Method for preparing ampicillin sodium salt
A technology of ampicillin sodium salt and ampicillin, applied in the field of compound preparation, can solve the problems of unfavorable market competition, high manufacturing cost, large recovery and the like, and achieve the effects of overcoming unstable product quality, low cost and low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
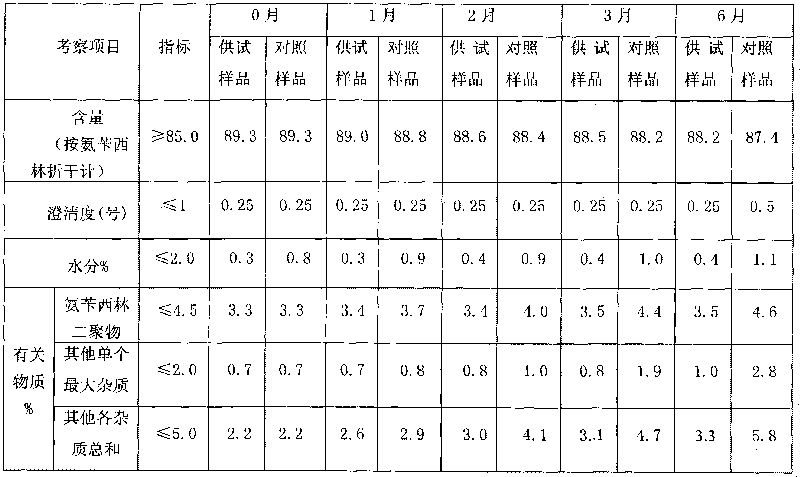
[0031] Add water for injection into the salt-forming tank, pre-cool to 3-5°C, put 50kg of ampicillin into the salt-forming tank under stirring, add 5.44kg of 10% sodium hydroxide solution until the solution is clear, and the final pH is 9.0-10.5. After the ampicillin is completely dissolved, add activated carbon and stir, go through preliminary filtration, prefiltration, and final sterile filtration, and then enter the aseptic room freeze dryer for freeze-vacuum drying. After freeze-drying and vacuum-drying, ampicillin sodium is ground into powder and loaded into the double cone, the manhole of the drier is installed, the vacuum is turned on, the temperature is raised, the temperature is controlled at 40-50°C, the holding time is 40 minutes, and the vacuum gauge pressure is ≤-0.085MPa. Mix the powder, pack and test to obtain the ampicillin sodium product.
Embodiment 2
[0033] Add water for injection into the salt-forming tank, pre-cool to 0-3°C, put 50kg of ampicillin into the salt-forming tank under stirring, add 5.44kg of 9.5% sodium hydroxide solution until the solution is clear, and the final pH is 9.0-10.5. After the ampicillin is completely dissolved, add activated carbon and stir, go through preliminary filtration, prefiltration, and final sterile filtration, and then enter the aseptic room freeze dryer for freeze-vacuum drying. After lyophilized and vacuum-dried, ampicillin sodium is ground into powder and loaded into a double cone, equipped with a manhole in the drier, turned on vacuum, heated up, controlled temperature 70-95°C, holding time 60 minutes, vacuum gauge pressure ≤ -0.075MPa. Mix the powder, pack and test to obtain the ampicillin sodium product.
Embodiment 3
[0035] Add water for injection into the salt-forming tank, pre-cool to 2-4°C, put 50kg of ampicillin into the salt-forming tank under stirring, add 5.44kg of 9.0% sodium hydroxide solution until the solution is clear, and the final pH is 9.0-10.5. After the ampicillin is completely dissolved, add activated carbon and stir, go through preliminary filtration, prefiltration, and final sterile filtration, and then enter the aseptic room freeze dryer for freeze-vacuum drying. After lyophilization and vacuum drying, the ampicillin sodium is ground into powder and loaded into the double cone, the manhole of the drier is installed, the vacuum is turned on, the temperature is raised, the temperature is controlled at 60°C, the holding time is 180 minutes, and the vacuum gauge pressure is ≤ -0.065MPa. Mix the powder, pack and test to obtain the ampicillin sodium product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com