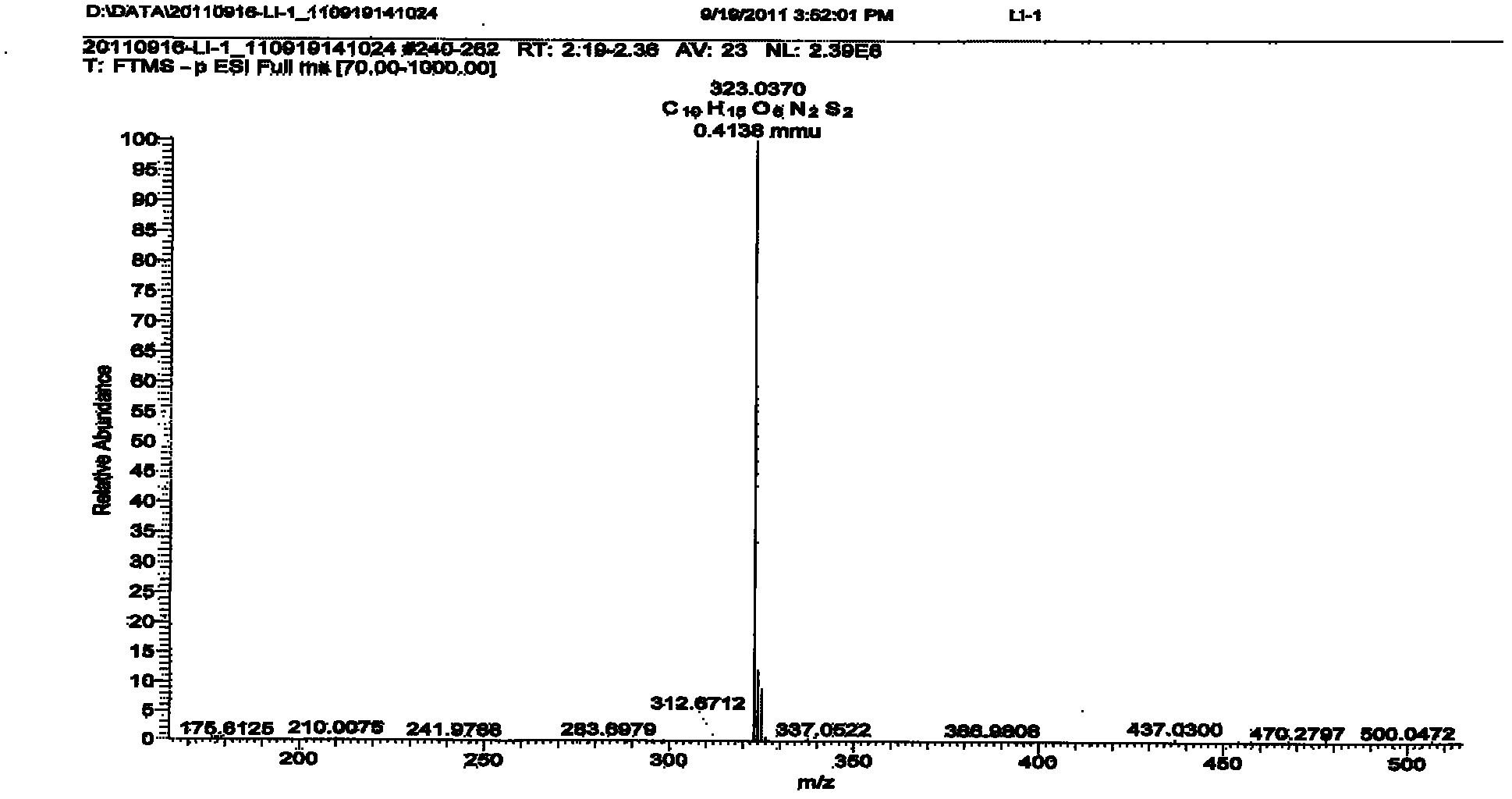
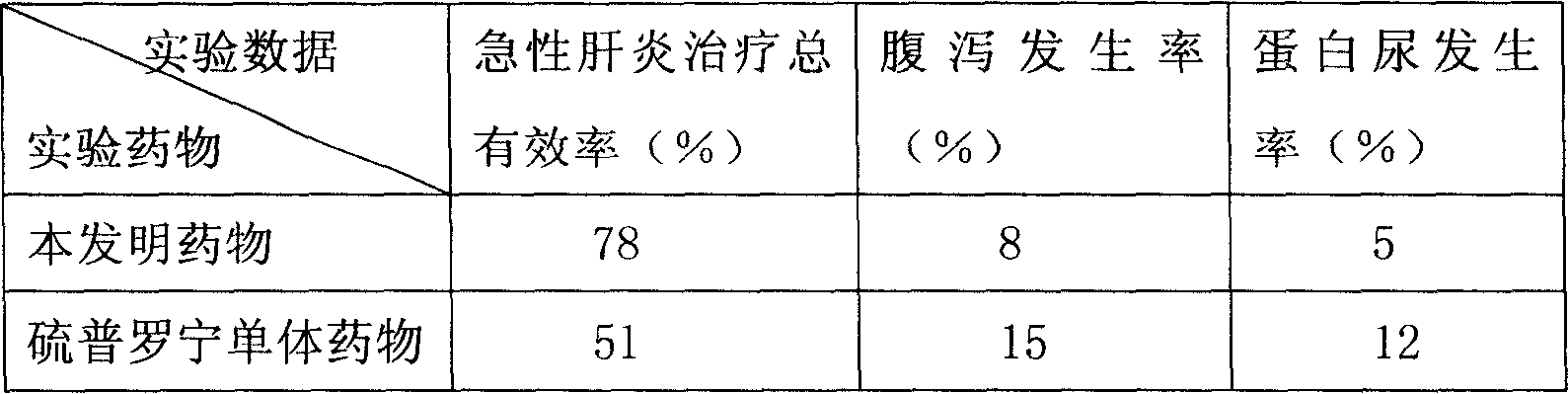
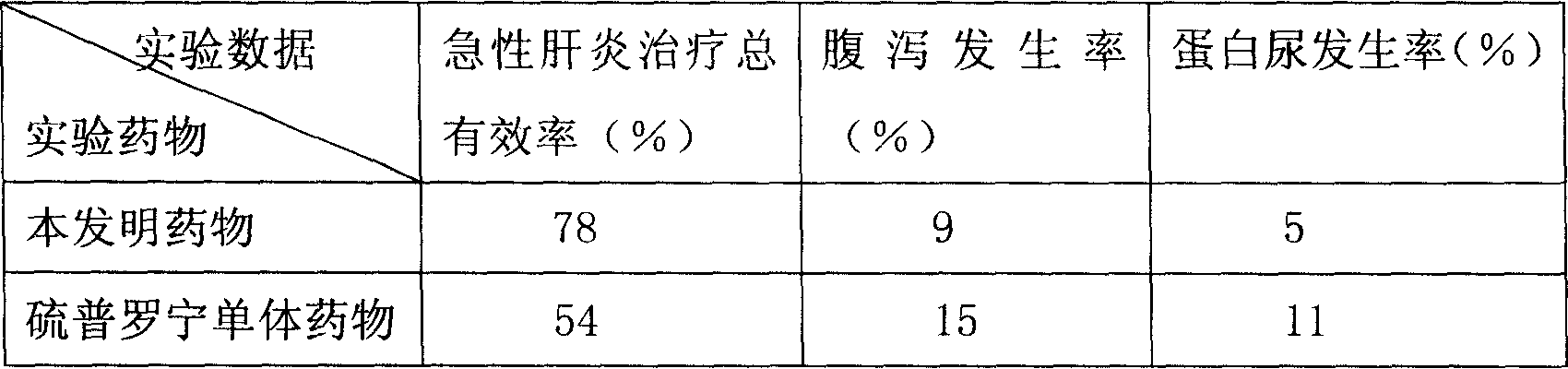
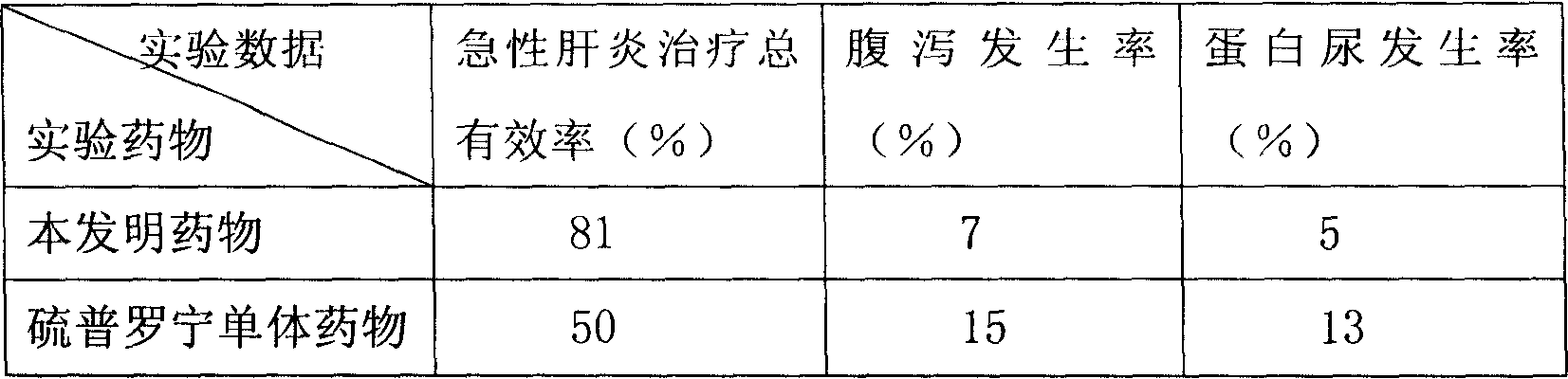
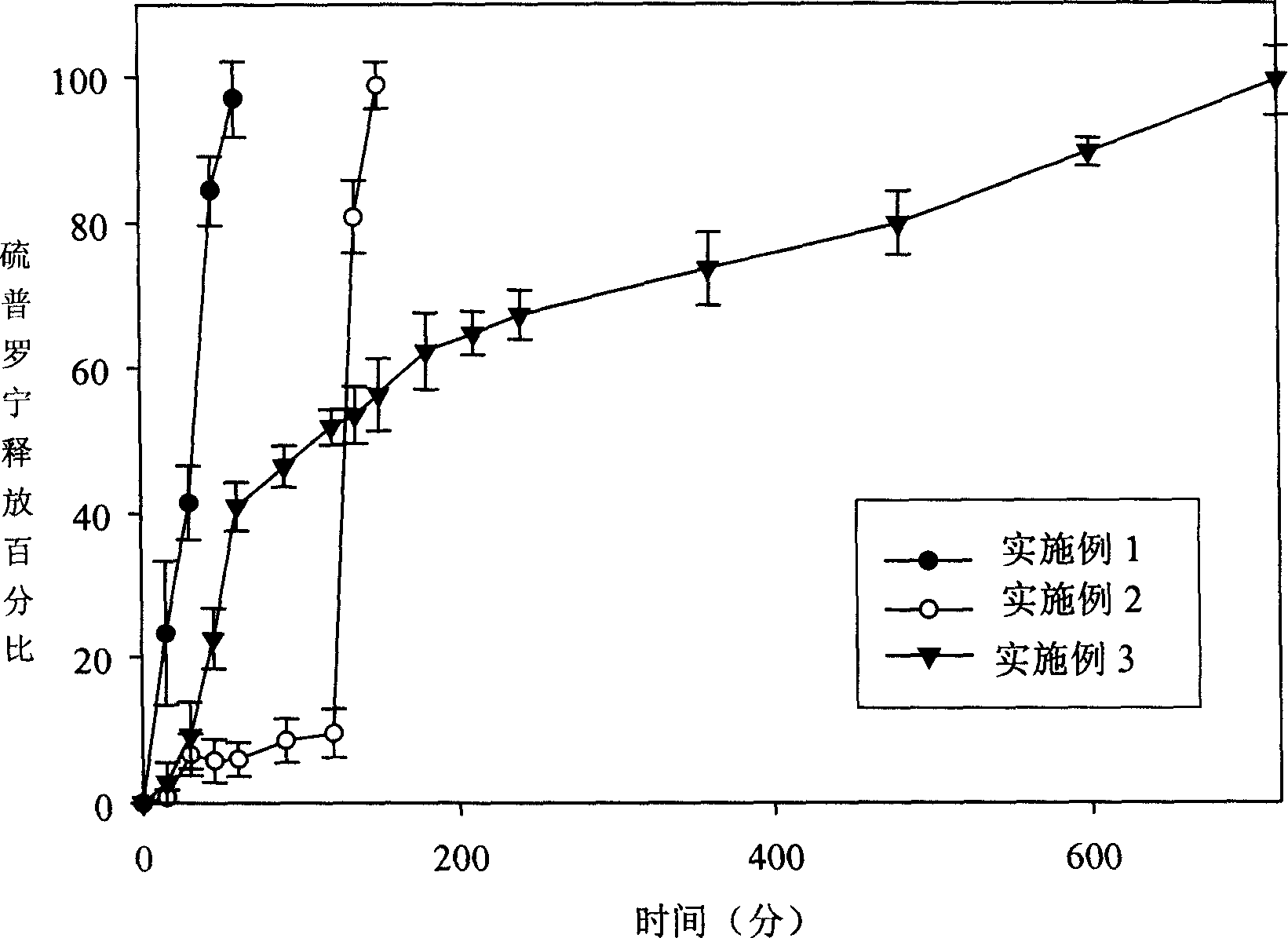
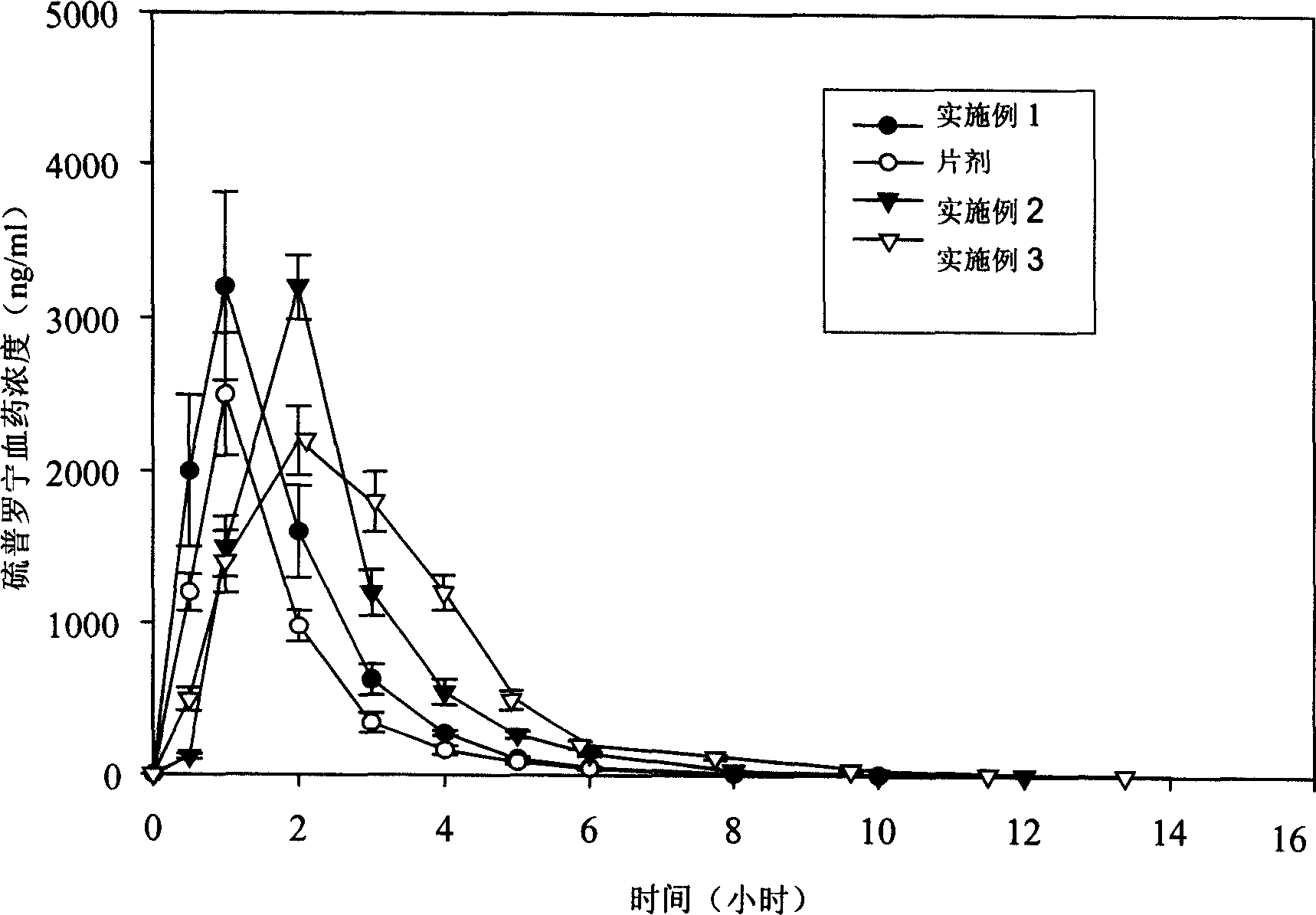
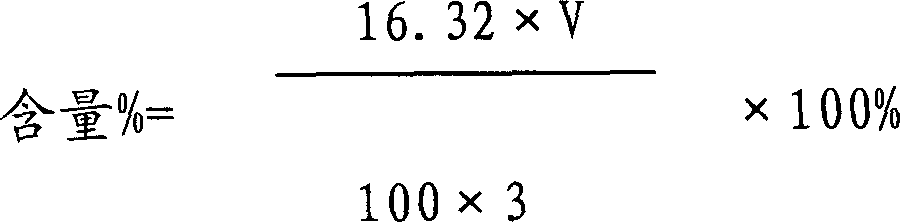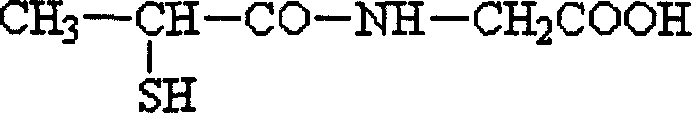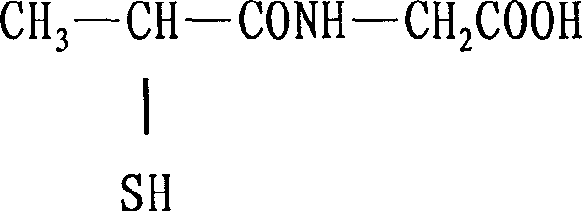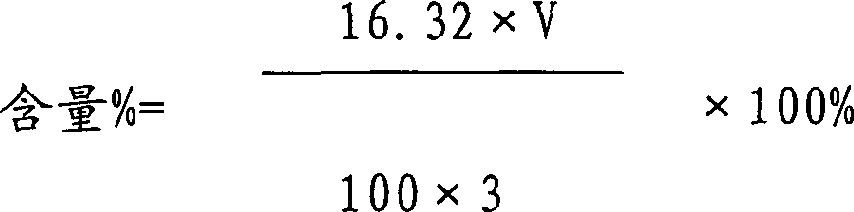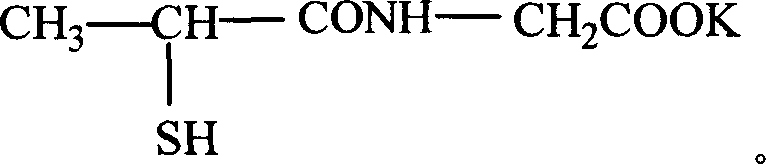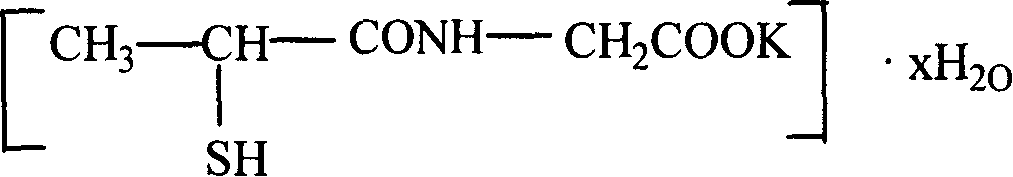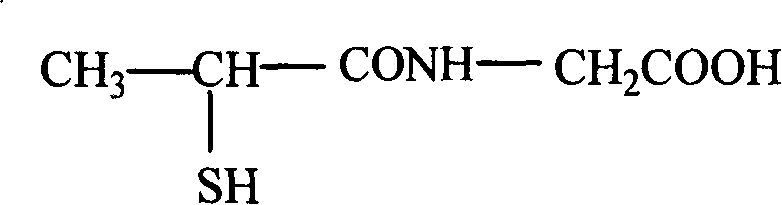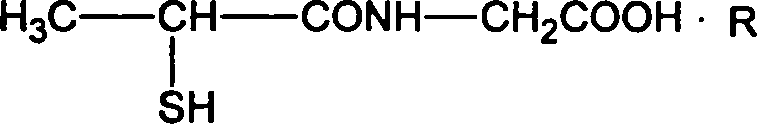