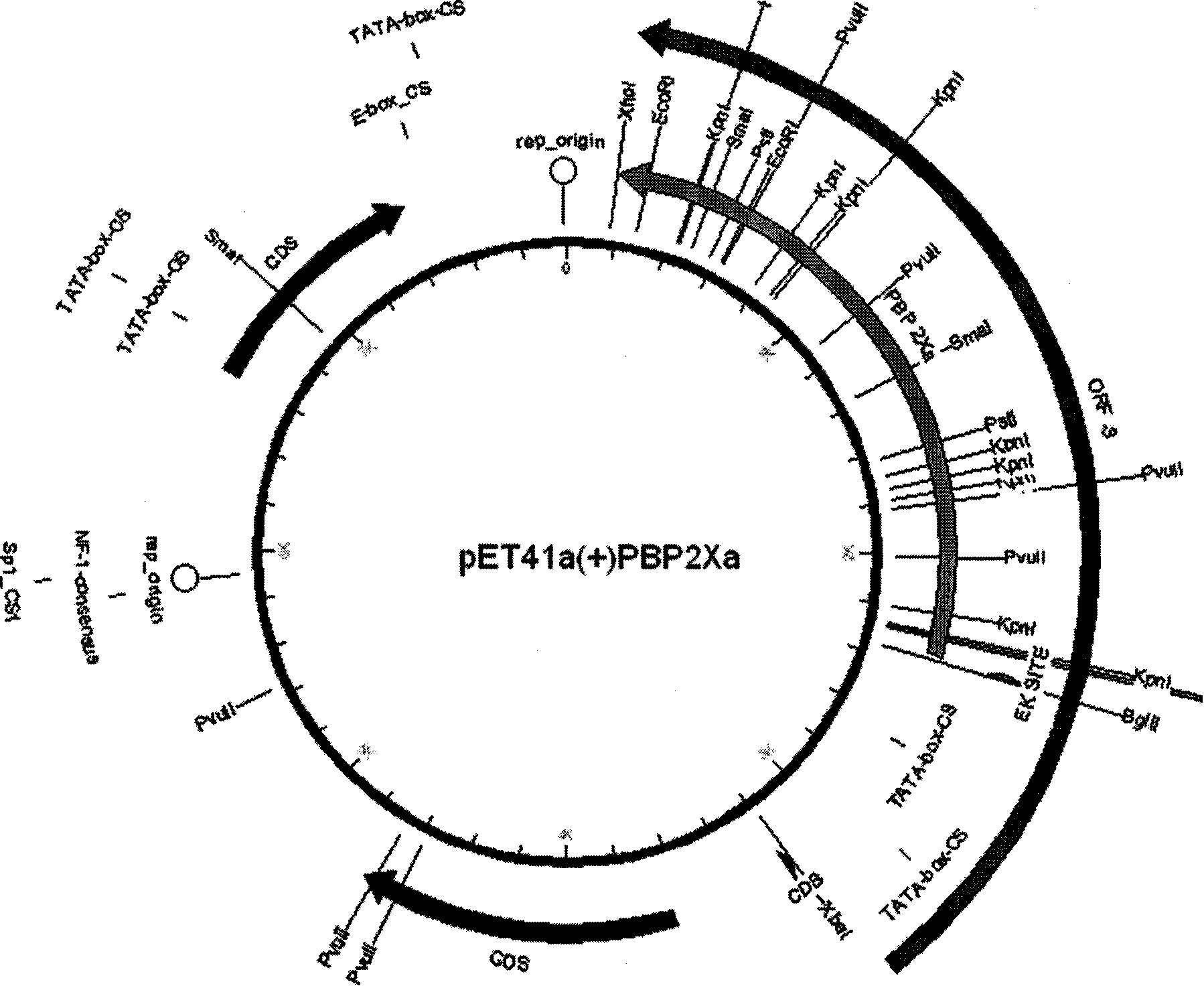
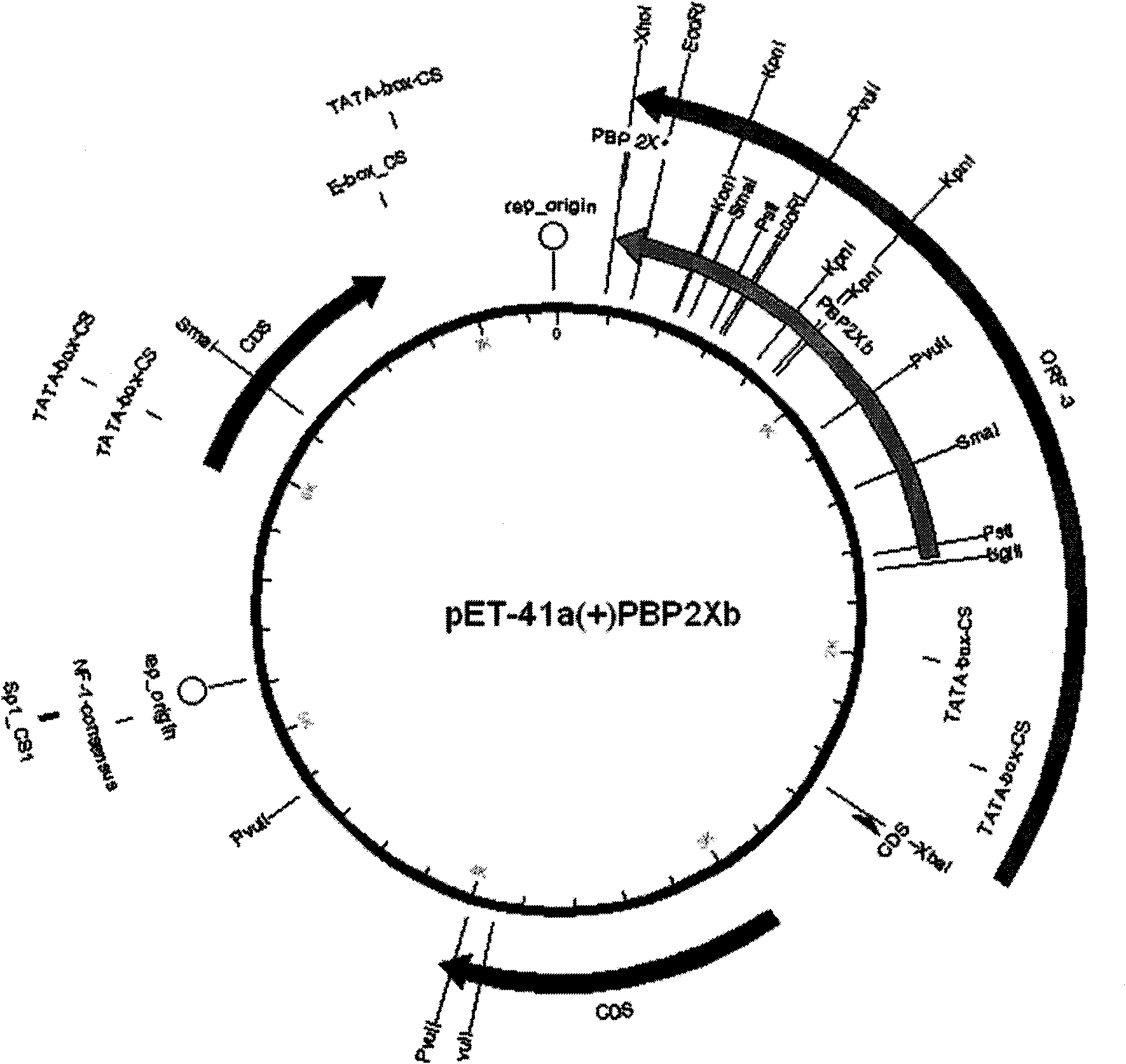
The invention discloses a kit for detecting beta-lactam antibiotic ligand in milk by a receptor method and a detection method thereof. The kit comprises the following components: (1) an enzyme-labeled plate which is coated by penicillin-binding protein PBP2xa or PBP2xb recombinant protein; (2) an enzyme-labeled marker; (3) standard solution of ampicillin sodium; (4) 20 times concentrated cleaning buffer solution; (5) enzyme-labeled diluent; (6) 20 times concentrated sample extract; (7) developing solution; and (8) reaction stop solution. The kit of the invention can simultaneously screen eight beta-lactam antibiotic residues in the milk; the detection limit is lower than the minimum residue limit of China and main developed countries in the world; the detection time is within 50 minutes; and the kit has the characteristics of simple, rapid and accurate operation, low cost and the like and is suitable for large-scale popularization and application.
Owner:上海溯源生物技术有限公司
Method for preparing ampicillin sodium
InactiveCN101486717AEasy to recycleEasy to produceAntibacterial agentsOrganic chemistryManufacturing cost reductionOrganic solvent
The invention discloses a preparation method of ampicillin sodium, which comprises the steps: (a) a 15%-50% sodium iso-octoate solution is prepared; (b) ampicillin amine slat is prepared: firstly, ampicillin is dissolved in an organic solvent to prepare an organic ampicillin solution which is then dehydrated till the moisture thereof is lower than 1% and then reacts with diisopropylamine; (c) the amine salt obtained from step (b) is pressed into a crystallizing tank and added with the sodium iso-octoate solution for two times, and after crystallization the growing of crystal is carried out; and (d) after the crystallization reaction is finished, separation, washing and menstruum recovering processes are carried out. The preparation method has simple processes and easy operation, is capable of further improving the quality of the finished ampicillin sodium products and being beneficial to the recovery of the menstruum in the technical system, and can effectively reduce the production costs simultaneously.
Owner:NORTH CHINA PHARMA COMPANY
Ampicillin sodium sulbactam sodium preparation for injection and preparation method thereof
InactiveCN104644629ARaise the reaction temperatureHigh reaction yieldAntibacterial agentsPowder deliveryAmpicillin Sodium/ Sulbactam SodiumAmpicillin Sodium
The invention discloses an ampicillin sodium sulbactam sodium preparation for injection and a preparation method thereof. The ampicillin sodium sulbactam sodium preparation for injection is formed by mixing high-purity ampicillin sodium crystals with high-purity sulbactam sodium uniformly, wherein the weight ratio of ampicillin sodium to sulbactam sodium is (2-4):1. The ampicillin sodium sulbactam sodium preparation for injection has the advantages of high purity, less impurity, high stability, difficult irritability and the like.
