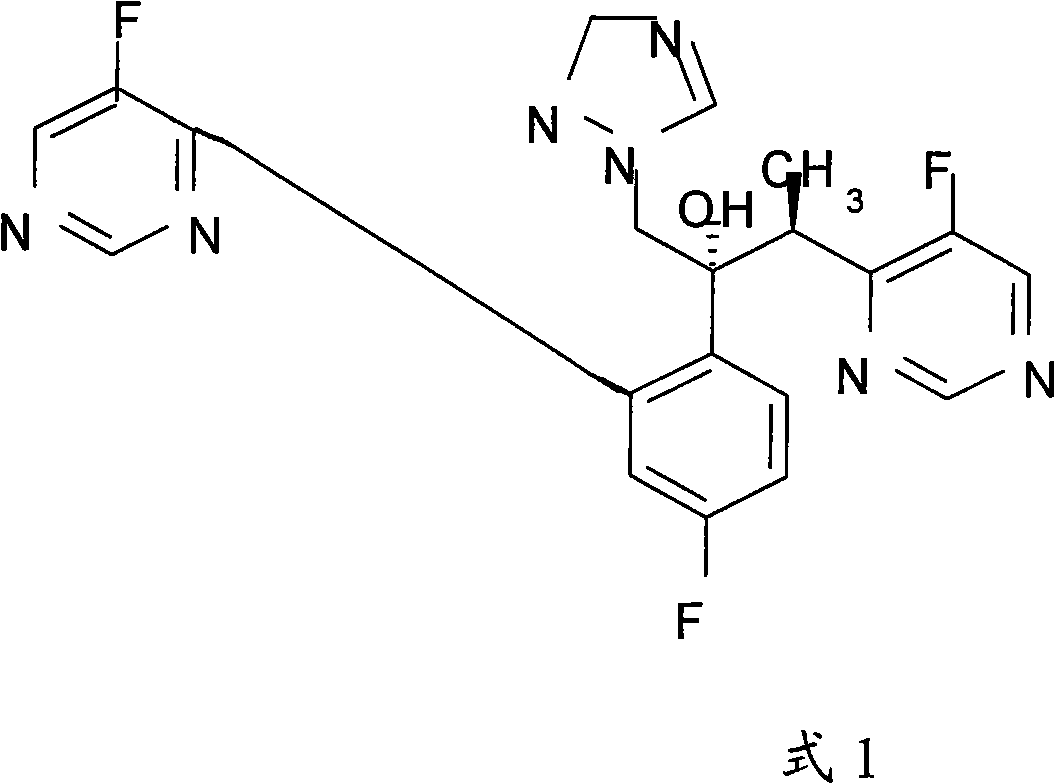
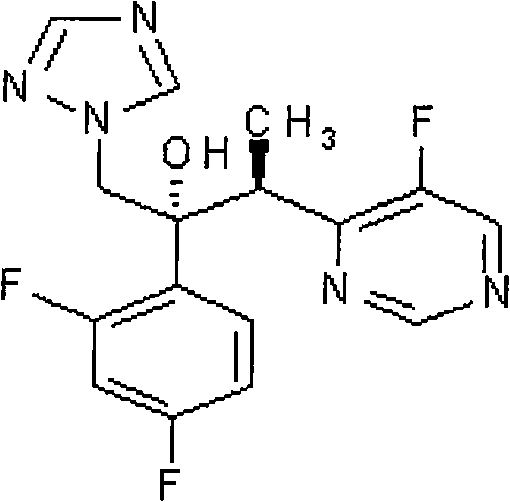
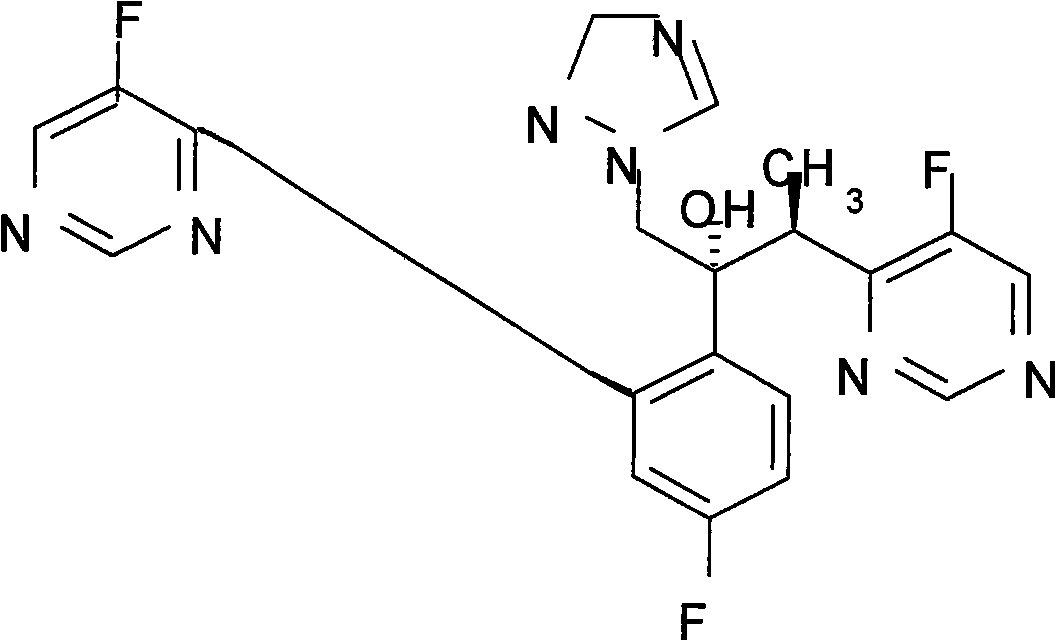
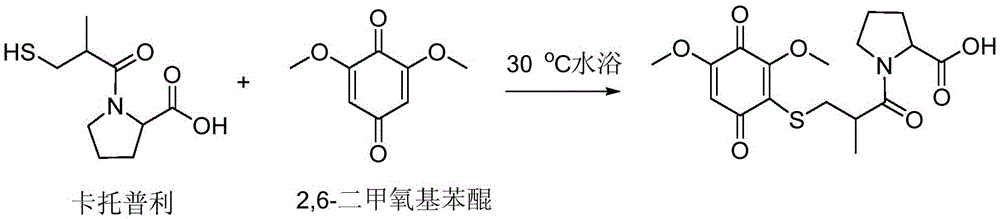
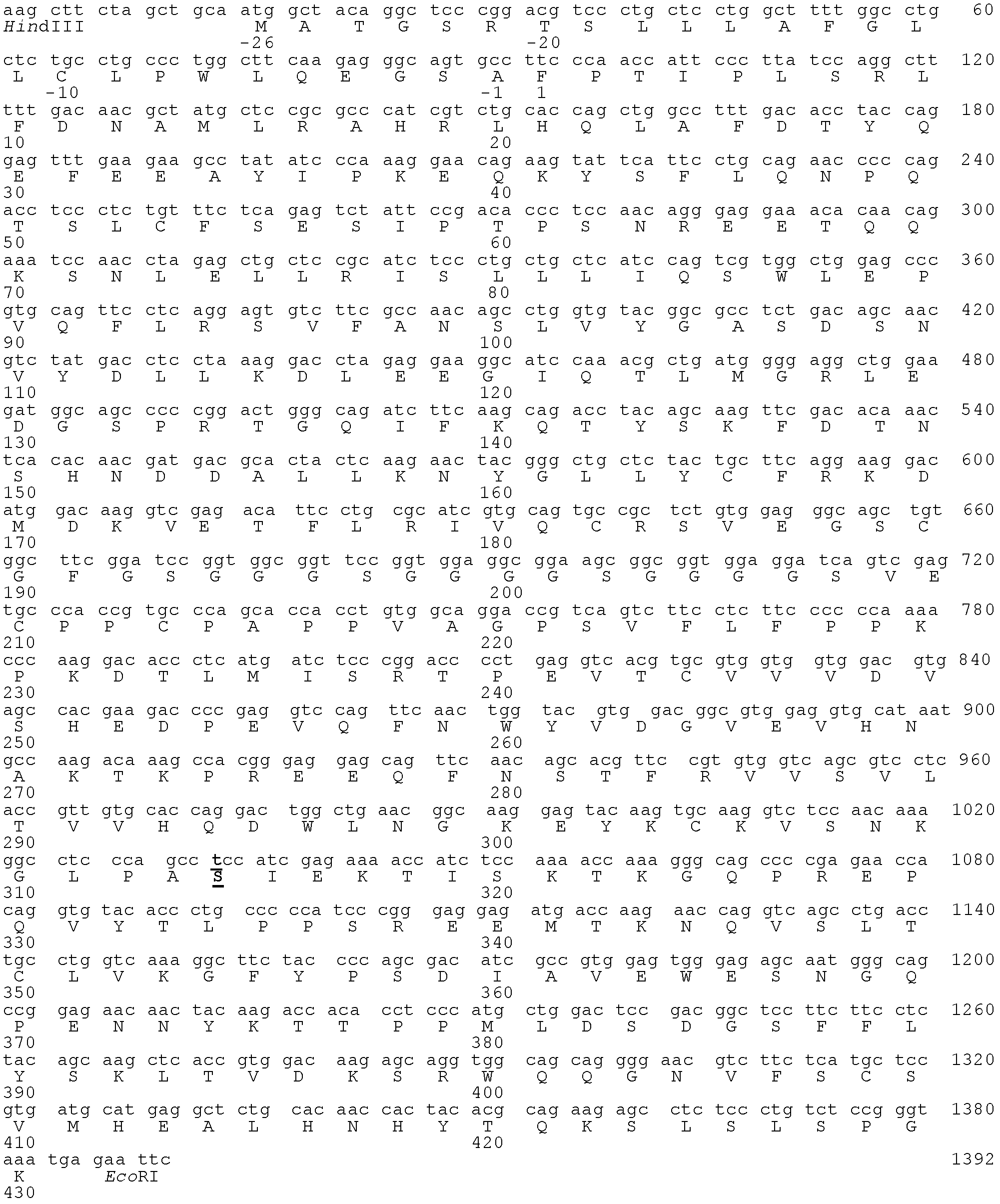Patents
Literature
126 results about "Drug Kinetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug administration is the giving of a drug by one of several means (routes). Drug kinetics (pharmacokinetics) describes how the body handles a drug and accounts for the processes of absorption, distribution, metabolism, and elimination.
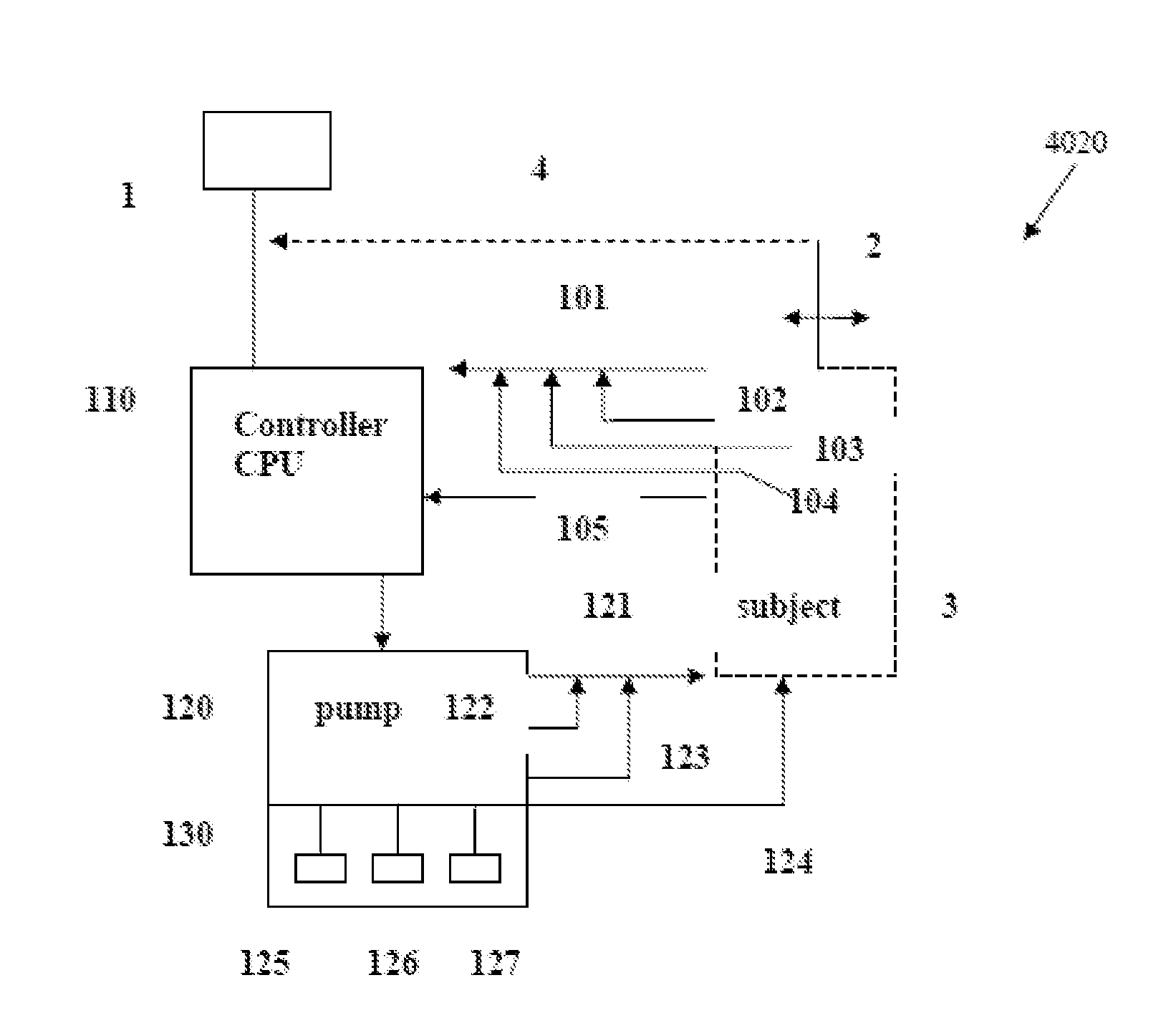
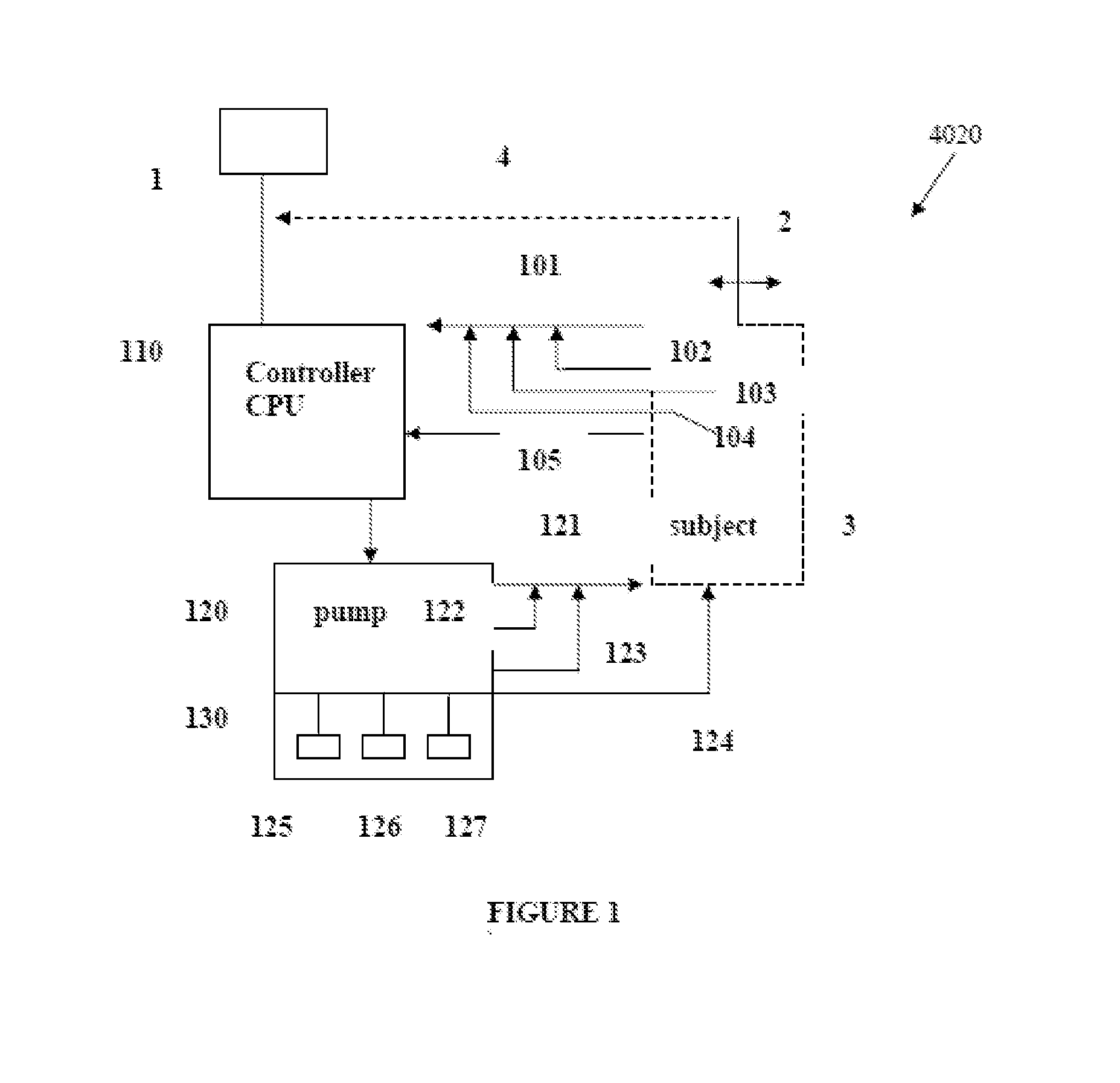
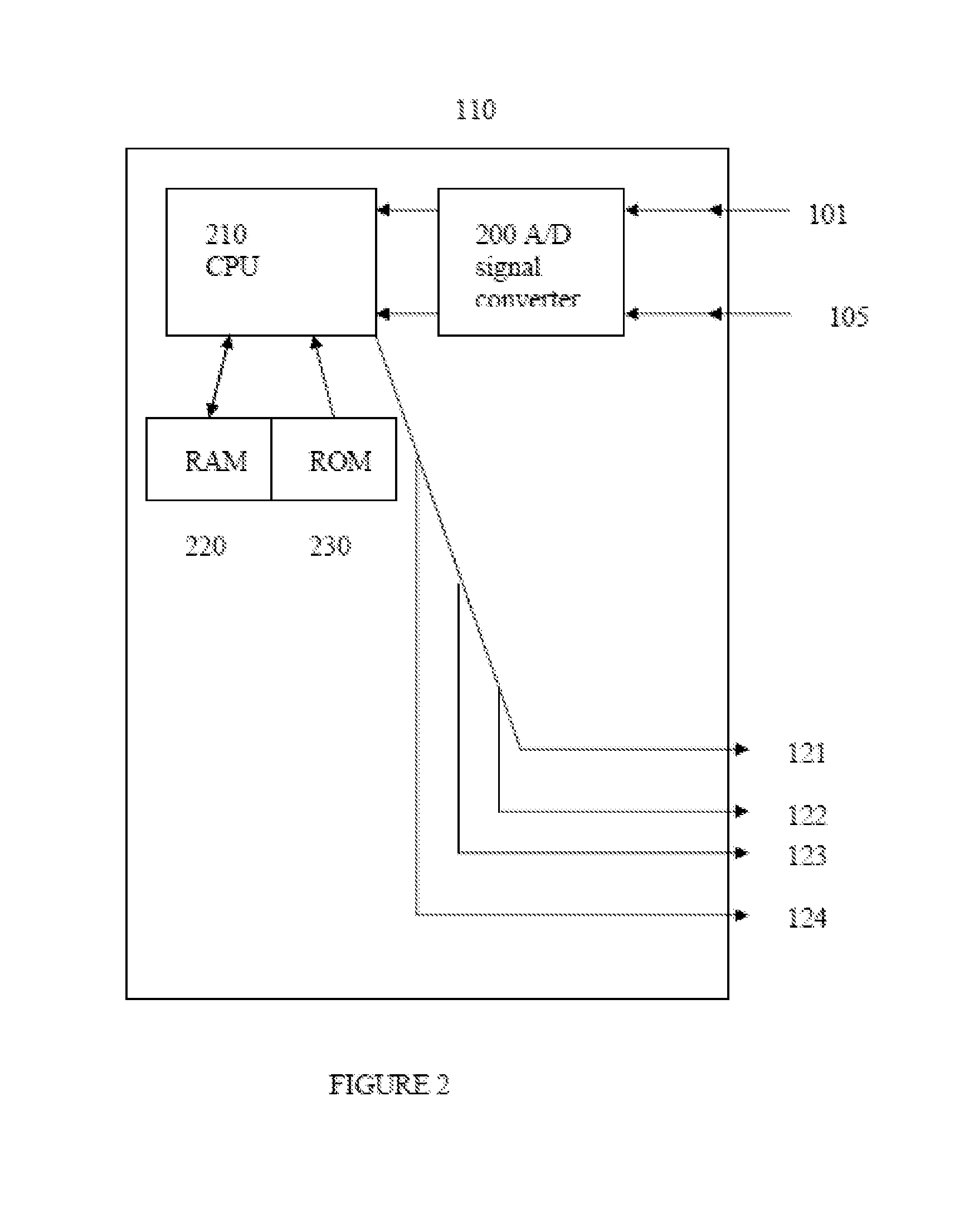
Intelligent Drug and/or Fluid Delivery System to Optimizing Medical Treatment or Therapy Using Pharmacodynamic and/or Pharamacokinetic Data
ActiveUS20130296823A1Improve securityStrengthen security controlData processing applicationsDrug and medicationsPharmacodynamic StudyDelivery system
Owner:XHALE ASSURANCE INC +1
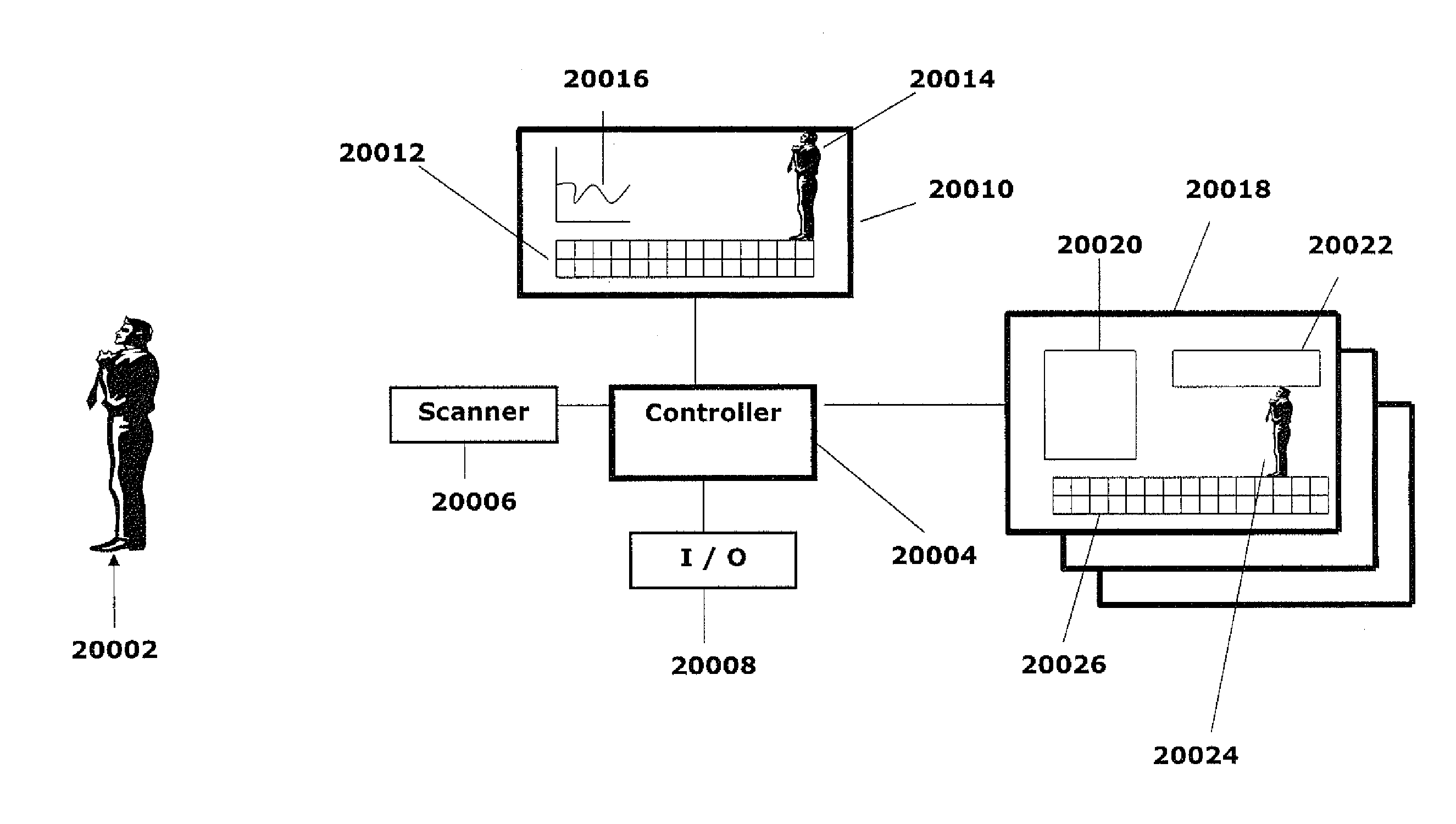
Multi-dimensional image reconstruction and analysis for expert-system diagnosis
ActiveUS7872235B2Maximize obtainedAvoid saturationMaterial analysis by optical meansMedical automated diagnosisDiseaseMulti dimensional
An electronic storage medium that comprises at least one radiopharmaceutical identity, SPECT measured values of at least one radiopharmaceutical kinetic parameter of a flow rate across a tissue membrane, for the radiopharmaceutical, and a set of instructions for associating the at least one radiopharmaceutical kinetic parameter with a disease signature.
Owner:SPECTRUM DYNAMICS MEDICAL LTD
Timed pulsatile drug delivery systems
A pharmaceutical dosage form such as a capsule capable of delivering therapeutic agents into the body in a time-controlled or position-controlled pulsatile release fashion, is composed of a multitude of multicoated particulates (beads, pellets, granules, etc.) made of one or more populations of beads. Each of these beads except an immediate release bead has at least two coated