Patents
Literature
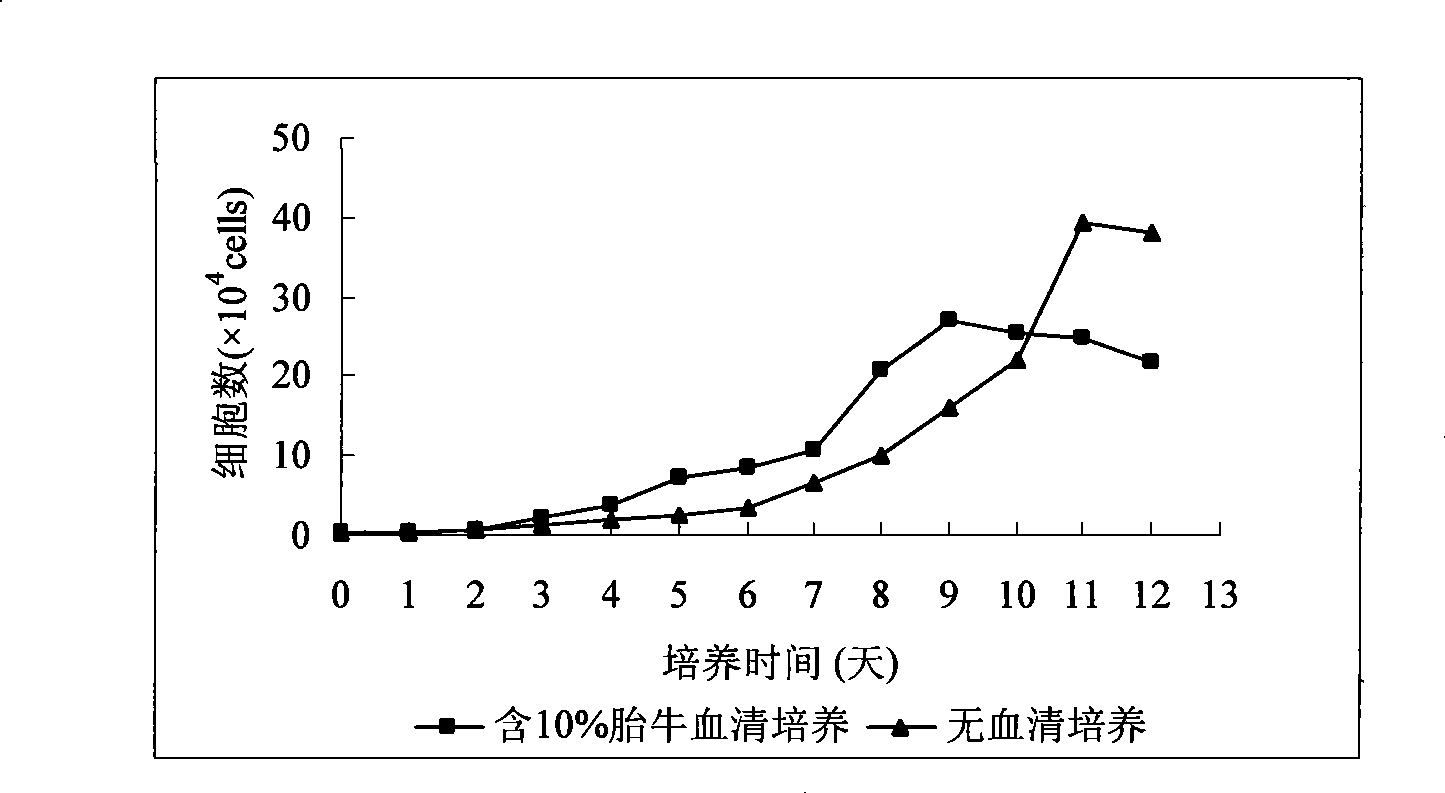
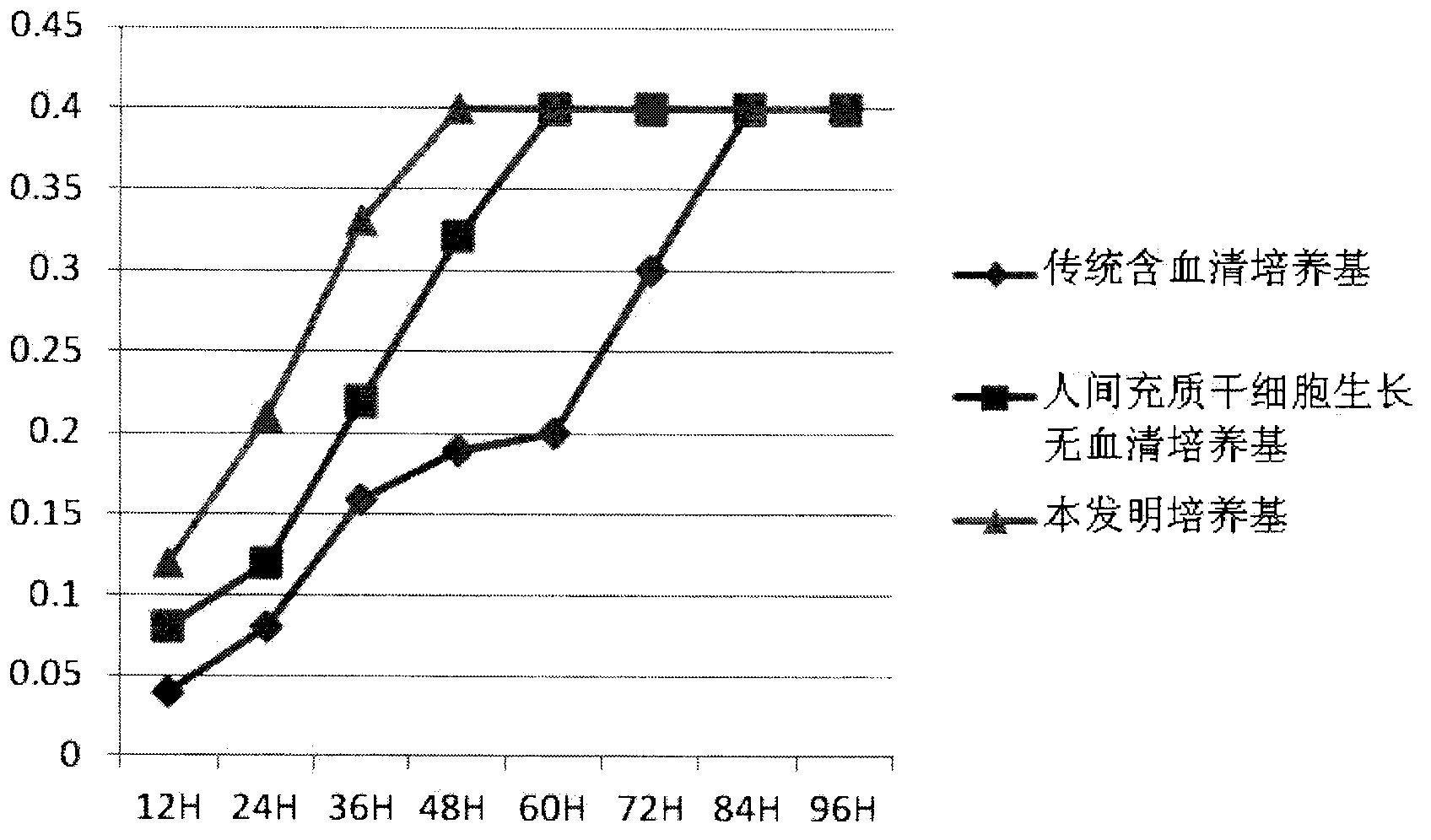
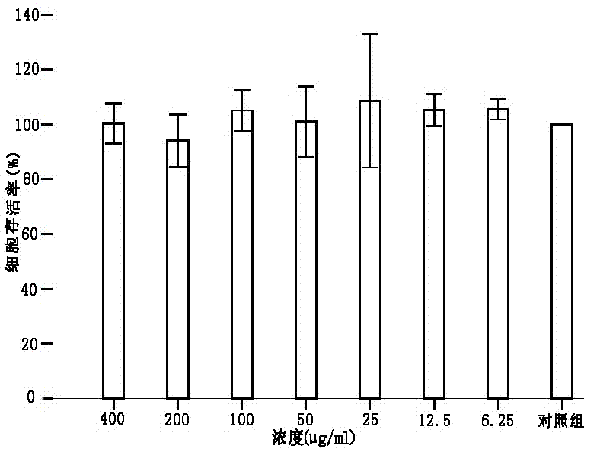
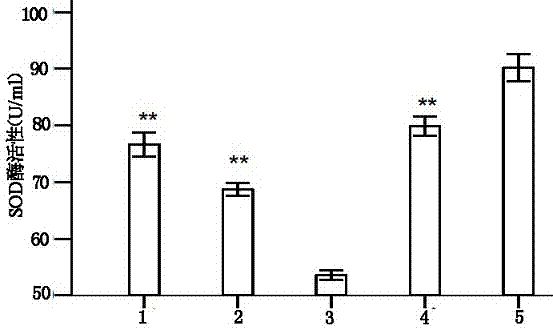
1144 results about "Serum free" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Defined media for stem cell culture