Owner:NORTH CHINA PHARMA COMPANY +2
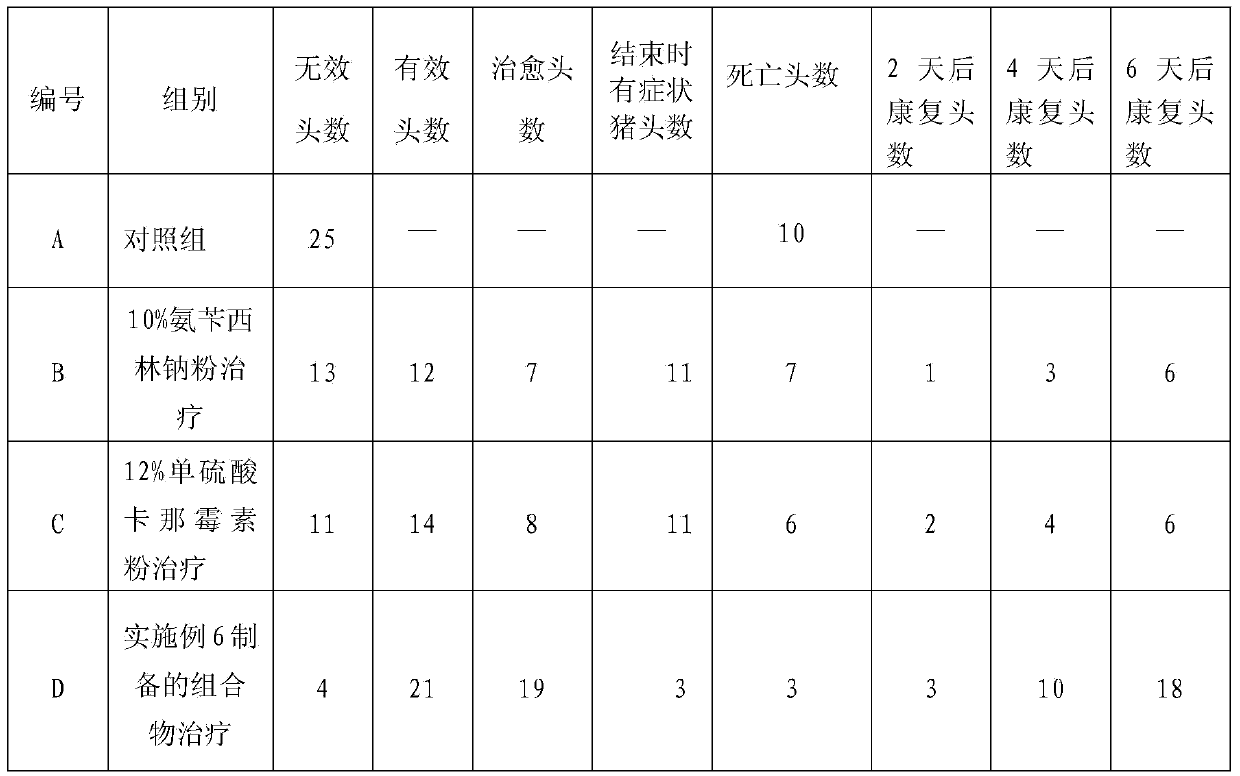
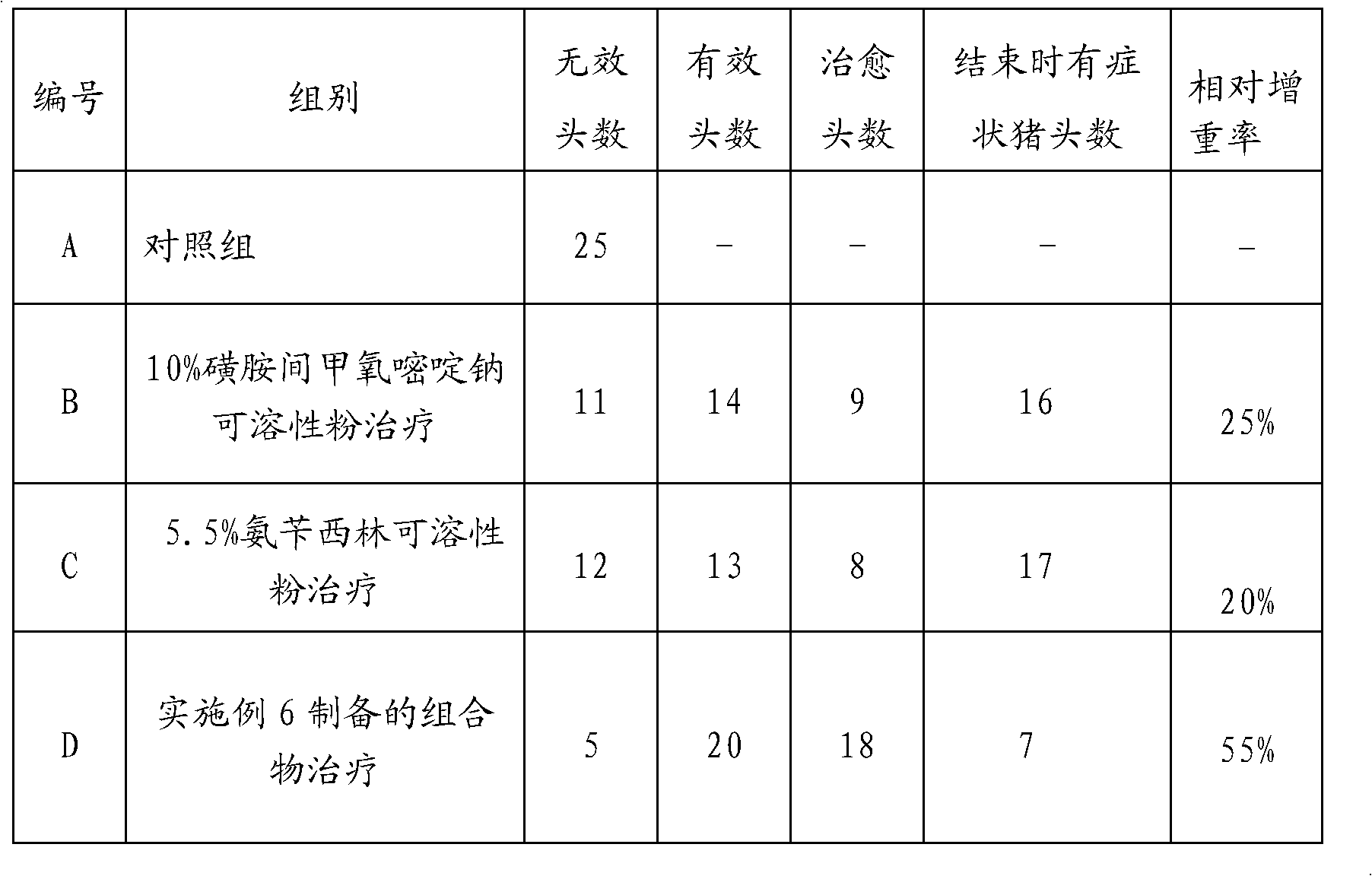
Composition for treating bacterial diarrhea of pigs and preparation method thereof
InactiveCN103417570AReduce chance of drug resistanceLittle side effectsAntibacterial agentsDigestive systemSodium bicarbonateSide effect
The invention discloses a composition for treating bacterial diarrhea of pigs and a preparation method of the composition. The composition comprises, by weight, 5-15% of ampicillin sodium, 5-20% of kanamycin sulfate, 5-15% of taurine, 5% of potassium chloride, 8% of sodium bicarbonate, 10% of sodium chloride and the balance sodium chloride. The composition for bacterial diarrhea reduces drug resistance of pathogens, takes effect rapidly, and is high in cure rate. Through reasonable matching, the composition combining the medicines has the obvious therapeutic effect on bacterial diarrhea of the pigs, can lower pathogenic drug resistance probability, reduces side effects of the medicines, treats both symptoms and root causes, and speeds up treatment.
Owner:TIANJIN SHENGJI GRP CO LTD
Method for preparing ampicillin sodium salt
ActiveCN101723957AOvercome the shortcomings of unstable product qualityHigh purityAntibacterial agentsOrganic chemistryOrganic solventSolvent
The invention discloses a method for preparing an ampicillin sodium salt. In the method, the general refrigerating and drying process has an additional post-treatment step of milling ampicillin sodium which is refrigerated and dried under vacuum into powder, filling the powder into a bipyramid, vacuumizing the bipyramid, heating the powder, keeping the temperature of the powder between 45 and 95 and the heat preservation time between 40 and 180 minutes and mixing the powder and separately packing the mixed powder. The method of the invention overcomes the drawback of instable quality of the products prepared by the traditional refrigerating and drying method and prepares high-purity, low-hygroscopicity and stable-quality ampicillin sodium raw material and preparation. The method is simple in process, avoids consuming a large amount of organic solvent like a solvent crystallization method and recovering the organic solvent and therefore has low cost.
Owner:华北制药集团先泰药业有限公司
Diluent for frozen cattle semens and preparation method of diluent
InactiveCN102948415AEasy to freezeImprove qualityDead animal preservationBiotechnologyAnimal science
The invention relates to the technical field of frozen cattle semen production and provides a diluent for frozen cattle semens. The diluent comprises a diluent A and a diluent B, wherein per 100ml of the diluent A comprises the following raw materials: 2.5-3.4g of trisodium citrate or sodium citrate, 0.1-0.2g of gentamycin, 0.03-0.1g of Ciprofloxacin, 50-100 million thousand units of ampicillin sodium, 50-100 million thousand units of streptomycin, 1-2g of levulose, 10-15ml of yolk and the balance of distilled water; and the diluent B is prepared by adding 12-16ml of glycerol or glycol into the 84-88 percent by volume of the diluent A used as a base solution. The preparation method mainly comprises the following steps of: weighting various raw materials and the distilled water for later use; preparing the base solution; preparing the diluent A; and preparing the diluent B. The diluent can be used for effectivlely reducing the bacterium content of the frozen cattle semens, is good in antibiotic effect, and can be used for greatly improving the frozen cattle sement production efficiency and the frozen cattle semen quality; and the preparation method is simple to operate and is economical and practical.
