Patents
Literature
120results about How to "Expand clinical application" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aptamer-based methods for identifying cellular biomarkers
InactiveUS20090117549A1Easy to fixEasy to synthesizeElectrolysis componentsParticle separator tubesBiotin-streptavidin complexCancer cell
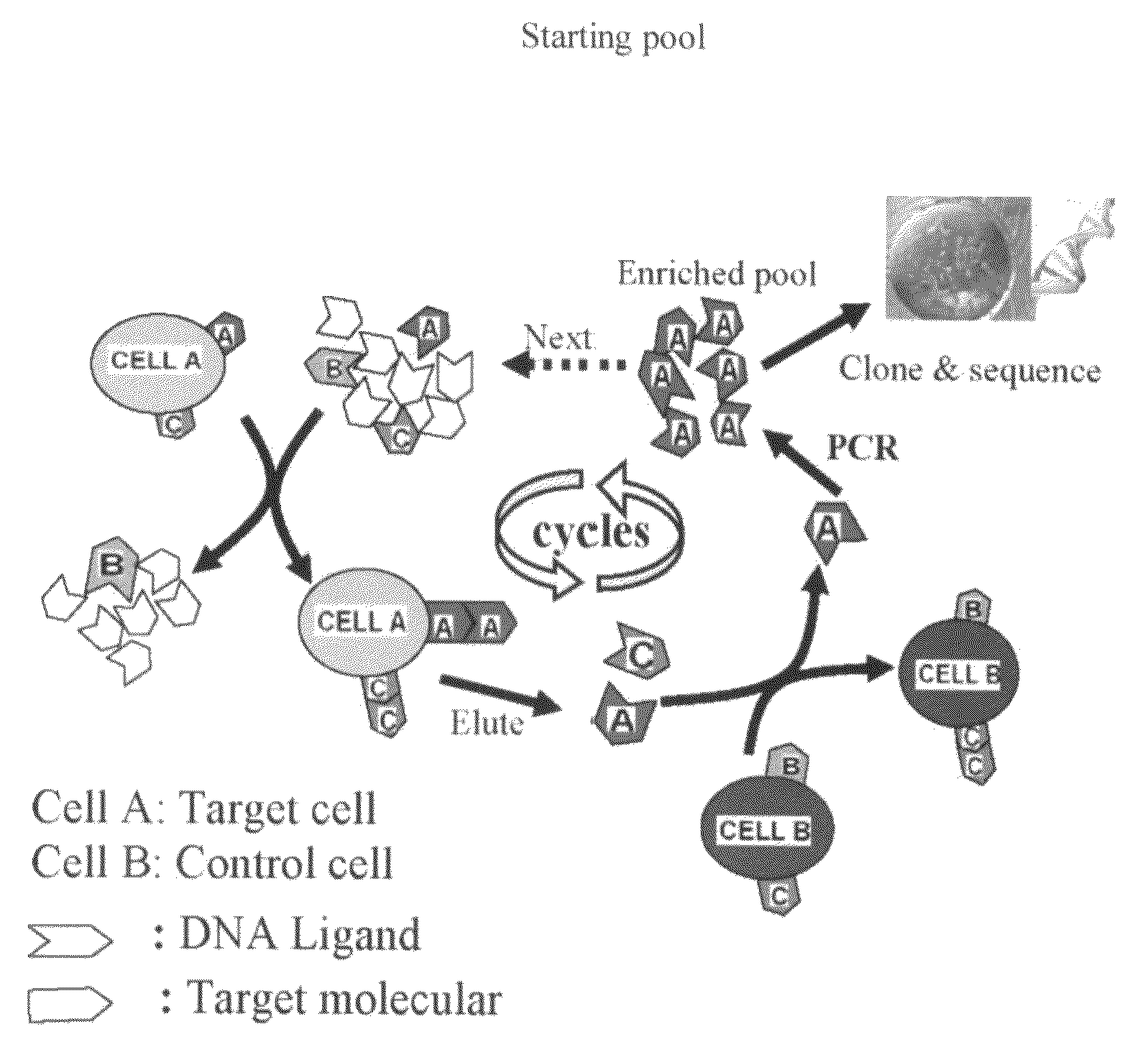
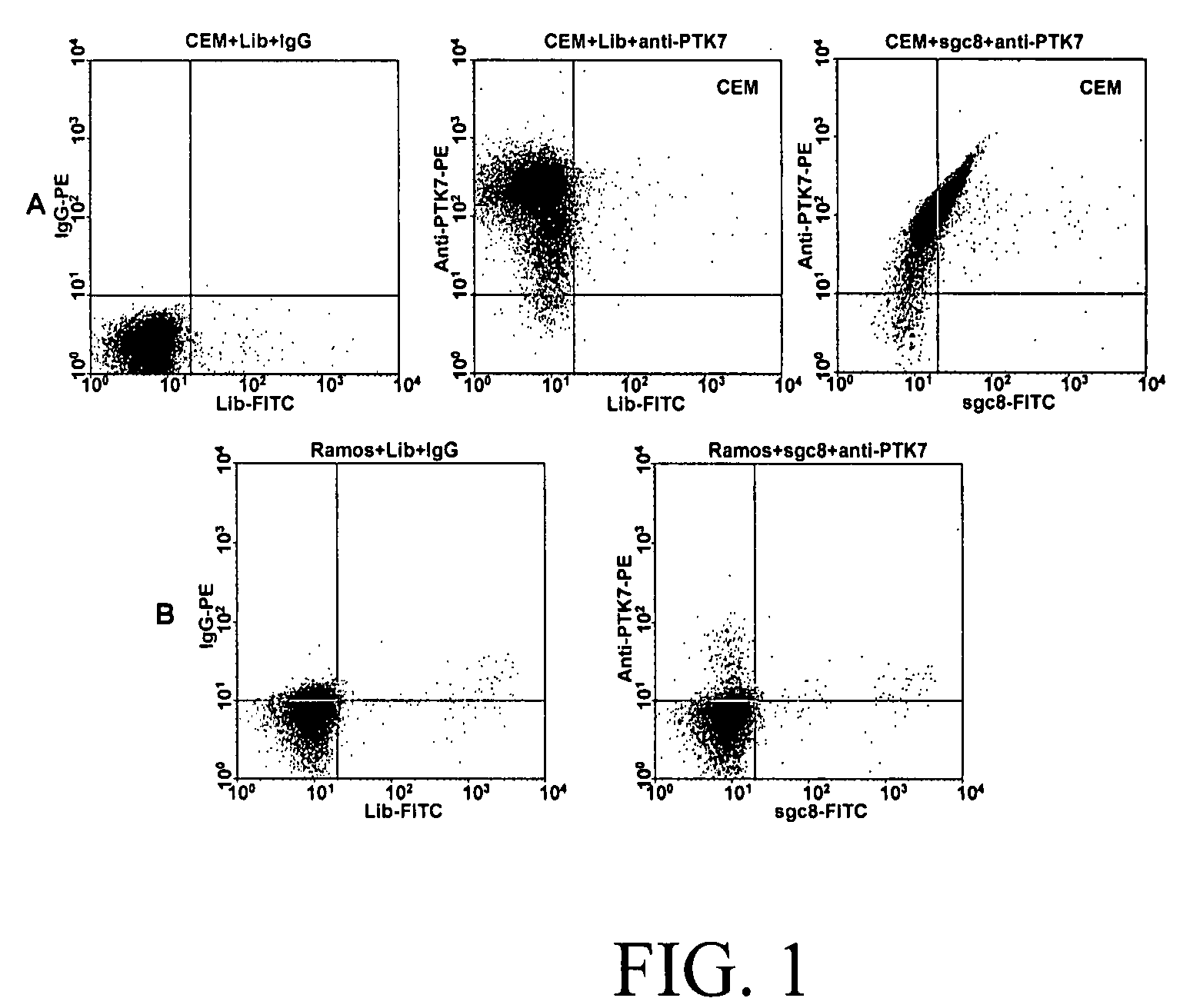
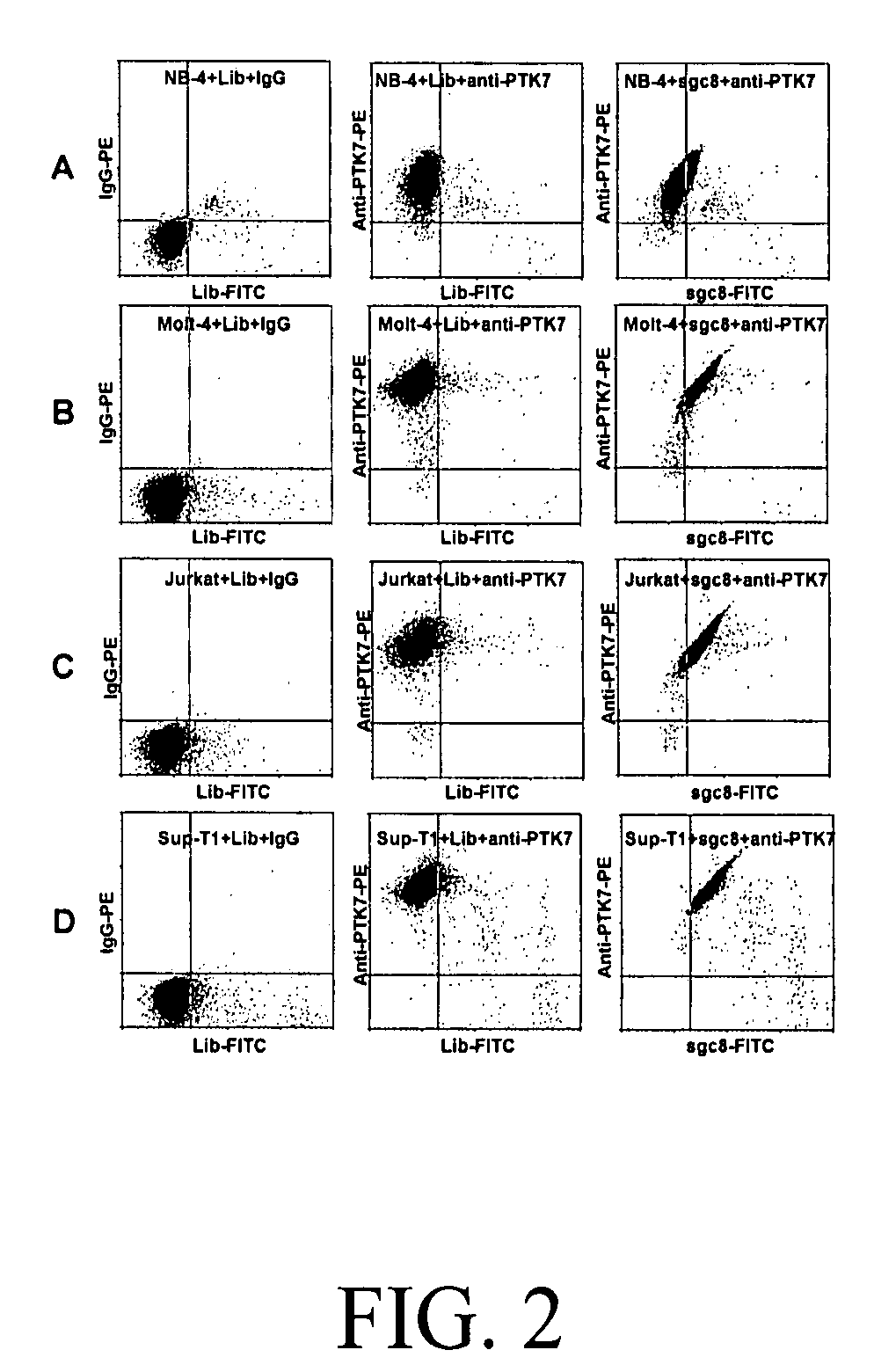
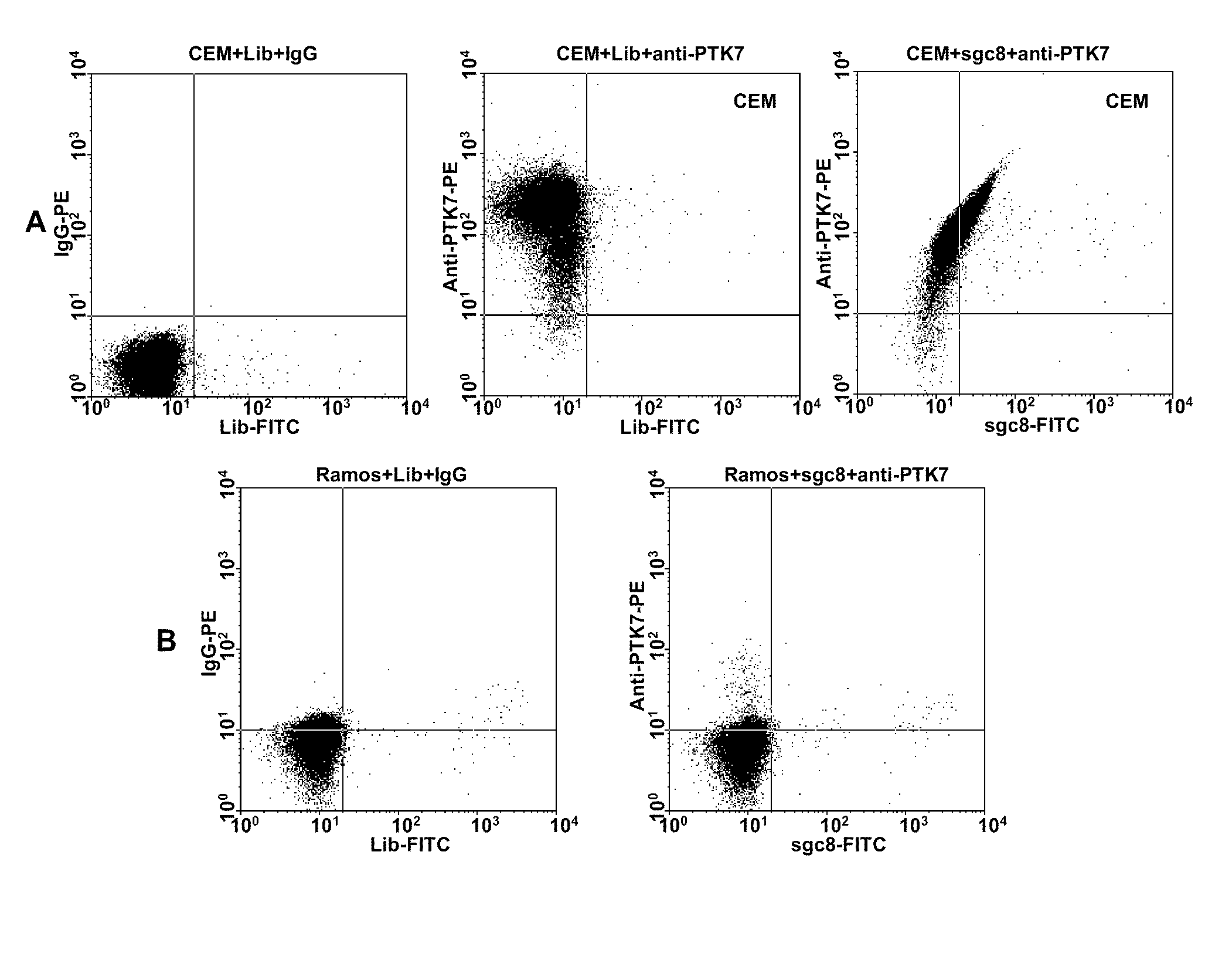
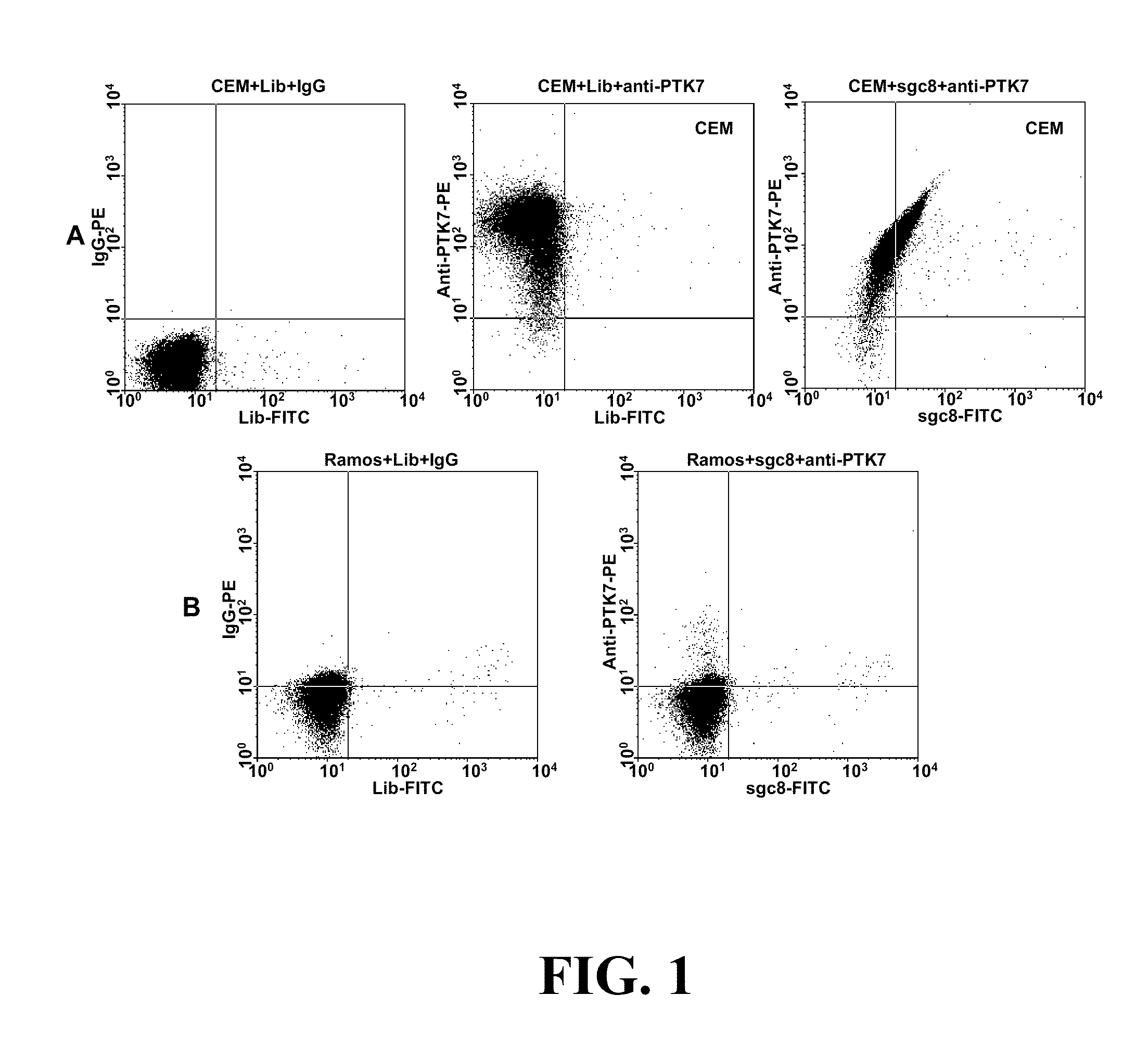
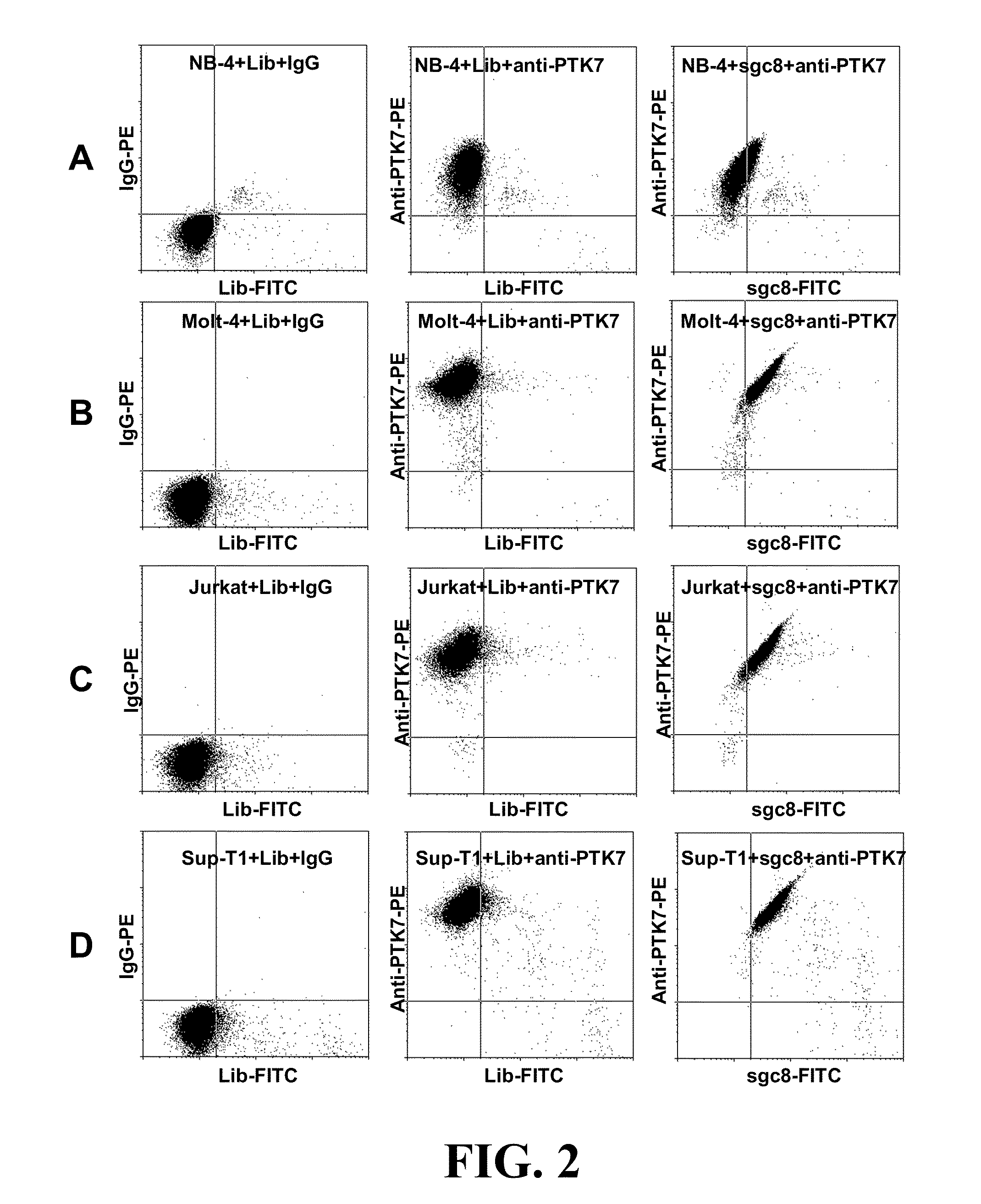
In this invention, a biomarker discovery method has been developed using specific biotin-labeled oligonucleotide ligands and magnetic streptavidin beads. In one embodiment, the oligonucleotide ligands are firstly generated by whole-cell based SELEX technique. Such ligands can recognize target cells with high affinity and specificity and can distinguish cells that are closely related to target cells even in patient samples. The targets of these oligonucleotide ligands are significant biomarkers for certain cells. These important biomarkers can be captured by forming complexes with biotin-labeled oligonucleotide ligands and collecting the complexes using magnetic streptavidin beads, whereupon the captured biomarkers are analyzed to identify the biomarkers. Analysis of biomarkers include HPLC-Mass Spectroscopy analysis, polyacrylamide gel electrophoresis, flow cytometry, and the like. The identified biomarkers can be used for pathological diagnosis and therapeutic applications. Using the disclosed methods, highly specific biomarkers of any kinds of cells, in particular cancer cells, can easily be identified without prior knowledge of the existence of such biomarkers.
