Thiol medicine content detection method
A detection method, the technology of thiols, applied in the detection field, can solve the problems of complex operation steps, low detection sensitivity, high cost, etc., and achieve the effects of accurate measurement results, short analysis time, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] To measure the amount of captopril in the solution:
[0054] (1) Adjust the pH of the captopril sample solution with a concentration of 35ppm to 7, add 45ppm reducing agent TCEP, stir rapidly for 5min, and the stirring intensity is 200r / min to obtain the reduced product;
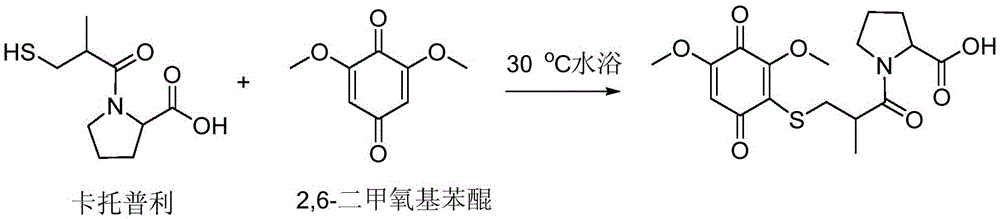
[0055] (2) Add 2,6-dimethoxybenzoquinone as a quinone derivatization reagent to the reduction product, the dosage is 40ppm, react in a water bath at 30°C for 15min, and the derivatization product can be obtained. The sample is 0.45μm Standby after filtering through the filter head, the generation mechanism of the derivatized product is as follows: figure 1 shown;
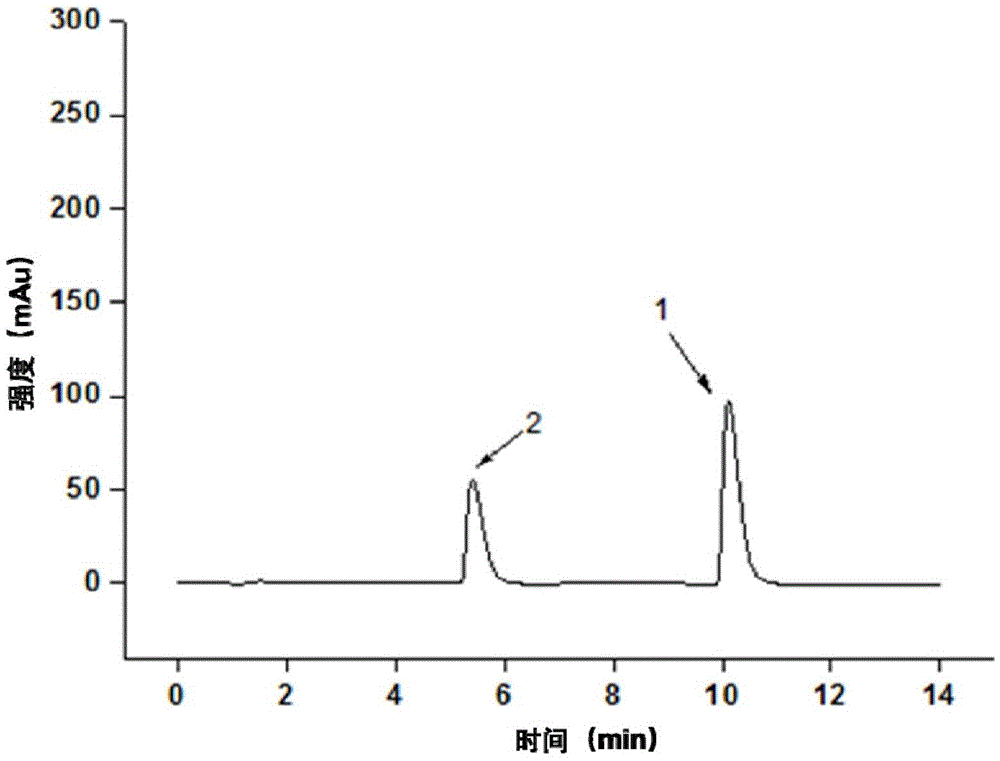
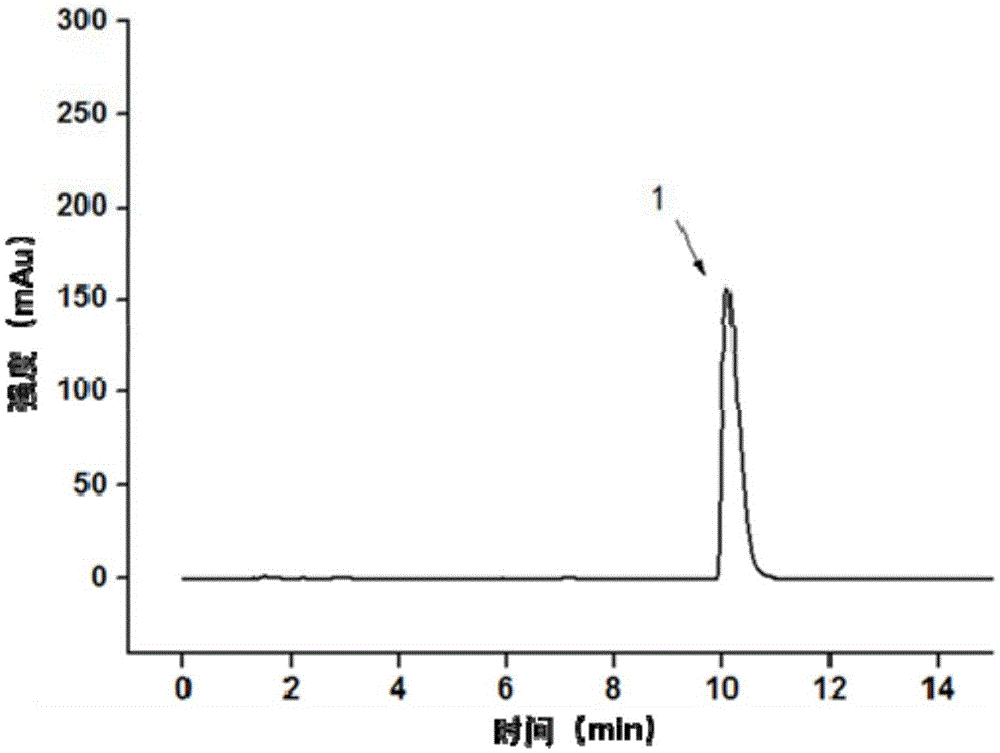
[0056] (3) Separation and detection of derivatized products using high-performance liquid chromatography in series with ultraviolet detectors. The chromatographic column is an AgilentPH5-C18 column (100×2.1mm1.d, 2.7μm), the column temperature is 35°C, and the mobile phase is 80% Containing 0.1wt% phosphoric acid aqueous solution + 20% ac...
Embodiment 2
[0063] To measure the content of tiopronin in the solution:
[0064] (1) The pH of the tiopronin sample solution with a concentration of 45ppm is adjusted to 6, and 40ppm reducing agent sodium borohydride is added, and the stirring speed is 3min, and the stirring intensity is 150r / min to obtain the reduced product;