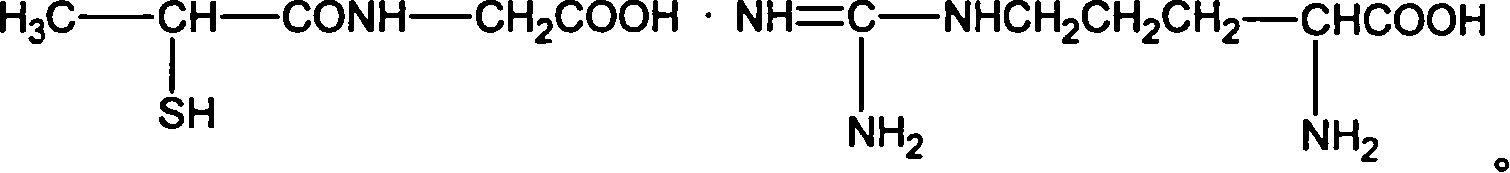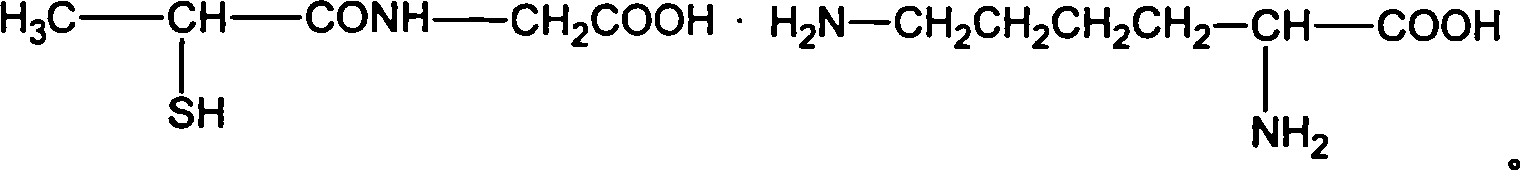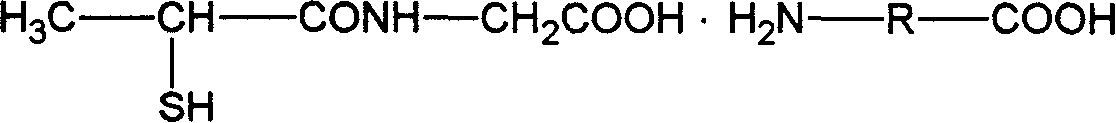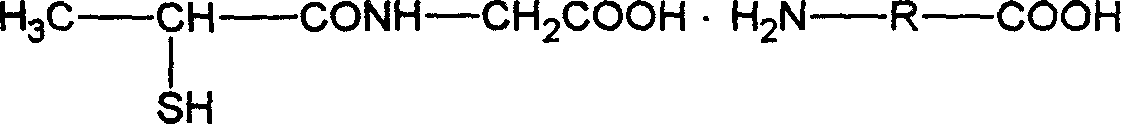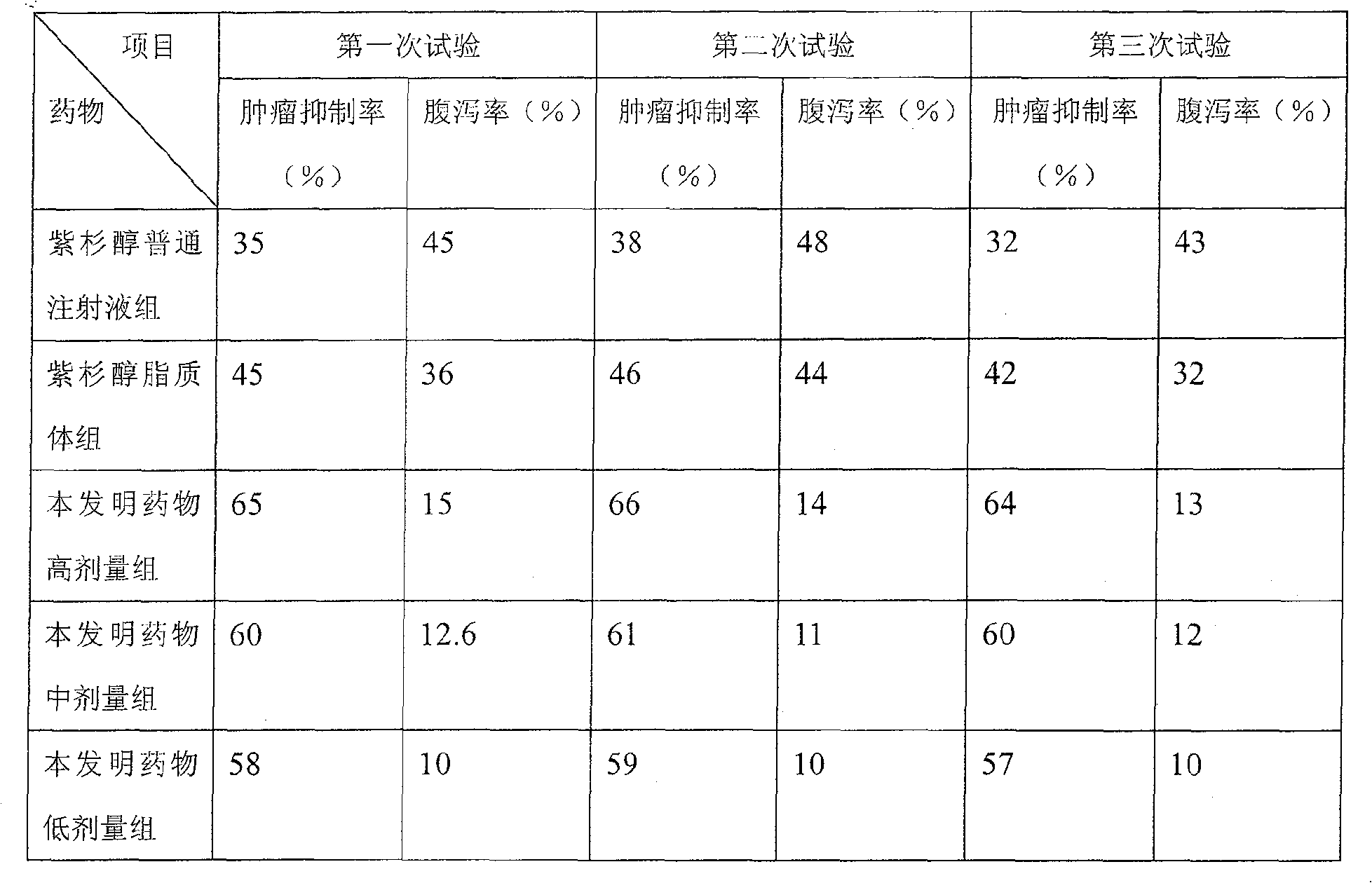Patents
Literature
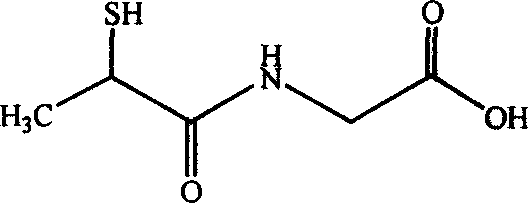
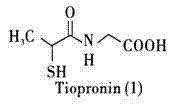
79 results about "Tiopronin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
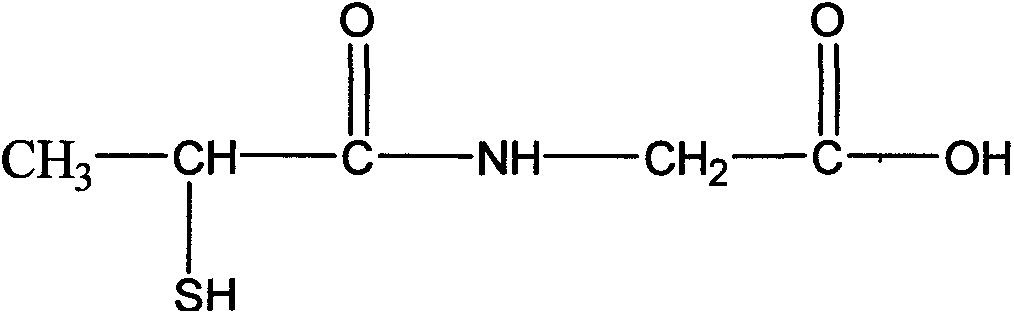
This medication is used to prevent kidney stones in patients with a certain inherited disorder (cystinuria).