Owner:XINJIANG TIANSHAN ANIMAL HUSBANDRY BIO ENG
Soluble powder for treating infection of domestic bird caused by sensitive bacteria
InactiveCN101467998APromote oral absorptionImprove securityAntibacterial agentsPowder deliveryPerihepatitisEscherichia coli
The invention relates to soluble powder for treating sensitive bacterial infections of poultry, comprising sodium ampicillin, sulbactam sodium and auxiliary materials. As an antibiotic medicine, the inventive medicament is mainly used to treat body infectious diseases such as perihepatitis, pericarditis, salpingitis, vitelline peritonitis, air sacculitis, enteritis, diarrhea and the like caused by sensitive bacteria such as escherichia coli, salmonella and the like. The sodium ampicillin and sulbactam sodium have compatibility when used together. The soluble powder is used to treat body infectious diseases caused by sensitive bacteria, has good absorption and relatively high safety when orally taken, and is suitable for being popularized in animal husbandry.
Owner:TIANJIN RINGPU BIO TECH
Compound medicament for treating proventriculitis and gizzard erosion of poultry and preparation method thereof
ActiveCN103394021AGuaranteed normal needsQuick cureHydroxy compound active ingredientsDigestive systemSnow moldTherapeutic effect
A disclosed compound medicament for treating proventriculitis and gizzard erosion of poultry comprises the following components in parts by weight: 5-20 parts of terbinafine hydrochloride, 10-40 parts of ampicillin sodium, 50-150 parts of amomum villosum, 50-100 parts of poria cocos, 5-15 parts of vitamin A and 10-20 parts of methionine. By aiming at the symptoms of proventriculitis and gizzard erosion of poultry and inducing factors, the medicines used for inhibiting fungus and mould, the traditional Chinese medicines capable of controlling gastric acid secretion and other medicines are selected for the medicament of the invention, and the formula is reasonable; and the medicament has the characteristics of production simplicity, low price, convenient production and the like.
Owner:CHENGDU CENTURY INVESTMENT
Modeling method of non-rodent animal model for studying intestinal flora
InactiveCN107898796AShorten the timeReduce mortalityMicrobiological testing/measurementSaccharide peptide ingredientsKanamycinMortality rate
The invention discloses a modeling method of a non-rodent animal model for studying intestinal flora. A male rhesus monkey of 12-months old is taken as a laboratory animal and fed with mixed antibiotic for 21 days to clear symbiotic intestinal flora in the body. The antibiotic includes neomycin, streptomycin, ampicillin sodium, kanamycin, metronidazole and vancomycin; different antibiotic formulasare adopted for the first 10 days and later 11 days of feeding. The modeling result is verified by combining a Realtime-PCR method and an amplicon sequencing method. The method disclosed by the invention has the advantages of simplicity and easiness in implementation, short time, high repeatability, low death rate and stable model. The method has an important application value in the treatment offlora-related metabolic diseases, inflammatory bowel diseases and infectious diseases, evaluation of intestinal flora in the immune system, development of new medicines and the like.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
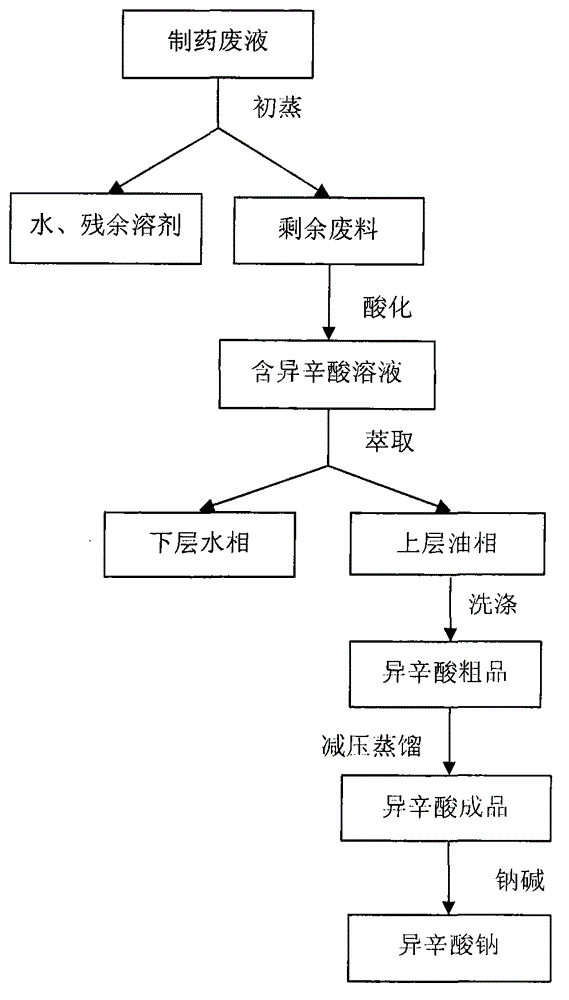
Process for recovering sodium isooctoate from pharmaceutical waste liquor
InactiveCN104672079ALow costEasy to operatePreparation from carboxylic acid saltsOrganic compound preparationDistillationOil phase
The invention discloses a process for recovering isooctanoic acid from pharmaceutical waste liquor and transforming isooctanoic acid into sodium isooctoate. The process comprises the following steps of: removing water and residual organic solvents which are contained in the waste liquor through preliminary distillation, then adding acid for acidification to transform isooctoate contained in a waste material into isooctanoic acid, adding an extracting agent to split the phase of the acidified waste material, and repeatedly washing an oil phase rich in the isooctanoic acid by using distilled water to obtain a crude isooctanoic acid product; then carrying out reduced pressure distillation to obtain a finished isooctanoic acid product; finally reacting the obtained finished isooctanoic acid product with sodium alkali to generate sodium isooctoate. The process disclosed by the invention can be used for effectively recovering isooctanoic acid contained in antibiotic production waste liquor, namely ampicillin sodium, amoxicillin sodium, cephalosporin sodium salt and the like which are obtained through a solvent crystallization method, and transforming isooctanoic acid into salt-forming agent sodium isooctoate capable of being recycled.
Owner:HEBEI UNIV OF TECH
Levofloxacin complex preparation and preparation method thereof
InactiveCN102961381ADelay drug resistanceExpanded antimicrobial spectrumAntibacterial agentsHeterocyclic compound active ingredientsEscherichia coliTherapeutic effect
The invention relates to a levofloxacin complex preparation. The levofloxacin complex preparation is characterized by comprising 10-20 parts of levofloxacin and auxiliary ingredients, and the auxiliary ingredients comprise 5-10 parts of ampicillin sodium, 3-5 parts of gentamicin sulphate or 3-5 parts of amoxicillin. According to the levofloxacin complex preparation, the levofloxacin and auxiliary ingredients are organically combined together, all components are coordinated and effected, the drug resistance of bacteria to the levofloxacin can be reduced, the antibacterial spectrum of the levofloxacin can be extended, the treatment effect can be improved, the clinical trials show that the levofloxacin complex preparation has stronger killing action on escherichia coli, salmonella, staphylococcus aureus, streptococcus pneumoniae, penicillin-resistant strain and the like, thereby being a preparation with high-efficiency and broad-spectrum antibacterial functions.
Owner:DINGZHENG ANIMAL PHARMA TIANJIN
Preparation method of ampicillin sodium for injection
ActiveCN114796131ALower the eutectic point temperatureReduce generationAntibacterial agentsPowder deliveryPhysical chemistryMesoporous silica
The preparation method comprises the following steps: S1, dissolving an ampicillin sodium crude product in deionized water, and adding a proper amount of alkali liquor to adjust the pH value of the solution to 6.7-6.9; s2, adding a magnetic nanotube into the alkalized solution, and stirring and adsorbing in a vacuum environment; s3, separating the magnetic nanotubes by using a magnetic field, and dispersing the magnetic nanotubes into deionized water for oscillation desorption; wherein the magnetic nanotube comprises a multi-layer structure, and the multi-layer structure sequentially comprises a compact silicon dioxide nanotube layer, a Fe3O4 layer and a mesoporous silicon dioxide layer from inside to outside; and S4, merging and crystallizing the ampicillin sodium solution obtained by oscillation desorption to obtain refined ampicillin sodium. The ampicillin sodium molecules are reversibly adsorbed by adopting the special structure of the magnetic nanotube in a weak acid environment, and the inner layer of the silicon dioxide nanotube and the outer layer of the mesoporous silicon dioxide layer can be subjected to physical adsorption reaction with the ampicillin sodium molecules through hydrogen bonds, surface atomic coordination and the like.
Owner:SICHUAN PHARMA
Method of quickly measuring content of Paenibacillus polymyxa in compound microbial agent
ActiveCN105200116AGrowth inhibitionAccurate measurementMicrobiological testing/measurementMicroorganism based processesMicroorganismMicrobial agent
The invention discloses a method of quickly measuring content of Paenibacillus polymyxa in a compound microbial agent. Active ingredients of the compound microbial agent include Bacillus subtilis, Paenibacillus polymyxa and Bacillus mucilaginosus. Ampicillin sodium of certain concentration is added into a culture medium to inhibit the growth of Bacillus subtilis but not inhibit the growth of Paenibacillus polymyxa, thereby avoiding the influence of quickly grown Bacillus subtilis upon statistic data of Paenibacillus polymyxa. The method allows the content of Paenibacillus polymyxa in the compound microbial agent to be measured quickly, simply and accurately.
