Freeze-dried metronidazole powder injection and preparation method thereof
A technology of freeze-dried powder injection and metronidazole, which is applied in the field of medicine, can solve the problems of increasing insoluble particles, long reconstitution time, and difficulty in compounding, etc., achieve high long-term stability, reduce insoluble particles, and shorten reconstitution time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
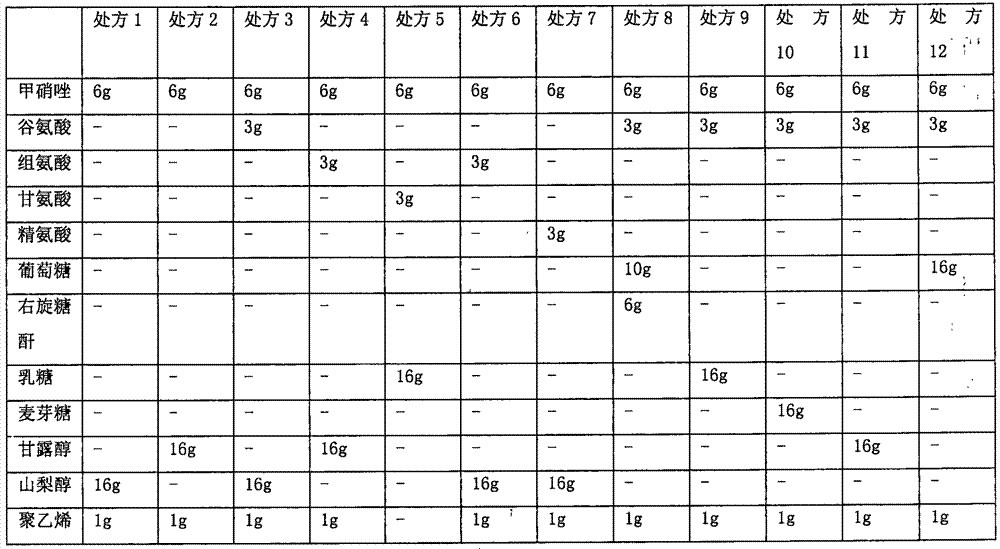
[0033] Example 1: Screening and optimization of freeze-dried powder prescription
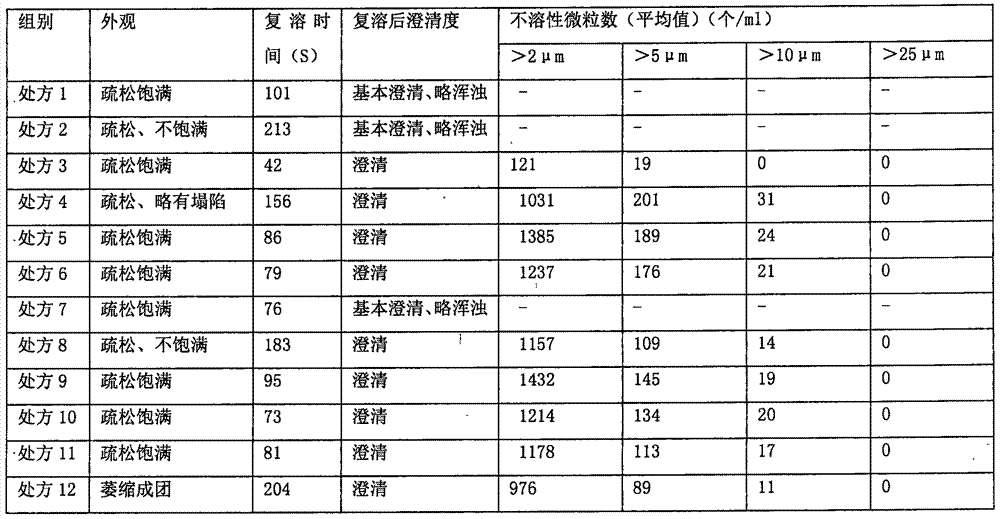
[0034] The metronidazole medicinal solution was prepared respectively according to the prescription in Table 1, and then freeze-dried to obtain a freeze-dried powder injection. The freeze-drying process is as follows: pre-freezing stage: keep warm at -40°C for 3 hours, and then vacuumize; primary sublimation: the temperature rises to -30°C for 4 hours, then the temperature rises to -20°C for 4 hours, and then the temperature rises to 0°C Keep it for 6 hours; secondary drying: the temperature rises to 30°C and keeps it for 6 hours. Then the appearance, reconstitution time, clarity after reconstitution and insoluble particle inspection of the lyophilized powder were detected respectively.
[0035] Appearance and clarity: visually inspect the appearance. Dissolve the powder injection in 100mL of sodium chloride injection and then inspect it. With reference to the second part of the "Chinese Pharm...
Embodiment 2
[0044] prescription:
[0045]
[0046] Preparation method: Take 85% of the prescription amount of water for injection, slowly add the prescription amount of metronidazole and polyvinylpyrrolidone into the water for injection, stir and dissolve at a temperature of 40-50°C, and then continue to add sorbitol and glutamic acid to stir and dissolve , and then make up the rest of the water for injection to prepare the intermediate drug solution, and then freeze-dry to obtain the freeze-dried powder injection after filtering through the filter membrane. ; Primary sublimation: temperature rises to -30°C for 4 hours, then temperature rises to -20°C for 4 hours, then temperature rises to 0°C for 6 hours; secondary drying: temperature rises to 30°C for 6 hours.
Embodiment 3
[0048] prescription:
[0049]
[0050] Preparation method: Take 85% of the prescription amount of water for injection, slowly add the prescription amount of metronidazole and polyvinylpyrrolidone into the water for injection, stir and dissolve at 50°C, then continue to add sorbitol and glutamic acid to stir and dissolve, and then put Make up the rest of the water for injection to prepare the intermediate drug solution, and then filter the membrane and freeze-dry to make the freeze-dried powder injection. The freeze-drying process is as follows: pre-freezing stage: keep at -45°C for 4 hours, vacuumize; once sublimated : The temperature is raised to -30°C for 4 hours, then the temperature is raised to -15°C for 5 hours, then the temperature is raised to 0°C for 5 hours; secondary drying: the temperature is raised to 28°C for 7 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com