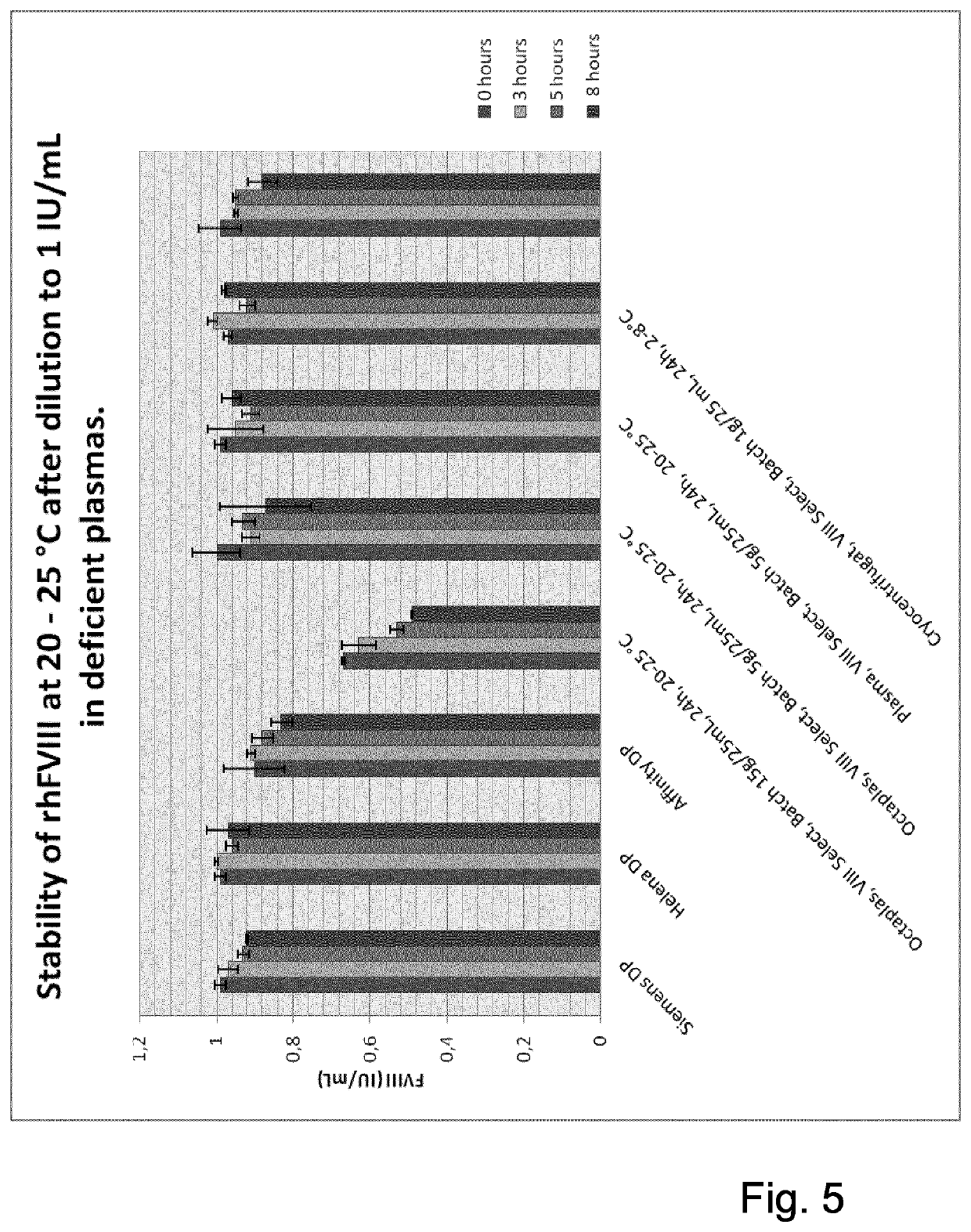
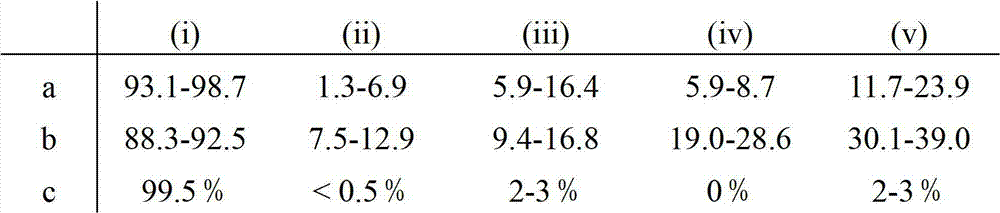
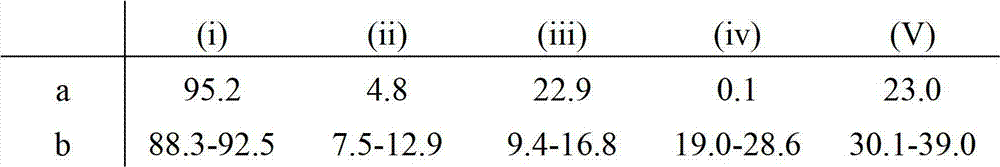Patents
Literature
45 results about "Blood coagulation factor XIII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of production and use of liquid formulations of plasma proteins
InactiveUSRE38431E1Enhanced quality of lifeReduce infectivityPeptide/protein ingredientsInorganic non-active ingredientsMethods of productionFactor XIIIa
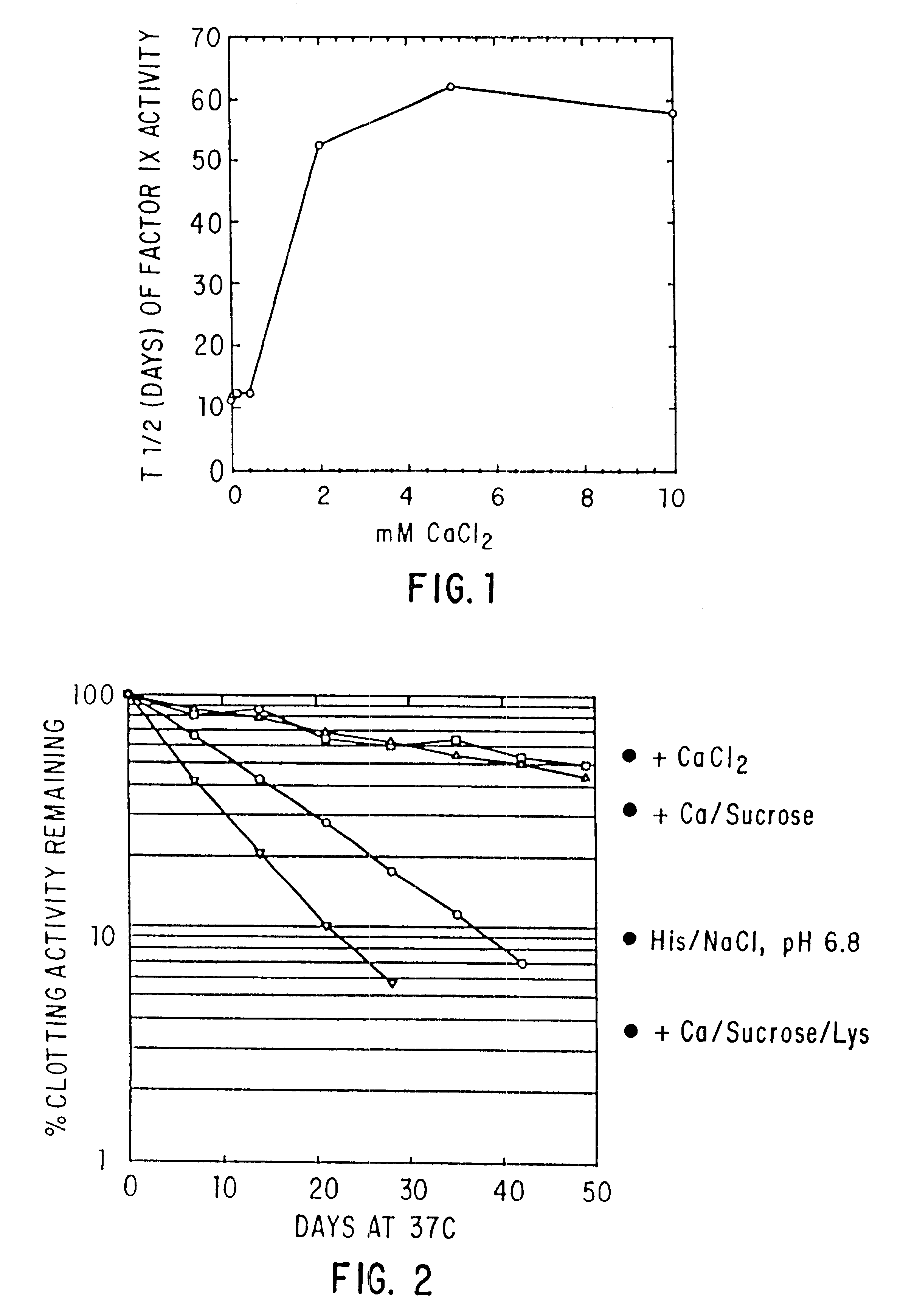
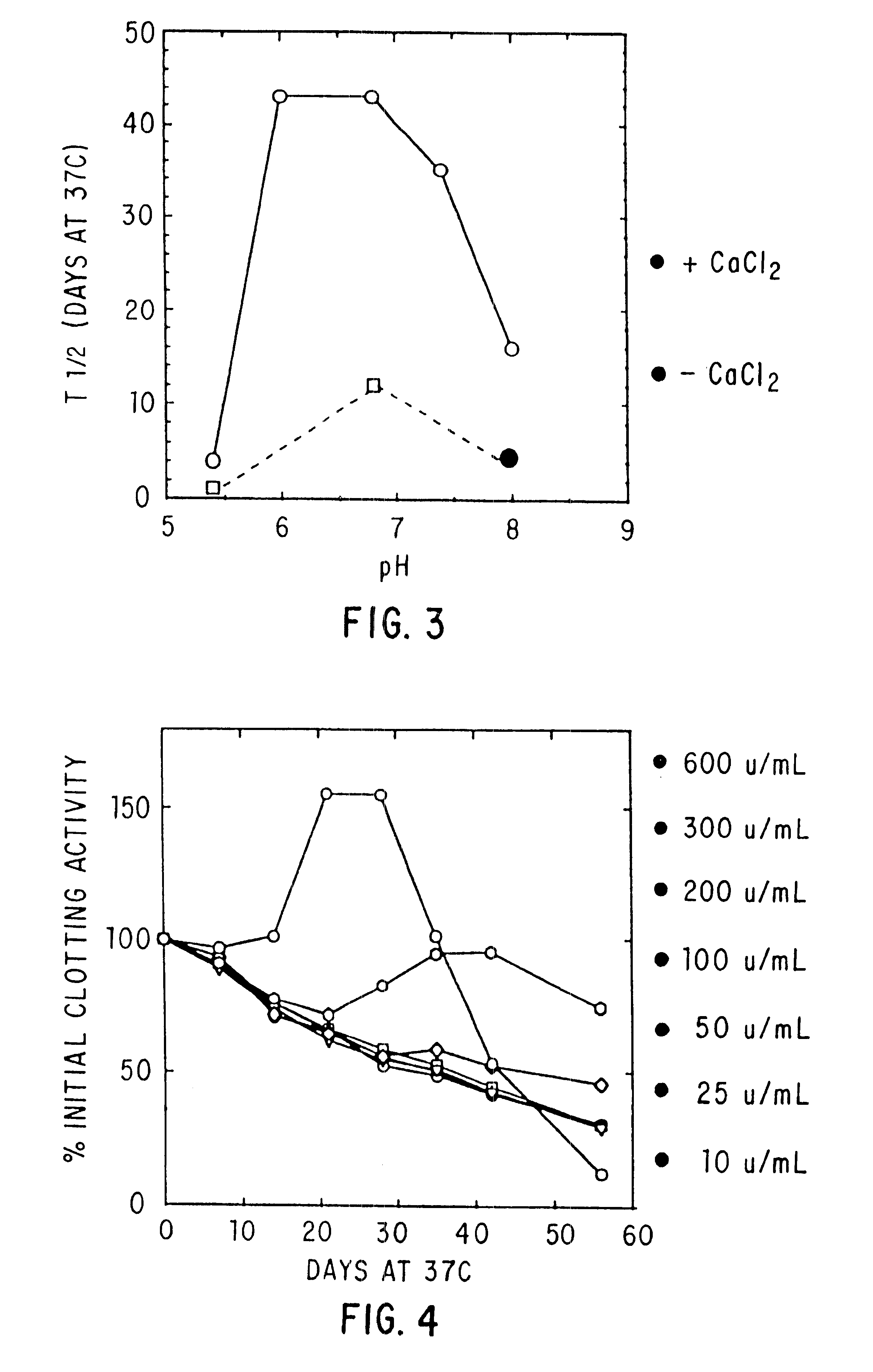
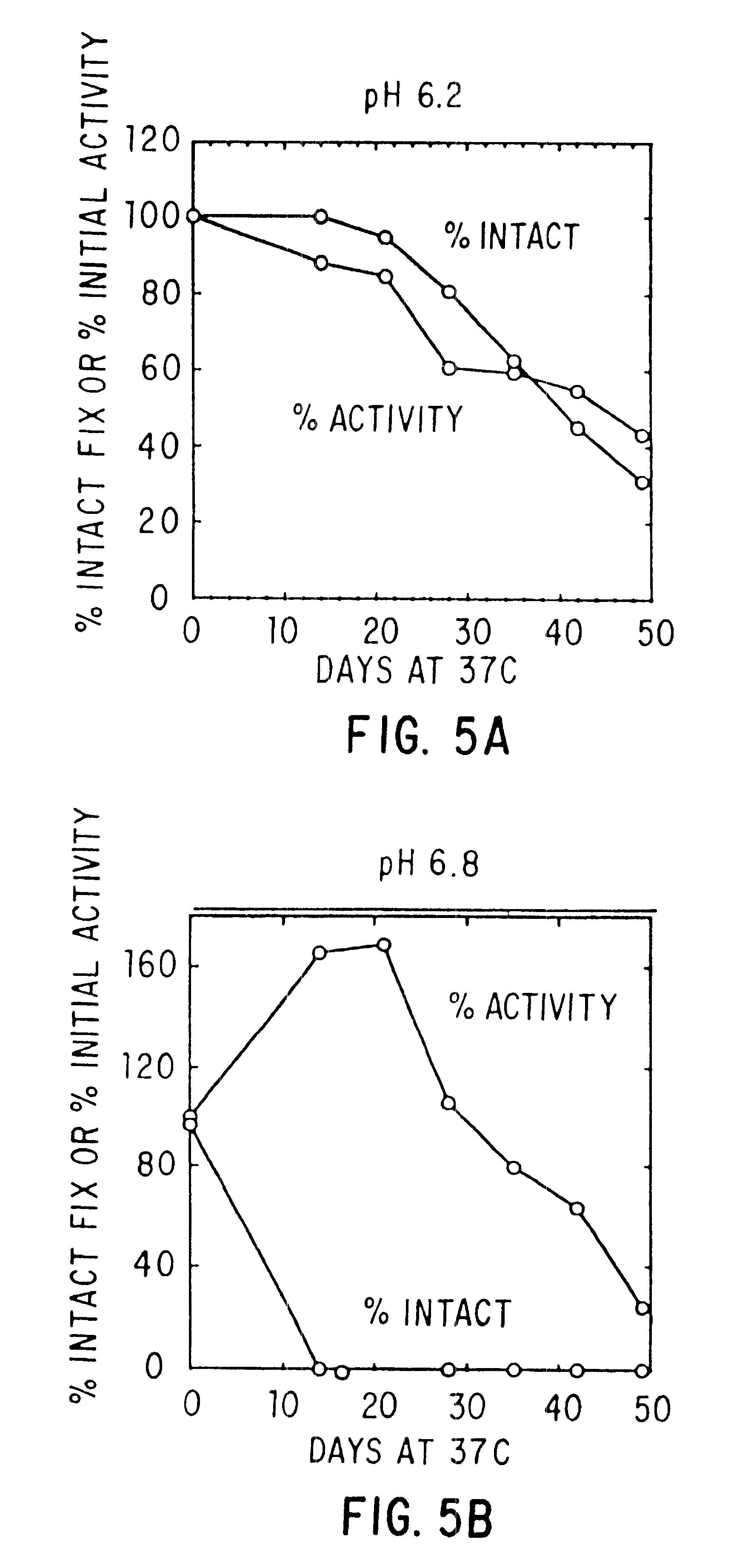
The present invention relates to the preparation and use of liquid formulations of plasma proteins, particularly blood coagulation factors. More specifically, the present invention relates to stable liquid formulations of Factor VIII and Factor IX that can be administered by injection or infusion to provide a constant level of the coagulation factor in the blood.