Owner:WUHAN KERNEL BIO-TECH CO LTD
Method for preparing ampicillin sodium by menstruum crystallization method
ActiveCN104151324AControl the speed of crystallizationControl the crystallization rateOrganic chemistryDiisopropylamineDissolution reaction
The invention discloses a method for preparing ampicillin sodium by a menstruum crystallization method. The method comprises the following steps: firstly, dissolving sodium 2-ethylhexanoate into methanol, controlling the temperature to 15 DEG C, adding ampicillin acid into dichloromethane, and dripping diisopropylamine for a dissolution reaction; adding a sodium 2-ethylhexanoate solution into a crystallization tank, then adding a dichloromethane liquation agent in a flowing manner, and adding a seed crystal for growing crystals in a standing manner; then adding the dichloromethane liquation agent for crystallization; controlling the crystallization temperature to 20-25 DEG C; controlling the flowing acceleration of the liquation agent to 100 ml / h; after the crystallization reaction is completed, discharging materials, filtering the discharged materials, and drying the filtered materials to obtain the ampicillin sodium. According to the method, the methanol solvent, in which the ampicillin sodium is easy to dissolve, is selected for use, and then the dichloromethane solvent is used for dissolving out products, so that the crystallization speed of the product can be controlled; in addition, measures of adding the seed crystal for growing the crystal, controlling the dissolving temperature and the crystallization temperature, controlling the flowing acceleration of the liquation agent and the like are taken, so that the crystallization speed can be controlled, the crystal nucleus of the product are enlarged and uniform, the product purity is high, the dissolving residue is low, and the quality is stable.
Owner:山东安信制药有限公司
Medicament for treating swine high fever
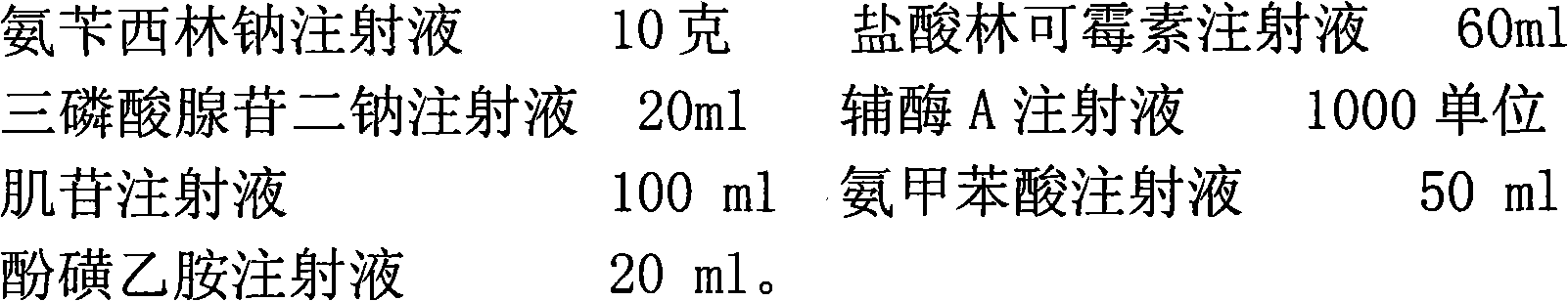
InactiveCN102048749AAlleviate the problem of slow growth processEffective treatmentAntibacterial agentsPeptide/protein ingredientsIntestinal structureCoenzyme A biosynthesis
The invention discloses a medicament for treating swine high fever. The medicament provided by the invention comprises a first group of active ingredients based on the following mixture ratio: 10g of ampicillin sodium injection, 60ml of lincomycin hydrochloride injection, 20ml of adenosine disodium triphosphate injection, 1000 units of coenzyme A injection, 100ml of inosine injection, 50ml of aminomethylbenzoic acid injection and 20ml of etamsylate injection; and the medicament has the advantage of reasonably and scientifically mediating and treating high heat and high fever generated by soft tissues injected with various bacteria and viruses which can cause high fever mainly, multiple symptoms that the skin becomes dry, red, purple and the like, fiber type bleeding of liver, kidney, spleen, stomach, intestine and the like and lymph node atrophy saliva.
Owner:胡友忠
Novel ampicillin sodium used for injection
InactiveCN107929260ASimple preparation processLow costAntibacterial agentsPharmaceutical non-active ingredientsLactideAdditive ingredient
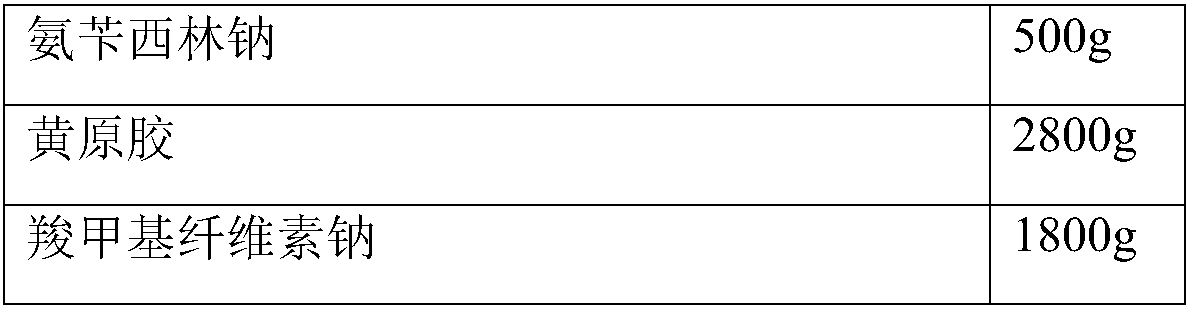
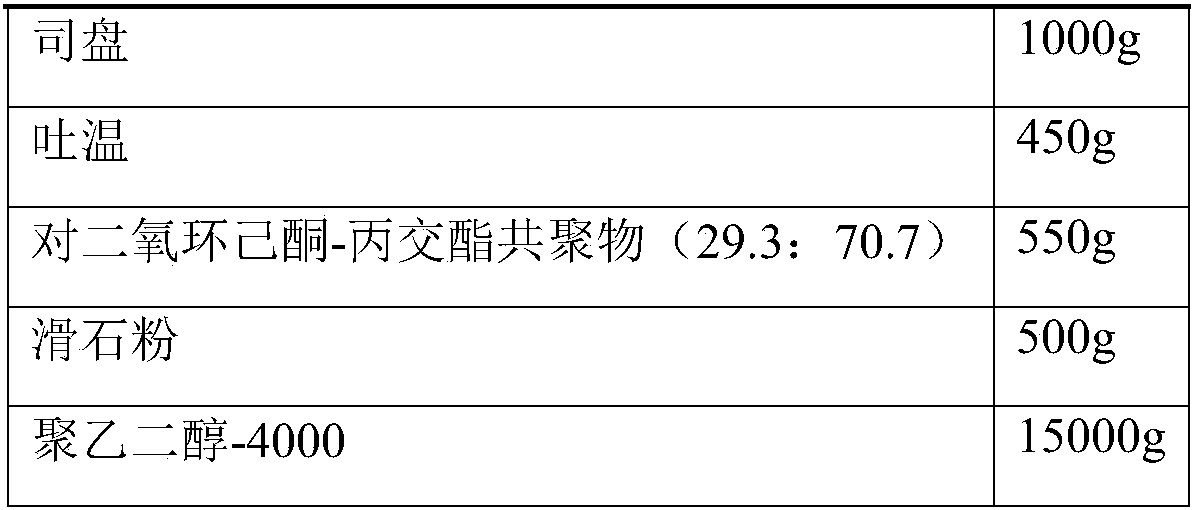
The invention relates to novel ampicillin sodium used for injection and a preparation method thereof, and belongs to the technical field of medicines. The novel ampicillin sodium used for injection comprises the following ingredients in parts by weight: 1 part of ampicillin sodium, 5.5-6 parts of xanthan gum, 3.5-3.7 parts of sodium carboxymethylcellulose, 2.8-2.9 parts of an emulsifying agent, 0.8-1 part of an anti-sticking agent, 1.1-1.2 parts of a stabilizing agent and 30-35 parts of a solvent, wherein the stabilizing agent dioxy-cyclohexanone-lactide copolymer is a copolymer with a specific ratio and has a crucial effect on the stability of the preparation. Through a slow-release effect of a micro-capsule, the injection preparation is administrated once instead of twice to four times every day, and the dosing interval is shortened, so that the injection preparation is convenient in use; the bioavailability is improved, and the dosage is reduced, so that the blood concentration is stable, the curative effect is improved, and the adverse reaction is reduced. The ampicillin sodium used for injection provided by the invention is simple in preparation process and low in cost, so that the ampicillin sodium is suitable for large-scale production and is a product having a good market prospect.
Owner:石药集团中诺药业(石家庄)有限公司
Making process of Chinese cypress paper with wide-spectrum antiseptic function for cigarette
InactiveCN1236141CSimple methodImprove applicabilityPaper/cardboardPolyvinyl alcoholAntiseptic solutions
In the present invention, China cypress paper is painted with the ink and rolled, so that wide-spectrum antiseptic ink with inorganic antiseptic, organic antiseptic and Chinese medicinal antiseptic is painted on the surface to produce wide-spectrum antiseptic China cypress paper. The present invention features the ink, which consists of modified rosin, PVA, dibutyl phthalate, butyl aldehyde condensate, anatase type titania, medical alcohol, ferrite yellow calcium carbonate and talcum powder; has silver ion, copper ion, Ampicillin sodium and Eight Health Restoring Medicine mixture; and is produced through reaction, grinding, and dispersion.