membrane barriers. One of the membrane barriers is composed of an enteric polymer while the second membrane barrier is composed of a mixture of water insoluble polymer and an enteric polymer. The composition and the thickness of the polymeric membrane barriers determine the lag time and duration of drug release from each of the bead populations. Optionally, an organic acid containing intermediate membrane may be applied for further modifying the lag time and / or the duration of drug release. The pulsatile delivery may comprise one or more pulses to provide a plasma concentration-time profile for a therapeutic agent, predicted based on both its pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:ADARE PHARM INC
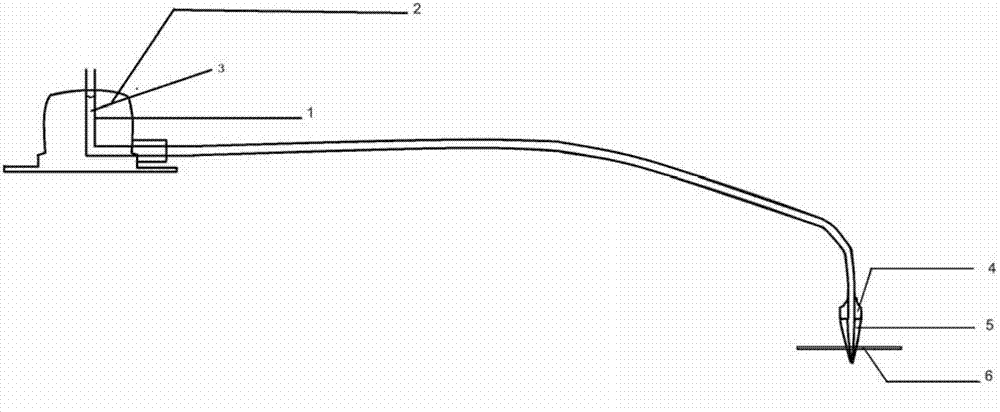
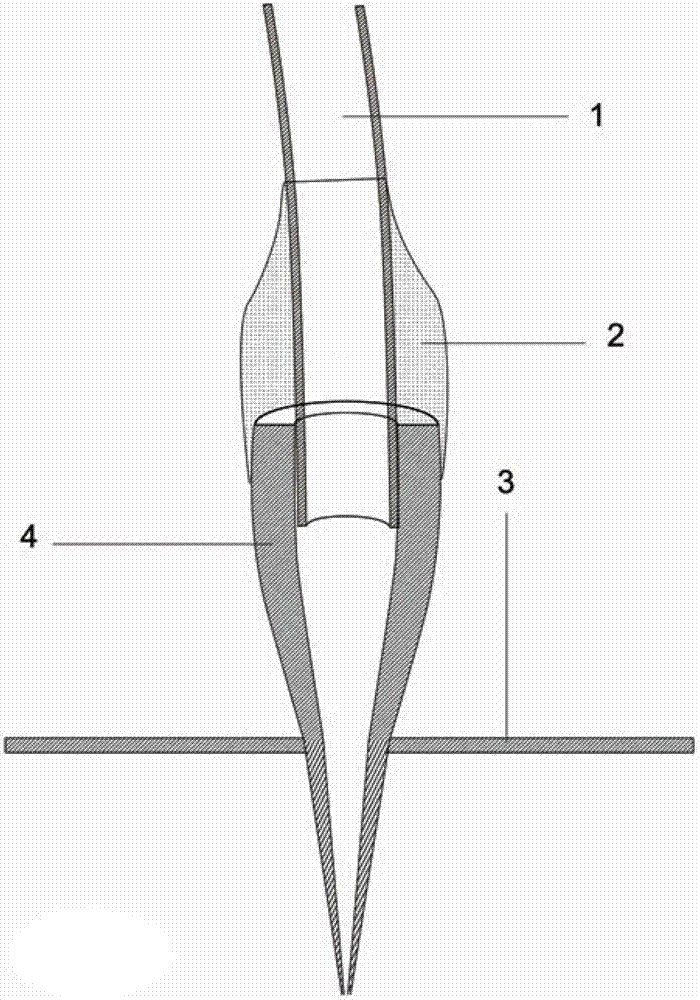
Delivery device, method of using and method of manufacturing
An active principle delivery device (1) comprising an inner capsule (4) within an outer capsule (2), the inner and outer capsules (4,2) containing the same active principle (5,3), with at least the outer capsule (2) being a hard capsule and the active principle (3,5) in at least one of the capsules (2,4), comprising a fluid. Also provided is a method of fabricating such a delivery device (1), as well as a method of controlling the pharmaco-kinetic profile of an active principle.
Owner:MW ENCAP
Device with engineered surface architecture coating for controlled drug release
In one embodiment of the present invention a coating topology, or engineered surface architecture that may be referred to as a microdroplet deposited engineered surface architecture is provided. A plurality of drops are placed on the stent with the purpose, of building up individual units of coating material on the outer stent surface. This architecture results in a coating that uses less material, i.e., polymer, solvent, medicine, while at the same time providing for better, and determinable, drug kinetics, approaching 100% delivery and better mechanical operation of the coating binding to the stent.