Owner:THE COALITION FOR HEMOPHILIA B +1
Pharmaceutical composition comprising factor VII polypeptides and protein C inhibitors
InactiveUS20060013812A1Improved and reliable and widely applicableGood coagulationFactor VIIPeptide/protein ingredientsMedicineProtein C inhibitor
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and a protein C inhibitor, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
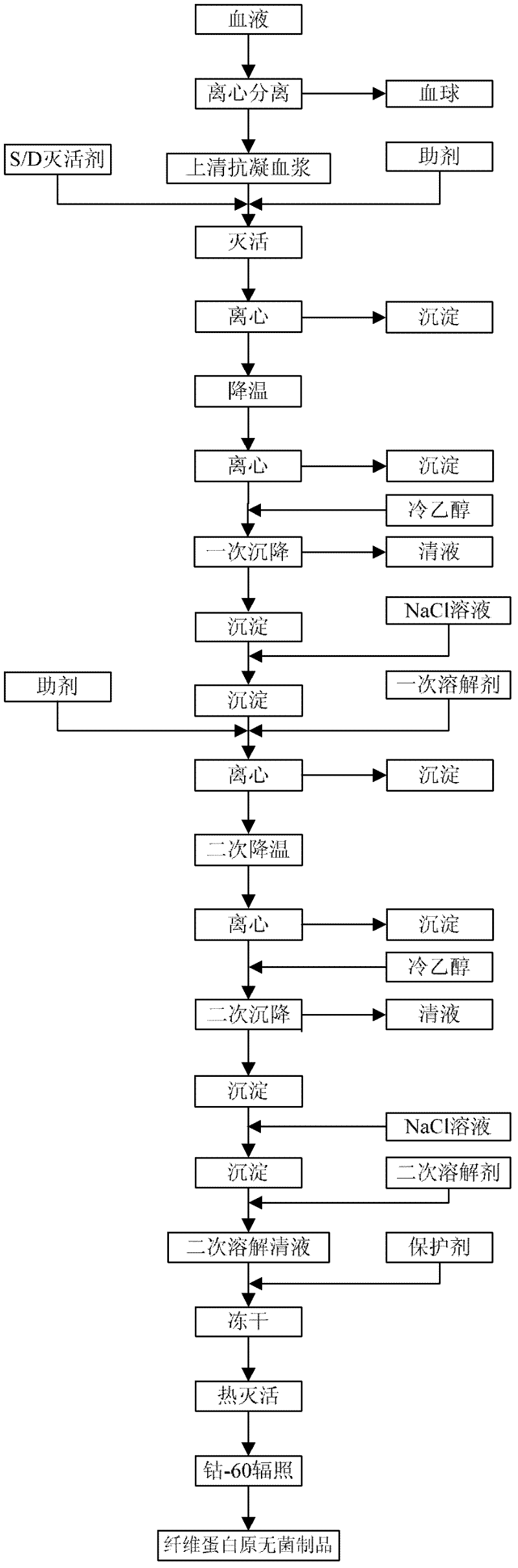
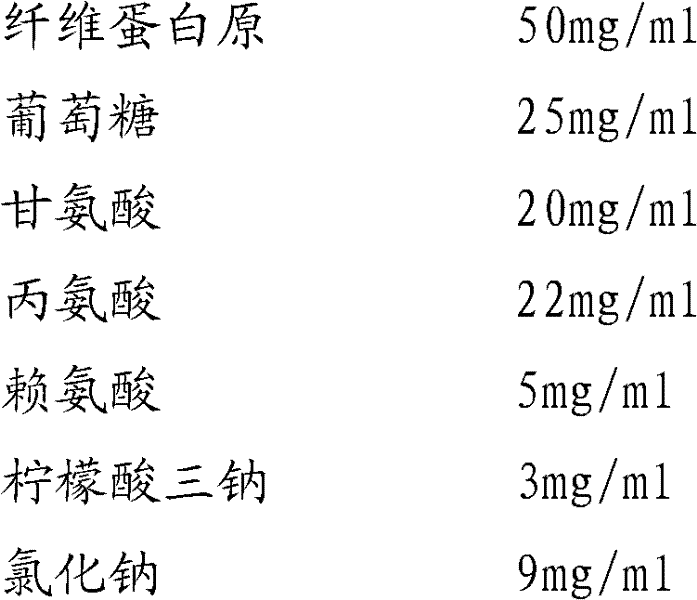
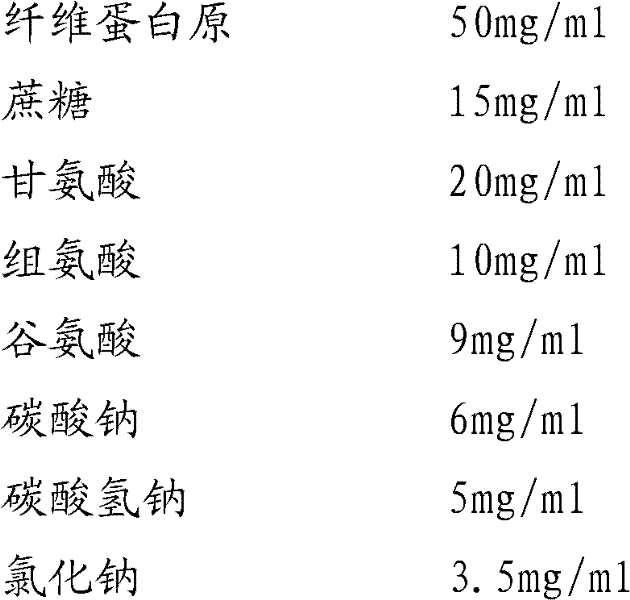
Preparation method of fibrinogen
ActiveCN102286095AHigh purityImprove extraction efficiencyFibrinogenPeptide preparation methodsSolubilityFreeze-drying
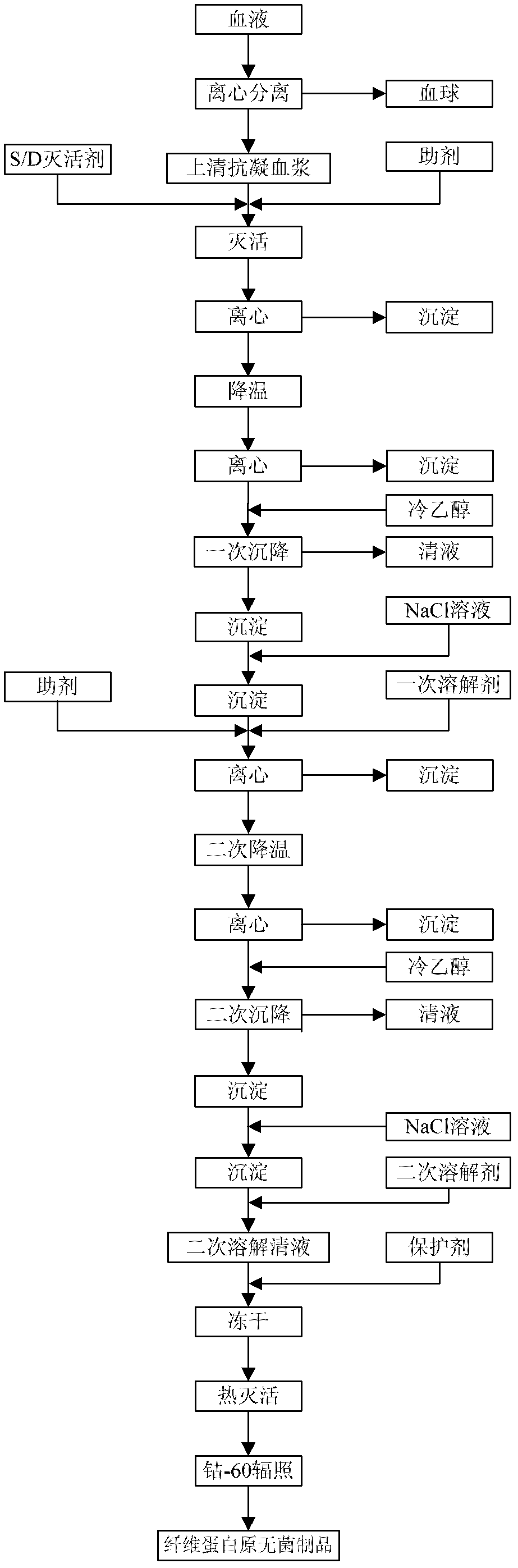
The invention provides a preparation method for fibrinogen with the advantages of high dissolution rate in the production process, short production period, fast solubility in clinical use, good biological activity, high safety in clinical use and high yield. An aid is added into supernate anticoagulant plasma. Compared with the prior art, the preparation method has the advantages that: 1, the preparation method provided by the invention is high in extraction efficiency; 2, the aid adopted in the method can improve the quality of the fibrinogen product, and plays a role in protecting the biological activities of the fibrinogen and blood coagulation factors XIII in virus inactivation and cold ethanol settlement processes; 3, in the process, the process solubility time of the fibrinogen is shortened to about 10 minutes; and 4, the solubility time of the freeze dried product of the fibrinogen is within 30 seconds, so precious time is won for rescuing patients in time in clinic.
Owner:DATIAN HUACAN BIO TECH
Factor VIII:Ca chromogenic assay
InactiveUS6100050AGood reproducibilityHigh degree of sensitivityHydrolasesMicrobiological testing/measurementBlood coagulation factor VIIIFactor ii
A chromogenic assay for determination of blood coagulation Factor VIII:Ca, using an indicator of Factor Xa simultaneously as a measure of Factor VIII:Ca concentration and as an inhibitor of Factor Xa. This technique can be applied to measure the concentration of an activating enzyme using the rate of conversion of the indicator molecule of the product of that enzyme as an indirect indicator of enzyme concentration.
Owner:DADE BEHRING
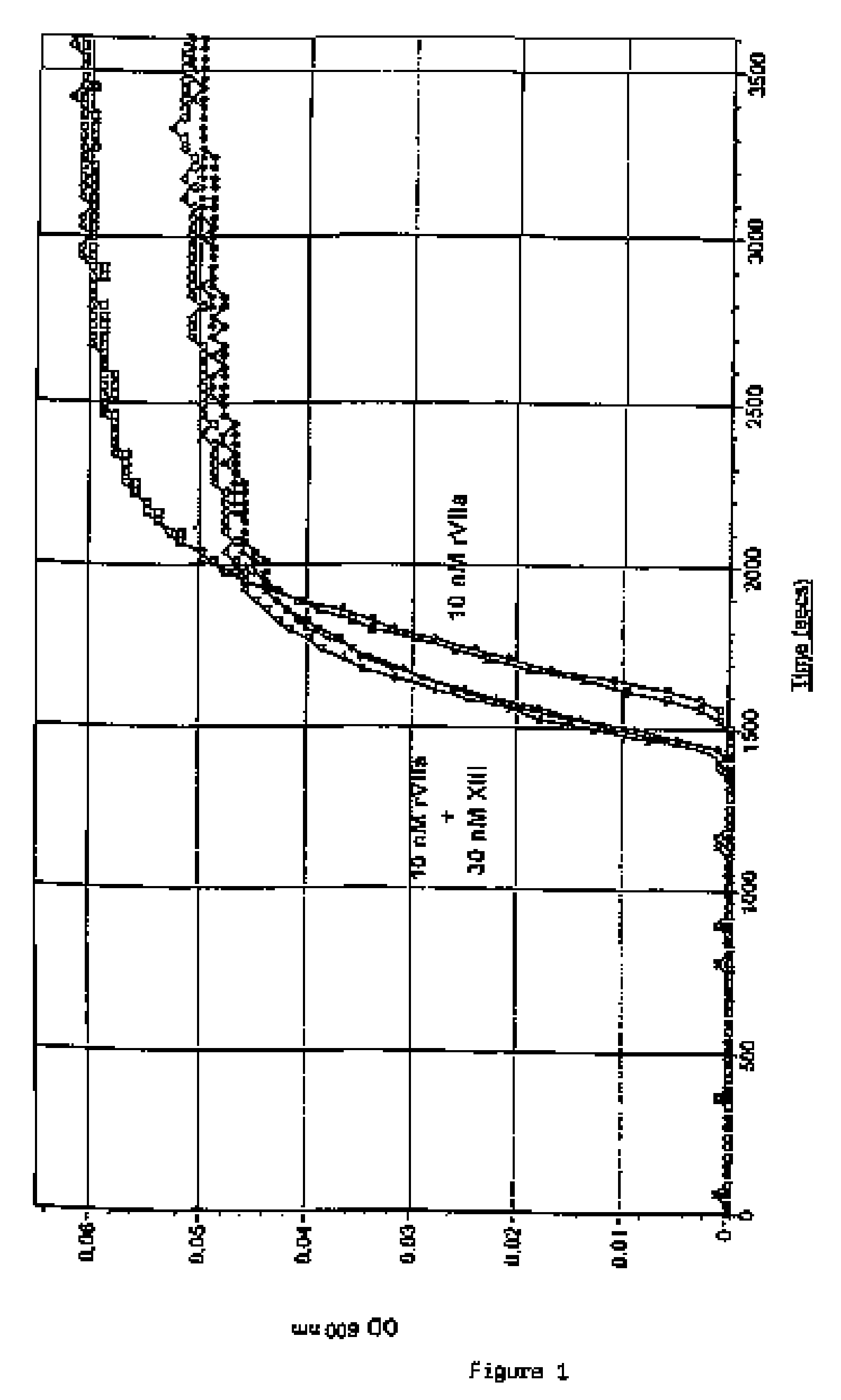
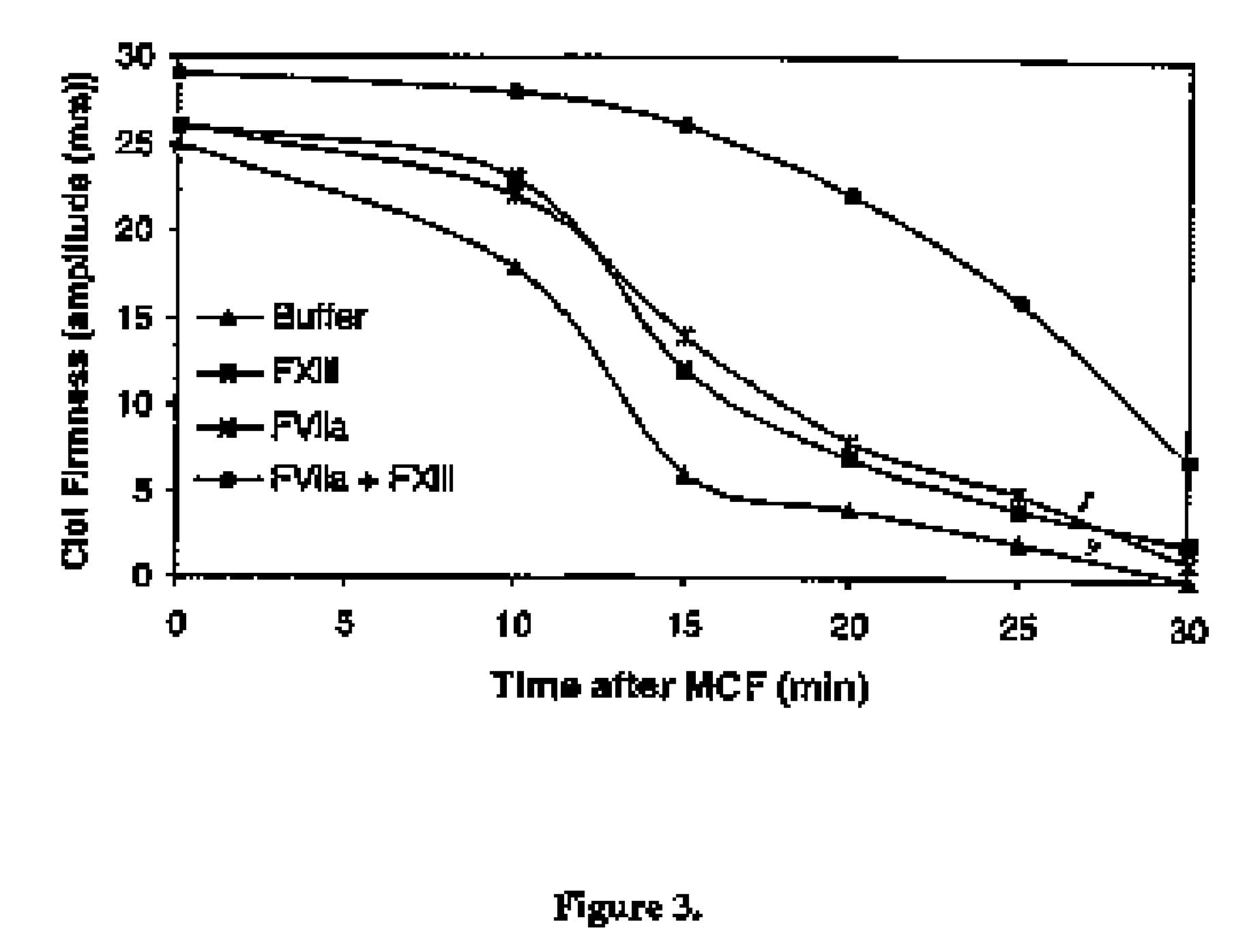
PHARMACEUTICAL COMPOSITION COMPRISING A FACTOR VIIa AND A FACTOR XIII
InactiveUS20070280920A1Effective treatmentHigh levelPeptide/protein ingredientsSurgical drugsFactor VIIaPharmaceutical drug
The present invention relates to the use of a factor VIIa and a factor XIII in the treatment or prophylaxis of bleeding episodes.