Quantum dots and their uses
InactiveUS7498177B2Easy to useEasy to produceMicrobiological testing/measurementChemiluminescene/bioluminescenceQuantum dotBiocompatibility Testing
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
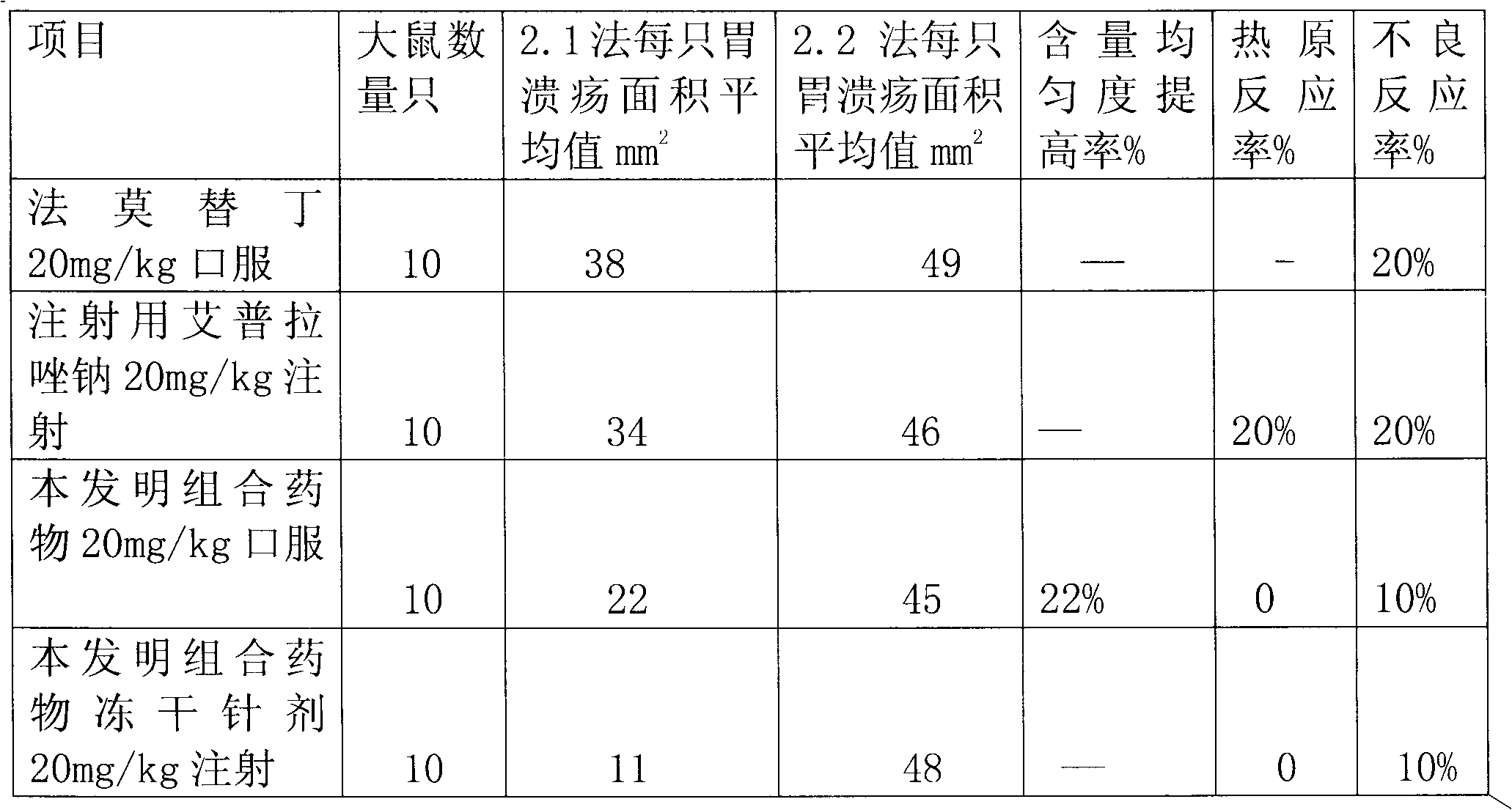
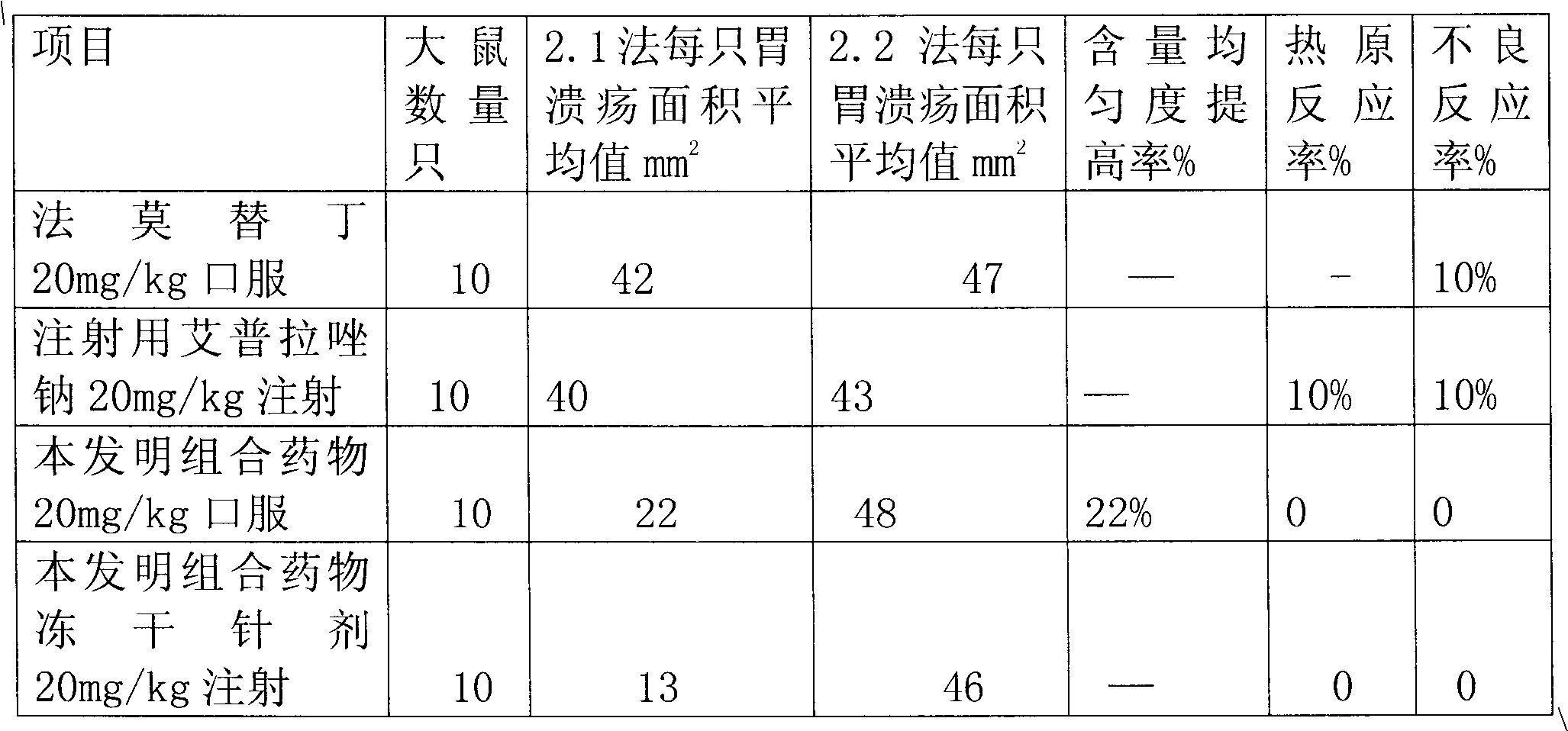
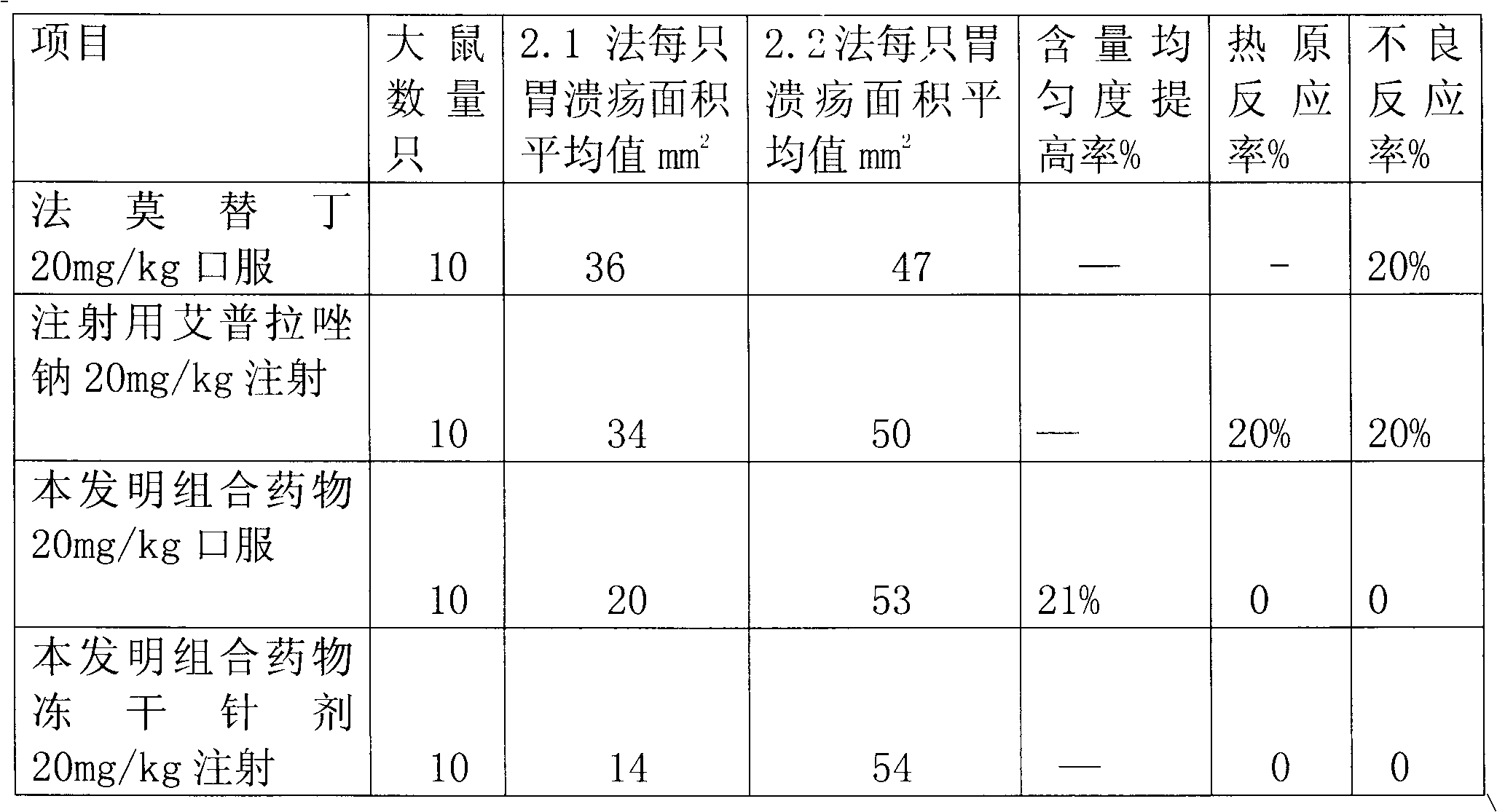
Combination medicament of ilaprazole sodium and preparation process thereof
InactiveCN102058593AEliminate adverse reactionsGood regeneration performanceOrganic active ingredientsDigestive systemCoated tabletsFreeze-drying
The invention provides a combination medicament of ilaprazole sodium. The medicament is characterized by comprising the following raw materials in percentage by weight: 5-10 percent of ilaprazole sodium, 25-35 percent of reduction composition containing glutathione and tiopronin with a weight ratio of 1:10 and 45-55 percent of diammonium glycyrrhizinate. The invention also provides a preparation process of the medicament. According to a pharmaceutically acceptable dosage of the ilaprazole sodium, the combination medicament can be respectively prepared into medical preparations of the dosage forms of injections of the combination medicament of the ilaprazole sodium, freeze-drying injections of combination medicament of the ilaprazole sodium, coated tablets of the combination medicament of the ilaprazole sodium, enteric capsules, sprays, and the like and is used for treating gastric ulcer.