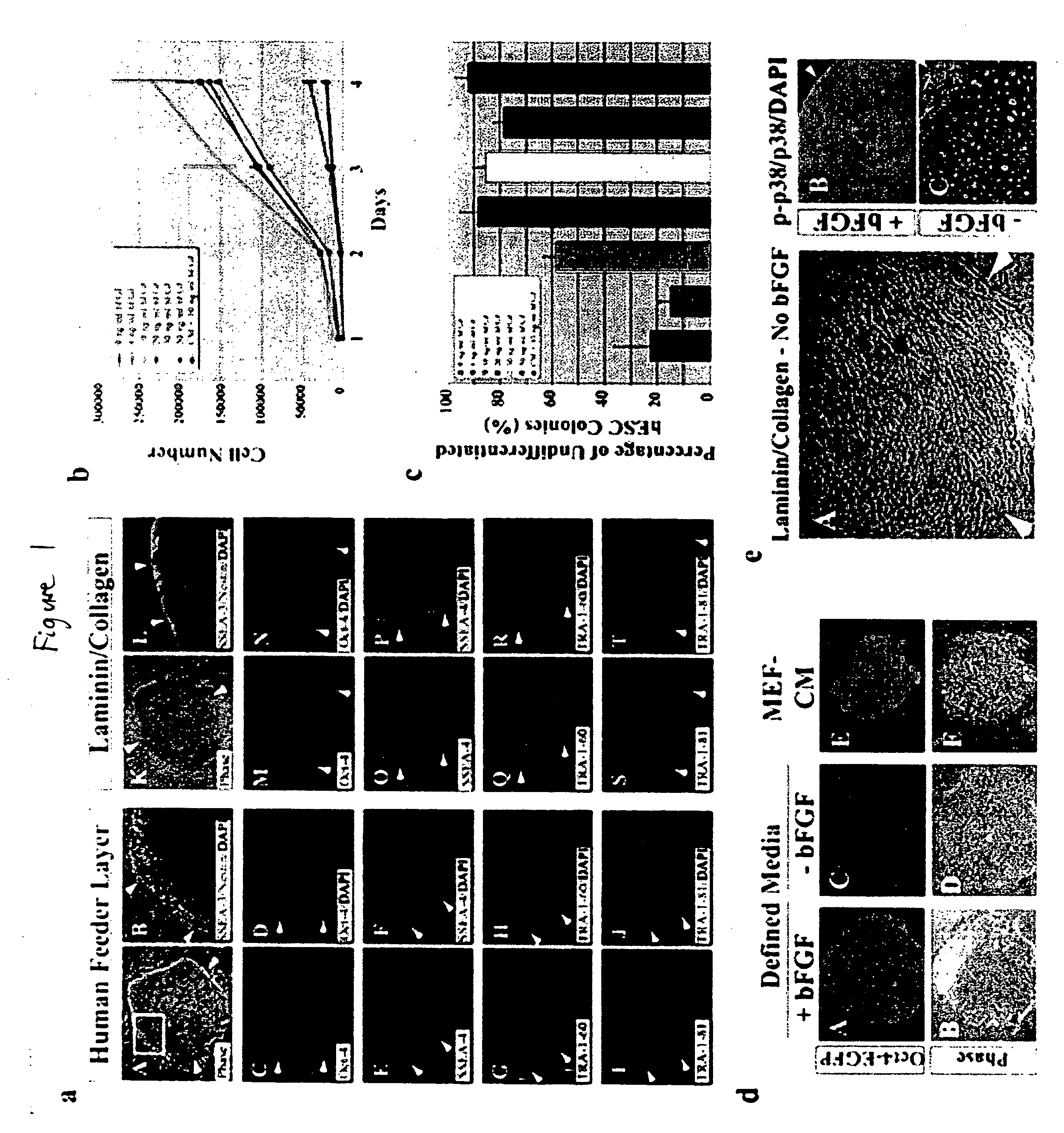
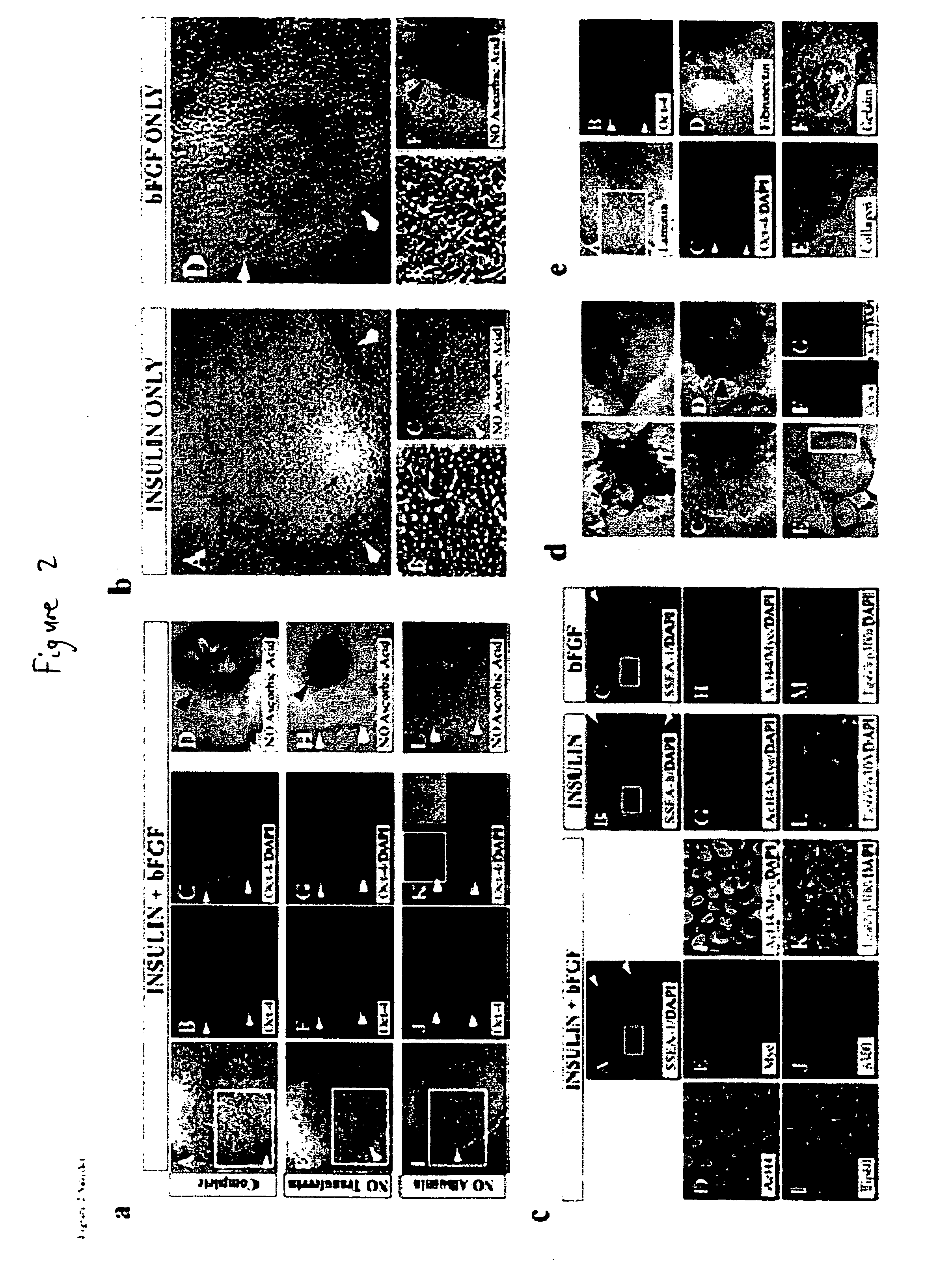
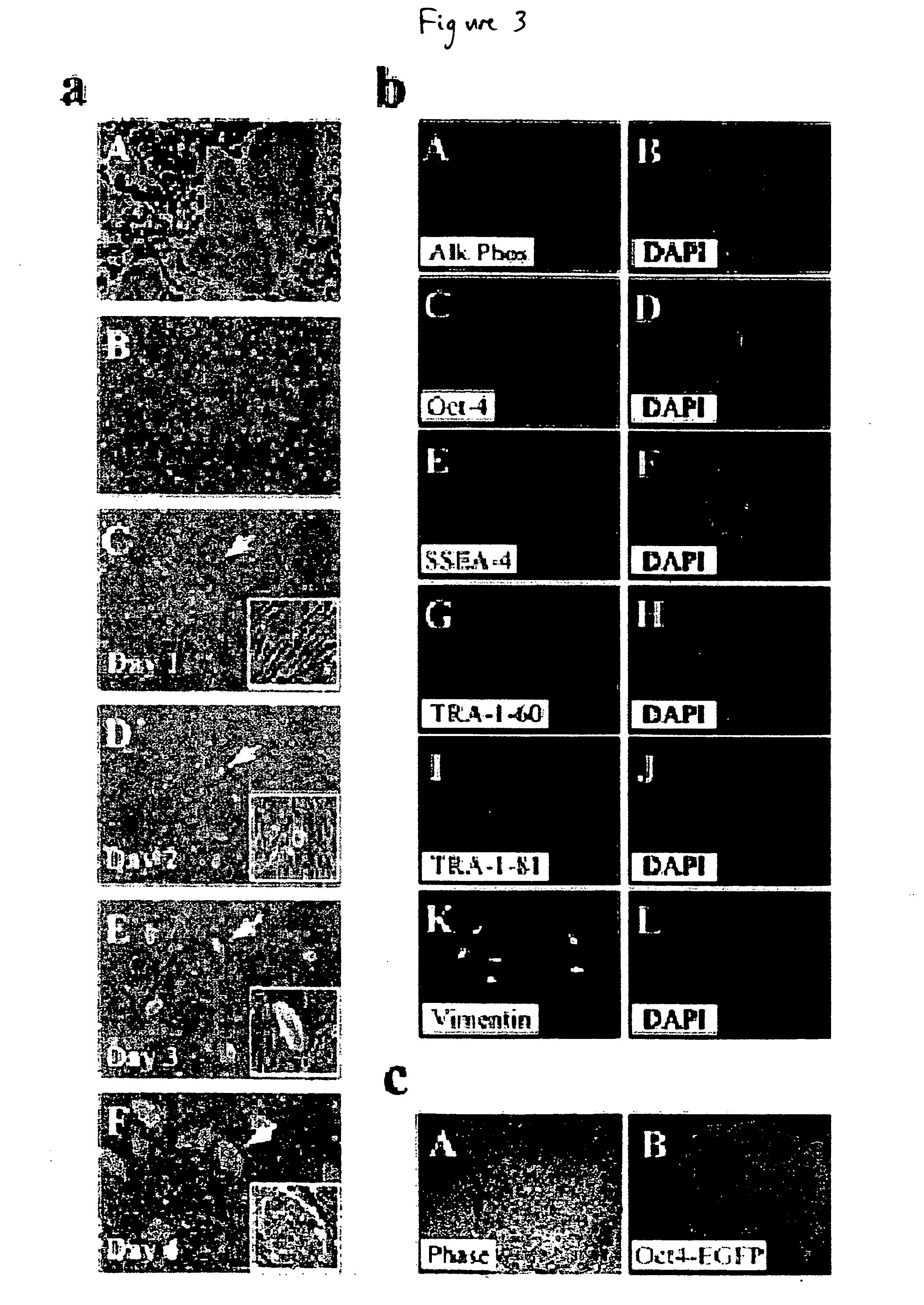
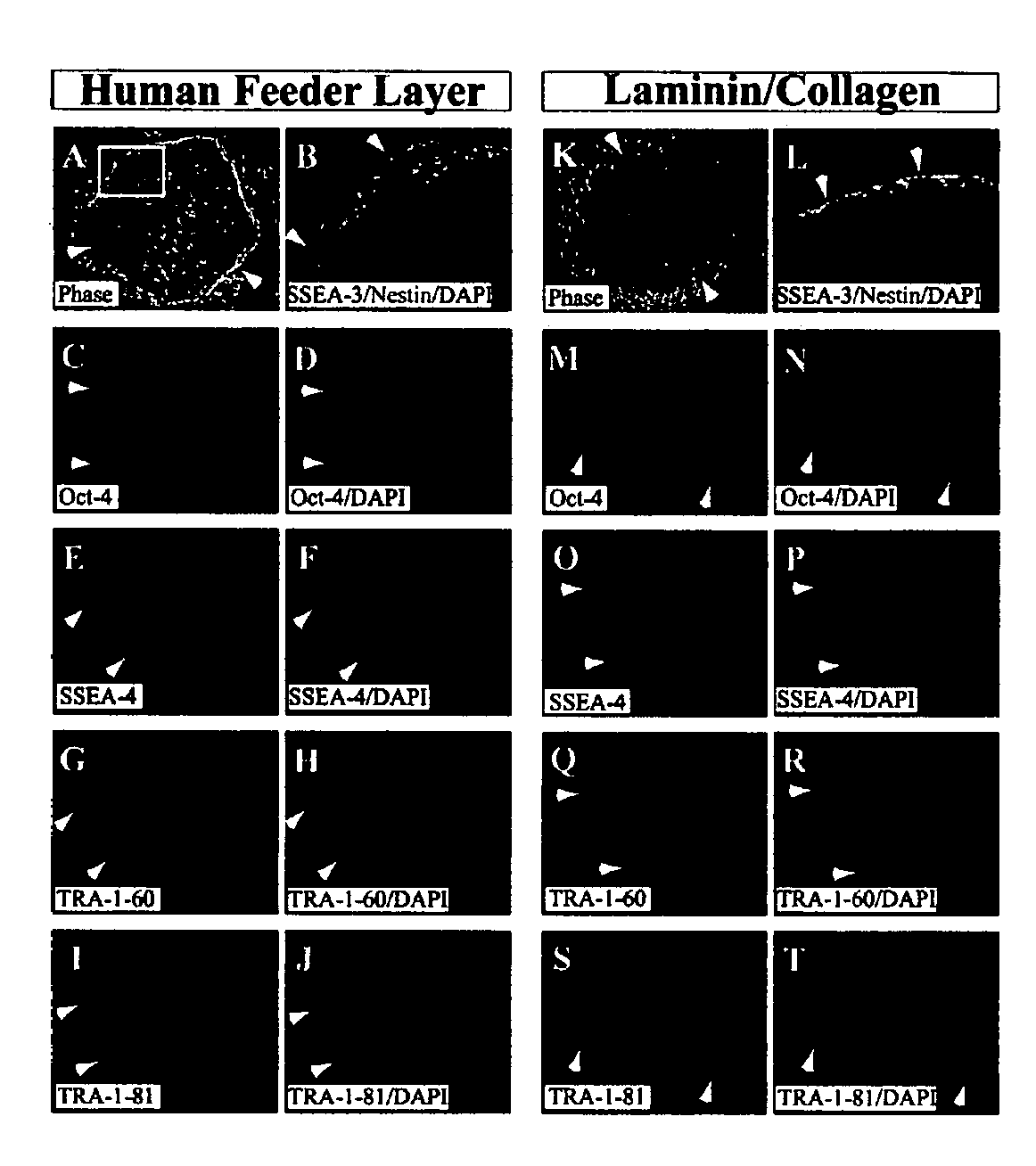
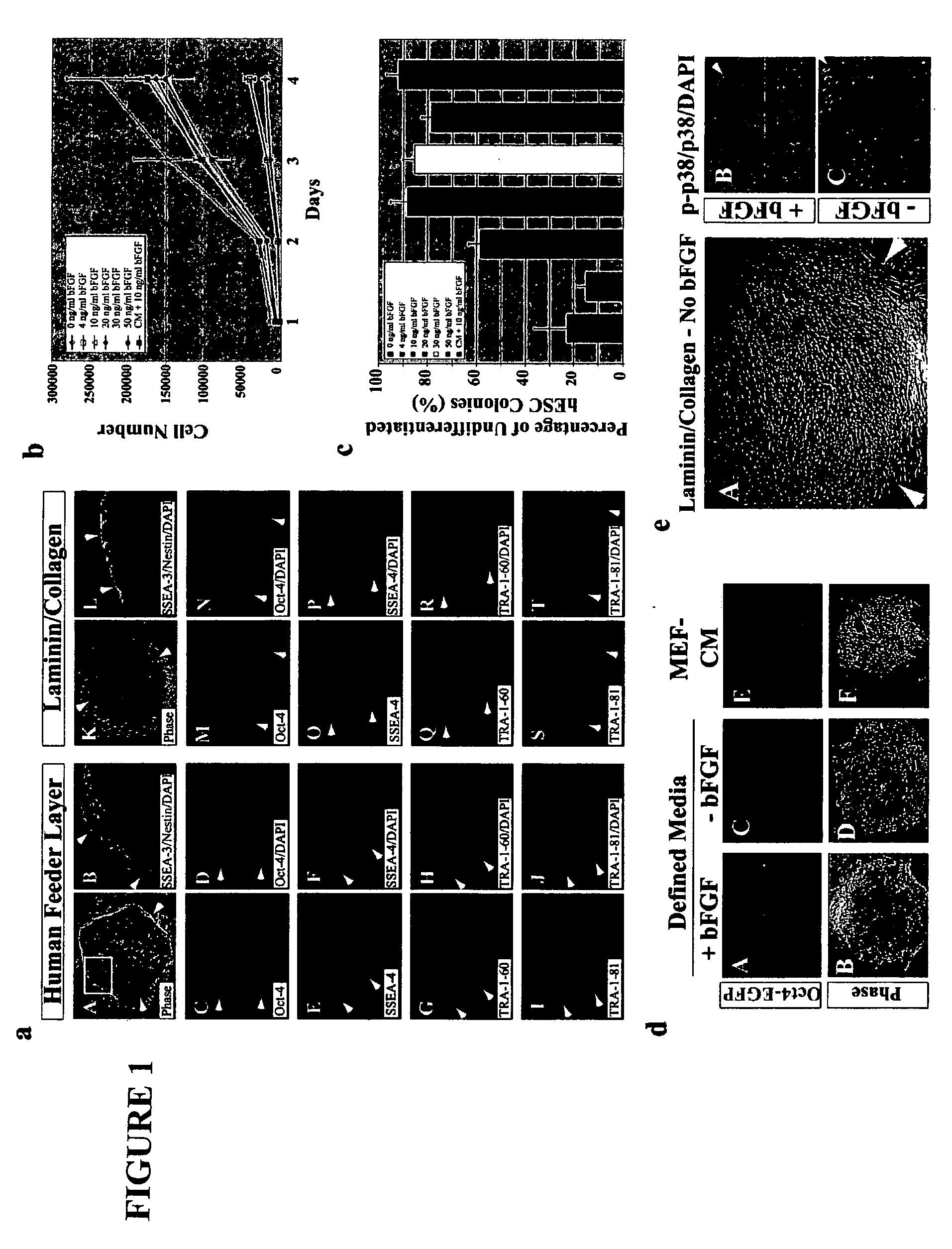
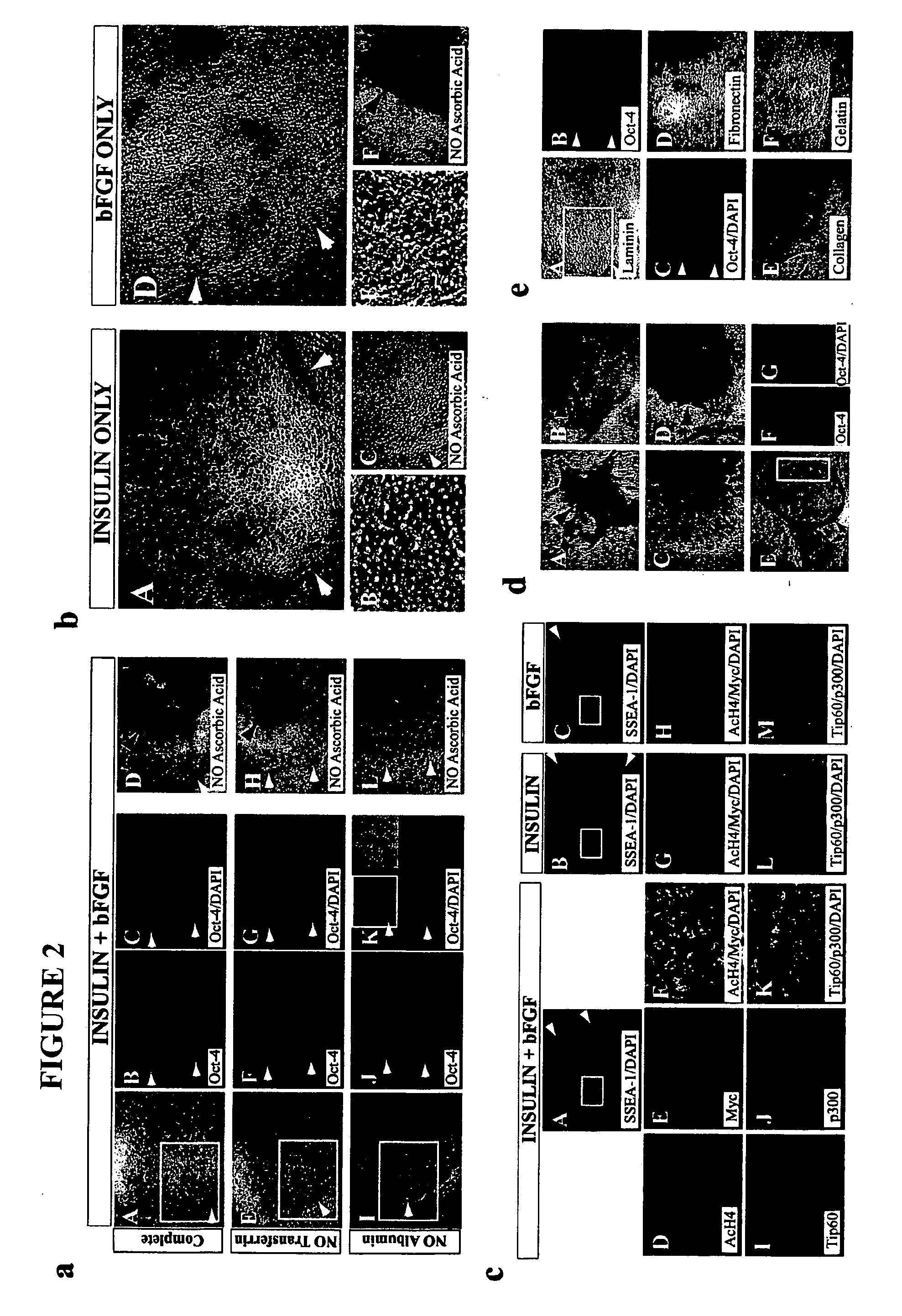
