Patents
Literature
33 results about "Plasma mhpg" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of production and use of liquid formulations of plasma proteins
InactiveUSRE38431E1Enhanced quality of lifeReduce infectivityPeptide/protein ingredientsInorganic non-active ingredientsMethods of productionFactor XIIIa
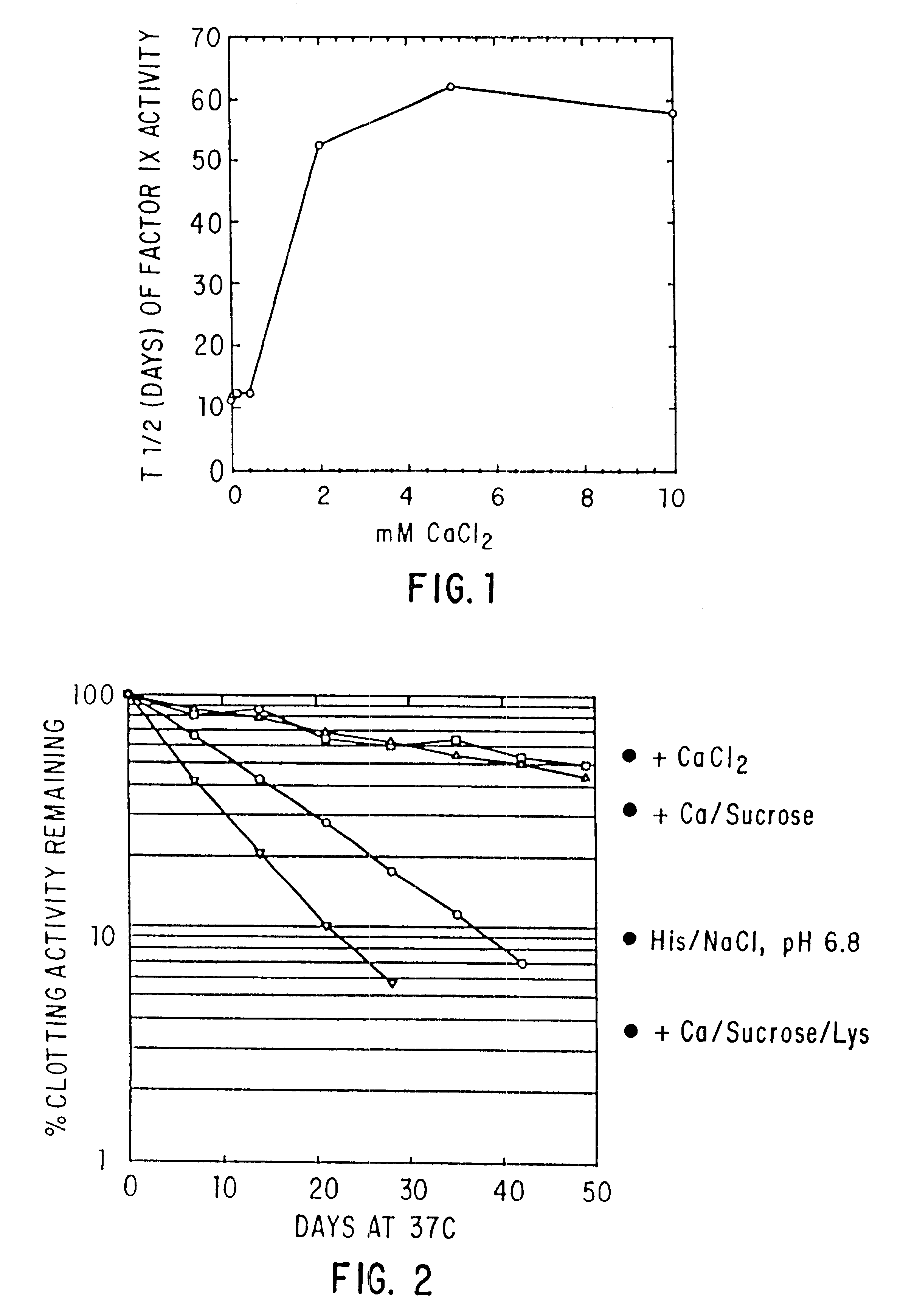
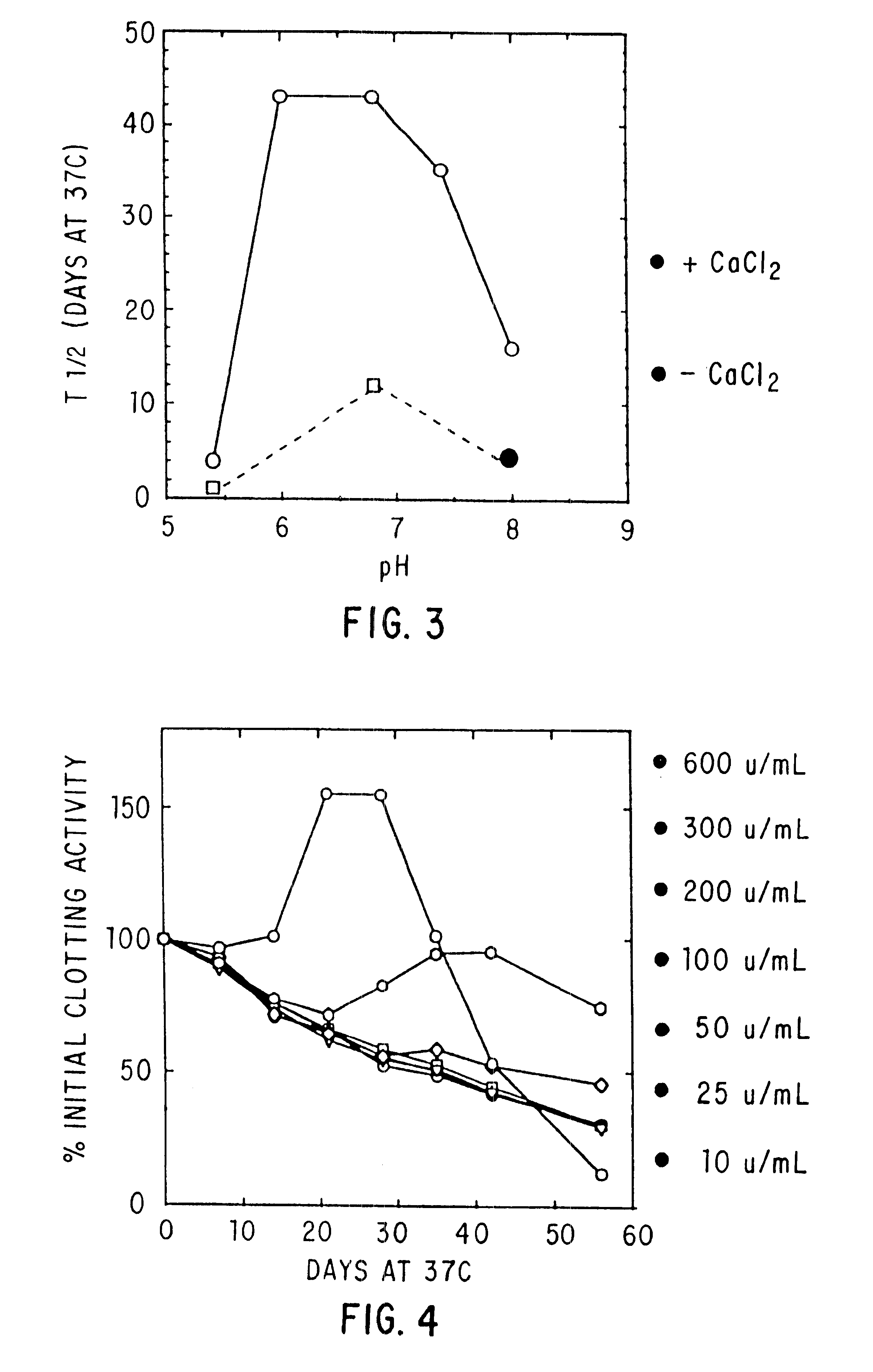
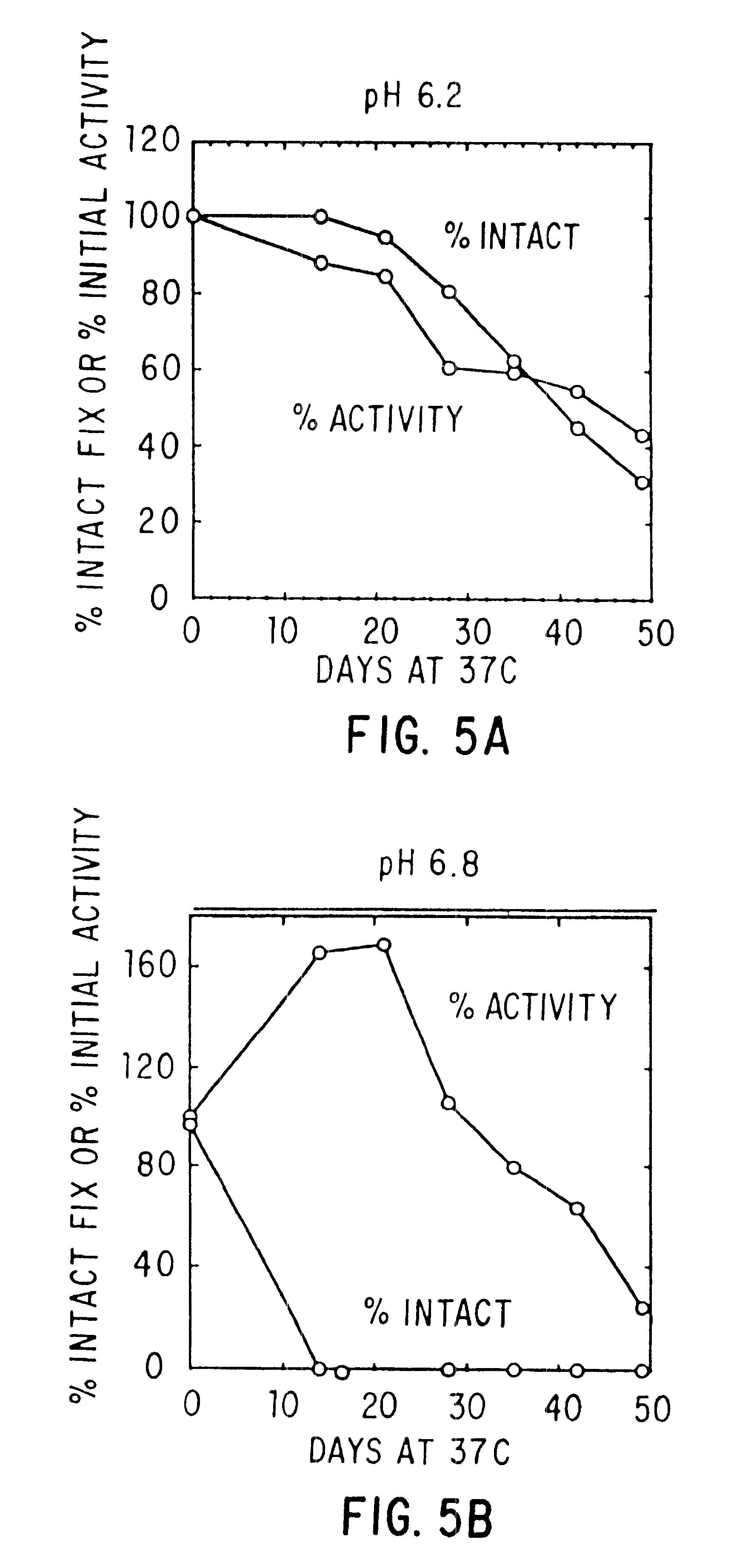
The present invention relates to the preparation and use of liquid formulations of plasma proteins, particularly blood coagulation factors. More specifically, the present invention relates to stable liquid formulations of Factor VIII and Factor IX that can be administered by injection or infusion to provide a constant level of the coagulation factor in the blood.
Owner:THE COALITION FOR HEMOPHILIA B +1
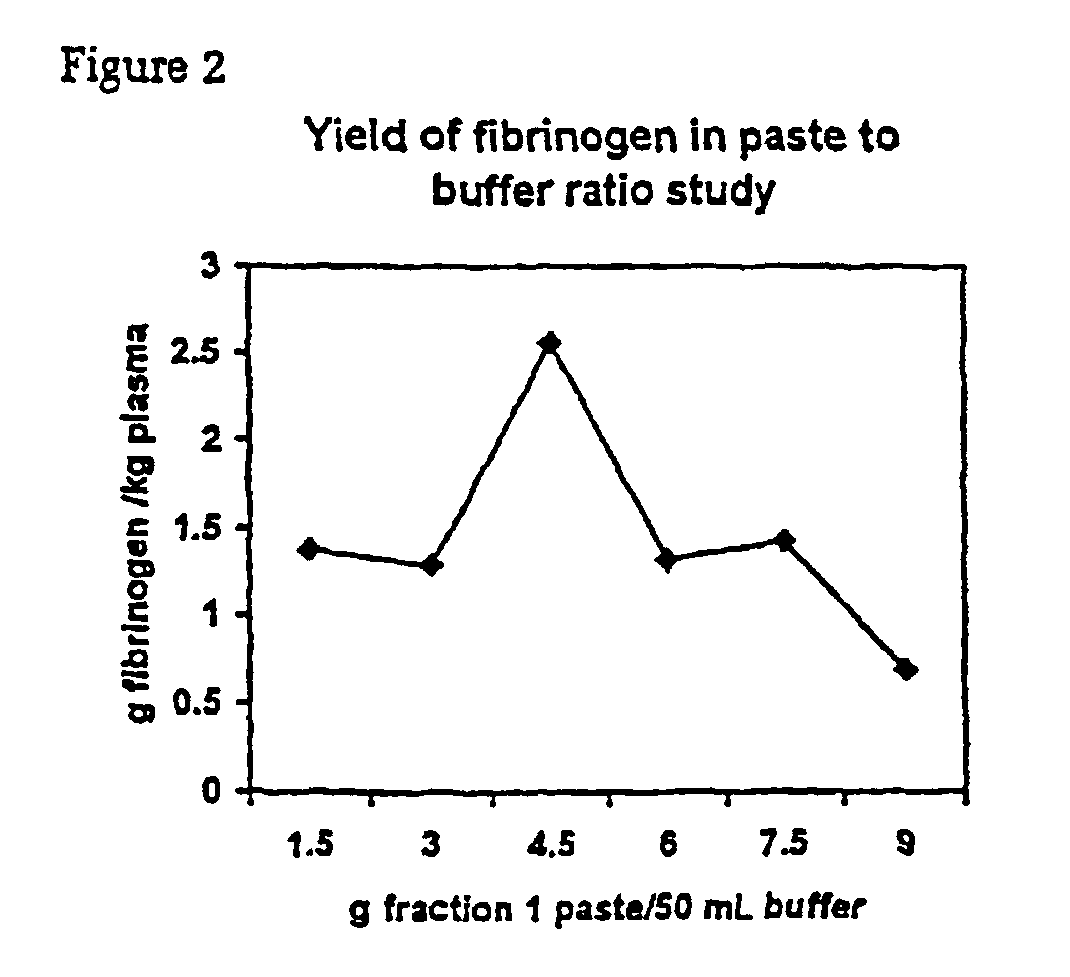
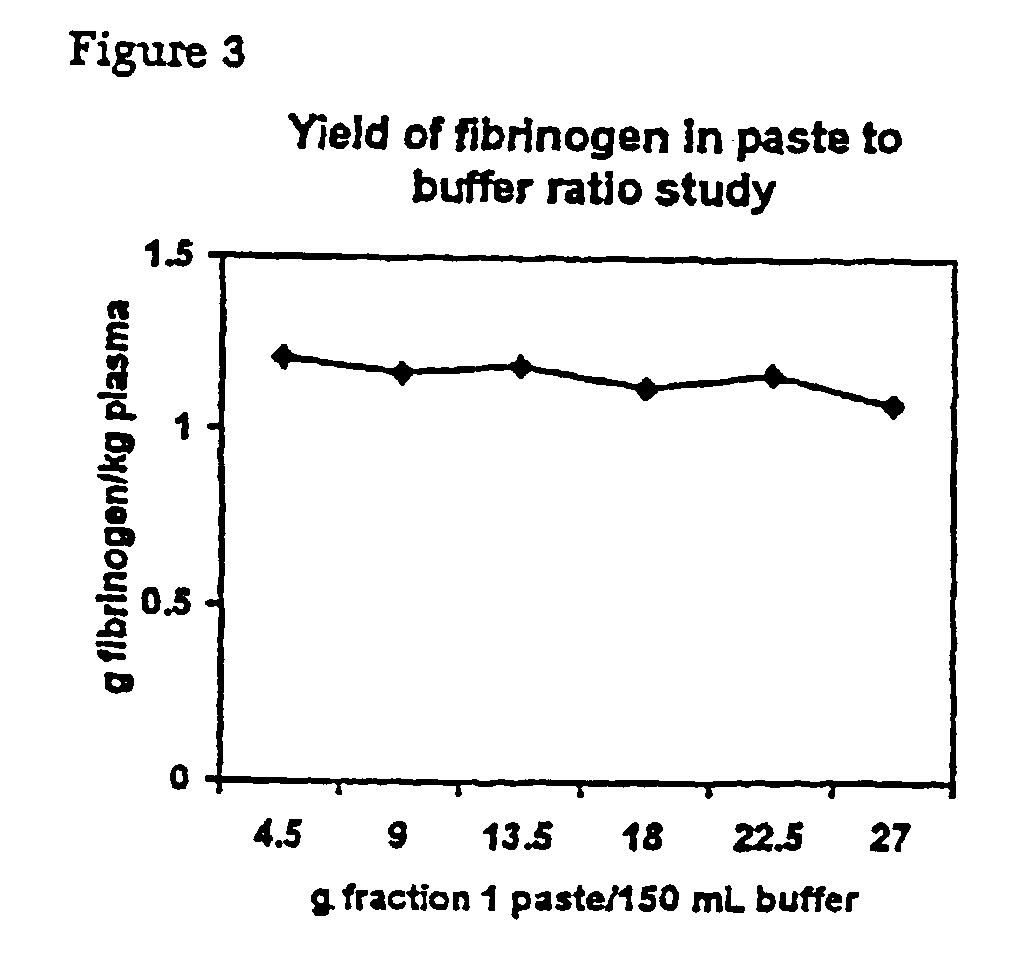
Separation of fibrinogen from plasma proteases
InactiveUS6960463B2Simple manufacturing methodHigh purityFibrinogenHydrolasesProteinase activityIon exchange
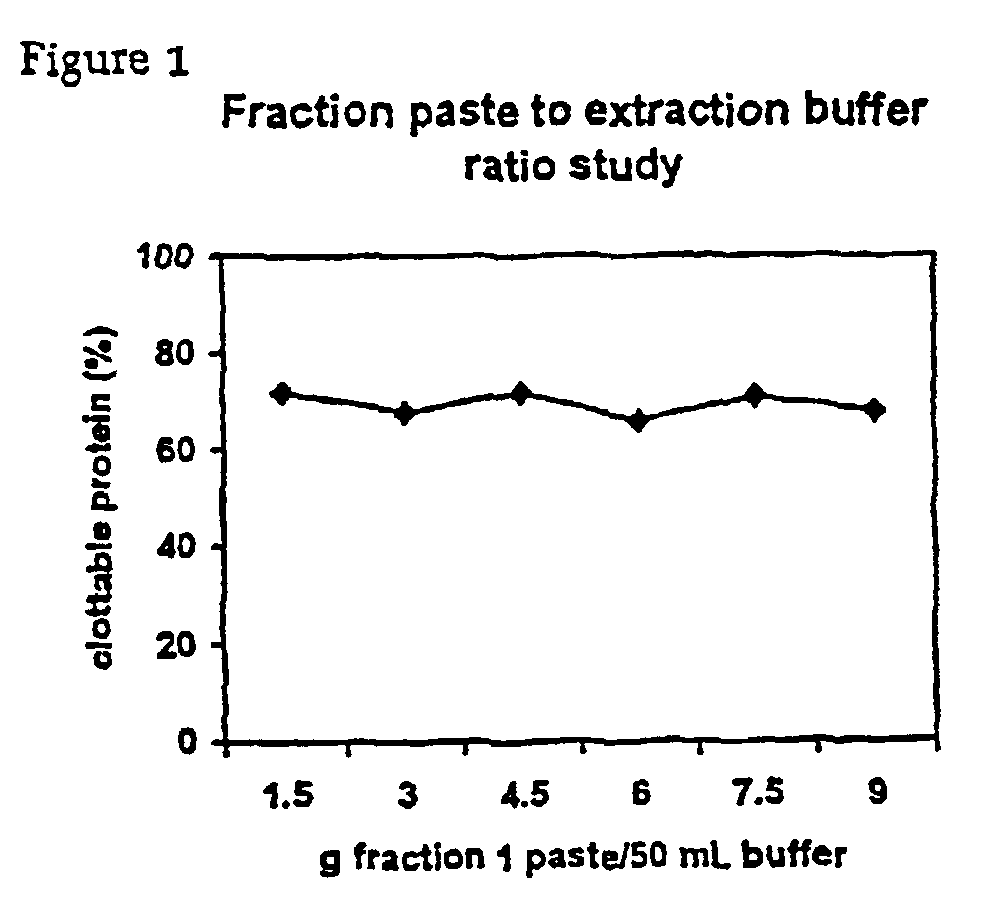
The present invention relates to methods for purifying fibrinogen. In one aspect, the present invention relates to a method of separating fibrinogen from plasma fraction I precipitate. In another aspect, the invention relates to the purification of fibrinogen using ion exchange chromatography.