Owner:BOSTON SCI SCIMED INC
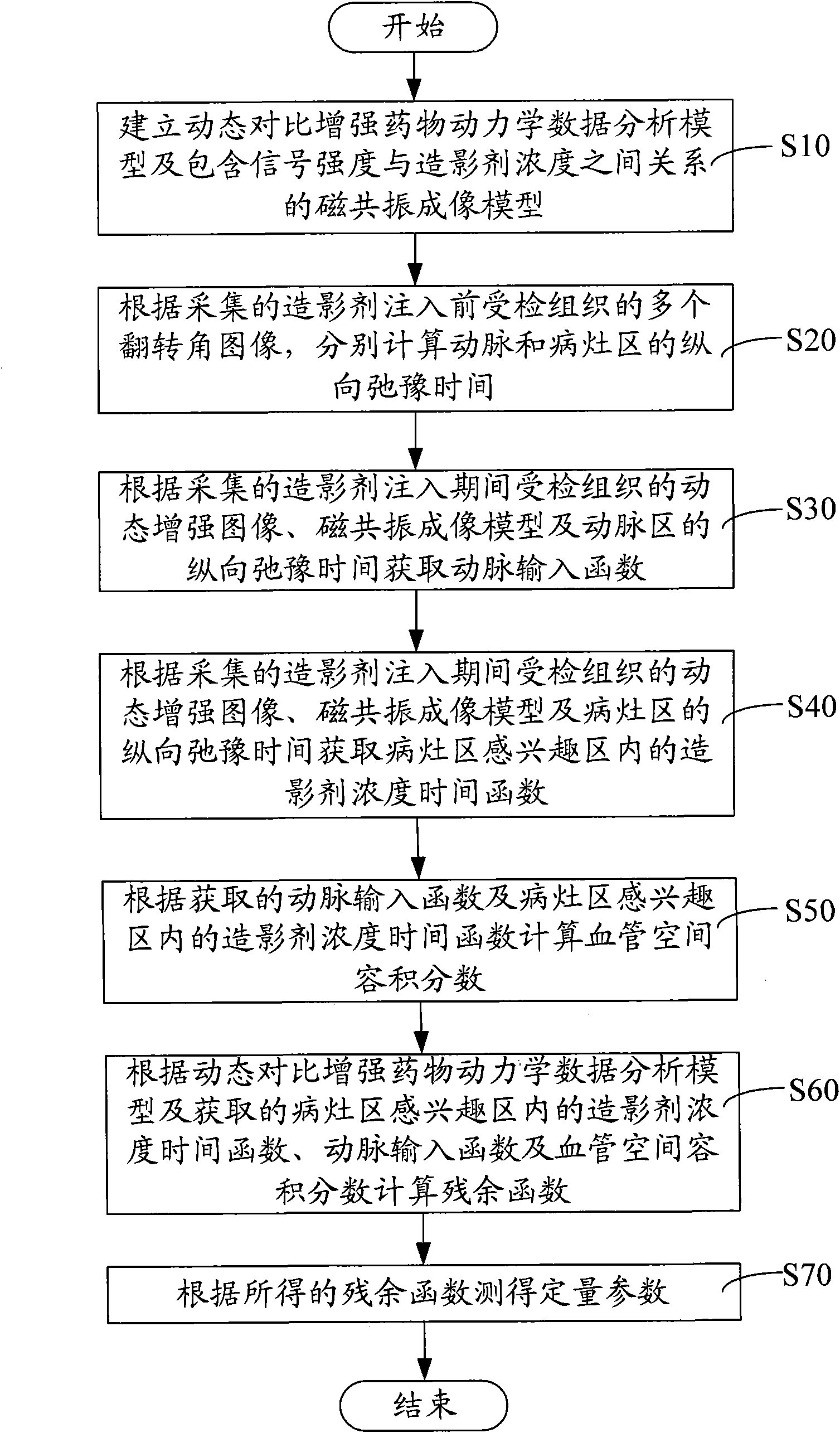
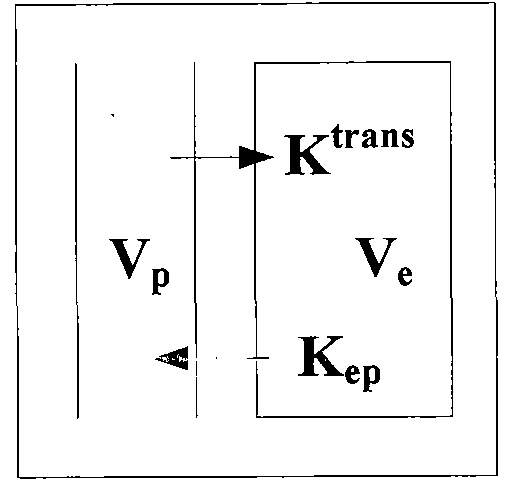
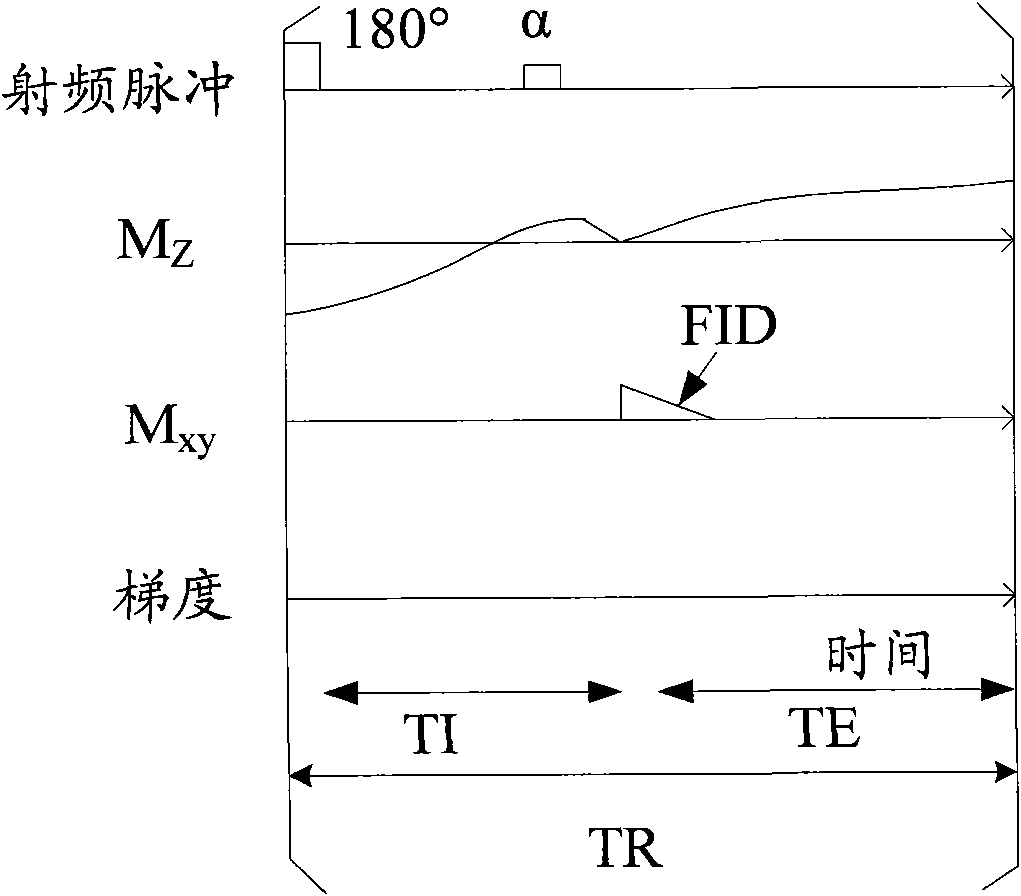
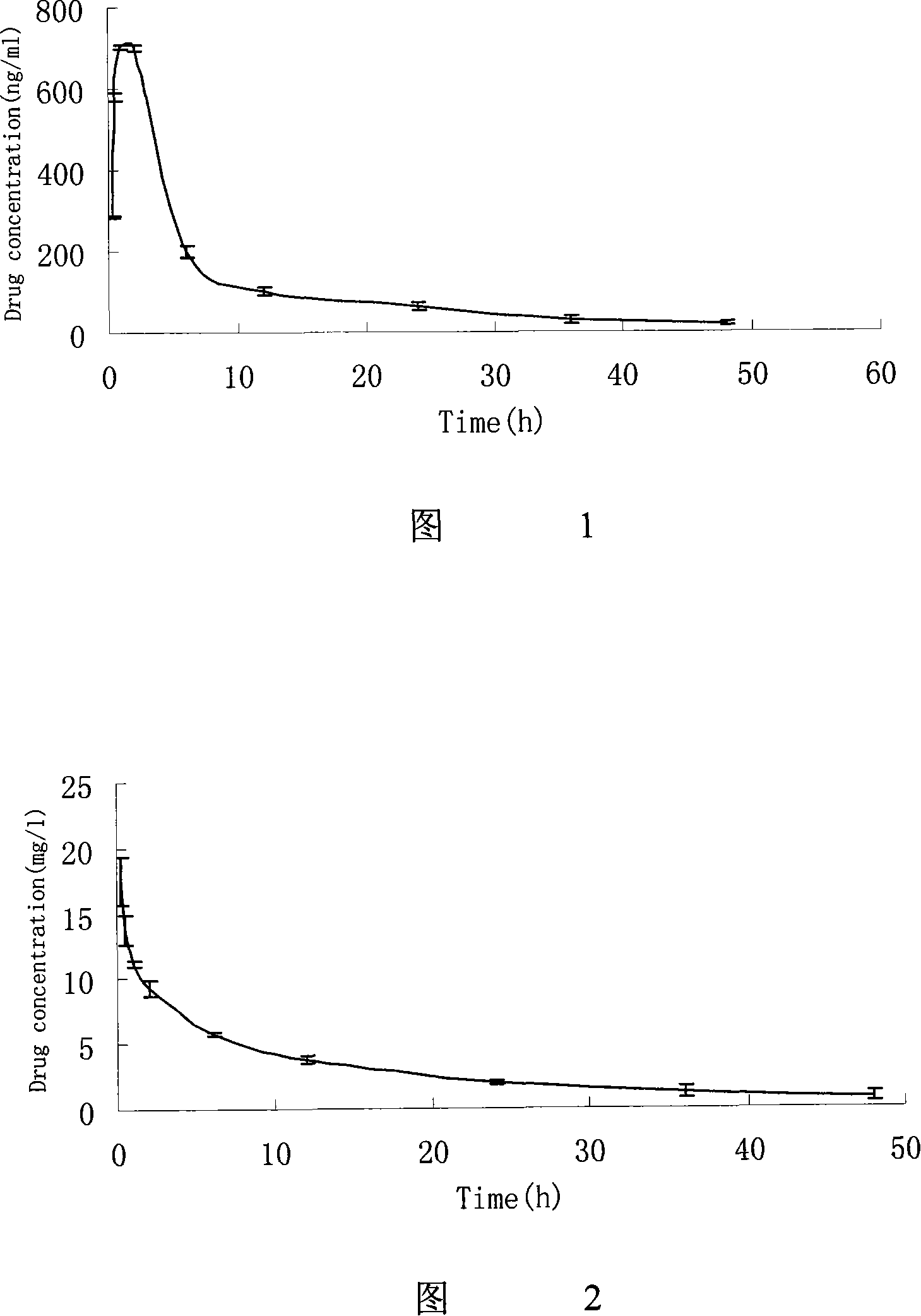
Method and system for calculating quantitative parameter of magnetic resonance imaging
The invention discloses a method and a system for calculating a quantitative parameter of magnetic resonance imaging. The method comprises the following steps of: establishing a dynamic comparison enhancement drug dynamics data analysis model and a magnetic resonance imaging model containing the relationship between the signal intensity and the concentration of a contrast mediummagnetic resonance imaging model; calculating a residual function according to the dynamic comparison enhancement drug dynamics data analysis model and the acquired quantitative parameter according to the acquired residual function. In the method and the system for calculating the quantitative parameter of magnetic resonance imaging, the quantitative parameter can be also improved.
Owner:SHANGHAI UNITED IMAGING HEALTHCARE
Sirolimus lipidosome freeze-dried acanthopanax powder and technique of preparing the same
InactiveCN101129361AGood curative effectExtension of timeOrganic active ingredientsPowder deliveryDiffusion methodsFreeze-drying
The invention discloses a xiluomosi liposome freeze dried and making technique, which comprises the following steps: selecting liposome component, buffer, organic solvent, antioxidant and freeze-drying protective as raw material; adopting film diffusion method to make the liposome turbid liquor with xiluomosi through high-pressure or hypersonic dispersing method evenly; drying the liposome to improve the storage stability obviously; dispersing the freeze dried at random proportion in the water evenly without any sediment and impurity; making the packing rate of liposome at 96% with the grain size at 50-250nm; improving the drug effect greatly in comparison with oral agent; lengthening the circulating time in the blood; elevating the biological utility of drug.
Owner:山东华诺生物科技有限公司
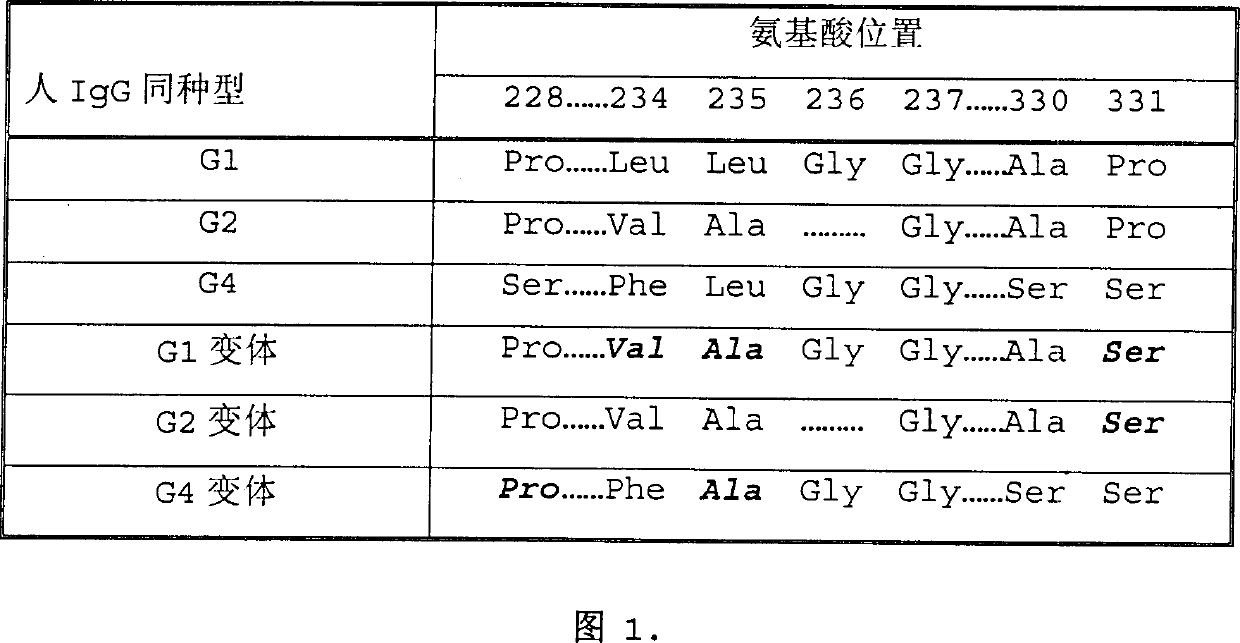
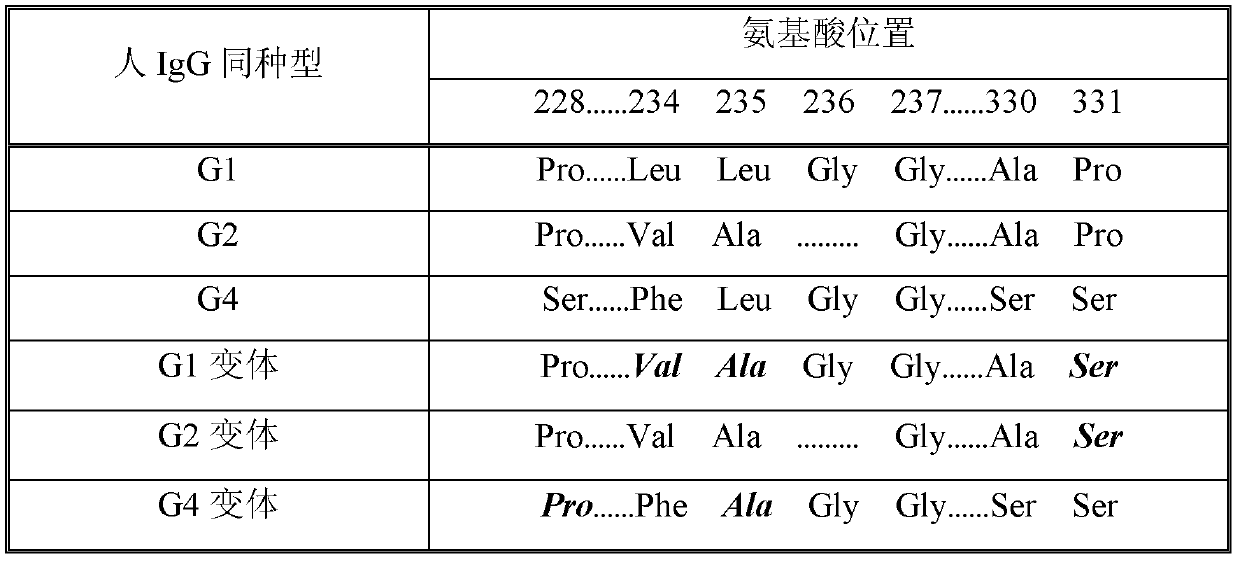