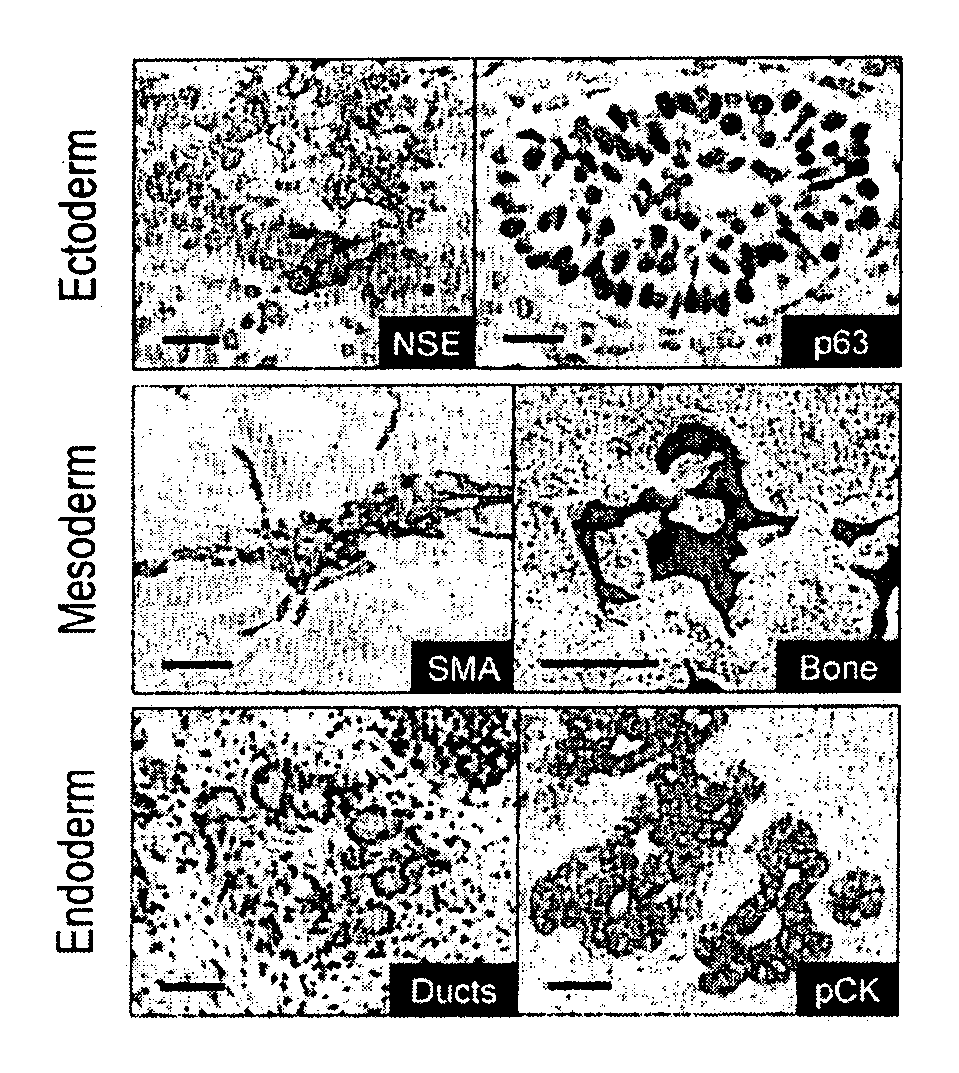
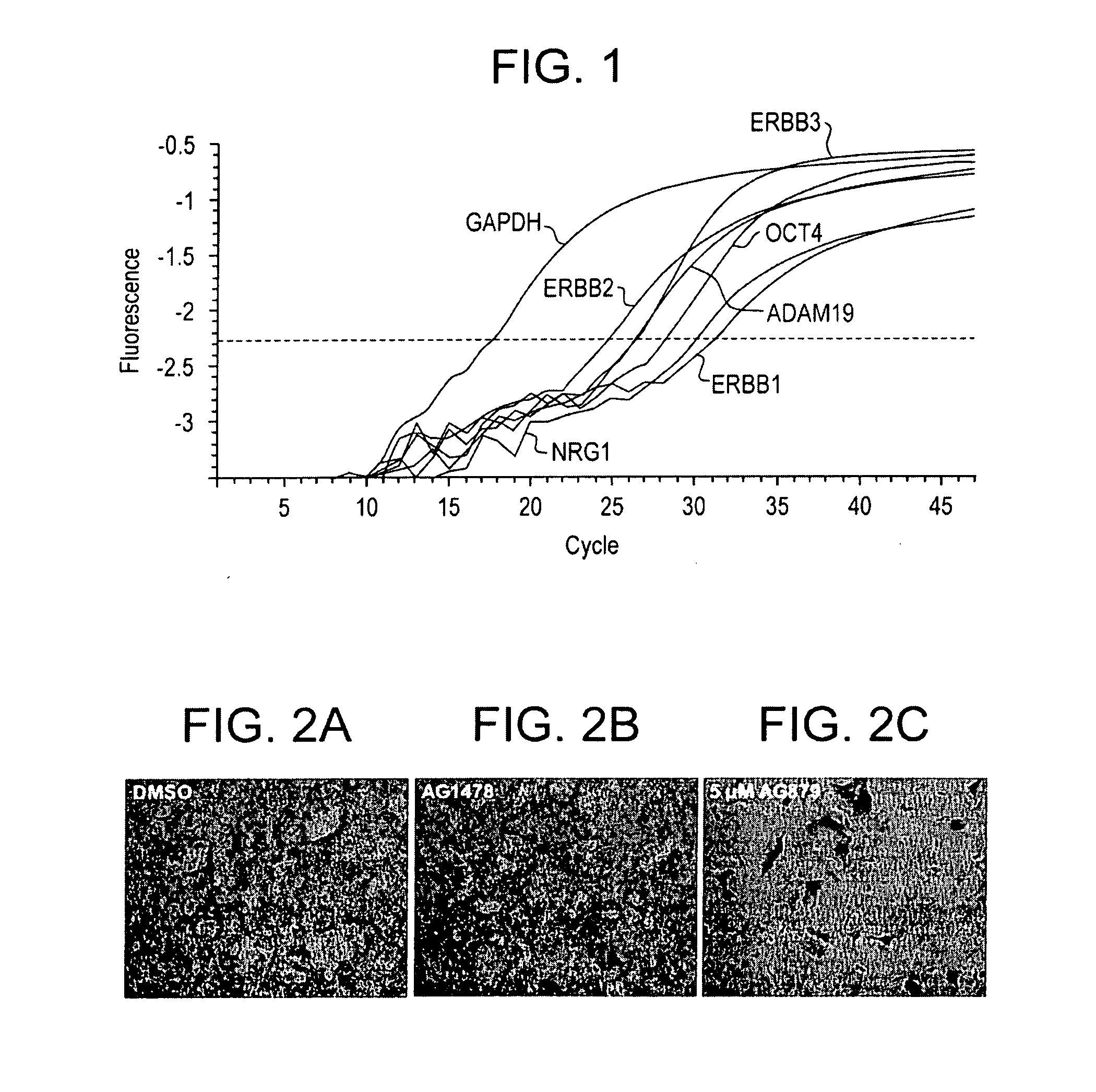
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Defined media for pluripotent stem cell culture
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
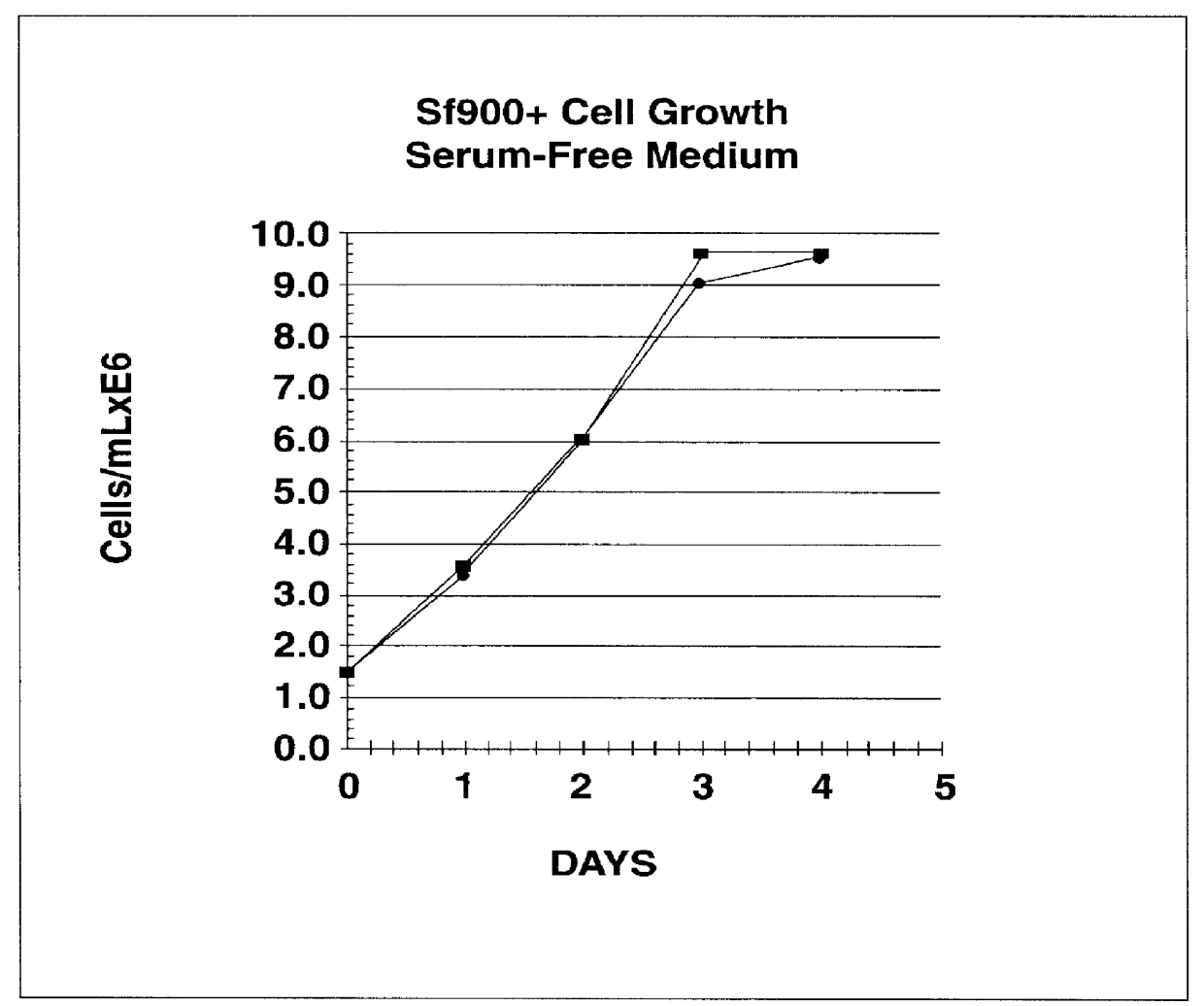