Owner:CSL LTD
Pad for alleviating and treating plasma protein exudation skin diseases including atopic diseases
InactiveUS20200030480A1Reduce the temperatureEasy dischargeCosmetic preparationsNon-adhesive dressingsDiseaseAtopic disease
The present invention relates to a pad for treating skin diseases, comprising agar gel and a fiber layer fixed to the inside of the gel, and a manufacturing method therefor. The pad of the present invention inhibits blood vessel exudation symptoms according to vasodilation by maintaining a low temperature of the affected area, reduces an itchy sensation and, at the same time, removes, from the skin, plasma proteins and various plasma-derived substances accumulated in skin tissue, thereby having an effect of alleviating the symptoms of the affected area.
Owner:LIPOBIOMED CORP
Novel plasma protein affinity tags
InactiveUS20070105750A1Reduce probabilityNervous disorderPeptide/protein ingredientsHalf-lifeBlood plasma
Owner:NOVO NORDISK AS
Medication for chronic renal failure
InactiveCN1481891AImprove fatigueImprove stool drynessUnknown materialsUrinary disorderChronic kidney failureRhizome
The present invention provides one kind of Chinese medicine for treating chronic kidney function failure is prepared with Chinese medicinal materials, including ginseng, astragalus root, white atractylodes rhizome, Chuanxiong rhizome, leech, etc. The medicine has high curative effect, can improve clinical symptoms, reduce urea nitrogen in blood, lower creatinine level, reduce exhausted urine protein, protect kidney function, promote the synthesis of plasma protein, raise plasma protein density, improve body's nutrient state and produce many other positive effects. It is safe clinically and low in production cost.
Owner:贾在金
Application of HBP protein as diagnostic marker of Kawasaki disease
ActiveCN110726846AHigh sensitivityImprove accuracyDisease diagnosisBiological testingCoronary arteriesKawasaki disease
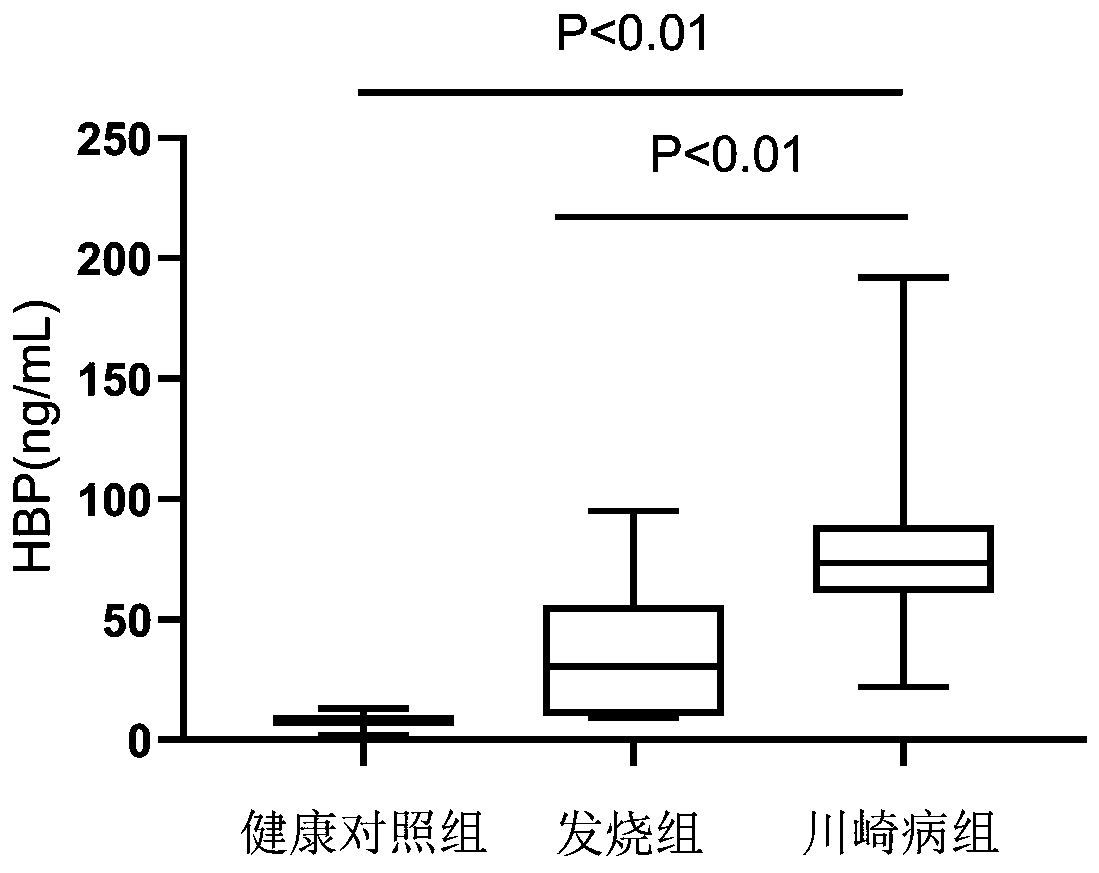
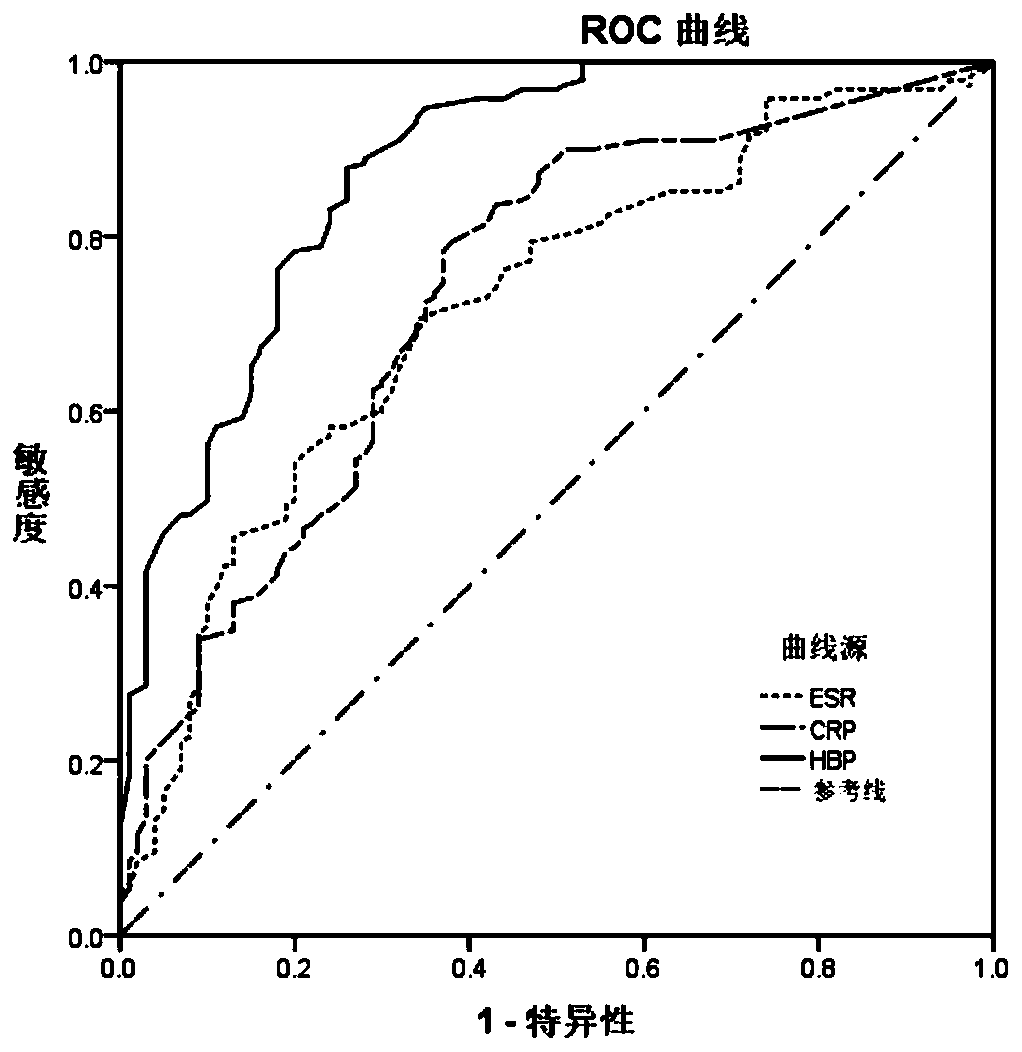
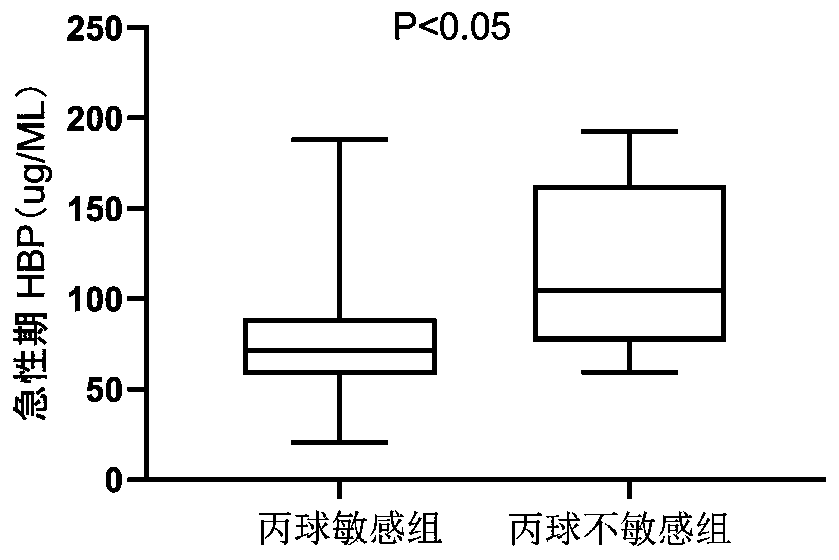
The invention provides application of HBP protein as a diagnostic marker of Kawasaki disease, and discloses application of HBP protein as a diagnostic marker of Kawasaki disease in preparing diagnostic products of Kawasaki disease and a kit for diagnosing the Kawasaki disease. The experimental results show that the plasma HBP level in acute Kawasaki patients is significantly higher than that in healthy children and general patients infected with fever, the higher HBP concentration is significantly correlated with coronary artery injury in Kawasaki disease, and the HBP level is higher in insensitive patients treated with IVIG than in sensitive patients treated with IVIG. The invention finds that plasma HBP can be used to differentiate Kawasaki disease from infected fever patients, wherein the area under ROC curve (area under the curve, AUC) is 0.88, the optimal diagnostic threshold is 52.5pg / mL, the sensitivity is 88.3%, and the specificity is 74.0%. Therefore, plasma HBP protein of patients can be used as a potential marker for distinguishing diagnosis of Kawasaki disease from infected fever.
Owner:NANJING CHILDRENS HOSPITAL
Clinical diagnosis of hepatic fibrosis using a novel panel of low abundant human plasma protein biomarkers
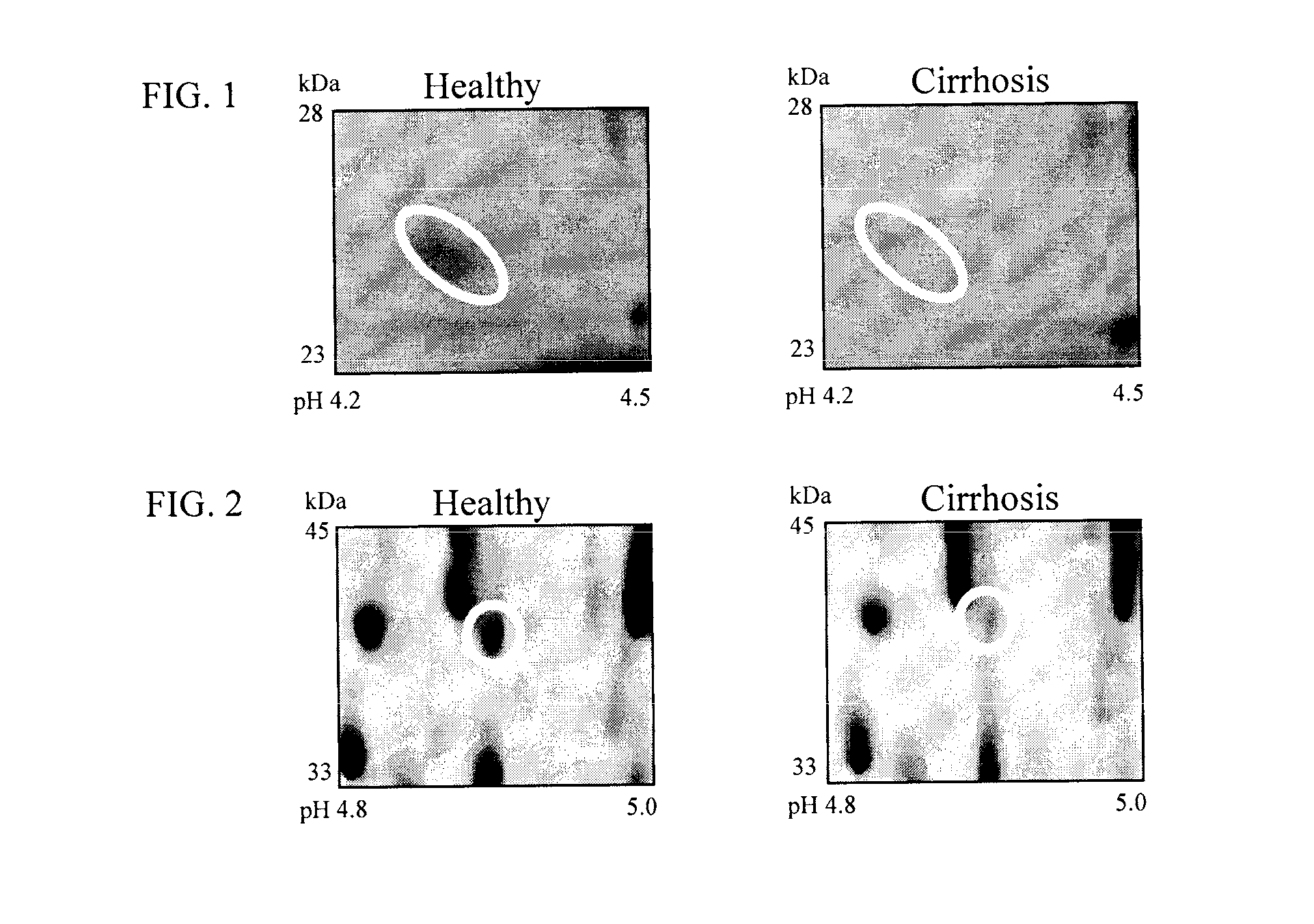
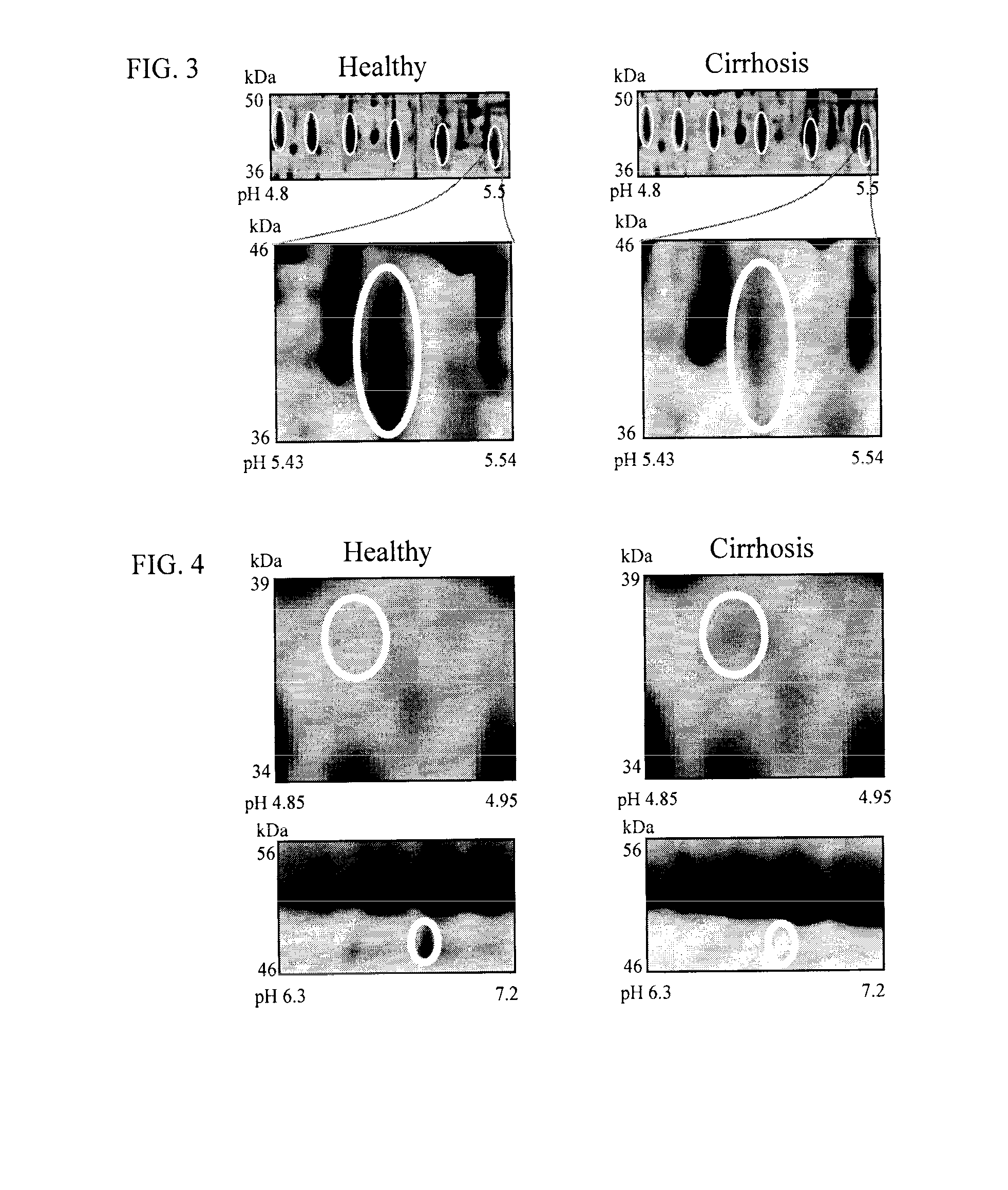
The inventors have proposed a novel panel of human plasma protein biomarkers for diagnosing hepatic fibrosis and cirrhosis. Presently there is no reliable non-invasive way of assessing liver fibrosis. A 2D-PAGE based proteomics study was used to identify potential fibrosis biomarkers. Plasma from patients with hepatic cirrhosis induced by infection with the hepatitis C virus (HCV) were analysed. Several proteins associated with liver scarring and potentially also related to viral infection were identified. These proteins include 14-3-3 protein zeta / delta, adiponectin, afamin, alpha-1-antitrypsin, alpha-2-HS-glycoprotein, apolipoprotein C-M, apolipoprotein E, C4b-binding protein beta chain, intact / cleaved complement C3dg, corticosteroid-binding globulin, fibrinogen gamma chain, beta haptoglobin at pH 5.46-5.49, haptoglobin-related protein, hemopexin, immunoglobulin J chain, leucine-rich alpha-2-glycoprotein, lipid transfer inhibitor protein, retinol-binding protein 4, serum paraoxonase / arylesterase 1, sex hormone-binding globulin and zinc-alpha-2-glycoprotein.
Owner:THE CHANCELLOR MASTERS & SCHOLARS OF THE UNIV OF OXFORD
Apparatus of handling fluids
InactiveUS20020131897A1Scattering properties measurementsColor/spectral properties measurementsBlood serumGeneral chemist
An apparatus and method for performing immunoturbidimetric measurements of plasma proteins on an apparatus used for measuring plasma and serum interferents are described. Immunoturbidometric measurements are made on a sample in a disposable dispensing tip which acts as cuvette and reaction chamber. These features allow tests which are not available on general chemistry analyzers, to become available, and at the same time the apparatus can provide a screening system for serum and plasma interferents.