Owner:吴赣英
Tiopronin freeze-dried powder injection
InactiveCN1488343AImprove stabilitySignificant effectPeptide/protein ingredientsDigestive systemCelluloseBenzoic acid
The invention refers to a Tiopronin freeze-dried powder-form injection for treating chronic liver diseases. It comprises active Tiopronin compound 1-1000mg, like 50mg, 100mg or 200mg, and the receivable carrier of drug is selected from one or multiple of low-molecular dextran, mannite, cyclodextrin, soluble amylum, glucide, NaCl, benzoic acid, cellulose or water. It also refers to the making method including the steps of preparing medical liquid, eliminating heat source, sterilizing, canning and freeze-drying.
Owner:上海凯宝新谊(新乡)药业有限公司
Anti-hepatitis medical combination
InactiveCN101062084AMeet urgent clinical needsImprove immunityOrganic active ingredientsDigestive systemDiseaseHepatic fibrosis
Disclosed is a novel pharmaceutical composition for treating liver diseases and process for preparation, wherein the pharmaceutical composition mainly comprises glycyrrhizic acid or its pharmaceutically acceptable salts, tiopronin, kurarinone, one or more of silybin or its derivatives or silymarin, rhodiola rosea or rhodiola rosea, the composition can be prepared into any one of the pharmaceutically acceptable dosage forms, preferably injections and oral preparations.
Owner:JIANGYIN TIANJIANG PHARMA
Frozen powder injection of tiopronin
A freeze-dried powder injection of tiopronin is prepared from tiopronin and the excipient chosen from amino acid, the salt of amino acid, and phosphate through proportionally and respectively dissolving them in the water for injecting, mixing, stirring, sterilizing, pouring in containers and freeze drying.
Owner:江西欧氏药业有限责任公司
Method for preparing tiopronin
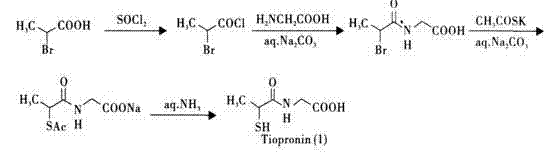
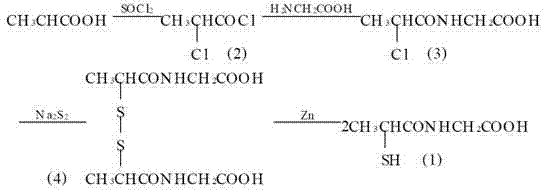
The invention provides a method for preparing tiopronin. The method comprises the following steps of: (1) making alpha-chloropropionic acid react with thionyl chloride to obtain alpha-chloropropionylchloride; (2) making alpha-chloropropionylchloride react with glycine to obtain alpha-chloroglycine; and (3) making alpha-chloroglycine react further to obtain the tiopronin. The method has the advantages of small quantity of steps, low pollution and high yield.
Owner:SUZHOU ERYE PHARMA CO LTD
Pantoprazole sodium combined drug
ActiveCN101766615AEliminate adverse reactionsImproved depyrogenation processOrganic active ingredientsDigestive systemDisodium EdetateCurative effect
The invention discloses a pantoprazole sodium combined drug and a preparation method thereof. The pantoprazole sodium combined drug contains the following active ingredients in parts by weight: 35-100 parts of pantoprazole sodium, 15-35 parts of tiopronin, 90-200 parts of calcium glutamate and 15-25 parts of edetate disodium. The pantoprazole sodium combined drug has the functions of preventing damages caused by pantoprazole sodium to liver and other adverse reactions. The invention has the advantages of high drug quality, stable curative effect and advanced preparation method.
Owner:安徽延寿堂药业有限公司
Refining method of tiopronin
Owner:GUANGDONG XIANQIANG PHARMA
Thipronin enteric-coated delayed-release agent
InactiveCN101045044AReduce direct irritationLittle side effectsOrganic active ingredientsMetabolism disorderSide effectMedicine
A slow-releasing enteric tiopronin without stimulation and by-effect is prepared from tiopronin (3-65 Wt%), slow-releasing material (5-90) and enteric coating material (3-30).
Owner:魏秀华
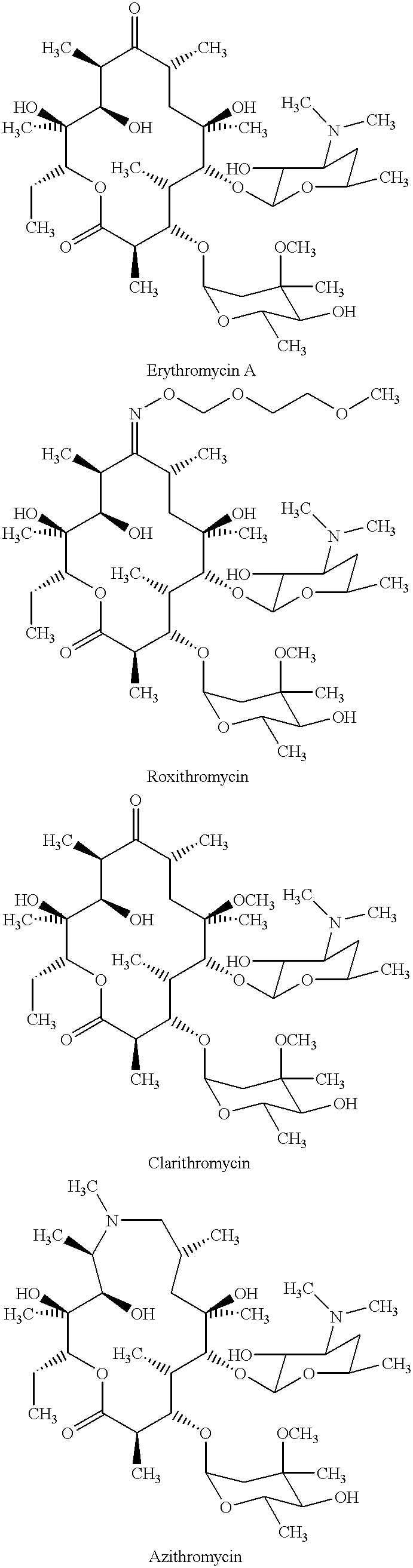
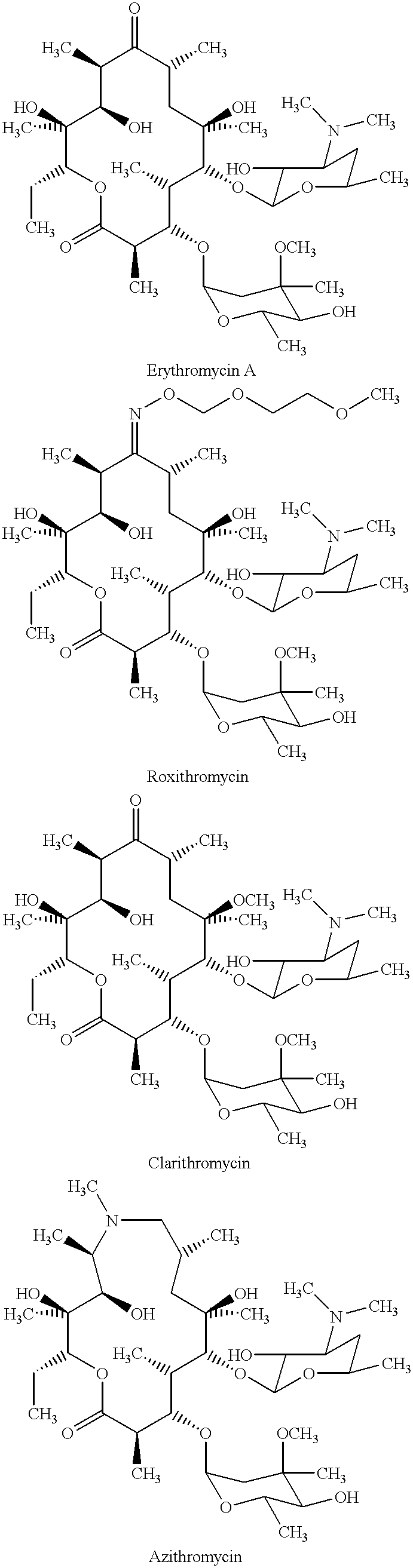
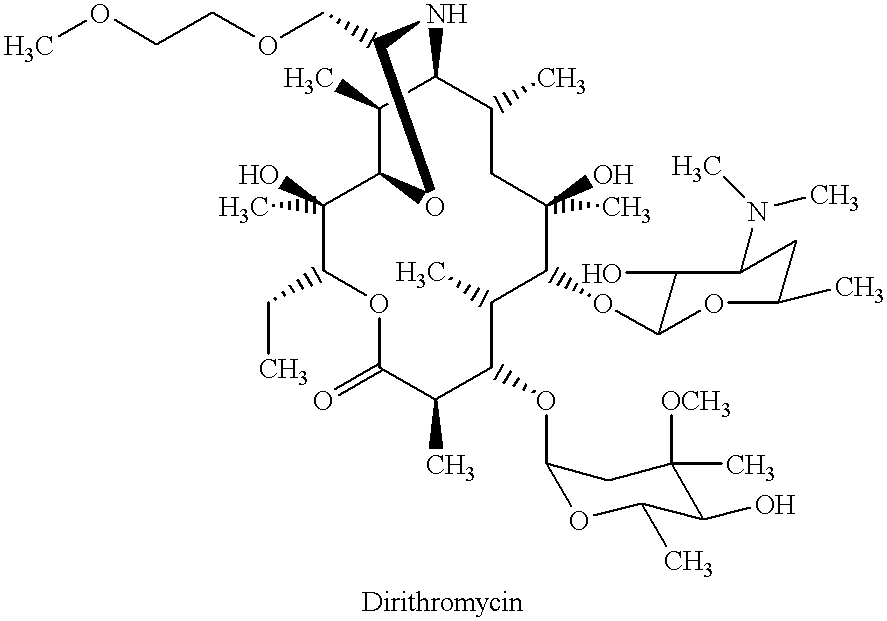
Derivatives of erythromycin, clarithromycin, roxithromycin or azithromycin with antibiotic and mucolytic activity
InactiveUS20010031736A1Improved pharmacological profileGood effectBiocideSugar derivativesRoxithromycinAzithromycin
A pharmaceutical with an enhanced pharmaceutical profile comprises a mucolytic and an antibiotic in which the mucolytic is present in an amount of greater than one molar equivalent of the antibiotic. The antibiotic may be selected from Erythromycin, Roxithromycin, Clarithromycin, Azithromycin, Dirithromycin; and pharmaceutically acceptable salts or esters thereof. The mucolytic is a mucolytically active thiol, especially N-acetylcysteine, mercaptoethanesulfonic acid, tiopronin or methylcysteine. The adducts can be isolated via a simple and efficient process.
