Preparation method of ampicillin sodium for injection
A technology of ampicillin sodium and ampicillin, applied in the field of pharmaceuticals, can solve the problems of time-consuming, increase of product impurities, long cooling and freezing time, etc., and achieve the effect of increasing the sublimation rate, improving stability, and promoting the volatilization of bound water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
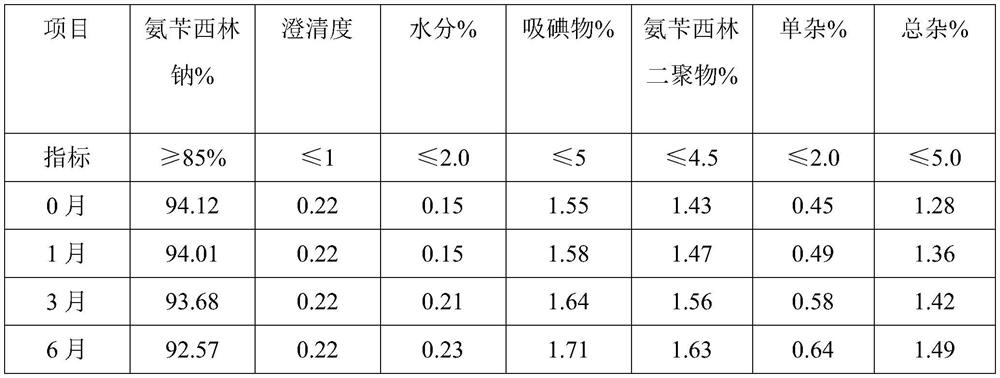
Embodiment 1
[0031] A preparation method of ampicillin sodium for injection provided in the embodiment of the present invention comprises the following steps:
[0032] 1) Weigh the raw materials: 70 g of ampicillin, 10% sodium hydroxide solution (density 1.1 g / cm 3 ) 63.5ml, water for injection 100ml, dry ice 10g, mannitol 15g;
[0033] 2) Alkali-soluble: add water for injection and mannitol to the reaction vessel, stir evenly, then add ampicillin and stir to obtain a suspension, then add 10% sodium hydroxide solution, control the pH value of the solution to be 7.8, and adjust the pH after the reaction is completed Add dry ice and mesoporous silica to coat Fe immediately after the value reaches 7.1 3 O 4 The magnetic material of the particles removes impurities;
[0034] 3) Freezing: The ampicillin sodium solution generated by alkali dissolution is separated from the magnetic adsorbent by magnetic field, and after precision filtration, it is poured into the freeze-drying equipment, firs...
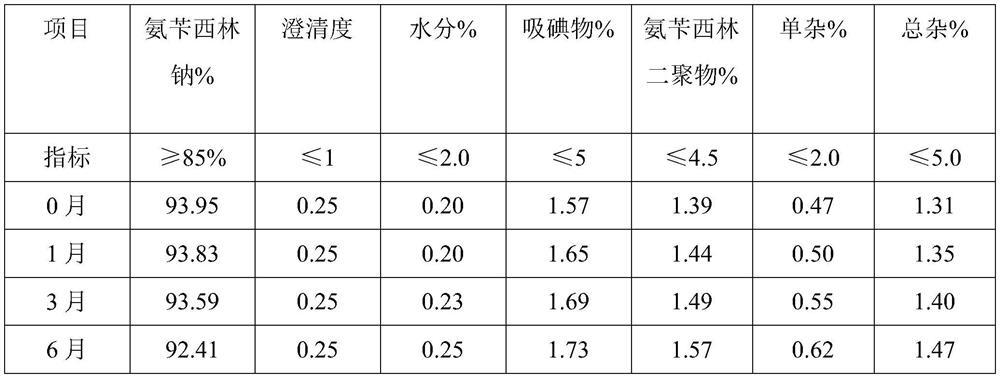
Embodiment 2
[0042] A preparation method of ampicillin sodium for injection provided in the embodiment of the present invention comprises the following steps:
[0043] 1) Weigh the raw materials: Ampicillin 60g, 10% sodium hydroxide solution (density 1.1g / cm 3 ) 54.5ml, water for injection 95ml, dry ice 10g, mannitol 14g;
[0044] 2) Alkali-soluble: add water for injection and mannitol to the reaction vessel, stir evenly, then add ampicillin and stir to obtain a suspension, then add 10% sodium hydroxide solution, control the pH value of the solution to be 7.7, and adjust the pH after the reaction is completed Add dry ice and mesoporous silica to coat Fe immediately after the value reaches 7.1 3 O 4 The magnetic material of the particles removes impurities;
[0045] 3) Freezing: The ampicillin sodium solution generated by alkali dissolution is separated from the magnetic adsorbent by magnetic field, and after precision filtration, it is poured into the freeze-drying equipment, firstly co...
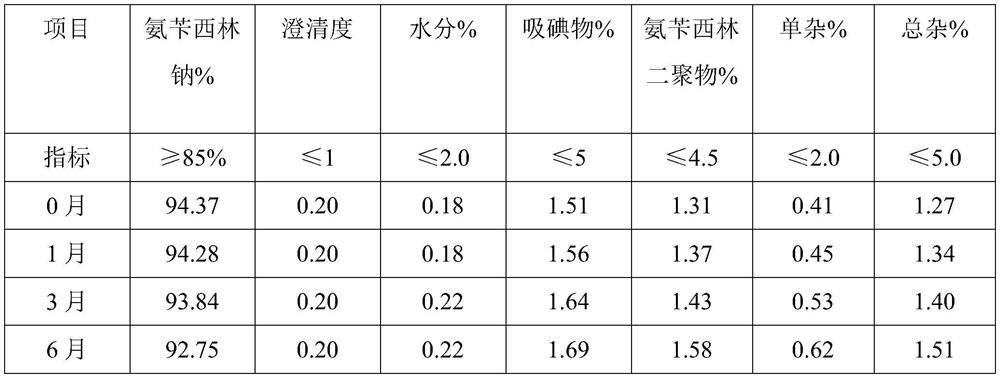
Embodiment 3
[0053] A preparation method of ampicillin sodium for injection provided in the embodiment of the present invention comprises the following steps:
[0054] 1) Weigh the raw materials: 80 g of ampicillin, 10% sodium hydroxide solution (density 1.1 g / cm 3 ) 63.5ml, water for injection 105ml, dry ice 10g, mannitol 15g;
[0055] 2) Alkali-soluble: add water for injection and mannitol to the reaction vessel, stir evenly, then add ampicillin and stir to obtain a suspension, then add 10% sodium hydroxide solution, control the pH value of the solution to be 7.8, and adjust the pH after the reaction is completed Add dry ice and mesoporous silica to coat Fe immediately after the value reaches 7.1 3 O 4 The magnetic material of the particles removes impurities;
[0056] 3) Freezing: The ampicillin sodium solution generated by alkali dissolution is separated from the magnetic adsorbent by magnetic field, and after precision filtration, it is poured into the freeze-drying equipment, firs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com