Owner:YUNNAN YUXI TIPPING PAPER FACTORY +1
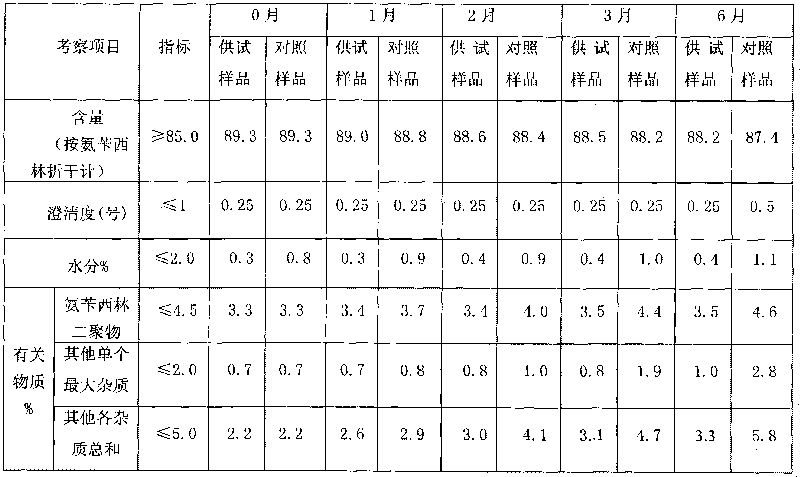
Ampicillin sodium and sulbactam sodium pharmaceutical composition
ActiveCN105520942AEasy to degradeHigh stability of clarityAntibacterial agentsPowder deliveryAmpicillin SodiumSulbactam Sodium
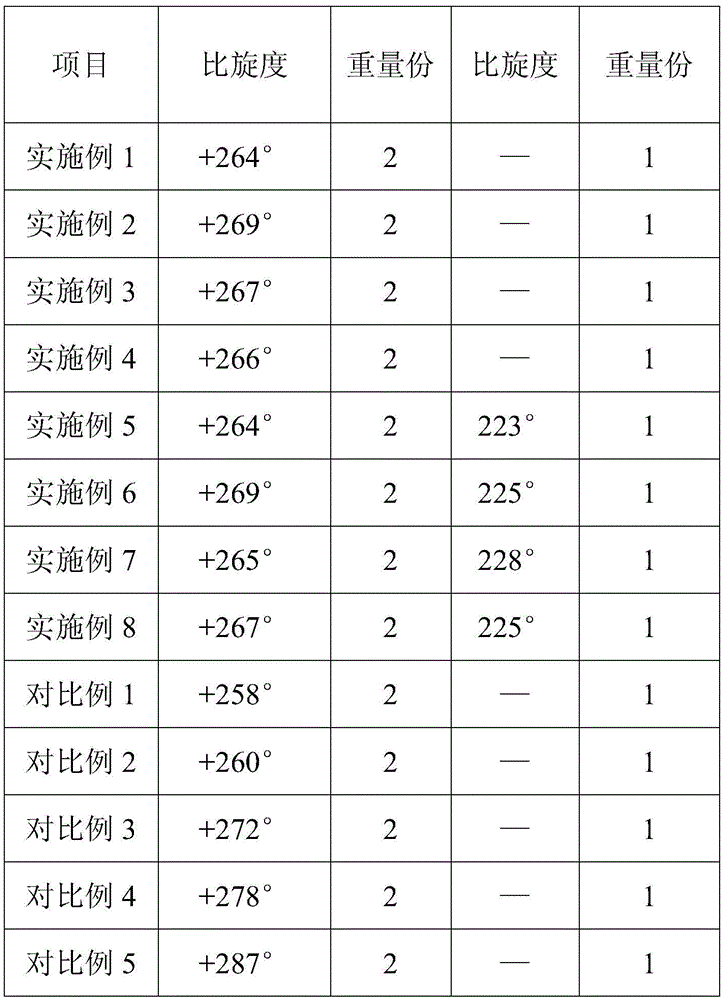
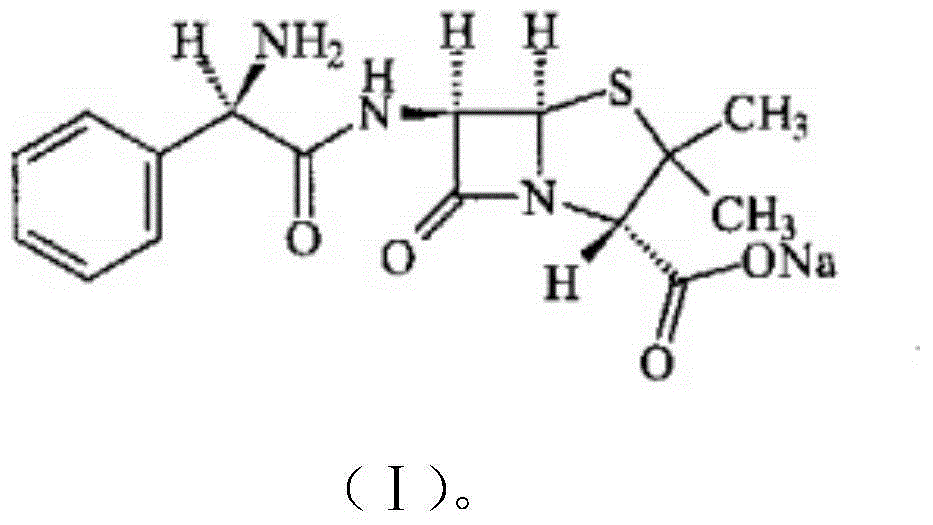
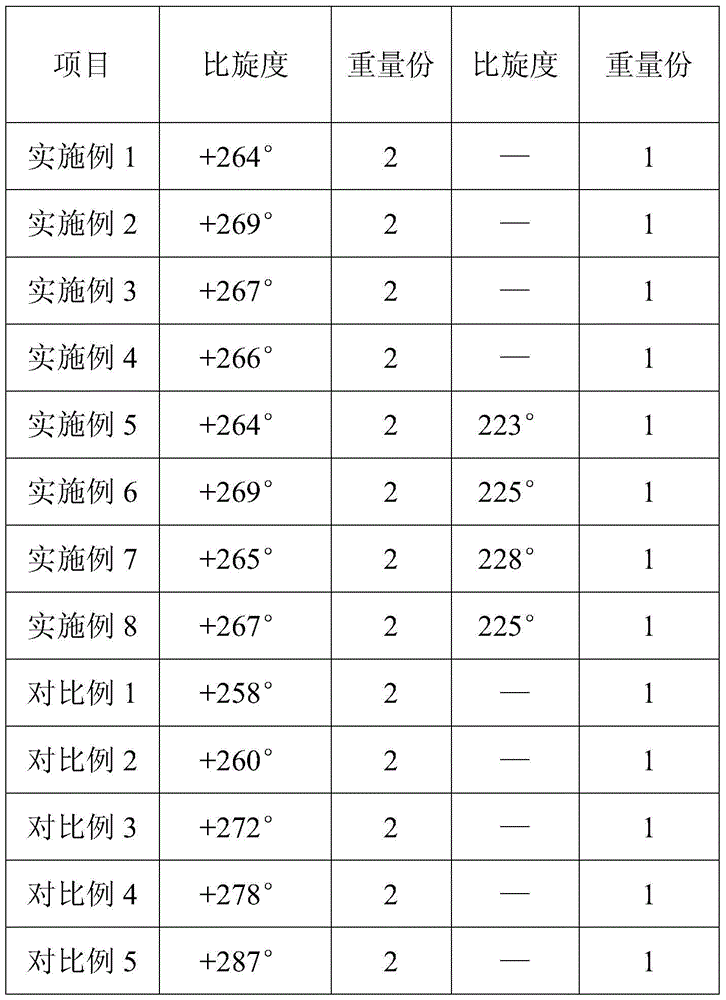
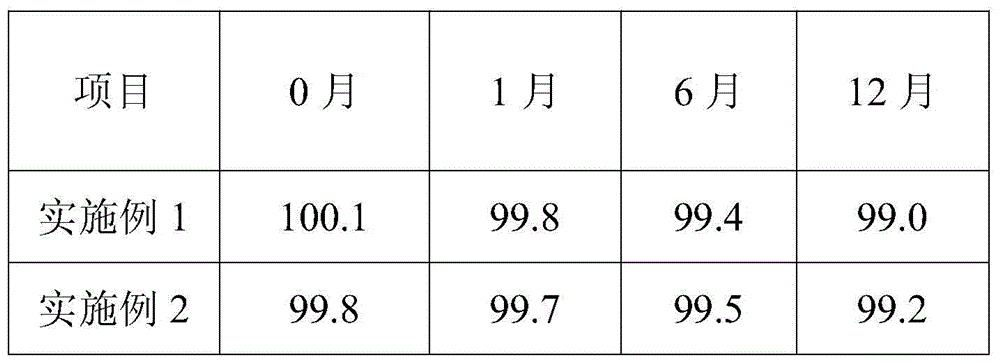
The present invention discloses an ampicillin sodium and sulbactam sodium pharmaceutical composition comprising sulbactam sodium and ampicillin sodium with the specific optical rotation of + 264 degrees to + 269 degrees, and the mass ratio of ampicillin sodium to sulbactam sodium is 2: 1. The ampicillin sodium and sulbactam sodium pharmaceutical composition is prepared from the sulbactam sodium and the ampicillin sodium with the particular specific optical rotation, the drug stability is improved, and drug safety is improved.