Owner:NOVO NORDISK HEALTH CARE AG
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
Device and method for preparing and administering one-component fibrin sealant
ActiveUS20150272562A1Improve concentrationReduce concentrationSurgical adhesivesPeptide/protein ingredientsFactor iiClot formation
Provided herein are systems for preparing and delivering fibrin sealant to a surface and methods of use thereof. In one embodiment, the system comprises: a. a quantity of a liquid mixture disposed within a container, the mixture comprising: I. fibrin or II. fibrinogen and Factor II; and b. a resin bed disposed within a vessel, the vessel capable of being in fluid communication with the container, wherein when in fluid communication, passage of the mixture through the vessel results in modification of the concentration of small molecules inhibitor(s) and / or inducer(s) within the mixture, favoring fibrin clot formation.
Owner:ETHICON INC +1
Factor IX variants with clotting activity in absence of their cofactor and their use for treating bleeding disorders
The present invention relates to variants of a vitamin K-dependent serine protease of the coagulation cascade, preferably variants of factor IX (F.IX), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Pharmaceutical Composition Comprising Factor VII Polypeptides and Protein C Inhibitors
InactiveUS20080102064A1Effective treatmentPeptide/protein ingredientsSurgical drugsMedicineProtein C inhibitor
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and a protein C inhibitor, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK HEALTH CARE AG
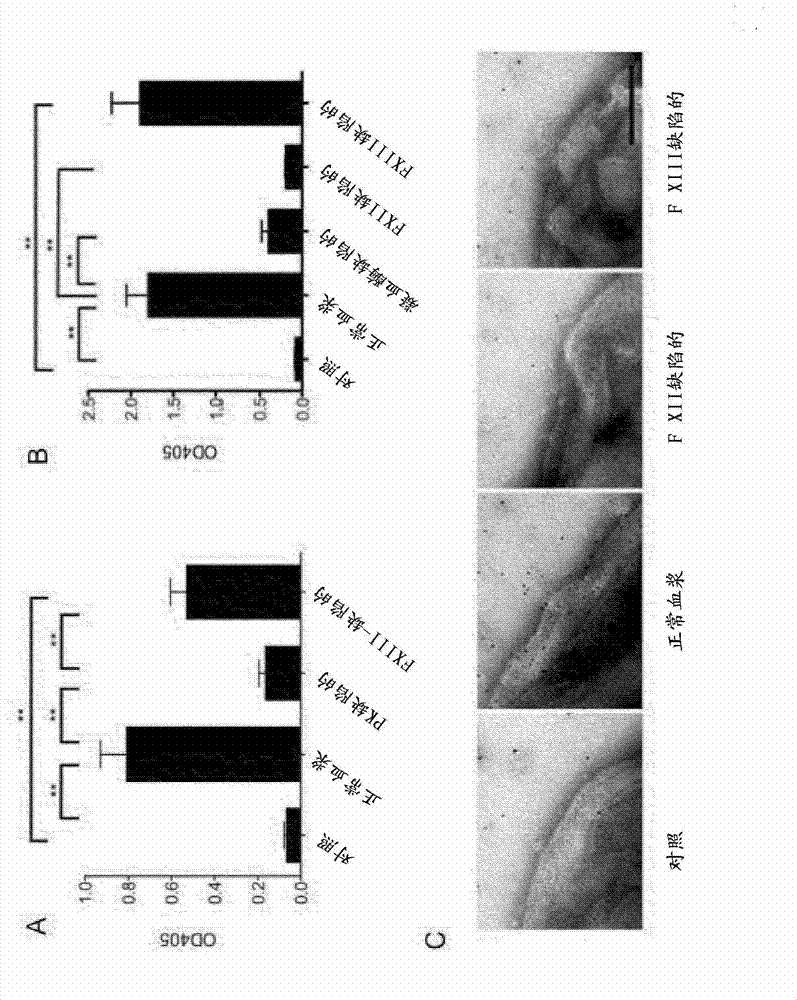
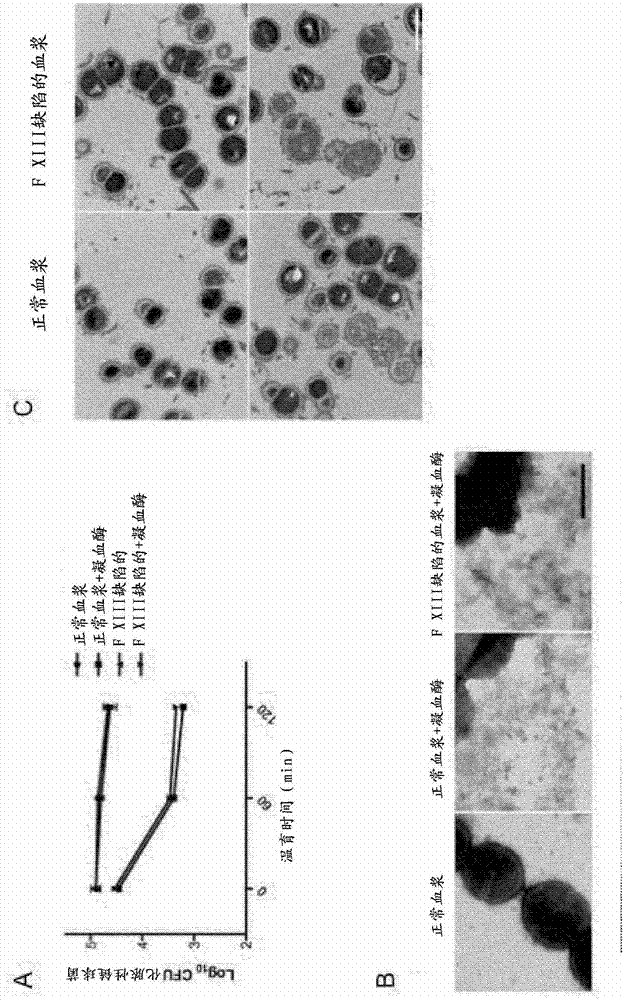
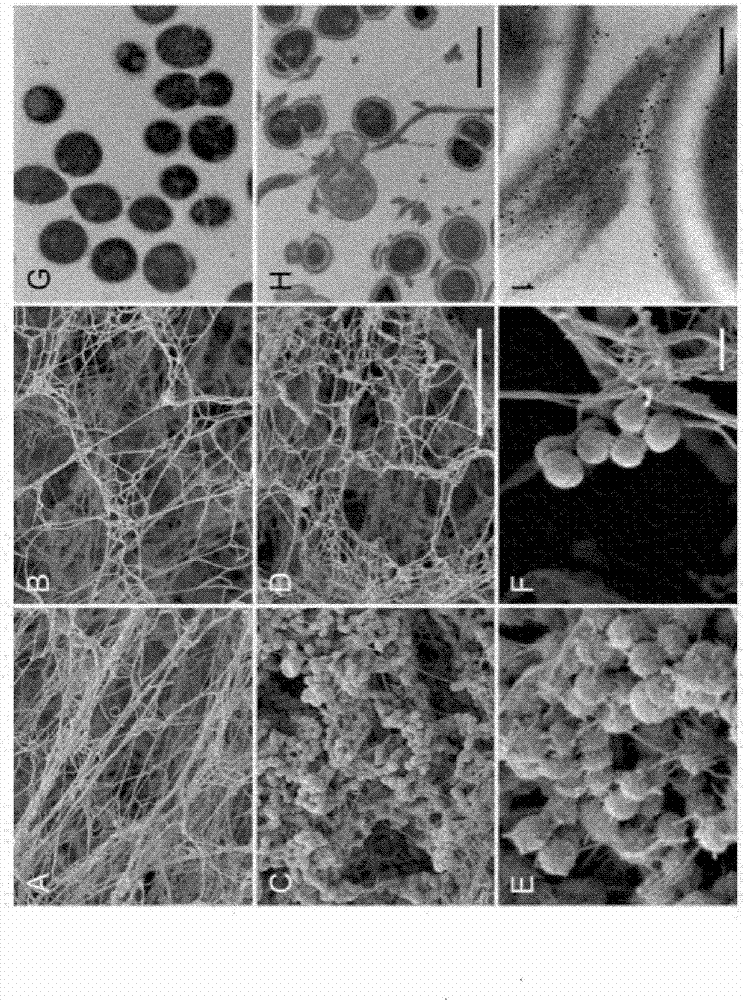
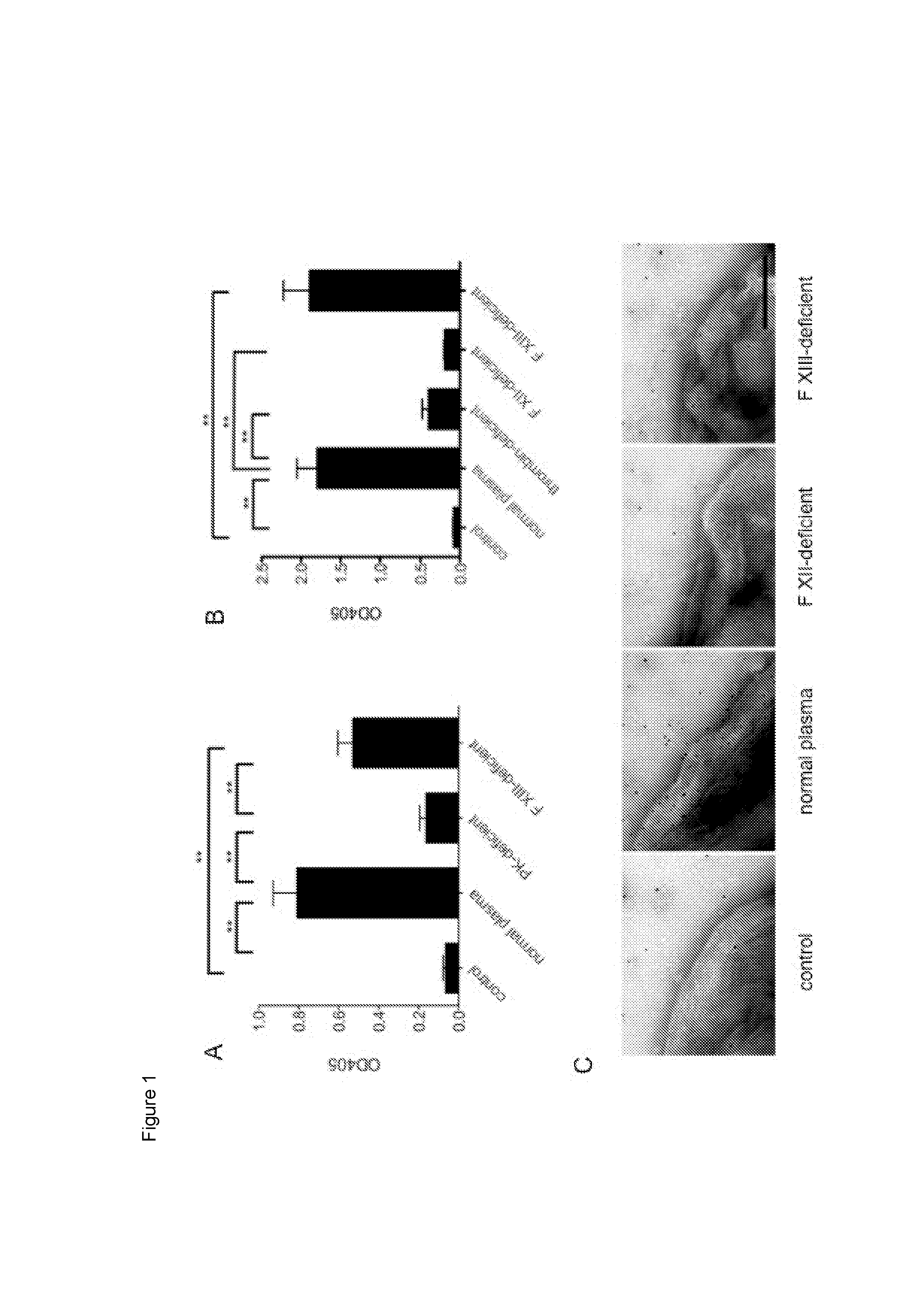
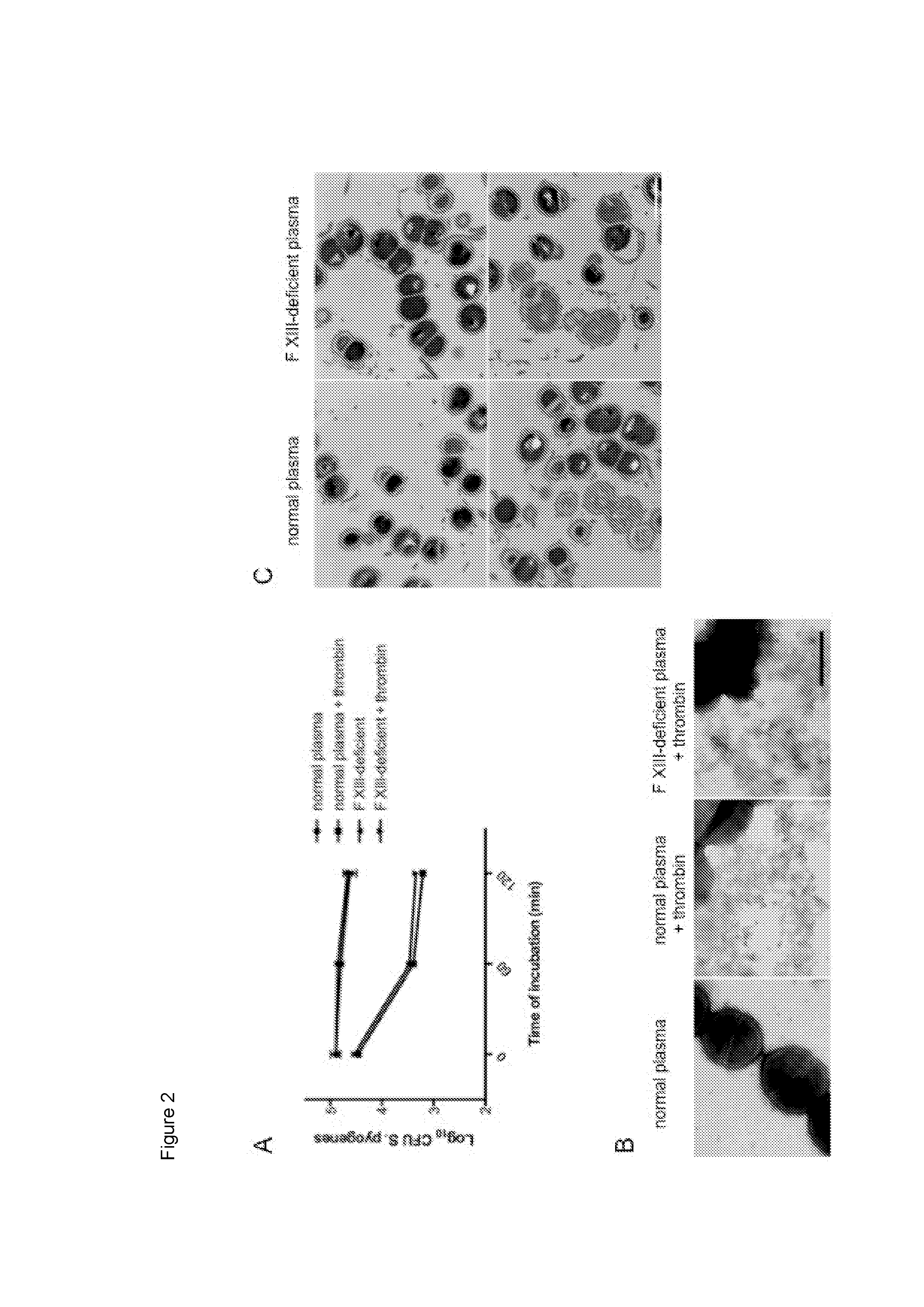
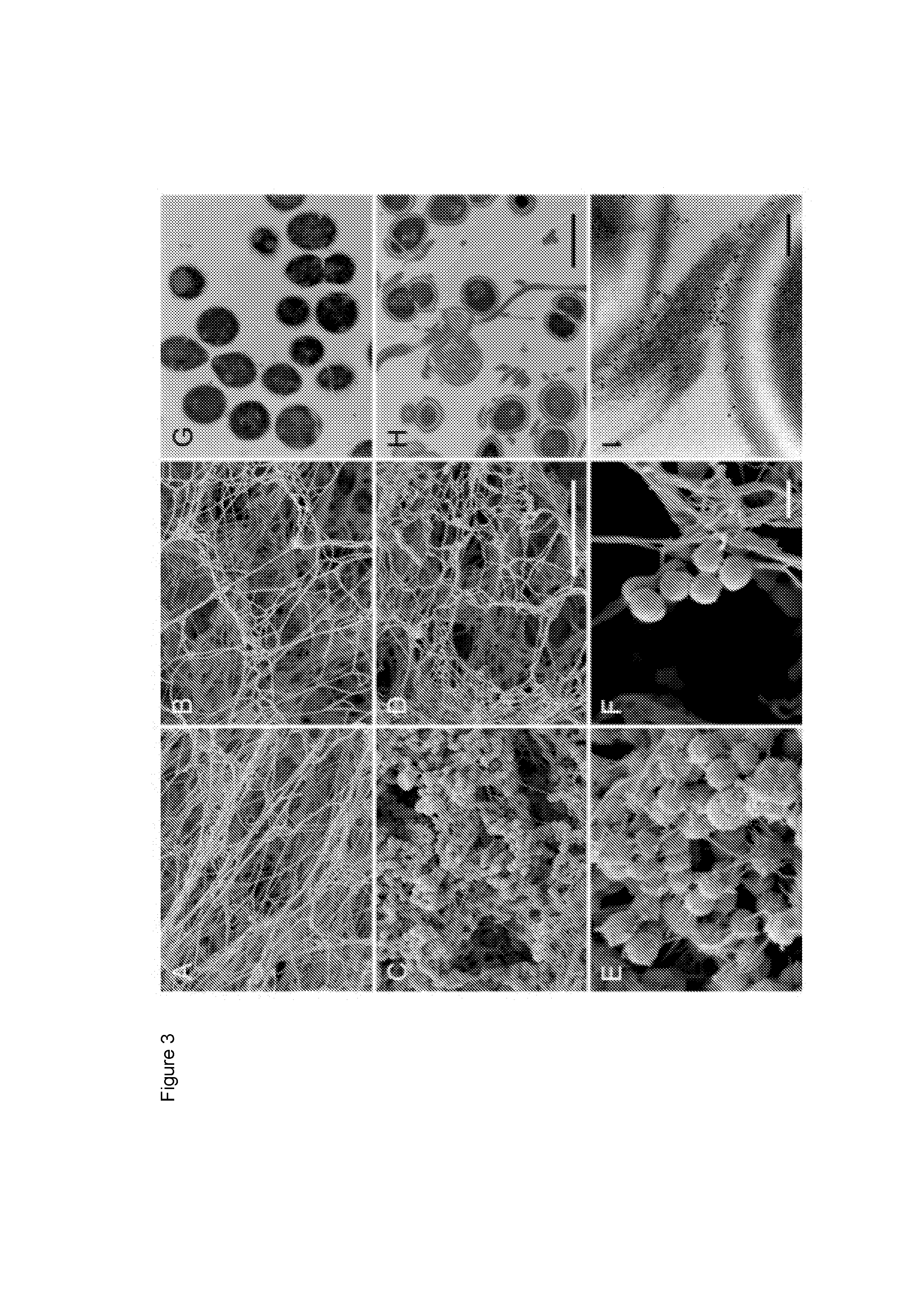
An ancestral serine protease coagulation cascade exerts a novel function in early immune defense
The present invention relates to blood coagulation factor XIII (FXIII) for treatment and / or prevention of an infection by a microorganism and / or the symptoms associated with said infection, a pharmaceutical composition comprising a pharmaceutically effective amount of said FXIII, a method for the manufacture of a medicament comprising a pharmaceutically effective amount of said FXIII, and a method of treatment comprising administering to a patient in need a pharmaceutically effective amount of said FXIII.
