Ampicillin sodium sulbactam sodium preparation for injection and preparation method thereof
A technology of ampicillin sodium sulbactam sodium and ampicillin sodium, which is applied in the field of medicine, can solve problems such as poor stability, easy allergies, and many impurities, and achieve the effects of good stability, increased reaction yield, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
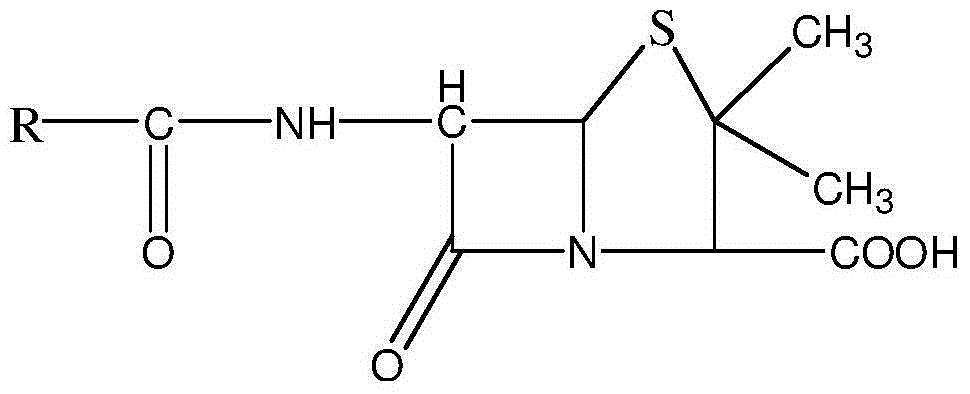
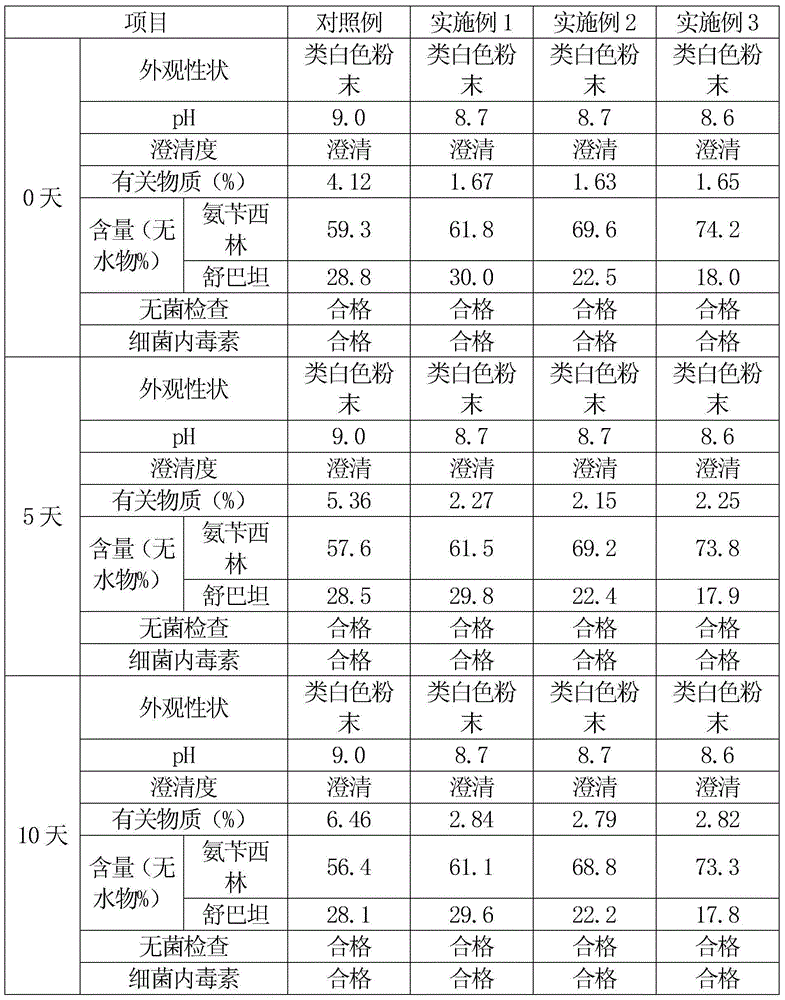
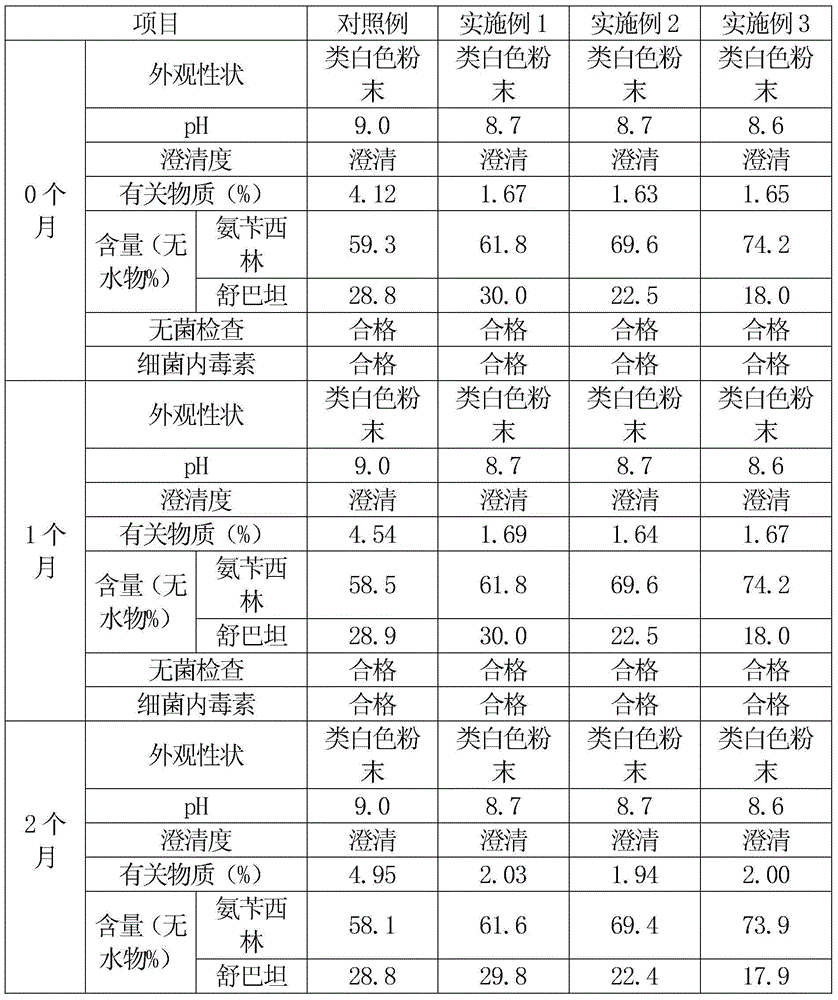
[0043] An ampicillin sodium sulbactam sodium preparation for injection is prepared by uniformly mixing high-purity ampicillin sodium and high-purity sulbactam sodium, and the weight ratio of ampicillin sodium to sulbactam sodium is 2:1.
[0044] The ampicillin sodium sulbactam sodium preparation for injection is prepared by the following steps:
[0045] (1) prepare high-purity ampicillin sodium; this high-purity ampicillin sodium is prepared with the following method:
[0046] ① Preparation of mixed acid anhydride: add 906ml of dichloromethane, add 298 grams of L-phenylglycine ethyl Deng potassium salt (Deng salt) under rapid stirring, add 130ml N, N-dimethylacetamide (DMA) at the same time, keep the temperature at 10- 15°C, cool down, when the temperature drops to 0°C, add 7.5 grams of 2,6-lutidine, continue to cool down to -30°C, add 119 grams of pivaloyl chloride at a uniform speed within 10 minutes, and control the temperature at -15°C, The reaction was incubated for 60 m...
Embodiment 2
[0060] An ampicillin sodium sulbactam sodium preparation for injection is prepared by uniformly mixing high-purity ampicillin sodium and high-purity sulbactam sodium, and the weight ratio of ampicillin sodium to sulbactam sodium is 3:1.