Owner:SPECTROMEDICAL
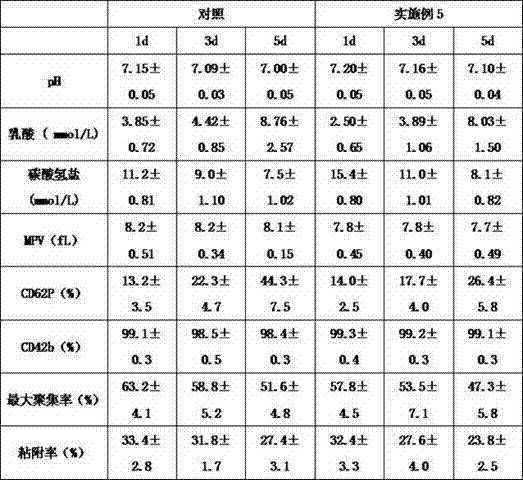
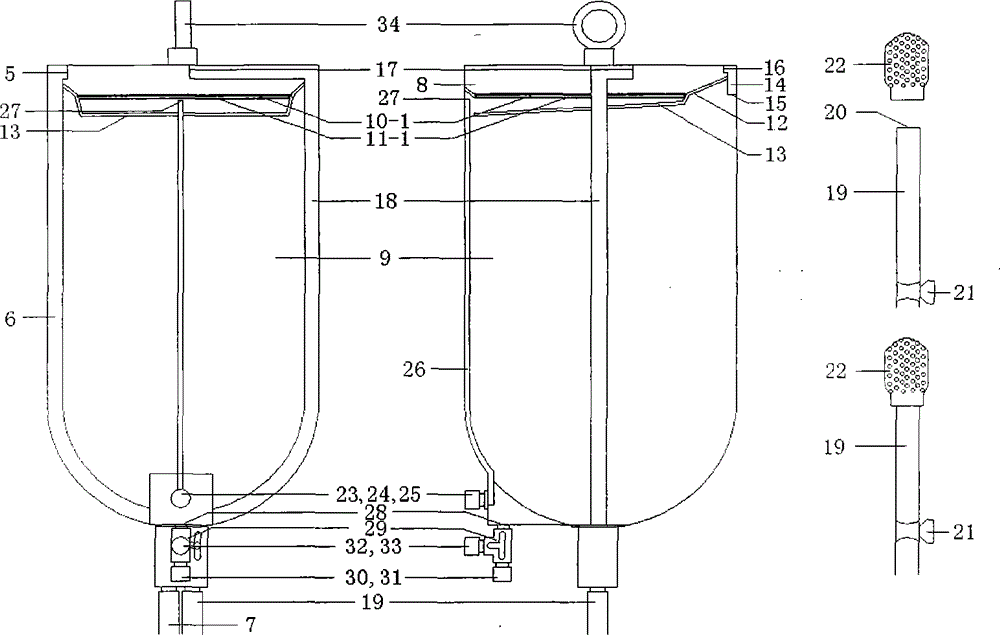
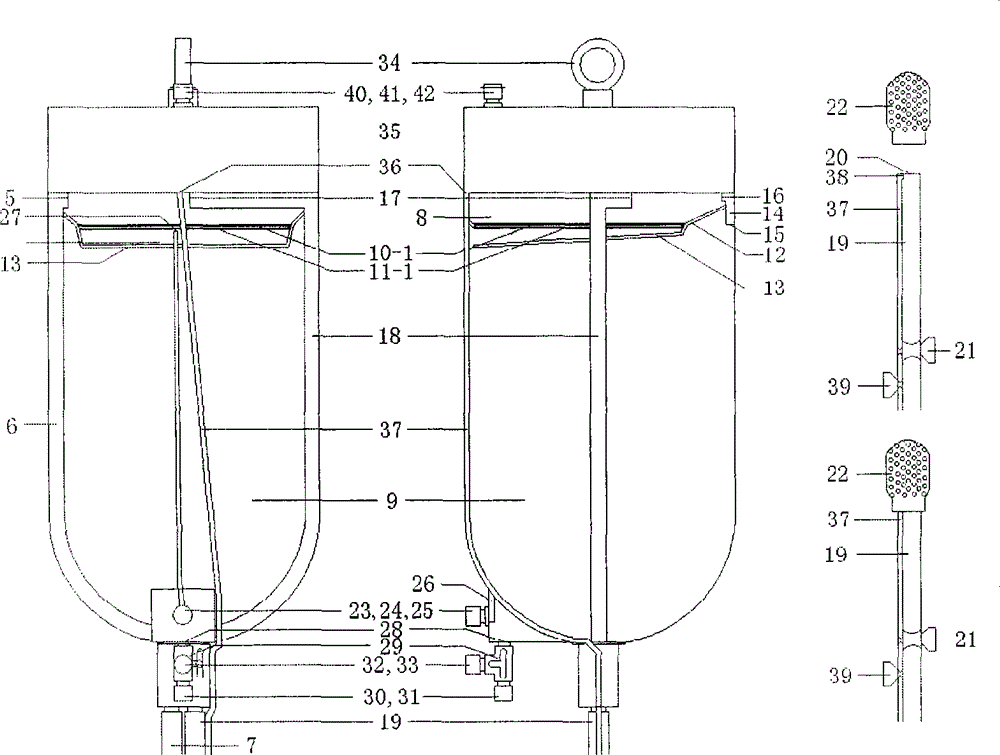
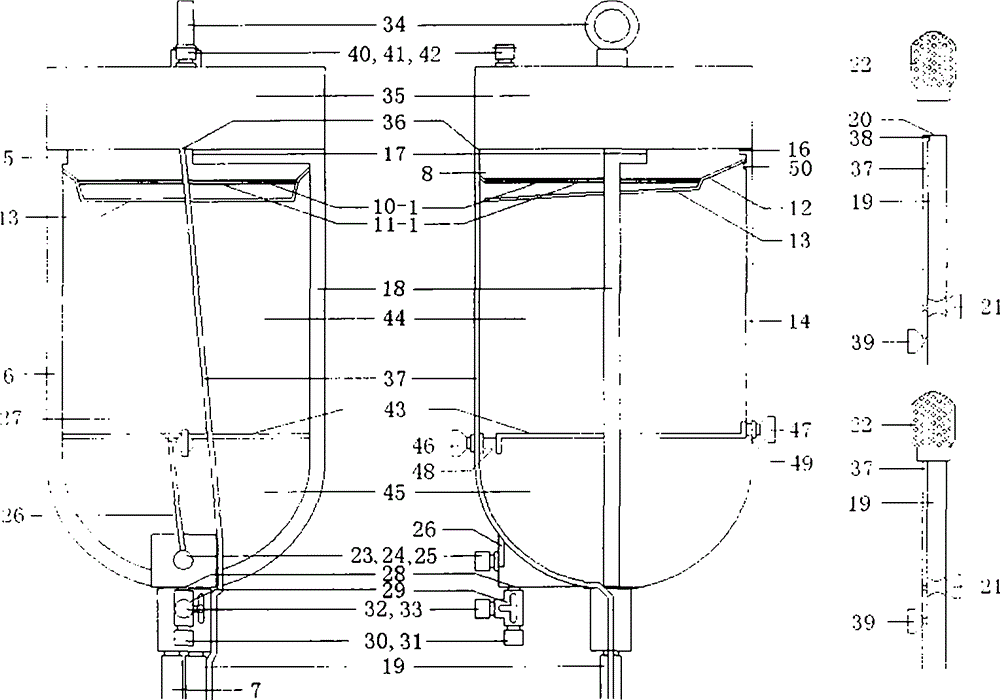
Simple device for preparing plasma protein products through batch adsorption, and method thereof
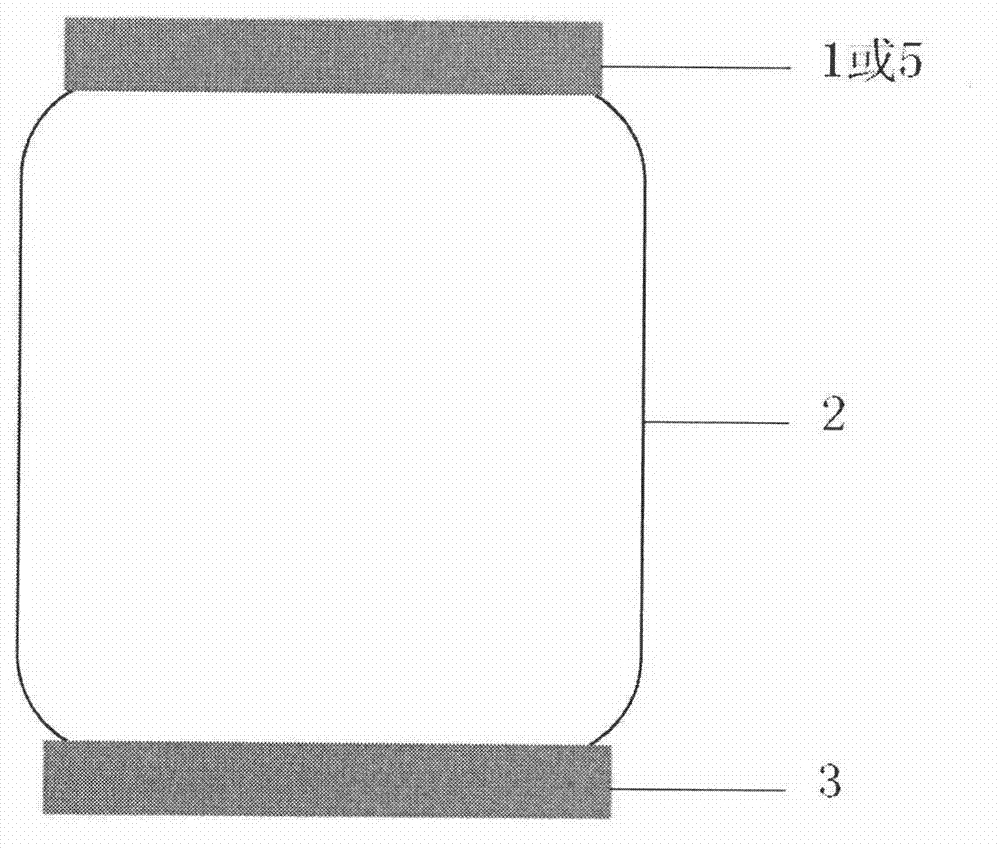
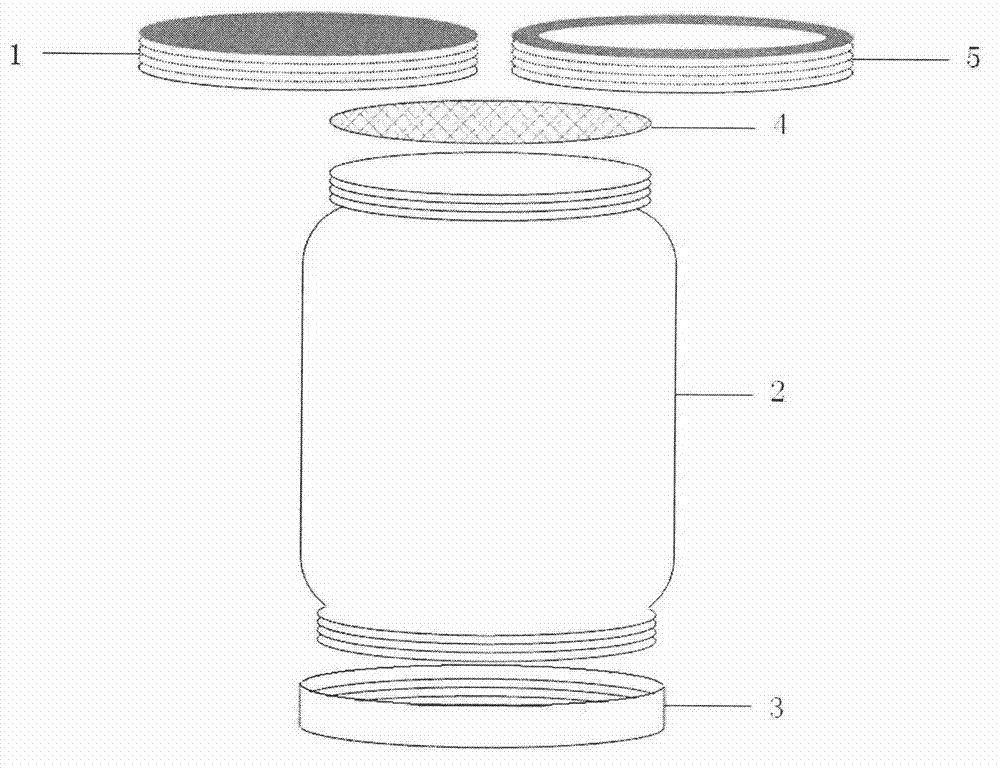
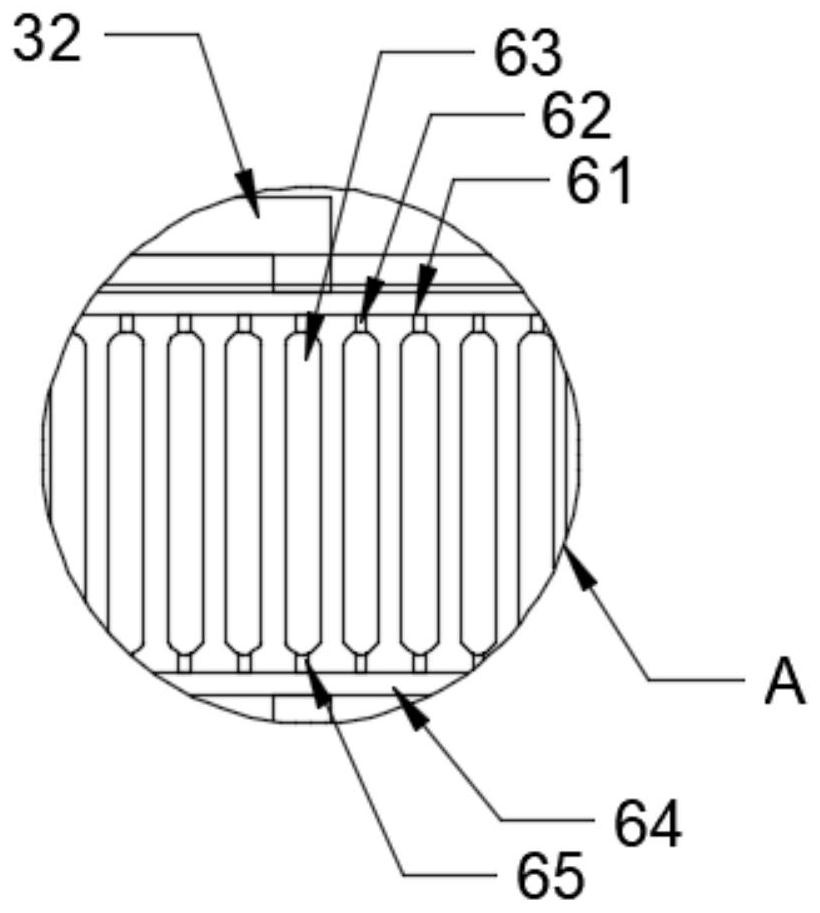
InactiveCN102786577AEasy to separateFactor VIIPeptide preparation methodsLiquid compositionPlasma protein adsorption
The invention relates to the fields of the medical and biological technologies, and discloses a simple device for preparing plasma protein products through batch adsorption, and a method thereof. The device comprises an upper end sealing cover (1), a reaction bottle (2), a lower end sealing cover (3), a filter cloth sheet (4) and an openmouthed cover (5). The method is characterized in that two ends of the reaction bottle (2) are sealed through the upper end sealing cover (1), the filter cloth sheet (4) and the lower end sealing cover (3); the addition of a material in the reaction bottle (2) is realized through opening the lower end sealing cover (3); a liquid component in the reaction bottle (2) is filtered by the filter cloth sheet (4) through replacing the upper end sealing cover (5) by the openmouthed cover (5) and through pressurizing the middle part of the reaction bottle (2); and the whole batch adsorption preparation process comprising medium balancing, plasma protein adsorption, other protein washing and target plasma protein eluting is carried out in the reaction bottle (2). The device and the method simplify equipment and operations required by the preparation of the plasma proteins through the batch adsorption, and can simply realize the efficient separation of various plasma proteins. Same-batch experiments enable the screening of various conditions of the batch-adsorption preparation technology to be completed by utilizing a plurality of the devices.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
Preparation method of transferrin, transferrin-containing composition and application
PendingCN113801219AImprove nesting abilityImprove self-care abilityNervous disorderPeptide/protein ingredientsBiopharmaceuticalBlood plasma
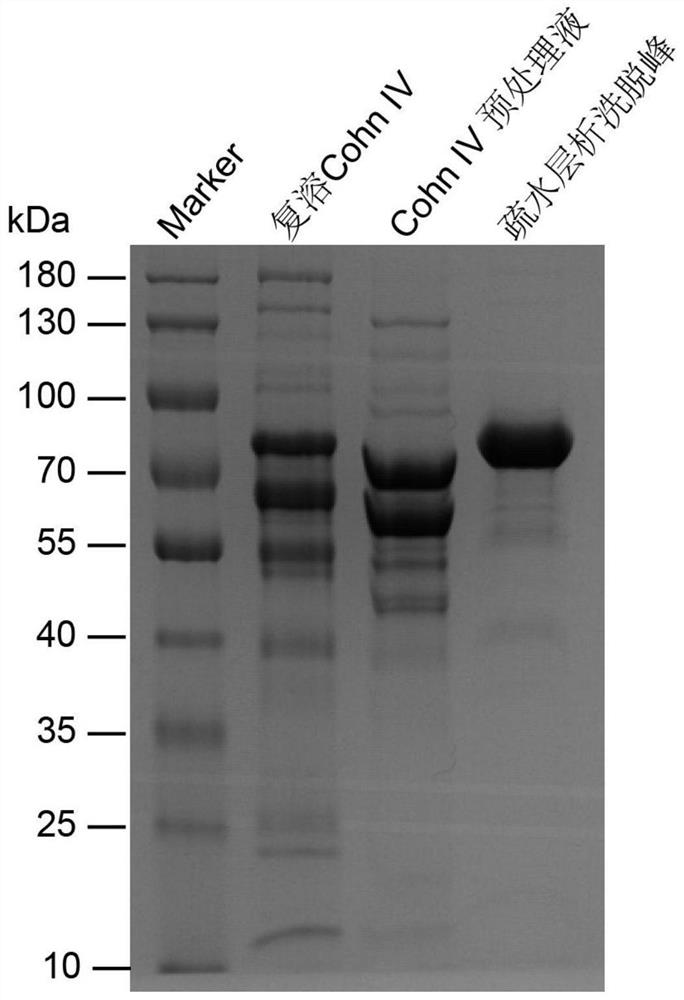
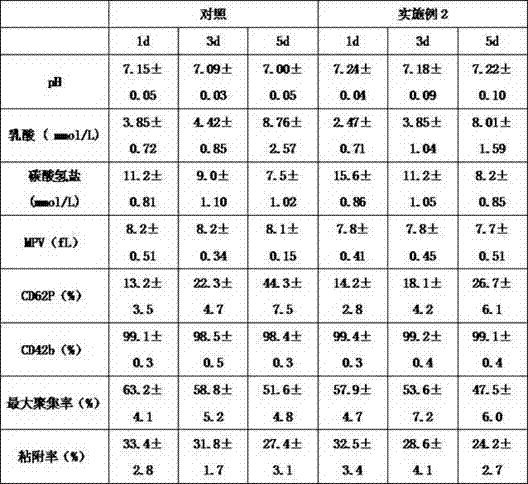
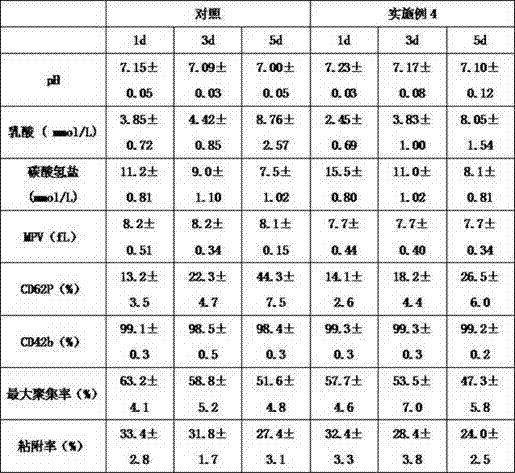
The invention belongs to the field of application of biological medicines, and particularly relates to a preparation method of transferrin, a transferrin-containing composition and application. Preparation raw materials of the transferrin comprise a human plasma CohnIV component, and the preparation method of the transferrin comprises steps of pretreatment liquid preparation, hydrophobic chromatographic purification and concentration replacement. The transferrin disclosed by the invention can be independently used or combined with other plasma proteins, thereby playing a role of enhancing memory and improving cognition.
Owner:江苏豪思睦可生物科技有限公司
Preparation of platelet washing solution
InactiveCN104711223AAddressing Adverse Infusion ReactionsHigh recovery rateBlood/immune system cellsRingers solutionAnticoagulant
The invention discloses a preparation of a platelet washing solution. The platelet washing solution comprises two solutions as follows: 500ml of a bicarbonate Ringer solution and 20-30ml of an ACD-A anticoagulant. The platelet washing solution prepared from 500ml of the bicarbonate Ringer solution and 25ml of the ACD-A anticoagulant is optimal in washing effect. According to the preparation of the platelet washing solution, two solutions with permission to the clinical application at present are mixed at a certain ratio; the platelet washing solution is used for washing and preparing plasma-poor platelet; by virtue of the clinical application of the plasma-poor platelet, the untoward effects of platelet transfusion caused by plasma proteins can be solved. The platelet washing solution is simple in components and liable to depolymerize; the recovery rate of the platelet is increased; the agglomeration force is improved; the safety is guaranteed.
