Pharmacokinetic Drug Dosing Regimen Devices and Methods
A technology of pharmacokinetics and dosing regimens, applied in the direction of drugs or prescriptions, drug combinations, medical equipment, etc., can solve problems such as stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
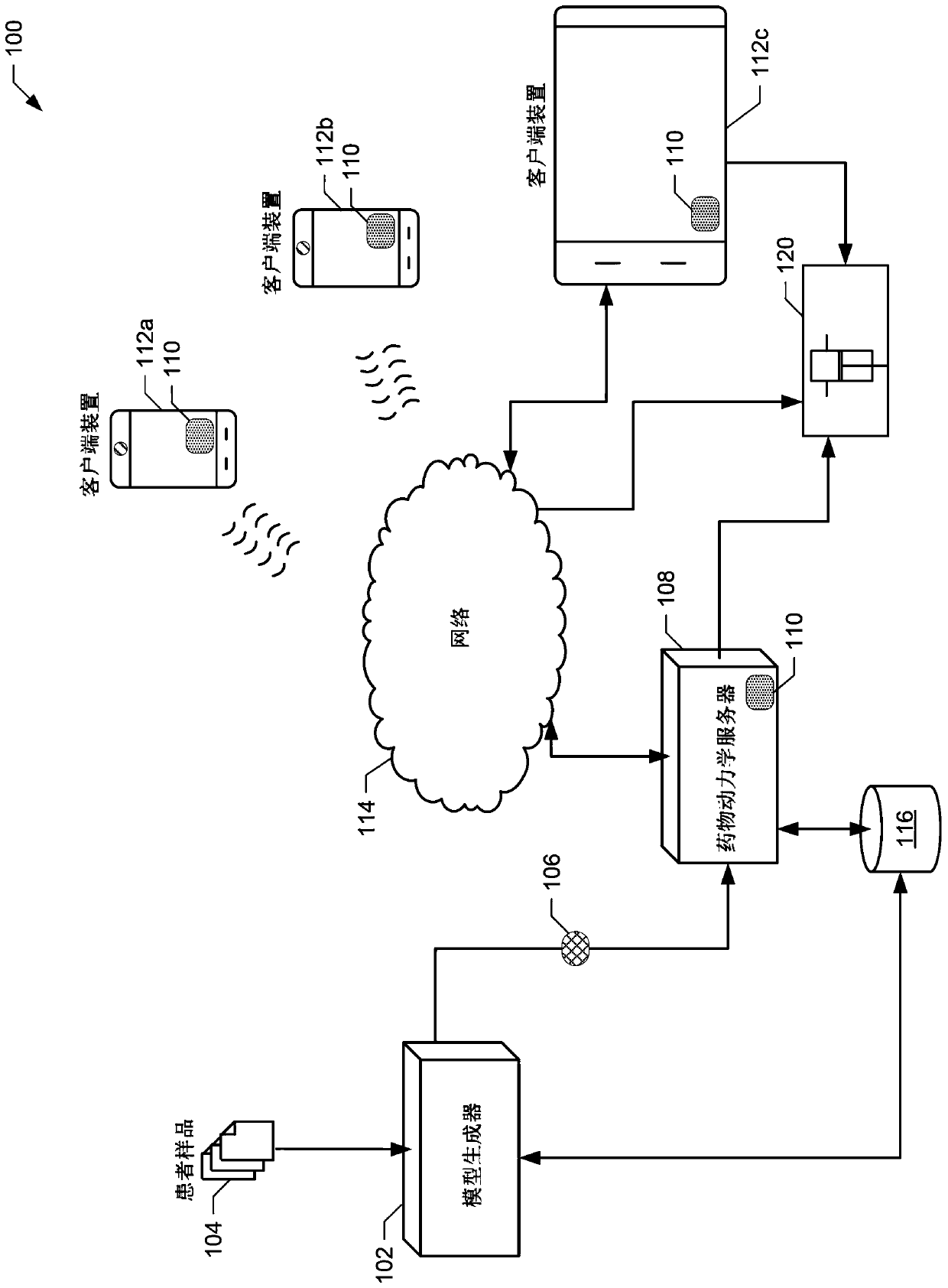
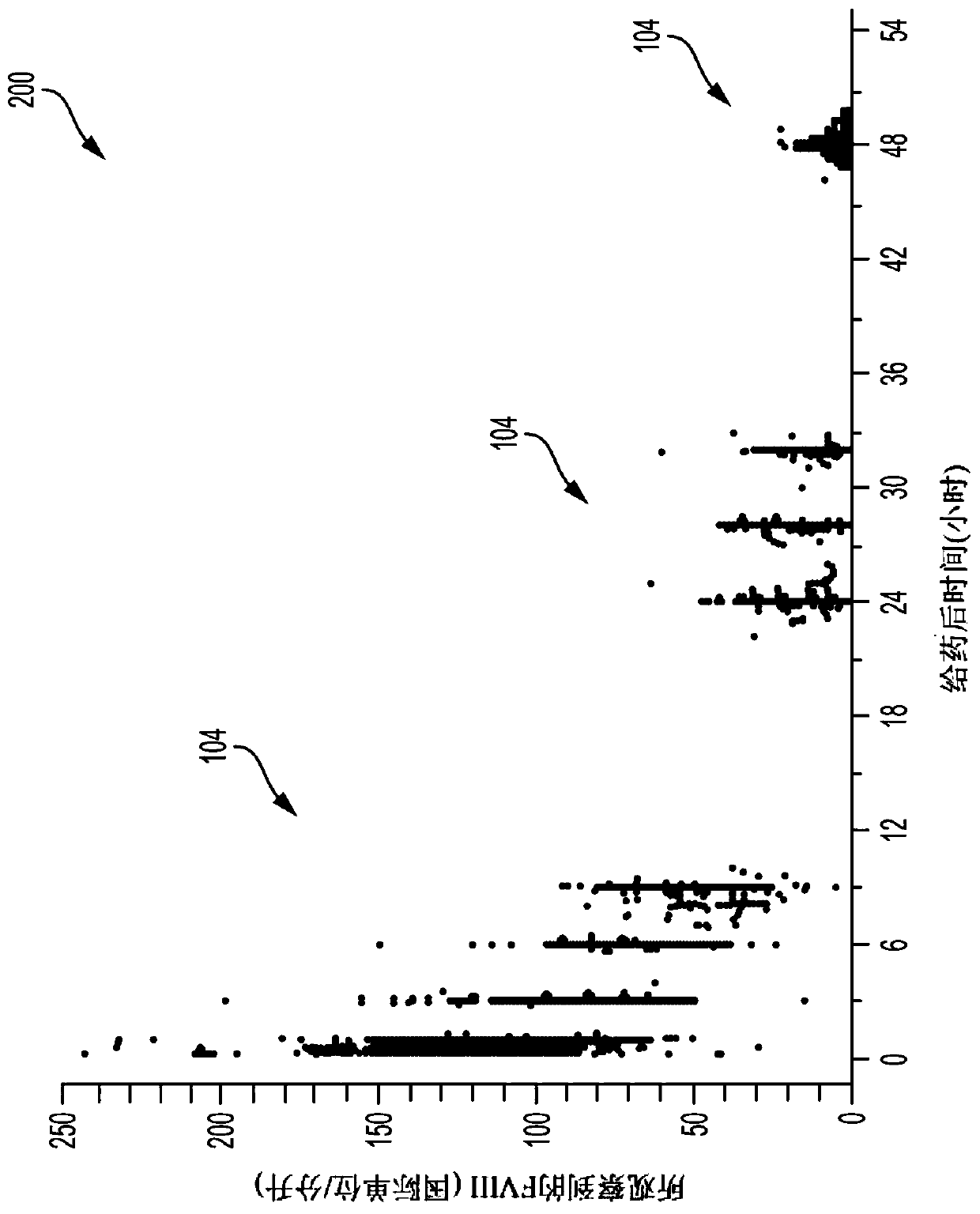
[0043] The present invention relates generally to methods, systems and devices for providing drug dosing regimens, and in particular providing pharmacokinetic drug dosing regimens based on models of the pharmacokinetic profile of a sampled patient. The pharmacokinetic drug dosing regimens described herein provide cost-effective use of therapeutic plasma proteins that can be tailored to individual patients. Thus, the exemplary pharmacokinetic drug dosing regimens described herein provide healthcare providers with the ability to achieve relatively rapid and accurate patient dosing without having to determine a patient-specific pharmacokinetic profile based (only) on blood tests suggested tool. The invention also encompasses coagulation factor FVIII products modified to prolong the mean residence time in patients beyond that of native FVIII, for example via the use of water soluble proteins or FC fusion technology, with dosing schedules / intervals longer than three days.
[0044]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com