Owner:CSL BEHRING GMBH +1
Use of blood coagulation factor XIII for treating hemophilia A
InactiveUS20070021340A1Peptide/protein ingredientsMicrobiological testing/measurementBlood coagulation factor XIIIHaemophilia
A patient having hemophilia A is treated by administering factor XIII generally in conjunction with factor VIII or desmopressin.
Owner:ZYMOGENETICS INC
Blood coagulation factor XIII for treating platelet disorders
Use of factor XIII for treating the symptoms of thrombocytopenia. A patient having thrombocytopenia, either chemically- or metabolically induced, is treated by administering factor XIII.
Owner:ZYMOGENETICS INC
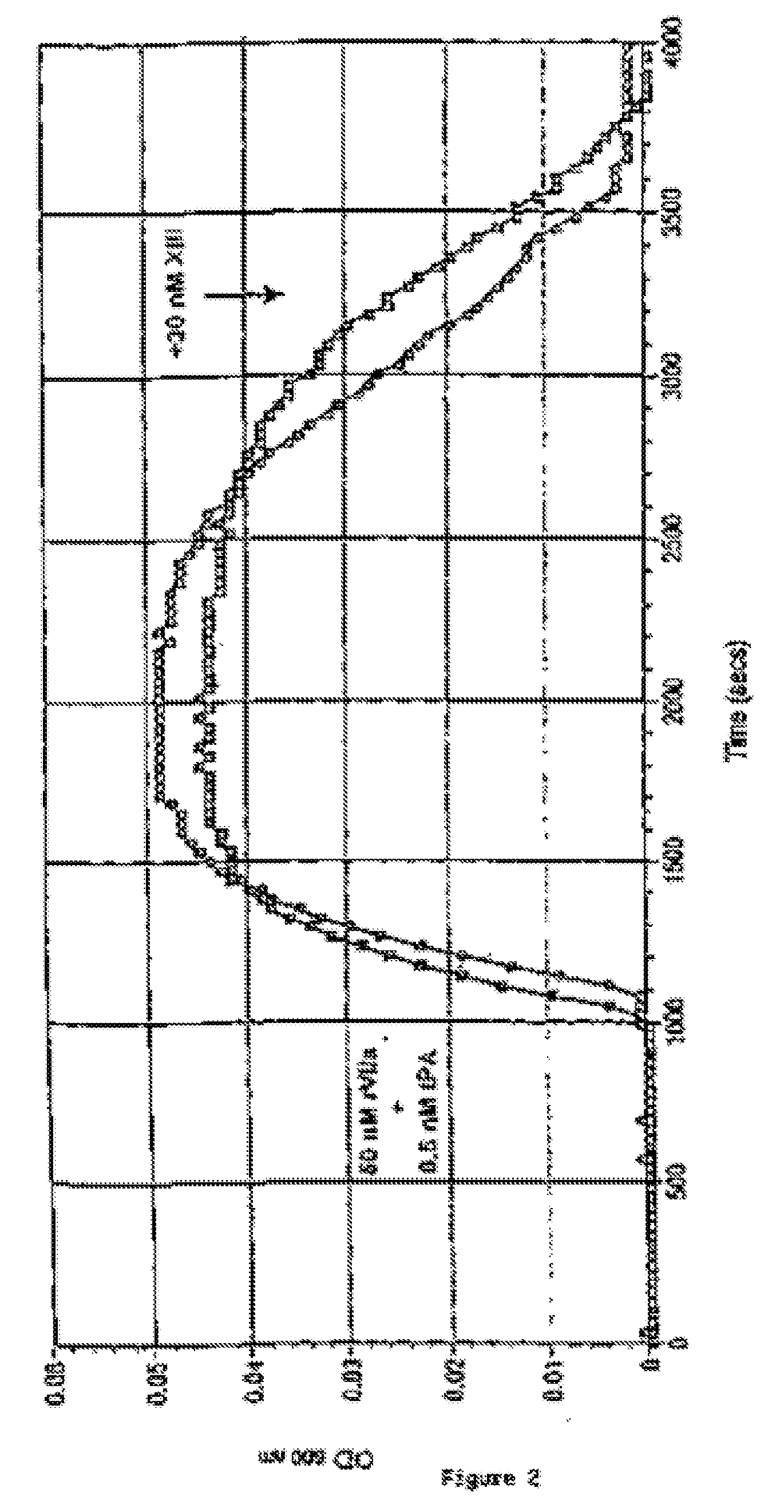
Method for differentiating between factor XIII deficiency states and fibrinogen deficiency states by means of thrombelastographic techniques
ActiveUS7939288B2Material analysis using sonic/ultrasonic/infrasonic wavesMicrobiological testing/measurementDeficiency stateMedicine
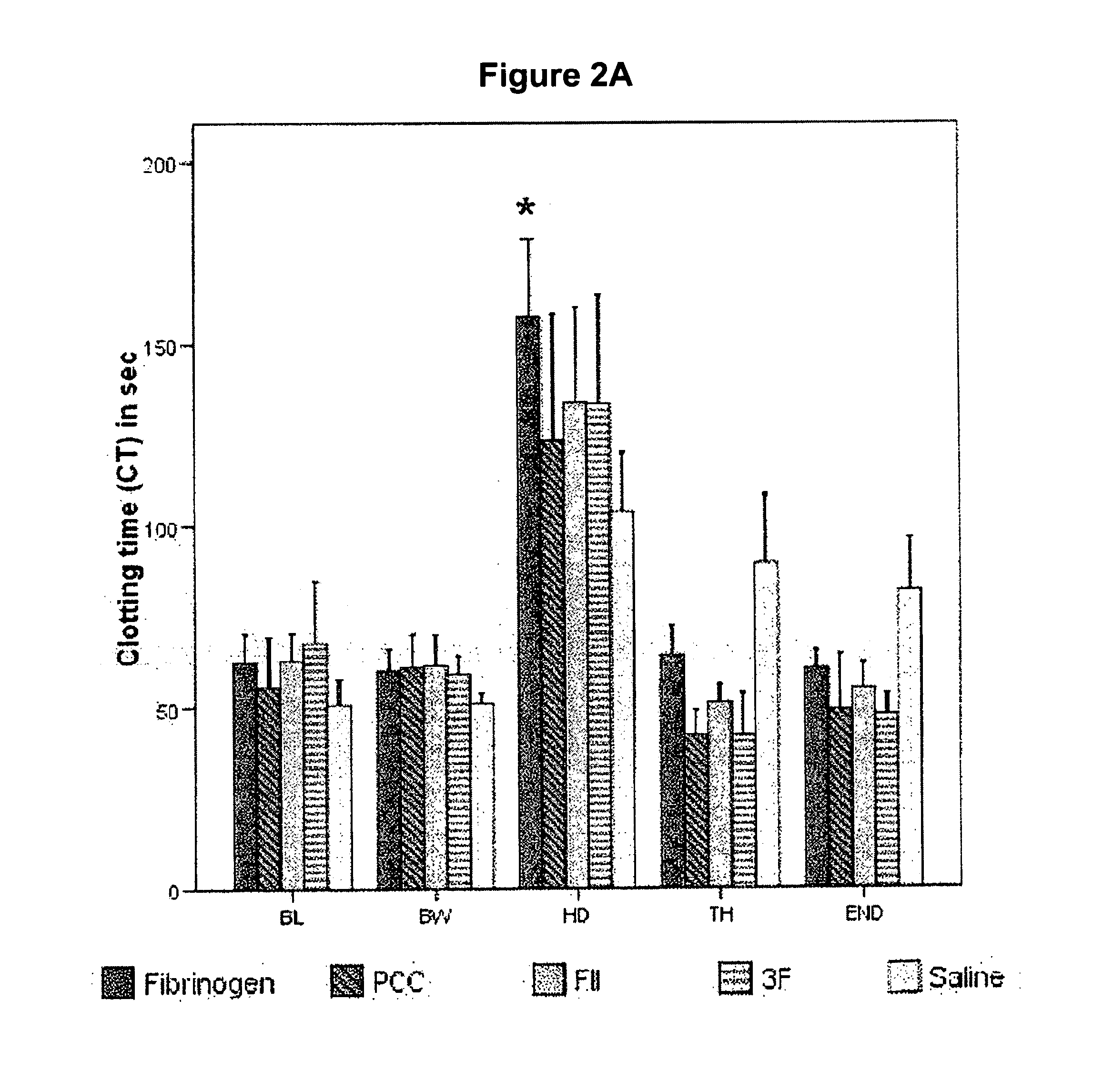
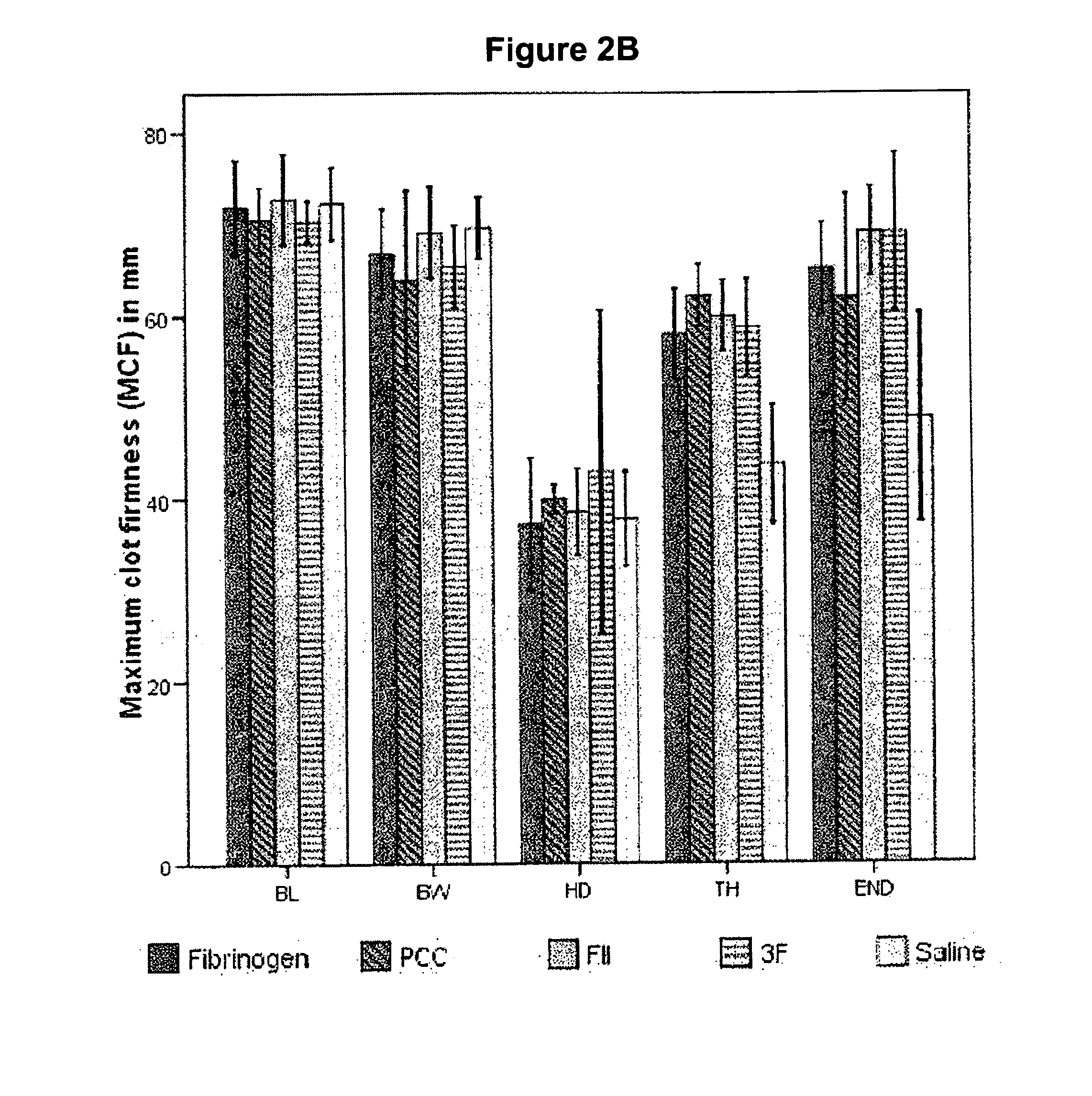
The invention relates to a method for determining a factor XIII deficiency, a method for determining a fibrinogen deficiency, and a method for differentiating between a factor XIII deficiency and a fibrinogen deficiency by means of thrombelastographic techniques. On the basis of the evaluation of the thrombelastographic parameters, a rapid and a selective substitution of factor XIII and / or of fibrinogen in deficiency states is possible.
Owner:KORTE WOLFGANG DR
Method for treating hemophilia B
Use of factor XIII for treating hemophilia B. A patient having hemophilia B is treated by administering factor XIII, generally in conjunction with factor IX.
Owner:ZYMOGENETICS INC
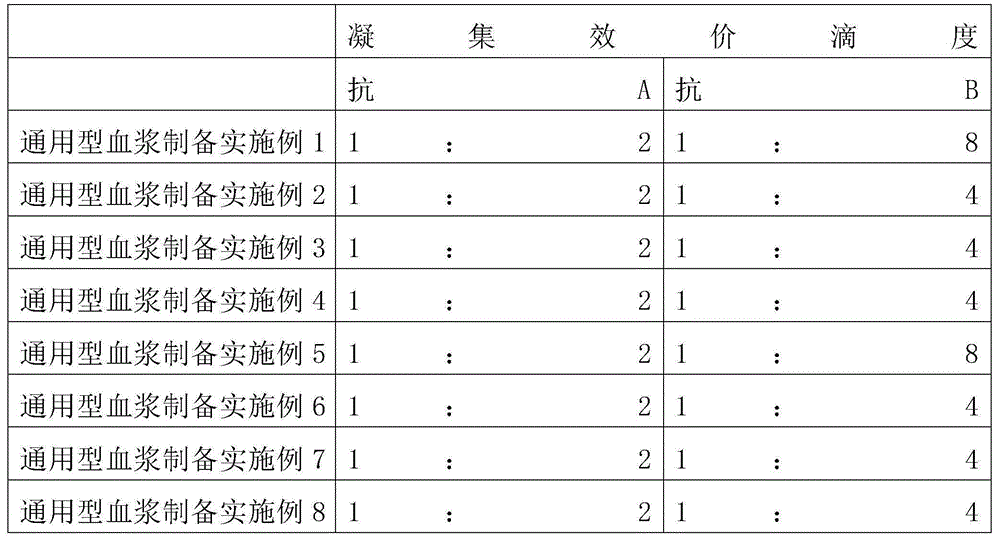
Human plasma with multiple functions and preparation method thereof
InactiveCN105832768ASave transfusion timeIncreased flexibility of usePeptide/protein ingredientsMammal material medical ingredientsPatient needBlood Coagulation Factor X
The invention provides a human plasma with multiple functions and a preparation method thereof, including general-purpose plasma and blood component additives; the general-purpose plasma is general-type fresh frozen plasma or general-purpose freeze-dried plasma; the blood component additive is selected from albumin, C Globulins, platelets, fibrinogen, factor II, factor V, factor VII, factor VIII, factor IX, factor X, factor XI, factor XII, factor XIII, and prothrombin complex any one or more of them. The advantages are: for patients who need to supplement plasma and at least one blood component additive at the same time, only the plasma including the corresponding blood component additive needs to be transfused, thereby increasing the scope and flexibility of use, saving the patient's blood transfusion time, and saving Blood transfusion equipment.