Fc fusion protein of human granulocyte colony stimulin with enhanced bioactivity
InactiveCN1410450AImprove biological activityAntibody mimetics/scaffoldsColony-stimulating factorHalf-lifePharmacodynamic Study
Owner:PHARMAB
Therapeutics dispensing device and methods of making same
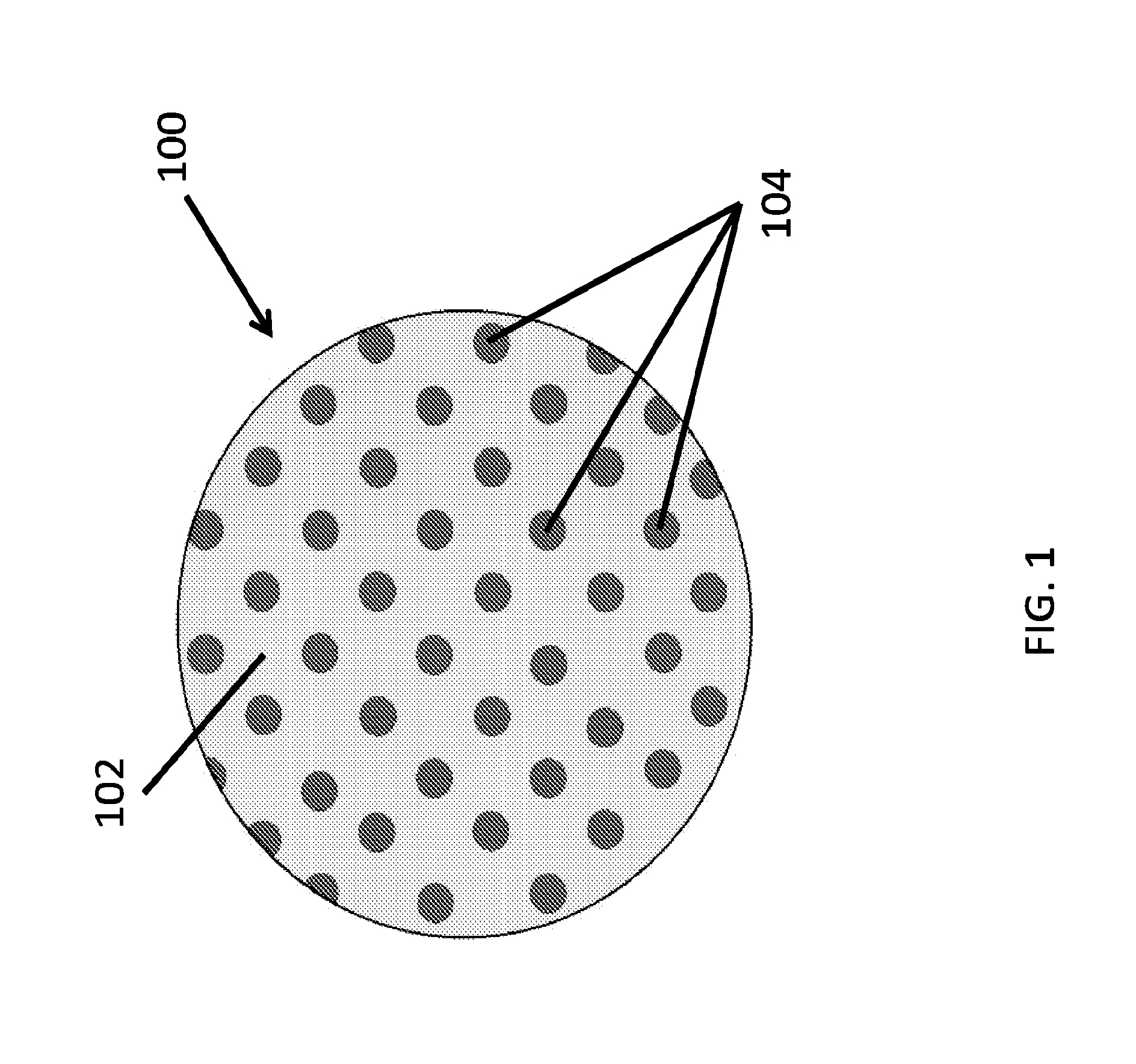
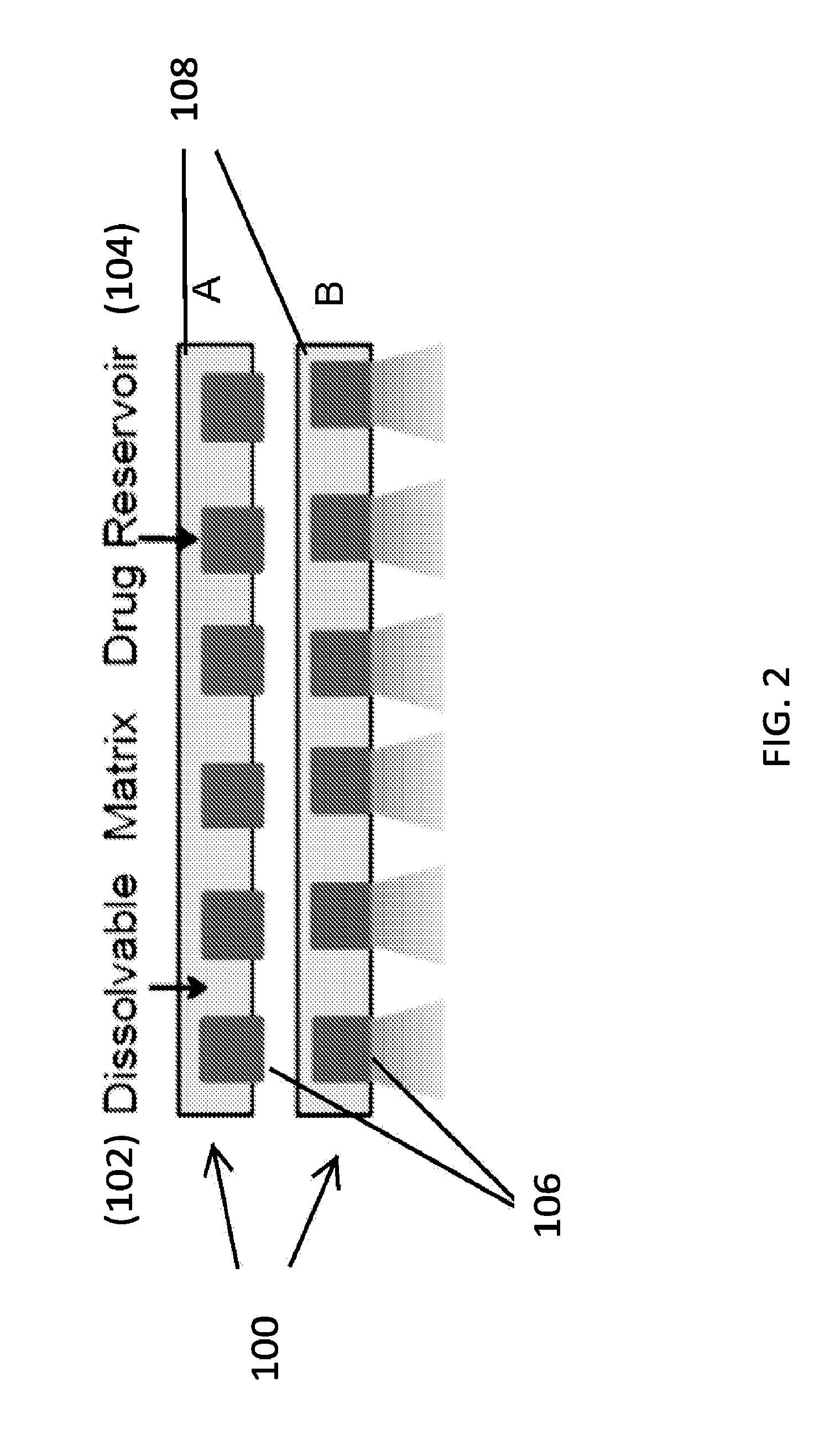
A therapeutics delivery system, and methods of making and using same, are disclosed for environments that rapidly clear any injected therapeutics, such as a patient's eye. The therapeutics delivery system releases the drug in a therapeutically effective concentration for a desired duration of time with a predefined drug kinetics. In one embodiment, the embodiments of the present disclosure release a therapeutically effective concentration for a longer time period than other delivery systems, for instance from a day to a week. Certain embodiments comprise a therapeutics dispensing device comprising a biodissolvable hydrogel matrix for long term drug release that allows the device to be placed directly at the injured site, e.g., onto the surface at or near the injury, and retained there rather than through injection, whether locally or systematically.
Owner:BAYLOR COLLEGE OF MEDICINE +1
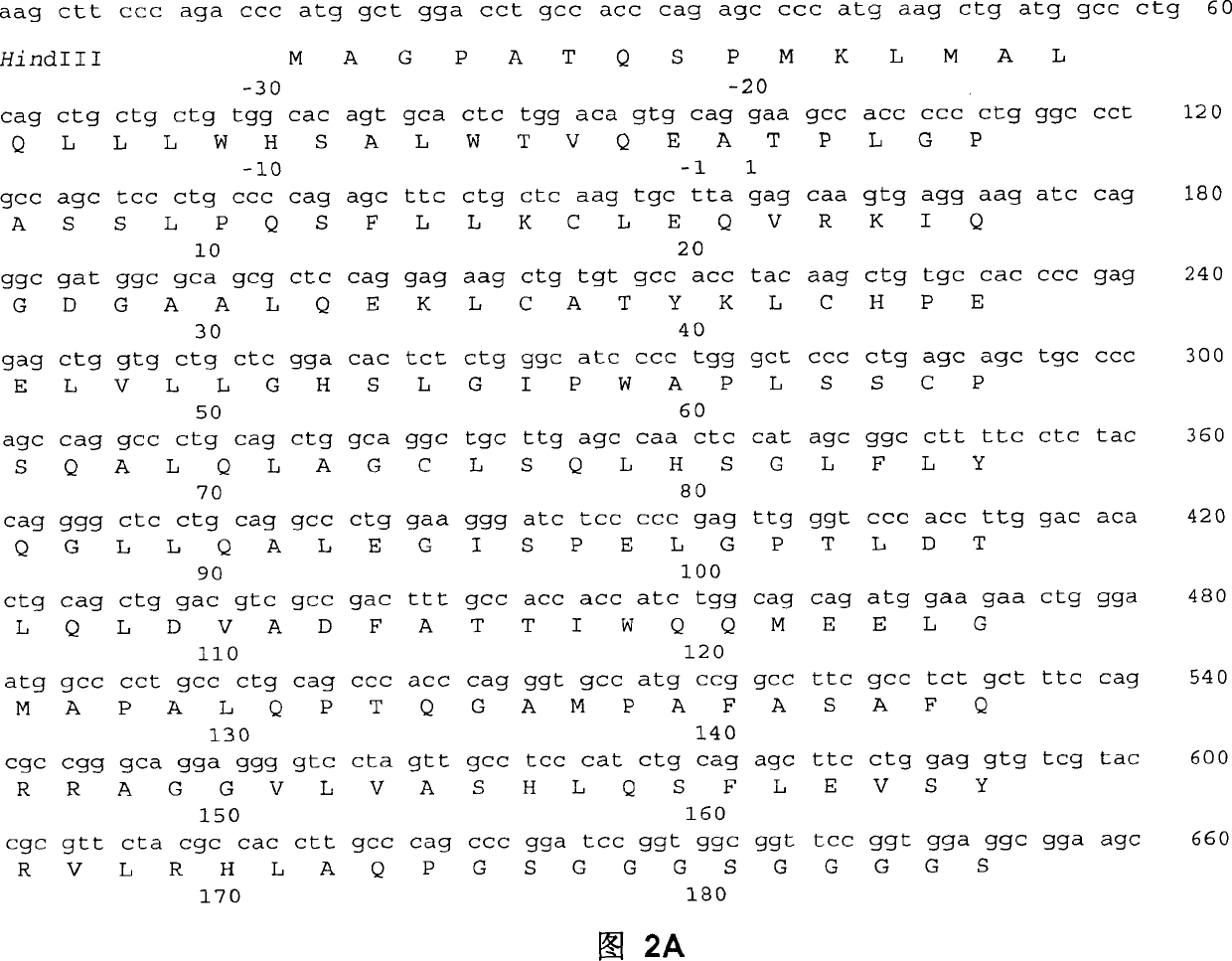
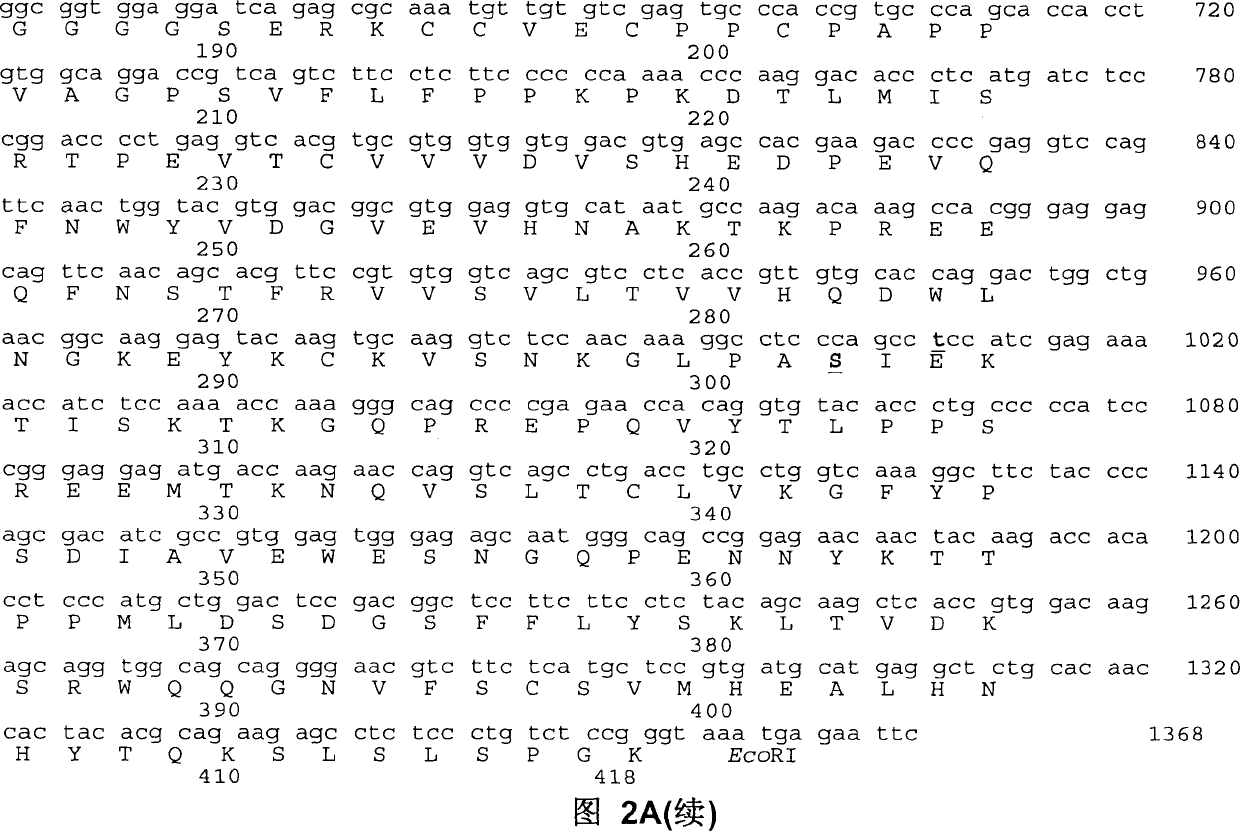
Fc fusion protein of long-acting recombinant human growth hormone
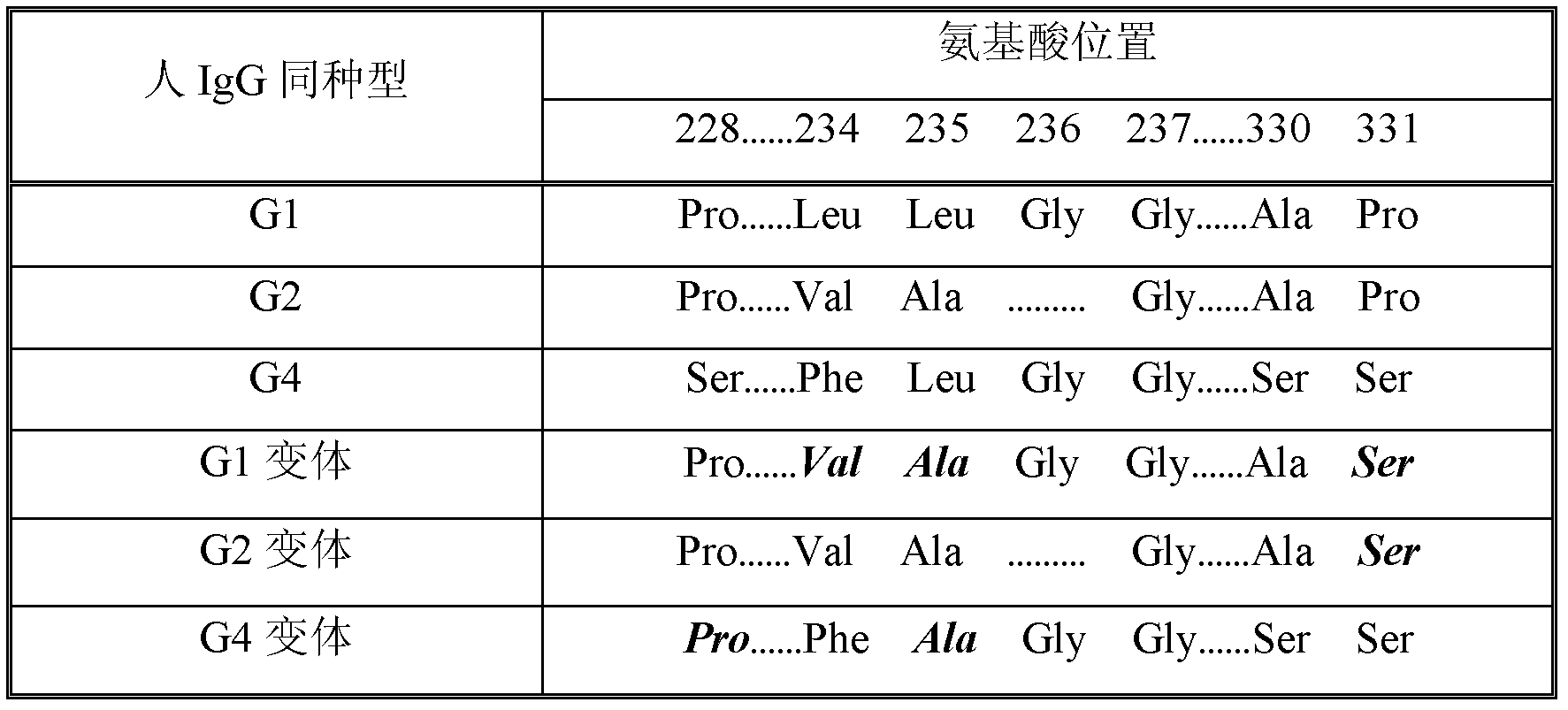
The invention discloses a Fc fusion protein of long-acting recombinant human growth hormone. The Fc fusion protein (hGH-L-vFc fusion protein) disclosed herein contains human growth hormone, flexible peptide linker of about 2-20 amino acids, and human IgG Fc mutant. The Fc mutant is not lytic and has tiny side effect of adverse Fc-mediator. The invention further discloses a method for preparing or generating the fusion protein with high expression level. The hGH-L-vFc fusion protein disclosed herein has prolonged serum half-life period and increased biological activity, so as to improve the pharmacokinetics and drug efficacy, and needs few times of injection required in treatment.