Owner:TAN WEIHONG +1
Full-automatic nucleic acid extraction and PCR amplification micro-fluidic chip and application method thereof
ActiveCN105316224AImprove reliabilityHigh yieldBioreactor/fermenter combinationsBiological substance pretreatmentsSiphonCentrifugal force
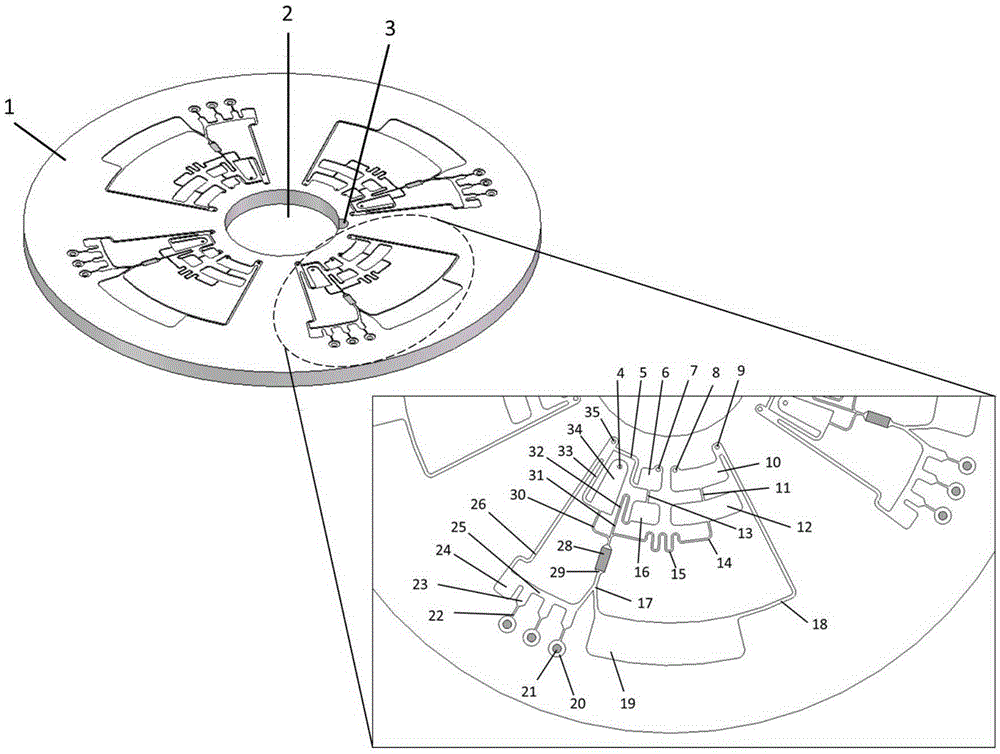
The invention provides a full-automatic nucleic acid extraction and PCR amplification micro-fluidic chip which comprises at least one main body, wherein the main body is distributed in equal distance around the chip; the main body comprises a nucleic acid extraction unit, a PCR amplification unit, a waste liquid unit and an exhaust unit, wherein the nucleic acid extraction unit is positioned on the main body and is used for extracting, washing and eluting nucleic acid in sequence under the driving of centrifugal force and capillary force; the PCR amplification unit is adopted for PCR reaction amplification; the waste liquid unit comprises a waste liquid cavity which is used for storing a waste liquid; the exhaust unit comprises exhaust holes and a plurality of exhaust channels; and the plurality of exhaust channels are communicated with the nucleic acid extraction unit, the PCR amplification unit and the waste liquid unit and are used for exhausting air. The invention discloses a micro-fluidic chip for achieving full-automatic nucleic acid extraction and PCR reaction through centrifugal force, capillary force and siphon phenomenon, the micro-fluidic chip has no integrated equipment such as pumps or valves, so that the manufacturing difficulty and cost of the chip are greatly reduced, and moreover the reliability of the chip is improved.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Medical device for recovering targeted components of cavity content
ActiveCN103330961ABenefit from Transplant TreatmentImprove positive diagnosis rateSuction devicesIntestinal structureSlag
The invention belongs to the field of medical devices, and relates to a medical device for recovering targeted components of a cavity content. The device recovers the targeted components of the human body cavity content in vitro. The device can be used for greatly preparing a fecal bacterium solution with parasitic ova removed to support a preparation procedure of standardized fecal bacterium transplantation, used for greatly collecting the parasitic ova in a fecal suspension to increase a positive detection rate of the parasitic ova, used for collecting deslagged secretions in intestine and stomach cavities by an endoscope to meet requirements of checking and diagnosis, used for collecting lavage liquid in a trachea and removing slag by the endoscope to meet requirements of a hormone test and cytological examination, and used for collecting liquid of biliary and pancreatic ducts and a urogenital canal to meet requirements of the checking and the cytological examination. The method solves the practical problems of clinical applications, and has important innovation and an application value.
Owner:NANJING FMT MEDICAL
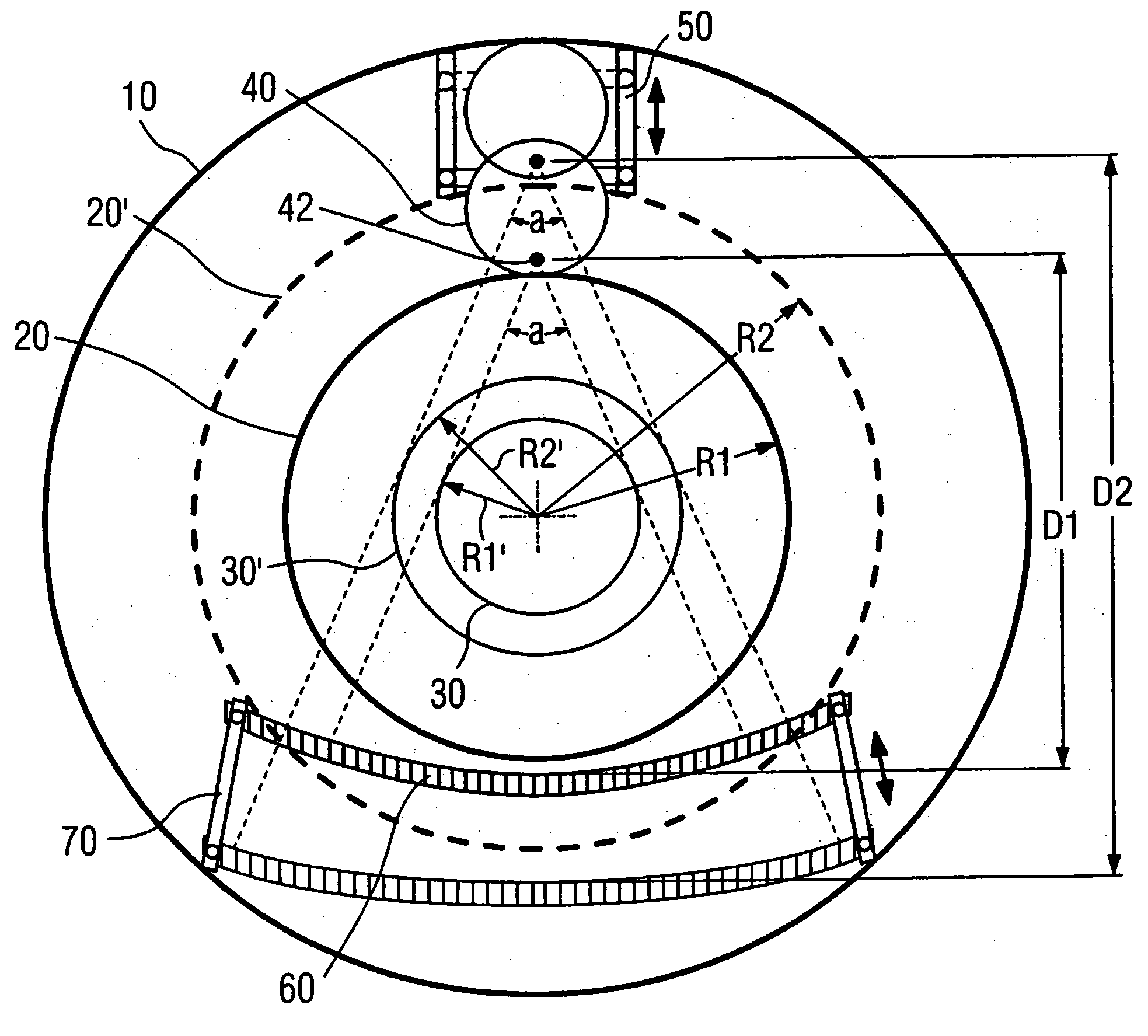
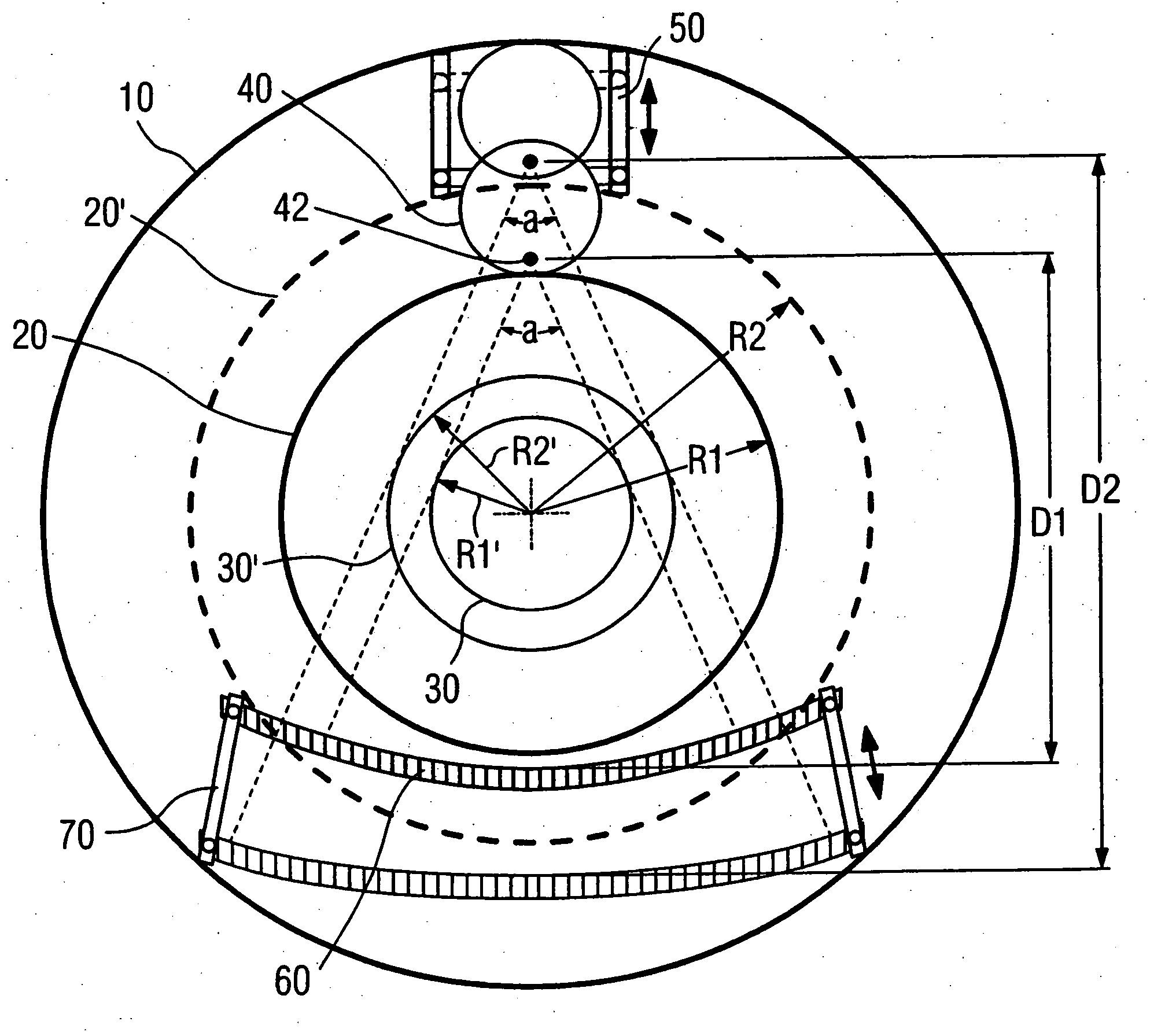
Computed tomography system with adjustable focal spot-to-detector distance
InactiveUS20060222143A1Improve accessibilityIncrease the diameterMaterial analysis using wave/particle radiationRadiation/particle handlingComputed tomographyX-ray
A computed tomography system with an adjustable focal spot-to-detector distance has a gantry provided with a patient opening, an x-ray tube and a detector, respectively mounted at opposite sides of the gantry. The x-ray tube has a focal spot and the x-ray fan beam radiated from the focal spot exhibits a fixed aperture angle. The x-ray tube is installed on a linear track and can be moved along the rail of the linear track.