Owner:王丹 +1
Suspended retransfusion set
InactiveCN104689403ANo assistance requiredBlood transfusion starts quicklyOther blood circulation devicesMedical devicesMedical equipmentBottle
The invention discloses a suspended retransfusion set, and belongs to the technical field of medical equipment and supplies. A clinical washing type autotransfusion technology has allogeneic transfusion shortcomings and plasma protein and coagulation factor loss; a simple method of manual filtering operation by multiple operators is frequently adopted for the conventional filtering type autotransfusion technology, and is exposed and high in pollution rate. The suspended retransfusion set consists of a negative-pressure suction pipeline, a blood recovery pipeline, a retransfusion pipeline and an automatic anticoagulant transfusion device, wherein the negative-pressure suction pipeline consists of a negative-pressure pipe, a negative-pressure conversion bottle, a negative-pressure indicator and a regulator, and is communicated with an upper filter screen gap through an internal opening of the negative-pressure pipe; the blood recovery pipeline comprises a suction head, a blood inlet pipe, a coarse filter screen, a fine filter screen, the upper filter screen gap, a blood storage tank, a blood storage tank air inlet pipe, a blood transfusion three-way valve and the like; the automatic anticoagulant transfusion device comprises a medicine storage tank, a medicine transfusion pipe, a medicine transfusion pipe grading valve, a blood inlet pipe-medicine transfusion pipe synchronization valve and the like. The suspended retransfusion set has the advantages of integrated design, automatic anticoagulant transfusion function, high recovered blood quality, high operability, high emergent treatment performance and the like.
Owner:杨海龙
High-speed filtration preparation process of polypeptide donkey-skin gelatin plasma substitutes
InactiveCN102218080BReduce viscosityLow viscosityUnknown materialsBlood disorderRenal glomerulusTryptophan
The invention combines the polypeptide donkey-skin gelatins with different weight-average molecular weights and then adds tryptophan into the combined polypeptide donkey-skin gelatins to prepare the plasma substitutes containing gelatin molecules with different molecular weights. The process has the advantages that: smaller molecules can easily pass through renal glomerulus, which contributes to diuretics at the early stage of introduction; bigger molecules are left in the blood vessels, and the dilatation effect is prolonged; and the increase of the tryptophan can stimulate the synthesis of albumins so as to replenish the plasma proteins in huge loss.
Owner:JIANGSU JIANZHONG INVESTMENT
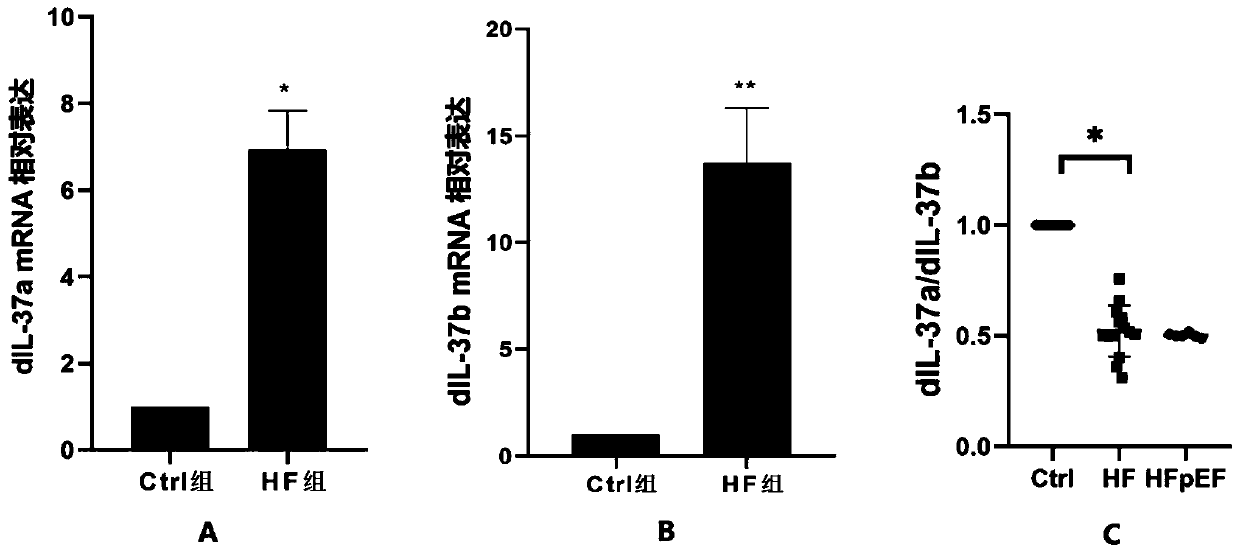
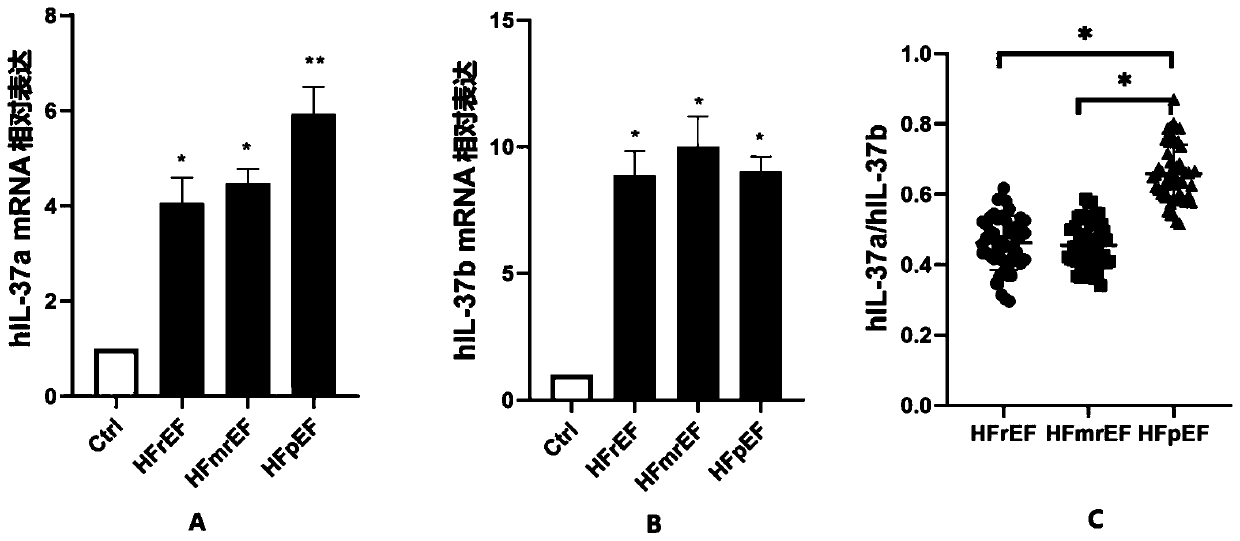
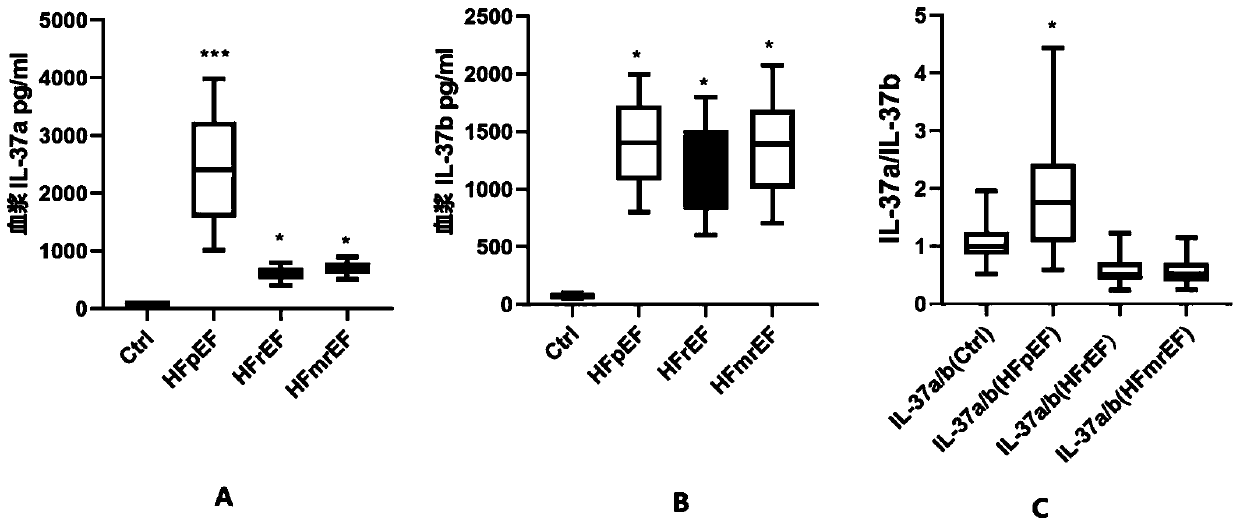
Heart failure biomarker and application thereof
The invention discloses a heart failure biomarker and application thereof. The biomarker is IL-37, particularly IL-37a and IL-37b. The inventor finds that IL-37a and IL-37b are highly expressed in heart failure patients from plasma mRNA and plasma protein levels, and can distinguish heart failure patients and healthy people. For three types of heart failure: HFrEF, HFmrEF and HFpEF, research and comparison are carried out on IL-37a and IL-37b from the plasma mRNA and plasma protein levels, and it is found that the IL-37a / IL-37b ratio can be used as an early screening and early diagnosis markerfor identifying the HFpEF. Theoretical basis and guiding significance can be provided for early screening, early diagnosis and the like of heart failure (especially HFpEF), and the survival rate of patients can be increased.
Owner:承启医学(深圳)科技有限公司
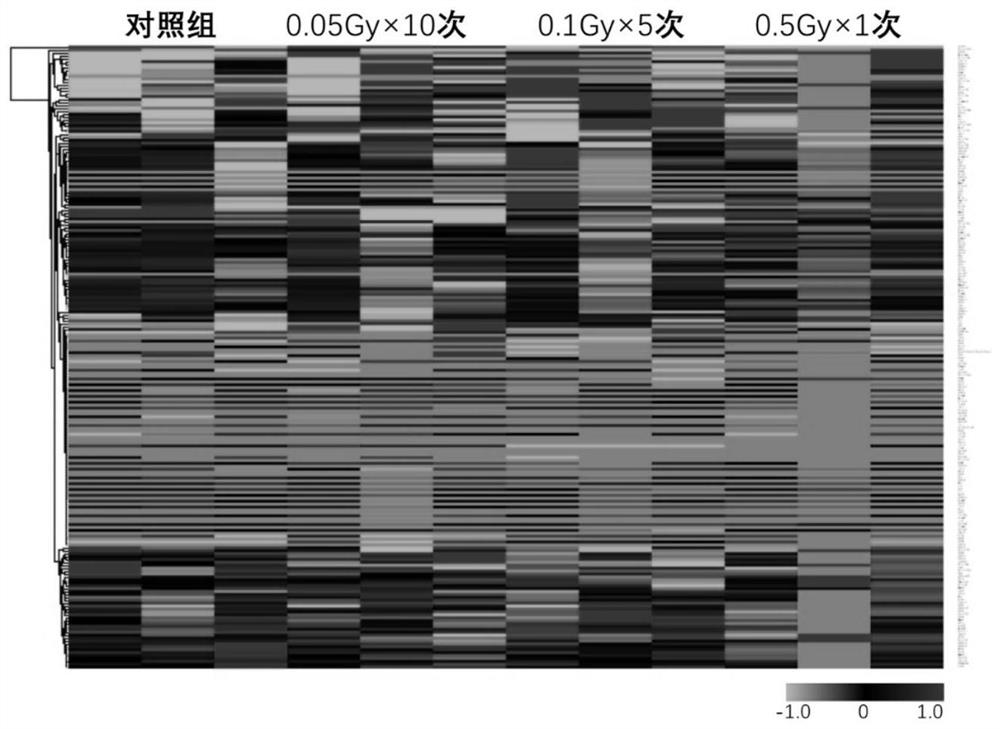
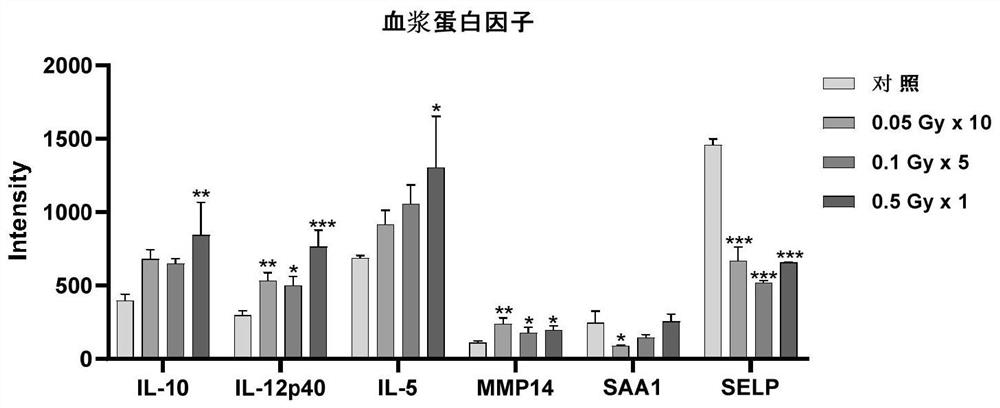
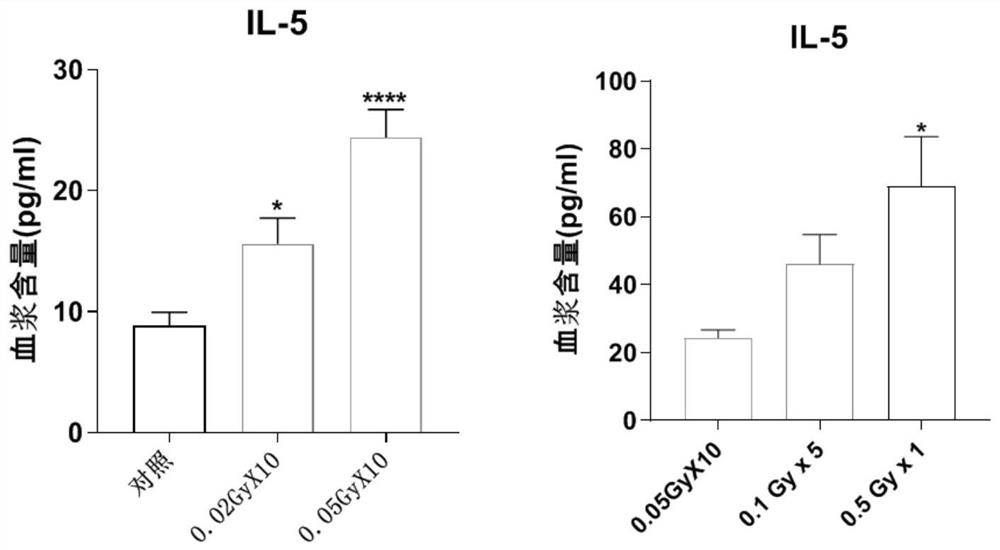
Application of plasma protein composition in preparation of product for predicting low-dose radiation irradiation dose
The invention discloses application of a plasma protein composition in preparation of a product for predicting a low-dose radiation irradiation dose. The invention provides the application of a substance for detecting IL-5, IL-12p40 and / or SELP content in blood plasma. The application comprises the following steps of: preparing the product for predicting the low-dose radiation irradiation dose; and preparing products for predicting the number and / or duration of low dose radiation irradiation. The plasma protein composition can be used as an index for predicting the long-term low-dose radiationaccumulated dose, and then the application of the plasma protein composition in predicting the long-term low-dose radiation accumulated dose is provided. The application has important significance indiagnosing the radiation accumulated dose of a radiological worker threatened by long-term low-dose radiation.
Owner:ACADEMY OF MILITARY MEDICAL SCI
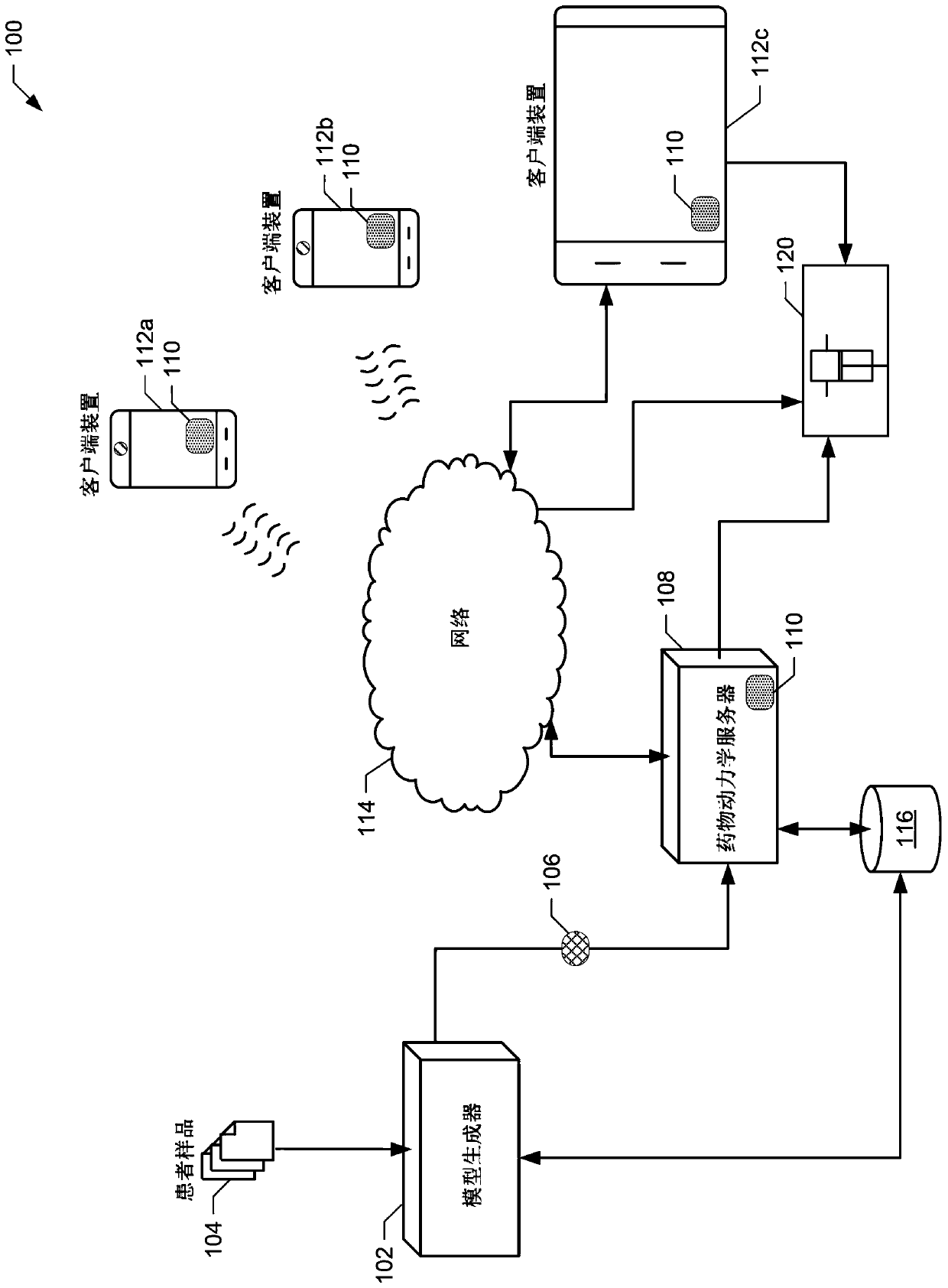
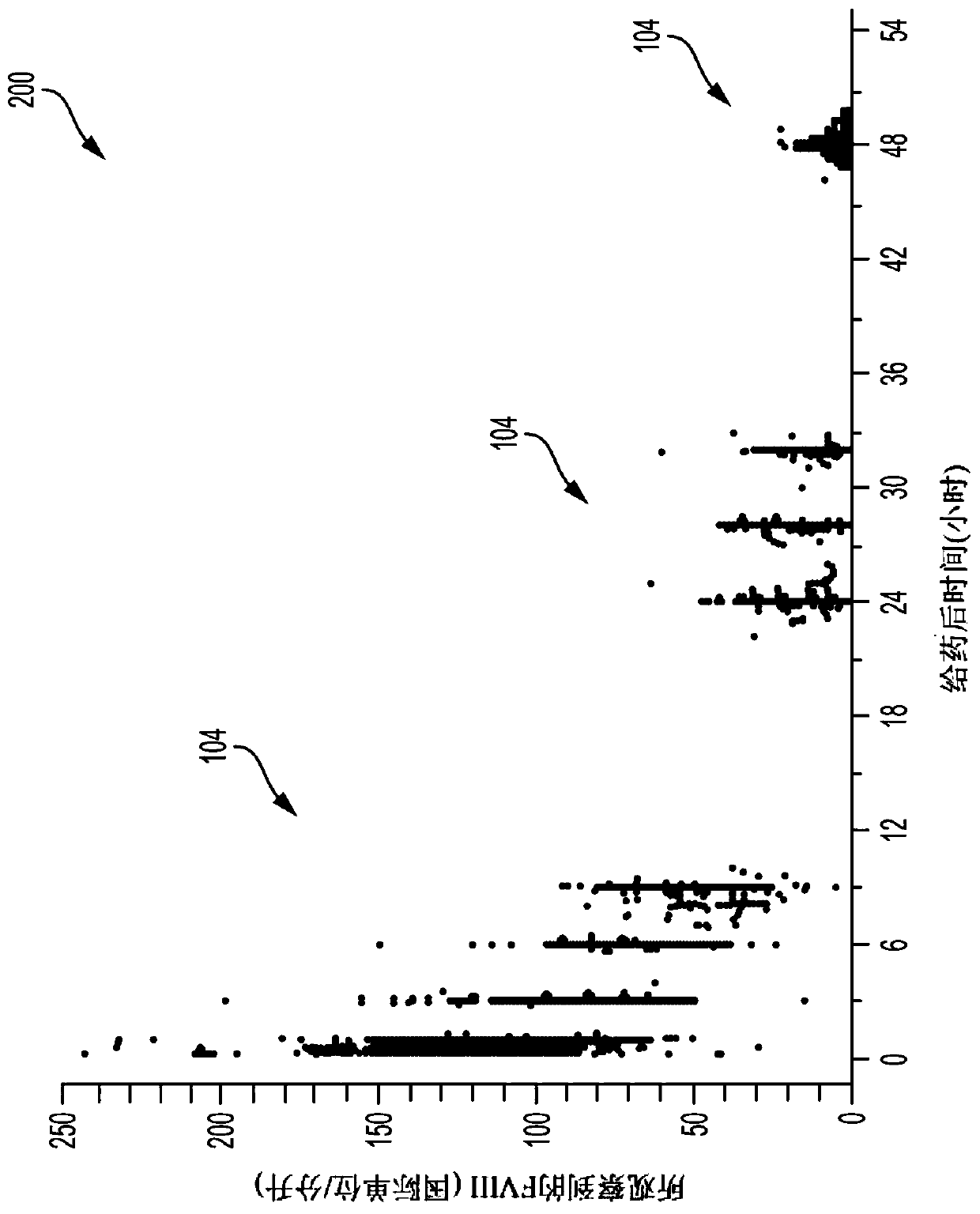
Pharmacokinetic Drug Dosing Regimen Devices and Methods
A system and method for providing a therapeutic plasma protein dosing regimen includes determining a patient's pharmacokinetic profile using a Bayesian model of the sampled patient's pharmacokinetic profile. The example systems and methods also include determining a first dosing regimen for a first prescribed dosing interval comprising (i) a first dose and (ii) the patient over a period of time based at least on the pharmacokinetic profile. and determining a second dosing regimen for a second prescribed dosing interval comprising (i) a second dose and (ii) at least based on said pharmacokinetic profile over said time The second therapeutic plasma protein level in the patient described in paragraph. The example systems and methods further include displaying the first dosing regimen and the second dosing regimen on a client device such that the first dosing regimen is displayed in conjunction with the second dosing regimen.