Owner:杜祖英
Preparation method for fibrinogen
ActiveCN102286095BHigh purityImprove extraction efficiencyFibrinogenPeptide preparation methodsSolubilityFreeze-drying
Owner:DATIAN HUACAN BIO TECH
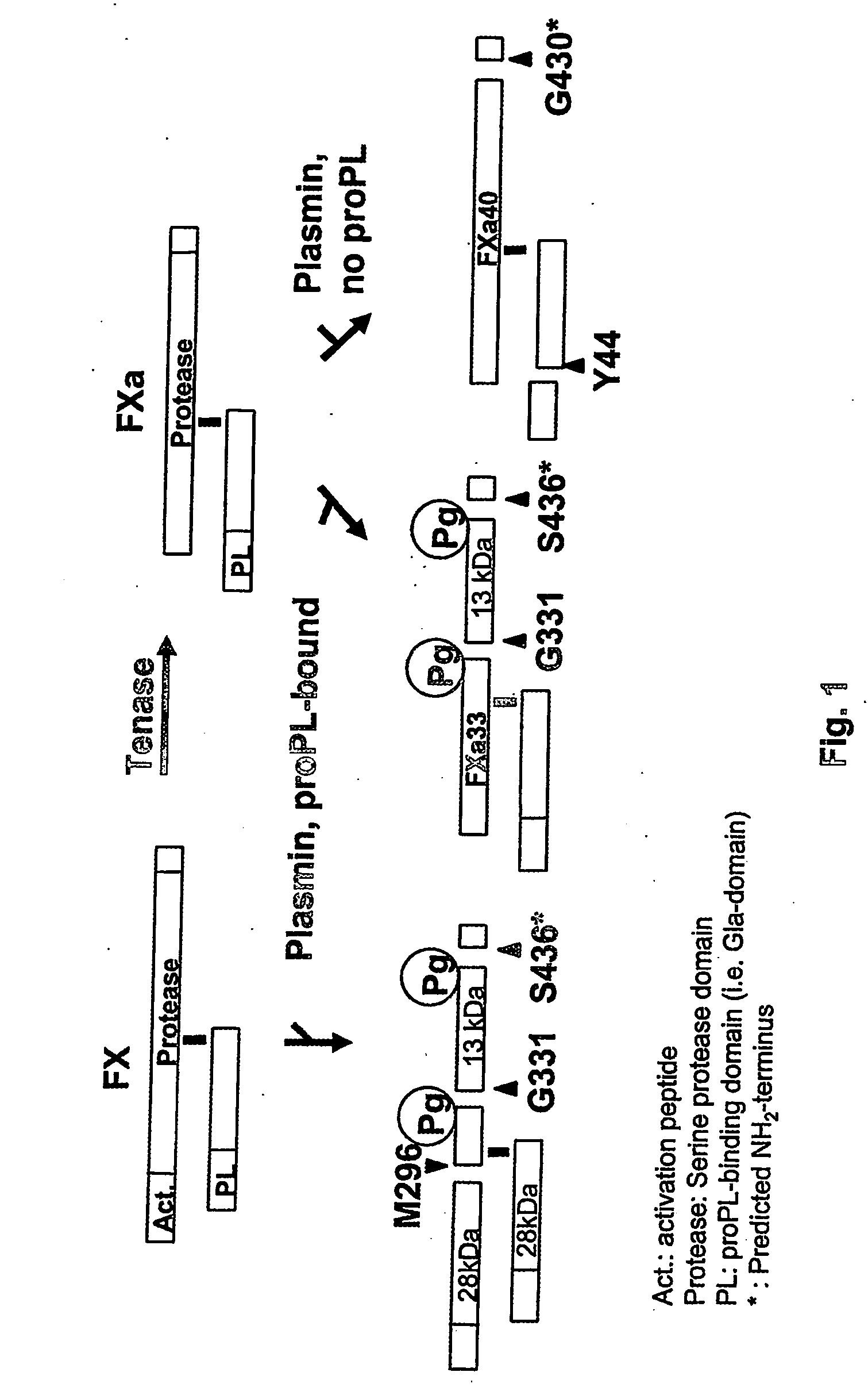
Crystal structure of factor Vai and method for identifying blood factor Va modulators
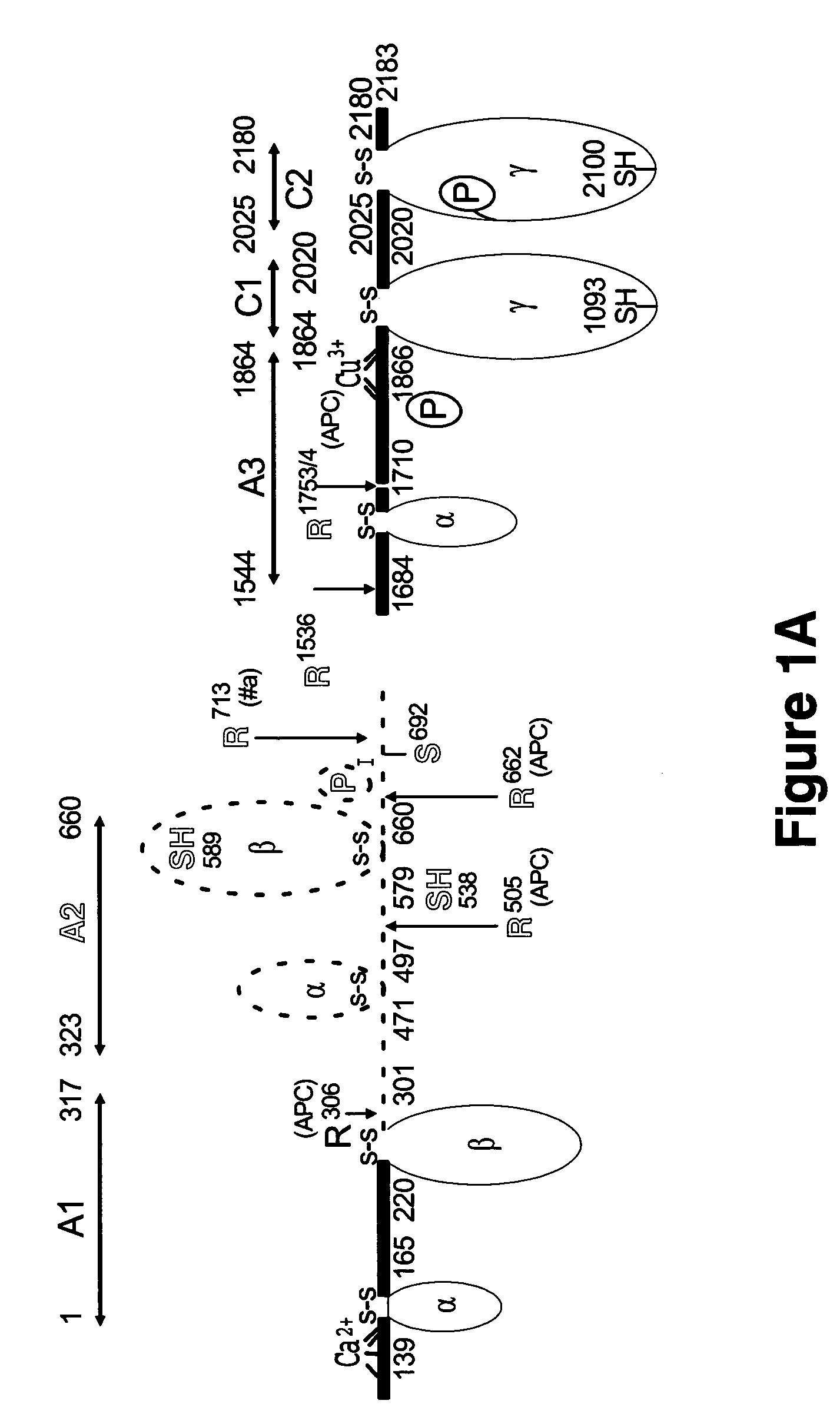
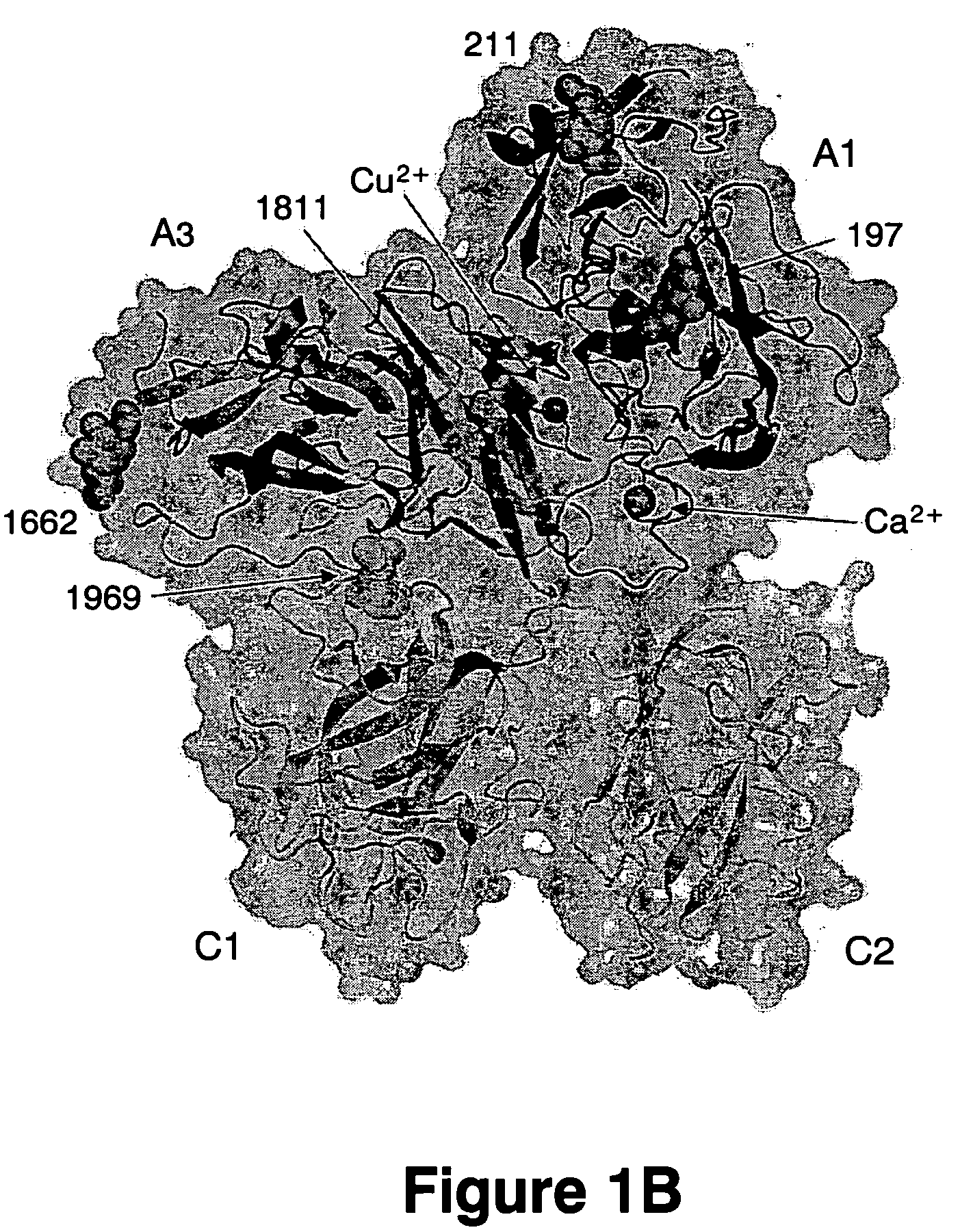
The present invention shows the crystal structure of protein C inactivated factor Va (A1-A3-C1-C2) that depicts a novel domain arrangement. The newly disclosed orientation has implications for binding to membranes essential for function. A high-affinity calcium binding site and a copper binding site have been identified, neither of which show a direct involvement in chain association. This structure represents the largest physiologically relevant fragment of factor Va solved to date and provides a new scaffold for generation of models of coagulation factors.
Owner:EVERSE STEPHEN J +3
Fusion proteins comprising factor ix for prophylactic treatment of hemophilia and methods thereof
InactiveCN105848669AExtended half-lifeIncremental Recovery HighPeptide/protein ingredientsSerum albuminDosing regimenPhysiology
Owner:JET
Uses of a2 domain of von willebrand factor
PendingUS20220193204A1Improve survivalImprove bleedingPeptide/protein ingredientsAntiviralsFactor VIII vWFVon willebrand
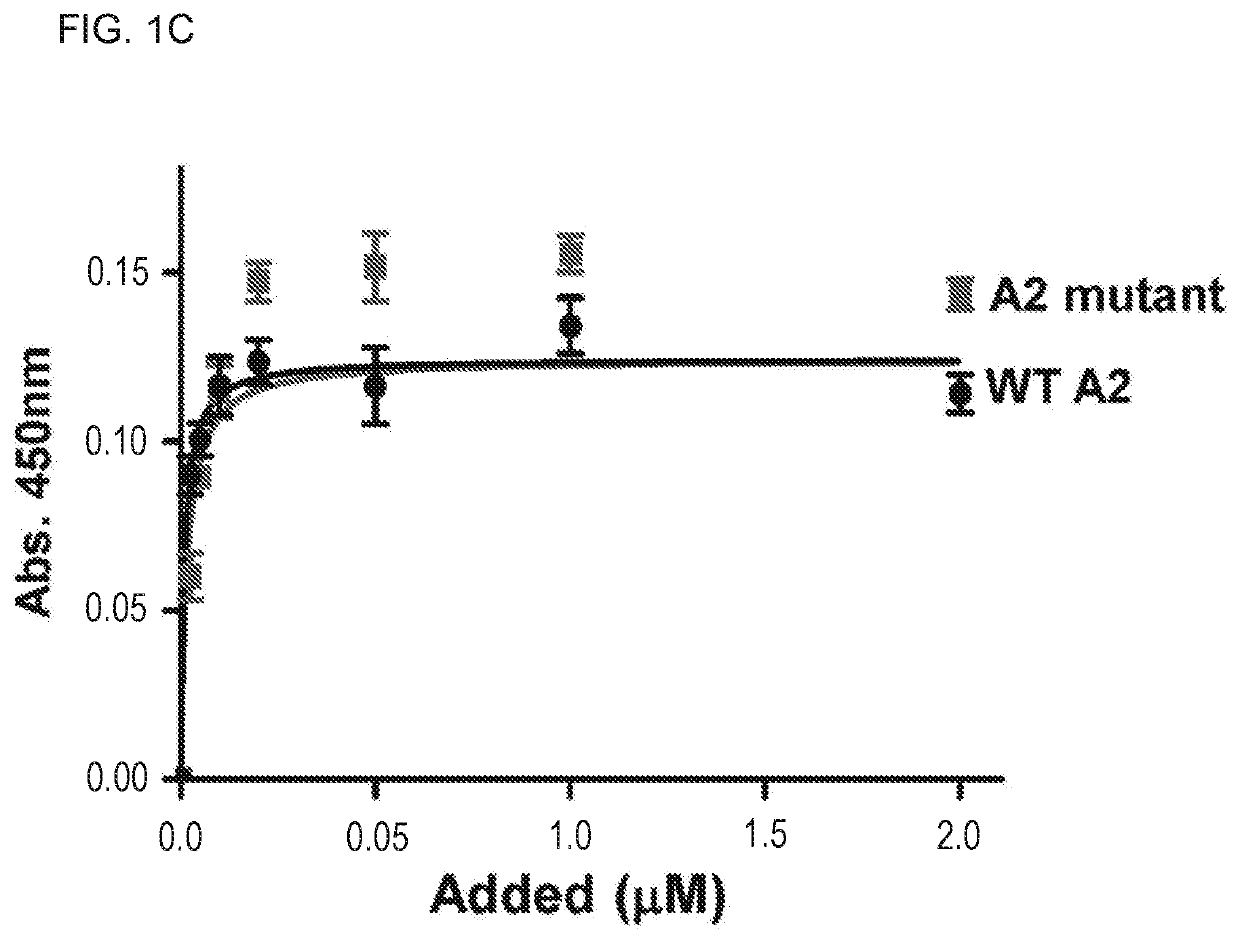
Embodiments of the disclosure encompass methods and compositions for maintaining a healthy fibrin network in an individual. The disclosure includes methods of targeting fibrin in an individual for the purpose of restoring fibrin that is subject to a level of fibrinolysis that is deleterious, such as excessive or reduced with respect to the general population. Such modifications of fibrin in an individual may include direct targeting of fibrin with the A2 domain of von Willebrand factor or a functional derivative or fragment thereof. In specific embodiments, the methods restore to a normal level any imbalance between coagulation and inflammation.