Owner:RUSSINSKY
Novel tiopronin freeze drying powder preparation and its prepn. process
InactiveCN1833637AImprove stabilityGood curative effectOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
A freeze-dried powder injection of tiopronin is prepared from tiopronin and the human body receptable non-active carrier chosen from dextran, glucose, mannitol, xylitol, lactose, amino acid, etc through preparing liquid medicine, regulating pH value, removing heat source and bacteria, filling in bottles and freeze drying.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD
Freeze dry tiopronin preparation without adjuvant for intravenous injection and its preparation process
InactiveCN1732912AAvoid joiningEnsure safetyOrganic active ingredientsPowder deliveryAdjuvantFreeze-drying
The invention relates to a freeze dry tiopronin preparation without adjuvant for intravenous injection and its preparation process, wherein the preparation contains only medicinal Tiopronin but no addition agent of any kinds, one dosage unit of the freeze-dried preparation contains 10-1000mg of Tiopronin. The ratio of the medicament and water in the freeze-dried preparation can be 1:100 to 1:1, the purity of the Tiopronin medicament can be 85-100%.
Owner:苑振亭
Tiopronin freeze-dried powder injection
InactiveCN1194678CImprove stabilitySignificant effectPeptide/protein ingredientsDigestive systemCelluloseBenzoic acid
The invention refers to a Tiopronin freeze-dried powder-form injection for treating chronic liver diseases. It comprises active Tiopronin compound 1-1000mg, like 50mg, 100mg or 200mg, and the receivable carrier of drug is selected from one or multiple of low-molecular dextran, mannite, cyclodextrin, soluble amylum, glucide, NaCl, benzoic acid, cellulose or water. It also refers to the making method including the steps of preparing medical liquid, eliminating heat source, sterilizing, canning and freeze-drying.
Owner:上海凯宝新谊(新乡)药业有限公司
Potassium tiopronin as well as preparation and uses thereof
InactiveCN101205206AHigh purityImprove stabilityOrganic active ingredientsThiol preparationBalance disturbancesOrganic solvent
The invention relates to a medicine-potassium tiopronin used for curing acute and chronic hepatopathy, a method for manufacturing the same and the application in medication. The tiopronin is prepared into potassium salt so as to increase the potassium salt supplement for hepatopaths, and thus the electrolyte balance disorder of hepatopaths is adjusted. The manufacturing method comprises the following steps of: adding tiopronin and potassic inorganic base in organic solvent to undergo the acids-bases salifying reaction with sulfhydryl protecting group assisted in the reaction process; adding precipitator in the system to precipitate at the end of reaction; filtering and drying; finally obtaining potassium tiopronin.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
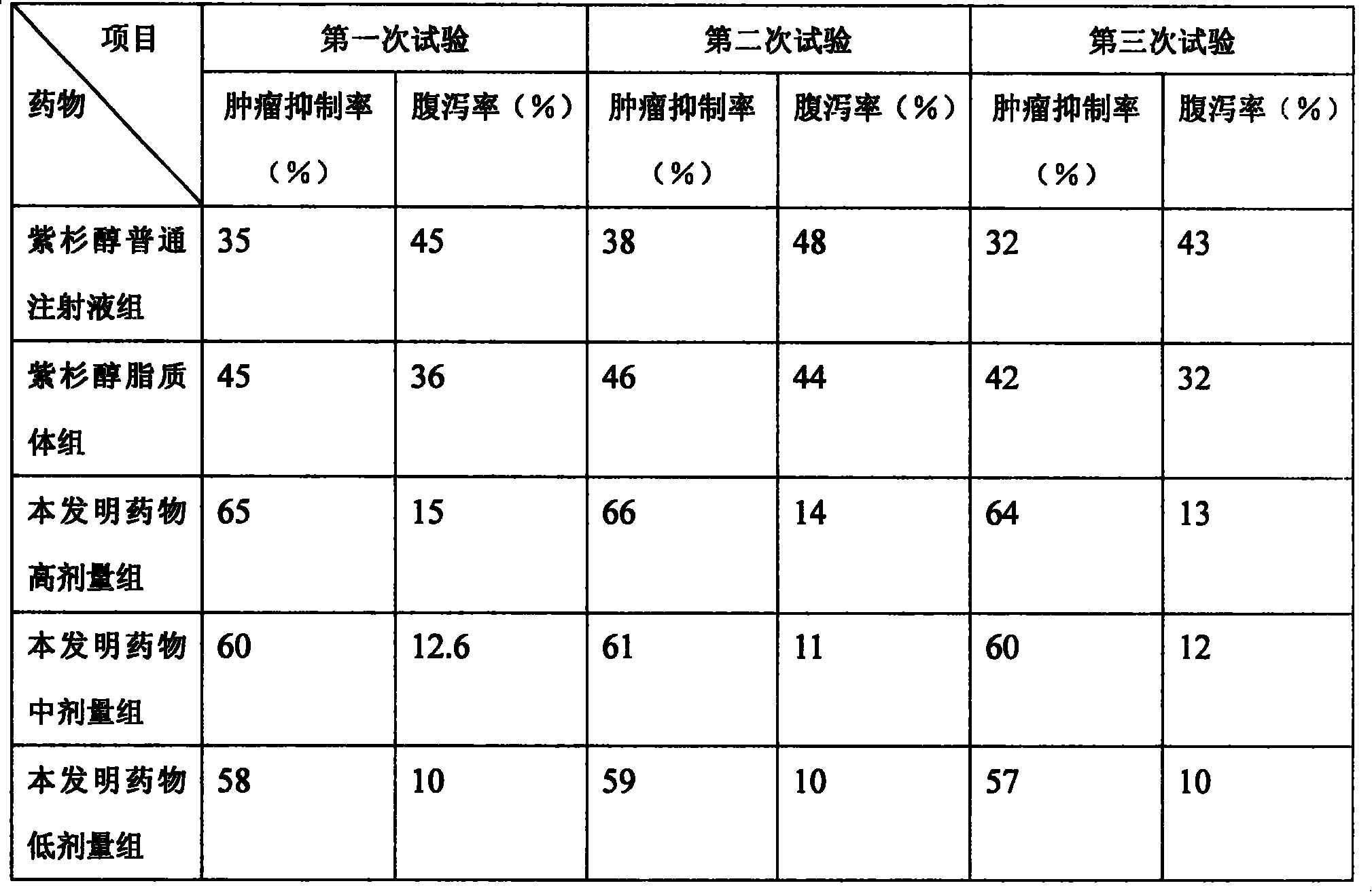
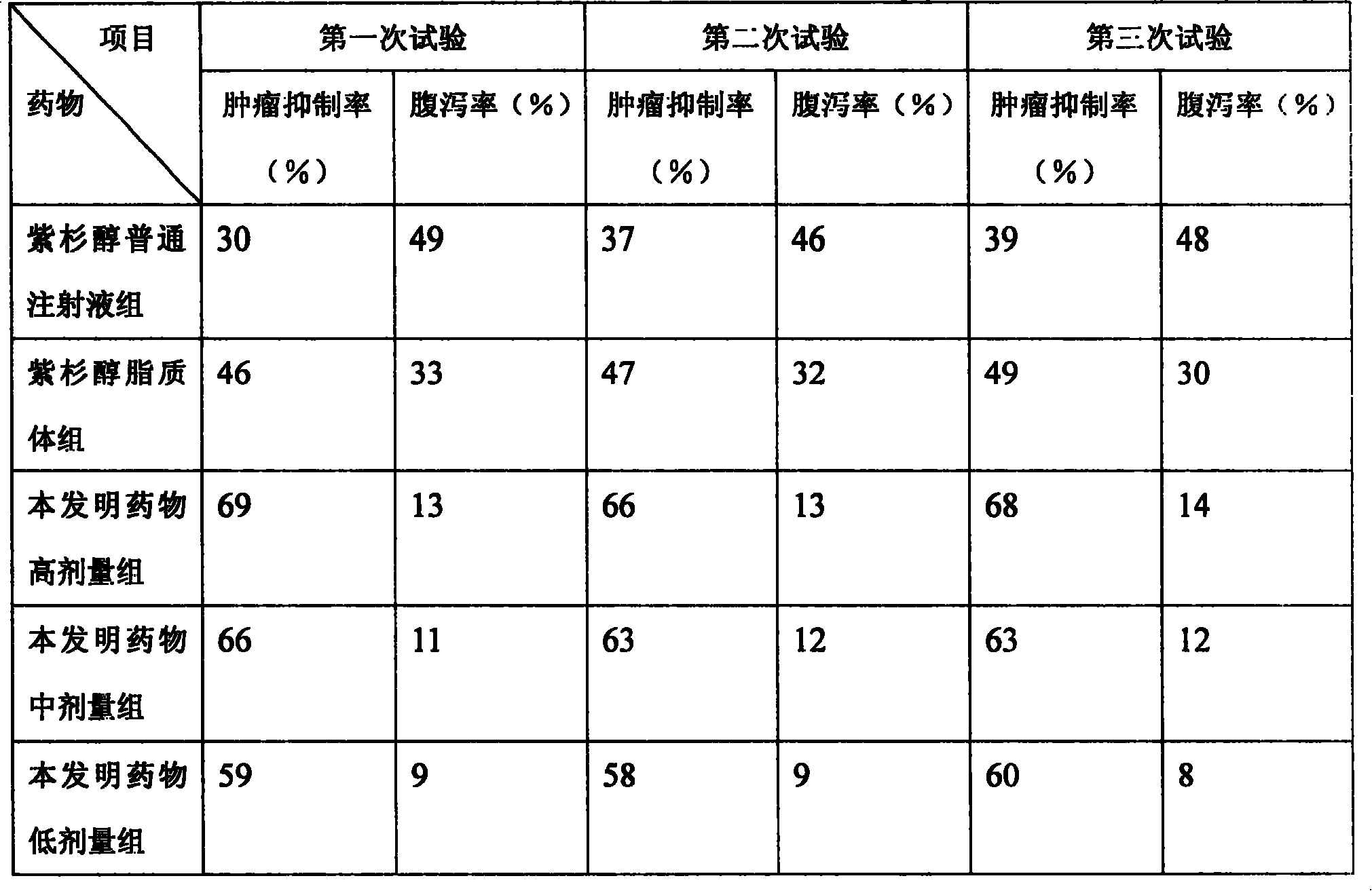
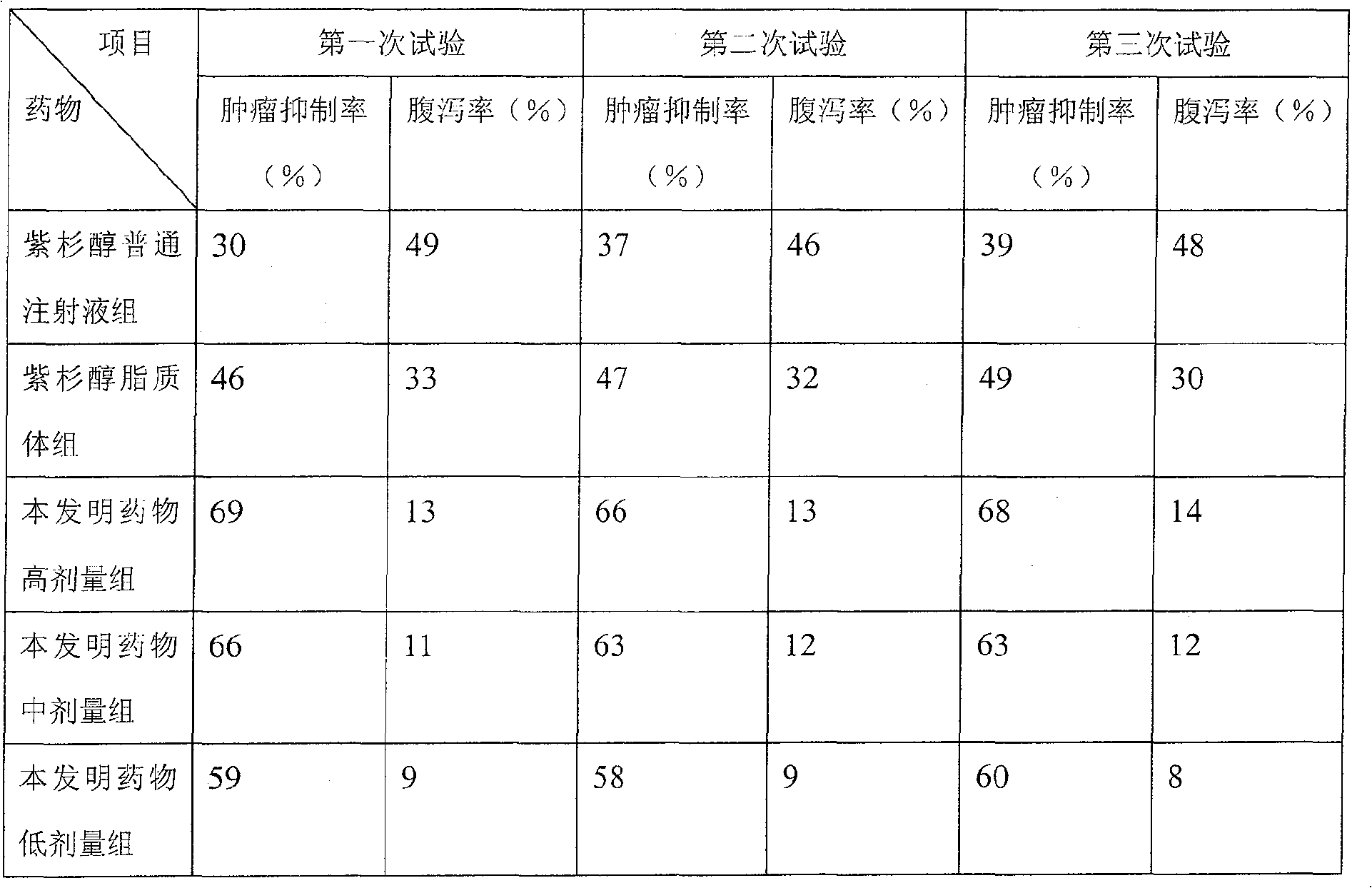
Paclitaxel/multialkene paclitaxel liposome composite medicine and preparation method thereof
InactiveCN101176719ASmall doseSmall toxicityOrganic active ingredientsAntineoplastic agentsSide effectVitamin C
The invention relates to a liposome drug combination and a preparation method of the drug combination. The weights of the effective drug components are listed as follows: the weight of the admixture for hydrogenated soybean phosphatide and sheep brain lecithin with a weight ratio of 2:1 is 300 to 550; the weight of the admixture for cholesterol, stearamide and beta-sitosterol with a weight ratio of 1:1:0.1 is 200 to 400; the weight of the admixture for tiopronin and vitamin E with a weight ratio of 4:1 is 100 to 200; the weight of the admixture for polyethylene glycol-4000 and polyethylene glycol-6000 with a discretional weight ratio is 150 to 200; the weight of vitamin C is 150 to 180; the weight of the admixture for tert-butyl alcohol and anhydrous alcohol with a weight ratio of 1.5 to 2.2:0.01 to 0.05 is 4000 to 7000; the weight of the admixture for paclitaxel and polyene paclitaxel with a discretional weight ratio is 20 and 40; the weight of the admixture for diphenhydramine and cimetidine with a weight ratio of 1:6 is 350 to 500. The invention has the advantages of reducing the drug dosage by nearly one time, increasing the curative effect by over 15 percent, and greatly reducing the side effect of the drugs.