Owner:TAKEDA PHARMA CO LTD
A plasma protein isolate for treating Alzheimer's disease and its preparation method and application
ActiveCN110724176BEnhance memoryRaise the level of awarenessNervous disorderPeptide/protein ingredientsDiseaseCoboglobin
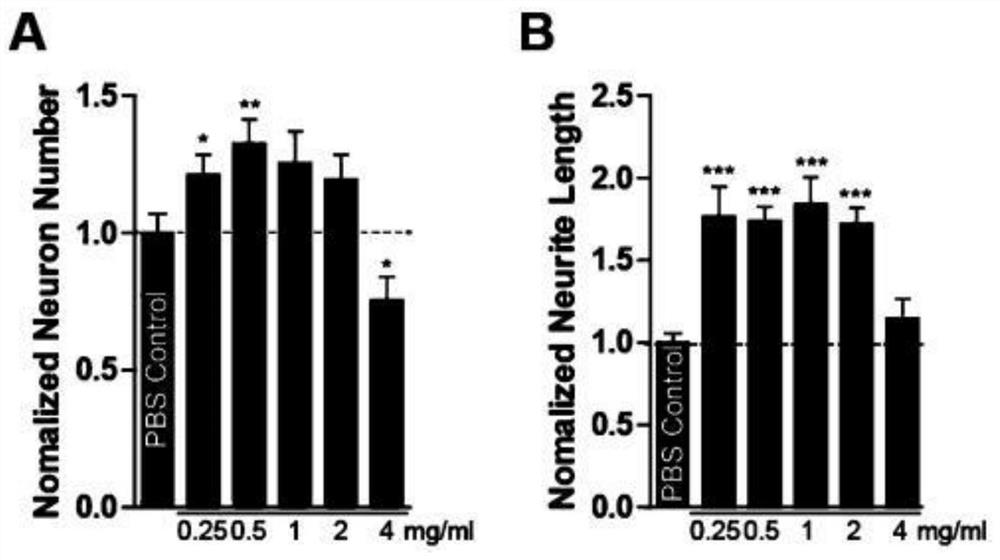
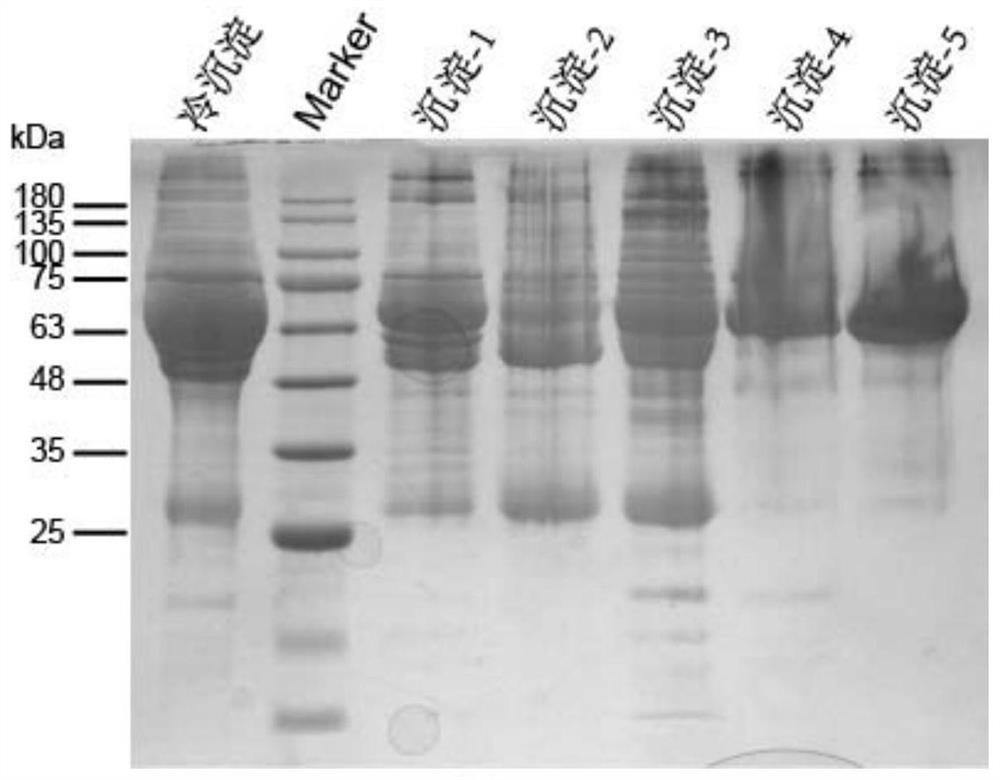
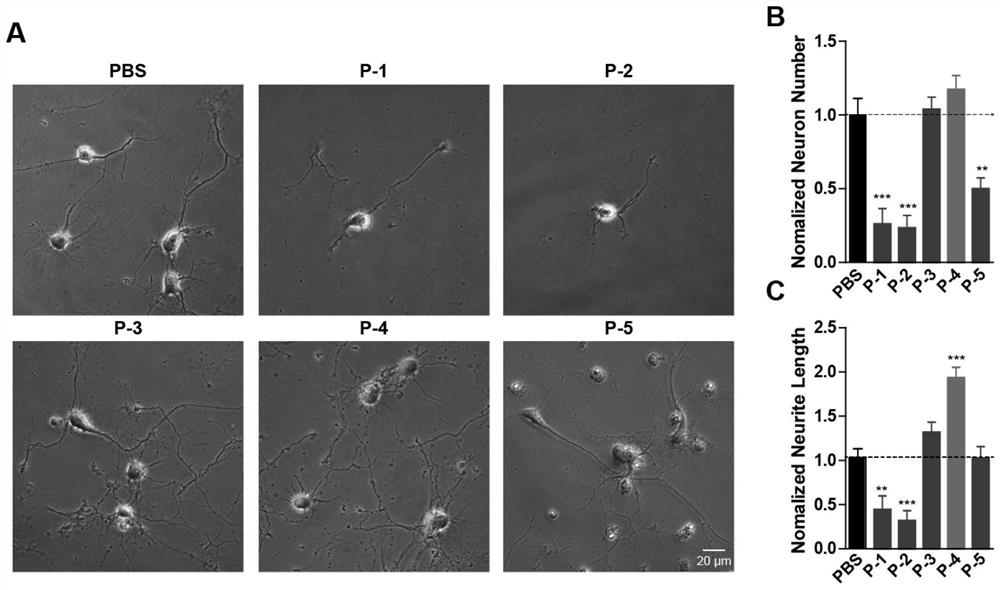
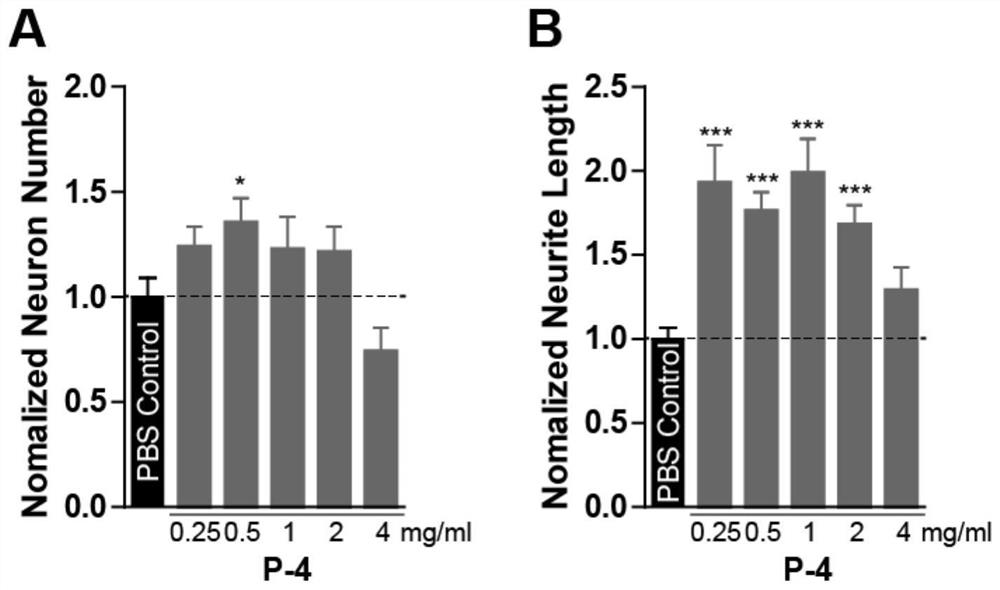
The invention belongs to the field of molecular biology, and relates to a plasma protein isolate for treating Alzheimer's disease, a preparation method and application thereof. The plasma protein isolate includes: α, β globulin, ceruloplasmin, transferrin, haptoglobin and albumin; the preparation method of the plasma protein isolate comprises in turn: separating plasma from blood, and performing The cryoprecipitate is removed, and the supernatant obtained by the cryoprecipitate removal is subjected to four low-temperature ethanol precipitations, and the precipitate obtained after the fourth low-temperature ethanol precipitation is used as the plasma protein isolate. In the present invention, through a series of reasonable operation steps, reasonable reagents and parameters are selected in each step, and various factors cooperate with each other to improve the curative effect of the plasma protein isolate on Alzheimer's disease and enhance memory. The preparation method of the invention is simple, can be directly scaled up for industrialized production, and can be applied to the pharmaceutical industry on a large scale.
Owner:北京豪思生物科技股份有限公司
Compositions and methods for treating and preventing proteinuria and endothelial erosion
ActiveUS10881686B2Inhibit and ameliorate proteinuriaInhibit and ameliorate and comorbiditiesPeptide/protein ingredientsSulfur/selenium/tellurium active ingredientsBlood flowBlood plasma
In some embodiments, a system and / or method may inhibit and / or ameliorate proteinuria and related comorbidities in a subject. In some embodiments, proteinuria and / or Endothelial Erosion (EE) and related comorbidities may be inhibited and / or ameliorated by maximizing a subject's serum zeta potential. A subject's serum zeta potential may be maximized by administering pharmaceutical compositions which: optimize the negative electrical surface charges of a subject's blood cells, plasma proteins, and / or endothelial surface layer; optimize the composition of a subject's bloodstream (e.g., ionic strength and ion concentrations), and / or optimize the properties of the subject's bloodstream (e.g., serum pH). In some embodiments, Proteinuria and / or EE and related comorbidities may be inhibited and / or ameliorated by: maximizing a subject's serum zeta potential; repairing damage to the endothelial surface layer in a subject; making up for a subject's urinary losses of plasma proteins; and / or optimizing blood flow and facilitating healing of damage caused by Proteinuria and / or EE.
Owner:ZETA BIOLONGEVITY INC
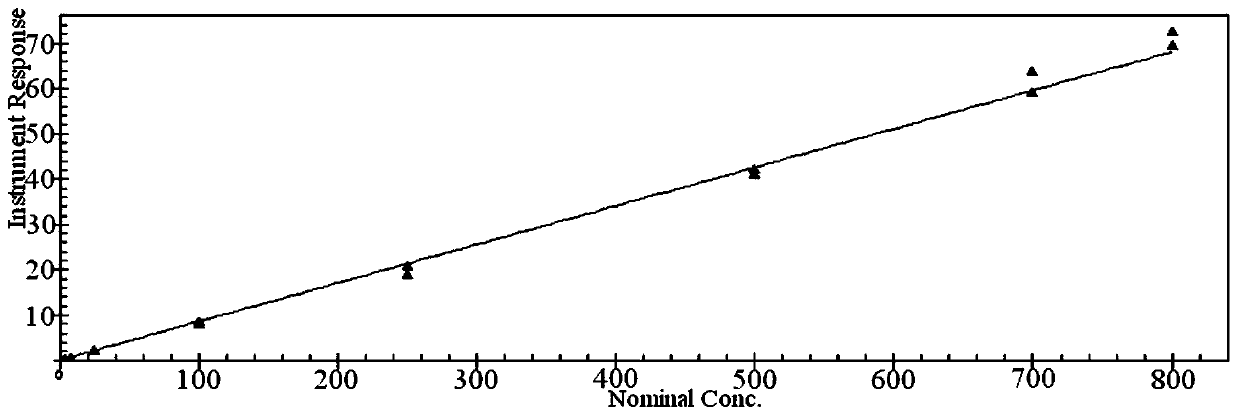
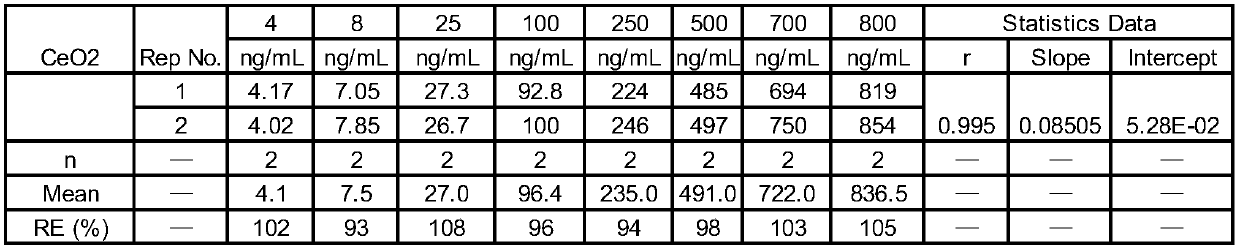
Sample pretreatment method for determining nanoscale cerium oxide in plasma or serum
ActiveCN107478709BEvenly distributedLower pHMaterial analysis by electric/magnetic meansPhysical chemistryBlood plasma
The invention discloses a sample pre-treatment device for determining nano-grade cerium oxide in blood plasma or blood serum by adopting an inductively coupled plasma mass spectrometer. The sample pre-treatment device comprises the following steps: step 1, uniformly distributing nano-grade cerium oxide particles; step 2, adding a reduction agent into the nano-grade cerium oxide particles; step 3, digesting blood plasma protein or blood serum protein and carrying out reduction treatment on the cerium oxide particles; step 4, carrying out post-treatment. According to the sample pre-treatment device disclosed by the invention, a stock and working solution is prepared by adopting a mixed solution of nitric acid and Triton-X-100 and the nano-grade cerium oxide particles stably exist in the solution; a manner of digesting in a water bath at 80 DEG C for one night is adopted and the blood plasma protein or the blood serum protein is digested to reduce cerium oxide into trivalent cerium ions, so that accurate detection of an inductively coupled plasma mass spectrum can be carried out. According to the sample pre-treatment device disclosed by the invention, a nano-grade cerium oxide particle solution is stable and the protein is completely digested; a method is simple to operate and the testing cost is low; the determination of a large batch of blood plasma samples can be realized in one step.