Owner:BAYLOR COLLEGE OF MEDICINE
Use of Blood Coagulation Factor XIII for Treating Hemophilia A
InactiveUS20090036361A1Peptide/protein ingredientsBlood disorderFactor iiBlood coagulation factor XIII
Owner:ZYMOGENETICS INC
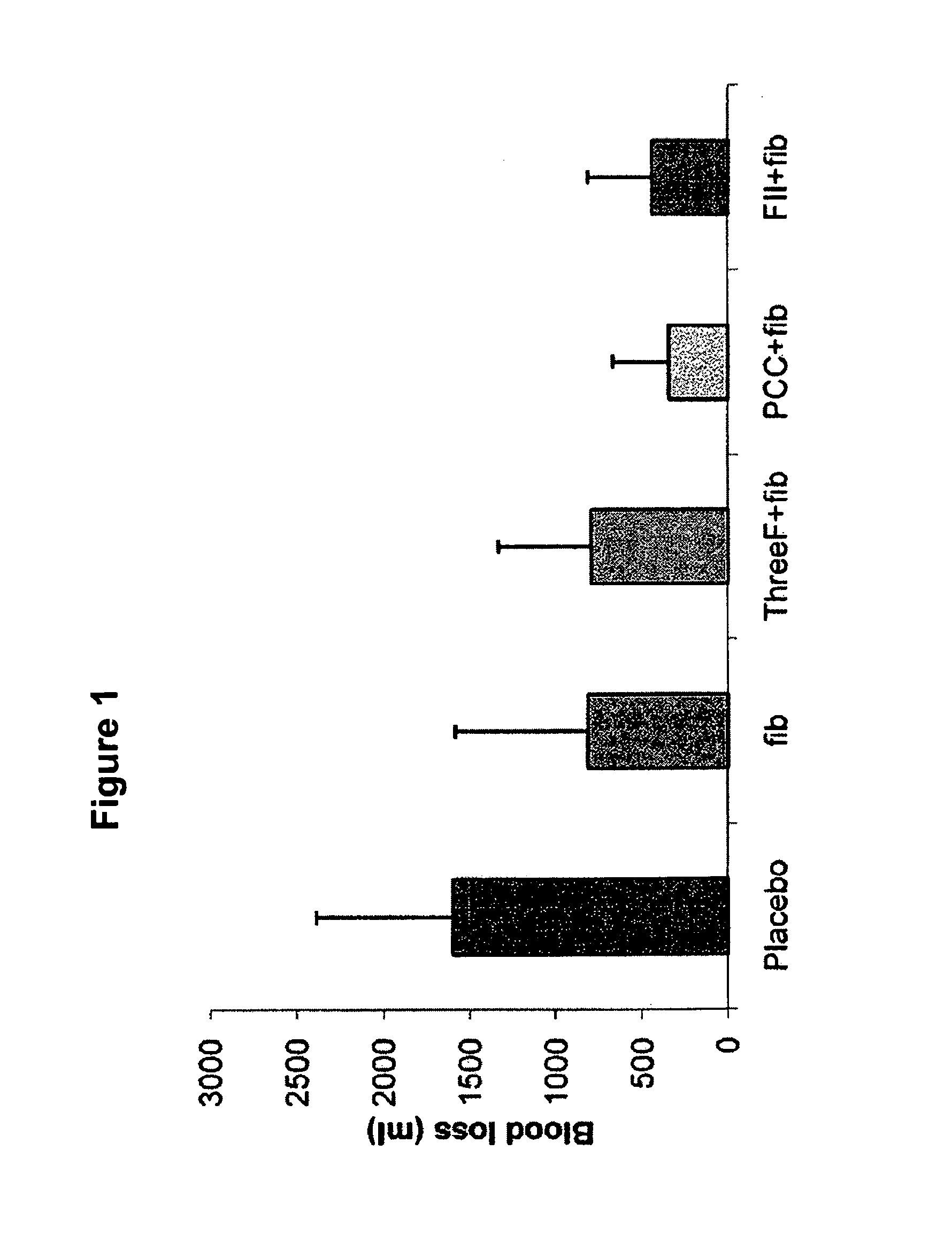
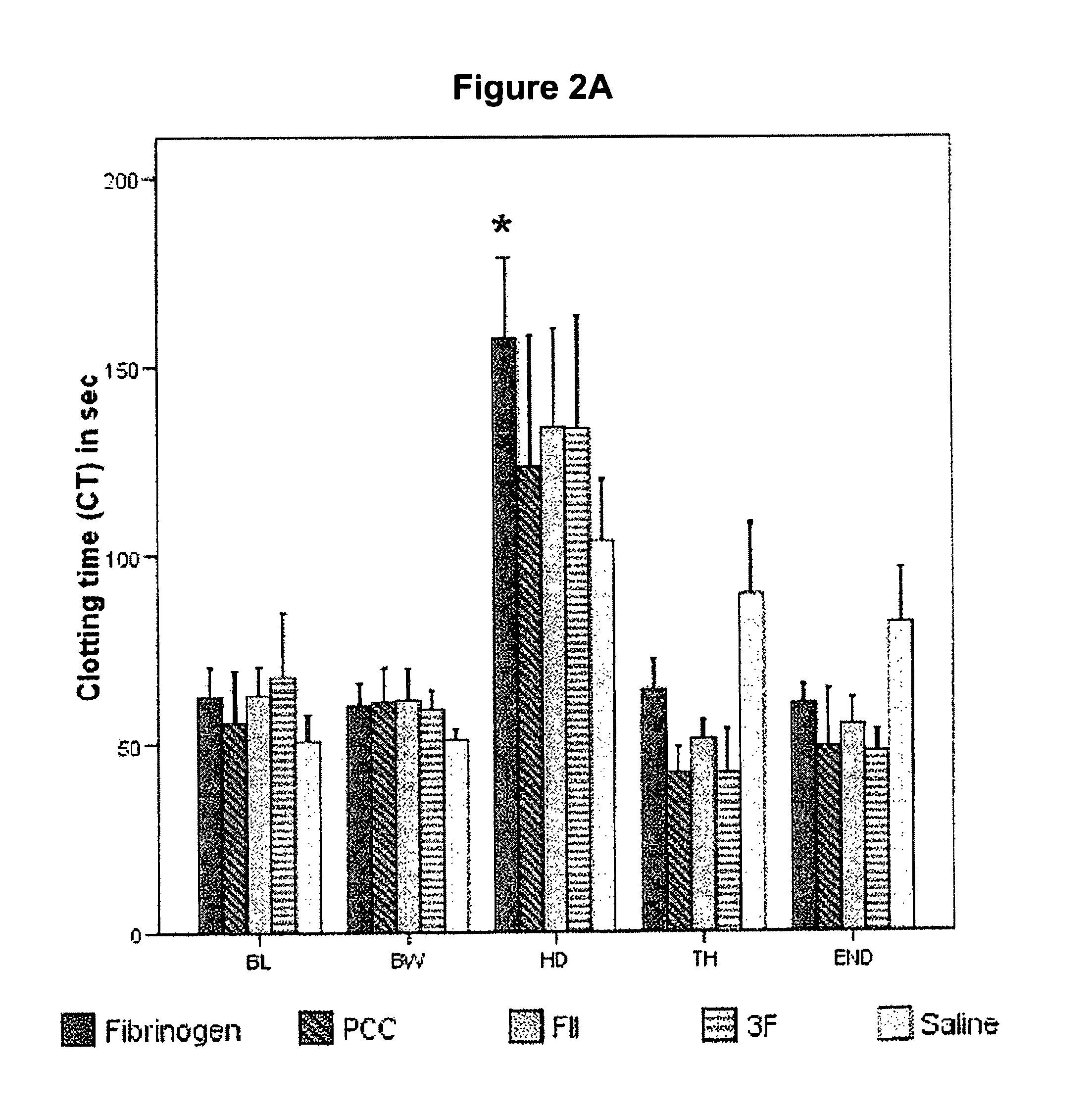
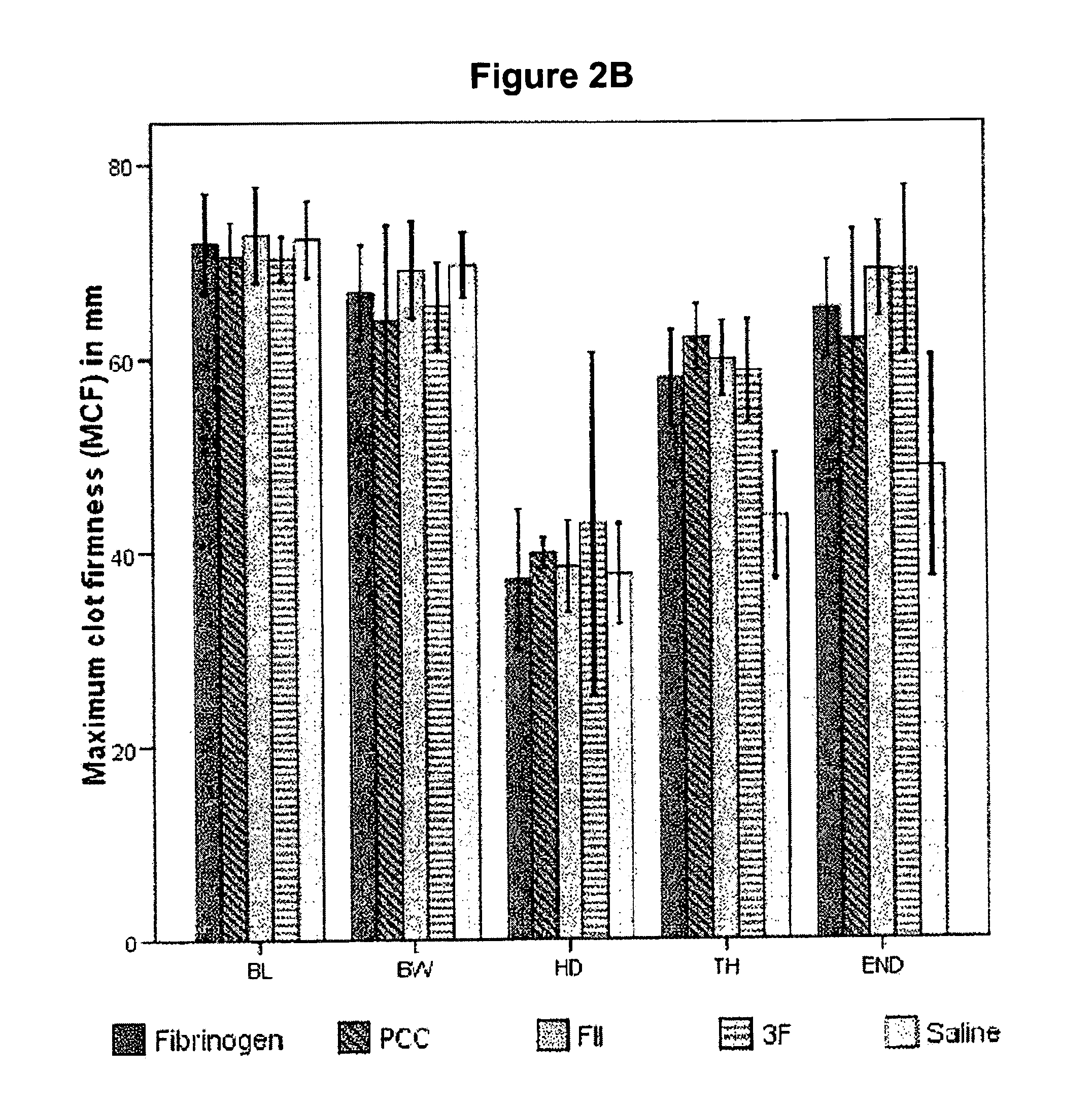
Factor ii and fibrinogen for treatment of haemostatic disorders
InactiveUS20130280236A1Effective treatmentEasy to adjustPeptide/protein ingredientsBlood disorderFactor iiClotting factor
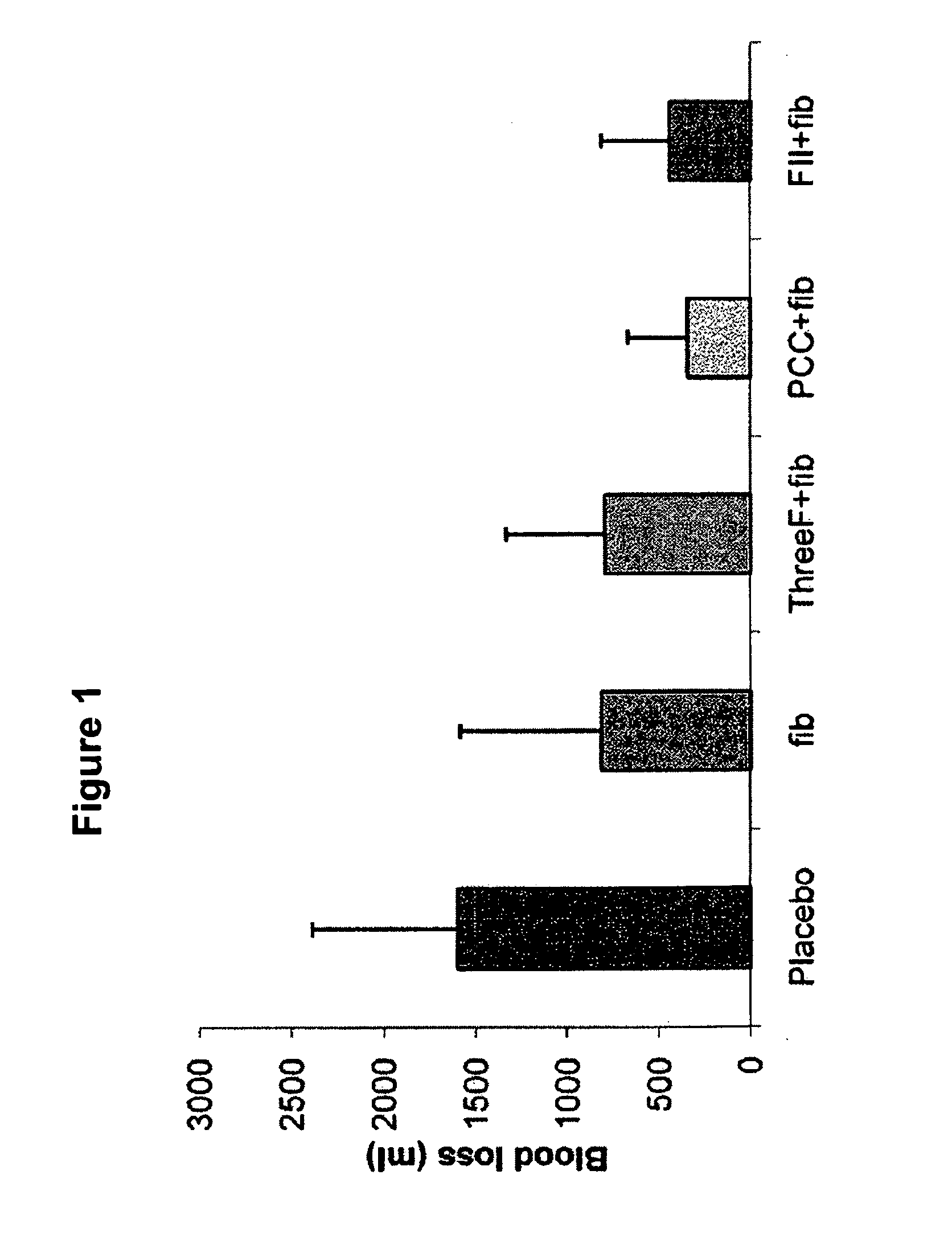
The present invention relates to normalizing impaired haemostasis comprising administering a clotting factor treatment selected from the group consisting of (1) FII; (2) PCC; and (3) a three factor combination of FH, FX and FVIIa. The clotting factor treatment can be administered in combination with fibrinogen. The clotting factor(s) can be recombinant human clotting factor(s).