Owner:SIEMENS HEALTHCARE GMBH
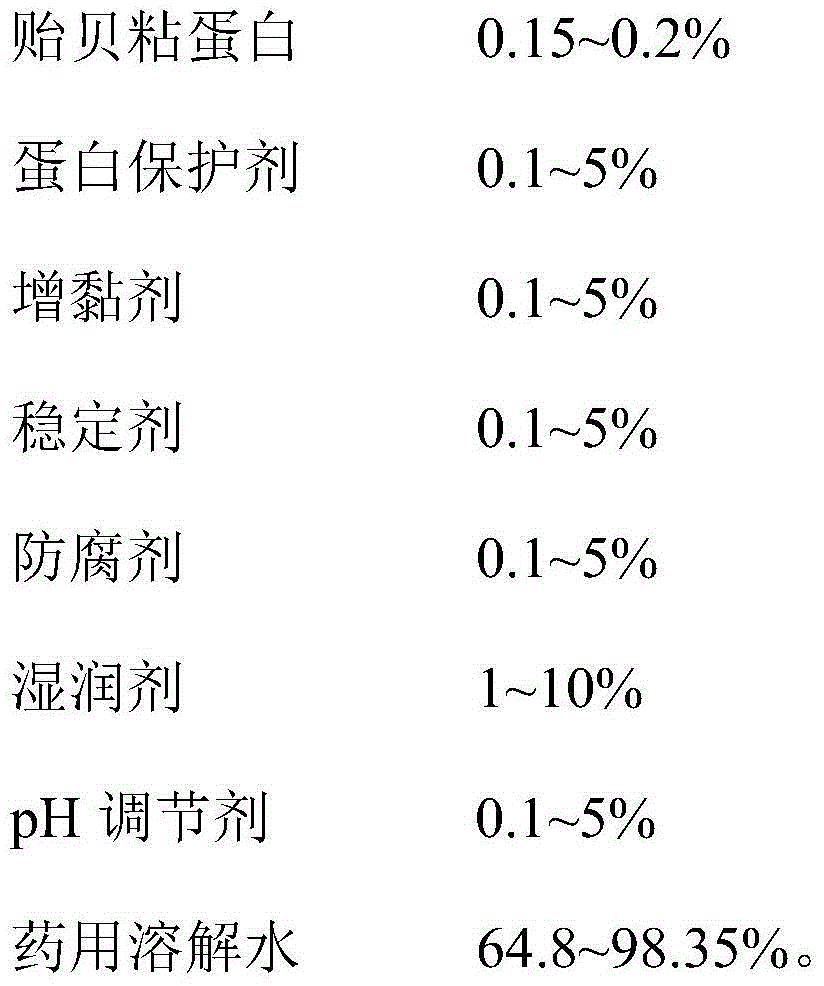
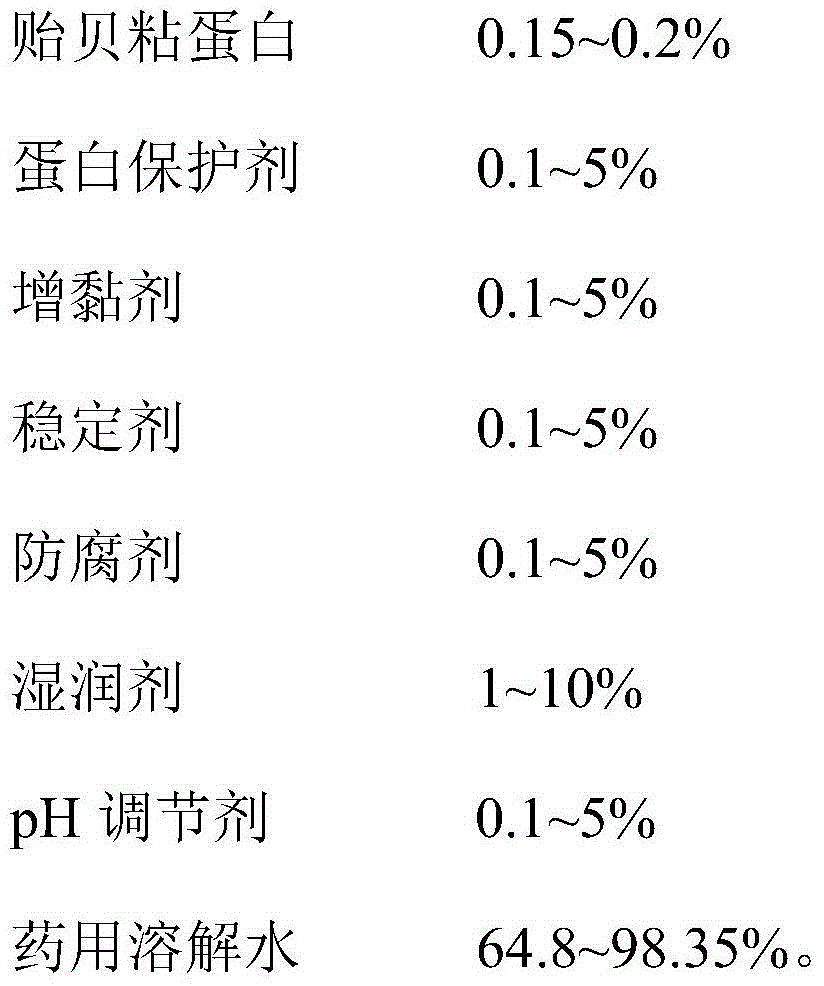
Mussel mucoprotein gel for wound repair, and preparation method and application thereof
InactiveCN104645320ABroaden applicationGood adhesionPeptide/protein ingredientsAerosol deliveryIrritationPreservative
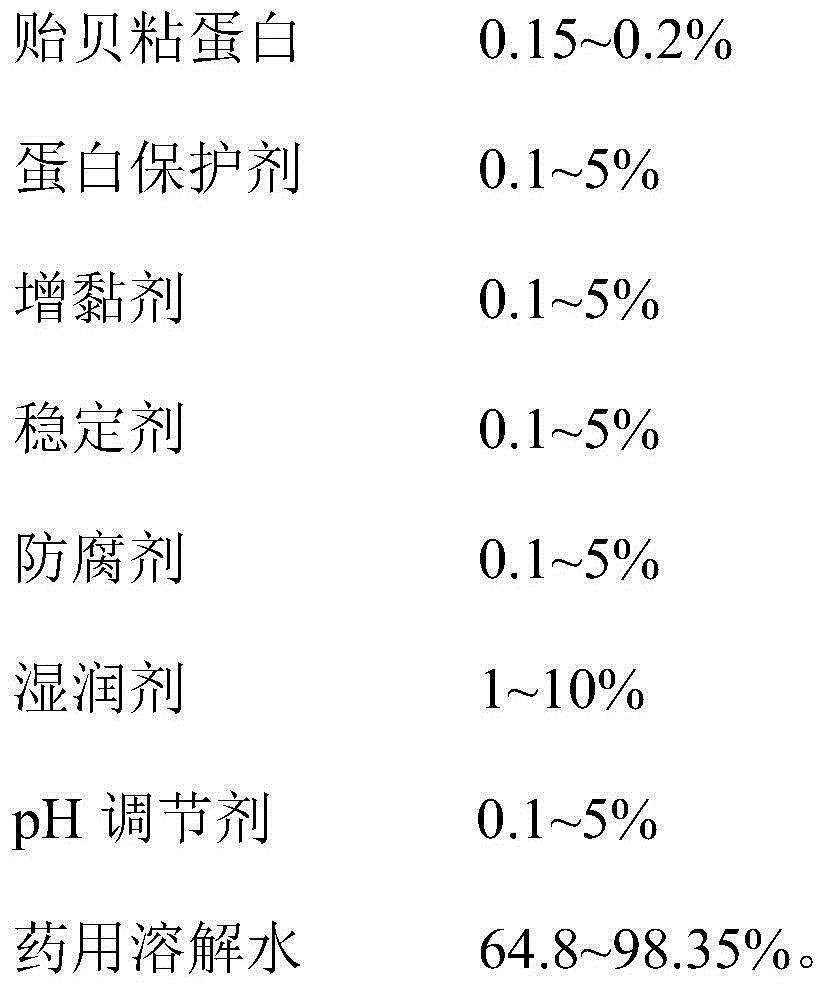
The invention provides a mussel mucoprotein gel for wound repair, and a preparation method and an application thereof. The mussel mucoprotein gel for wound repair comprises the following raw materials in percentage by weight: 0.15-0.2% of mussel mucoprotein, 0.1-5% of protein protector, 0.1-5% of tackifier, 0.1-5% of stabilizer, 0.1-5% of preservative, 1-10% of wetting agent, 0.1-5% of pH (potential of hydrogen), and 64.8-98.35% of medical dissolving water. With Carbomer as a substrate, the mussel mucoprotein gel has good extending property, is easy to coat and adhere on a skin, has no irritation to skin and mucosa and can absorb tissue penetrating fluid, so secretions can be exhausted conveniently; the gel is not greasy, the medicines are released fast, and the coupling effect with skin tissues is excellent.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Metabonomics-based early diagnosis marker for chronic obstructive pulmonary disease and application thereof
InactiveCN111289736AExpand clinical applicationHigh promotional valueComponent separationDisease diagnosisDihydrouracilDimethylaniline N-oxide
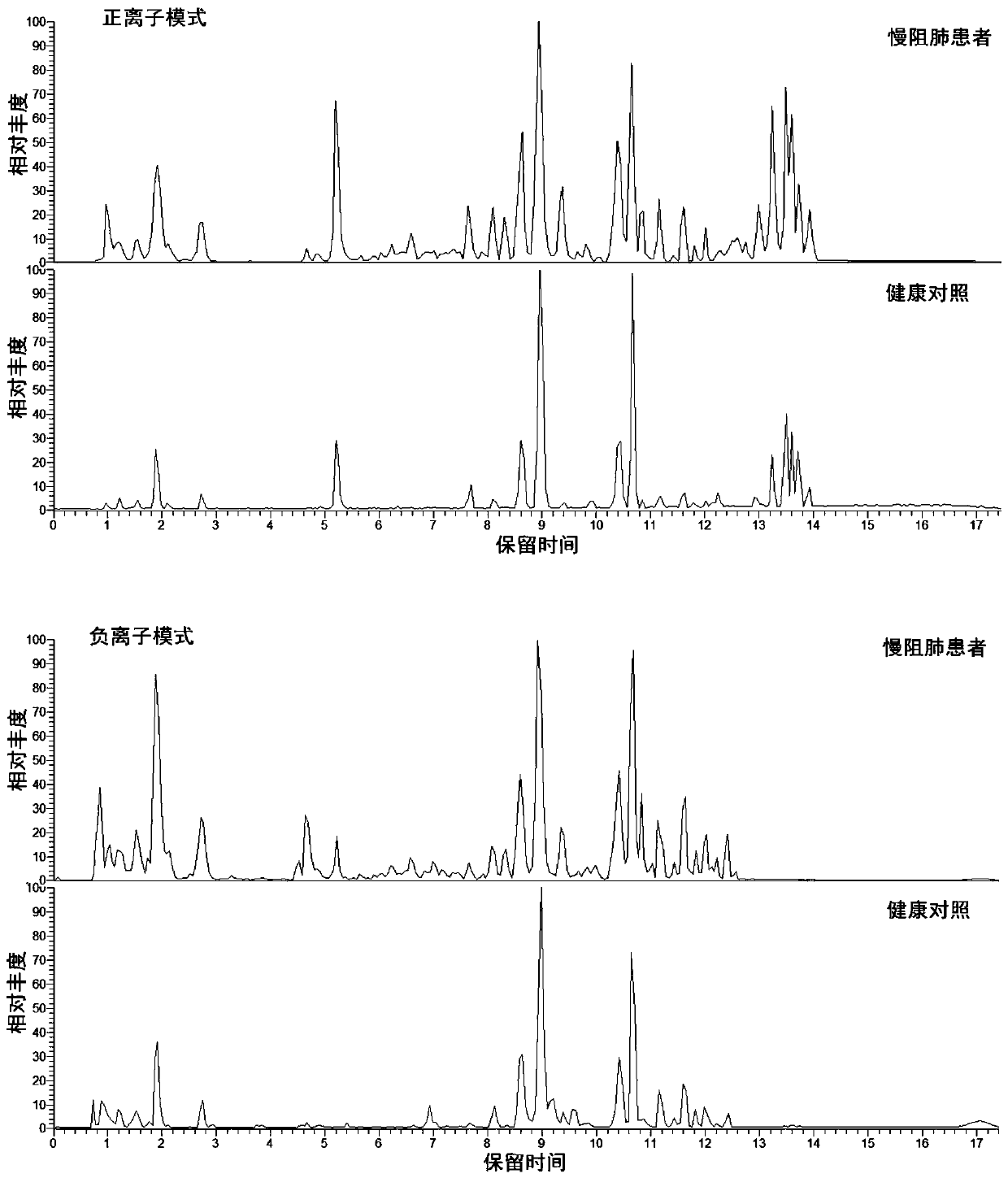
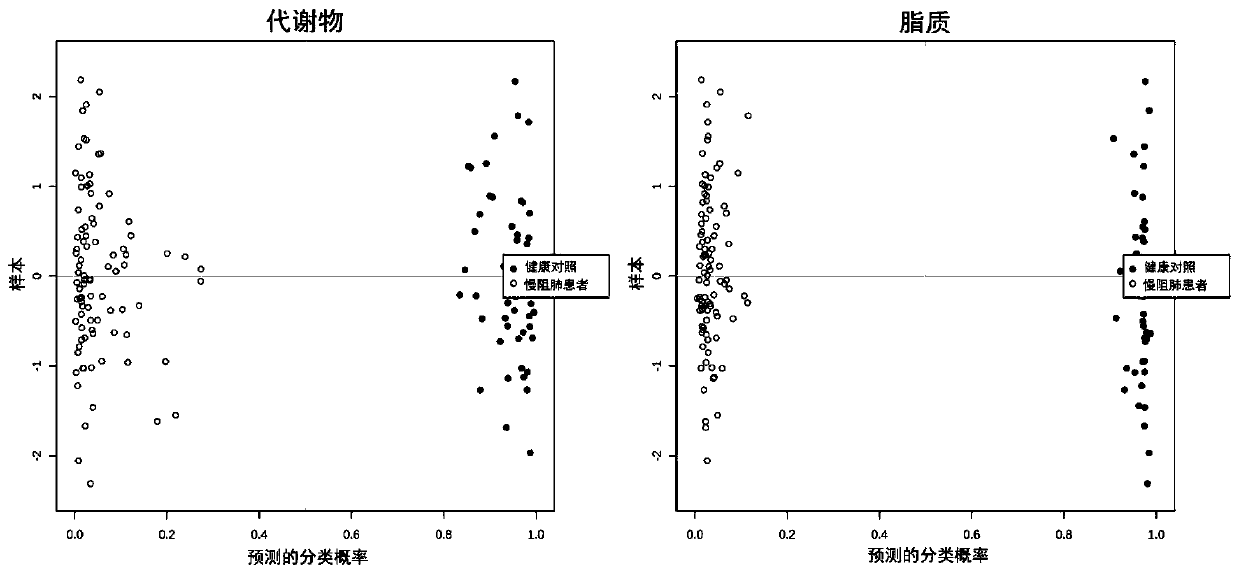
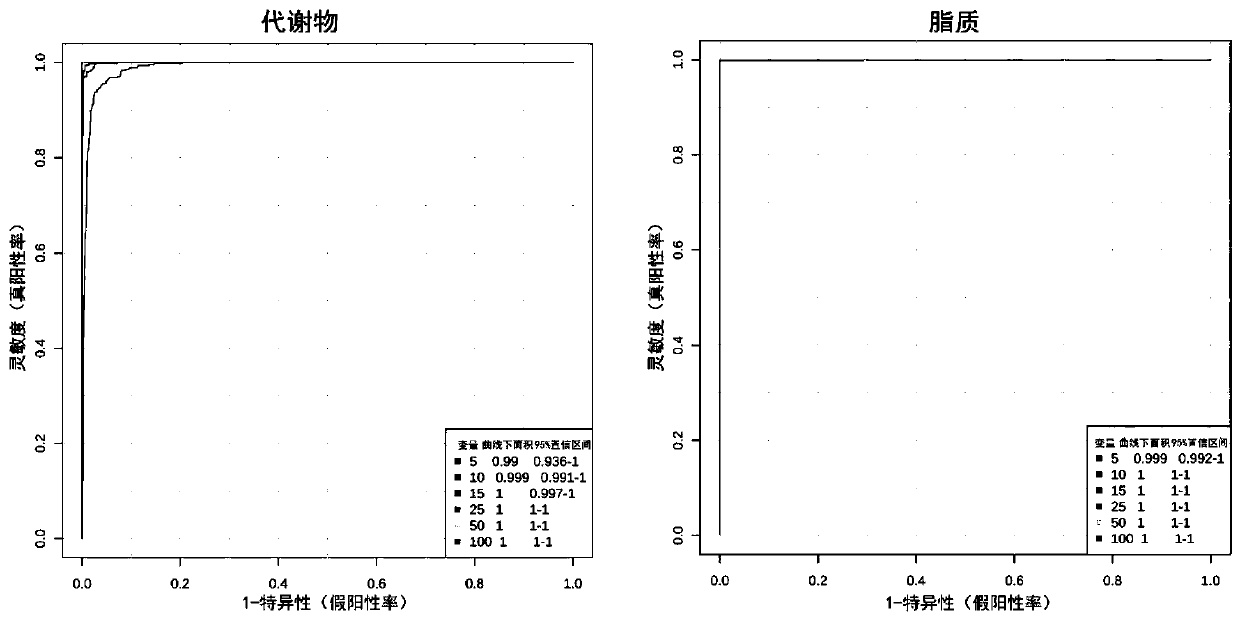
The invention discloses a metabonomics-based diagnosis marker for chronic obstructive pulmonary disease and application thereof. The diagnosis marker comprises the following 28 blood plasma metabolismmarkers: phosphatidylcholine PC 16:1-36:3, phosphatidylcholine PC 26:0-22:4, phosphatidylcholine PC 26:0-22:3, phosphatidylcholine PC 47:5e, phosphatidylcholine PC 44:11e, phosphatidylcholine PC 16:0-24:5, phosphatidylcholine PC 20:2-20:3, phosphatidylcholine PC 40:10, phosphatidylcholine PC 38:9e, phosphatidylcholine PC 18:1-20:4, phosphatidylcholine PC 16:0-20:3, phenylacetaldehyde, dihydrouracil, nicotinamide, 4-methoxycinnamic acid, ketovaline, threonine, DL-3-aminoisobutyric acid, pyruvic acid, stachyose, caffeic acid, N, N-dimethylaniline, maltotriose, D(-)-gulonate-gamma-lactone, and L-asparaginase. The marker provided by the invention has good classification on metabolome data of patients suffering chronic obstructive pulmonary disease and healthy people, and can accurately distinguish the patients suffering chronic obstructive pulmonary disease from the healthy people.
Owner:PEKING UNIV +1
Aptamer-based methods for identifying cellular biomarkers
InactiveUS20110124015A1Easy to fixEasy to synthesizeElectrolysis componentsParticle separator tubesBiotin-streptavidin complexAptamer
Owner:TAN WEIHONG +1
Calf serum protein-removing extract for injection and its preparation method
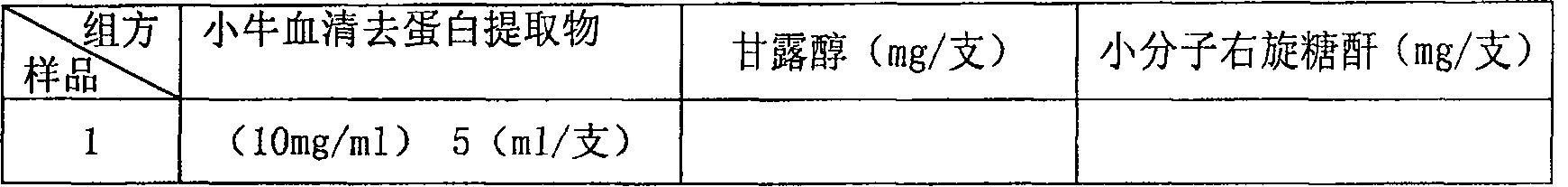
ActiveCN100531723CDelicate appearanceImprove product qualityPowder deliveryMammal material medical ingredientsMannitolDextran
The invention discloses a calf serum deproteinized extract freeze-dried powder injection and a preparation method thereof. The calf serum deproteinized extract freeze-dried powder injection contains the following components by weight and ratio: 1 part of dry matter of calf serum deproteinized extract; 9-11 parts of mannitol; and 4.5 parts of small molecule dextran -5.5 parts. The deproteinized calf serum extract freeze-dried powder injection of the present invention overcomes the defects of unstable water injection and infusion quality, prolongs the valid period of the product, expands clinical application, and reduces the probability of adverse reactions.
Owner:JINZHOU AHON PHARM CO LTD
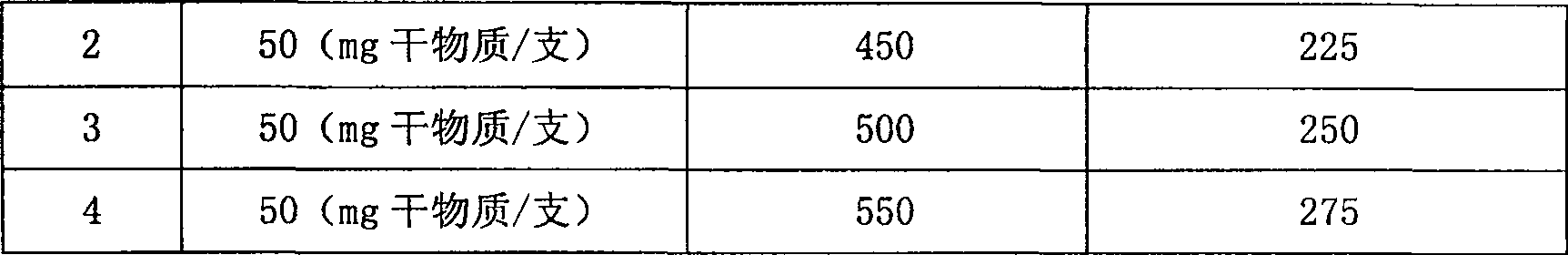
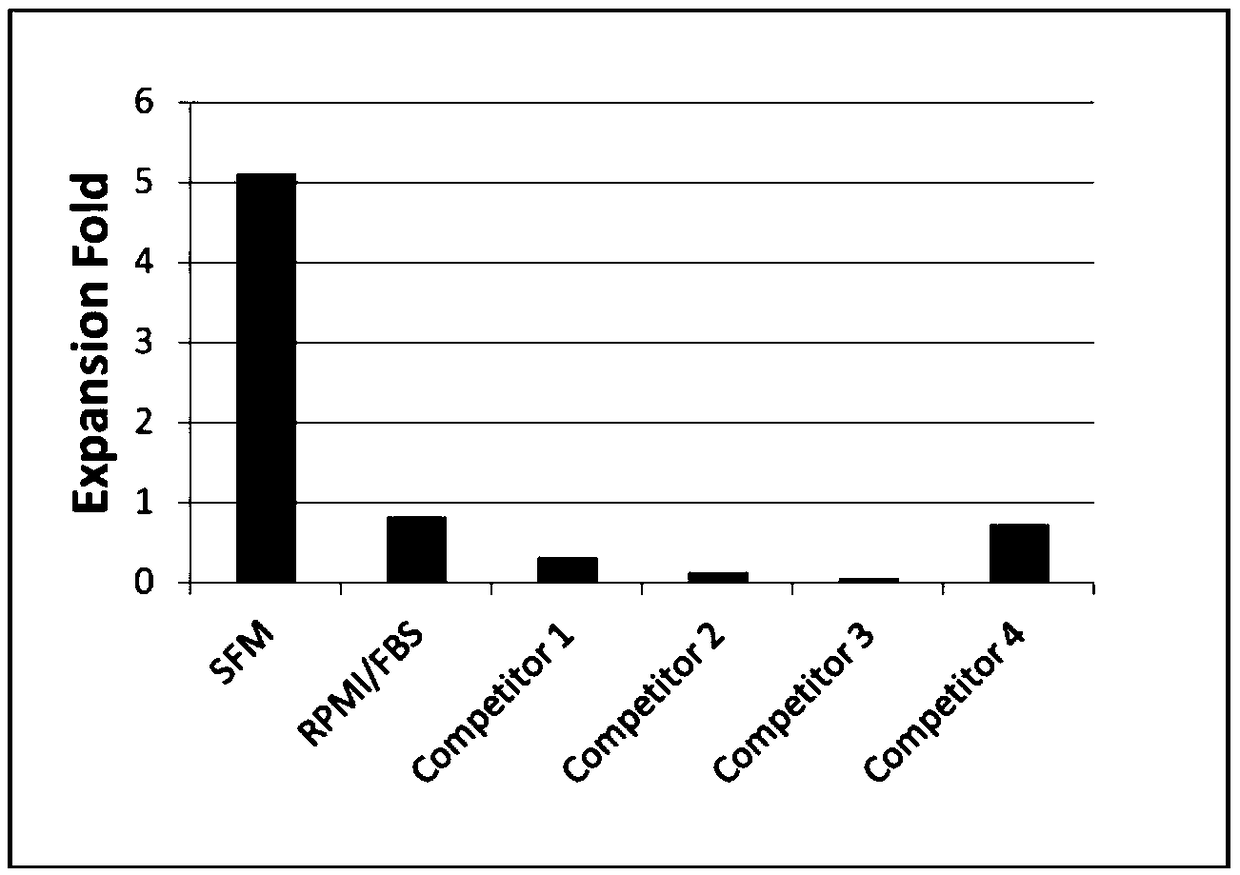
T cell serum-free medium and using method thereof
InactiveCN108642006AStrong PH buffering capacityHigh in nutrientsCulture processBlood/immune system cellsSerum free mediaVitamin C
The invention belongs to the field of culture mediums, and in particular relates to a T cell serum-free medium and a using method thereof. The T cell serum-free medium consists of a basal culture medium applied to growth culture of cells, as well as the following addition ingredients: ethanolamine, copper sulfate, ferric nitrate, zinc sulfate, sodium selenite, sodium pyruvate, insulin, transferrin, glutamine, serum albumin, thioglycerol and L-vitamin C. The T cell serum-free medium provided by the invention breaks through shortcomings of the prior art, and according to the T cell serum-free medium, the entire medium system is free from the introduction of non-human proteins, and the T cells in the blood sample, which is safe and reliable in source, undergo efficient selective amplification; and therefore, higher clinical application and scientific research values are achieved.