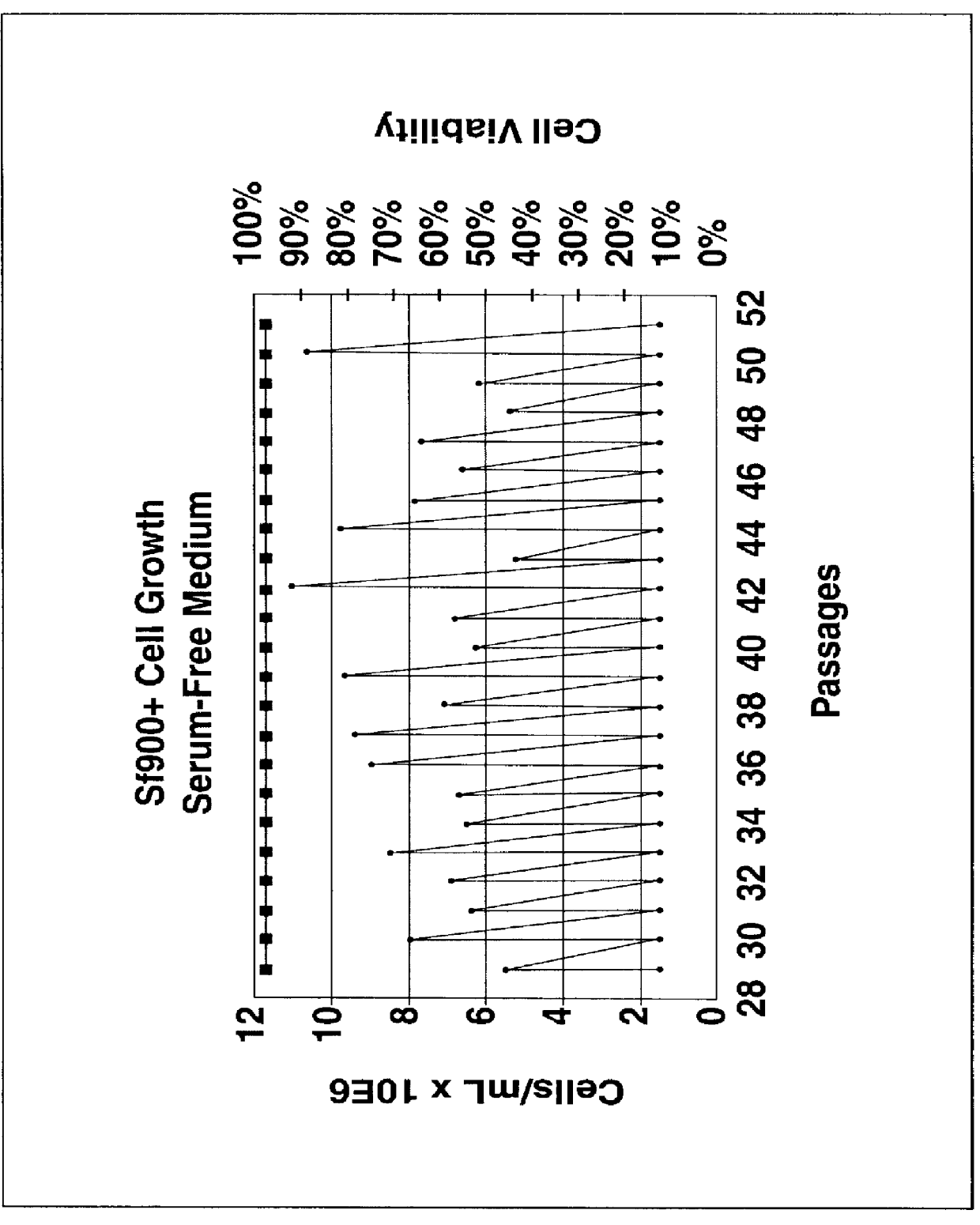
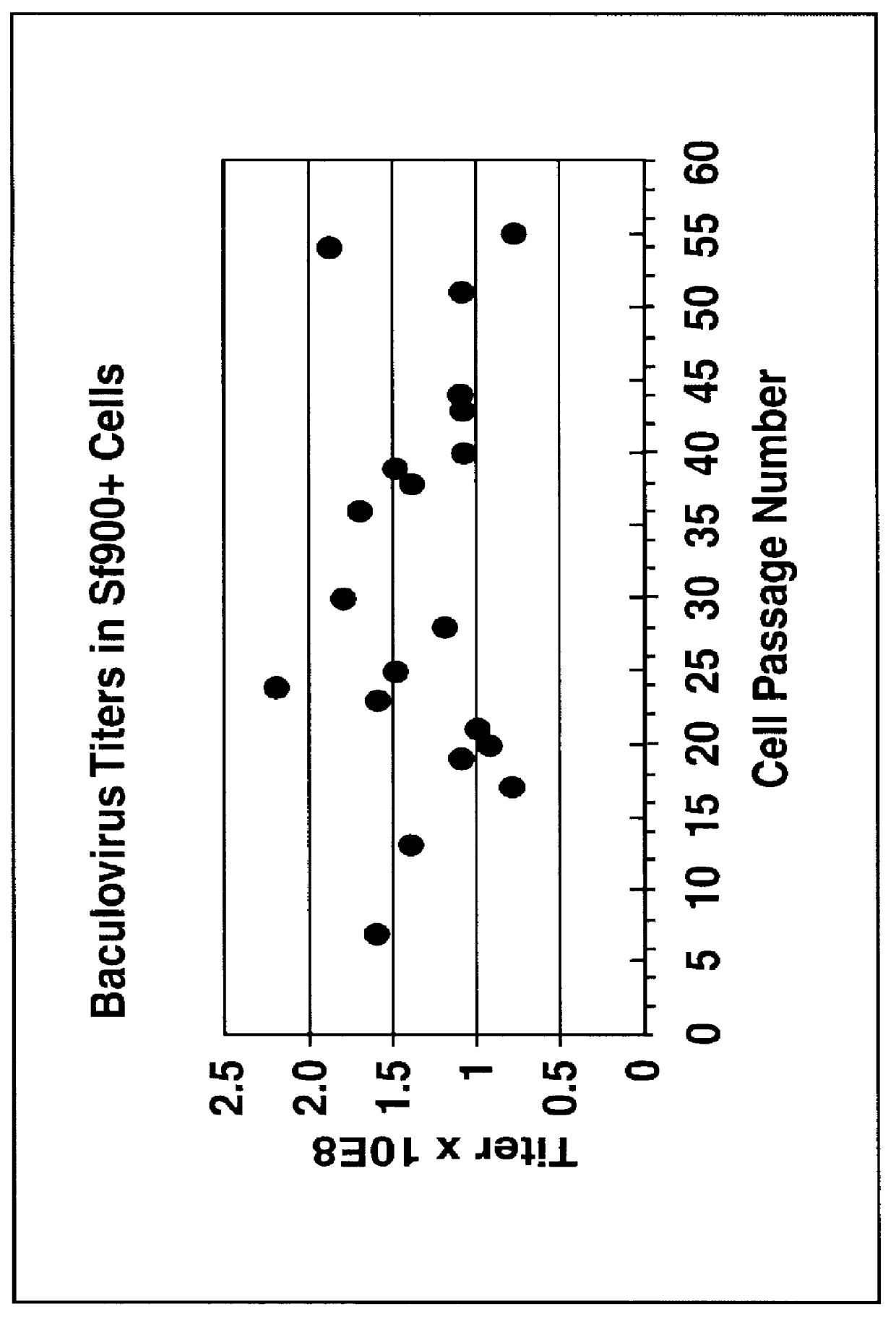
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
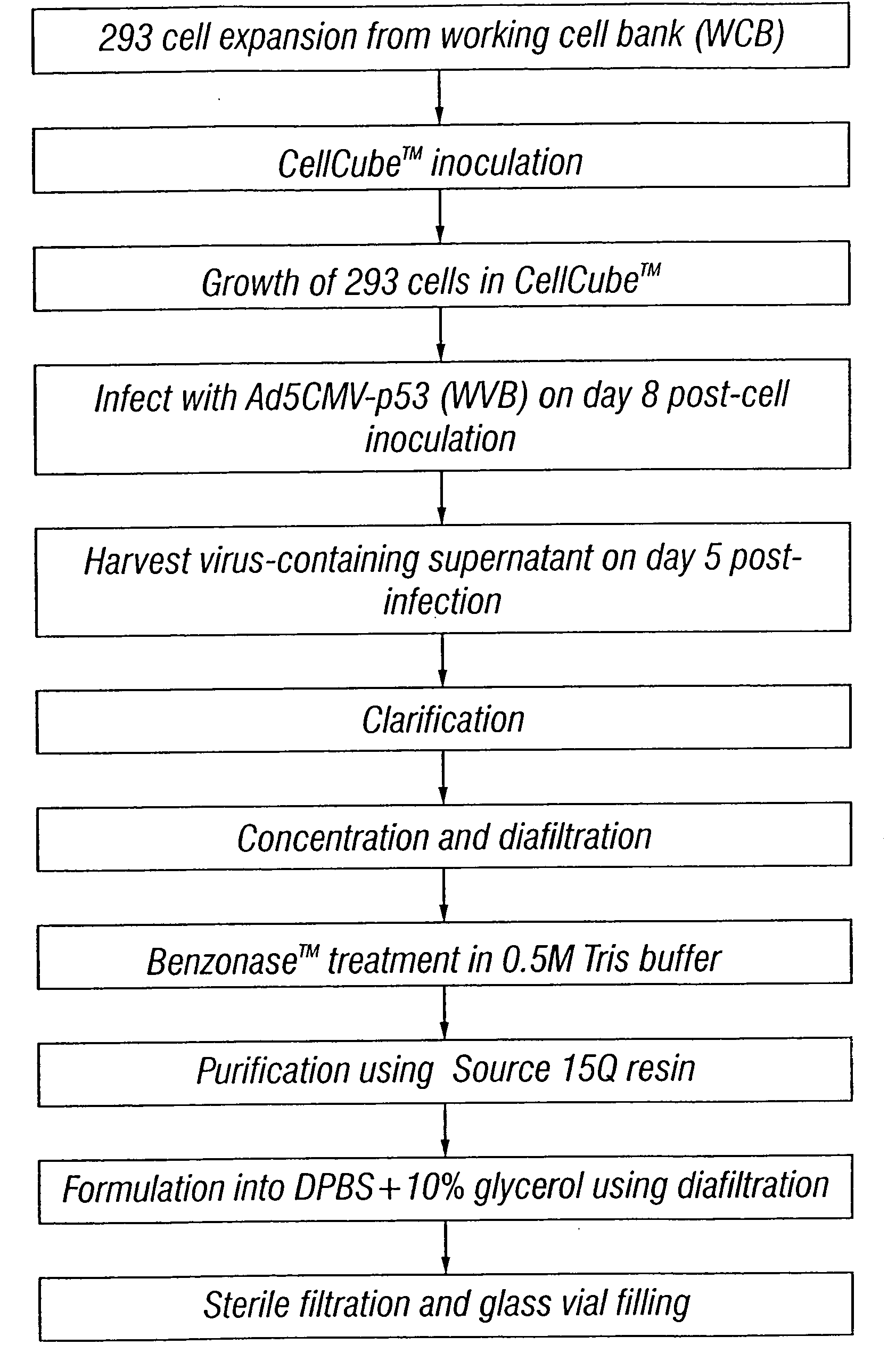
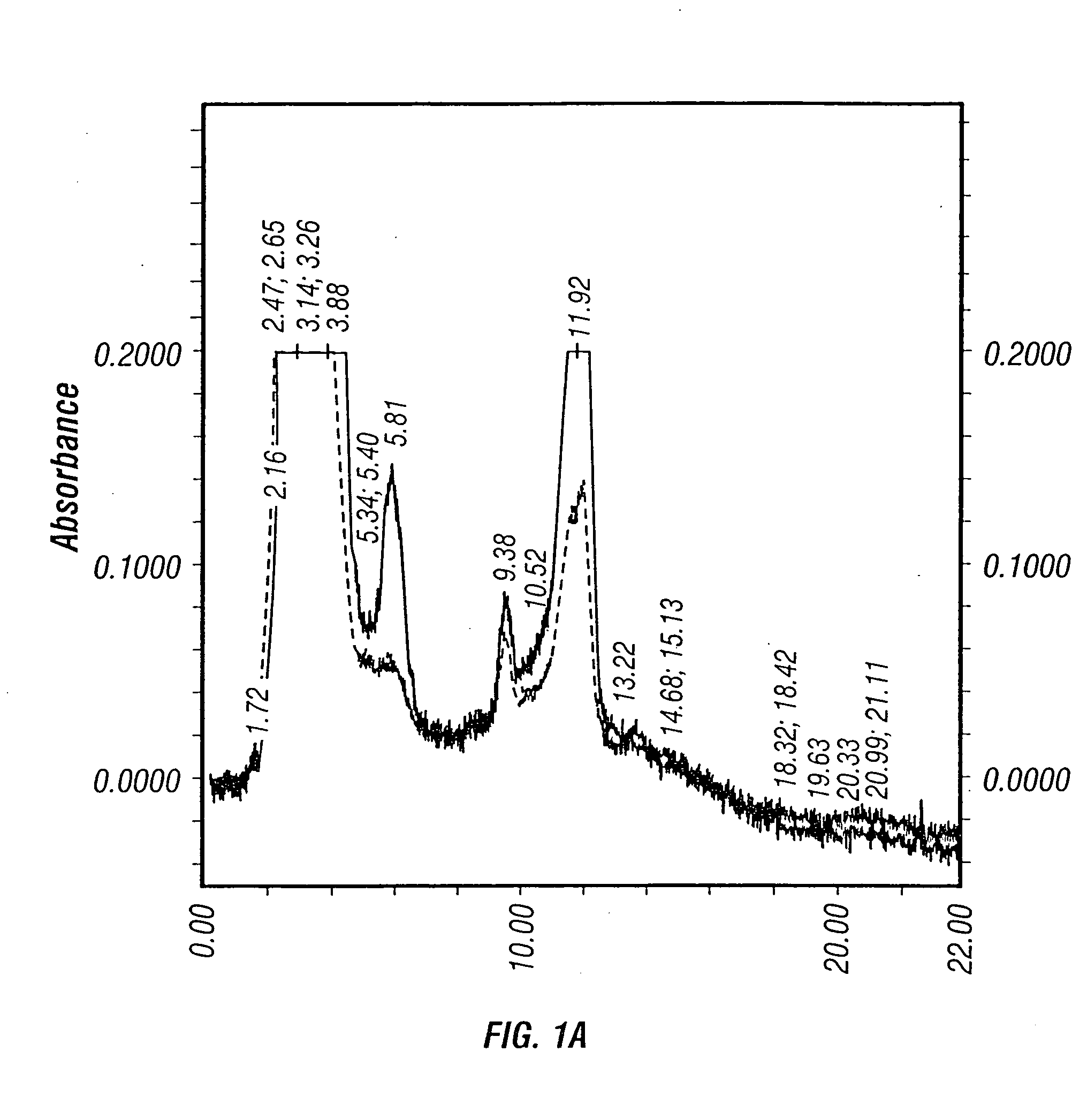
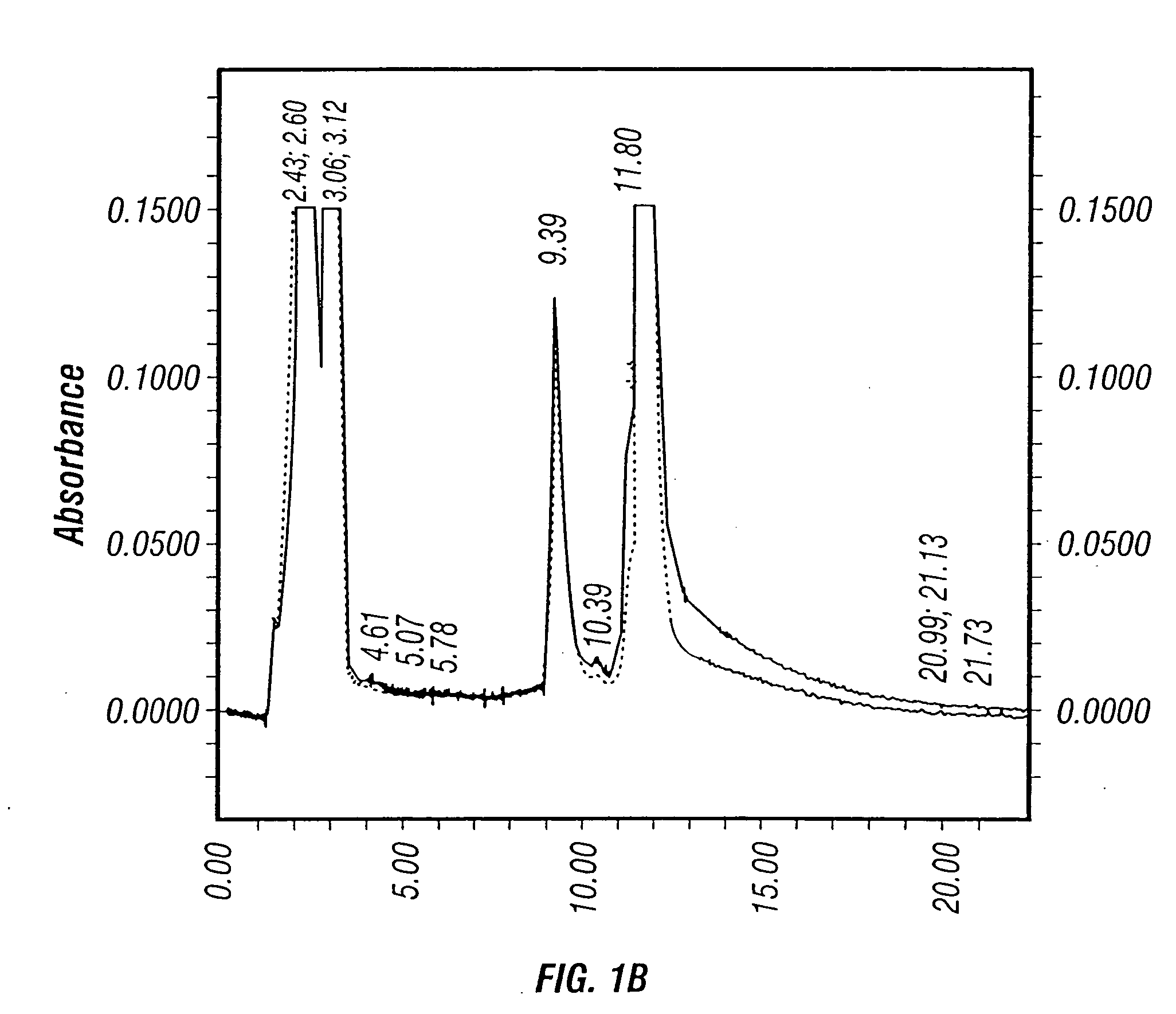