[0061] The ampicillin sodium sulbactam sodium preparation for injection is prepared by the following steps:
[0062] (1) prepare high-purity ampicillin sodium; this high-purity ampicillin sodium is prepared with the following method:
[0063] ① Preparation of mixed acid anhydride: add 1000ml of dichloromethane, add 298 grams of L-phenylglycine ethyl Deng potassium salt (Deng salt) under rapid stirring, and add 150ml N,N-dimethylacetamide (DMA) at the same time, keep the temperature at 10- 15°C, lower the temperature, when the temperature drops to 0°C, add 8.2 grams of 2,6-lutidine, continue to cool down to -34°C, add 125 grams of pivaloyl chloride at a uniform speed within 10 minutes, and control the temperature at -18°C, The reaction was in...
Embodiment 3
[0077] An ampicillin sodium sulbactam sodium preparation for injection is prepared by uniformly mixing high-purity ampicillin sodium and high-purity sulbactam sodium, and the weight ratio of ampicillin sodium to sulbactam sodium is 4:1.
[0078] The ampicillin sodium sulbactam sodium preparation for injection is prepared by the following steps:
[0079] (1) prepare high-purity ampicillin sodium; this high-purity ampicillin sodium is prepared with the following method:
[0080] ① Preparation of mixed acid anhydride: Add 1132ml of dichloromethane, add 298 grams of L-phenylglycine ethyl Deng potassium salt (Deng salt) under rapid stirring, add 160ml N, N-dimethylacetamide (DMA) at the same time, keep the temperature at 10- 15°C, lower the temperature, when the temperature drops to 0°C, add 9.0 grams of 2,6-lutidine, continue to cool down to -38°C, add 131 grams of pivaloyl chloride at a uniform speed within 10 minutes, and control the temperature at -20°C, The reaction was incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com