Owner:SICHUAN PHARMA
Children ampicillin sodium compound entity and pharmaceutical preparation thereof
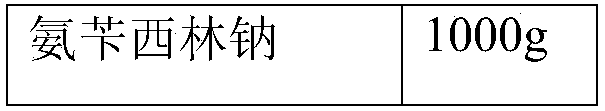
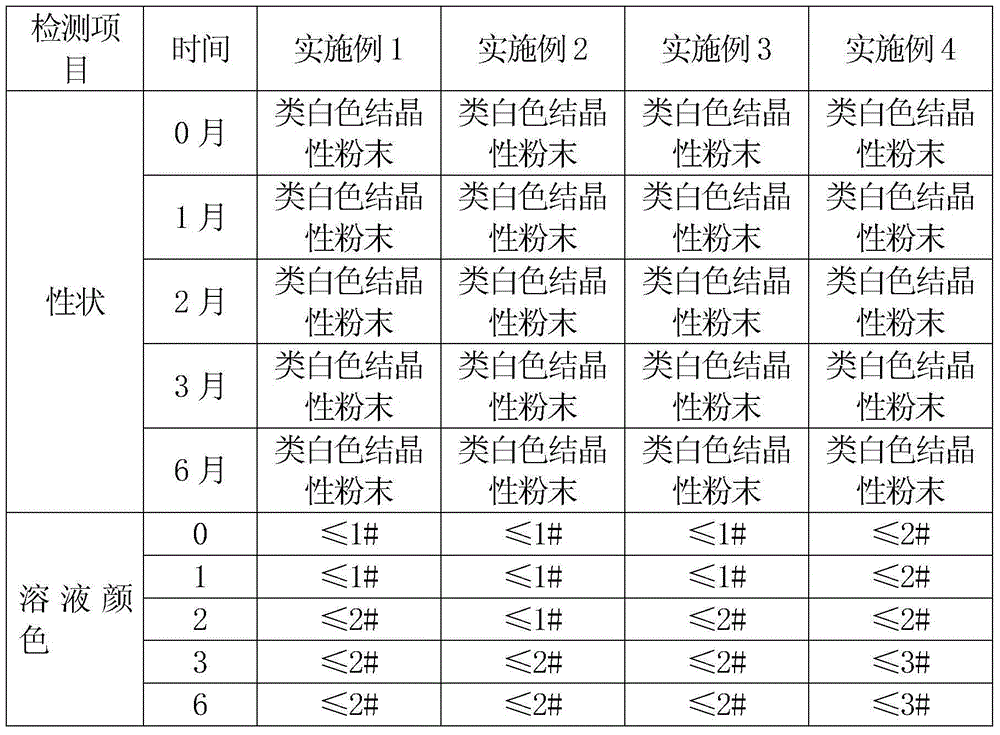
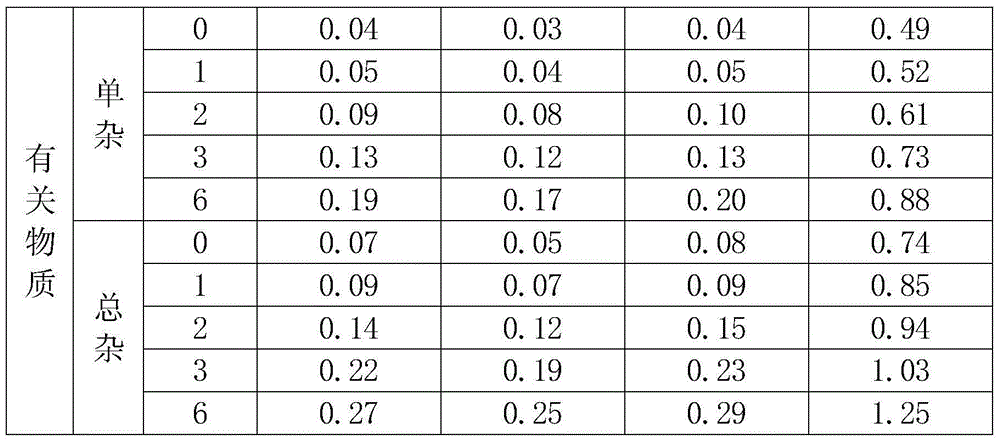
InactiveCN104945418ALess impuritiesHigh purityAntibacterial agentsOrganic chemistrySolubilitySide effect
The present invention discloses a children ampicillin sodium compound entity, wherein the preparation method comprises: (1) adding ampicillin to a mixed solution of dichloromethane and triethylamine, cooling, adding a small amount of a sodium 2-ethylhexanoate solution, increasing the solution temperature, adding the remaining sodium 2-ethylhexanoate solution, carrying out suction filtration, washing, and carrying out vacuum drying to obtain an ampicillin sodium crude product; (2) dissolving the ampicillin sodium crude product in purified water, adding active carbon, carrying out stirring decolorizing, and filtering; (3) adding an extractant to the filtrate under the stirring, transferring and filling into a pressure resistance container, removing air bubbles, carrying out sealing oscillation, carrying out temperature control freezing, and taking out; and (4) carrying out liquid-solid separation, discarding the extractant, adding acetone in a dropwise manner at a temperature of 5 DEG C after the solid melts, stirring at a slow speed, growing the grain, filtering, washing, and carrying out vacuum drying to obtain the ampicillin sodium finished product. The children ampicillin sodium compound entity of the present invention has advantages of good solubility, good clarity, low related substance content, good stability, low toxic-side effect, and the like.
Owner:ZHEJIANG CHANGDIAN PHARMA
A kind of preparation method of the pharmaceutical composition of ampicillin sodium sulbactam sodium
ActiveCN105520942BImprove stabilityImprove securityAntibacterial agentsPowder deliverySpecific rotationAmpicillin Sodium
The present invention discloses a pharmaceutical composition of ampicillin sodium and sulbactam sodium, wherein a sulbactam sodium is combined with an ampicillin sodium with a specific rotation of +264° to +269°, wherein the ampicillin sodium and the sulbactam sodium have a mass ratio of 2:1. The invention is produced by combining the sulbactam sodium with an ampicillin sodium with a selected specific rotation, thereby improving pharmaceutical stability and user safety of the pharmaceutical composition.
Owner:SICHUAN PHARMA
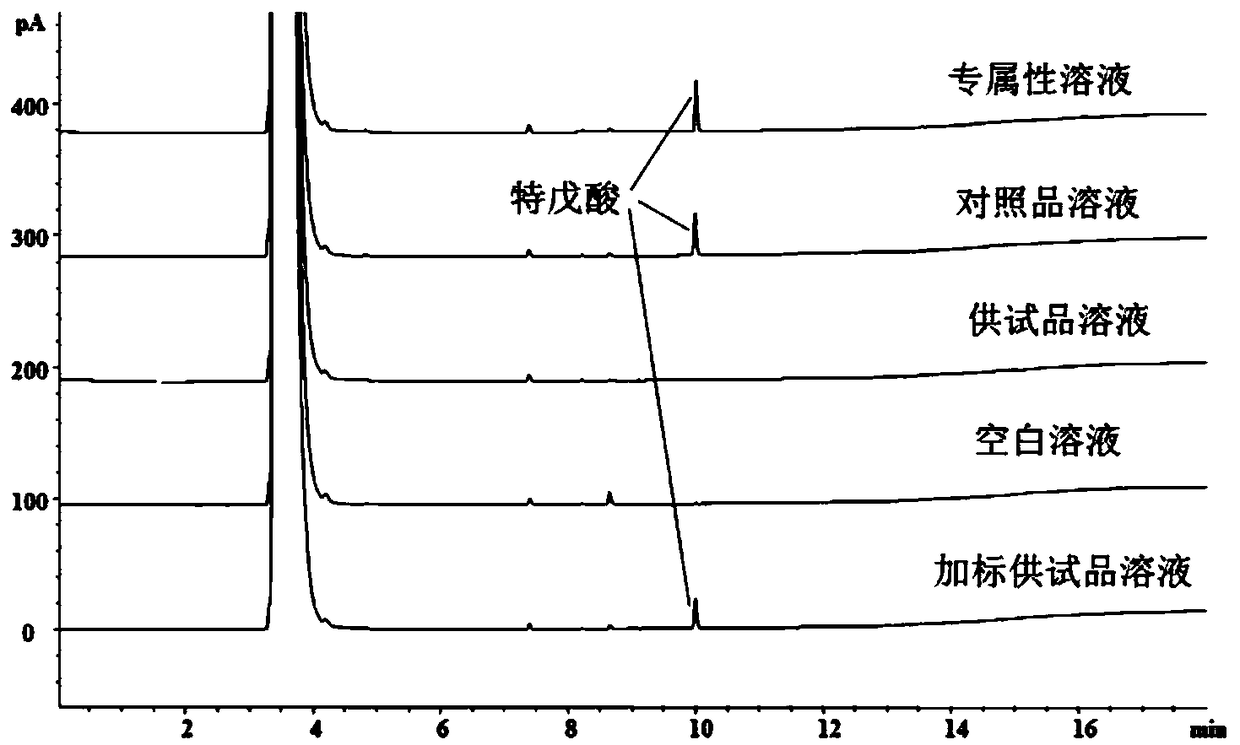
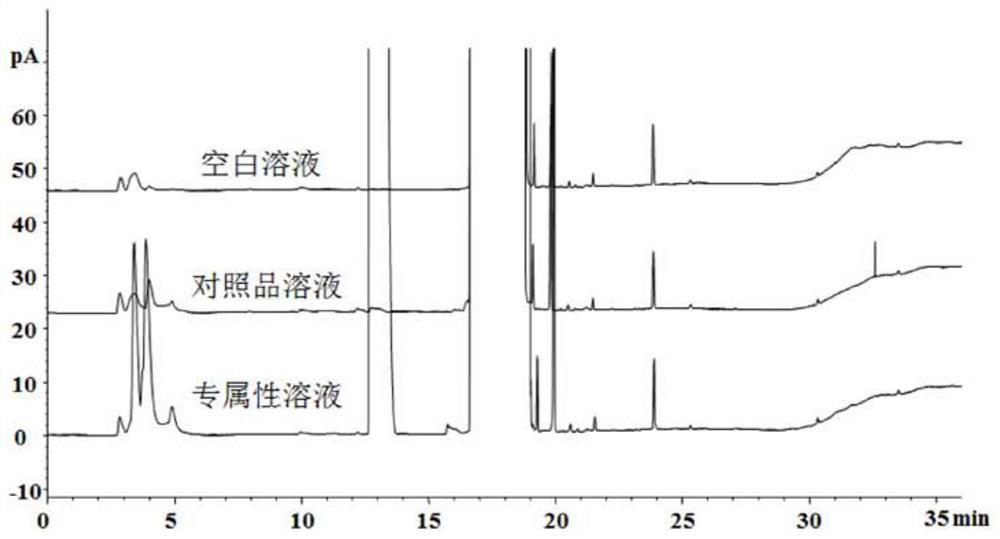
Detection method of trimethylacetic acid in ampicillin and/or ampicillin sodium
ActiveCN109490439AMeet monitoringMeet impurity control requirementsComponent separationPolyethylene glycolColumn temperature
The invention discloses a detection method of remaining trimethylacetic acid in ampicillin and / or ampicillin sodium. A sample to be detected is detected by a gas chromatographic method, nature or quantity is determined according to chromatographic results, chromatographic conditions include that a chromatographic column is a capillary chromatographic column taking nitroterephthalic acid modified polyethylene glycol as stationary liquid or a chromatographic column with similar or same polarity, a detector is an FID (flame ionization detector), column temperature increase procedures include thatinitial temperature is 60 DEG C and increased to 180 DEG C at the rate of 20 DEG C / min, and the temperature is kept for 5 minutes, increased to 200 DEG C at the rate of 20 DEG C / min and kept for 3 minutes. The detection method is simple to operate, strong in specificity, good in peak shape, high in sensitivity, good in accuracy and repeatability, the results are reliable, the trimethylacetic acidcan be rapidly analyzed, impurities in the technical process can be monitored, and the control requirement of the impurities in finished products can be met.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Compound medicament for treating proventriculitis and gizzard erosion of poultry and preparation method thereof
ActiveCN103394021BGuaranteed normal needsQuick cureHydroxy compound active ingredientsDigestive systemTherapeutic effectAmomum villosum
Owner:CHENGDU CENTURY INVESTMENT
Composition for treating swine infectious atrophic rhinitis and preparation method thereof
InactiveCN101972266AImprove side effectsEliminate side effectsAntibacterial agentsInorganic non-active ingredientsSodium bicarbonateSide effect
The invention discloses a composition for treating swine infectious atrophic rhinitis and a preparation method thereof, and aims to provide the composition for treating the swine infectious atrophic rhinitis caused by infection of swine bordetella bronchiseptica and toxigenic pasteurella multocida, which reduces pathogenic medicament resistance and is convenient to use, and the preparation method thereof. The composition comprises the following components in percentage by weight: 5 to 15 percent of sulfamonomethoxine sodium, 1 to 3 percent of lactic acid trimethoprim (TMP1), 3 to 8 percent of ampicillin sodium, 5 percent of sodium carbonate, 5 to 15 percent of sodium bicarbonate and the balance of anhydrous dextrose. By adopting the sulfamonomethoxine sodium and the ampicillin sodium as anti-pathogenic components, the pathogenic microorganism can be effectively killed, the probability of medicament resistance can be reduced, the composition has an effect on secondary bacterial infection, the lactic acid TMP has obvious synergistic effect on the sulfamonomethoxine sodium and the ampicillin sodium, the sodium carbonate can contribute to dissolving the sulfamonomethoxine sodium and the ampicillin sodium, and the sodium bicarbonate relieves the side effect of the sulfamonomethoxine sodium and prevents crystalluria.