Owner:安徽中盛溯源生物科技有限公司
Atractylone lipidosome and preparation method thereof
InactiveCN101703549ANarrow and uniform particle size distributionExpand clinical and application valueOrganic active ingredientsDigestive systemSolventChemistry
The invention discloses an atractylone lipidosome containing atractylone and a lipidosome membrane material, wherein the atractylone is an Atractylis ovata extract, and the lipidosome membrane material is phosphatidylcholine or cholesterol. A method for preparing the atractylone lipidosome by adopting a supercritical fluid dispersing precipitation technology comprises the following steps of: (1) dissolving the atractylone and the lipidosome membrane material in a supercritical CO2 / alcohol mixed solvent, then obtaining a supercritical solution; (2) dissolving a surface active agent as a stabilizer in an aqueous medium, then obtaining a stabilizer solution; (3) and rapidly jetting the supercritical solution to the stabilizer solution at a certain pre-swelling pressure and pre-swelling temperature, and then obtaining an atractylone lipidosome mixed suspension by forcible dispersion and precipitation. The preparation process of the lipidosome is completed in the chemically inert CO2 medium; toxic organic solvents are not used, and the envelop rate of the lipidosome is improved, thereby effectively avoiding the agglomeration phenomenon caused by colliding among lipidosome particles, and ensuring the stability of the atractylone lipidosome in the preparation process.
Owner:SHENZHEN UNIV
Externally applied liquid asiaticoside preparation and its preparing process
InactiveCN1857291AGood curative effectQuick resultsOrganic active ingredientsDermatological disorderCentella asiatica extractMedicine
The externally applied liquid asiaticoside preparation has effective component comprising asiaticoside 0.1-30.0 wt% and medicinal solvent 70.0-99.9 wt%. Its preparation process includes dissolving asiaticoside in the medicinal solvent, filtering, filling, sterilizing and packing. The present invention expands the clinical application of asiaticoside, and the abacterial preparation has high medicine concentration, high medicinal effect, no wound infection, and other advantages.
Owner:浙江茵诺邦健康科技有限公司
Visual laser therapeutic apparatus with dot matrixes
ActiveCN104921805AClear visionRealize visual operationDiagnostics using lightSurgical needlesPhysicsLaser scanning
The invention discloses a visual laser therapeutic apparatus with dot matrixes. The visual laser therapeutic apparatus comprises a positioning insertion tube, a beam combination mirror assembly, a camera, a laser scanning assembly and a control system. The positioning insertion tube is a hollow tube and is used for positioning lesion positions and defining laser paths, and openings are formed in two ends of the hollow tube; the beam combination mirror assembly is a hollow tube, openings are formed in the hollow tube, a side opening is formed in a side surface of the beam combination mirror assembly, and one end of the beam combination mirror assembly is connected with an end of the positioning insertion tube; the camera is connected with the beam combination mirror assembly by the side opening, so that images of the lesion positions can be formed; the laser scanning assembly is connected with the other end of the beam combination mirror assembly, so that laser beams for scanning the lesion positions can be generated according to the formed images of the lesion positions; the control system is connected with the laser scanning assembly and the camera. The visual laser therapeutic apparatus with the dot matrixes has the advantages that the visual laser therapeutic apparatus is simple in operation, the cervical lesion positions can be automatically scanned by the controlled laser beams according to the preset paths and can be quickly burned through and removed, the operation time is short, the work intensity can be greatly relieved for doctors, the therapeutic efficiency can be improved, and the therapeutic success rate can be increased.
Owner:ZHONGWEI XIANGGUANG BEIJING TECH CO LTD
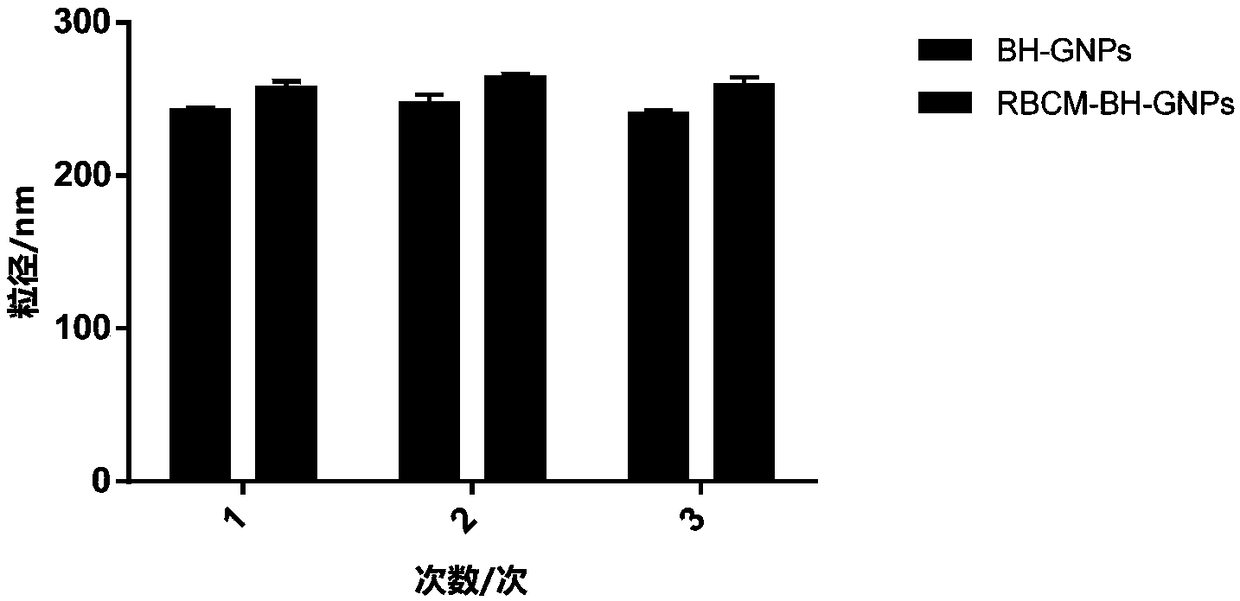
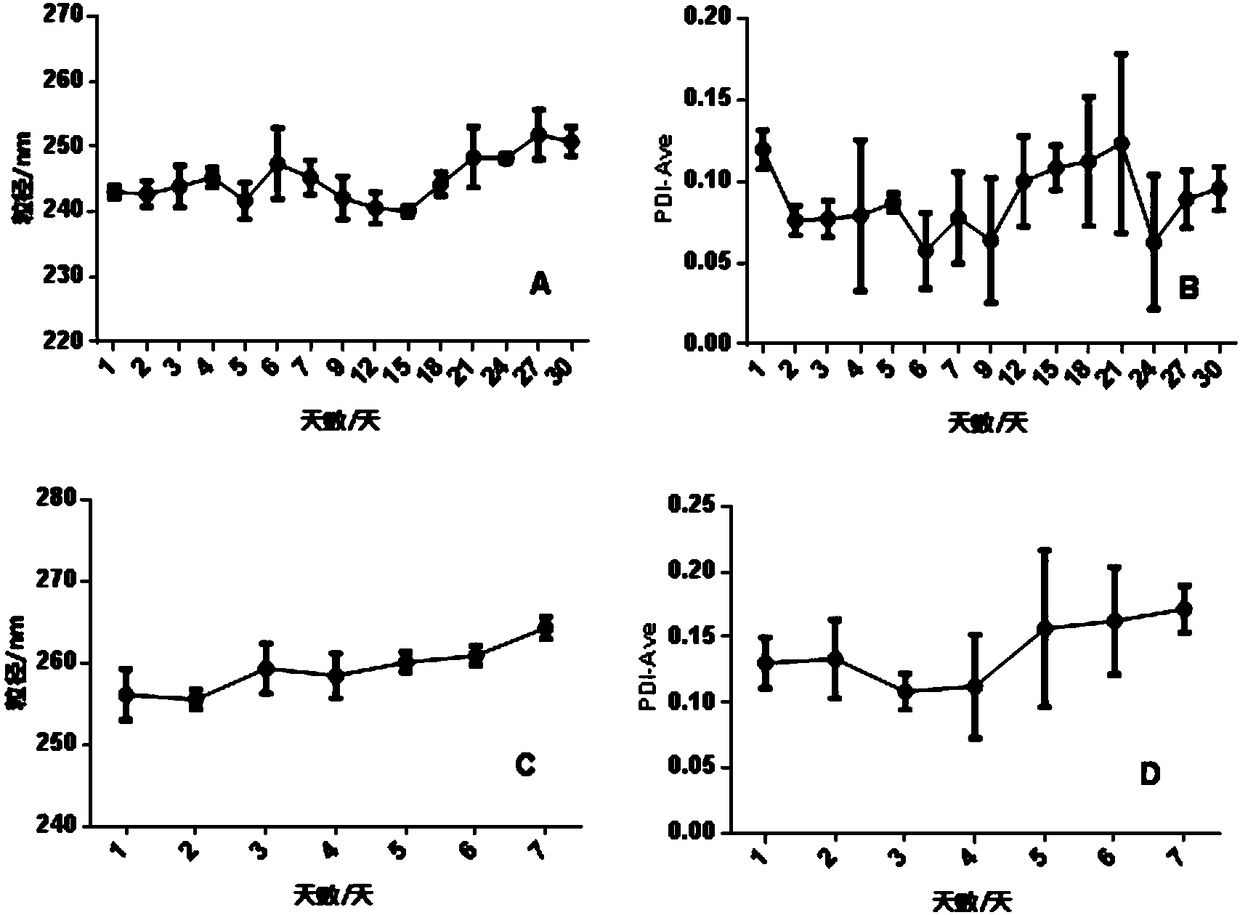
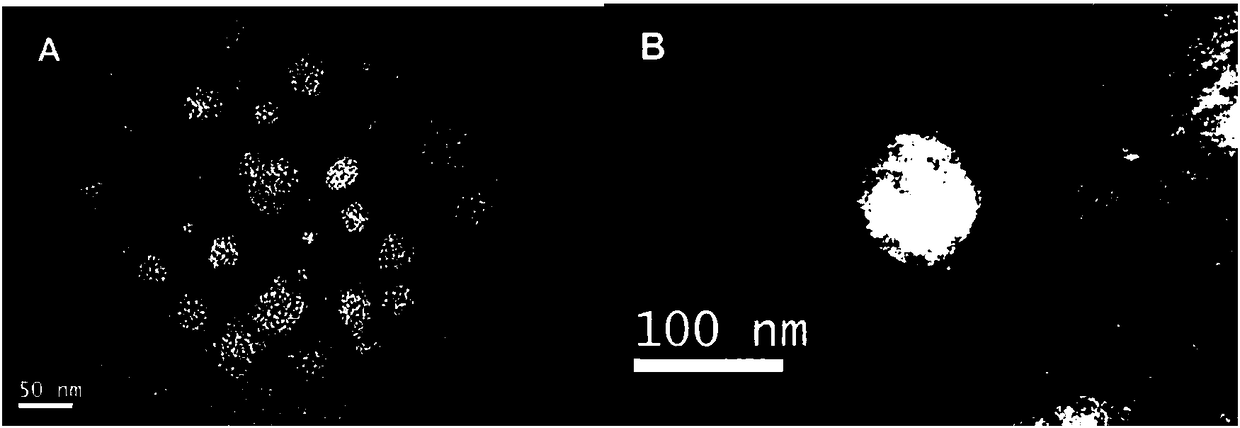
Preparation method of red blood cell membrane-coated gelatin-loaded berberine hydrochloride gold nanoparticles and application thereof
ActiveCN108113977AHigh encapsulation efficiencyHigh drug loadingAntibacterial agentsOrganic active ingredientsErythrocyte membraneHemangiectasis
The invention belongs to the technical field of a pharmaceutical, and particularly relates to an red blood cell membrane-coated gelatin-loaded berberine hydrochloride gold nanoparticles, as well as apreparation method and application thereof. The RBCM-BH-GNPs prepared through the method is uniform in particle size distribution and favorable in stability, and shows a remarkable shell-core structure; a single-layer erythrocyte membrane coats spherical BH-GNPs. The RBCM-BH-GNPs cannot cause erythrocytes hemolysis or agglutination in vitro, and can be used for intravenous injection. In addition,the RBCM-BH-GNPs prepared through the invention has a remarkable in vitro sustained release effect on BH in vitro and the sustained release effect is superior to the BH-GNPs, the RBCM-BH-GNPs solves the problems of hemangiectasis, fall of blood pressure, heart inhibition and side reaction caused by excessive plasma drug peak concentration since BH common injection is short of slow release, and provides an idea for expanding clinical application of the BH and developing new traditional Chinese medicine preparations of the BH.
Owner:SHANGHAI JIAO TONG UNIV
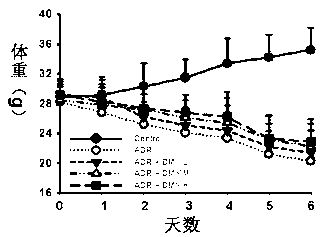
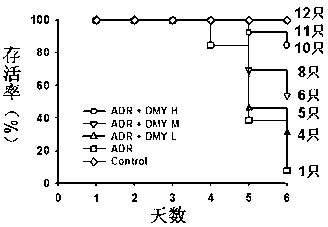
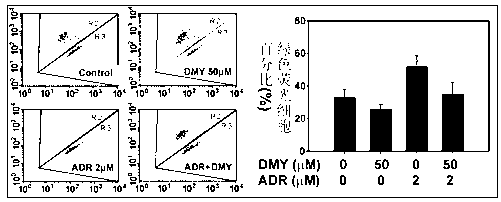
Application of dihydromyricetin in preparation of medicines for inhibiting adriamycin cardiotoxicity
InactiveCN103315993ACardiotoxicity Prevention and MitigationImprove protectionOrganic active ingredientsAntineoplastic agentsApoptosisIn vivo
The invention provides an application of a dihydromyricetin composition in preparation of medicines for inhibiting adriamycin cardiotoxicity. The composition is composed of dihydromyricetin and adriamycin; as shown by in vivo and in vitro experimental studies, the dihydromyricetin can inhibit cell apoptosis activated by the adriamycin and dependent to mitochondrial membrane potential so as to reduce lethality caused by adriamycin cardiotoxicity, and the composition provided by the invention has excellent protection effect on cardiotoxicity induced by adriamycin. The composition provided by the invention can be used for preventing and alleviating the cardiotoxicity educed by adriamycin and has clinical practicability.
Owner:ZHEJIANG UNIV
A device for recovering target components of medical cavity contents
ActiveCN103330961BBenefit from Transplant TreatmentImprove positive diagnosis rateSuction devicesReproductive tractBiology
The invention belongs to the field of medical devices, and relates to a medical device for recovering targeted components of a cavity content. The device recovers the targeted components of the human body cavity content in vitro. The device can be used for greatly preparing a fecal bacterium solution with parasitic ova removed to support a preparation procedure of standardized fecal bacterium transplantation, used for greatly collecting the parasitic ova in a fecal suspension to increase a positive detection rate of the parasitic ova, used for collecting deslagged secretions in intestine and stomach cavities by an endoscope to meet requirements of checking and diagnosis, used for collecting lavage liquid in a trachea and removing slag by the endoscope to meet requirements of a hormone test and cytological examination, and used for collecting liquid of biliary and pancreatic ducts and a urogenital canal to meet requirements of the checking and the cytological examination. The method solves the practical problems of clinical applications, and has important innovation and an application value.
Owner:NANJING FMT MEDICAL
Automatic nucleic acid extraction and pcr amplification microfluidic chip and its application method
ActiveCN105316224BImprove reliabilityHigh yieldBioreactor/fermenter combinationsBiological substance pretreatmentsSiphonProduct gas
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Sterile and freeze dried hypophysin preparation for injection and its preparing process
InactiveCN1557477AImprove stabilityImprove bioavailabilityPowder deliveryPeptide/protein ingredientsPituitrinFreeze-drying
The freeze dried bacteria-free hypophysin preparation for injection consists of hypophysin, excipient, acidity regulator, and injection water. The preparation process includes preparing hypophysin solution, adding excipient, fixing volume, packing the solution in bacteria-free freeze drying bottle, and freeze drying. The freeze dried bacteria-free hypophysin preparation thus prepared may be used in intramuscular injection or intravenous transfusion to raise the hypophysin level clinically, and has high light and heat stability, high bioavailability and less oxidation and reduction degradation.