Owner:PHARMAB
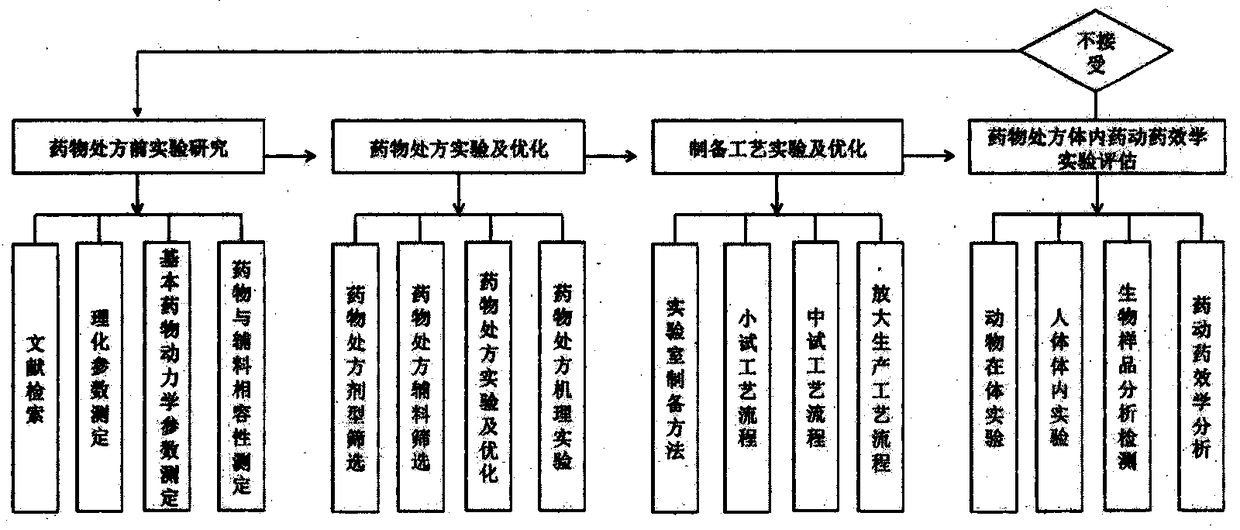
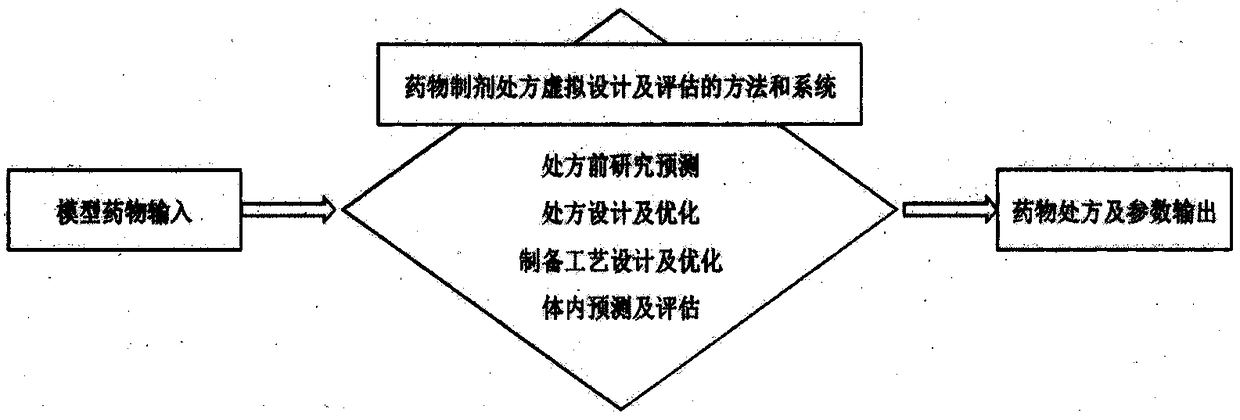
A method and a system for virtual design and evaluation of pharmaceutical formulation
InactiveCN108984811AObtain basic physical and chemical parametersGain stabilityComputing modelsDesign optimisation/simulationPre formulationIn vivo
A method and a system for virtual design and evaluation of pharmaceutical formulation are provided. The invention discloses the method and the system for virtual design and evaluation of pharmaceutical preparation prescriptions, and the system mainly comprises a pre-prescription research module, a pharmaceutical prescription design and optimization module, a pharmaceutical preparation process design and optimization module, and a pharmaceutical prescription in-vivo prediction and evaluation module. The method and the system for virtual design and evaluation of pharmaceutical formulation predict the whole process of pharmaceutical formulation from four aspects of pharmaceutical pre-formulation research, formulation design, preparation process design and pharmacokinetics prediction in vivo.Compare with a conventional empirical drug prescription screening and preparation method in a traditional laboratory, the method and the system can accelerate the development of drug product, do not consume any laboratory equipment, complete the virtual design and evaluation of drug formulation prescription through a computer platform, shorten the drug development cycle and save the development cost of new drugs.
Owner:欧阳德方
F-triazole ring-polyethylene glycol-metronidazole compound and preparation method thereof
InactiveCN101709060AImprove metabolic propertiesImprove mechanical propertiesOrganic chemistryRadioactive preparation carriersNitroimidazolePolyethylene glycol
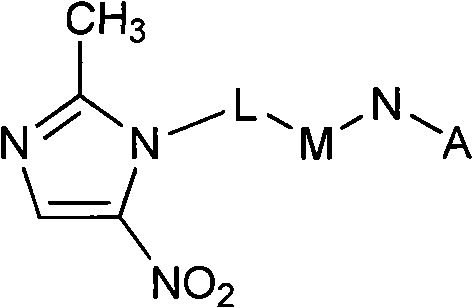
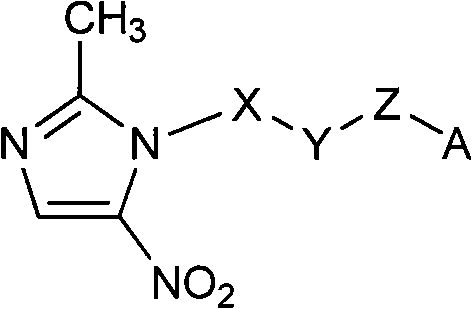
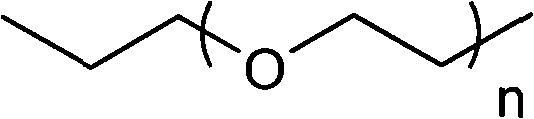
The invention relates to an F-1,2,3-triazole ring-polyethylene glycol-metronidazole compound which has the following structural general formula: L is ethyl or polyethylene glycol with the following structure: n is equal to 0, 1, 2 or 3; M is a triazole ring with the following structure: or, N is ethyl or polyethylene glycol with the following structure; n is equal to 0, 1, 2 or 3; and A is 19F or18F. Under a changeless condition that the invention keeps the metronidazole as a targeting base group, the polyethylene glycol is introduced to modify and change the molecular structure of a medicine, and the aims of improving medicine dynamics and pharmacodynamical properties and increasing the curative effect of the medicine are achieved. The triazole ring, a PEG base group, 2- metronidazole and 18 / 19F nuclide are connected by a click method, and a series of novel F-triazole ring-PEG-metronidazole derivatives are designed and synthesized. The nitro imidazole derivatives can quicken removing from normal tissues by lowering fat solubility so as to enhance a target / non-target ratio and is used for the development research of tumours, cardiac muscles or brain anoxia.
Owner:BEIJING NORMAL UNIVERSITY
Recombined dimerization antithrombin III-Fc fusion protein and mammalian cell efficient expression system thereof
ActiveCN102690354AHigh expressionSimplified purification stepsPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectHalf-life
The invention discloses a recombined dimerization anti-thrombin III-Fc fusion protein, of which the in vitro biological activity is similar to or higher than that of the serum derived anti-thrombin III, and the in vivo half-life period is prolonged. The fusion protein provided by the invention contains human anti-thrombin III (hAT), a flexible peptide joint (L) containing about 20 or less amino acids, and a human IgG Fc mutant (vFC) which is represented by hAT-L-vFC (Fc). Such Fc mutant excludes cracking property and shows extremely low bad-Fc-induced side effect. Such hAT-L-vFC fusion protein is prolonged in serum half-life period and enhanced in the biological activity, so that the pharmacokinetics effect and the pesticide effect are improved. The invention further discloses a method for efficiently expressing or producing such recombined fusion proteins by adopting the mammalian cells.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Novel voriconazole broad-spectrum antifungal medicine compound, broad-spectrum antifungal medicine composition and application thereof
ActiveCN101575330ABroad and potent antifungal activityImprove mechanical propertiesOrganic active ingredientsAntimycoticsMonilinia laxaItraconazole
The invention relates to a novel broad-spectrum antifungal medicine compound, a broad-spectrum antifungal medicine composition, an application of the compound or the composition in the preparation of a broad-spectrum antifungal medicine, and an application of the compound or the composition in the preparation of a medicine used for treating severe invasive infection (comprising Candida krusei) caused by invasive aspergillosis and / or Fluconazole-resistant Monilia and / or severe infection caused by Scedosporium and fusarium. The novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition have extensive and strong antifungal activity and better dynamic property and safety. For deep mycotic infection, the novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition are better than the original voriconazole in the aspects of the antibiotic activity and the medicine resistance and is better than amphotericin B in the aspects of safety and effectiveness; and compared with the formerly applied voriconazole, Fluconazole, itraconazole and amphotericin B, the novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition have reinforced medicine effect, less adverse reaction and good safety.
Owner:LIVZON PHARM GRP INC
Thiol medicine content detection method
InactiveCN105628813AOxidative side reaction inhibitionAccurate dataComponent separationDrug contentThiol
The invention provides a thiol medicine content detection method. The thiol medicine content detection method includes the following steps that 1, a reducing reagent is added in a thiol medicine solution, and a dithio polymer in the solution is reduced to obtain a reduced product solution; 2, the reduced product solution and a quinones derivatization reagent perform derivatization reaction to obtain a derivatization product solution; 3, the content of a derivatization product is detected to obtain the content of a thiol medicine. According to the method, the thiol medicine is subjected to derivatization pretreatment by using a quinone compound after being reduced, the stable derivatization product is quickly generated, then the content of the derivatization product is separated and determined by adopting a detection means, and the thiol medicine content in a sample can be accurately calculated. The thiol medicine content detection method is high in recovery rate, simple and easy to operate, high in accuracy rate, quick to detect and high in sensitivity and can be used in the fields of detection of the thiol medicine content, study of human body fluid pharmacokinetics and the like.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Antimicrobial lubricious coating
The present disclosure provides lubricious antimicrobial coating vehicles for medical devices capable of reducing the coefficient of friction of such devices upon exposure thereof to moisture and imparting antimicrobial properties to said devices. The coating vehicle allows the introduction of a pharmacological additive having a release rate that is within acceptable pharmacokinetic criteria.