Method for the production and purification of adenoviral vectors
InactiveUS20080050770A1Peptide/protein ingredientsGenetic material ingredientsSerum igeUltracentrifuge
Owner:JANSSEN VACCINES & PREVENTION BV
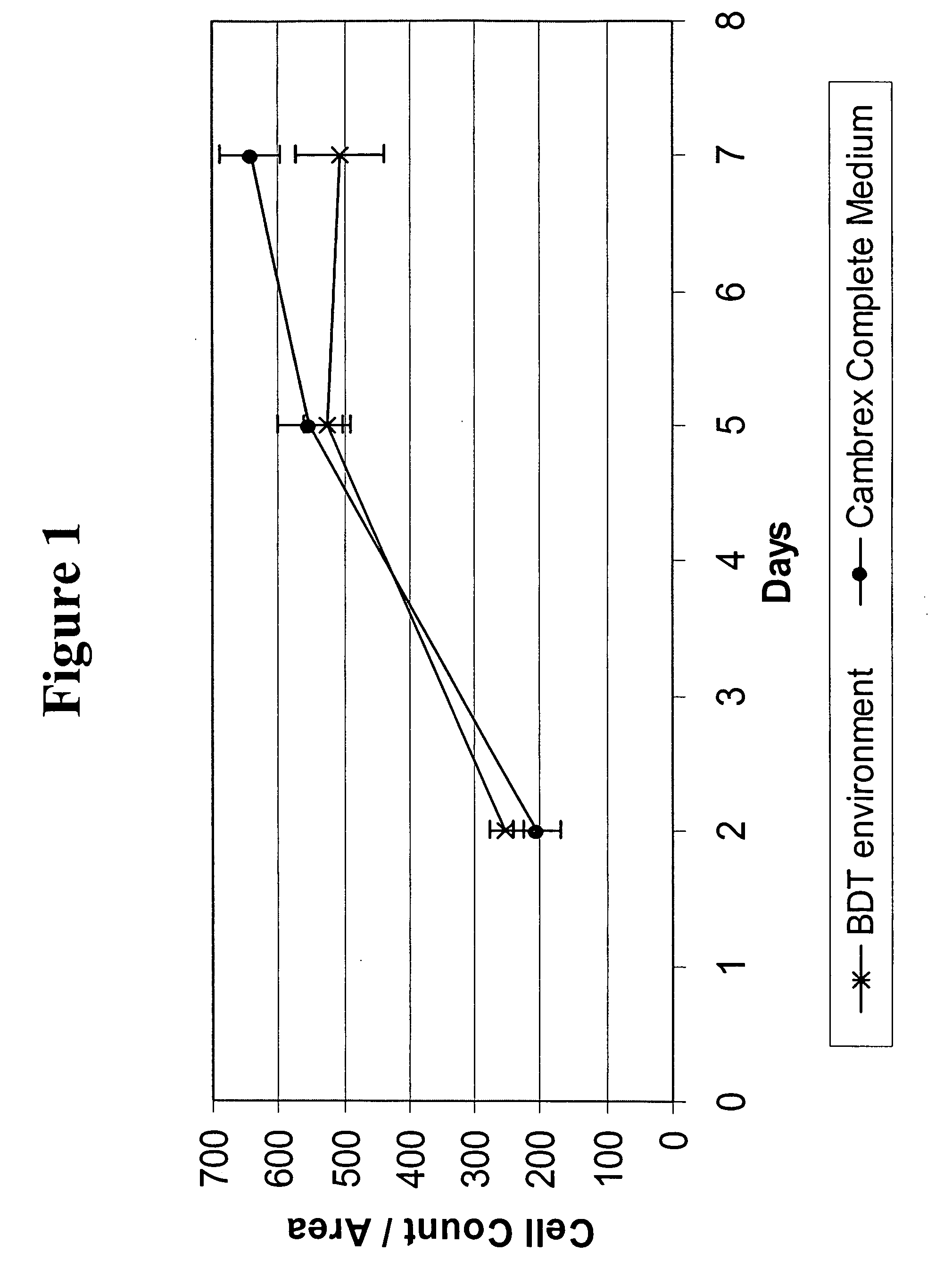
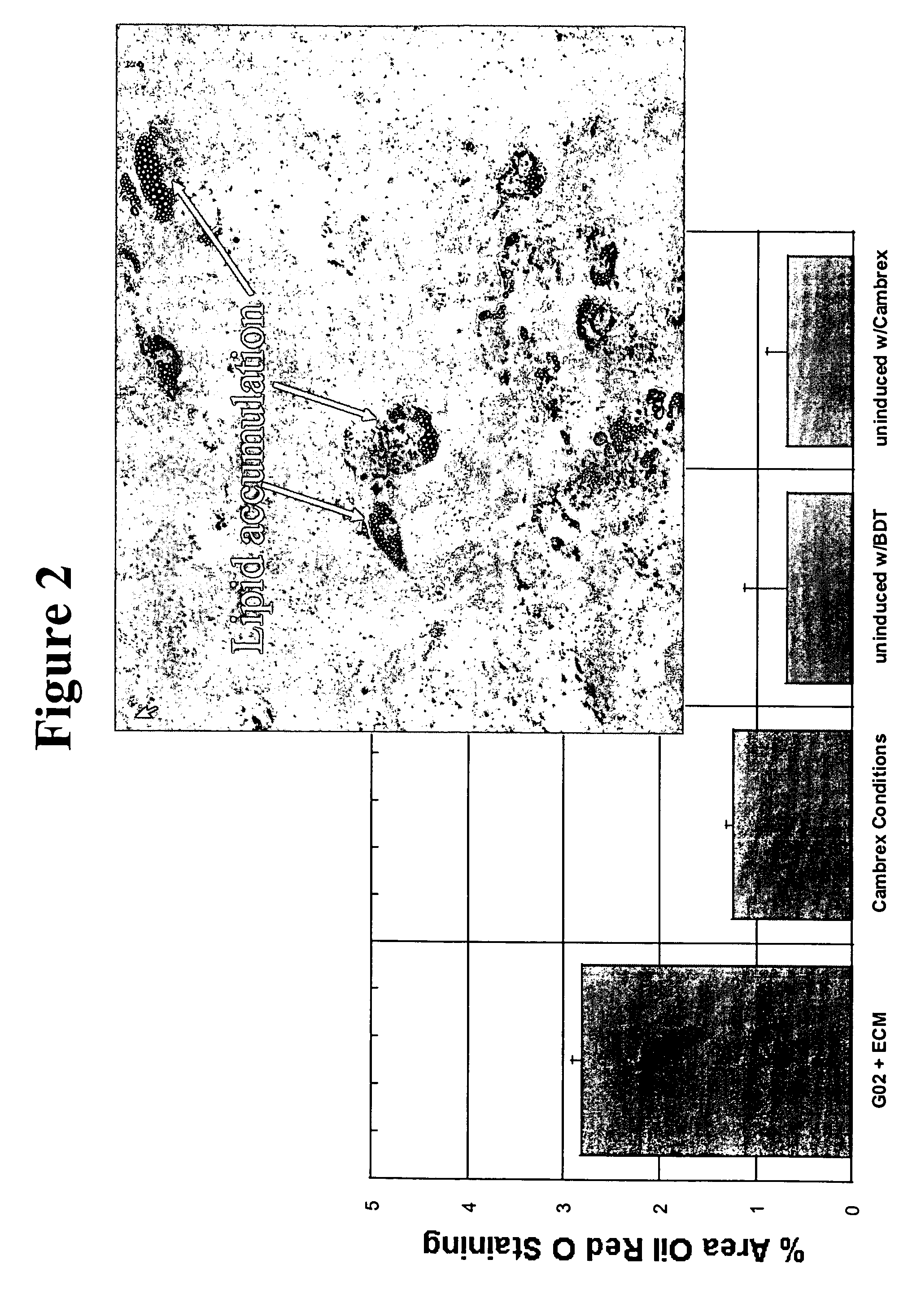