Owner:刘红 +1
Method for producing total flavonoids of chrysanthemum
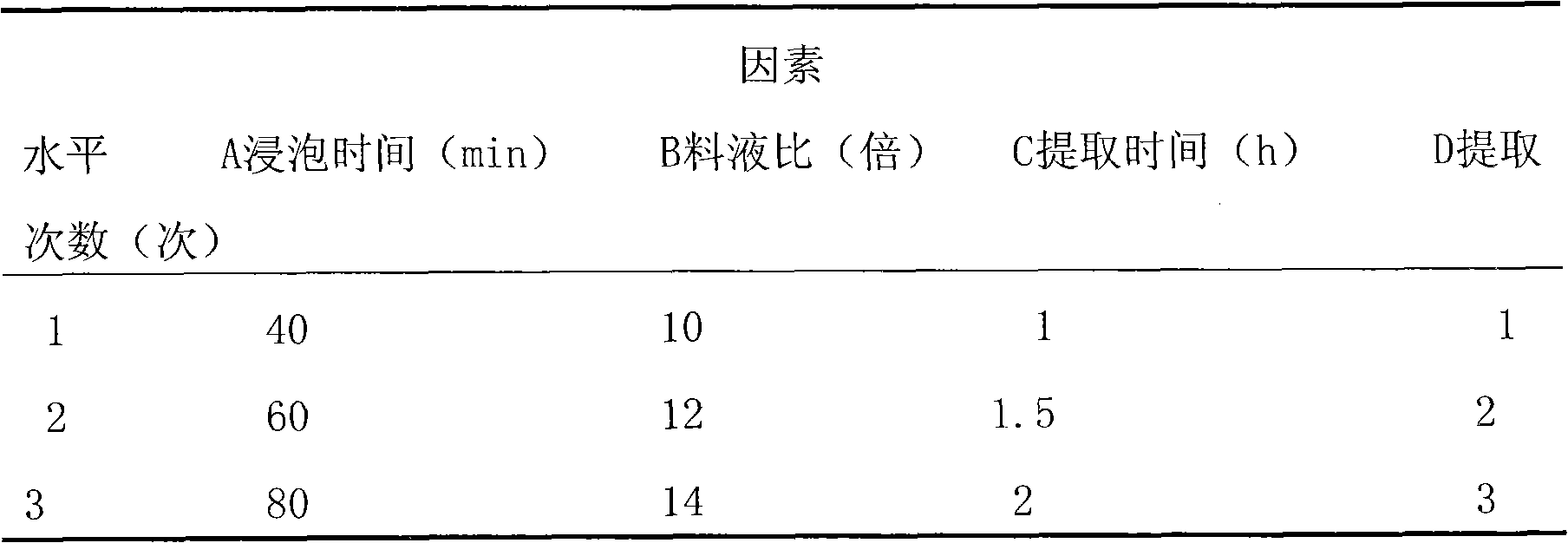
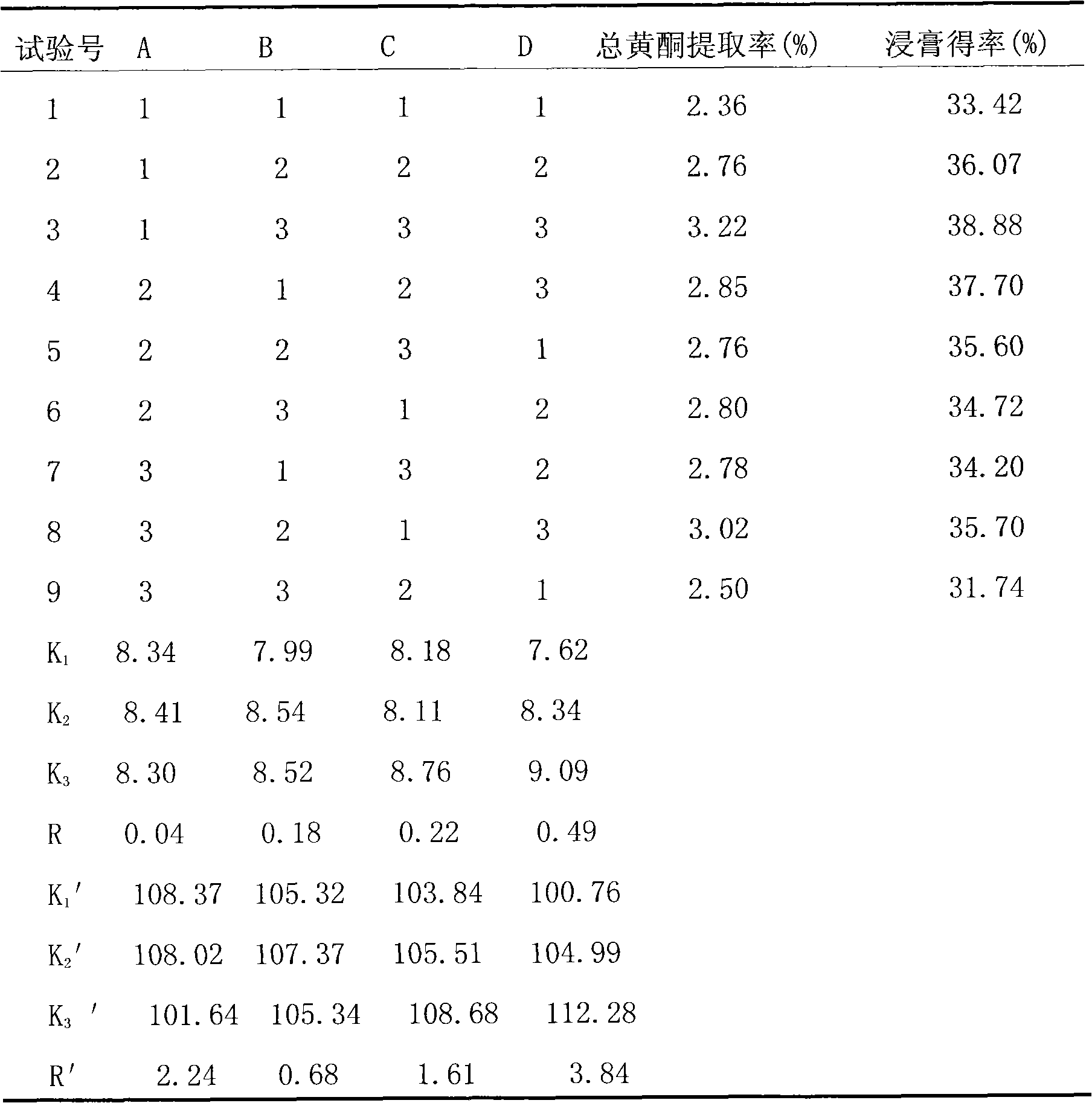
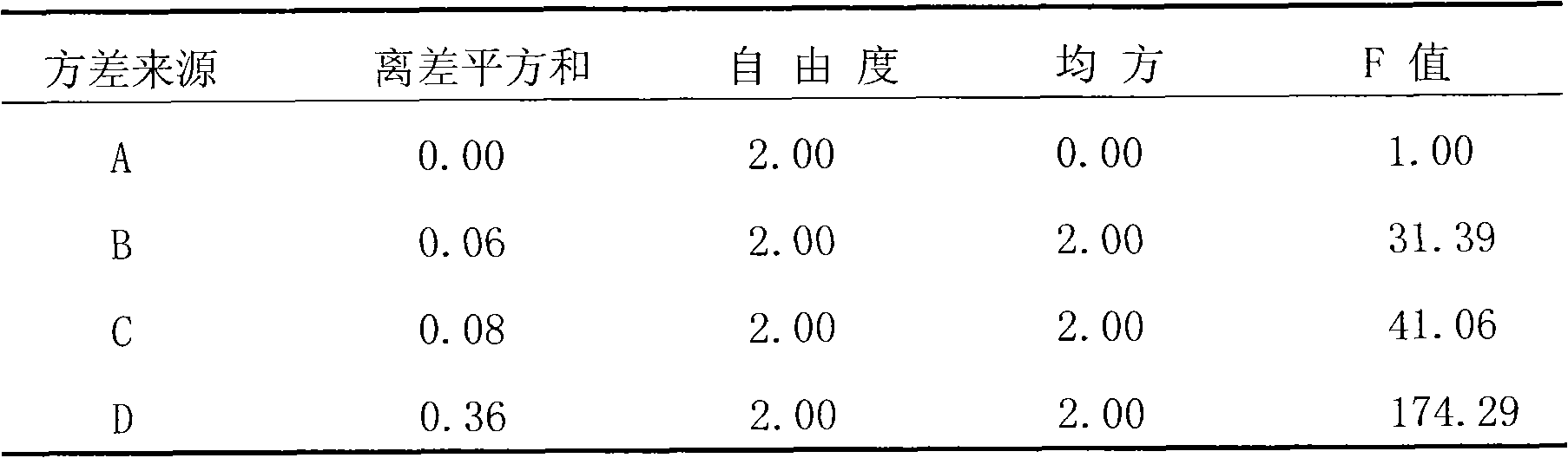
InactiveCN102100721ARaise the preparation levelExpand clinical applicationAntinoxious agentsFood preparationMacroporous resinEnvironmentally friendly
The invention relates to a method for producing total flavonoids of chrysanthemum. The method comprises the following steps of: extracting crude extracts from the chrysanthemum, and purifying the crude extracts to obtain the total flavonoids of the chrysanthemum. The method is characterized in that: the macroporous resin which is AB-8 type macroporous resin is used for purification. The content of the total flavonoids of the chrysanthemum, prepared by the method, is over 50 percent, nearly reaches 70 percent; the total flavonoids are high in purity and yield; moreover, the method is environmentally-friendly and applicable to industrial mass production.
Owner:安徽协和成药业饮片有限公司 +1
Lung cancer DNA methylation molecular markers and application thereof in preparation of kit for early diagnosis of lung cancer
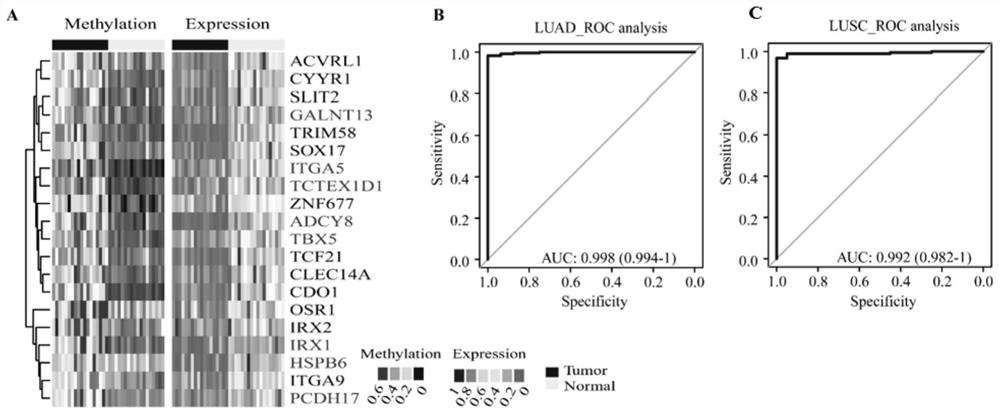
PendingCN112941180AHigh sensitivityGood specificityMicrobiological testing/measurementBiostatisticsLung cancer early detectionMolecular biology
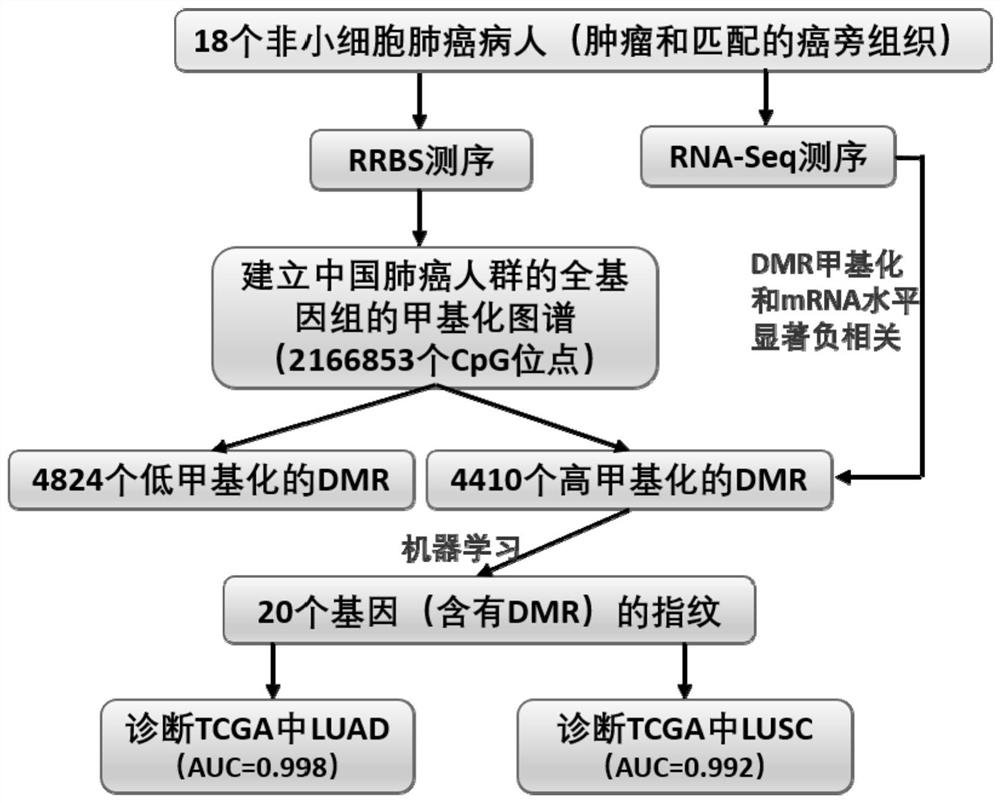
The invention discloses a group of DNA methylation molecular markers for lung cancer and application of the DNA methylation molecular markers in preparation of a kit for early diagnosis of lung cancer. The marker is obtained through methylation of 20 gene sequences of CDO1, SOX17, TCF21, TRIM58, ITGA9, CYYR1, CLEC14A, SLIT2, ZNF677, IRX2, ACVRL1, OSR1, ADCY8, GALNT13, HSPB6, IRX1, ITGA5, PCDH17, TBX5 and TTEX1D1. Eight of the gene sequences are methylated fingerprint genes newly found in lung cancer, and the methylated fingerprint genes comprise ADCY8, GALNT13, HSPB6, IRX1, ITGA5, PCDH17, TBX5 and TTEX1D1. On the basis of the markers, a mathematical model for lung cancer diagnosis is constructed; and the model is high in sensitivity and good in specificity, the AUC can reach 0.998, and the diagnosis effect is good. The invention also discloses a method for detecting the DNA methylation marker. The DNA methylation molecular marker disclosed by the invention has good diagnostic index characteristics, can be effectively used for lung cancer diagnosis, and has relatively high clinical use and popularization values.
Owner:ATTACHED OBSTETRICS & GYNECOLOGY OSPITAL MEDICALCOLLEGE ZHEJIANG UNIV
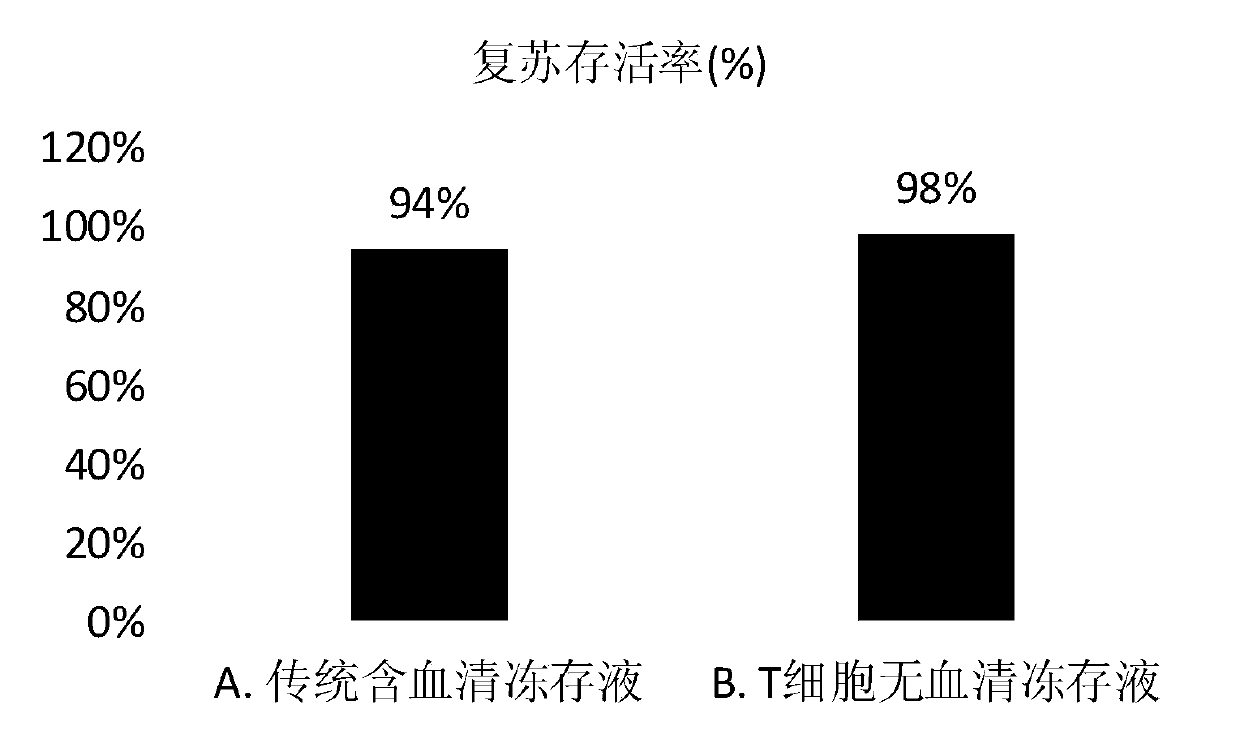
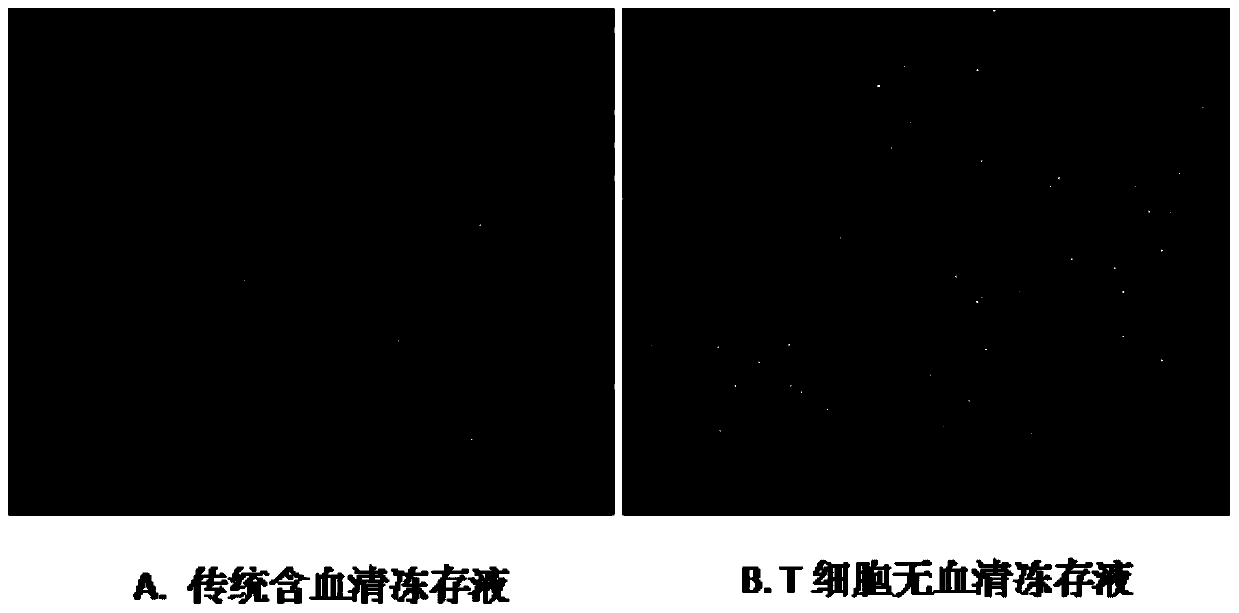
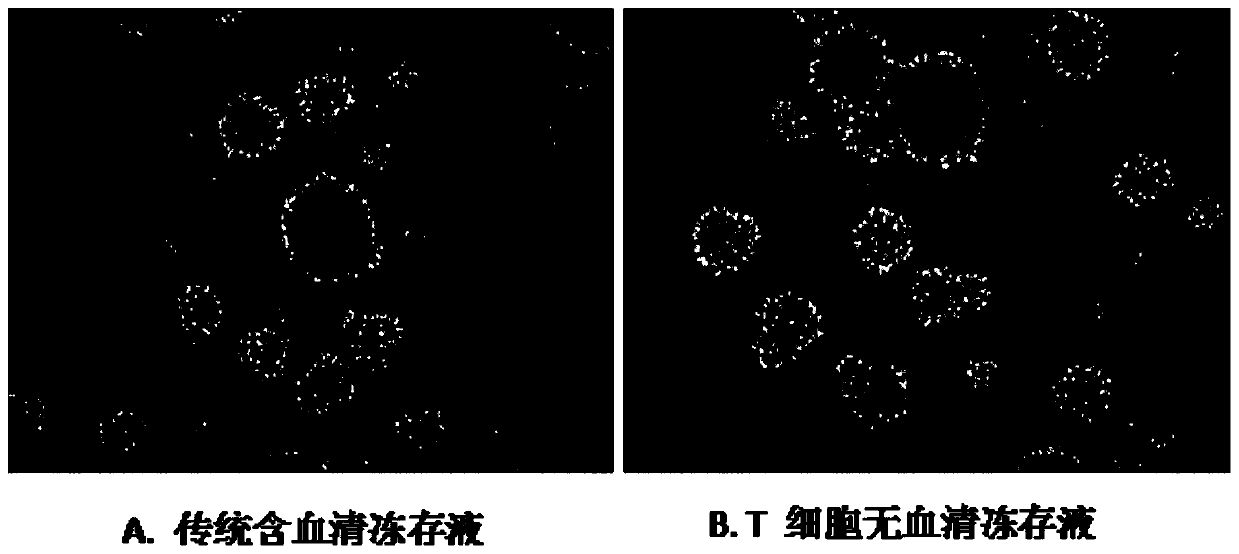
T cell serum-free cryopreservation solution and using method thereof
InactiveCN110583622AClear chemical compositionLow DMSO contentDead animal preservationVitamin CCell membrane
The invention relates to a T cell serum-free cryopreservation solution and a using method thereof. The cryopreservation solution comprises a basic culture medium, a cryopreservation protective agent and adding components which are ethanolamine, insulin, transferrin, human serum albumin, vitamin C and a cell membrane stabilizer. The cryopreservation solution disclosed by the invention has no serum,no human source and no animal source components, and is clear in chemical composition and low in DMSO content, the defect of high DMSO content in the prior art is overcome, the toxicity risk to cellsis avoided, meanwhile, possible clinical application of the cryopreservation solution is not affected, the cryopreservation solution has excellent cryopreservation performance on T cells, the resuscitation survival rate of the T cells is 90% or above after cryopreservation of the T cells, subsequent activation and amplification are not affected, and the T cell serum-free cryopreservation solutionhas high clinical application and scientific research value.
