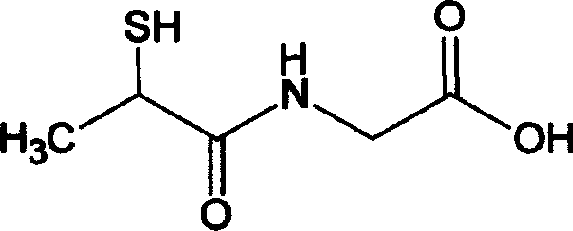
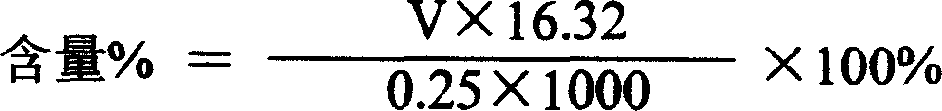Tiopronin injection and preparation method thereof
A technology of tiopronin and injection, which is applied in the field of medicine and can solve problems such as influence on drug application, danger, harsh production conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Take 100g of tiopronin, 0.1g of calcium sodium edetate as auxiliary material, and 2000ml of water for injection; nitrogen gas is passed through the water for injection for 30 minutes, and set aside. Add all the tiopronin into the prepared water for injection that has been purged with nitrogen, stir to dissolve, add all the auxiliary materials edetate calcium sodium, stir to dissolve; adjust the pH value of the above mixed solution to 5 with sodium hydroxide solution Add 0.02% (g / ml) activated carbon for needles according to the liquid volume, stir under nitrogen for 20 minutes, filter with a 0.45 μm microporous membrane until the filtrate clarity meets the requirements of the Chinese Pharmacopoeia 2005 edition; take the conventional process Fill the cleaned ampoule with the filtrate. After filling, pass nitrogen gas into the ampoule until the air in the ampoule is emptied, and seal it quickly after ventilating; sterilize the sealed ampoule at 100°C for 15 minutes under t...
Embodiment 2
[0065] Get tiopronin 150g, adjuvant calcium sodium edetate 0.1g, water for injection 2000ml, other steps are with embodiment 1.
Embodiment 3
[0067] Get tiopronin 80g, auxiliary material edetate disodium 0.1g, water for injection 2000ml, other steps are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com