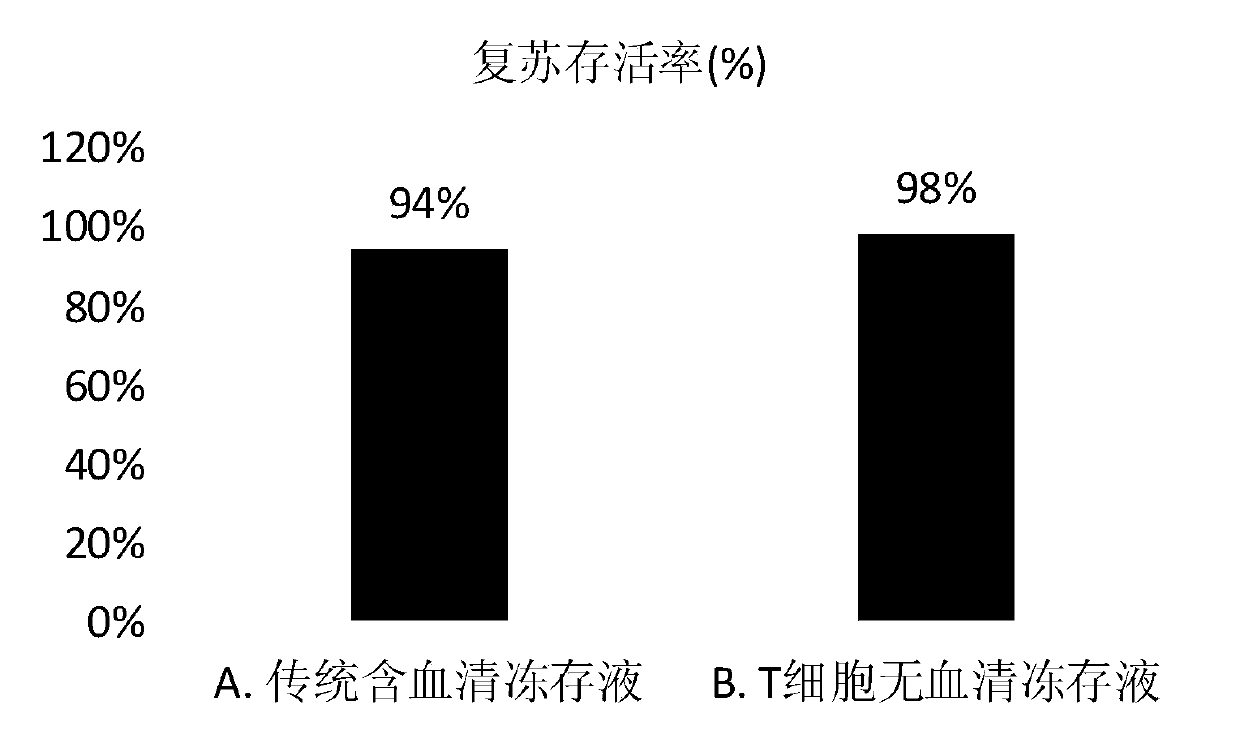
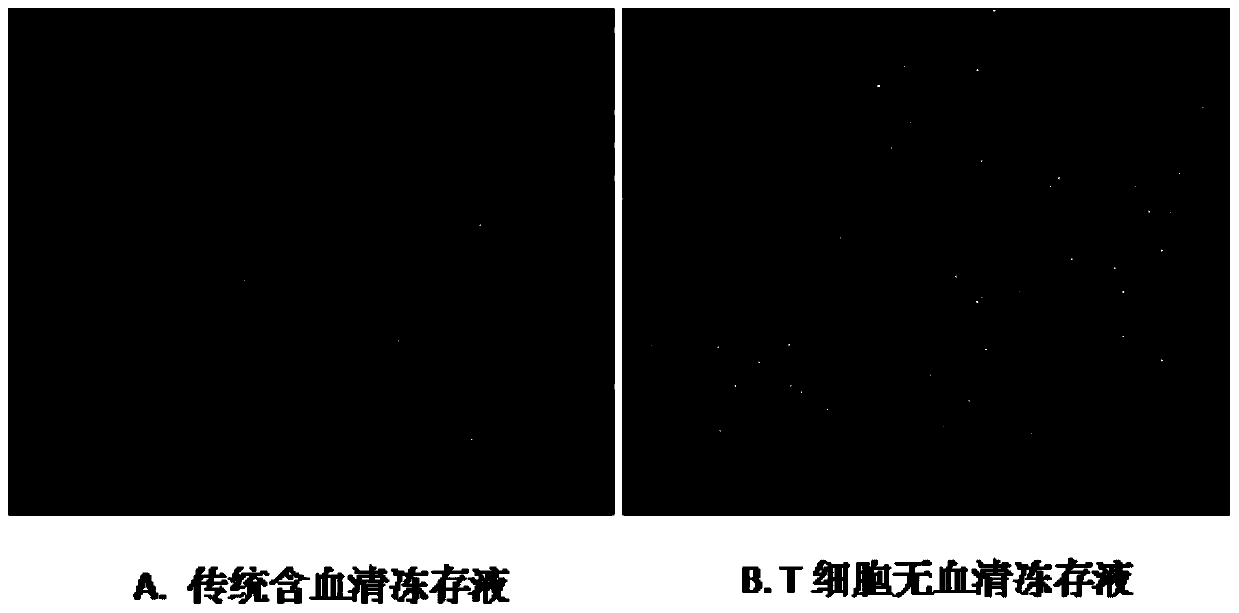
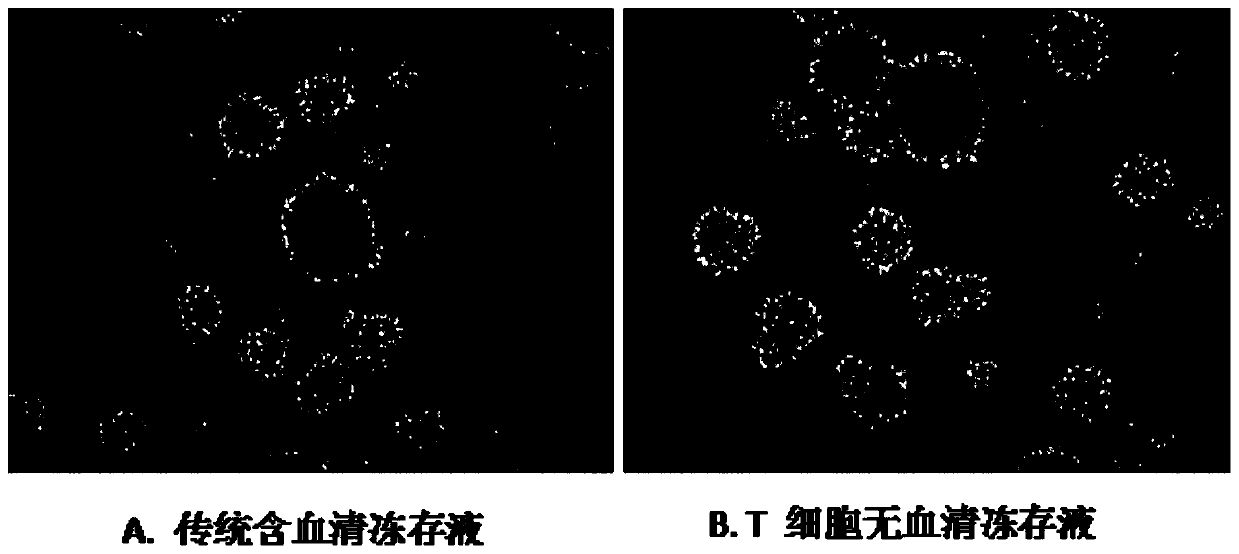
T cell serum-free cryopreservation solution and using method thereof
A cryopreservation, serum-free technology, applied in the application, preservation of human or animal body, animal husbandry, etc., can solve the problems of uncertainty of clinical use, complex serum composition, infection of xenogeneic animal pathogens, etc., and achieve high clinical application. and scientific research value, low DMSO content and clear chemical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. The specific configuration method of the cryopreservation solution used in this embodiment is as follows:
[0044] Use IMDM, DMEM / F12 and RPMI1640 three kinds of culture medium to mix by volume ratio 1:1:1 to prepare the basal medium of cryopreservation liquid, then add ethanolamine, 2.5 The recombinant human insulin of g / L, the recombinant human transferrin of 5mg / L, the recombinant human serum albumin of 0.2g / L, the vitamin C of 1mg / L and the Pluronic F-68 of 1g / L; 10% DMSO to prepare a serum-free freezing solution.
[0045] 2. Use the cryopreservation solution in this example to freeze T cells in human peripheral blood mononuclear cells (PBMC). The specific cryopreservation method is as follows:
[0046] PBMC were freshly isolated from human whole blood using density centrifugation for cryopreservation.
[0047] First, count the PBMC, then take the PBMC cell suspension corresponding to 1x10^6 cells in a 15mL centrifuge tube, centrifuge at 400g for 5 minutes to pel...
Embodiment 2
[0057] 1. The specific configuration method of the cryopreservation solution used in this embodiment is as follows:
[0058] Use IMDM and RPMI1640 two kinds of culture medium to mix by volume ratio 1:1 to prepare the basal culture medium of cryopreservation liquid, then add the ethanolamine that final concentration is 1mmol / L to it, the recombinant human insulin of 5g / L, the recombinant human insulin of 20mg / L Recombinant human transferrin, recombinant human serum albumin of 1g / L, vitamin C of 5mg / L, Pluronic F-68 of 1g / L and Kolliphor P 188 of 1g / L; DMSO to prepare a serum-free freezing solution.
[0059] 2. Use the cryopreservation solution in this example for cryopreservation of activated T cells. The specific cryopreservation method is as follows:
[0060] T cells cultured up to day 9 after initial activation were used for cryopreservation.
[0061] First, count the T cells on the 9th day of activation, then take the T cell suspension corresponding to 1x10^6 cells in a 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com