Owner:MEDIMMUNE LTD
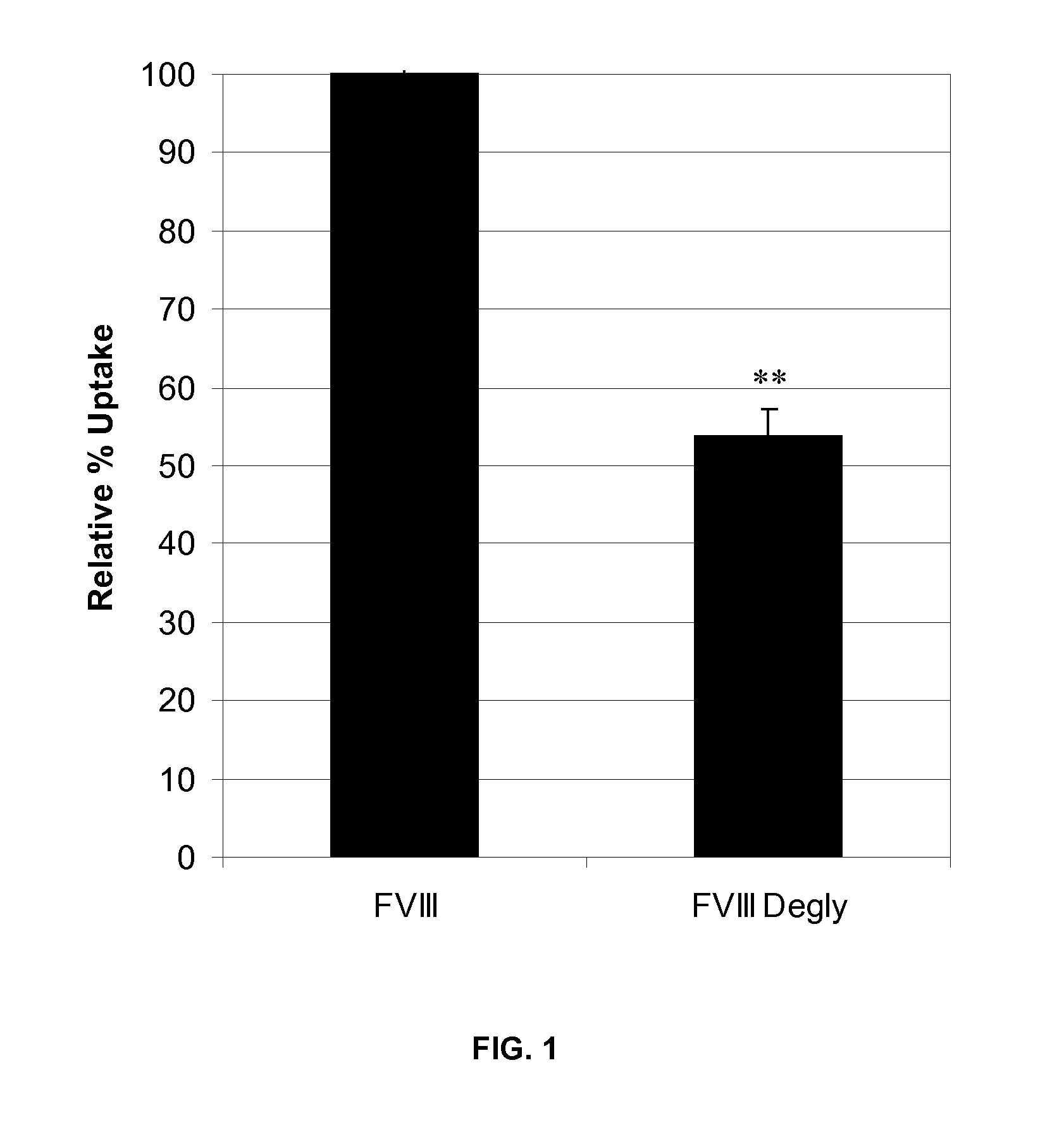
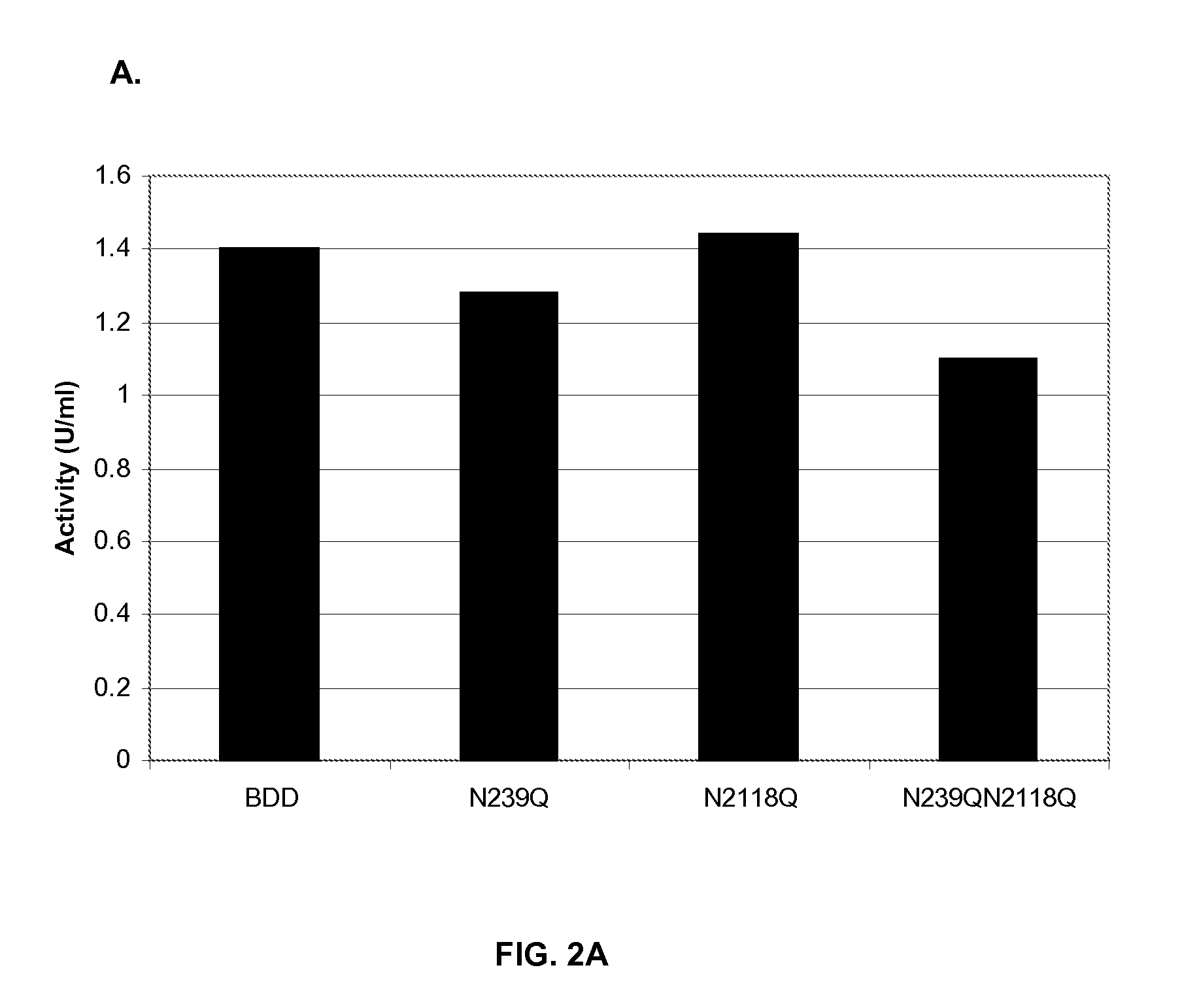
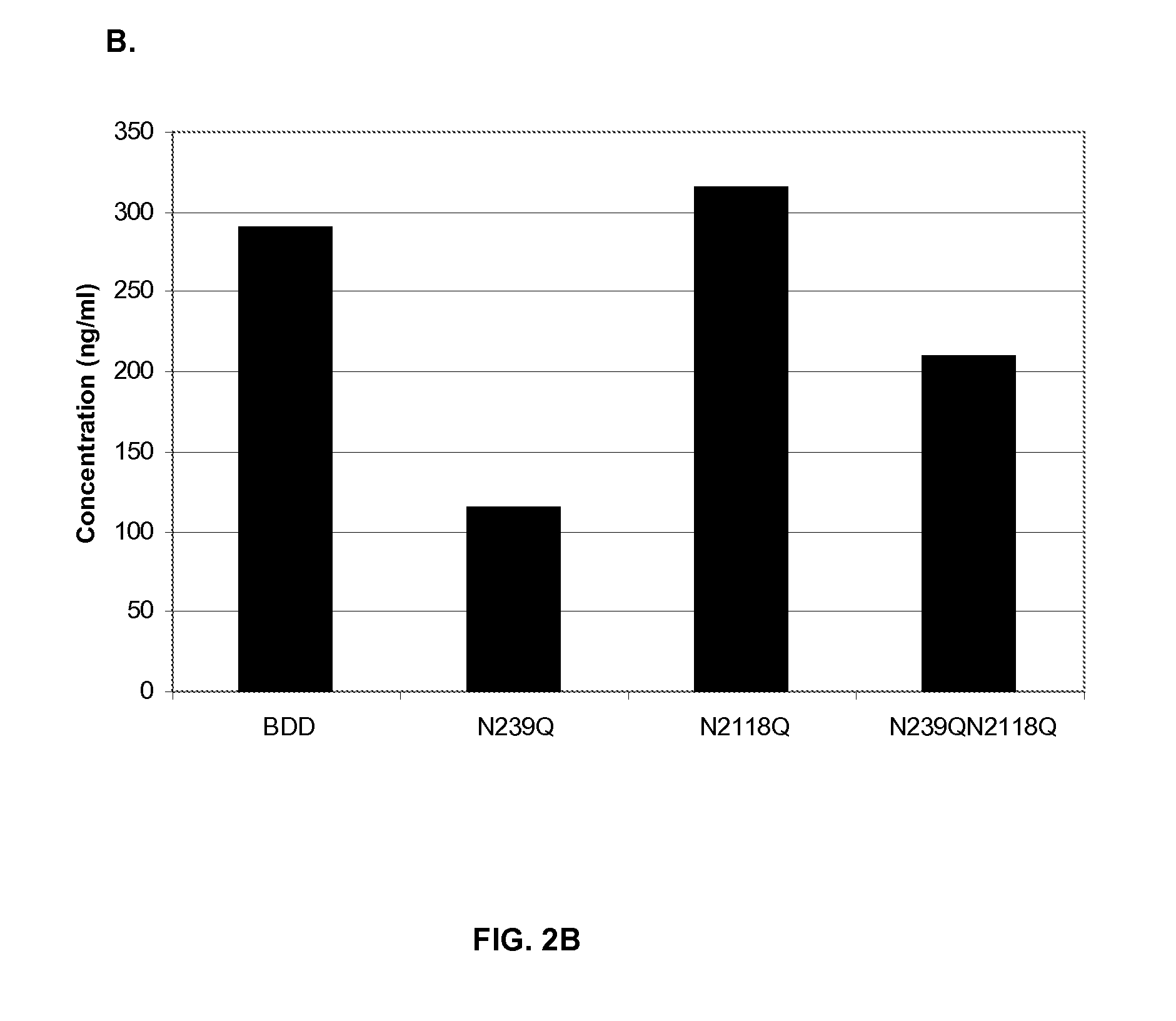
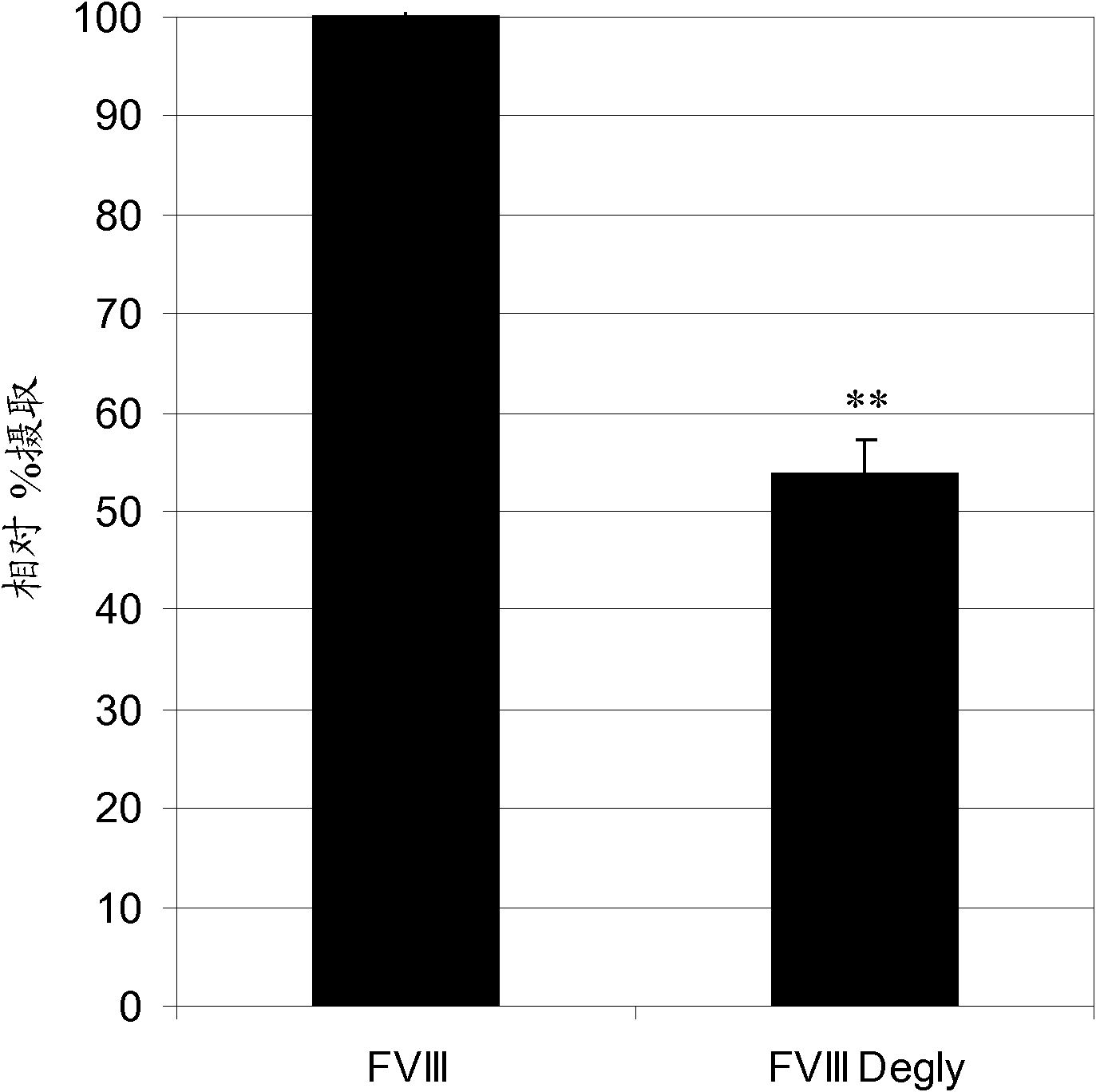
Factor VIII Muteins with Reduced Immonugenicity
The invention relates to modified Factor VIII molecules with reduced N-linked glycosylation and reduced immunogenicity. The invention also relates to methods of using modified Factor VIII molecules, for example, to treat patients afflicted with hemophilia.
Owner:BAYER HEALTHCARE LLC
Compositions, devices and methods for factor VII therapy
PendingCN114222590APeptide/protein ingredientsGenetic material ingredientsImplanted devicePharmaceutical formulation
Owner:СИГИЛОН ТЕРАПЬЮТИКС ИНК
Prodrug of triazolone compound
ActiveUS20130053563A1Improve bioavailabilityEnhanced inhibitory effectBiocideOrganic active ingredientsBlood levelOral medication
By oral administration of a compound represented by the following Formula (I): the blood level of Compound (IV): which has an excellent inhibitory action against blood coagulation factor VIIa and the anticoagulant action, reaches a level sufficient for expression of its pharmacological actions. Therefore, the compound of the present invention is useful as a therapeutic and / or prophylactic agent for diseases caused by thrombus formation.
Owner:EISIA R&D MANAGEMENT CO LTD
Factor VIII muteins with reduced immunogenicity
The present invention relates to modified Factor VIII molecules with reduced N-linked glycosylation and reduced immunogenicity. The invention also relates to methods of using modified Factor VIII molecules, for example, to treat patients afflicted with hemophilia.
Owner:BAYER HEALTHCARE LLC
Factor II and fibrinogen for treatment of haemostatic disorders
InactiveUS9433664B2Easy to adjustAvoid complicationsPeptide/protein ingredientsBlood disorderFactor iiClotting factor
The present invention relates to normalizing impaired haemostasis comprising administering a clotting factor treatment selected from the group consisting of (1) FII; (2) PCC; and (3) a three factor combination of FH, FX and FVIIa. The clotting factor treatment can be administered in combination with fibrinogen. The clotting factor(s) can be recombinant human clotting factor(s).