Owner:COVIDIEN LP
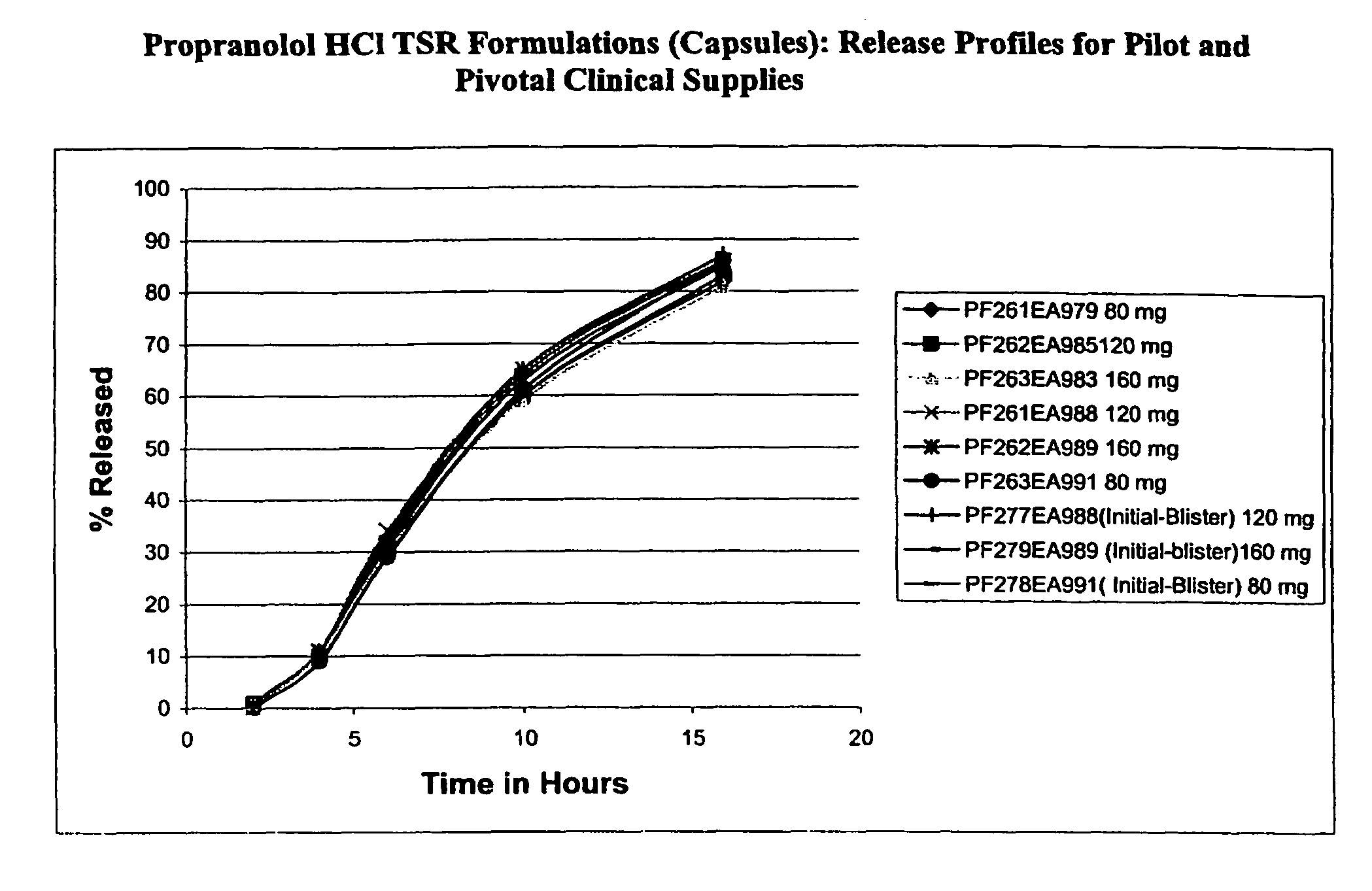
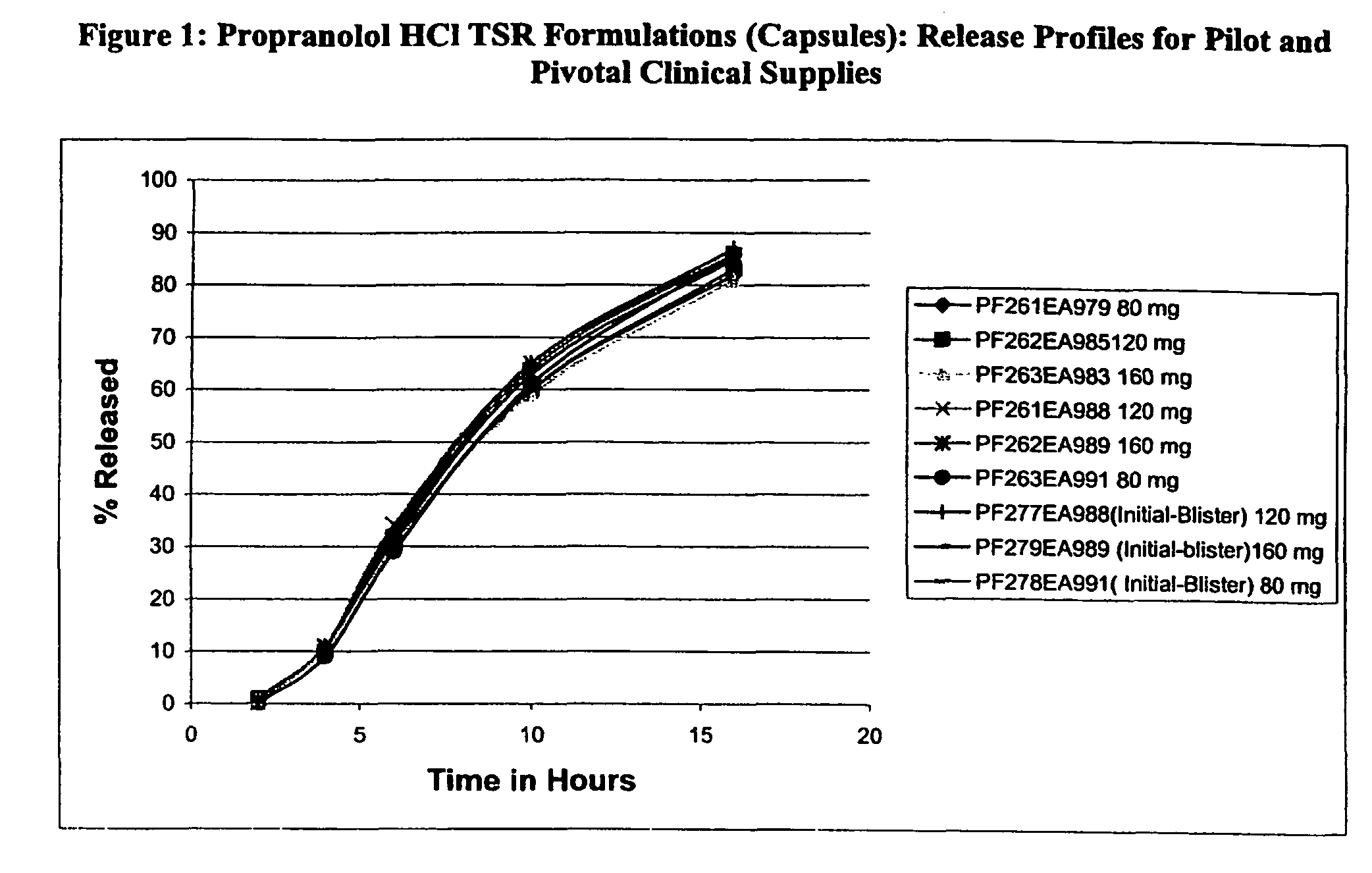
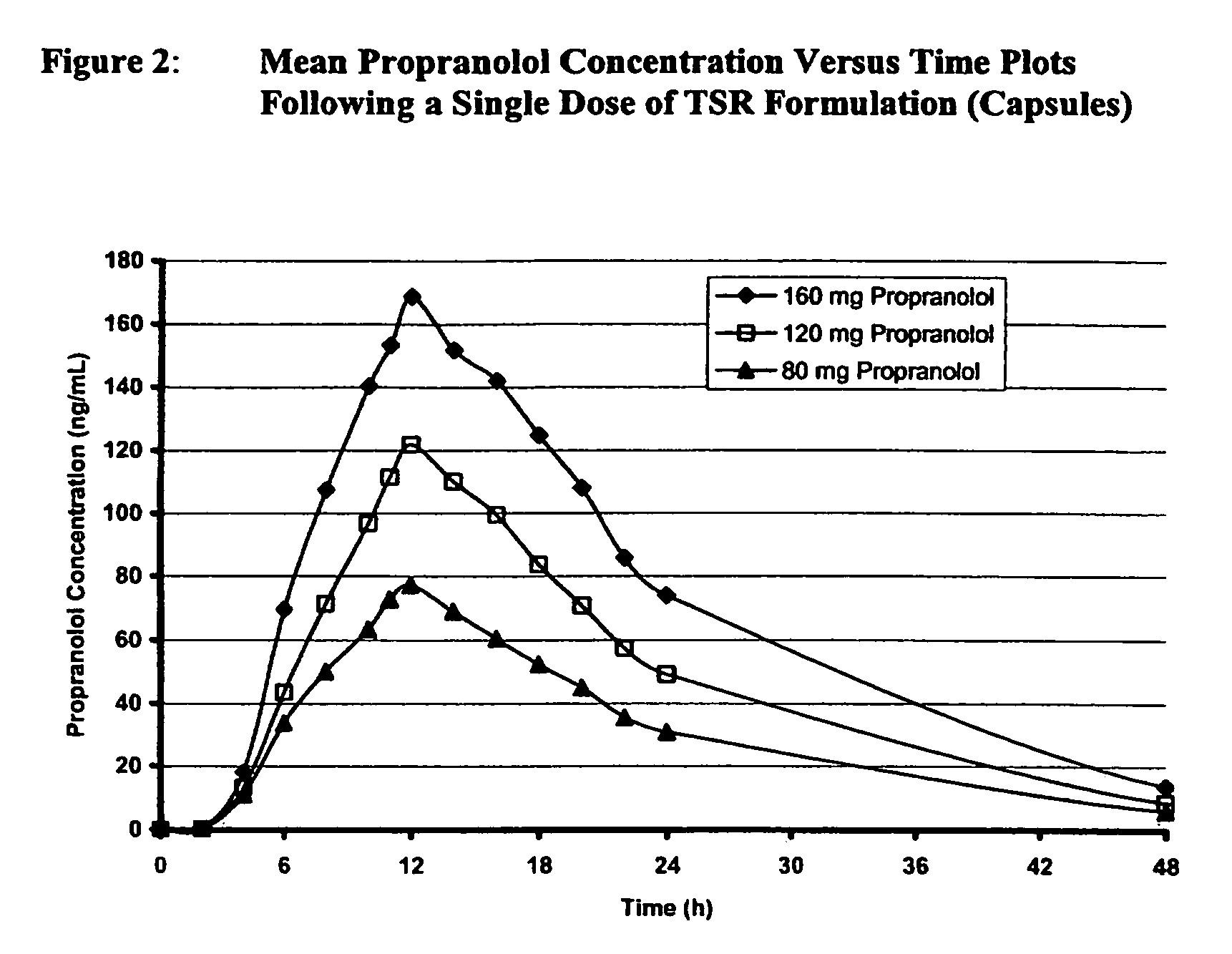
Timed, sustained release systems for propranolol
A unit dosage form, such as a capsule or the like for delivering drugs into the body in a circadian release fashion, is comprising of one or more populations of propranolol-containing particles (beads, pellets, granules, etc.). Each bead population exhibits a pre-designed rapid or sustained release profile with or without a predetermined lag time of 3 to 5 hours. Such a circadian rhythm release cardiovascular drug delivery system is designed to provide a plasma concentration-time profile, which varies according to physiological need during the day, i.e., mimicking the circadian rhythm and severity / manifestation of a cardiovascular disease, predicted based on pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:ADARE PHARM INC
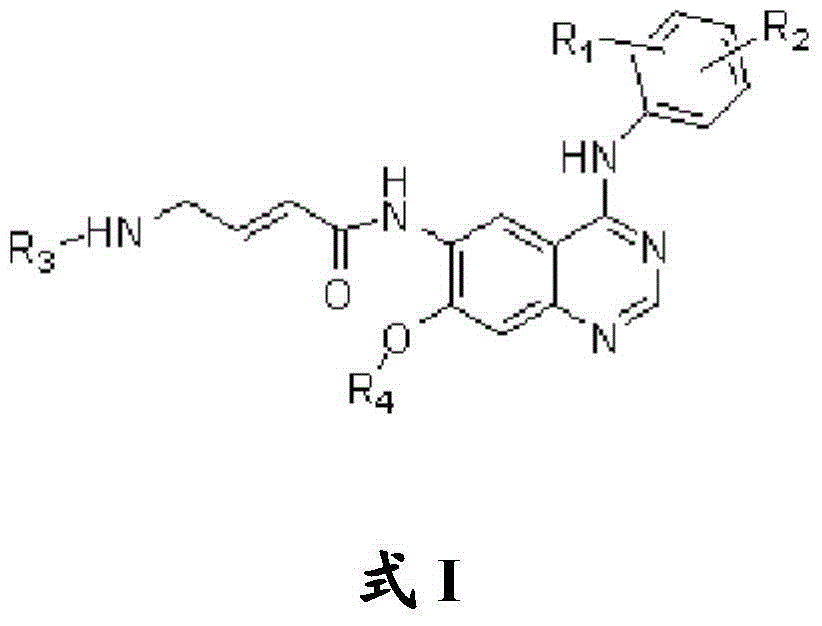
Quinazoline compound, preparation method and application thereof
The invention belongs to the medicine chemical field, and more specifically relates to a novel quinazoline compound having antineoplastic activity in a formula I and a preparation method. The compound 1 has effective protein tyrosine kinases irreversible inhibition effect and / or has good internal pharmacokinetics behavior, and is an effective protein tyrosine kinases irreversible inhibitor.
Owner:QILU PHARMA CO LTD
System and method for ranking options for medical treatments
ActiveUS20160210436A1Facilitate communicationEnhanced advantageData processing applicationsDrug and medicationsComputerized systemEfficacy
A computer system, computer program product and method for determining a probability of attaining a PK-PD target associated with efficacy for a patient that includes a processor obtaining information identifying an infection and based on the information, generating and displaying, by the processor, a list comprising one or more pathogens consistent with the information, the processor then obtaining a first indication designating at least one pathogen from the list comprising one or more pathogens and based on at the obtaining of the least one pathogen, generating a list comprising one or more drug therapies utilized to treat the at least one pathogen. The method also includes the processor obtaining, descriptive information relating to a patient and based on the one or more drug therapies, selecting a pharmacokinetic model and the processor applying the pharmacokinetic model and utilizing the information relating to the patient to determine, for each of the one or more drug therapies, a probability of attaining a PK-PD target associated with efficacy for the patient with the infection.
Owner:PRXCISION INC
Novel prodrug of steroid CYP 17 inhibitor as well as application and preparation method thereof
ActiveCN104017045AHigh doseGood antitumor activityOrganic active ingredientsSteroidsDiseaseAbiraterone
The invention discloses a precursor compound of a novel abiraterone drug, wherein the precursor compound provides improved oral bioavailability and drug dynamic characteristic. The drug is an irreversible inhibitor of human body CYP17 enzyme, can be used for treating urogenital system or androgen-associated cancers, diseases or sickness, for example, human prostate cancer, breast cancer and prostatic hyperplasia.
Owner:广州艾格生物科技有限公司
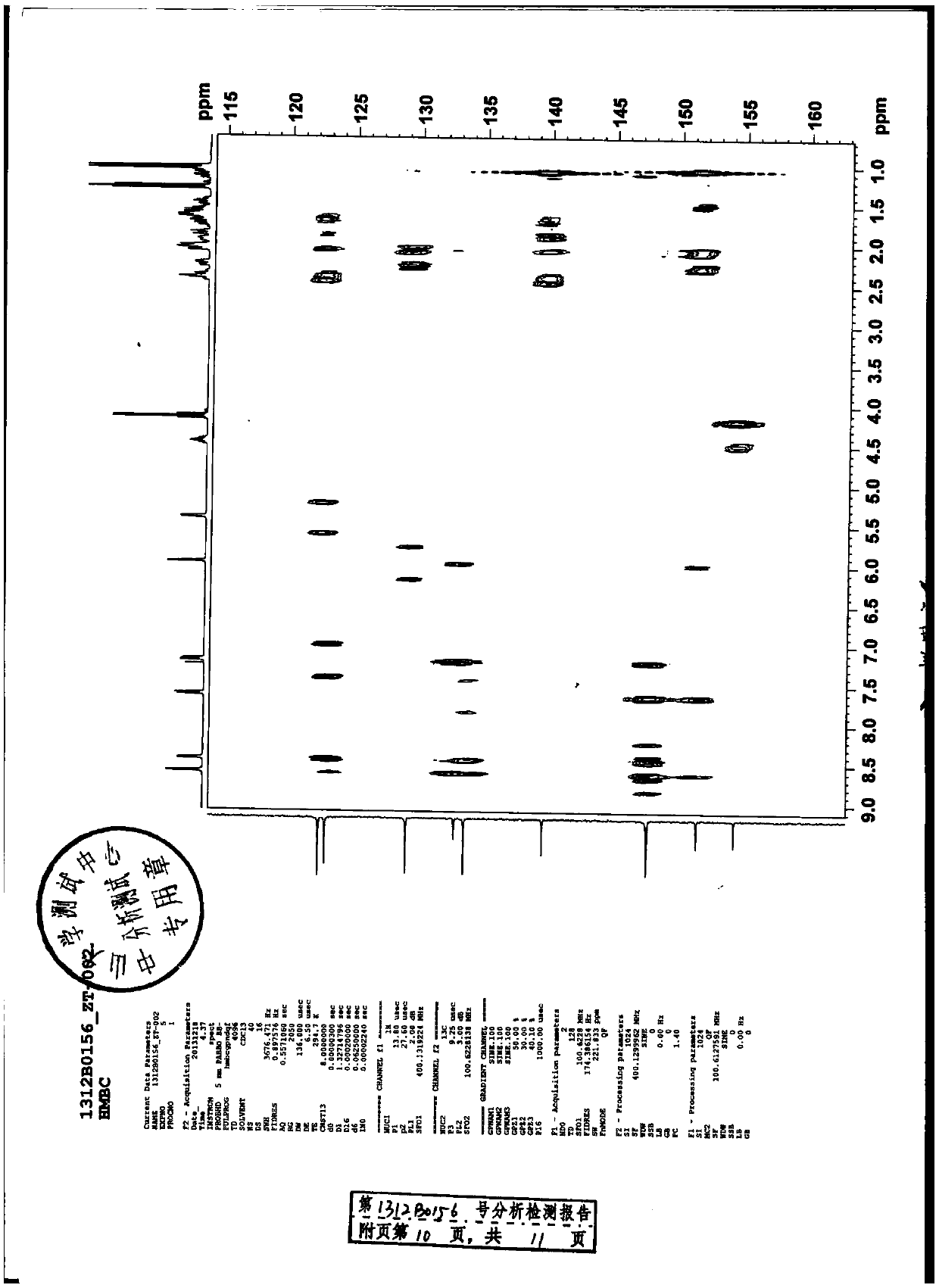
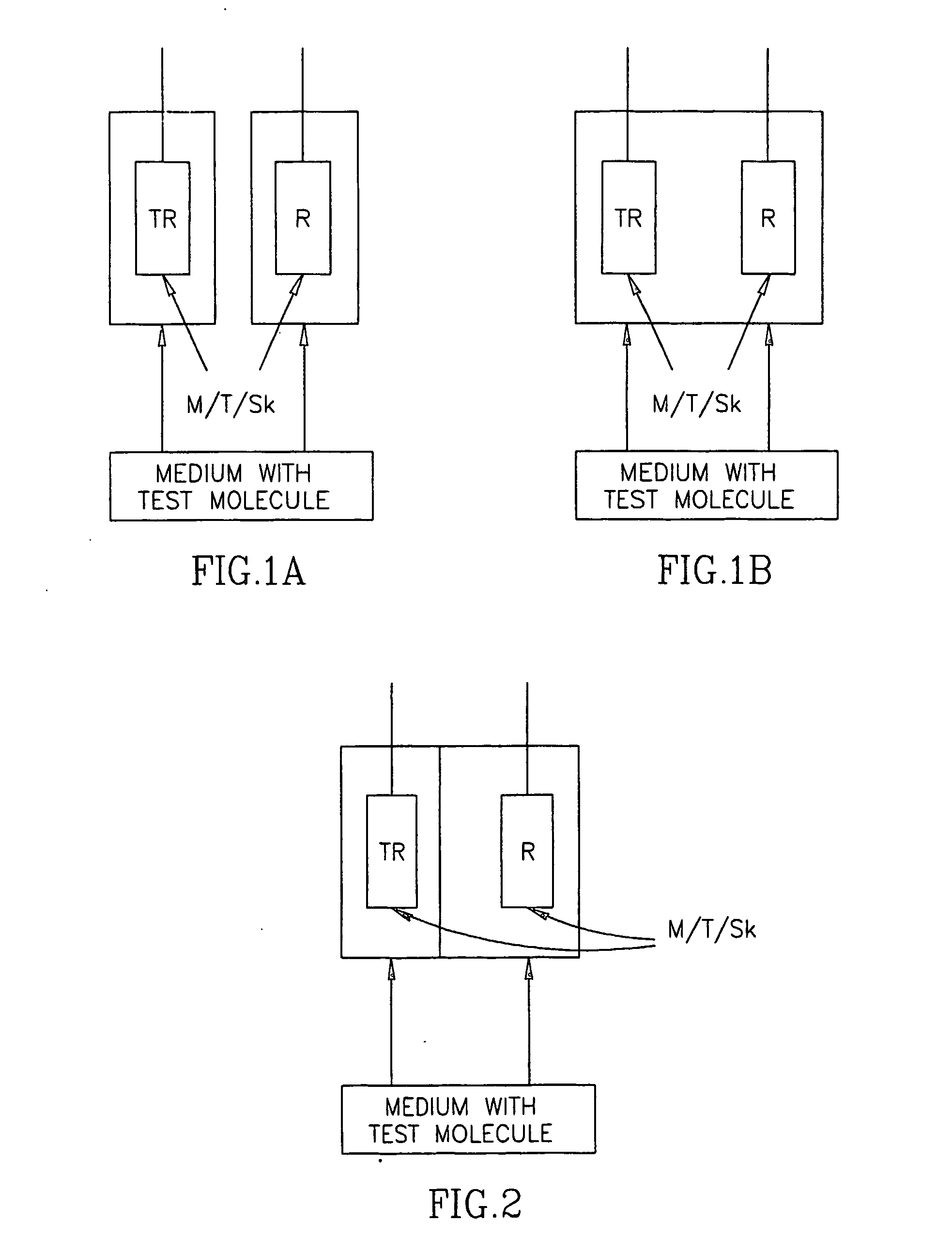
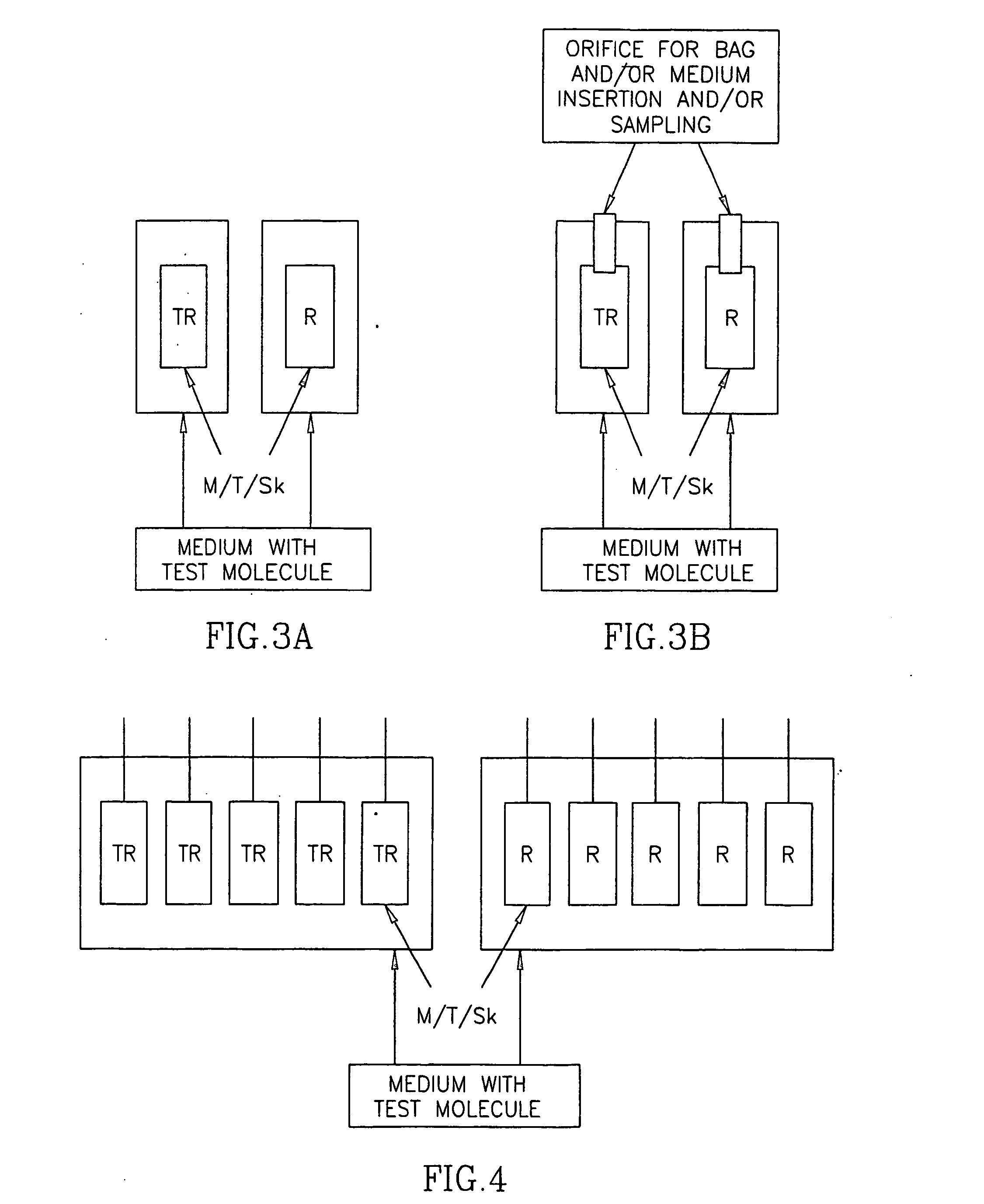
In vitro test for studying compound predicting pharmacologic and/or harmacokinetic and/or pharmacodynamic parameters of a compound
The invention provides a method and an assay for predicting the pharmacologic and / or pharmacokinetic and / or pharmacodynamic activity and / or effective concentration of a test material using cell and / or protozoa and / or micro-organism by assessing the effect of the test material on the and / or protozoa and / or micro-organism. Further, the invention provides an apparatus or a system, which comprises a donor and a receiver compartments, separated by membrane, wherein cell and / or protozoa and / or micro-organism are present in the receiver compartments, for predicting the pharmacologic of pharmacokinetic effects such as effective concentration of a test material, by assessing the effect of the test material, on the cell and / or protozoa and / or micro-organism. In addition, the invention provides use of an artificial human skin for measuring the diffusion or the penetration of a test material through the skin.