Owner:上海药明康德新药开发有限公司
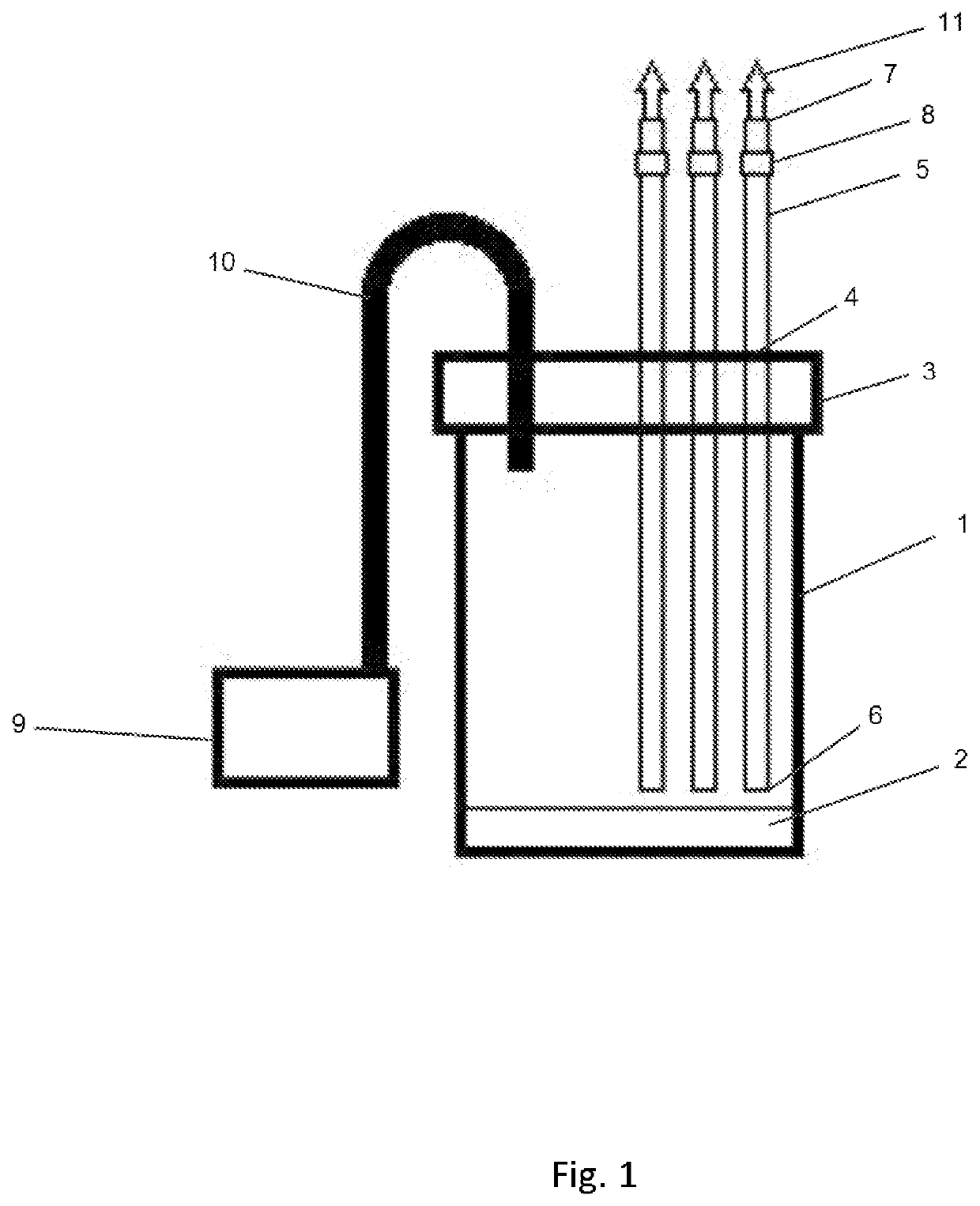
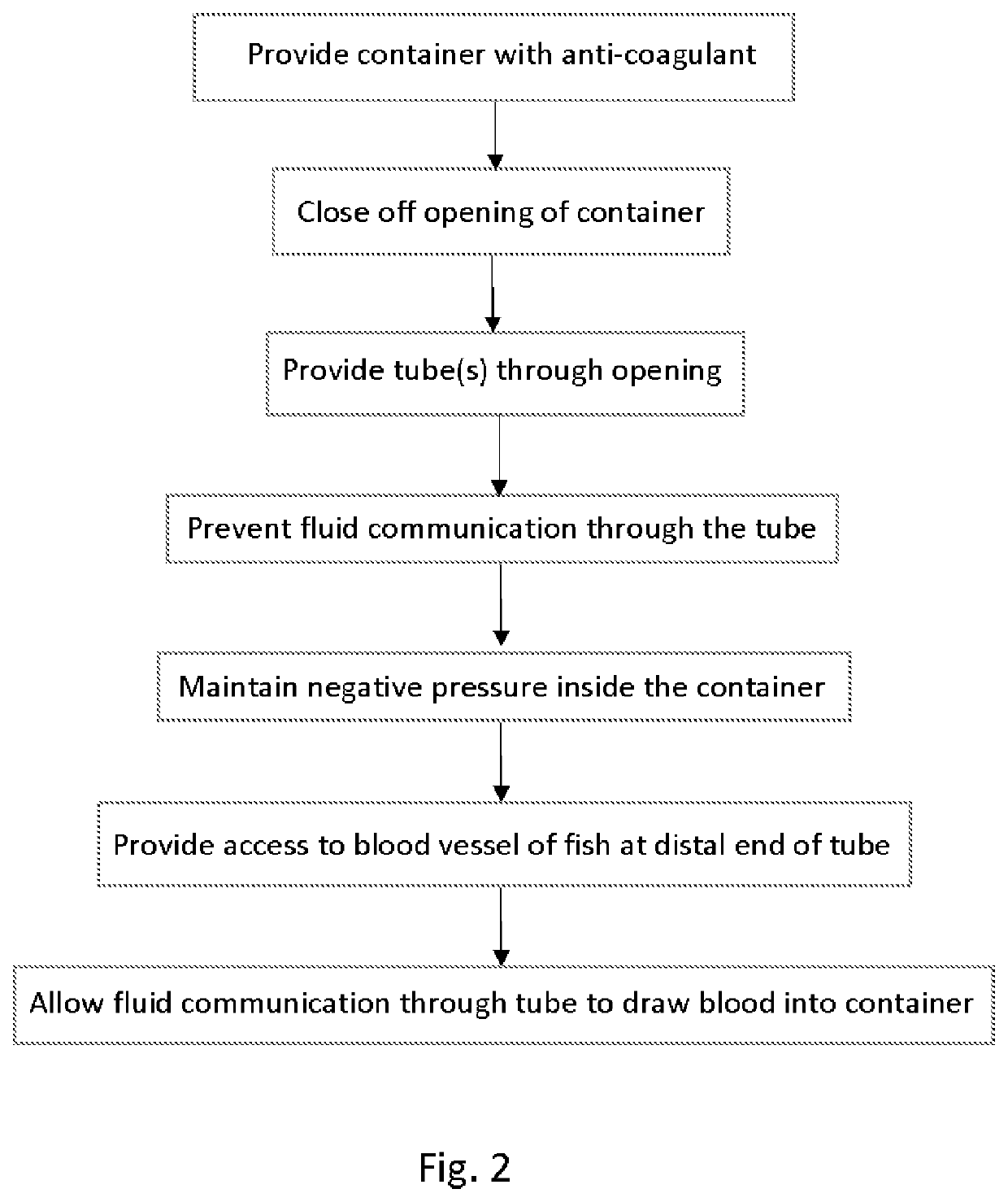
Device and Method of Obtaining Aseptic Blood from Fish
PendingUS20210290123A1Preventing fluid communicationClear clotSensorsBlood sampling devicesAnimal scienceMedicine
A method for obtaining large volumes of blood from farmed fish especially farmed salmon includes providing a container to which a negative internal pressure is applied. Input tubes to the container include valves to control the flow of fluid into the container through the input tubes. Needles at the distal ends of the tubes are used to access blood vessels of a fish. On opening of the valve associated with the tube connected to the needle accessing the fish blood vessel, the negative pressure in the container draws blood from the fish into the container. Multiple input tubes can be used to access the blood vessels of a corresponding number of fish at the same time to rapidly collect blood from an entire harvest of fish. The method preserves valuable plasma proteins, and prevents contamination of the blood.
Owner:SALMONICS LLC
A heat stable oxygen carrier-containing pharmaceutical composition for different treatment applications
ActiveCN103687609AReduce sizeTransfer minimizationPeptide/protein ingredientsPharmaceutical non-active ingredientsParanasal Sinus CarcinomaNasopharyngeal cancer
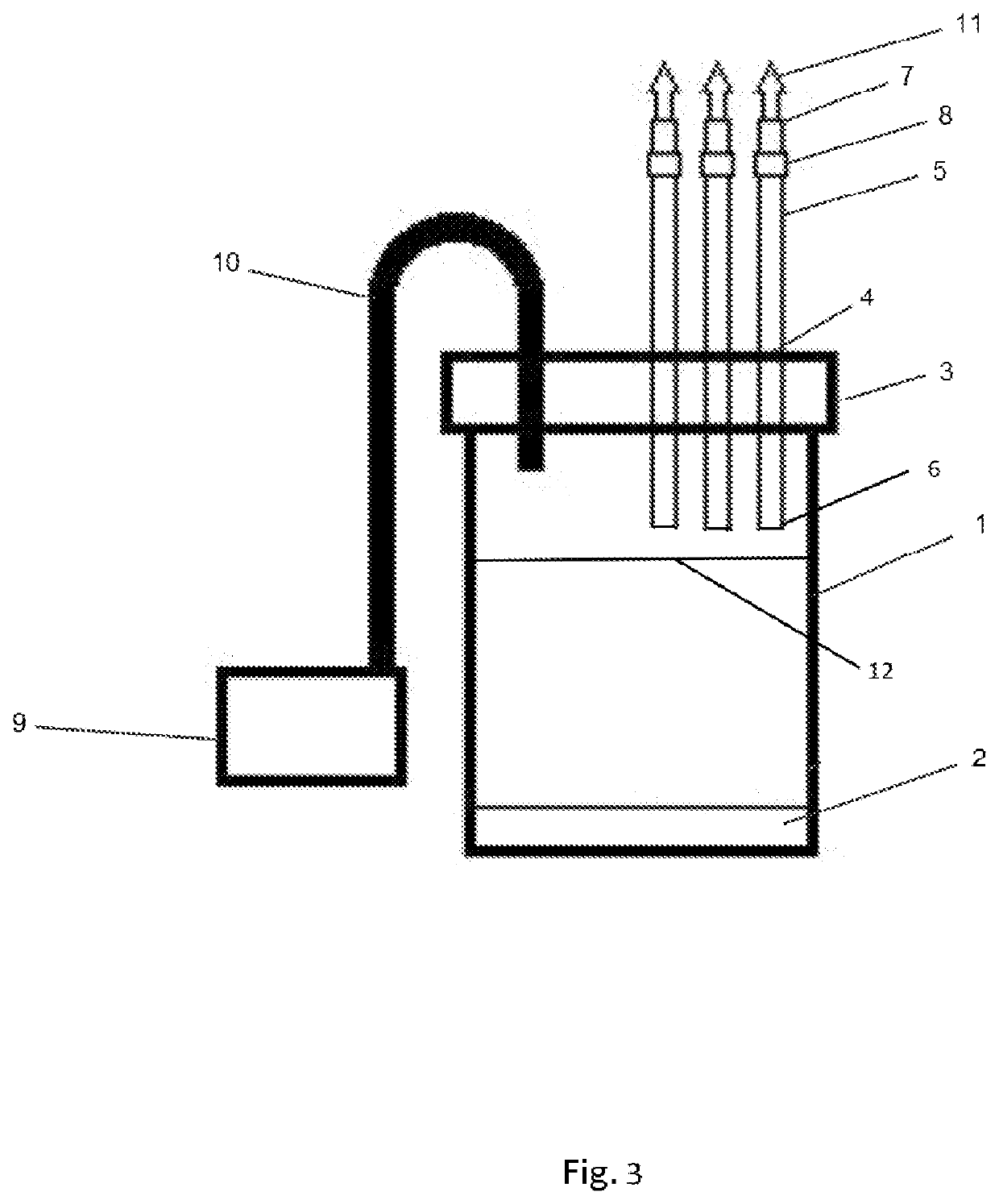
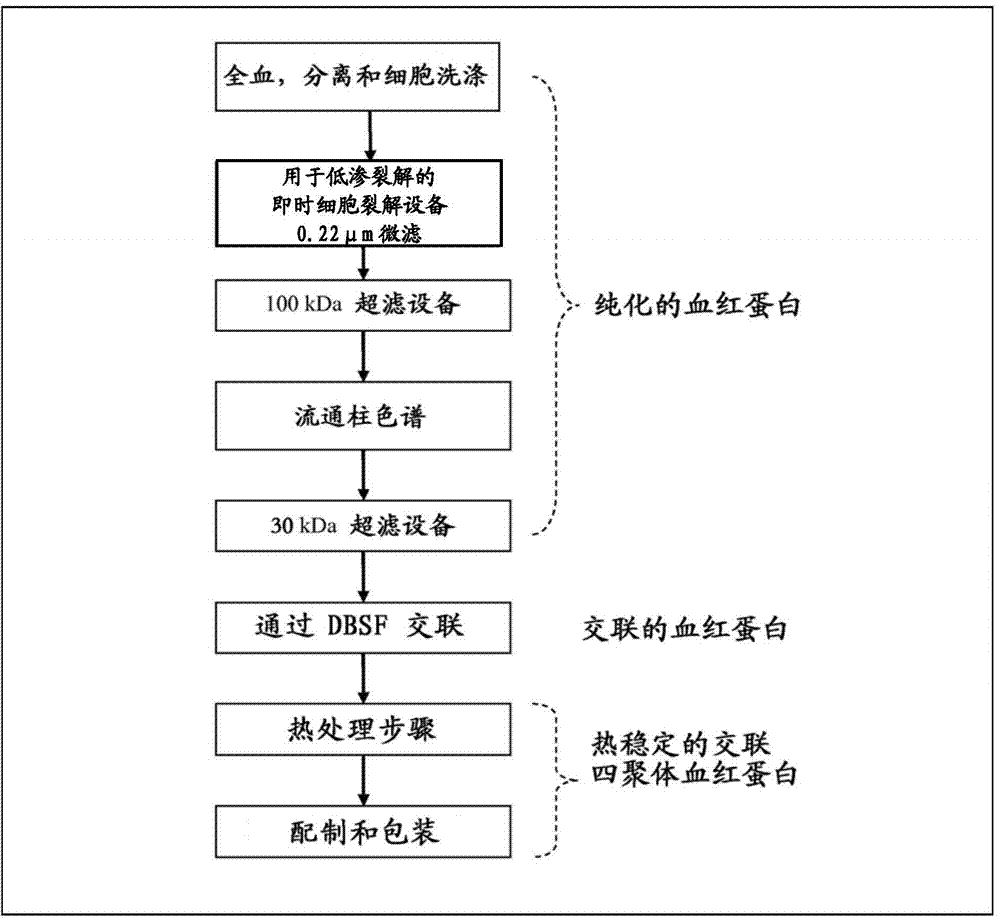
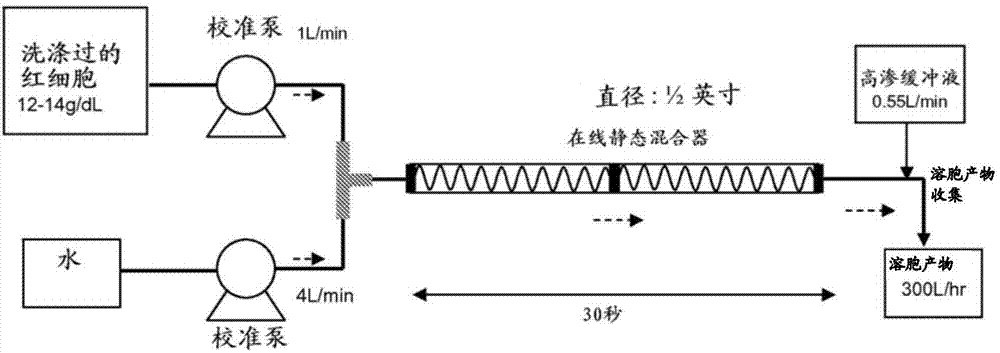
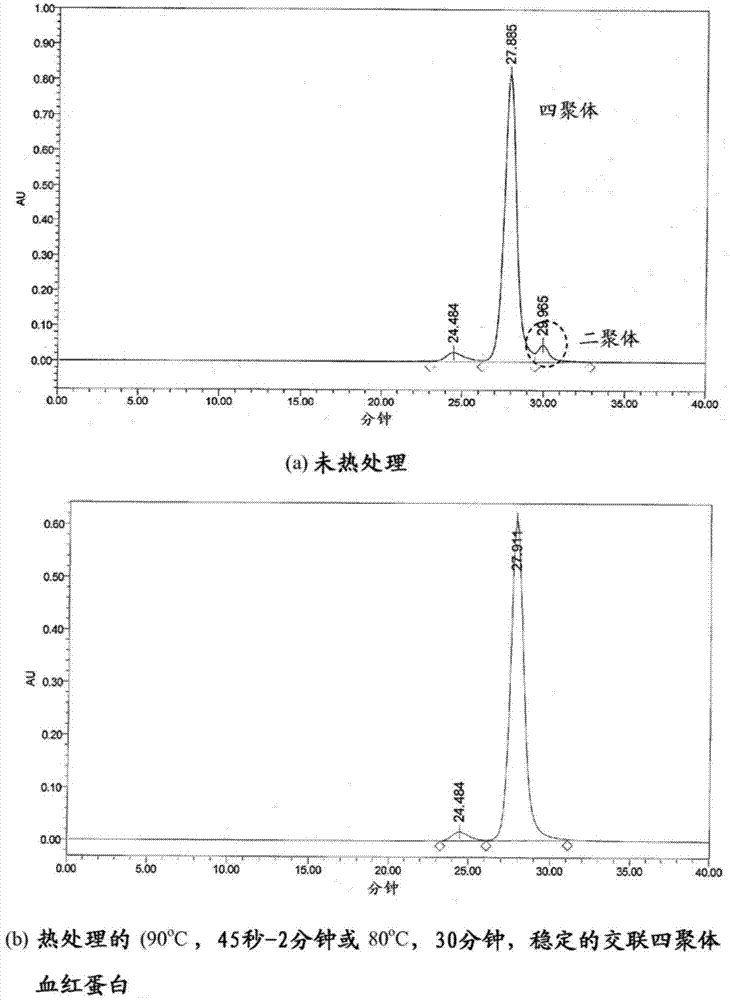
A highly purified and heat stable cross-linked nonpolymeric tetrameric hemoglobin suitable for use in mammals without causing renal injury and vasoconstriction is provided. A high temperature and short time (HTST) heat processing step is performed to remove undesired dimeric form of hemoglobin, uncross-linked tetrameric hemoglobin, and plasma protein impurities effectively. Addition of N-acetyl cysteine after heat treatment and optionally before heat treatment maintains a low level of met-hemoglobin. The heat stable cross-linked tetrameric hemoglobin can improve and prolong oxygenation in normal and hypoxic tissue. In another aspect, the product is used in the treatment of various types of cancer such as leukemia, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal carcinoma and esophageal cancer.The inventive tetrameric hemoglobin can also be used to prevent tumor metastasis and recurrence following surgical tumor excision. Further the inventive tetrameric hemoglobin can be administered to patients prior to chemotherapy and radiation treatment.
Owner:BILLION KING INT
Limulus plasma proteolytic peptide with antioxidant activity and its preparation method and application
ActiveCN106148465BImprove cleanlinessImprove antioxidant capacityAntinoxious agentsPeptide preparation methodsAntioxidant capacityHydrolysate
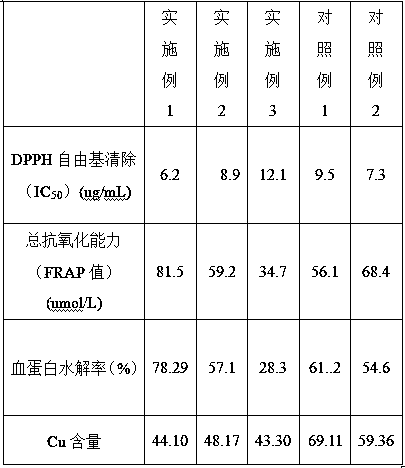
The invention relates to a limuloid plasma protein hydrolysis-peptide with antioxidative activity and a preparation method and application thereof. The preparation method comprises the following steps: a step S1 of causing limuloid plasma to undergo thermostatic waterbath for 2-4 h at 70-100 DEG C, performing suction filtration on the limuloid plasma and using water to wash the limuloid plasma, and reserving the limuloid plasma; a step S2 of using protease to hydrolyze the limuloid plasma obtained through the step S1, and then performing centrifugal separation on the limuloid plasma hydrolysate to obtain supernatant for standby application; a step S3 of causing the supernatant obtained through the step S2 to pass a SephadexG-50 column, and collecting a component with the maximum absorbance at the position of the wavelength of 230 nm, namely obtaining the limuloid plasma protein hydrolysis-peptide. The limuloid plasma is taken as a raw material, the limuloid plasma protein hydrolysis-peptide obtained through hydrolysis has high DPPH free radical scavenging capacity and total antioxidant capacity, and the limuloid plasma protein hydrolysis-peptide is non-toxic and can be prepared into a health care product which maintains beauty and delays senescence. The reutilization of the limuloid plasma is achieved, and the use value of the limuloid plasma is improved.