Owner:TIANJIN SHENGJI GRP CO LTD
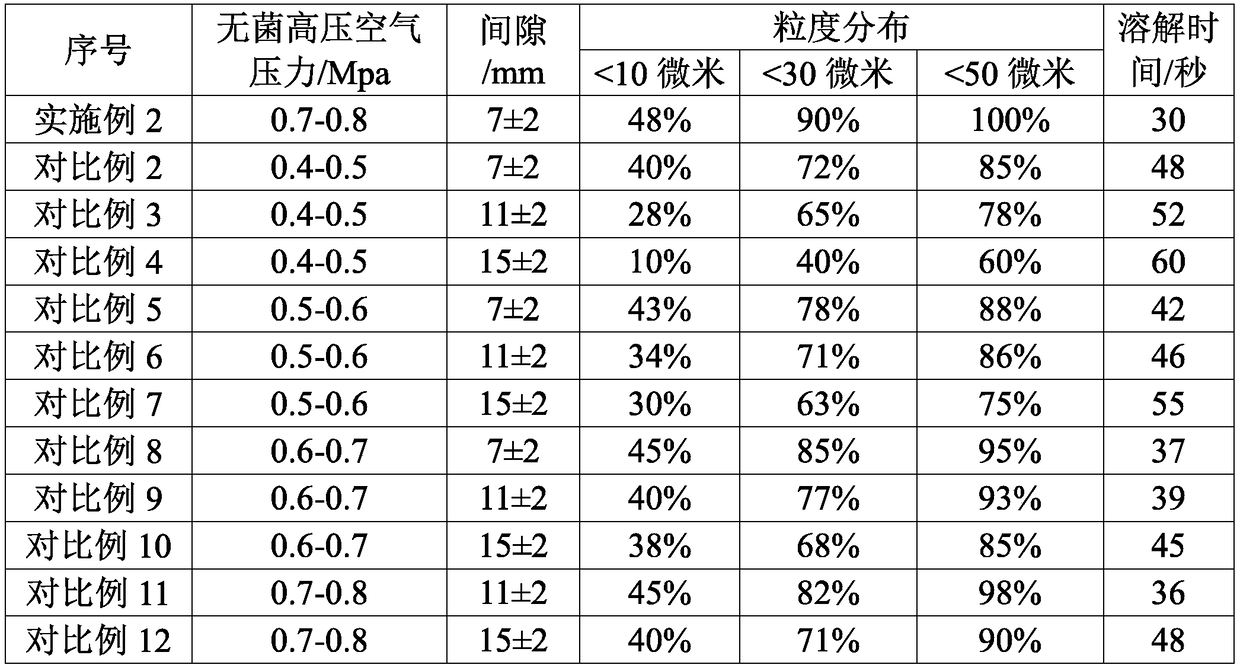
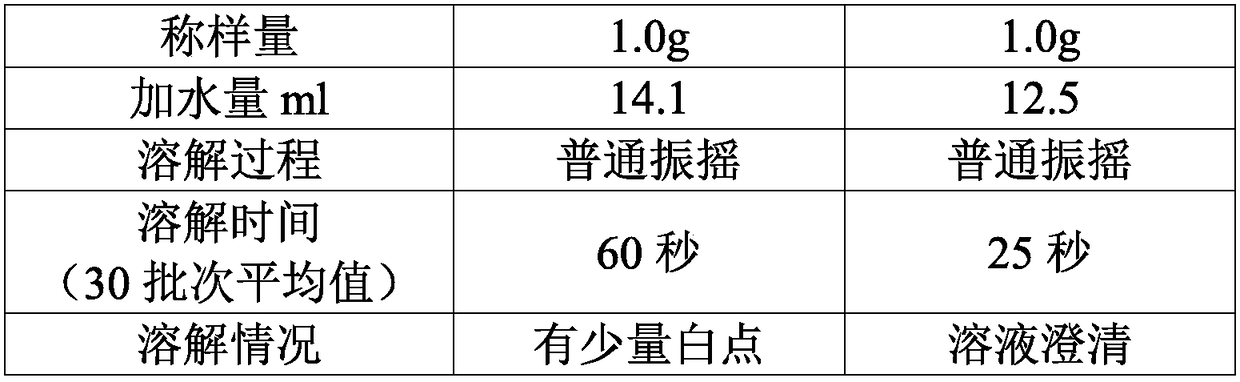
A kind of high-purity mezlocillin sodium preparation and preparation method thereof
ActiveCN105902543BFast dissolutionHigh purityOrganic chemistry methodsHeterocyclic compound active ingredientsMicroparticleAmpicillin Sodium
The invention discloses a high-purity mezlocillin sodium preparation. The high-purity mezlocillin sodium preparation comprises uniform granules with the grain sizes smaller than 50 micrometers. The content of total impurities in the high-purity mezlocillin sodium preparation is lower than or equal to 0.68%, and the content of polymers in the high-purity mezlocillin sodium preparation is lower than or equal to 0.06%. The invention further discloses a method for preparing the high-purity mezlocillin sodium preparation. The method includes six steps of preparing ampicillin sodium solution; preparing mezlocillin sodium condensation liquid; preparing mezlocillin dry powder; preparing mezlocillin sodium solution; preparing mezlocillin sodium active compounds; preparing the high-purity mezlocillin sodium preparation. The high-purity mezlocillin sodium preparation and the method have the advantages that existing problems can be solved by the aid of the high-purity mezlocillin sodium preparation prepared by the aid of novel crystallization technologies, and the quality of the high-purity mezlocillin sodium preparation can be greatly improved; the high-purity mezlocillin sodium preparation is high in dissolving rate and purity, low in impurity content and insoluble particle content, good in flowability and easy to separately package, and the like.
Owner:NORTH CHINA PHARMA COMPANY
Drug for treating pig high-fever high-temperature diseases
InactiveCN102552305AEffective treatmentAlleviate the problem of slow growth processAntipyreticTetracycline active ingredientsHigh energyMedicine
The invention discloses a drug for treating pig high-fever high-temperature diseases, which comprises the following raw materials according to the proportional ratio by weight: 10 g of ampicillin sodium injection, 1000 units of coenzyme A injection, 100 ml of inosine injection, 1000 g of terramycin, 1000 particles of chloromycetin and 500 g of high-energy electrolytic multivitamins. The drug has the advantages that high fever and high temperature caused by various bacteria and viral infection soft tissues based on high-fever diseases, skin drying, red turning, purple turning and other multiple diseases, fibrous bleeding of livers, kidneys, spleens, stomachs, intestinal tracts and the like, and lymph gland atrophic saliva are reasonably and scientifically mediated and treated.