Owner:TOUITOU ELKI +1
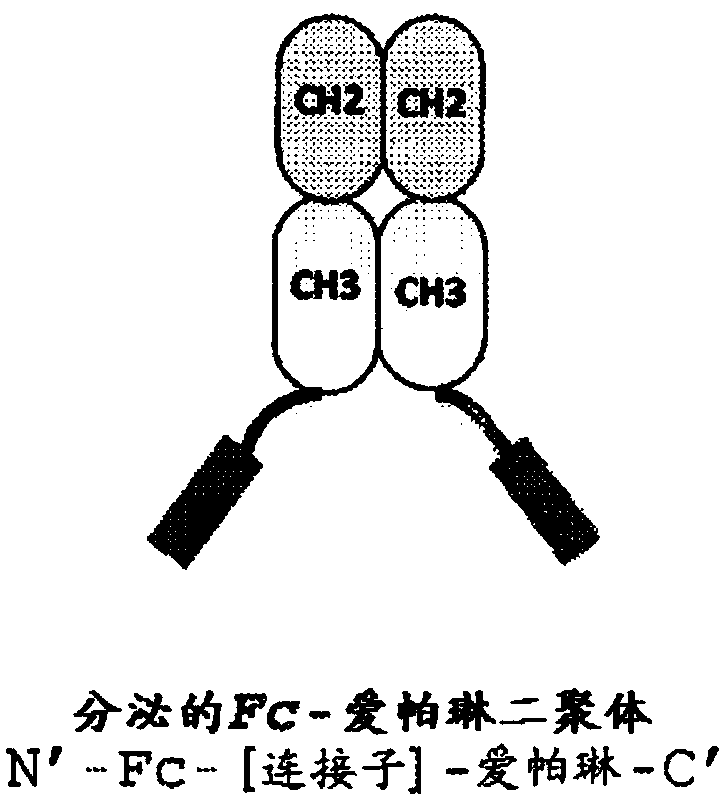
Apelin fusion proteins and uses thereof
InactiveCN105026423APeptide/protein ingredientsAntibody mimetics/scaffoldsDiabetes mellitusApelin receptor
The invention provides a fusion protein or polypeptide comprising an apelin peptide fused to a multimerizing component. The invention also provides a fusion protein or polypeptide comprising an apelin peptide fused to an Fc domain, a fragment of an Fc domain, or a variant of an Fc domain. Apelin Fc-fusion polypeptides are capable of binding to the apelin receptor (APLNR). Apelin Fc-fusion polypeptides are capable of activating the APLNR and have improved pharmacokinetic properties compared to apelin peptides that are not fused to an Fc or an Fc fragment. Apelin Fc-fusion polypeptides are useful in diseases and conditions related to cardiovascular function, diabetes, cancer, obesity and other apelin-related conditions.
Owner:REGENERON PHARM INC
Fc fusion protein of long-acting recombinant human growth hormone
The invention discloses a Fc fusion protein of long-acting recombinant human growth hormone. The Fc fusion protein (hGH-L-vFc fusion protein) disclosed herein contains human growth hormone, flexible peptide linker of about 2-20 amino acids, and human IgG Fc mutant. The Fc mutant is not lytic and has tiny side effect of adverse Fc-mediator. The invention further discloses a method for preparing or generating the fusion protein with high expression level. The hGH-L-vFc fusion protein disclosed herein has prolonged serum half-life period and increased biological activity, so as to improve the pharmacokinetics and drug efficacy, and needs few times of injection required in treatment.
Owner:PHARMAB
Blood brain barrier pharmacokinefic continuous dosing system and detecting system
InactiveCN102784012ASimple structureLow costSurgeryVaccination/ovulation diagnosticsMedicineDrug Kinetics
The invention discloses a detecting system capable of detecting whether drugs permeate through blood brain barrier in real time, and particularly discloses a continuous dosing device and a pharmacokinefic system which continuously extracts cerebrospinal fluid to perform high performance liquid chromatography (HPLC) analysis to detect blood brain barrier in real time under the waking state of an experimental animal. The detecting system overcomes shortcomings of existing monitoring means, is simple in operation and high in sensitivity, and has wide medical application prospects.
Owner:SUN YAT SEN UNIV
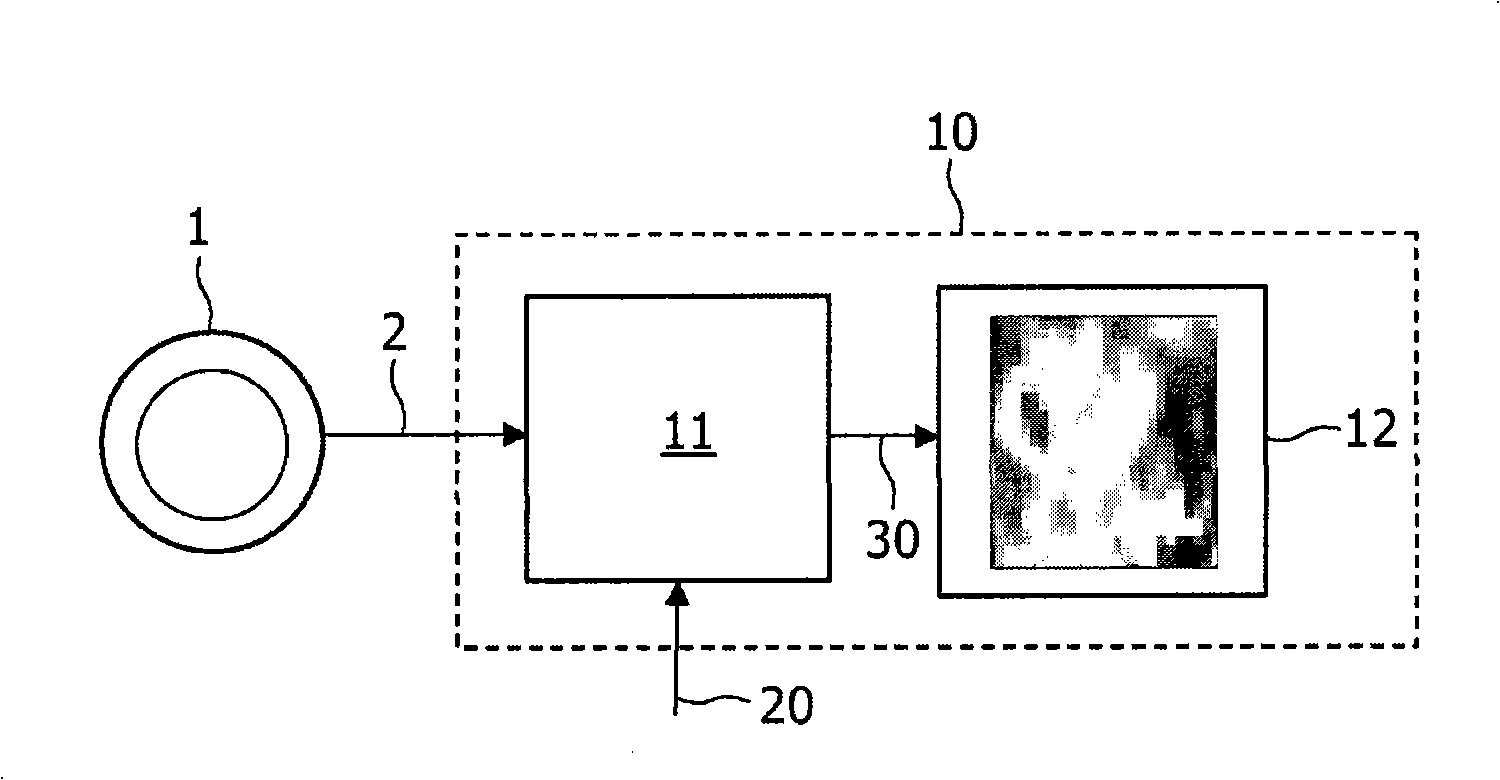
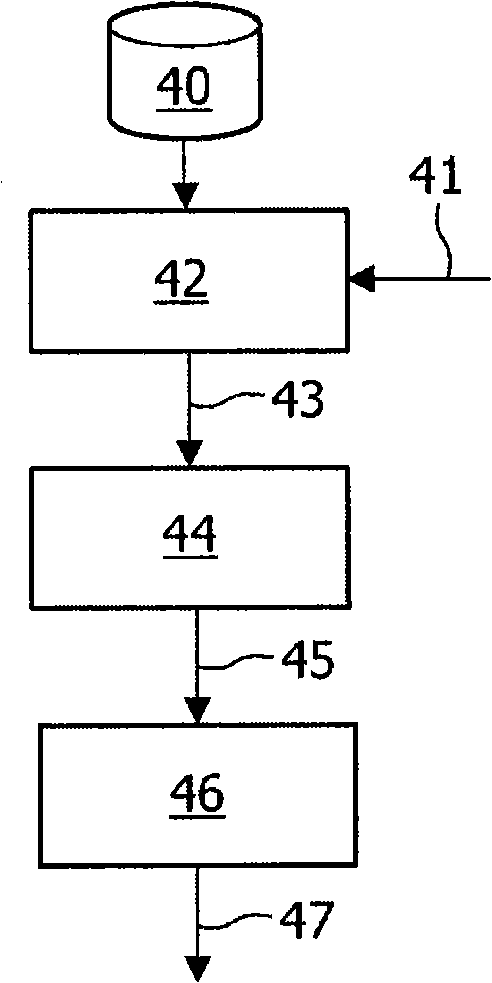
Image generation based on limited data set
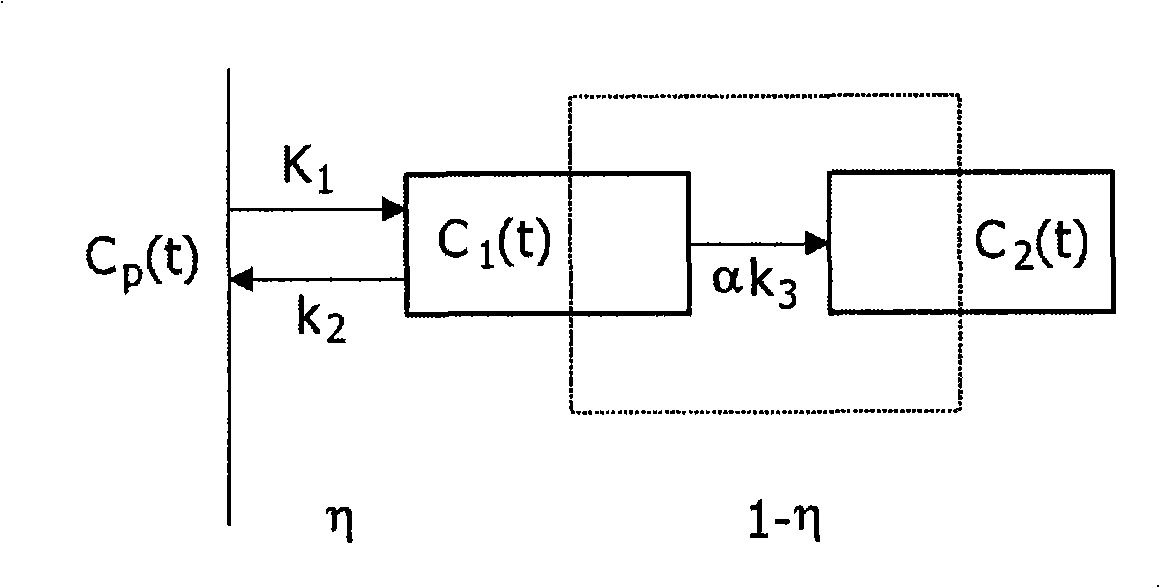
A method, signal processor, device, and system for estimating a parametric or functional image 47 mapping a biological process on the basis of a limited or incomplete sequence of biological process images 40 recorded as a function of time, e.g. by a PET or SPECT scanner after injection of a radio tracer. One or more kinetic parameters 43 are first extracted by applying a pharmacokinetic model 42 (compartmental model of the underlying tracer kinetics) to the sequence of biological process images 40. Additional data 41 are used in the model, comprising at least a predetermined kinetic parameter range (e.g. from the literature), and optionally an input function or a blood clearance function. Next, an iterative algorithm 44 is applied to arrive at a modified sequence of images 45, e.g. by inserting an estimated image into the incomplete sequence of images, utilizing the one or more kinetic parameters 43. After a stop criterion has been fulfilled, the resulting image 47 is finally estimated 46 from the modified sequence of images 45. The method can be used e.g. to estimate a hypoxia parameter k3 image in the case of a FMISO data set where only late-time images are available. The method may be implemented as part of existing PET, SPECT, CT, MR or Ultrasound scanner software, and since only a limited amount of late time post injection images are necessary to provide a reliable result, the method helps to increase patient comfort and clinical throughput.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
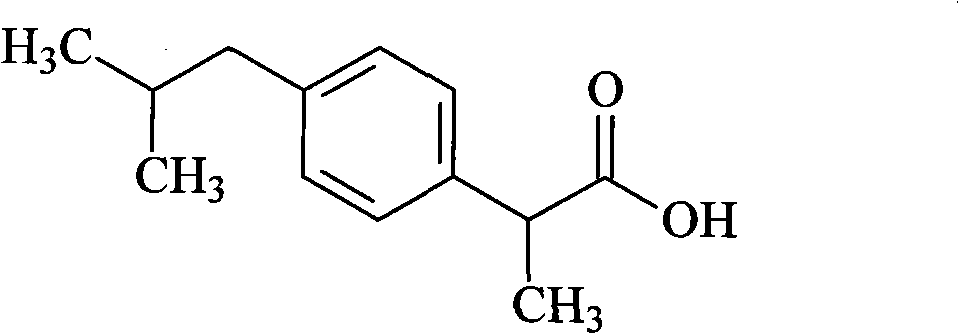
Ibuprofen injection preparation and preparation method thereof
ActiveCN102370615AFast playFully playOrganic active ingredientsPowder deliveryIbuprofen InjectionFreeze-drying
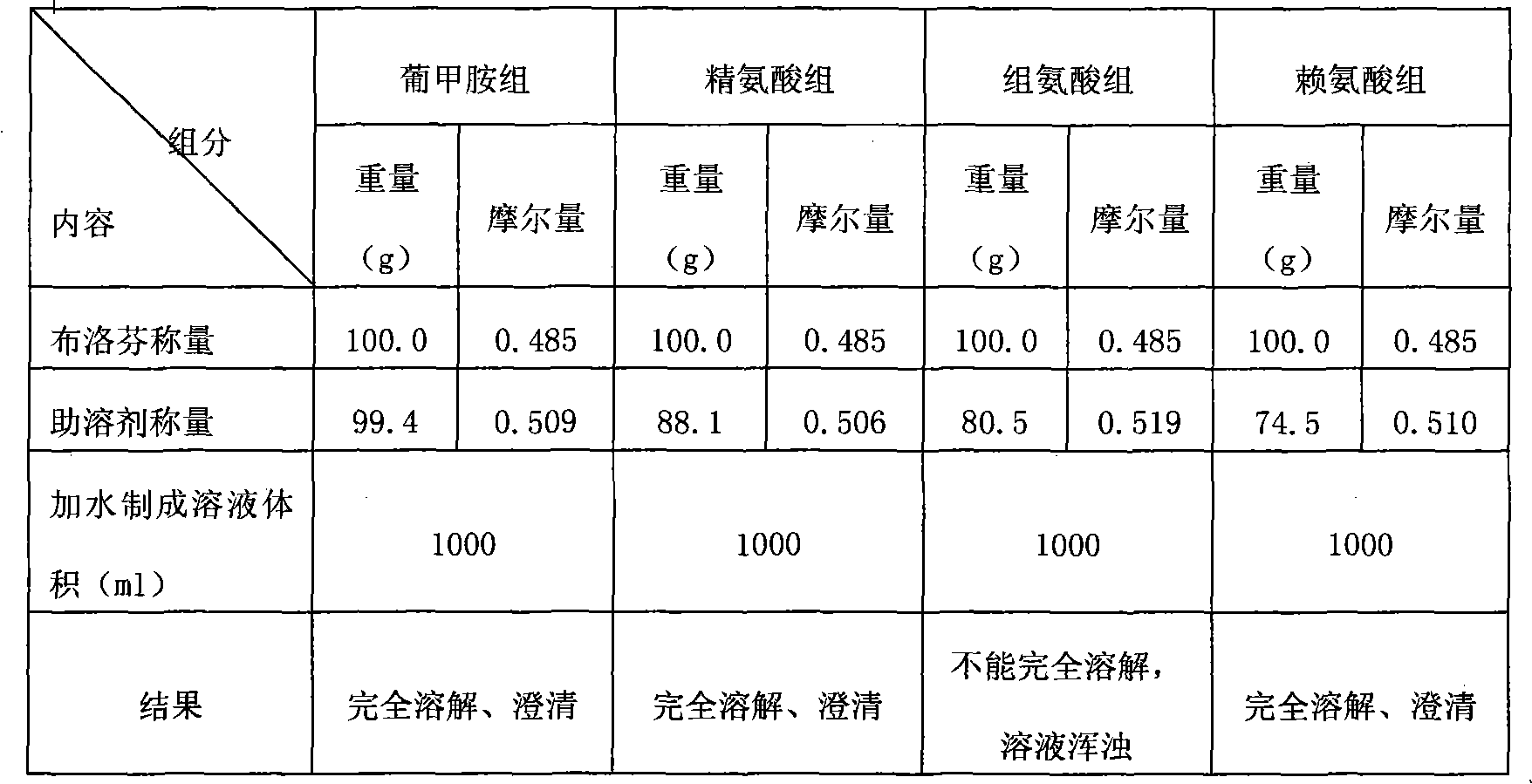
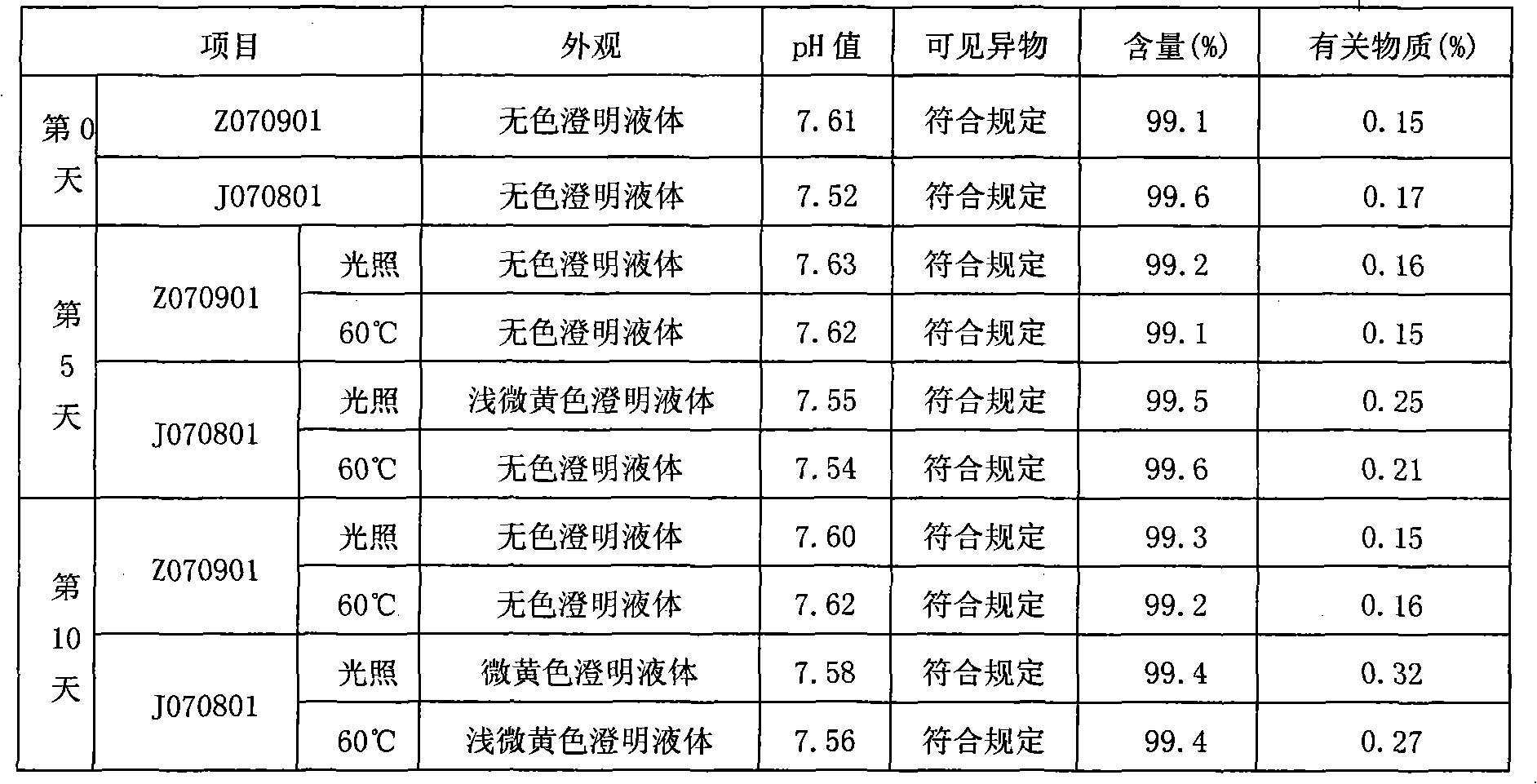