Owner:HUNAN KANGDU PHARMA
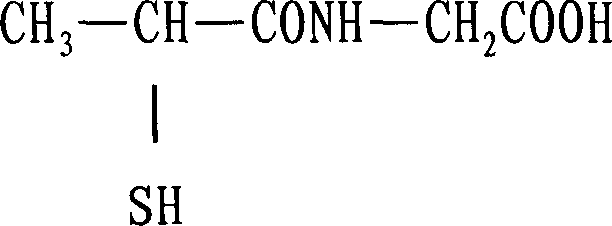
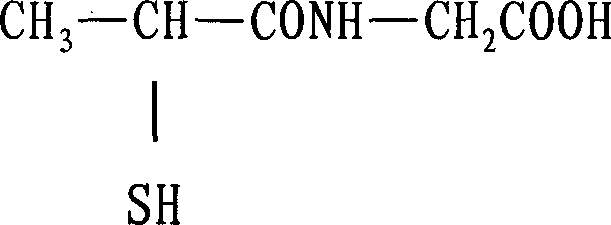
Tiopronin amidate and its prepn
InactiveCN1887859AOvercome stabilityAvoid defectsOrganic active ingredientsOrganic chemistryMedicineDecomposition
The present invention relates to one kind of Tiopronin amidate for treating acute and chronic hepatosis and its preparation process. The general expression of Tiopronin amidate is shown. The present invention changes Tiopronin with easy oxidation, degradation, volatilation and decomposition into stable Tiopronin amidate with raised smelting point and raised medicinal effect. The Tiopronin amidate may be further used to prepare other medicine preparation.
Owner:GRAND PHARM (CHINA) CO LTD
Nitrogen and sulfur co-doped carbon dot as well as preparation method and application thereof
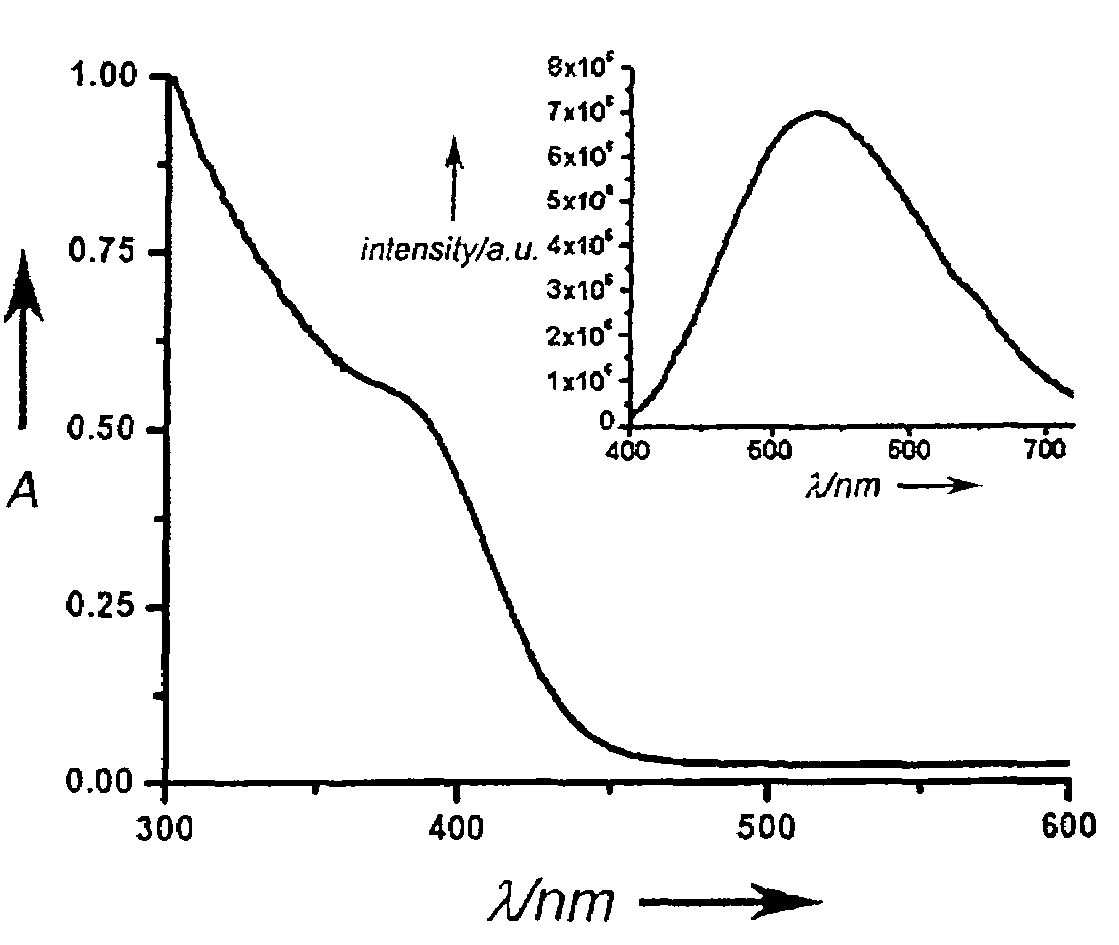
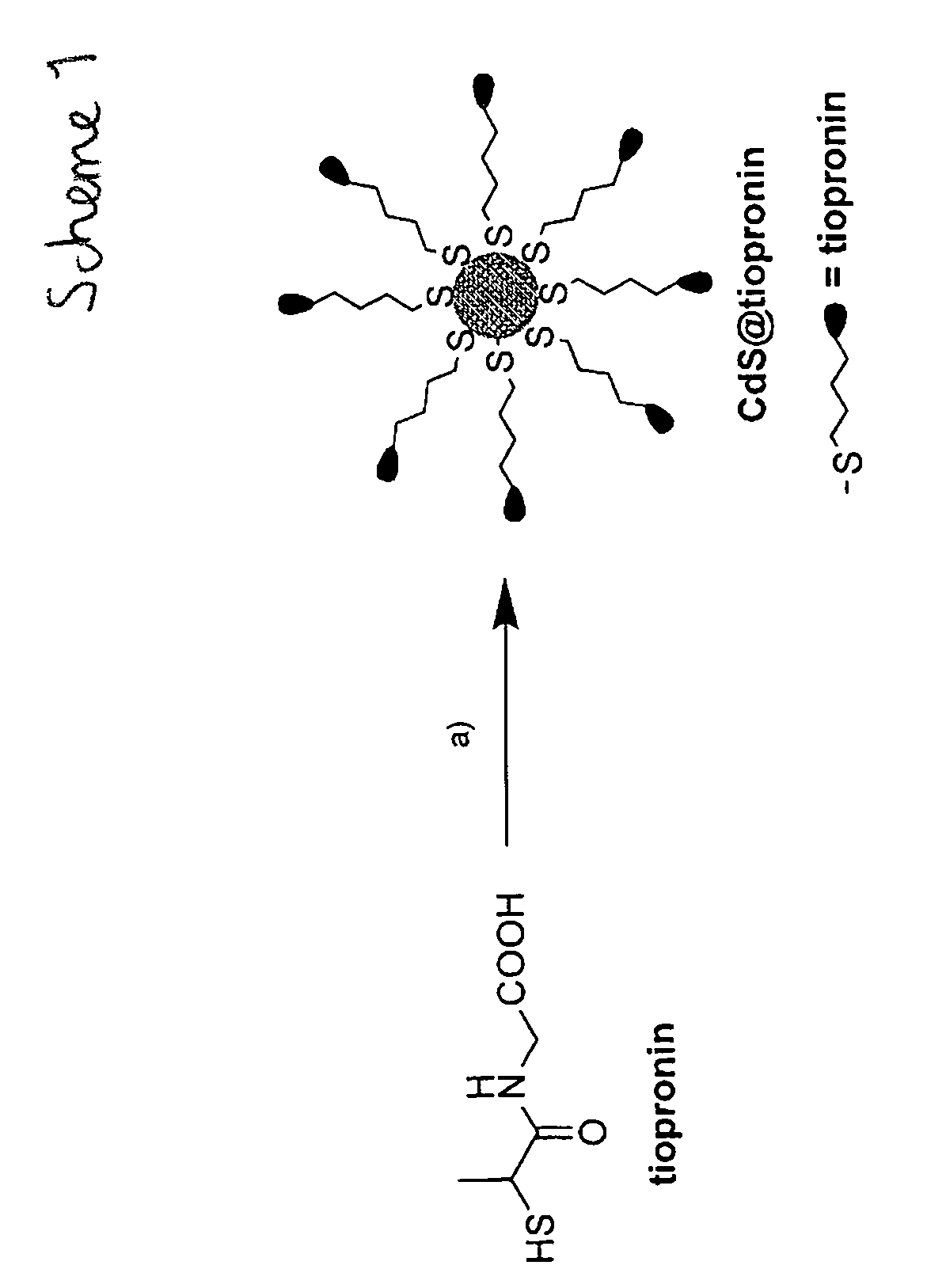
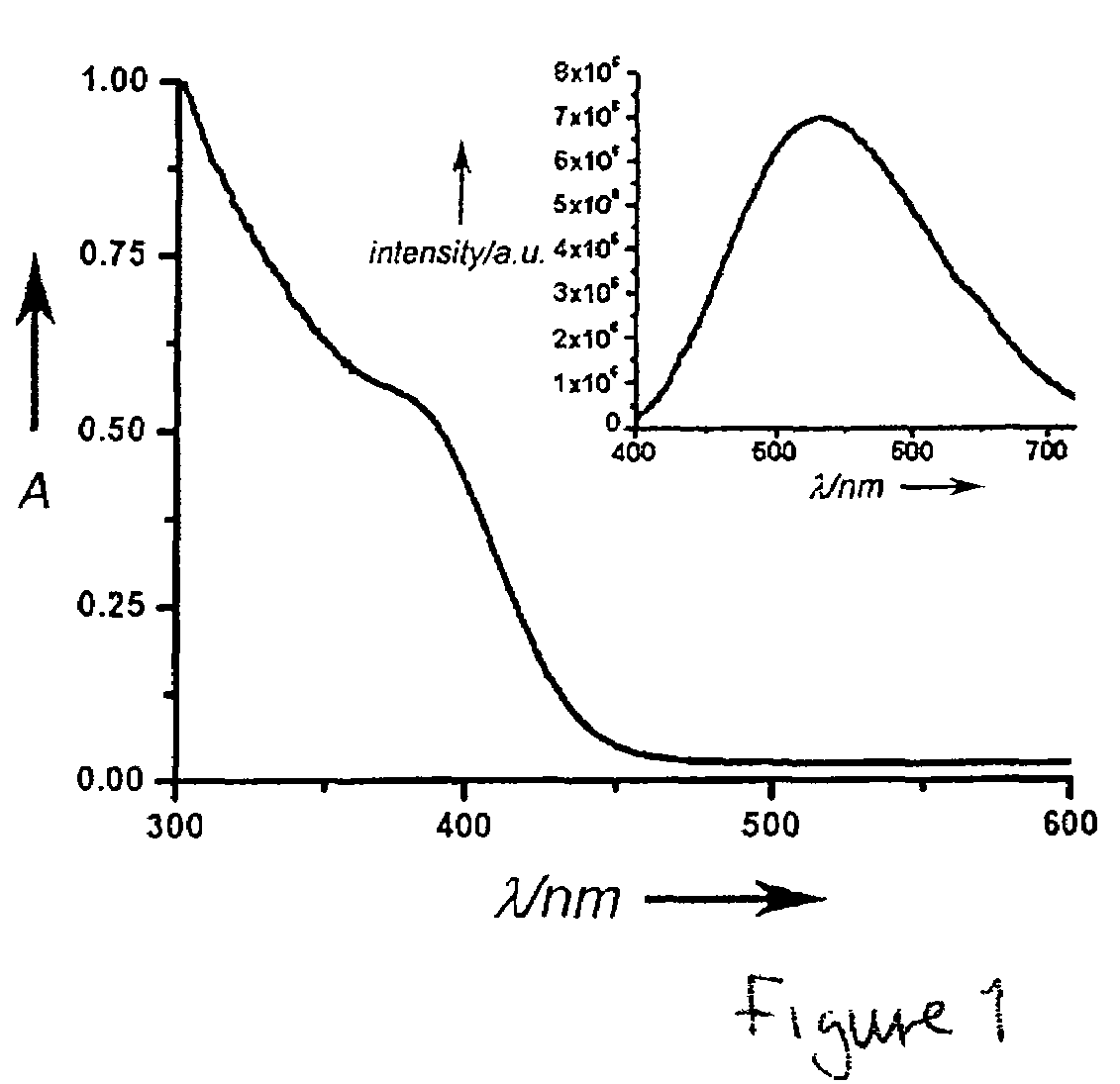
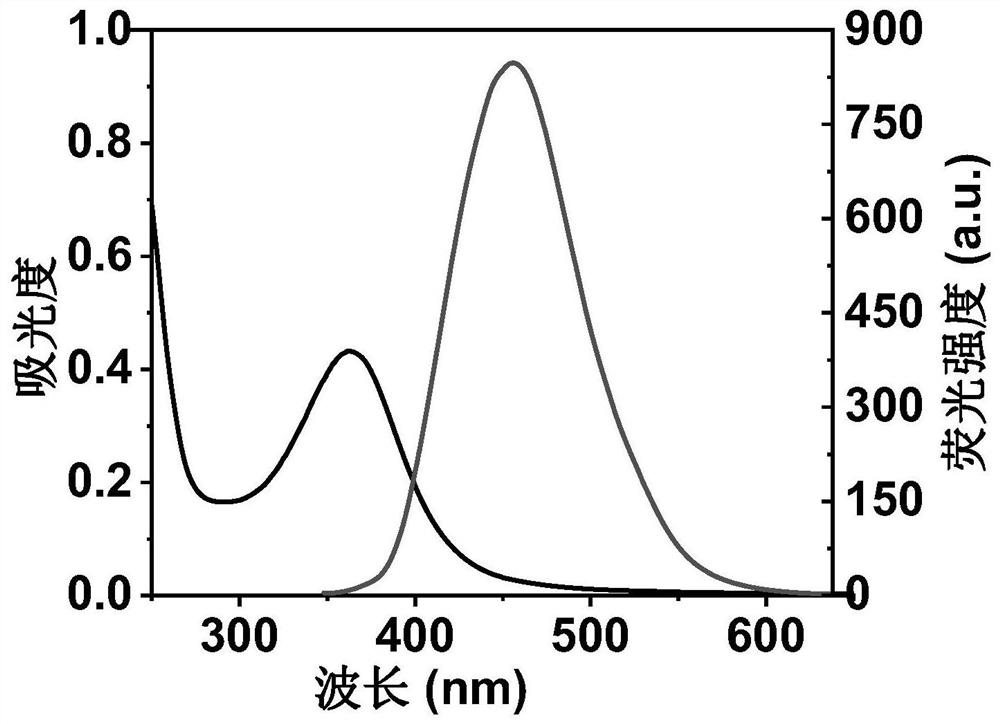
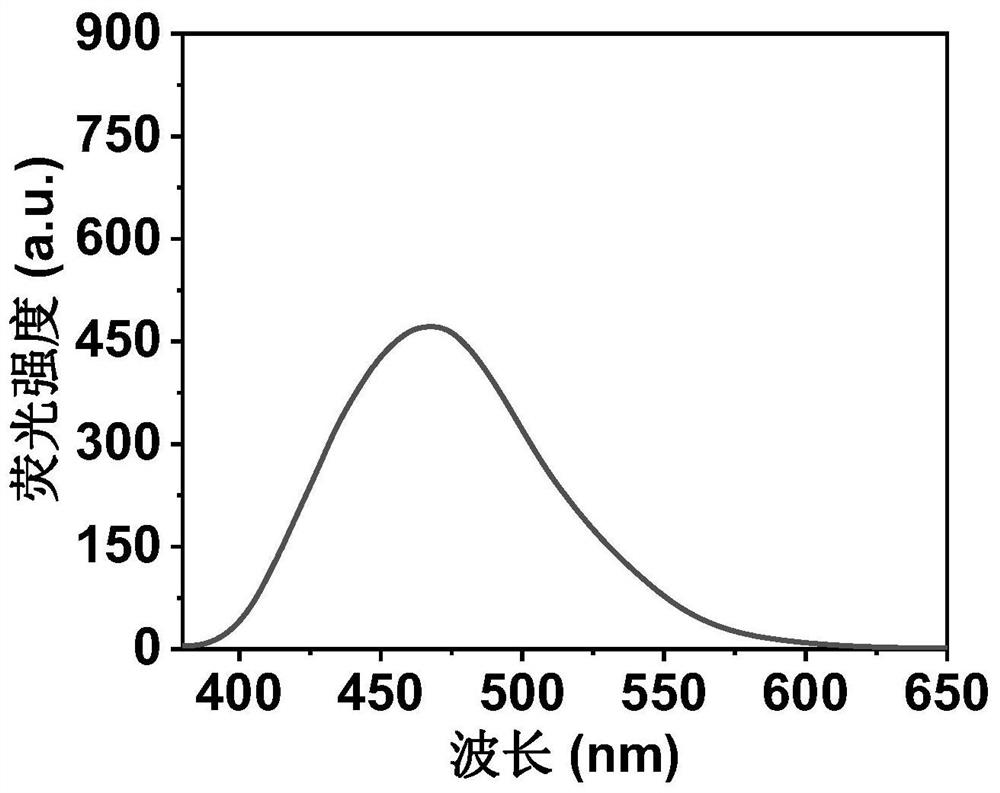
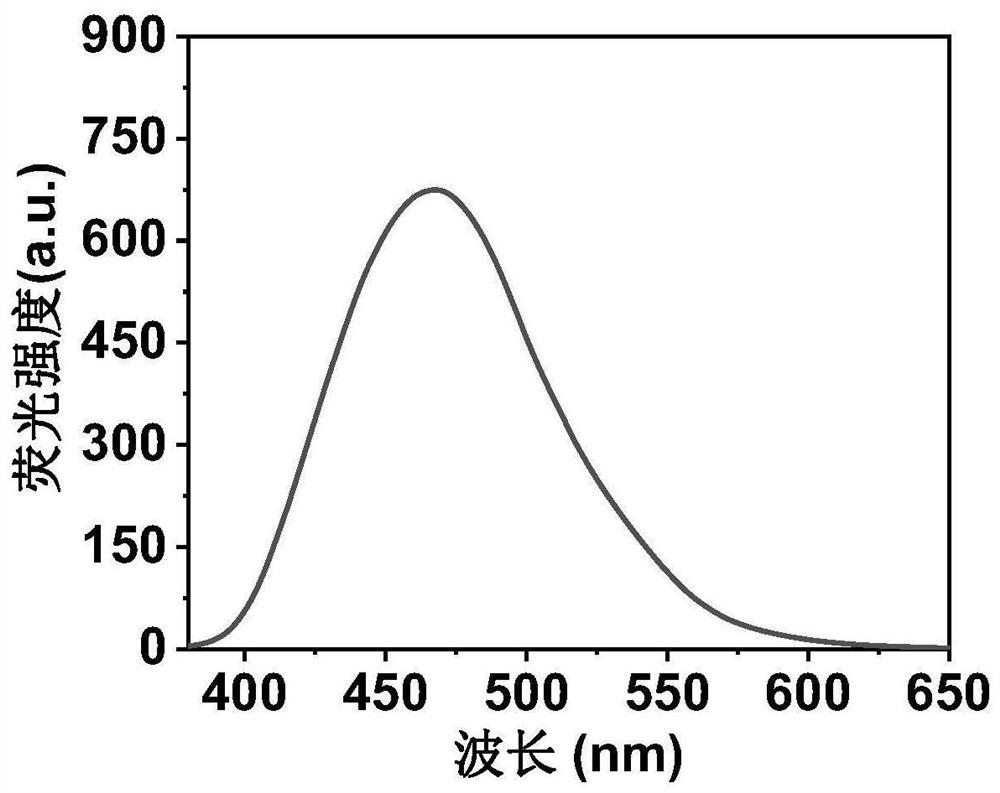
ActiveCN111849475AImprove dispersion compatibilityGood dispersionBiocideMaterial nanotechnologyBiologyCarbon dot
The invention discloses a nitrogen and sulfur co-doped carbon dot as well as a preparation method and application thereof. The preparation method comprises the steps: heating a carbon source and a nitrogen source in a solvent containing mercaptoglycerol to prepare the carbon dot. Sulfur-containing mercaptoglycerol is used as a sulfur source to participate in the reaction, and mercaptoglycerol canalso be used as a solvent of a reaction system, so that dispersion compatibility among various substances participating in the reaction is good, hydrophilic mercaptan is combined to carbon dot surfaceatoms, and dispersion of carbon dots in a polar solvent is stabilized at the same time. The nitrogen and sulfur co-doped carbon dots are prepared by adopting mercaptoglycerol as the sulfur source, sothat not only can copper ions be detected in a trace amount, but also tiopronin can be further detected, and therefore, the nitrogen and sulfur co-doped carbon dots can be used for constructing a dual-detection sensor capable of simultaneously detecting the contents of the copper ions and tiopronin. The carbon dots are also proved to have low toxicity and good biocompatibility, have differentialantibacterial ability to gram-positive bacteria (including drug-resistant bacteria) and gram-negative bacteria, and have the potential of treating biological bacterial infection.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
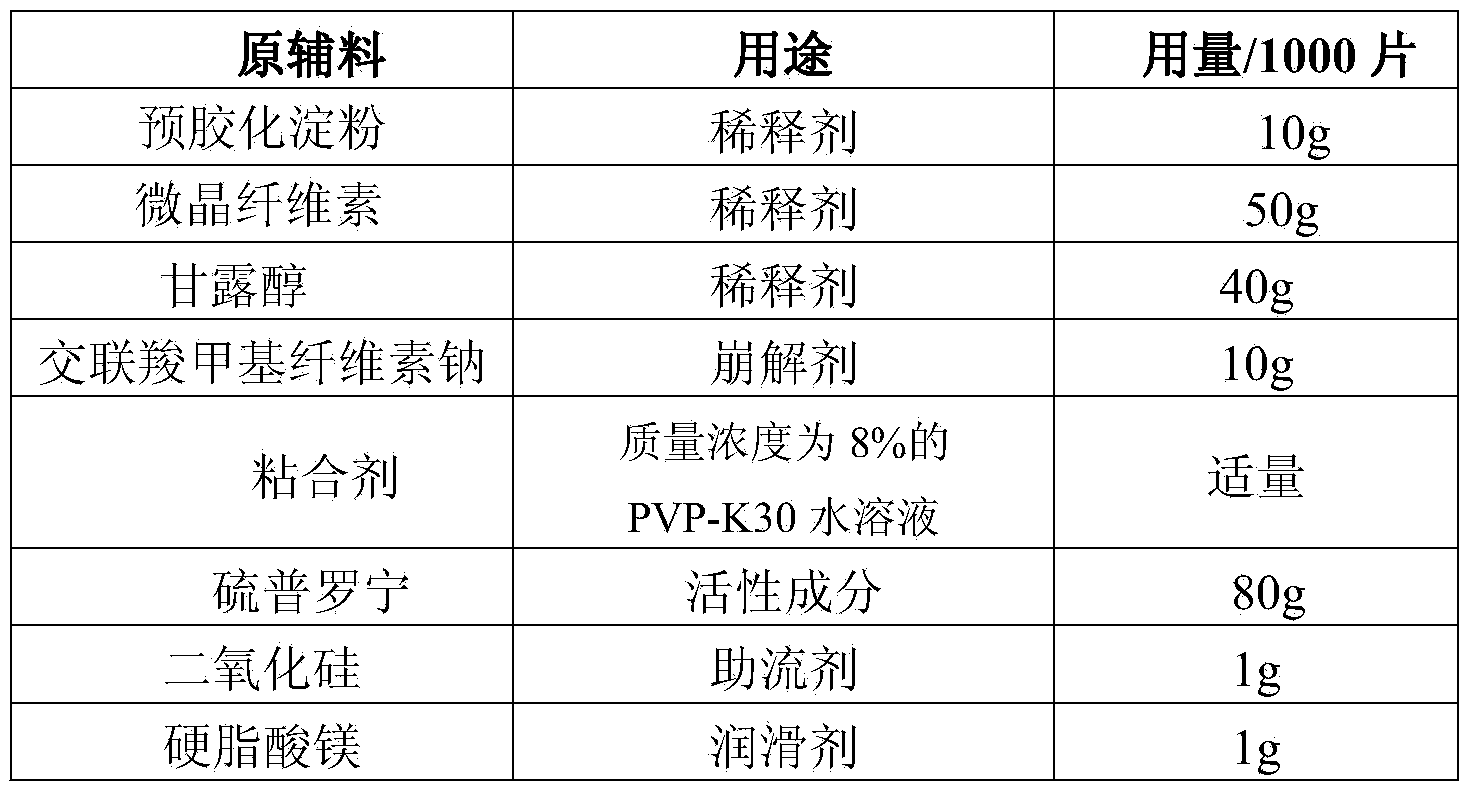
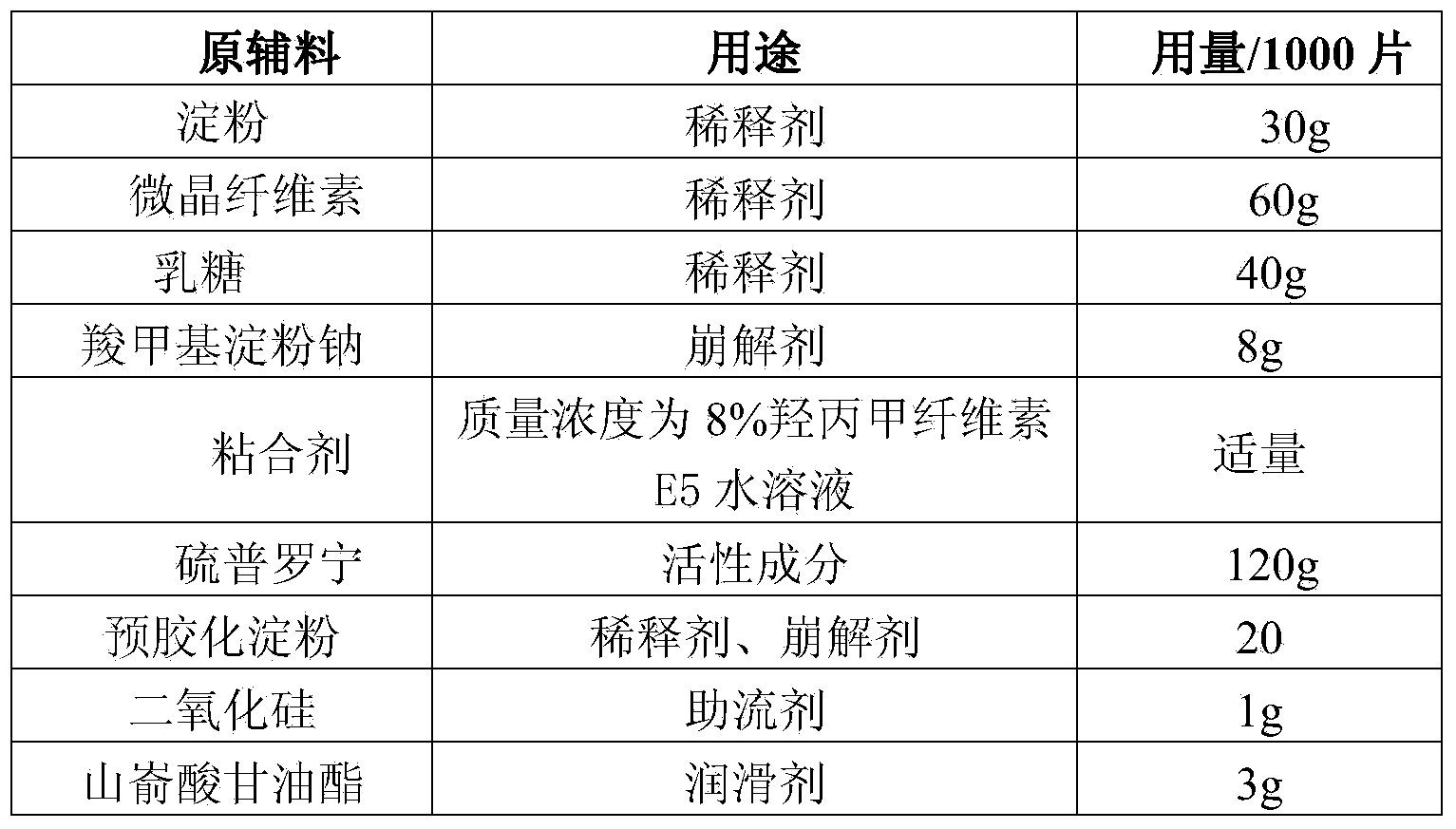
Preparation method of tiopronin tablets
InactiveCN103705482AReduce manufacturing costEasy to operatePeptide/protein ingredientsDigestive systemCoated tabletsDiluent
The invention relates to a preparation method of tiopronin tablets. The method comprises the following steps: (1) pelleting auxiliary materials, namely adding a diluent and a disintegrating agent into a binder, and pelleting the mixture by adopting a wet process; (2) drying and finishing pellets; (3) adding raw material tiopronin and additional auxiliary materials, and mixing; (4), tabletting to form tiopronin blank tablets; and (5) coating to form the tiopronin coated tablets. According to the preparation method of the tiopronin tablets, which is provided by the invention, the auxiliary materials, the diluent and the disintegrating agent are pelleted with the binder and are dried, and the raw material tiopronin and the additional auxiliary materials are added, mixed and tabletted, therefore, active ingredients in tiopronin can be protected against destroy due to wet and heat in a traditional wet method pelleting process, good stability of the tiopronin tablets is kept, and the problem that the traditional wet method pelleting process is not suitable for producing the tiopronin tablets is solved. The prepared tiopronin tablets are low in production cost, and the preparation operation is simple.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Method for preparing Tiopronin enteric-coated tablet