[0065] (2) Add 2-hydroxy-1,4-naphthoquinone to the reduction product as a quinone derivatization reagent, the dosage is 50ppm, and react in a water bath at 25°C for 10min to obtain the derivatization product. The sample is 0.45μm After the filter head is filtered, it is ready for use;
[0066] (3) Separation and detection of derivatized products using high-performance liquid chromatography in series with ultraviolet detectors. The chromatographic column is an AgilentPH5-C18 column (100×2.1mm1.d, 2.7μm), the column temperature is 30°C, and the mobile phase is 70% Containing 0.2wt% phosphoric acid aqueous solution + 30% acetonitrile, the flow rate is 0.5mL / min, the...
Embodiment 3
[0070] To measure the amount of mesna in a solution:
[0071] (1) Adjust the pH of the mesna sample solution with a concentration of 25ppm to 7.5, add 30ppm reducing agent tributylphosphine, stir rapidly for 4 minutes, and the stirring intensity is 175r / min to obtain the reduced product;
[0072] (2) Add 1,2-anthraquinone as a derivatizing agent to the reduced product, the dosage is 63ppm, and react in a water bath at 28°C for 18 minutes to obtain the derivatized product, and the sample is filtered through a 0.45 μm filter head for later use;
[0073] (3) The derivatized products were separated and detected by high-performance liquid chromatography with a series ultraviolet detector. The chromatographic column was an AgilentPH5-C18 column (100×2.1mm1.d, 2.7μm), the column temperature was 40°C, and the mobile phase was 60% Containing 0.1wt% phosphoric acid aqueous solution + 40% acetonitrile, the flow rate is 0.6mL / min, the injection volume is 30μL, and the detection wavelength...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com