The invention relates to an ibuprofen injection preparation and a preparation method thereof, and especially relates to an ibuprofen injection preparation with meglumine as a cosolvent and a preparation method thereof, wherein the molar ratio of meglumine and ibuprofen is 0.1-2.0:1. The invention provides a new effective method which allows ibuprofen to be dissolved, the drug effect of ibuprofen to be rapidly and fully performed, and the solution formed by ibuprofen to be used for preparing a solution for injection or freeze-dried powder for injection. By carrying out pharmic stability tests,animal safety tests, pharmacokinetics and the like to realize the multitime investigation of the stability, the safety and the effectiveness of the ibuprofen injection preparation, results show that the ibuprofen injection preparation has a strong stability. So compared with the prior part, the preparation method of the invention allows the safety and the effectiveness of drugs to be guaranteed.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
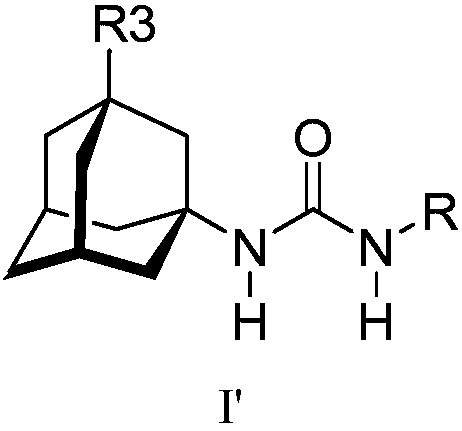
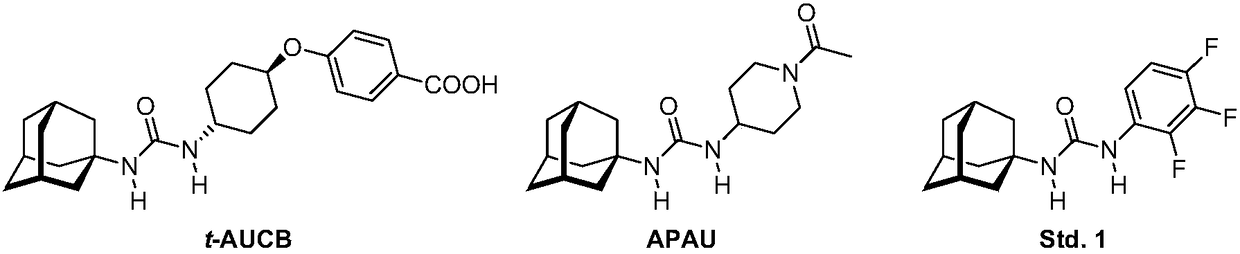
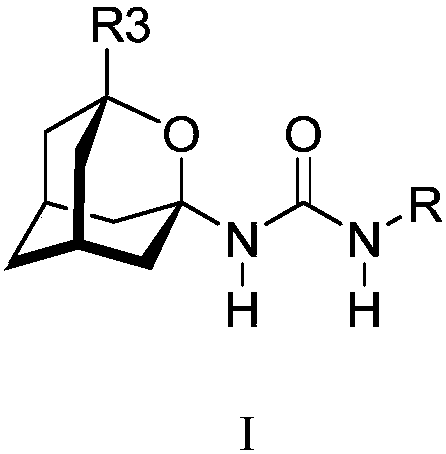
Analogs of adamantylureas as soluble epoxide hydrolase inhibitors
N-(2-oxaadamantan-1-yl)ureas of formula I, where R3 is H, C1-C3 alkyl, cyclohexyl or phenyl; R is -[CH2]n -Y; n is 0-15; in -[CH2]n - 0-n / 3 of the methylene groups are optionally replaced by non adjacent oxygen atoms; and Y is a 3- or 4-substituted phenyl, a 3- or 4-substituted cyclohexyl, a N-substituted piperidin-4-yl, a N-substituted piperidin-3-yl, a di- or tri-fluorosubstituted phenyl, 4-chloro-3-trifluoromethylphenyl, 3-chloro-4-trifluoromethylphenyl, 4-fluoro-3-trifluoromethylphenyl, or 3-fluoro-4-trifluoromethylphenyl; have epoxide hydrolase (sEH) inhibitory activities similar to thoseof their N-(adamantan-1-yl)urea analogs. Thus, compounds I are useful as API for the treatment of sEH mediated diseases. Besides, in general, compounds (I) have higher water solubilities and lower melting points, what make them more promising from the point of view of pharmacokinetics and formulation.
Owner:UNIV DE BARCELONA
Whole-human HER2 antibodies, and coding genes and application thereof
ActiveCN104530236AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAbnormal tissue growthHeavy chain
The invention relates to the field of medicinal chemistry, and particularly relates to two whole-human HER2 antibodies, and coding genes and an application thereof. The invention provides the two whole-human HER2 antibodies, wherein an amino acid sequence of a heavy chain variable region of one HER2 antibody is SEQ ID NO:1, and an amino acid sequence of a light chain variable region is SEQ ID NO:2; an amino acid sequence of a heavy chain variable region of the other HER2 antibody is SEQ ID NO:10, and an amino acid sequence of a light chain variable region is SEQ ID NO:11. The whole-human HER2 antibodies can reduce transfusion reactions and immunogenicity, improve drug safety and have better pharmacokinetic characteristics. In addition, the whole-human antibodies can be combined with other HER2 positive tumor therapeutic agents for use in treatment of HER2 positive tumors.
Owner:GENOR BIOPHARMA
Improved human blood coagulation factor FVII-Fc fusion protein and preparation method and application thereof
ActiveCN104774269APeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectPharmaceutical drug
Disclosed are a recombinant fusion protein of human blood coagulation factor FVII-Fc, preparation method therefor and use thereof. The fusion protein comprises a human FVII, flexible peptide linker, and IgG2 Fc variant sequentially from the N-terminal to the C-terminal. The Fc variant has on lytic activity, and shows minimal Fc-mediated adverse side-effects. The fusion protein has a bioactivity similar to or higher than that of human FVII and a greatly prolonged plasma half-life, thereby improving the pharmacokinetics and pharmaceutical effect.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Sulfonamide derivatives and methods of use thereof for improving the pharmacokinetics of a drug
The present invention relates to Sulfonamide Derivatives of Formula (I): and pharmaceutically acceptable salts thereof, wherein A, W, X, R1, R2, R3, R4 and R5 are as defined herein. The present invention also relates to compositions comprising at least one Sulfonamide Derivative, and methods of using the Sulfonamide Derivatives for improving the pharmacokinetics of a drug.
Owner:MERCK & CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com