ActiveCN101590026AReduce specific odorSlight tastePeptide/protein ingredientsDigestive systemEngineeringEnteric coated tablets
The invention provides a method for preparing Tiopronin enteric-coated tablet. The method comprises the steps of preparing Tiopronin tablet, tablet wrapping isolation clothes, wrapping enteric coating and the like. The Tiopronin enteric-coated tablet produced according to the method of the invention features simple technique and low cost, and can effectively cover the odor of Tiopronin.
Owner:ベイジンカインバイオテックシーオーエルティーディー +1
Method for preparing and purifying tiopronin disulphide
InactiveCN102491926AReduce dosageResidue reductionOrganic compound preparationHydropoly/poly sulfide preparationBisulfidePurification methods
The invention relates to a method for producing and purifying tiopronin disulphide. The method comprises the following steps of: using hydrogen peroxide to oxidize tiopronin to prepare tiopronin disulphide; and using a chromatographic column with C18 as the filling material, and using 0.1% of formic acid aqueous solution which adopts 5-10% of acetonitrile by volume concentration ratio as mobile phase to purify. The tiopronin which is easy to purchase is used as material, and the hydrogen peroxide which is easy to remove is used as the oxidant in the method; the aqueous solution is only adopted in the preparation process, and 0.1% of formic acid aqueous solution which contains only 5-10% of acetonitrile, is primarily adopted in the purification process, so that the dosage of the organic solvent is a little, the environment pollution is reduced and the cost is reduced; simultaneously, residue organic solvent in the tiopronin disulphide reference substance is reduced; moreover, the whole process has simple process steps, is easy to operate with good reproduction quality.
Owner:青岛市药品检验所
Pronin medicinal composition and its preparing method
InactiveCN101062024AIncrease the amount of distributionGood curative effectOrganic active ingredientsPowder deliveryVitamin CAntioxidant
The invention discloses a pharmaceutical composition of tiopronin and its preparing process, wherein the composition is prepared from the following raw materials: tiopronin 100-300, anti-oxidizing agent 2-10. liposome 100-400, vitamin C 100-300, sodium glutamate 400-1000. The invention also provides the process for preparing the pharmaceutical composition.
Owner:HUNAN KANGDU PHARMA
Tiopronin soft capsule
InactiveCN1615835AImprove bioavailabilityLess irritating to the gastrointestinal tractOrganic active ingredientsDigestive systemDispersed mediaMetal chelate
The present invention relates to a kind of new preparation of hepatitis B treating medicine, and is especially hepatitis B treating soft Tiopronin capsule preparation suitable for being taken orally. The soft capsule contains physiologically effective Tiopronin and medicine carrier suitable for soft capsule, and the pharmaceutically acceptable carrier includes dispersing medium, antioxidant, metal chelate and / or medicine release regulating material.
Owner:上海华源医药科技发展有限公司 +1
Application of coptisine or coptisine salts in preparation of medicines for preventing and treating fatty liver damage
ActiveCN103622959AAvoid accumulationLower triglyceride levelsOrganic active ingredientsMetabolism disorderTG - TriglycerideLipid lowering
The invention discloses an application of coptisine or coptisine salts in the preparation of medicines for preventing and treating fatty liver damage. The pharmacological research proves that the coptisine has the effects of lowering lipid, improving lipid metabolism and alleviating the hepatic lesion caused by hepatic tissue fat accumulation and is capable of inhibiting hepatocyte triglyceride accumulation; for experimental hyperlipemia symptoms, the coptisine has the effects of obviously reducing triglyceride, cholesterol and LDL-C (low-density lipoprotein cholesterol) in blood and reducing the content of triglyceride in hepatic tissues; for fatty liver caused by the overuse of cortisol, the coptisine is capable of reducing the accumulation of triglyceride in the liver, the rise of activity of glutamic-pyruvic transaminase (GPT) and glutamic oxalacetic transaminase (GOT) and alleviating the hepatic lesion and has an excellent fatty liver prevention effect; part indexes of the coptisine are obviously superior to those of lipid reducing medicines, namely simvastatin and fenofibrate and liver protecting medicines, namely tiopronin and glucurolactone; the coptisine can be applied to clinical fatty liver diseases.