Owner:孔繁采
Pharmaceutical composition for preventing and treating avian salmonellosis and preparation method thereof
ActiveCN113577112AImprove bioavailabilityImprove performanceAntibacterial agentsDigestive systemBiotechnologyEfficacy
The invention relates to a pharmaceutical composition for preventing and treating avian salmonellosis and a preparation method of the pharmaceutical composition. The pharmaceutical composition comprises the following components: artemisinic acid, ampicillin sodium, ciprofloxacin and polysaccharide and probiotic microcapsules. According to the pharmaceutical composition disclosed by the invention, monomer traditional Chinese medicine effective components are selected to be combined with the antibiotics, so that the use amount of traditional Chinese medicines and antibiotics can be reduced on the premise of improving the antibacterial inhibition and killing effects, and the bioavailability and efficacy of medicines are improved. Meanwhile, immune enhancement regulation is taken as an auxiliary treatment means, polysaccharide and probiotics are selected to regulate intestinal flora of the diseased chickens and reconstruct a good microbial system, so that the immune function of the chickens is enhanced, and the production performance is improved. The pharmaceutical composition disclosed by the invention can be administrated through mixed feeding, is suitable for clinical application, has an exact curative effect and is suitable for being applied to intensive large-scale farms.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Kit for detecting beta-lactam antibiotic ligand in milk by receptor method and detection method thereof
ActiveCN101813698BLow detection limitHigh sensitivityTesting dairy productsDepsipeptidesPenicillin bindingDiluent
Owner:上海溯源生物技术有限公司
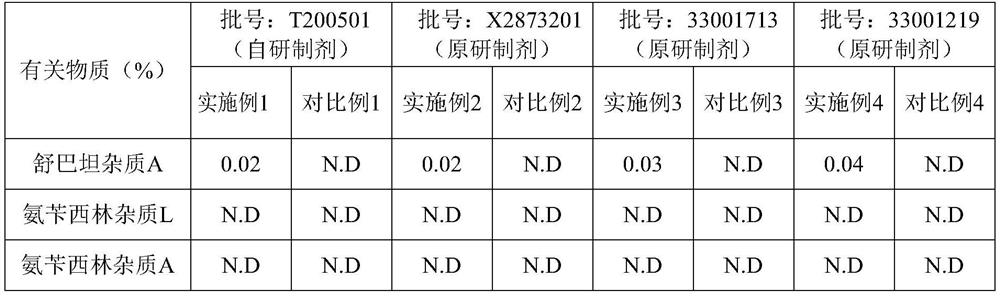
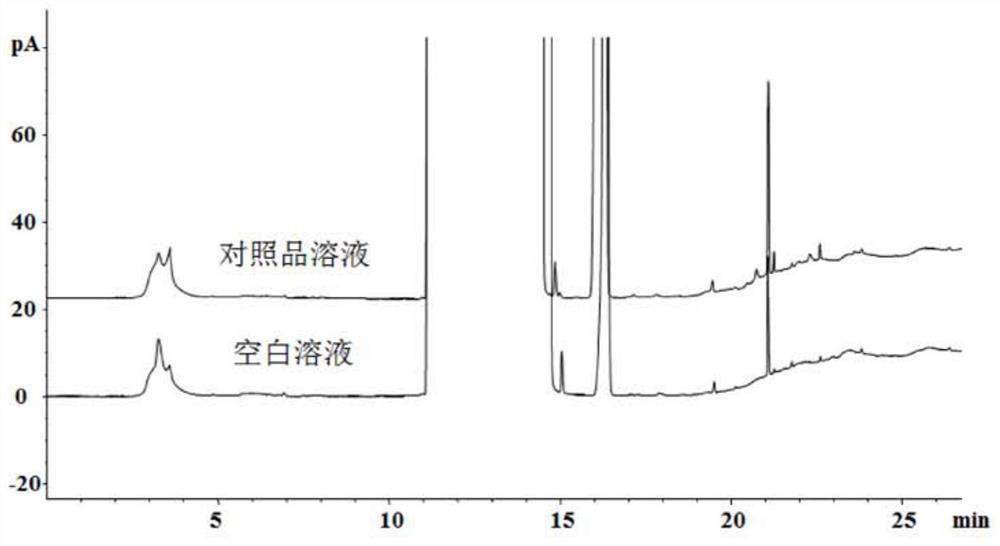
Method for determining related substances of ampicillin sodium and sulbactam sodium for injection
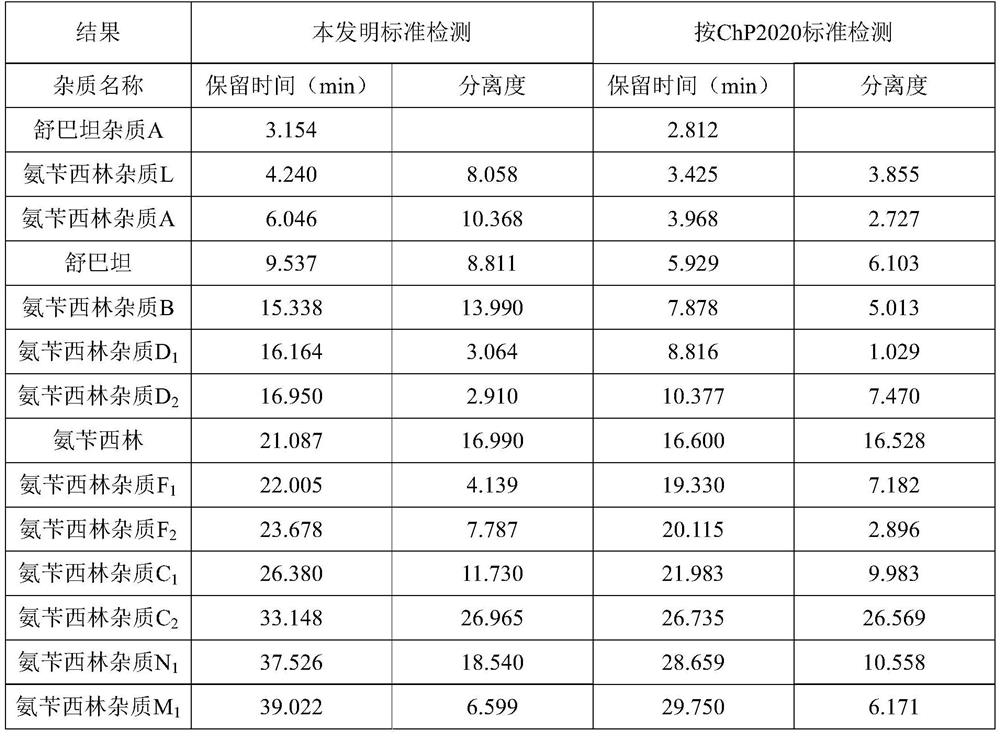
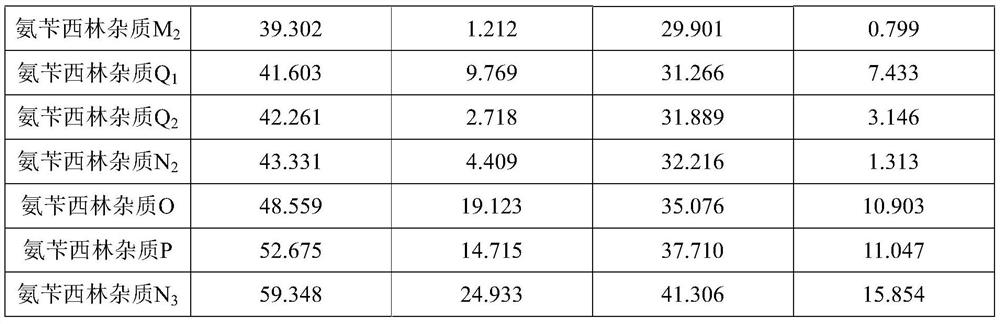
ActiveCN112986435AEfficient separationAvoid interferenceComponent separationFluid phaseAmpicillin Sodium
The invention discloses a method for determining related substances of ampicillin sodium and sulbactam sodium for injection, a high performance liquid chromatography is adopted to detect an ampicillin sodium and sulbactam sodium sample solution for injection, and mobile phases of the high performance liquid chromatography comprise a mobile phase A which is a sodium dihydrogen phosphate solution and a mobile phase B which is acetonitrile, on the basis of the standard of ampicillin sodium and sulbactam sodium for injection in Chinese Pharmacopoeia 2020, the pH value and linear gradient of the mobile phase A are modified, so that main degradation impurity peaks as well as main peaks and adjacent impurity peaks can be effectively separated, meanwhile, a solvent peak and a sulbactam impurity A peak can be effectively separated, and therefore, the interference of a solvent peak on the determination of the sulbactam impurity A is avoided, and the method disclosed by the invention has better specificity and sensitivity and can better control the quality of a product.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
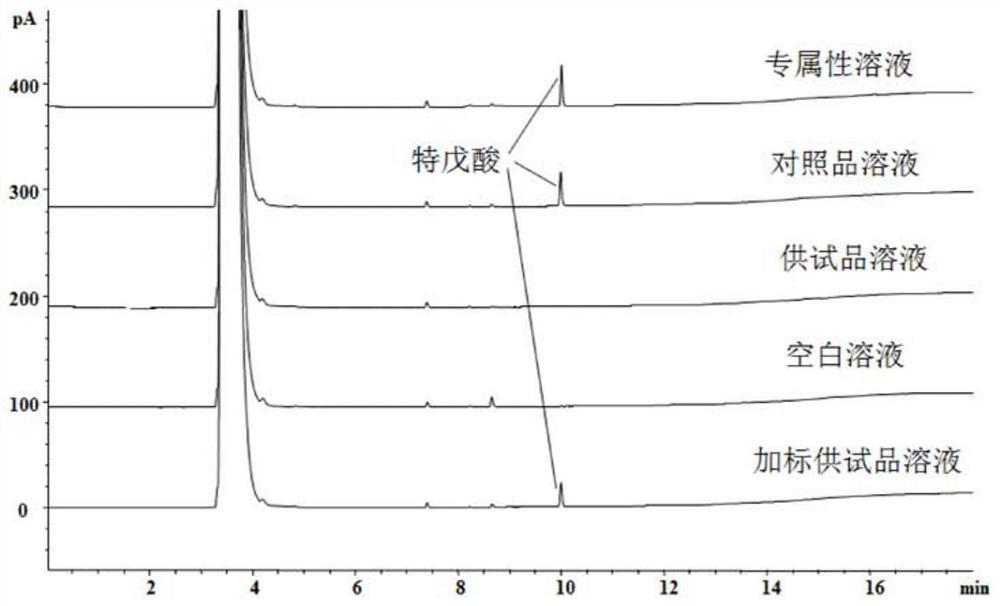
Determination method of pivalic acid in ampicillin and/or ampicillin sodium
ActiveCN109490439BMeet monitoringMeet impurity control requirementsComponent separationPivalic acidPhysical chemistry
The invention discloses a method for detecting residual pivalic acid in ampicillin and / or ampicillin sodium. Gas chromatography is used to detect the sample to be inspected, and the chromatographic results are qualitative or quantitative. The chromatographic conditions include: the chromatographic column adopts nitro-p-phenyl Diformic acid-modified polyethylene glycol is a capillary chromatographic column with fixed liquid or a chromatographic column with similar or equal polarity, and the detector adopts FID detector; The rate was increased to 180°C for 5 minutes, and then increased to 200°C at a rate of 20°C / min, and maintained for 3 minutes. The detection method of the present invention is simple in operation, strong in specificity, good in peak shape, high in sensitivity, good in accuracy and reproducibility, reliable in results, can realize rapid analysis of pivalic acid, and can meet the requirements of impurity monitoring in the process and monitoring of finished products. Impurity control requirements.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com