Owner:GUANGDONG OCEAN UNIVERSITY
A panel of plasma protein markers for predicting the risk of metastasis in nasopharyngeal carcinoma
ActiveCN111999499BPredict riskPredict prognosisDisease diagnosisBiological testingProtein markersParanasal Sinus Carcinoma
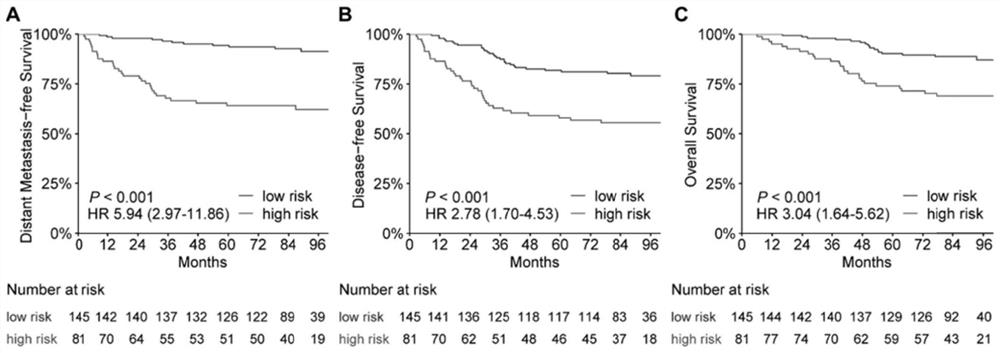
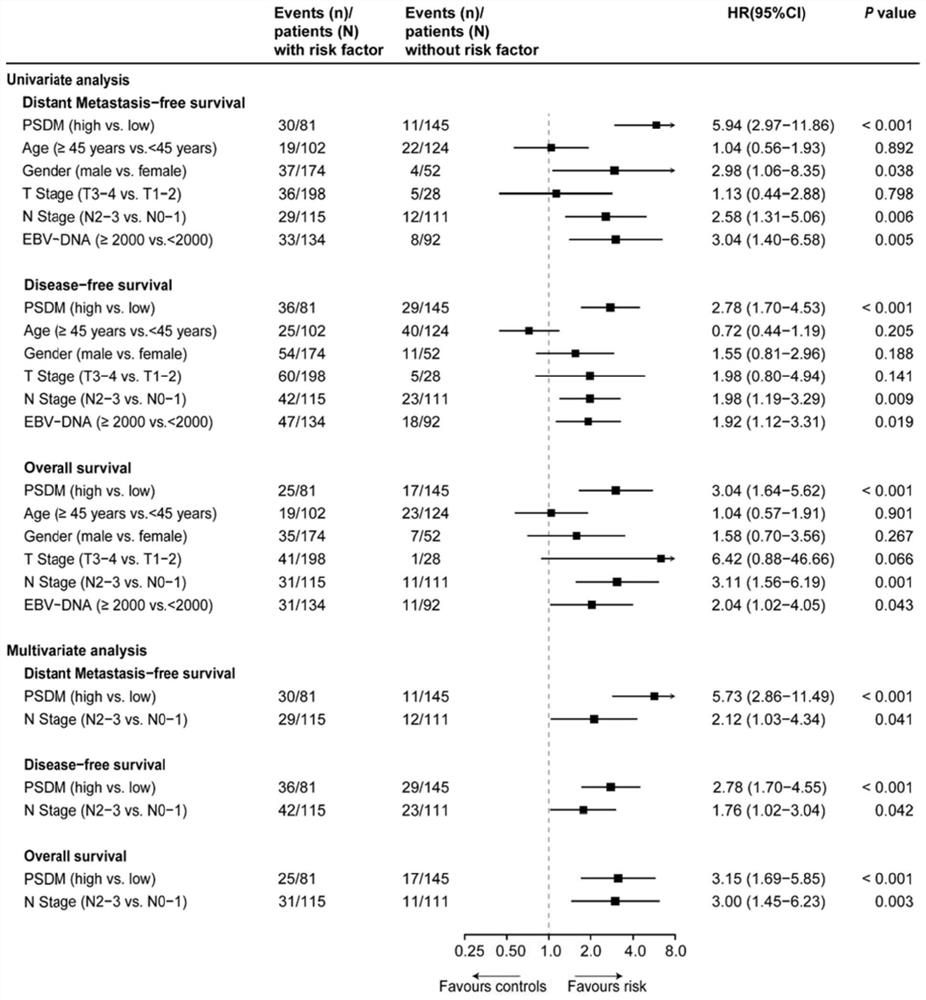
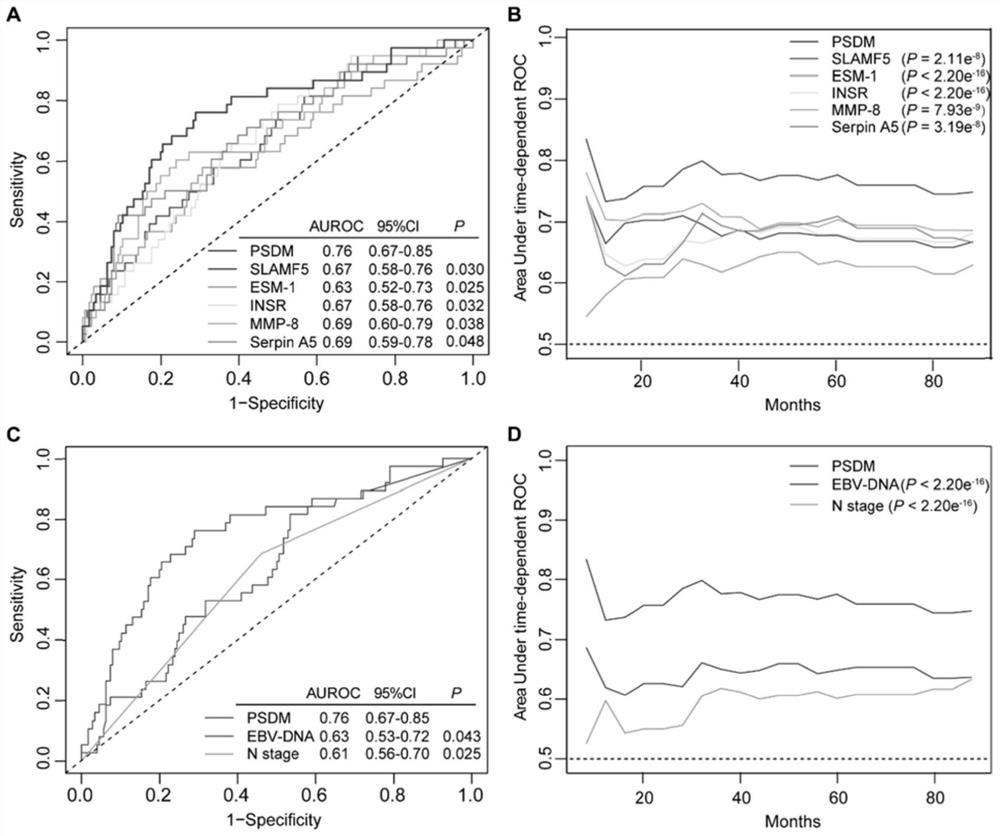
The invention discloses a group of plasma protein markers for predicting the risk of nasopharyngeal carcinoma metastasis and its application. Markers include SLAMF5, ESM‑1, MMP‑8, INSR, Serpin A5. The protein tags formed by the expression of five proteins in the present invention can reflect the biological specificity of nasopharyngeal carcinoma patients, can more accurately predict the metastasis risk and prognosis of nasopharyngeal carcinoma patients, better guide clinical medication, and have a positive impact on patients without distant survival. The predictive power was better than that of individual proteins, tumor N stage and EBV‑DNA levels.
Owner:SUN YAT SEN UNIV CANCER CENT
Device for detecting ultrafiltrate plasma protein of uremia patients after glomerular filtration
The invention provides a device for detecting ultrafiltrate plasma protein of uremia patients after glomerular filtration. The device comprises a body; the upper surface of the inner included angle ofthe side end of the body is fixedly connected to the top surface of a movable plate; clamping shafts are fixedly connected to the top end and the bottom end of the movable plate; the inner sides of the clamping shafts are movably connected to the right ends of movable rods; the inner sides of the movable rods are movably connected to the outer sides of movable shafts; suction stones penetrate through the movable shafts; the bottoms of the movable shafts are slidably connected to the top ends of shelves; and the outer sides of the shelves are fixedly connected to the inner sides of movable pieces. One part of a drying agent will be clamped on the inner side wall of a limiting pipe in an absorbing process, the drying agent which is not clamped can continuously fall to the limiting pipe at the bottom layer by layer to adsorb moisture, and the moisture in the sponge is gradually adsorbed by the drying agent, so that the interior of the machine can be kept dry, the condition of uneven drying area is avoided, inaccurate display is avoided, the reflecting mirror is prevented from becoming dirty, and the accuracy of the light ray rate is guaranteed.
Owner:陈晓艳
Fully automatic plasma processing equipment and processing method
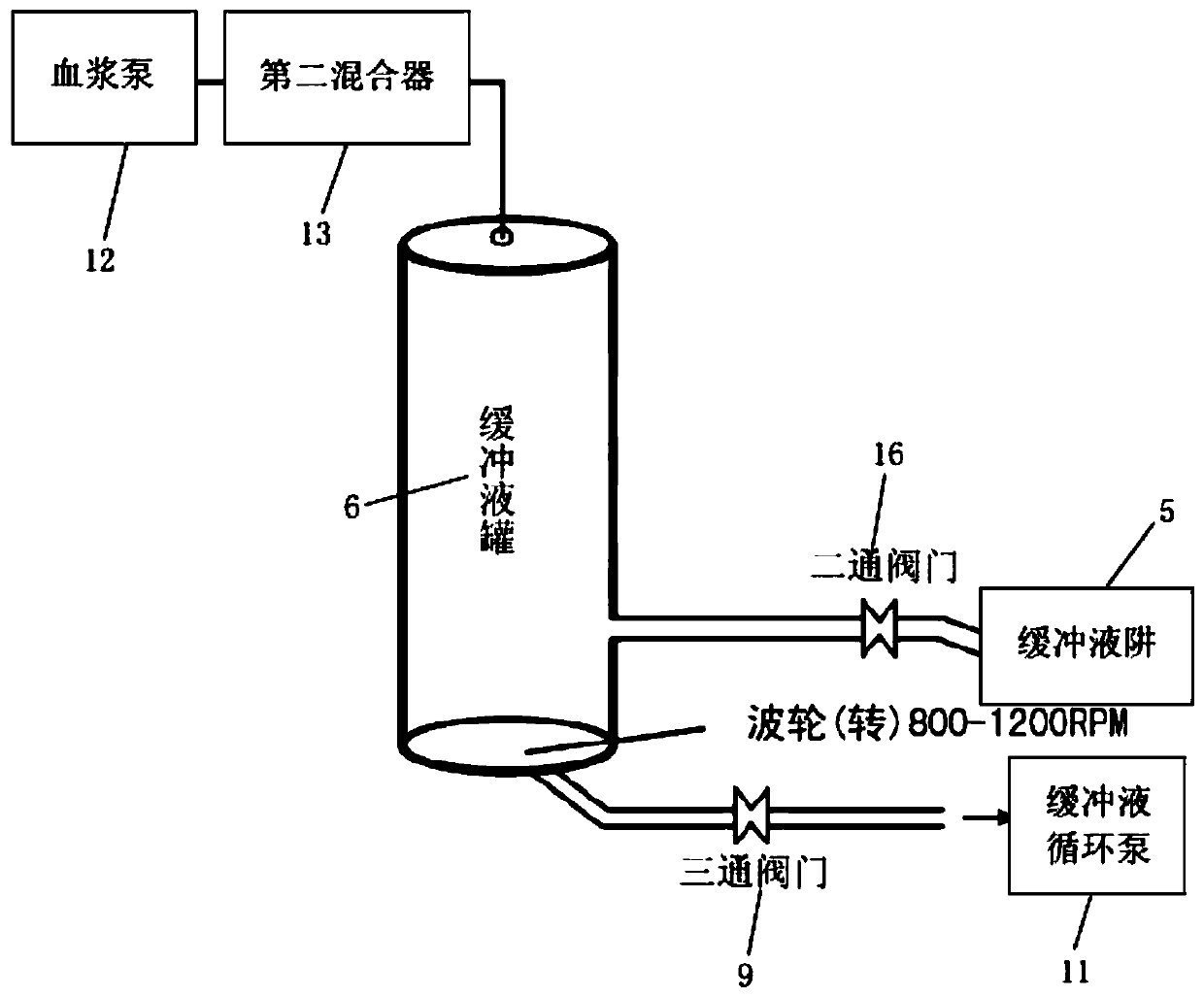
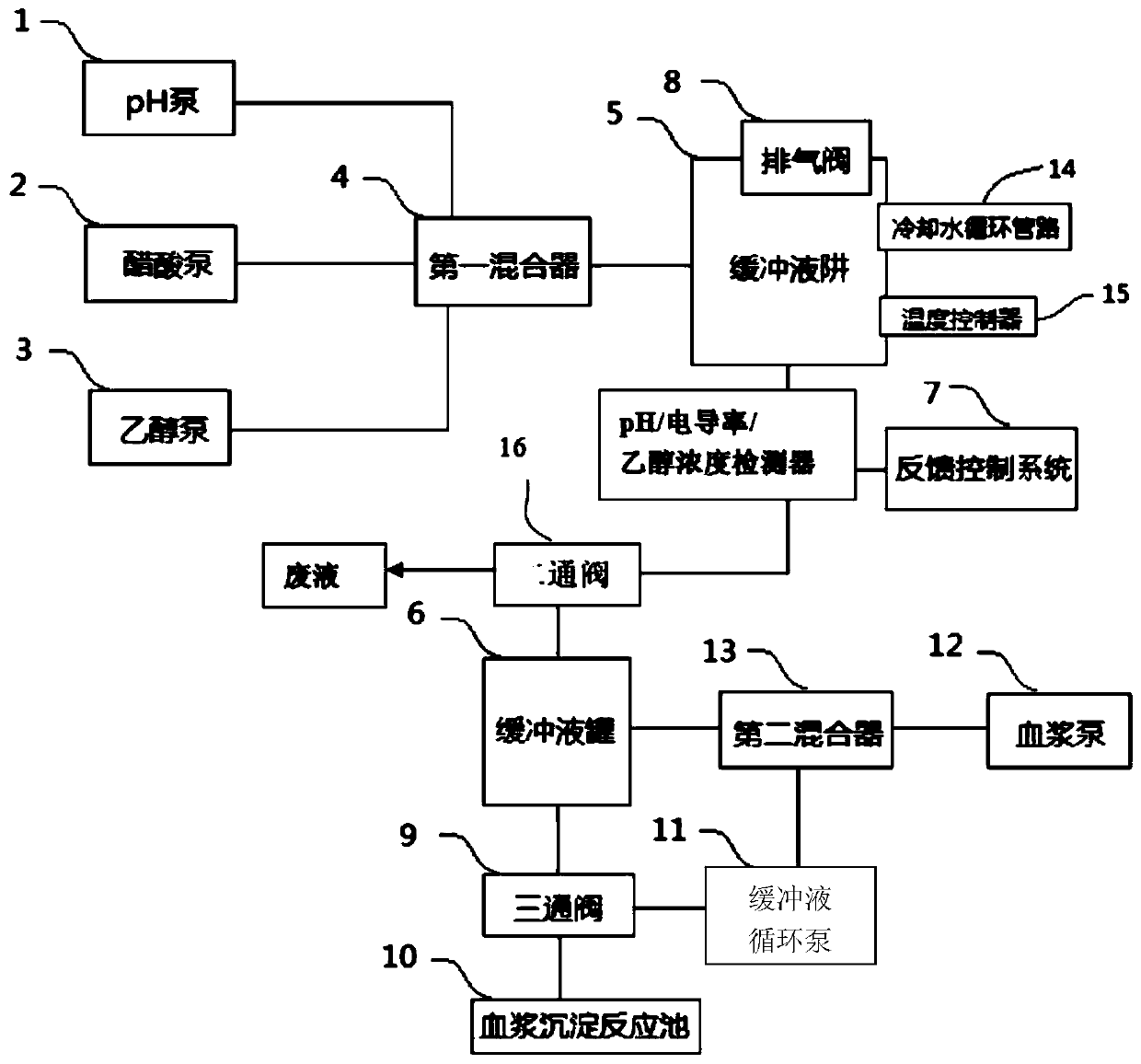
ActiveCN106699835BPrecise Control of ConductivityPrecise control of concentrationPeptide preparation methodsControl systemBlood plasma
The invention discloses a full-automatic plasma processing equipment and method. The equipment comprises a pH pump, an acetic acid pump, an ethanol pump, a plasma pump, a plasma precipitation reaction pool, a buffer solution circulating pump, a buffer solution trap, a buffer solution tank, a three-way valve and a feedback control system; the pH pump, the acetic acid pump and the ethanol pump are communicated with the buffer solution trap and the buffer solution tank respectively through a first mixer, the feedback control system is arranged between the buffer solution trap and the buffer solution tank, and the feedback control system is electrically connected with the pH pump, the acetic acid pump and the ethanol pump; the first port of the three-way valve is communicated with the buffer solution tank, the second port of the three-way valve is communicated with the plasma precipitation reaction pool, the third port of the three-way valve is communicated with the buffer solution circulating pump, and the buffer solution circulating pump and the plasma pump are communicated with the buffer solution tank through a second mixer. Accordingly, full automation can be achieved, the conductivity, ethanol concentration and acidity and alkalinity of a buffer solution are accurately controlled, protein in the plasma is precipitated through online verification, the recovery rate and yield of the plasma protein can be increased, and profit rate is increased.
Owner:CHANGSHU NANOMICRO BIOTECHNOLOGY CO LTD
Preparation process and device of low-ash pig plasma protein powder
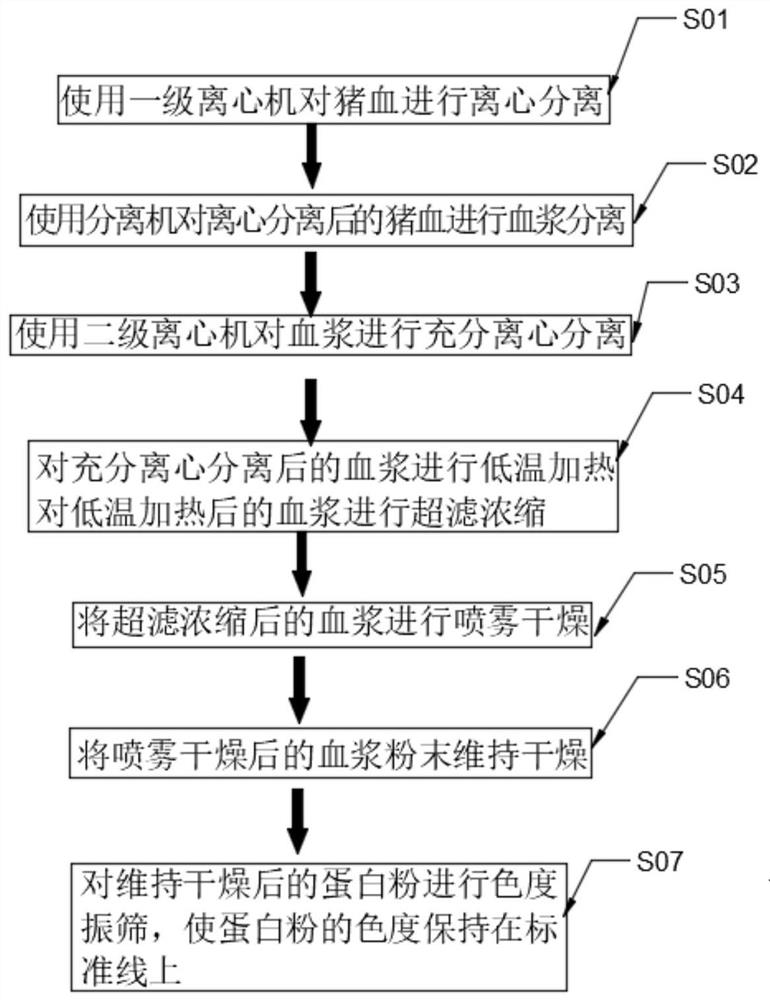
InactiveCN111871625AHigh whitenessQuality assuranceProtein composition from bloodCentrifugesUltrafiltrationPhysical chemistry
The invention discloses a preparation process and device of low-ash pig plasma protein powder. The preparation process comprises the following steps of S01, carrying out centrifugal separation on pigblood by using a first-stage centrifugal machine; S02, carrying out plasma separation on the pig blood subjected to centrifugal separation by using a separator; S03, carrying out sufficient centrifugal separation on plasma by using a second-stage centrifugal machine; S04, carrying out low-temperature heating on the plasma subjected to sufficient centrifugal separation, and carrying out ultrafiltration concentration on the plasma subjected to low-temperature heating; S05, carrying out spray drying on the plasma subjected to ultrafiltration concentration; S06, keeping the plasma powder subjectedto spray drying dry; and S07, carrying out chromaticity vibration screening on the protein powder subjected to dry keeping, so that the chromaticity of the protein powder is kept on a standard line.According to the preparation process and device, the plasma protein can be fully separated from the blood through first-stage centrifugal separation, separation with the separator and second-stage centrifugal separation, the purity of the plasma protein can be fully improved through ultrafiltration concentration, the gray scale of the plasma protein powder is reduced, and the plasma protein powdercan be made to be in a uniform powder shape through spray drying.