Owner:MEDIMMUNE LTD
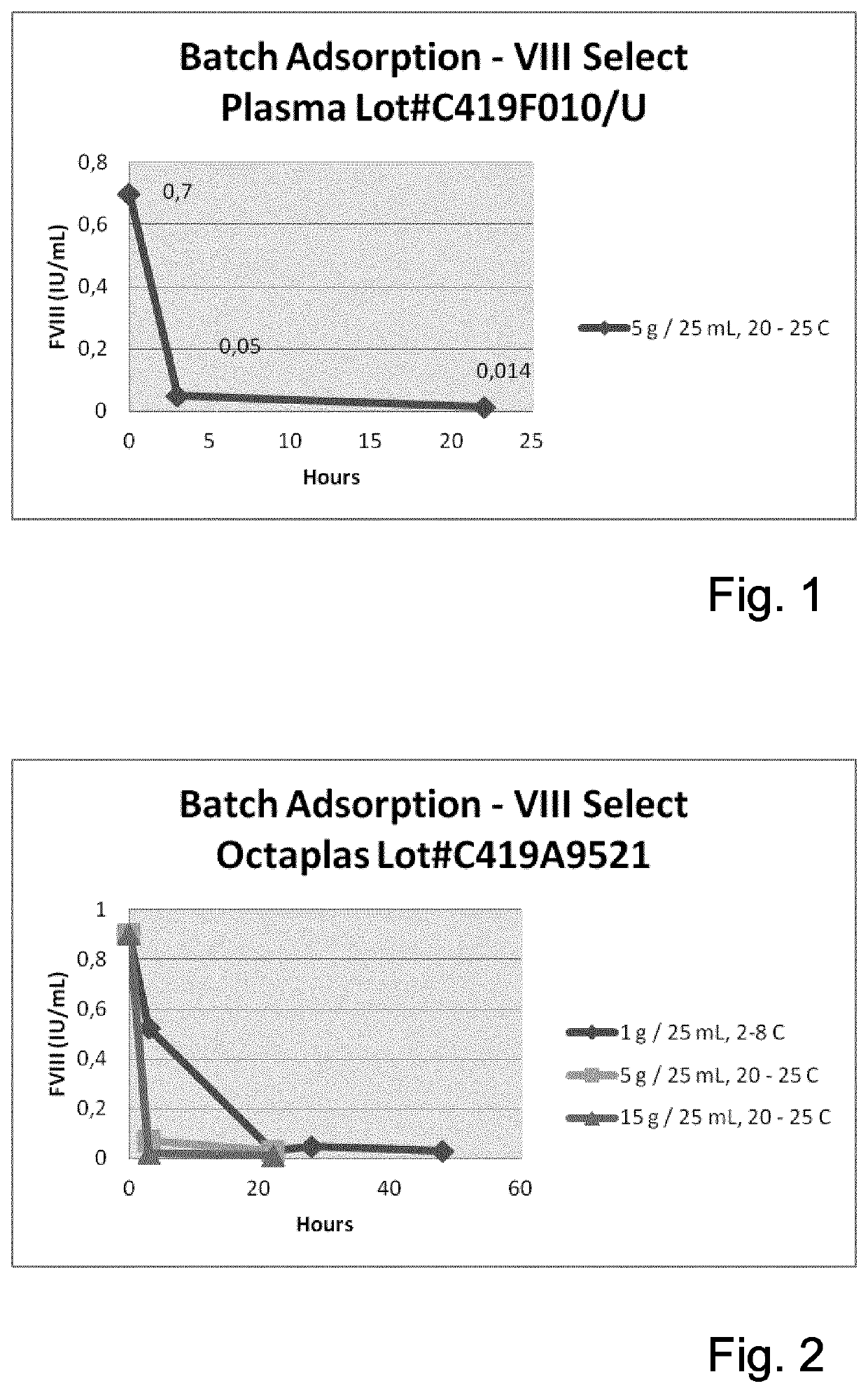
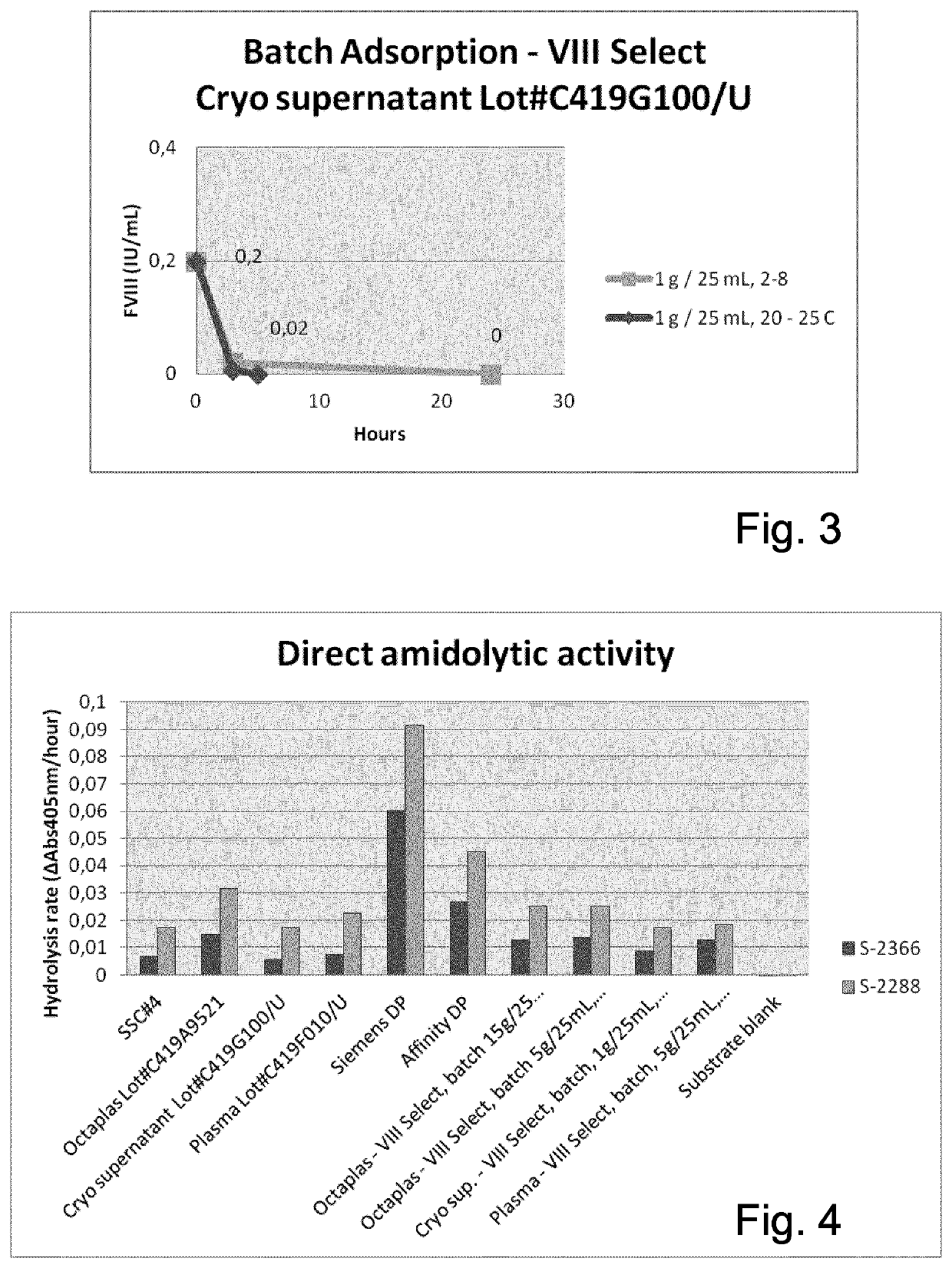
Method of separating Factor VIII from blood products
ActiveUS10889630B2Immunoglobulins against blood coagulation factorsFactor VIIWhole blood productVon Willebrand factor
The invention provides a method for separating a Factor VIII (FVIII) protein from a first composition comprising the FVIII protein, which contains at least the light chain of FVIII, and a von-Willebrand-Factor (vWF) protein which comprises at least the FVIII binding domain of vWF, wherein the FVIII protein can form a complex with the vWF protein, the method comprising the steps: contacting the first composition with an affinity resin comprising a ligand and a matrix, wherein the ligand has an affinity to the light chain of FVIII, and separating the affinity resin from the mixture to obtain a modified first composition and a second composition, wherein the second composition contains the affinity resin, and a complex of the FVIII protein and the vWF protein.
Owner:OCTAPHARMA
Factor VII glycoforms
The present invention provides preparations of Factor VIIa polypeptides or Factor VIIa-related polypeptides that exhibit predetermined glycoform patterns. The preparations of the invention exhibit improved functional properties and are useful for treating Factor VII-mediated conditions.
Owner:NOVO NORDISK AS
Ancestral serine protease coagulation cascade exerts a novel function in early immune defense
InactiveUS20130177547A1Promote bacterial infectionReduced inhibitory activityAntibacterial agentsNervous disorderMicroorganismSerine protease
The present invention relates to blood coagulation factor XIII (FXIII) for treatment and / or prevention of an infection by a microorganism and / or the symptoms associated with said infection, a pharmaceutical composition comprising a pharmaceutically effective amount of said FXIII, a method for the manufacture of a medicament comprising a pharmaceutically effective amount of said FXIII, and a method of treatment comprising administering to a patient in need a pharmaceutically effective amount of said FXIII.
Owner:CSL BEHRING GMBH +1
Use of coagulation proteins to lyse clots
InactiveUS20060275277A1Enhance dissolving said blood clotHigh dissolution rateOrganic active ingredientsPeptide/protein ingredientsSide effectLysis
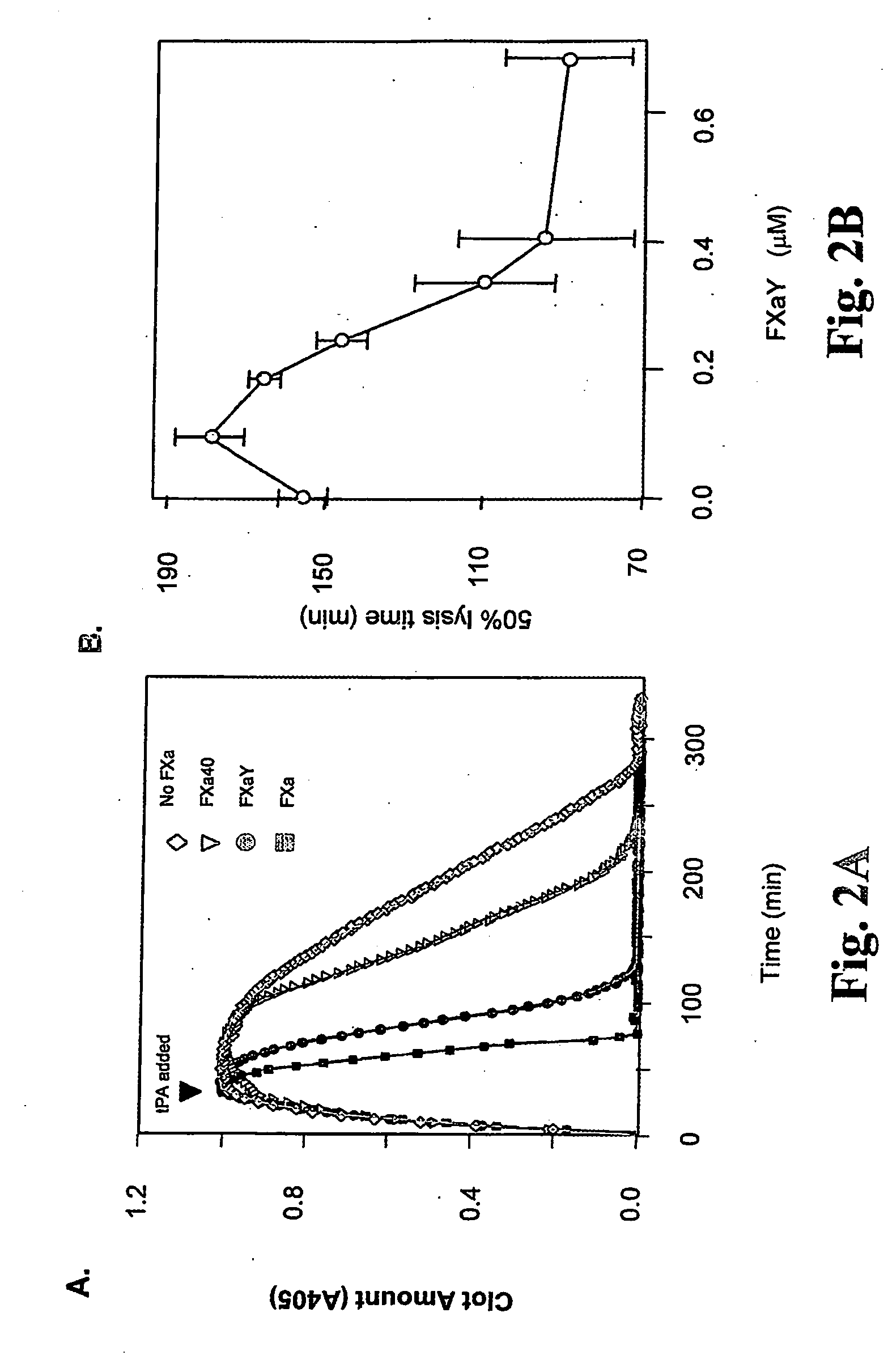
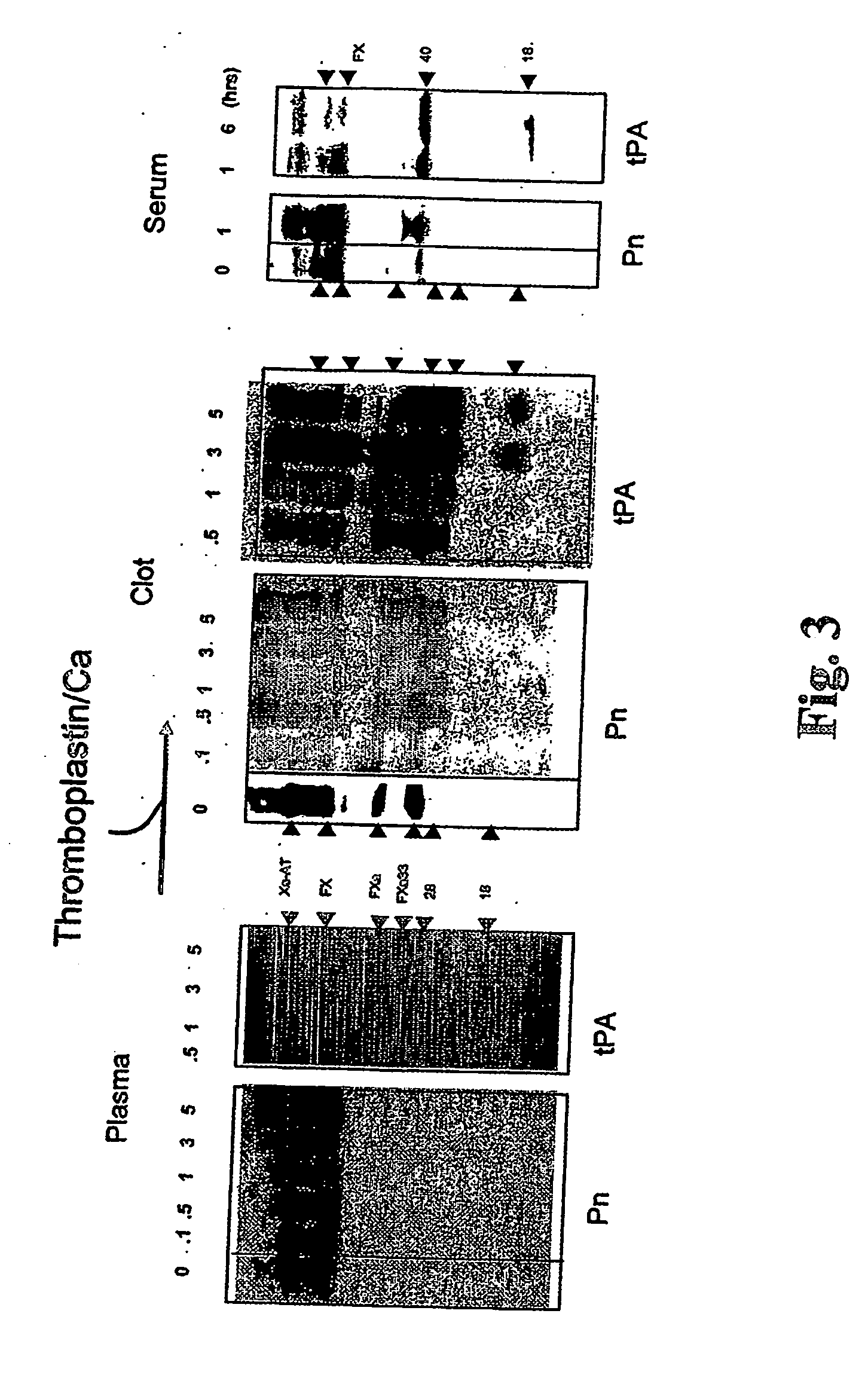
The present invention relates to the use of coagulation proteins for the lysis of blood clots. More specifically, the present invention provides a method for accelerating the dissolution of a blood clot through the administration of at least one coagulation protein comprising a basic C-terminal amino acid, wherein the coagulation protein may be a derivative of Factor X or Factor V or a combination thereof. Pharmaceutical compositions for the treatment and prophylaxis of blood clots are also provided, wherein, the methods and products of the present invention advantageously accelerate clot dissolution while potentially minimizing the adverse side-effects, such as hemorrhaging, seen with other clot dissolving agents. The present invention also provides a method for detecting a fibrinolytic potential in a subject.
Owner:CANADIAN BLOOD SERVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com