Owner:苏州依科赛生物科技股份有限公司
Apramycin liposome and preparation method thereof
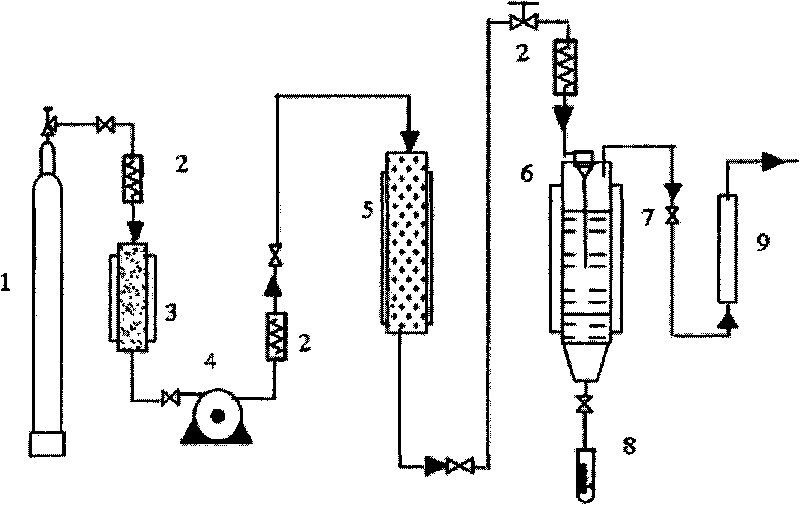
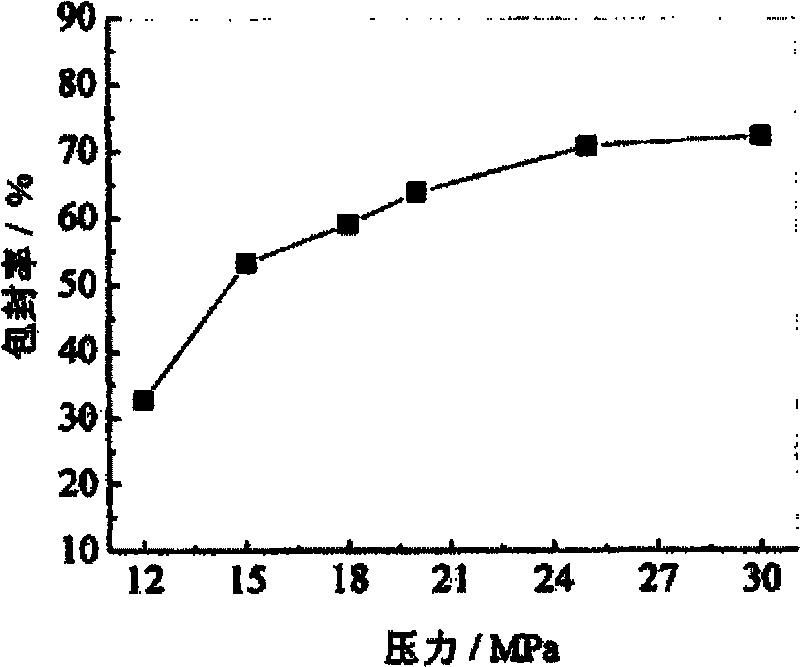
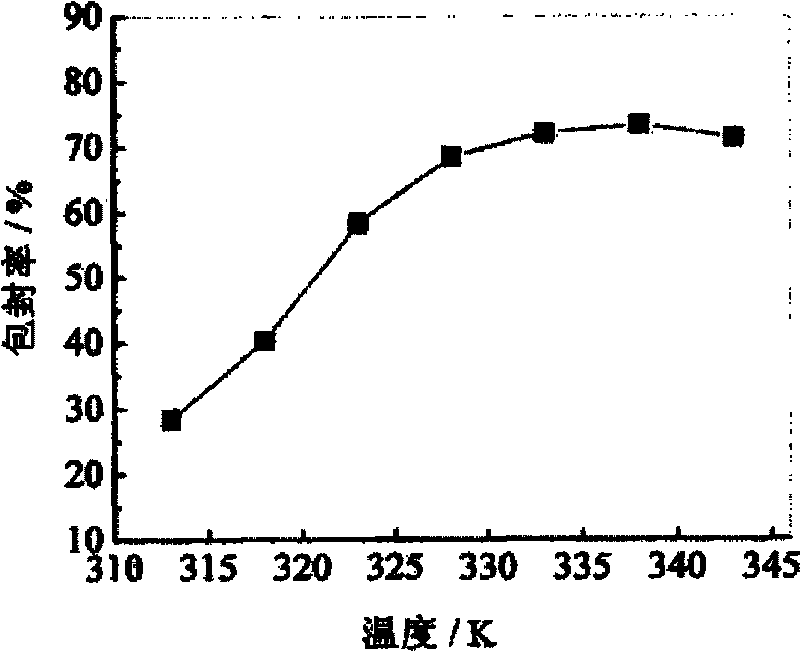
ActiveCN103565746AEasy reunionGood encapsulation effectOrganic active ingredientsPharmaceutical product form changePre expansionMass ratio
The invention discloses an apramycin liposome. The apramycin liposome is a composition with a spherical or ellipsoidal double-film structure, and is formed by encapsulating apramycin with a liposome film. A preparation method comprises the following steps: 1) dissolving the apramycin and the liposome film with ethanol to obtain a mixed solution, and dissolving the mixed solution into supercritical CO2 to obtain a supercritical solution, wherein the mass ratio of the apramycin to the liposome film is (1 : 1) to (1 : 30); the liposome film is phosphatidylcholine, or a mixture of phosphatidylcholine and cholesterol; 2) dissolving a stabilizer into an aqueous medium to obtain a stabilizer solution; 3) spraying the supercritical solution to the stabilizer solution at an expansion pressure of 15-30MPa and a pre-expansion temperature of 323-343K, dispersing and precipitating to obtain an apramycin liposome suspension.
Owner:HENAN SOAR VETERINARY PHARMA
Application of hedan preparation in preparation of diabetes medicine
ActiveCN103191222AExpand clinical applicationMetabolism disorderPlant ingredientsDiabetes mellitusRemove blood
The invention relates to application of a hedan preparation in preparation of a diabetes medicine. The hedan preparation adopts lotus leaves as the main medicine and adopts lotus leaves, red-rooted salvia roots, hawthorns, folium sennae and fructus psoraleae as the prescription medicines; the hedan preparation has the functions of reducing phlegm and descending the turbid and promoting blood circulation to remove blood stasis, and is clinically used for a patient with hyperlipemia phlegm stagnation symptoms. According to the research and the accidental discoveries, the hedan preparation has the effect of treating diabetes mellitus.
Owner:NANCHANG JISHUN PHARMA CO LTD
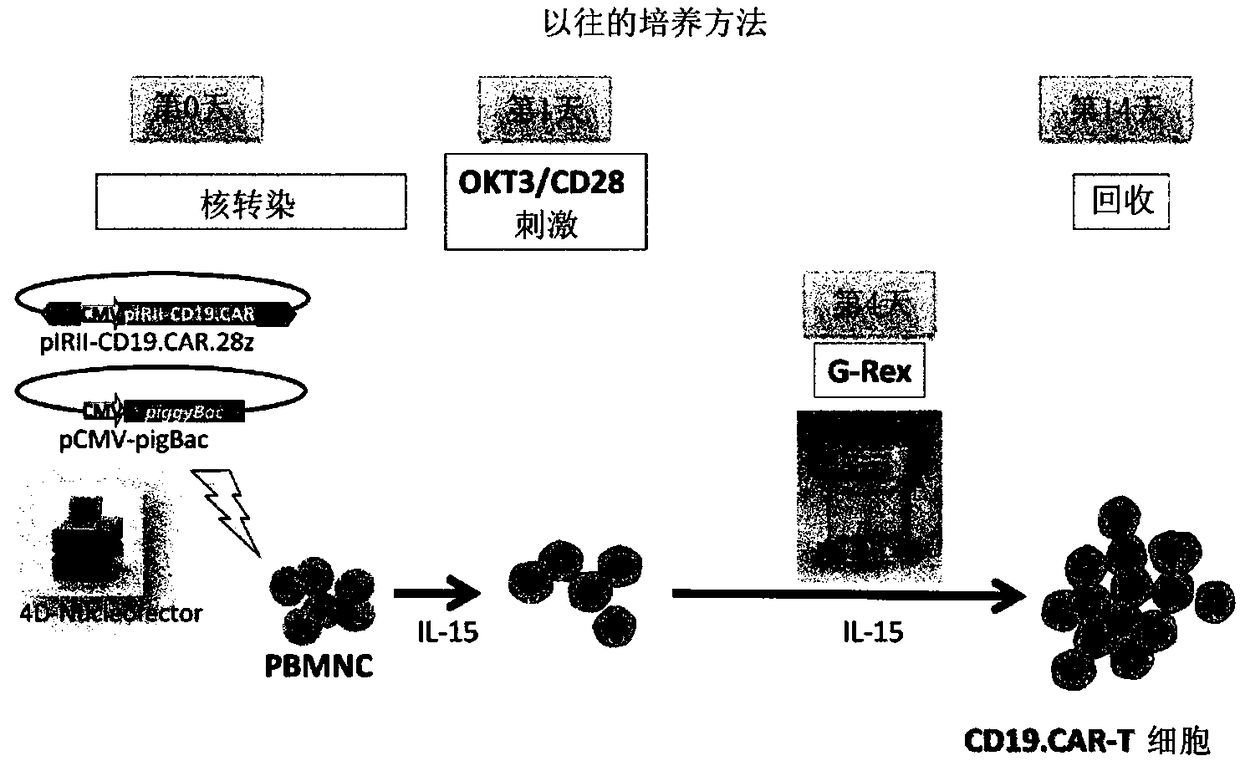
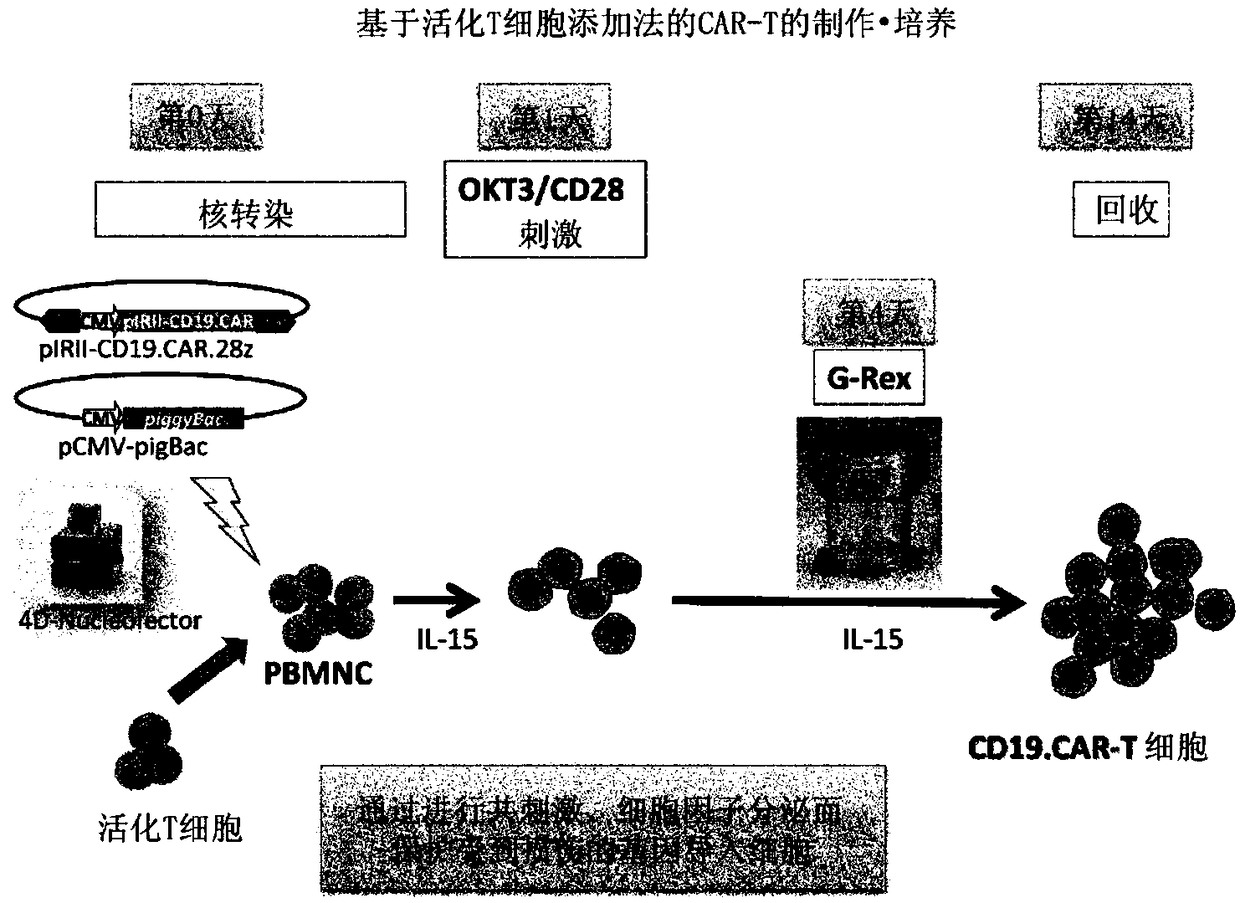
Method for preparing genetically-modified t cells which express chimeric antigen receptor
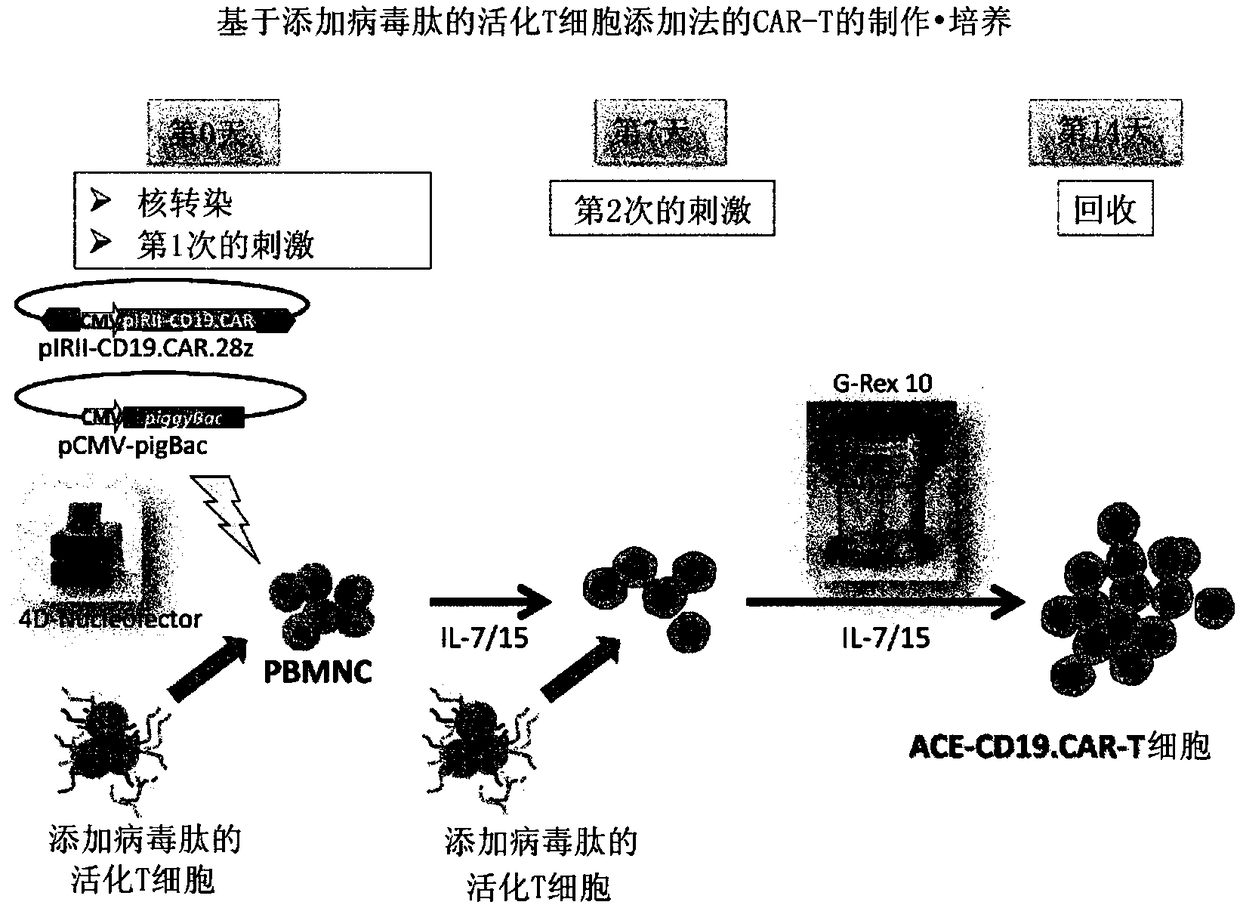
ActiveCN108138171AExpand clinical applicationGood treatment effectImmunoglobulin superfamilyMammal material medical ingredientsPeptide antigenAntiendomysial antibodies
In order to improve the efficiency of gene introduction in CAR therapy employing a transposon technique, provided is a method for preparing genetically-modified T cells which express a chimeric antigen receptor, comprising: (1) a step for preparing non-proliferative cells obtained by stimulating a group of cells including T cells using an anti-CD3 antibody and an anti-CD28 antibody, and thereafter, subjecting the cells to a treatment for causing the cells to lose their proliferation capability; (2) a step for obtaining genetically-modified T cells into which a target antigen-specific chimericantigen receptor gene has been introduced using a transposon technique; (3) mixing the non-proliferative cells prepared at step (1) with the genetically-modified T cells obtained at step (2), and co-culturing the mixed cells while stimulating the mixed cells using the anti-CD3 antibody and the anti-CD28 antibody; and (4) a step for collecting the cultured cells. Also, provided is a method for preparing genetically-modified T cells which express a chimeric antigen receptor, comprising: (i) a step for preparing non-proliferative cells holding a viral peptide antigen, which cells are obtained bystimulating a group of cells including T cells using an anti-CD3 antibody and an anti-CD28 antibody, and thereafter, subjecting the cells to culturing in the presence of the viral peptide antigen anda treatment for causing the cells to lose their proliferation capability; (ii) a step for obtaining genetically-modified T cells into which a target antigen-specific chimeric antigen receptor gene hasbeen introduced using a transposon technique; (iii) mixing the non-proliferative cells prepared at step (i) with the genetically-modified T cells obtained at step (ii), and co-culturing the mixed cells; and (iv) a step for collecting the cultured cells.
