Preparation method of coagulation factor xiii
A blood coagulation factor and blood technology, which is applied in the field of medical bioengineering, can solve the problems of increasing the content of pyrogens and other toxins, the reproduction of bacteria and other microorganisms, and the long time of tertiary dissolution, so as to reduce the growth of bacteria, shorten the dissolution time and save time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
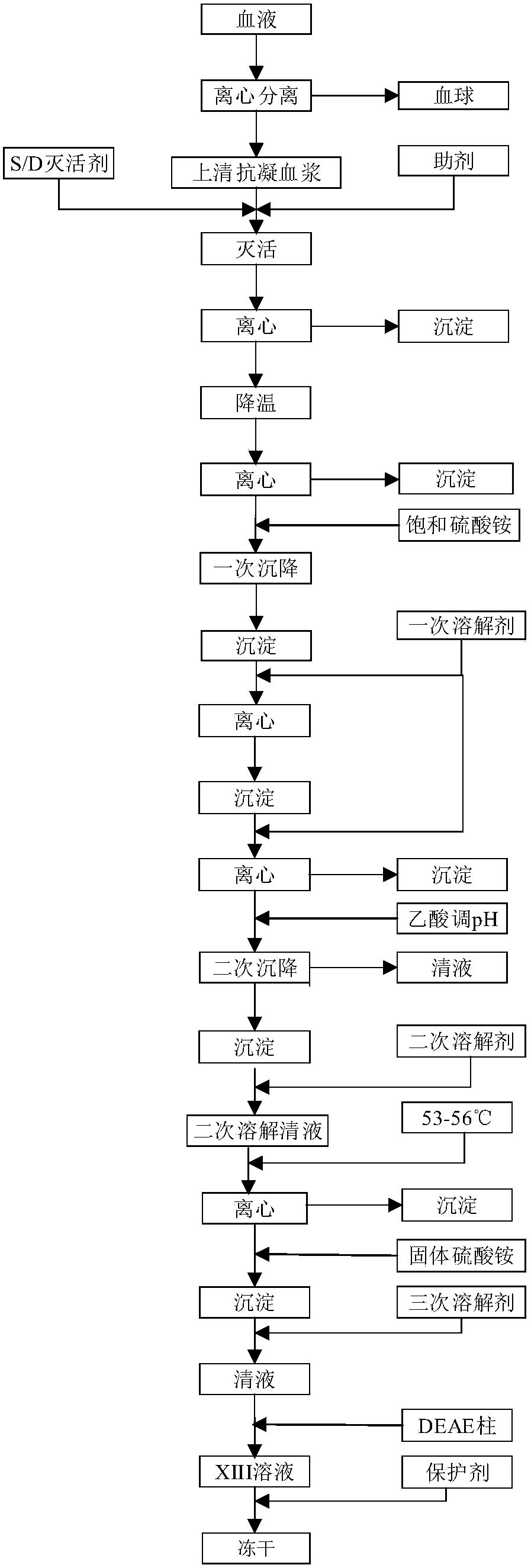
[0024] Please refer to figure 1 , the preparation method of blood coagulation factor XIII, comprises the steps:
[0025] Step 1. After adding anticoagulant to the blood, centrifuge and take the supernatant anticoagulant plasma;
[0026] Step 2, adding tributyl phosphate and Tween-80 as virus inactivating agents to supernatant anticoagulated plasma, and adding auxiliary agents to carry out S / D virus inactivation to obtain inactivated plasma;
[0027] Step 3, centrifuging the inactivated plasma obtained in step 2, and taking the supernatant;
[0028] Step 4. Pre-cool the clear liquid obtained in step 3 to 0-4°C, add a saturated ammonium sulfate solution at 0-4°C, and take the precipitate;
[0029] Step 5. Wash the precipitate obtained in step 4 with a saturated ammonium sulfate solution at 0-4°C, break the washed precipitate, add a primary dissolving agent at 0-4°C and stir, and take the clear liquid. The primary dissolving agent is chlorine Potassium chloride solution;
[0...
Embodiment 1
[0052] Please refer to figure 1 , All operations of the process are carried out in a sterile ten thousand-level purification area, among which sub-package and freeze-drying operations are all carried out in a local one-hundred-level purification area, and the operations performed are in line with the operating requirements of the clean area. The production specification is 1.5ml.
[0053] (1) Get 10000ml of aseptically collected pig blood;
[0054] (2) Centrifuge and take 4000ml of supernatant anticoagulated plasma;
[0055] (3) Add 0.3% w / v tributyl phosphate and 1% w / v Tween-80 as virus inactivator to the supernatant anticoagulated plasma of step (2) gained, add the glucose of 0.2mol / L as auxiliary agent, virus inactivation was carried out at 25°C for 6 hours;
[0056] Centrifuge the inactivated plasma, discard the precipitate, and take the supernatant;
[0057] (4) Primary sedimentation: Precool the clear liquid obtained in step (3) to 0-4°C, add 1000ml of saturated amm...
Embodiment 2
[0077] Please refer to figure 1 , All operations of the process are carried out in a sterile ten thousand-level purification area, among which sub-package and freeze-drying operations are all carried out in a local one-hundred-level purification area, and the operations performed are in line with the operating requirements of the clean area. The production specification is 1.5ml.
[0078] (1) Get 24000ml of aseptically collected pig blood;
[0079] (2) Centrifuge and take 12000ml of supernatant anticoagulated plasma;
[0080] (3) Add 0.3% w / v tributyl phosphate and 1% w / v Tween-80 as virus inactivator to the supernatant anticoagulated plasma of step (2) gained, add the glucose of 0.2mol / L as auxiliary agent, virus inactivation was carried out at 25°C for 6 hours;
[0081] Centrifuge the inactivated plasma, discard the precipitate, and take the supernatant;
[0082] (4) Primary sedimentation: precool the clear liquid obtained in step (3) to 0-4°C, add 3000ml of saturated am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com