Owner:江苏永盛生物科技有限公司
Preparation method of freeze-dried plasma
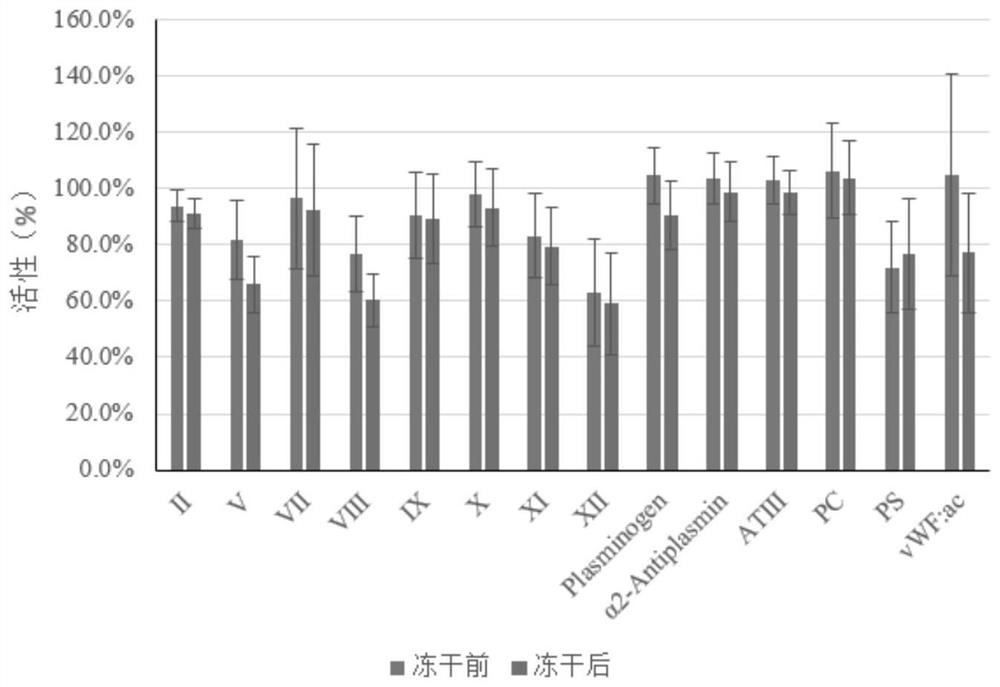
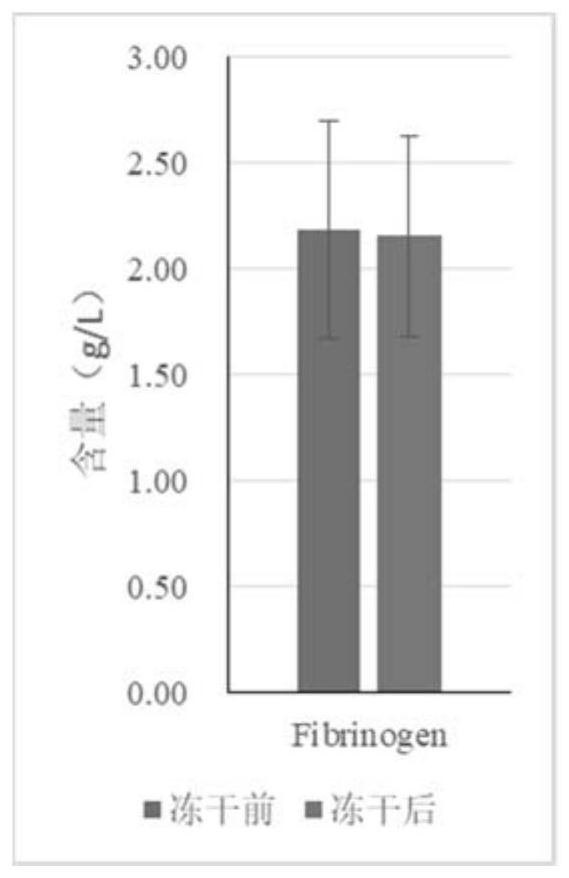
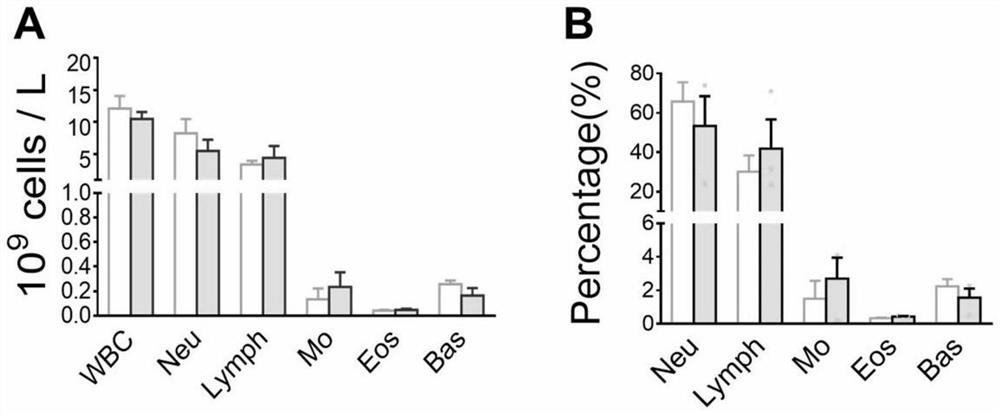
The invention discloses a preparation method of freeze-dried plasma. According to the method, a protective agent is not added, spin freezing is not carried out before freeze-drying, the recovery rate of the blood coagulation factor VIII and von Willebrand factor (vWF) can reach 70% or above, the recovery rate of other plasma proteins can reach 80% or above, and the method has important application value.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method for evaluating hypoimmunity of methamphetamine addict
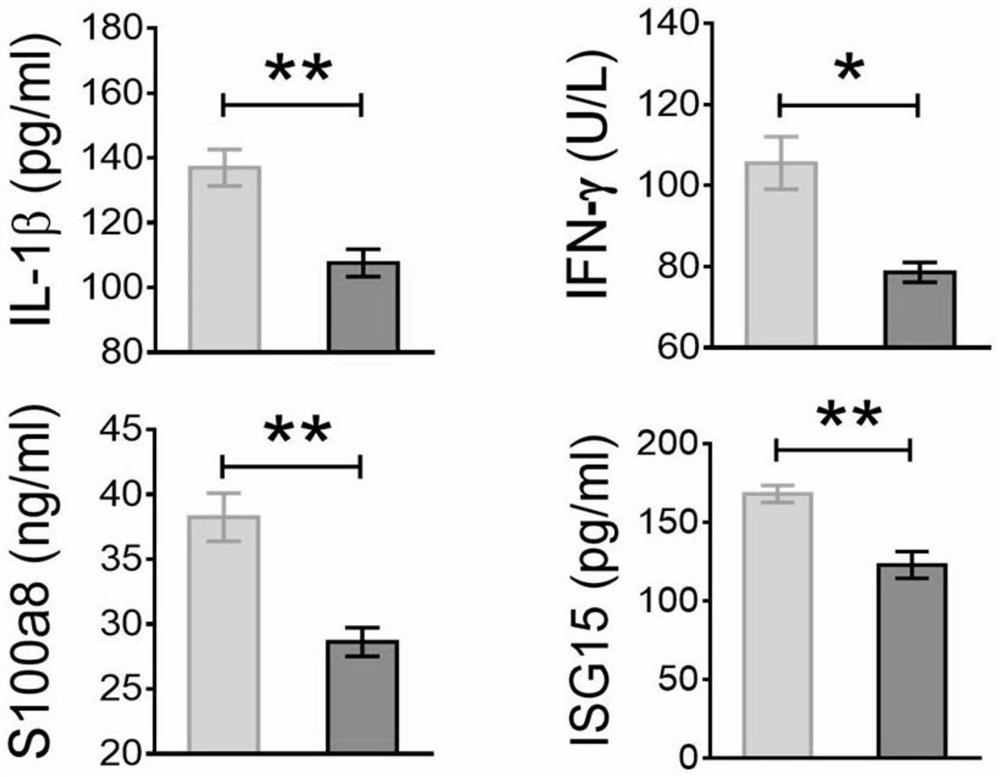
PendingCN114487440AQuick and easy assessmentReduce dosageBiological material analysisBiological testingISG15Venous blood
The invention relates to a method for evaluating hypoimmunity of a methamphetamine addict, which comprises the following steps: S1, collecting peripheral venous blood of a subject, and centrifugally separating the peripheral venous blood to obtain plasma; s2, carrying out enzyme-linked immunosorbent assay (ELISA) detection on IL-1beta, S100A8, IFN gamma and ISG15 on the plasma to obtain corresponding plasma protein levels of the IL-1beta, the S100A8, the IFN gamma and the ISG15; and S3, comparing the detected plasma protein levels of the IL-1beta, the S100A8, the IFN gamma and the ISG15 with the plasma protein levels of the IL-1beta, the S100A8, the IFN gamma and the ISG15 of a normal person, and when the detected plasma protein levels are reduced by more than 50% compared with the plasma protein levels of the normal person, considering that the immunity of the subject is reduced and the infection risk is increased. A detection sample is a conventional blood sample, the dosage is small, and sampling is convenient; the detection reagent, the instrument and the detection process are simple and convenient.
Owner:西安华壹健康医学检验实验室有限公司
Plasma protein markers for predicting the risk of acute mountain sickness and their application in the preparation of diagnostic ams susceptibility kits
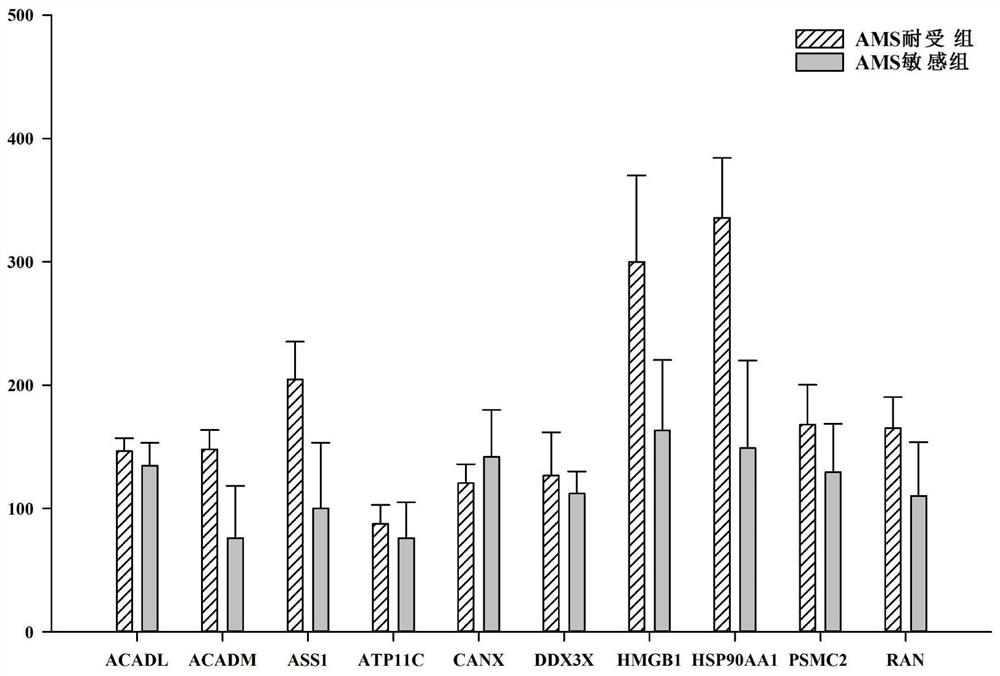
ActiveCN109212226BInsufficient preventive measuresAvoid side effectsDisease diagnosisBiological testingDisease riskBlood plasma
The invention relates to the technical field of medical biological detection, and provides a plasma protein marker for predicting the risk of acute mountain sickness and the application of the plasma protein marker in preparing a diagnostic reagent or a diagnostic kit for susceptibility to acute mountain sickness. The plasma protein marker of the present invention is a combination of plasma proteins ACADL, ACADM, ASS1, ATP11C, CANX, DDX3X, HMGB1, HSP90AA1, PSMC2 and RAN. The present invention further provides a kit and a detection method utilizing the susceptibility diagnosis of acute mountain sickness. The kit and detection method of the present invention are simple, reliable, short in cycle, high in specificity, and easy for clinical promotion.
Owner:中国人民解放军联勤保障部队第九四〇医院
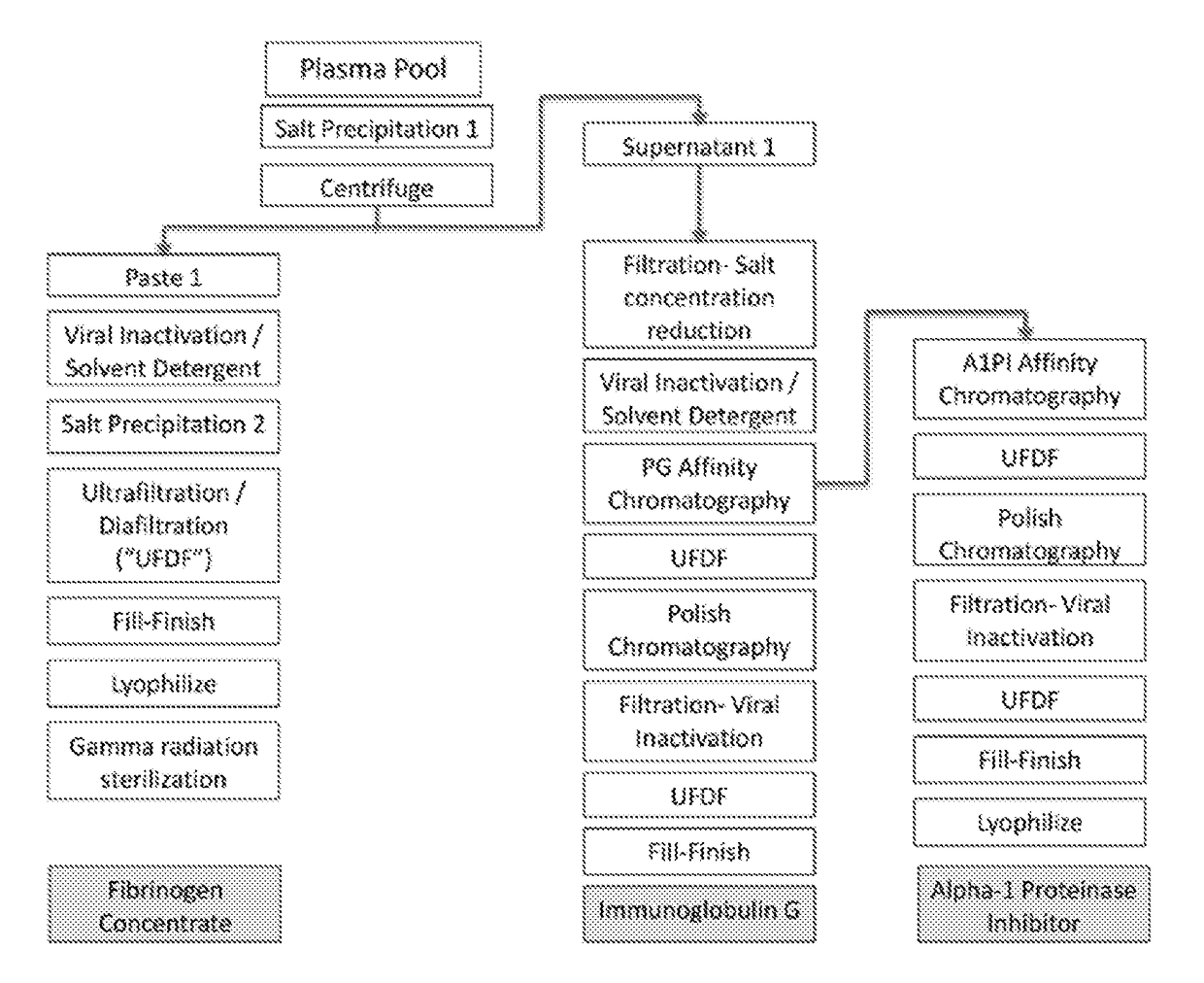
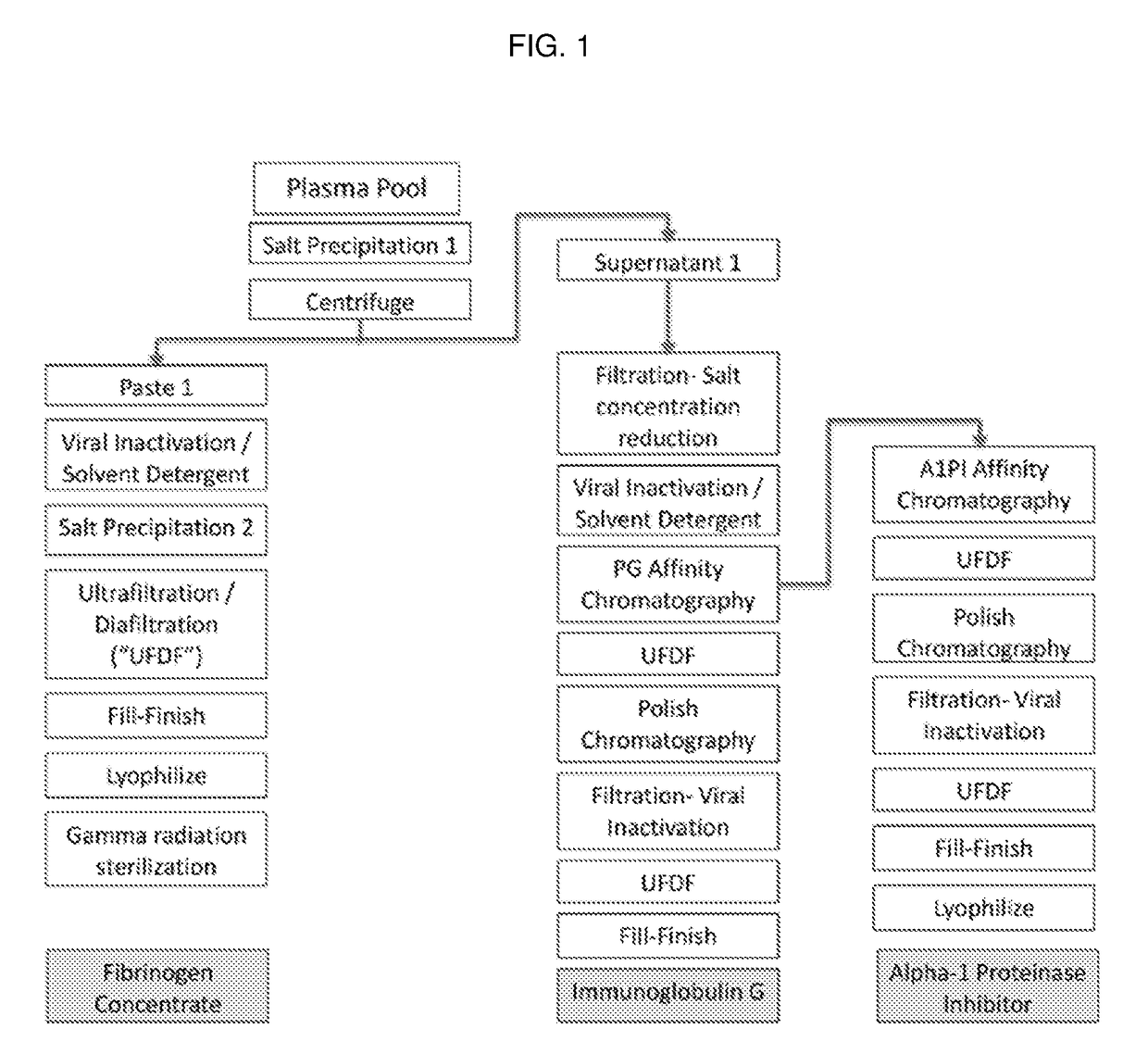
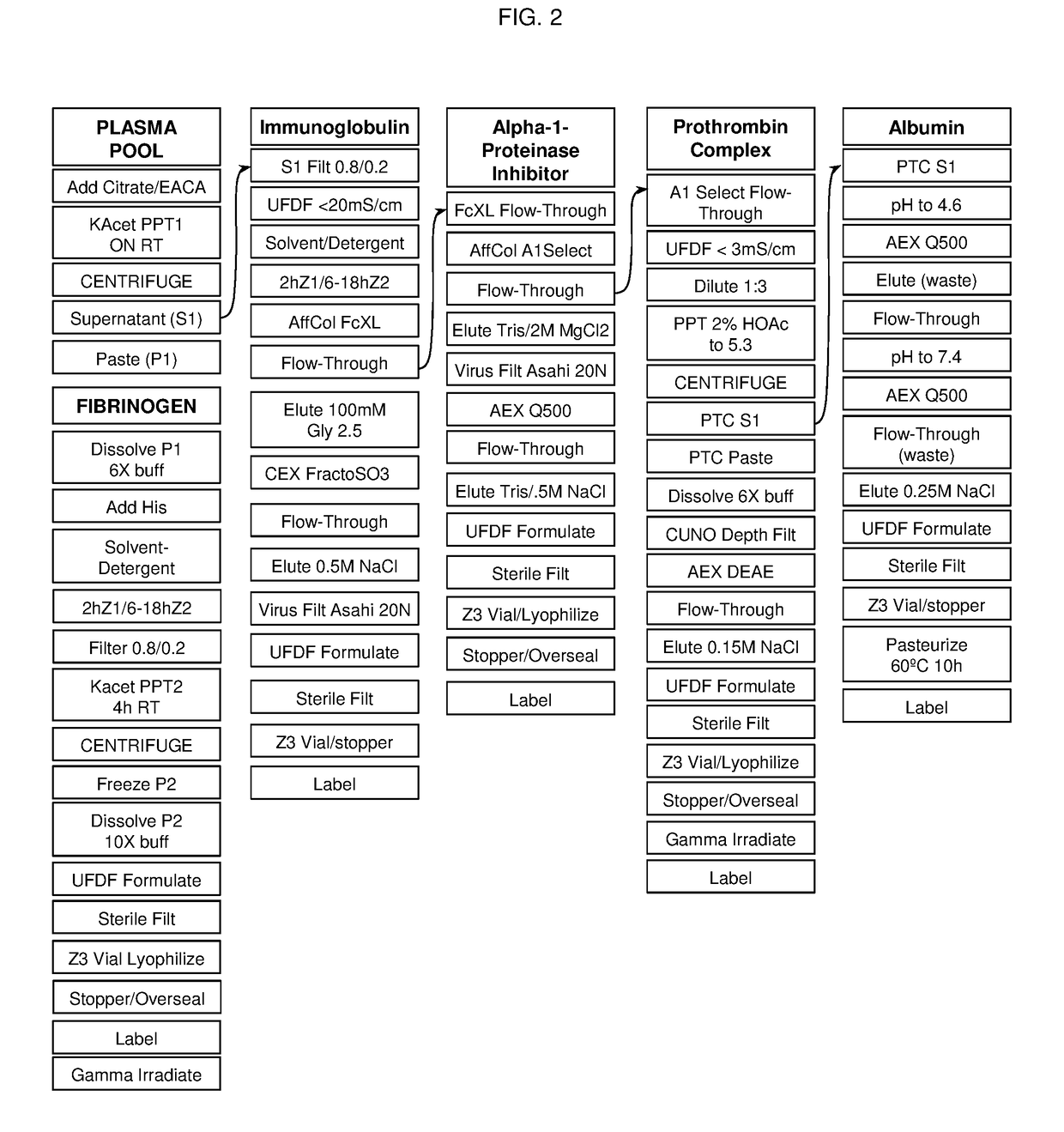
Processes for purifying proteins from plasma
InactiveUS20180305401A1Improved and scalable processReduce salt contentSerum immunoglobulinsPeptide preparation methodsChemistryBlood plasma
The invention provides processes for producing preparations (e.g., plasma preparations or fibrinogen (Fg)-depleted preparations) containing one or more proteins (e.g., plasma proteins). Processes of the invention can be used to obtain enriched preparations of one or more proteins (e.g., Fg, immunoglobulin (Ig; e.g., IgG), alpha-1 proteinase inhibitor (A1 PI), albumin, plasminogen, prothrombin complex, and / or other plasma proteins). Multiple enriched preparations can be obtained from a single sample (e.g., a whole blood or plasma sample) using the processes of the invention.
Owner:CAMBRYN BIOLOGICS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com