Owner:NAT UNIV CORP TOKAI NAT HIGHER EDUCATION & RES SYST
Enhanced dihydromyricetin soluble complex and preparation method thereof
InactiveCN108014104AImprove stabilityEnhance pharmacological effectsOrganic active ingredientsSenses disorderSolubilityAdjuvant
The invention discloses a dihydromyricetin complex with synergistically enhanced pharmacodynamic effects. The dihydromyricetin complex comprises dihydromyricetin as a main drug and is characterized inthat the dihydromyricetin complex comprises rebaudioside A as a drug adjuvant, a mass ratio of the dihydromyricetin main drug to the rebaudioside A drug adjuvant is 1: 15 to 1: 30, and the rebaudioside A (with a CAS register number of 58543-16-1, a molecular formula C44H70O23 and molecular weight of 967.03) has purity of greater than or equal to 98%. In the aqueous solution, the rebaudioside A spontaneously forms micelles to solubilize dihydromyricetin, the solubility of dihydromyricetin is 16.57 mg / ml, the micelle has small particle sizes, a distribution range is uniform, the drug stabilityis good, the dihydromyricetin absorption after oral administration is observably improved, the oral bioavailability is improved, the rebaudioside A has activity to resist diabetes and diminish inflammation and the dihydromyricetin complex has good synergistic pharmacological activity. A preparation method of the dihydromyricetin complex has simple processes, is suitable for large-scale industrialproduction and has good economical efficiency.
Owner:QINGDAO UNIV OF SCI & TECH
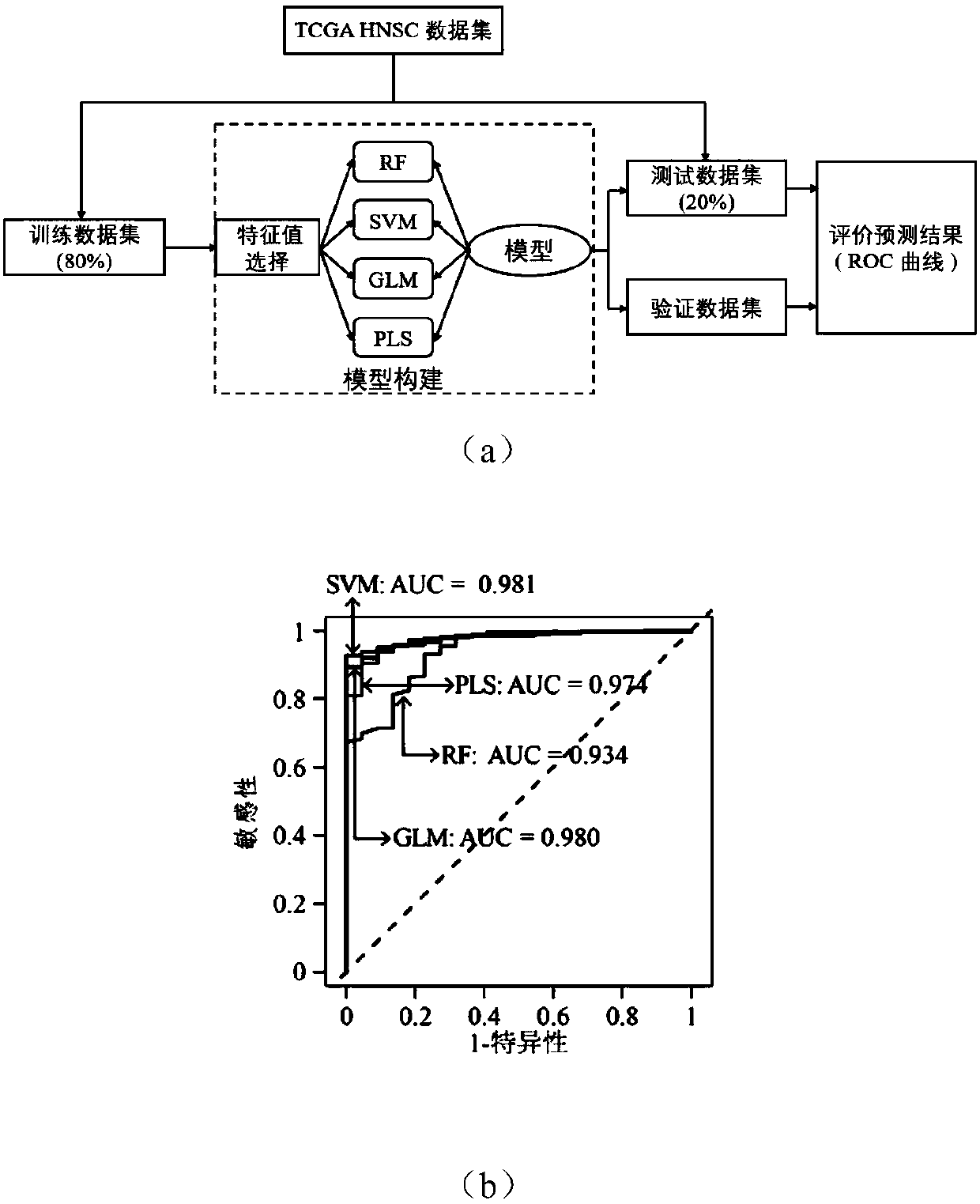
Novel molecular marker and application thereof in preparing kit for diagnosis and prognosis of head and neck cancer
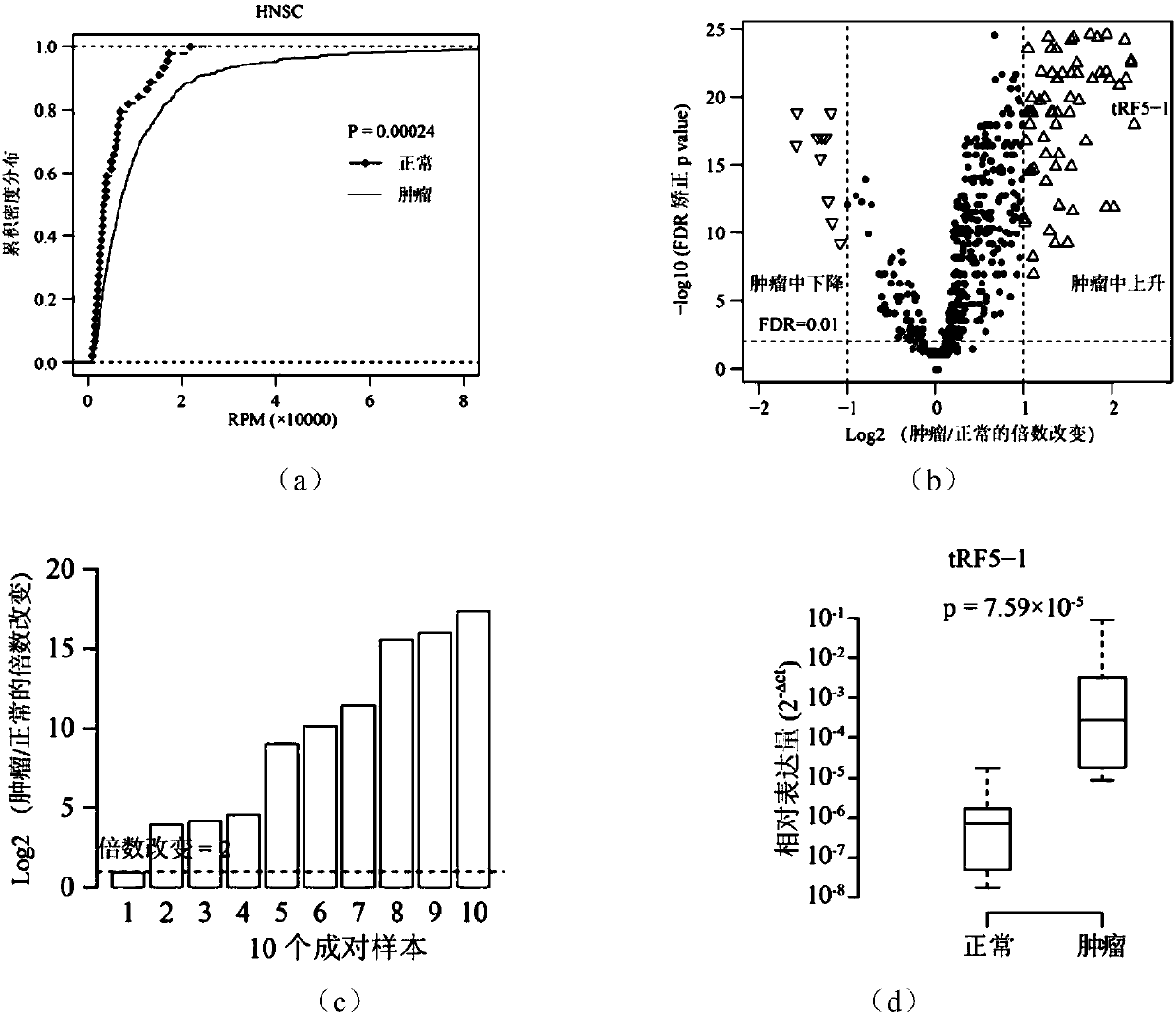
ActiveCN108048460AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMathematical modelMedicine
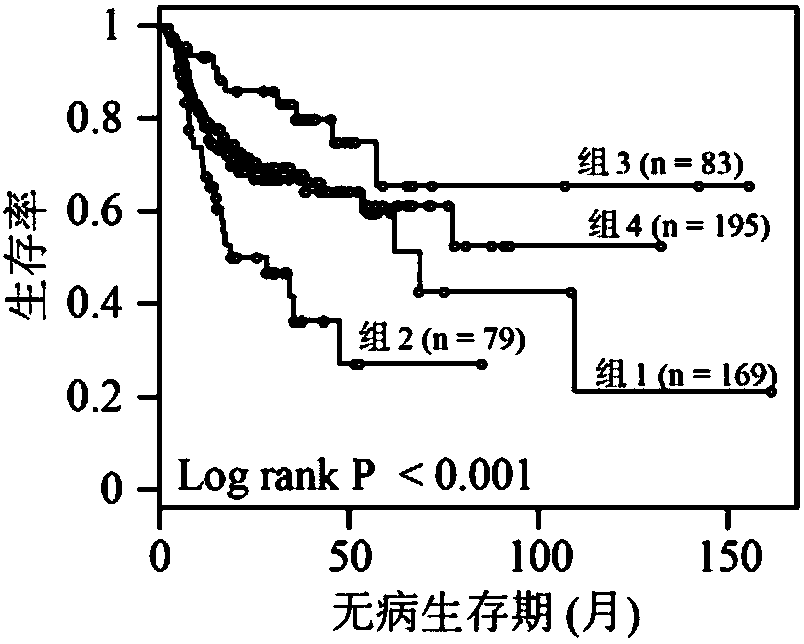
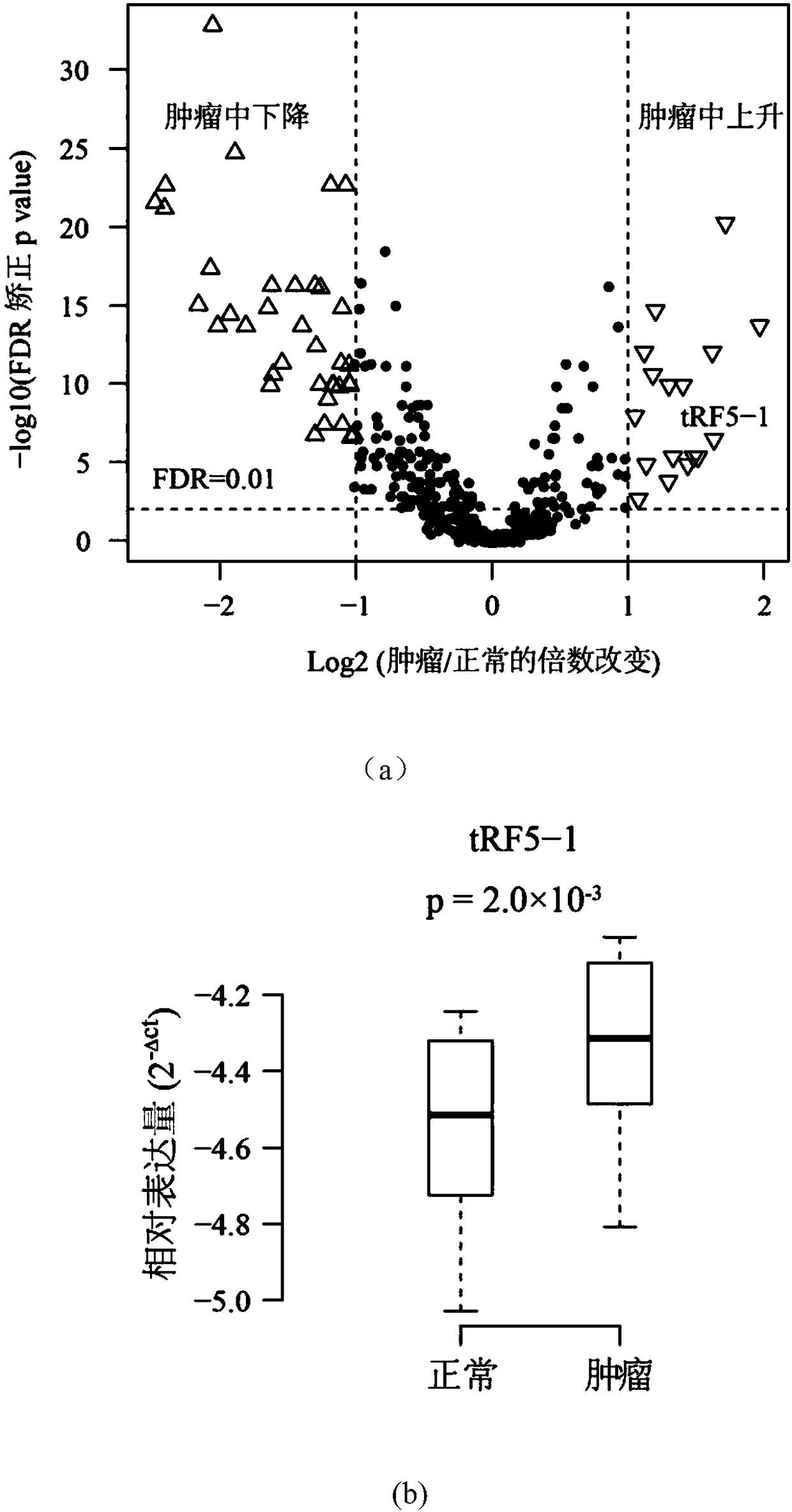
The invention discloses a novel molecular marker and application thereof in preparing a kit for diagnosis and prognosis of head and neck cancer. RNA (Ribonucleic Acid) sequences of the novel molecularmarker are shown as SEQ ID NO:1 to SEQ ID NO:48. A mathematical model for diagnosing the head and neck cancer is constructed by taking the marker disclosed by the invention as a basis, and has the advantages of high sensitivity, good specificity and good diagnosis effect; AUC can be as high as 0.981; besides, 48 tRF fragments can be used as molecular markers for typing the head and neck cancer and predicting life expectancy of patients; head and neck cancer tumor samples are clustered into four subtypes according to tRFs expression in test data; the analysis of a survival curve shows that thelife expectancies of the subtypes have significant difference. The novel tRF molecular marker disclosed by the invention has good characteristics of diagnostic indicators and higher clinical use andpopularization value, and can be effectively used for diagnosis, typing and prognosis of the head and neck cancer.
Owner:ZHEJIANG UNIV
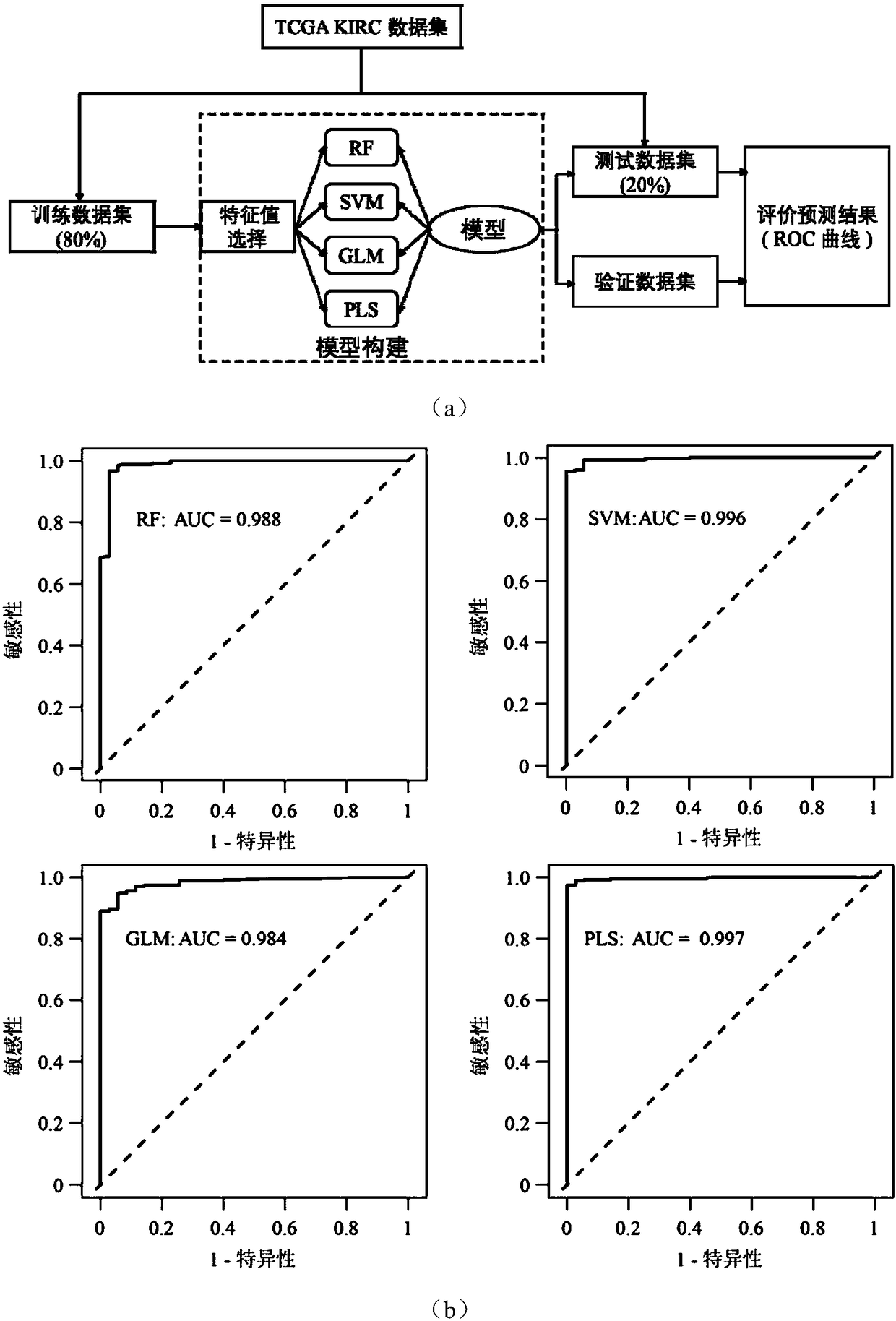
Application of novel molecular marker to preparation of kit for diagnosis and prognosis of renal clear cell carcinoma
ActiveCN108559777AHigh sensitivityStrong specificityMicrobiological testing/measurementProteomicsMathematical modelTumor Sample
The invention discloses application of a novel molecular marker to the preparation of a kit for diagnosis and prognosis of renal clear cell carcinoma. The RNA sequence of the novel molecular marker isshown in SEQ ID NO: 1 to SEQ ID NO: 57. A mathematical model for diagnosis of renal clear cell carcinoma is constructed by taking the marker as a base; the model is high in sensitivity and good in specificity, AUC can be as high as 0.997, and the diagnosis effect is good. In addition, 57 tRF fragments can serve molecular markers for classifying renal clear cell carcinoma and predicating the survival periods of patients; in test data, a renal clear cell carcinoma tumor sample is clustered into 3 subtypes according to rRFs expression, and survivorship curve analysis shows that the survival periods of the subtypes have obvious difference. The novel tRF molecular marker has excellent diagnosis index characteristic, can be effectively applied to the diagnosis, classification and prognosis of renal clear cell carcinoma, and has high clinical application and promotion values.
Owner:ZHEJIANG UNIV
Inclusion compound of higher fatty alcohol and its prepn
InactiveCN101066258AImprove solubilityHigh dissolution rateHydroxy compound active ingredientsMetabolism disorderDissolutionCyclodextrin derivative
The present invention relates to medicinal intermediate containing higher fatty alcohol and its preparation process. The medicinal intermediate is inclusion compound containing octacosyl alcohol in 0.65-50 wt% and cyclodextrin or cyclodextrin derivative. It is prepared through a grinding process, an ultrasonic process or a saturated water solution process. The higher fatty alcohol including compound has obviously raised dissolution of octacosyl alcohol in leaching medium and thus high bioavailability. It may be further prepared into tablet, suspension or other solid or liquid preparation forms for clinical application of octacosyl alcohol.