Owner:QINGDAO BAILI CAIXIN MEDICAL TECH CO LTD
Special ultrafine tiopronin powder lyophilized preparation and preparation method thereof
InactiveCN104163781AHigh clarityImprove stabilityOrganic active ingredientsPowder deliverySolubility2-Chloropropionic acid
The invention discloses a special ultrafine tiopronin powder lyophilized preparation and a preparation method thereof. The preparation method comprises the following steps: reacting 2-chloropropionic acid with phosphorus trichloride to generate 2-chloropropionyl chloride, reacting 2-chloropropionyl chloride with glycine to generate 2-chloropropionyl glycine, reacting 2-chloropropionyl glycine with sodium disulfide, reducing by zinc powder to obtain crude tiopronin, purifying, carrying out air jet ultrafine crushing, and lyophilizing to prepare the special ultrafine tiopronin powder lyophilized preparation. The special ultrafine tiopronin powder lyophilized preparation has the advantages of good clarity, high stability, high purity, few impurities, small particle size, large specific surface area, good solubility, small toxic side effects, difficult allergy and the like.
Owner:杭州长典老一元健康管理有限公司
Derivatives of erythromycin, clarithromycin, roxithromycin or azithromycin with antibiotic and mucolytic activity
A pharmaceutical with an enhanced pharmaceutical profile comprises a mucolytic and an antibiotic in which the mucolytic is present in an amount of greater than one molar equivalent of the antibiotic. The antibiotic may be selected from Erythromycin, Roxithromycin, Clarithromycin, Azithromycin, Dirithromycin; and pharmaceutically acceptable salts or esters thereof. The mucolytic is a mucolytically active thiol, especially N-acetylcysteine, mercaptoethanesulfonic acid, tiopronin or methylcysteine. The adducts can be isolated via a simple and efficient process.
Owner:RUSSINSKY
Stable solution containing tiopronin for injection
InactiveCN1704049AGuaranteed stabilityImprove stabilitySenses disorderPeptide/protein ingredientsBENZYL ALCOHOL/WATERAcidity regulator
Owner:合肥霄云科技有限公司
Tiopronin amino salt, and its preparing method
This invention relates tiopronin amino acid salt used to cure chronic hepatitis and its preparation method. Its constitutional formula is shown as follow; R is 1-20 carbon atoms straight chain or branched chain alkyl radical. This invention solves the problem of tiopronin oxidation degradation in water, and volatilization decomposition in heat. The tiopronin in this invention is prepared into amino acid salt, it prevent the tiopronin combining with oxygen, and its oxidation. Medical using substance melting point is improved, so its thermal stability is greatly improved, so its medical effect as effective constituent is greatly improved.
Owner:WUHAN WUYAO PHARMA
Paclitaxel/multialkene paclitaxel liposome composite medicine and preparation method thereof
InactiveCN100586430CSmall doseSmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsSide effectVitamin C
Owner:HUNAN KANGDU PHARMA
Frozen powder injection of tiopronin
A freeze-dried powder injection of tiopronin is prepared from tiopronin and the excipient chosen from amino acid, the salt of amino acid, and phosphate through proportionally and respectively dissolving them in the water for injecting, mixing, stirring, sterilizing, pouring in containers and freeze drying.
Owner:江西欧氏药业有限责任公司
Tiopronin injection and preparation method thereof
ActiveCN1698595ASolve the existing technology deficiencies of poor stabilityImprove stabilityOrganic active ingredientsDigestive systemState of artAnalytical chemistry
Owner:CISEN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com