Owner:深圳海创医药科技发展有限公司
Cyclic snoRNA biomarker used for diagnosing renal clear cell carcinoma, kit and applications
ActiveCN110229912AAvoid damageReduce medical costsMicrobiological testing/measurementDNA/RNA fragmentationDiseaseRenal clear cell carcinoma
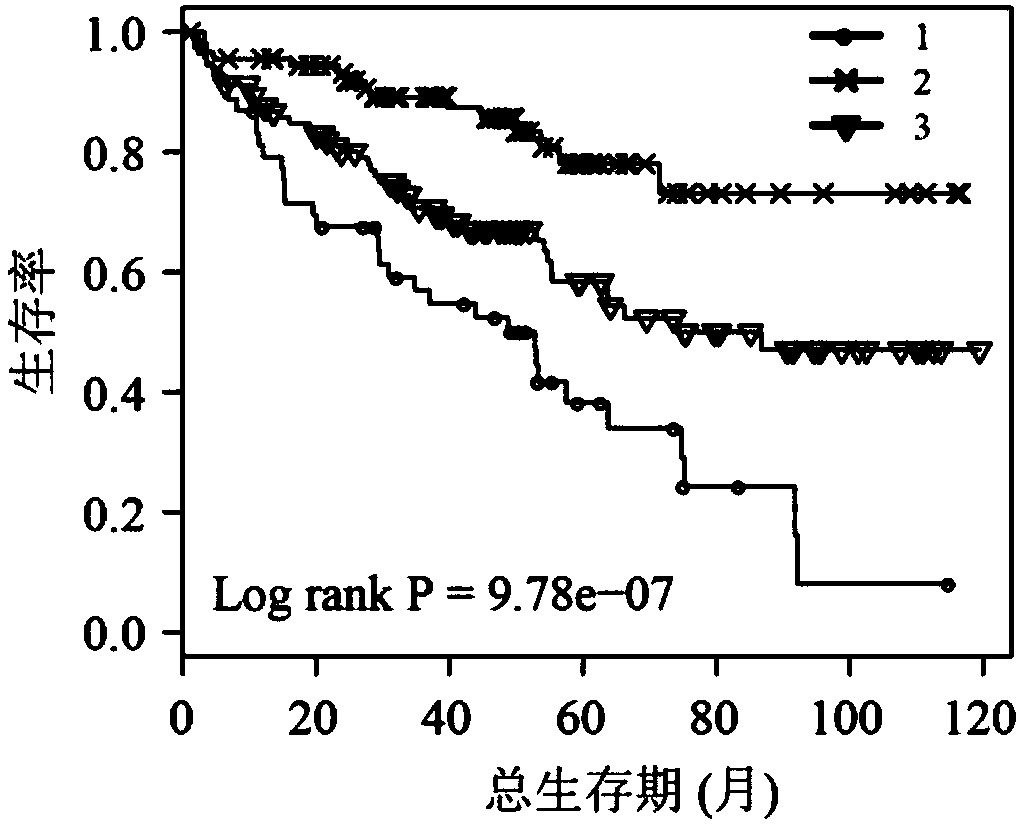
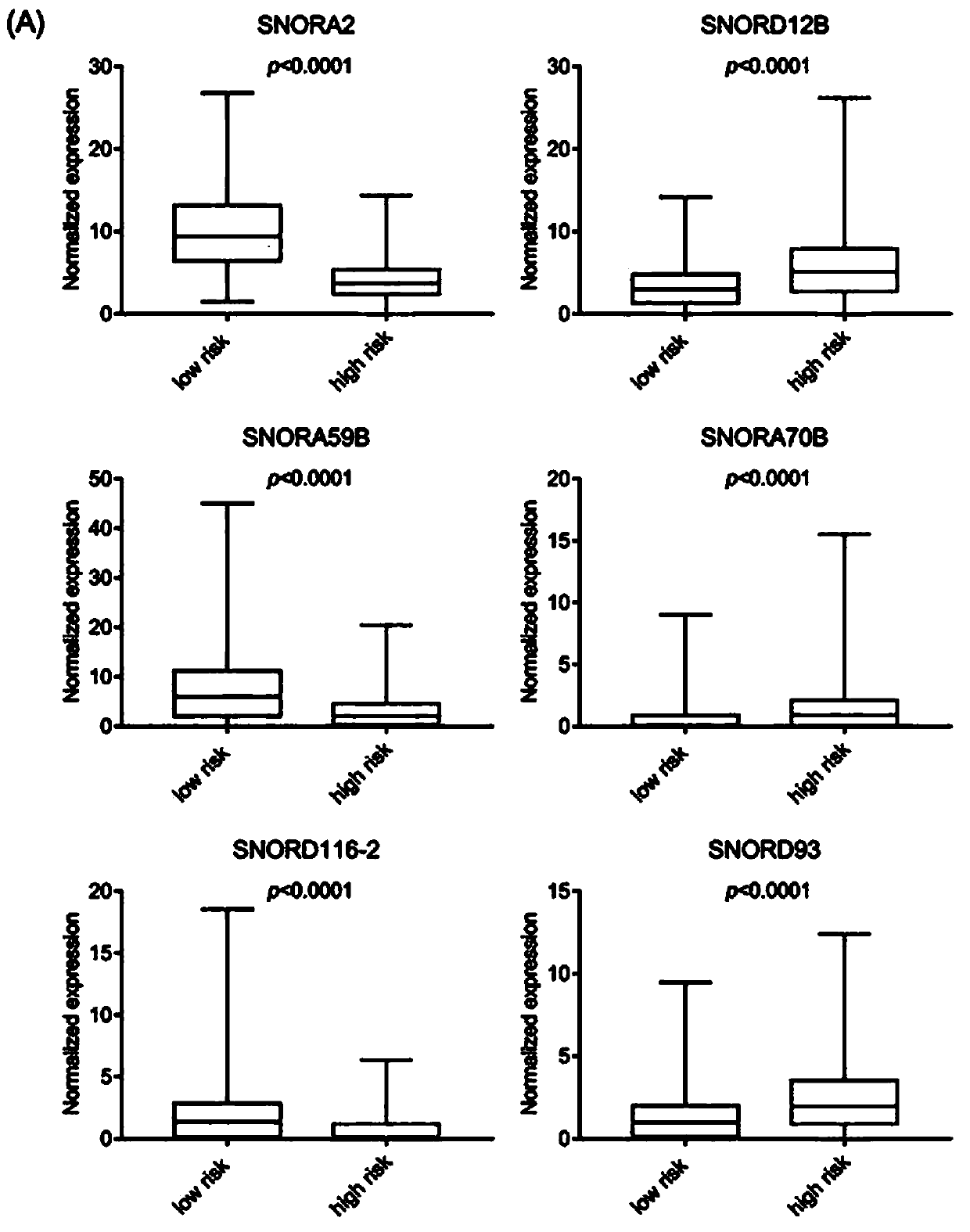
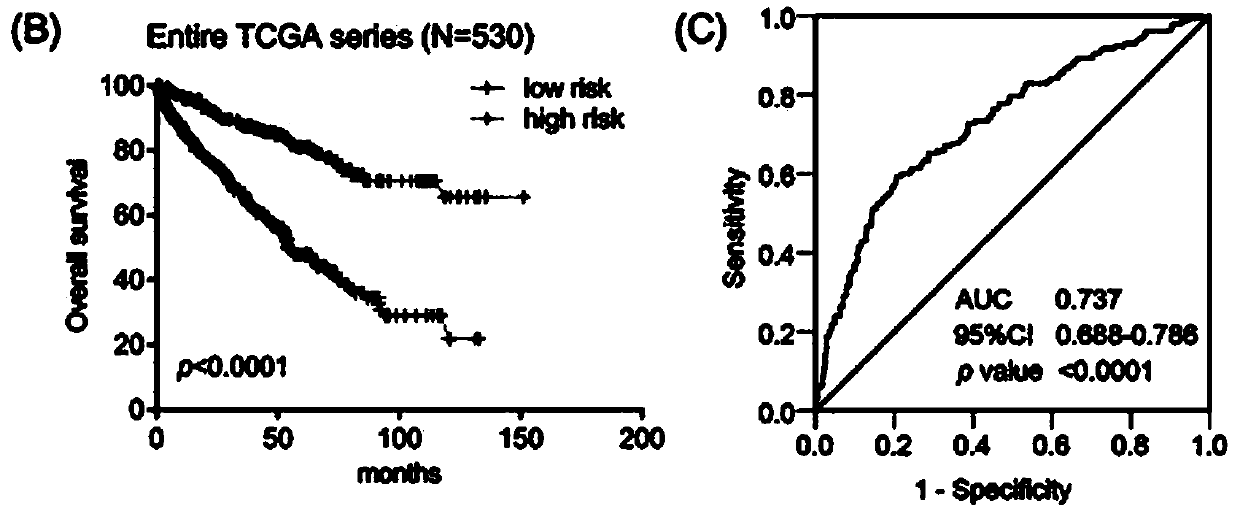
The invention belongs to the field of biological detection, and specifically relates to a cyclic snoRNA biomarker used for diagnosing renal clear cell carcinoma, a kit and applications. The biomarkerconsists of the following cyclic snoRNA: SNORA2, SNORD12B, SNORA59B, SNORA70B, SNORD93 and SNORD116-2. The snoRNA that can obviously influence the lifetime of renal clear cell carcinoma patients can be screened, and differential expression and diagnosis capabilities can be verified in tissues and serum; and the biomarker can be effectively used for the diagnosis and prognosis of renal clear cell carcinoma, and the development and utilization of the biomarker can provide a novel direction for the diagnosis of tumor and other diseases and further treatment.
Owner:中国医科大学
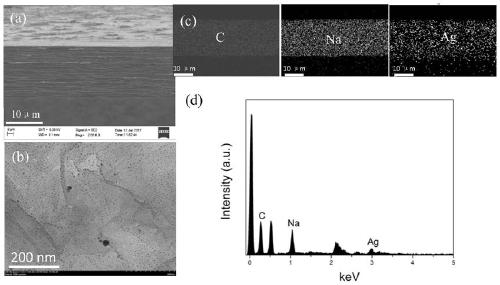
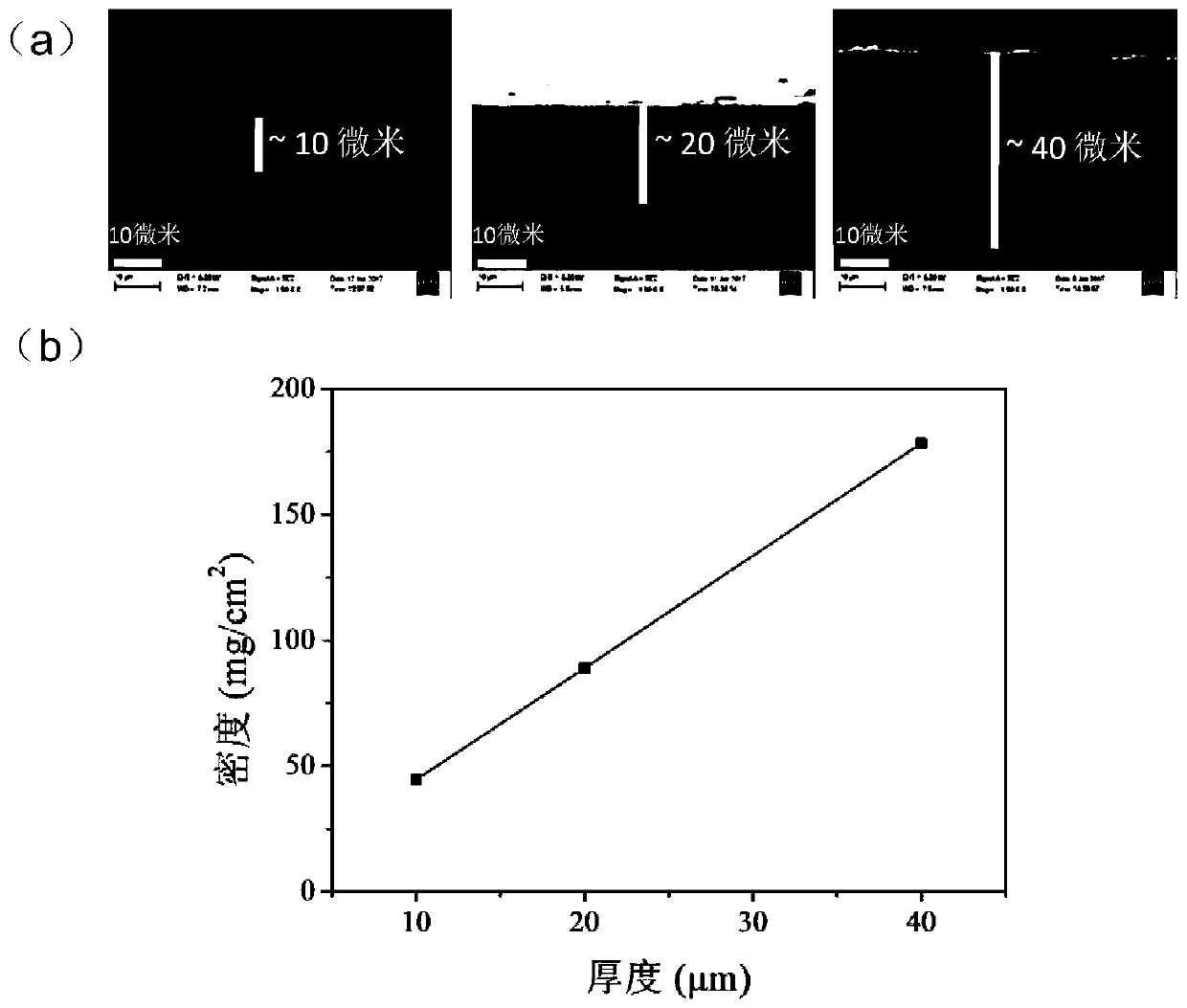
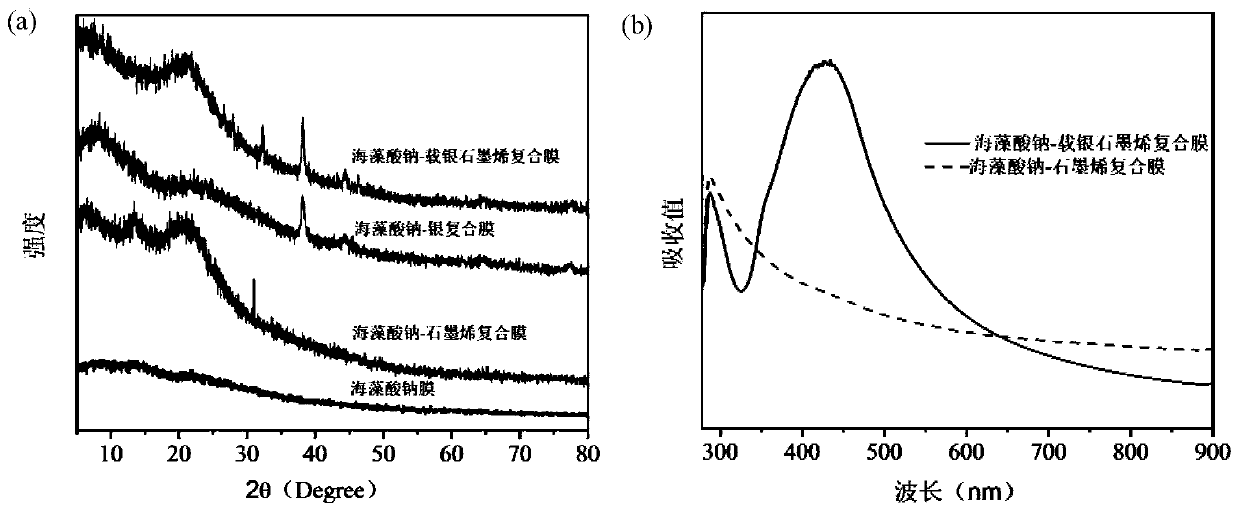
Sodium alginate-silver-loaded graphene composite film with antibacterial and wound healing functions and its application
The invention discloses a sodium alginate / silver loaded graphene composite film having bacterium resistance and wound healing promotion functions and application thereof. The composite film is characterized by being prepared through the following steps: uniformly mixing a silver loaded graphene solution and a sodium alginate solution, spraying and assembling. The composite film provided by the invention is simple in preparation method, simultaneously has bacterium resistance and wound healing promotion functions, and achieves an obvious effect when being used as a wound dressing, thereby having great clinical application potentials.
Owner:HEFEI UNIV OF TECH
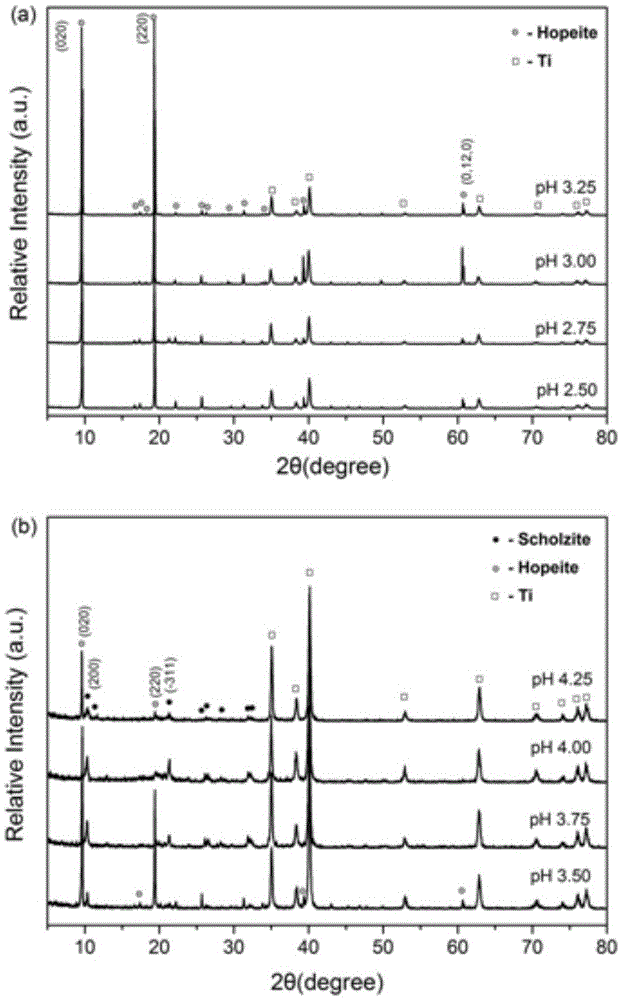
Method for regulating and controlling physical phases of zinc and calcium conversion films on pure-titanium surfaces by aid of pH (potential of hydrogen) value
ActiveCN105568272AAchieving controllable equipmentSimple processMetallic material coating processesProsthesisAcid etchingZinc phosphate
The invention discloses a method for regulating and controlling physical phases of zinc and calcium conversion films on pure-titanium surfaces by the aid of a pH (potential of hydrogen) value. The method includes steps of preparing chemical conversion liquid; regulating the pH value of the chemical conversion liquid by the aid of phosphoric acid and sodium hydroxide until the pH value reaches 2.50-4.50; soaking pure-titanium samples in the chemical conversion liquid after the pure-titanium samples are subjected to acid etching and surface modification and converting the pure-titanium samples for 15-45 min to obtain the zinc and calcium conversion films on the pure-titanium surfaces. The concentration of a component Zn(H2PO4)2 2H2O in the chemical conversion liquid is 22-30 g / L, the concentration of a component Ca(NO3)2 4H2O in the chemical conversion liquid is 8-14 g / L, and the concentration of a component NaNO2 in the chemical conversion liquid is 1.5-2 g / L. The method for regulating and controlling conversion of zinc and calcium salt on the pure-titanium surfaces by means of changing the pH value of the chemical conversion liquid has the advantages that the conversion films with zinc phosphate phases [Zn3(PO4)2 4H2O] and the conversion films with zinc and calcium phosphate phases [CaZn2(PO4)2 2H2O] can be respectively obtained by the aid of the method, film layers are uniform and dense, structures and textures are patterned, preparation processes are simple, a formula for the chemical conversion liquid does not need to be changed, accordingly, resources can be saved, the structures and the physical phases of the calcium and zinc conversion films on the pure-titanium surfaces can be doubly controllably prepared, and the method has important scientific research value in the technical field of metal surface modification and important significance on expanding clinical application of titanium.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com