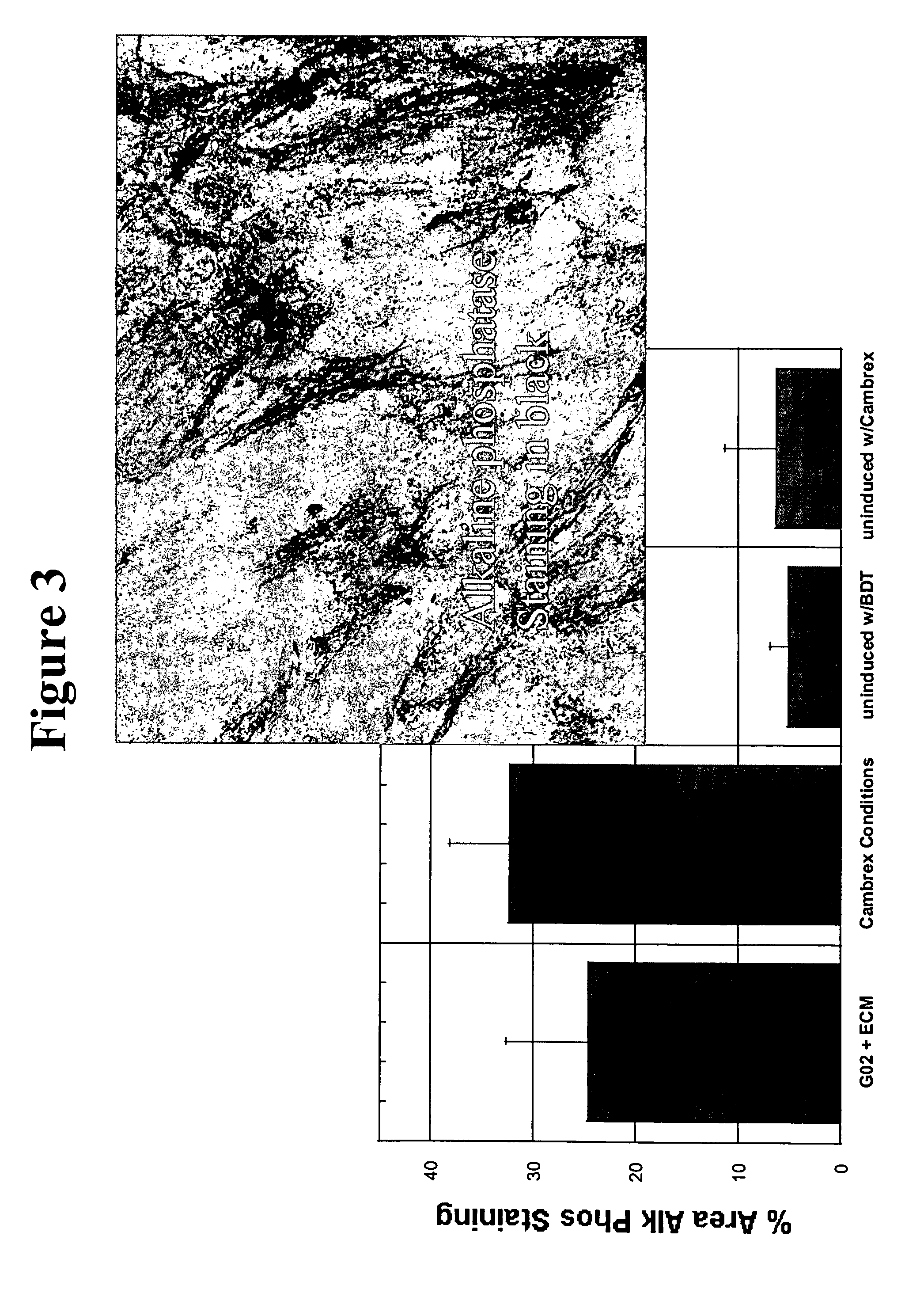
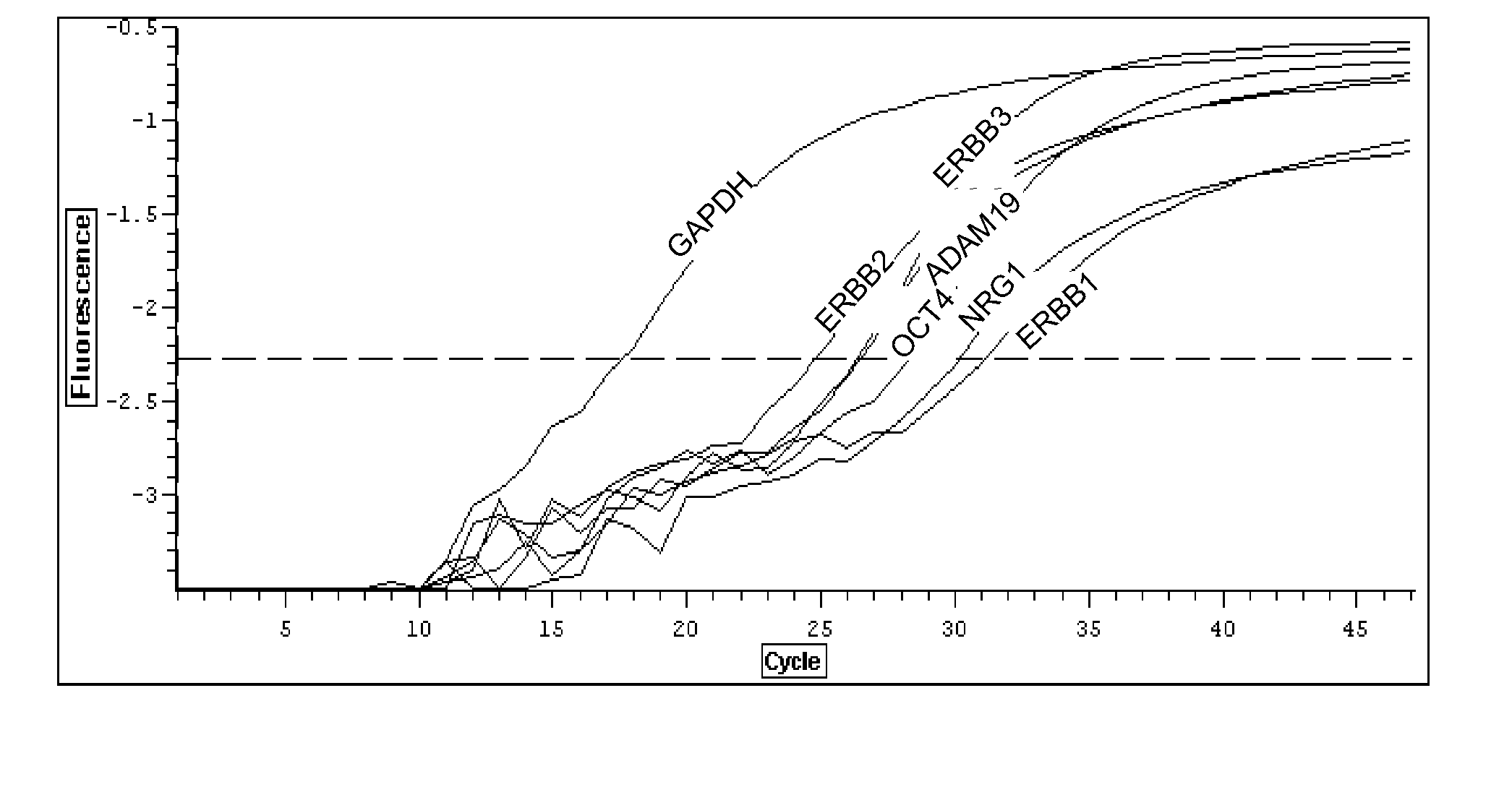
Cell culture environments for the serum-free expansion of mesenchymal stem cells
InactiveUS20050265980A1Promoting mesenchymal stem cell (MSC) expansionBiocideCulture processSerum freeMesenchymal stem cell
Compositions and methods for promoting mesenchymal stem cell expansion while maintaining a pluripotent phenotype are disclosed. Serum-free cell culture systems and kits and methods of use for mesenchymal stem cell expansion are provided. Methods also comprise the use of the expanded mesenchymal stem cells to treat various disorders or diseases, particularly those of the cardiovascular system, bone, or cartilage.
Owner:BECTON DICKINSON & CO
Method for the production and purification of adenoviral vectors
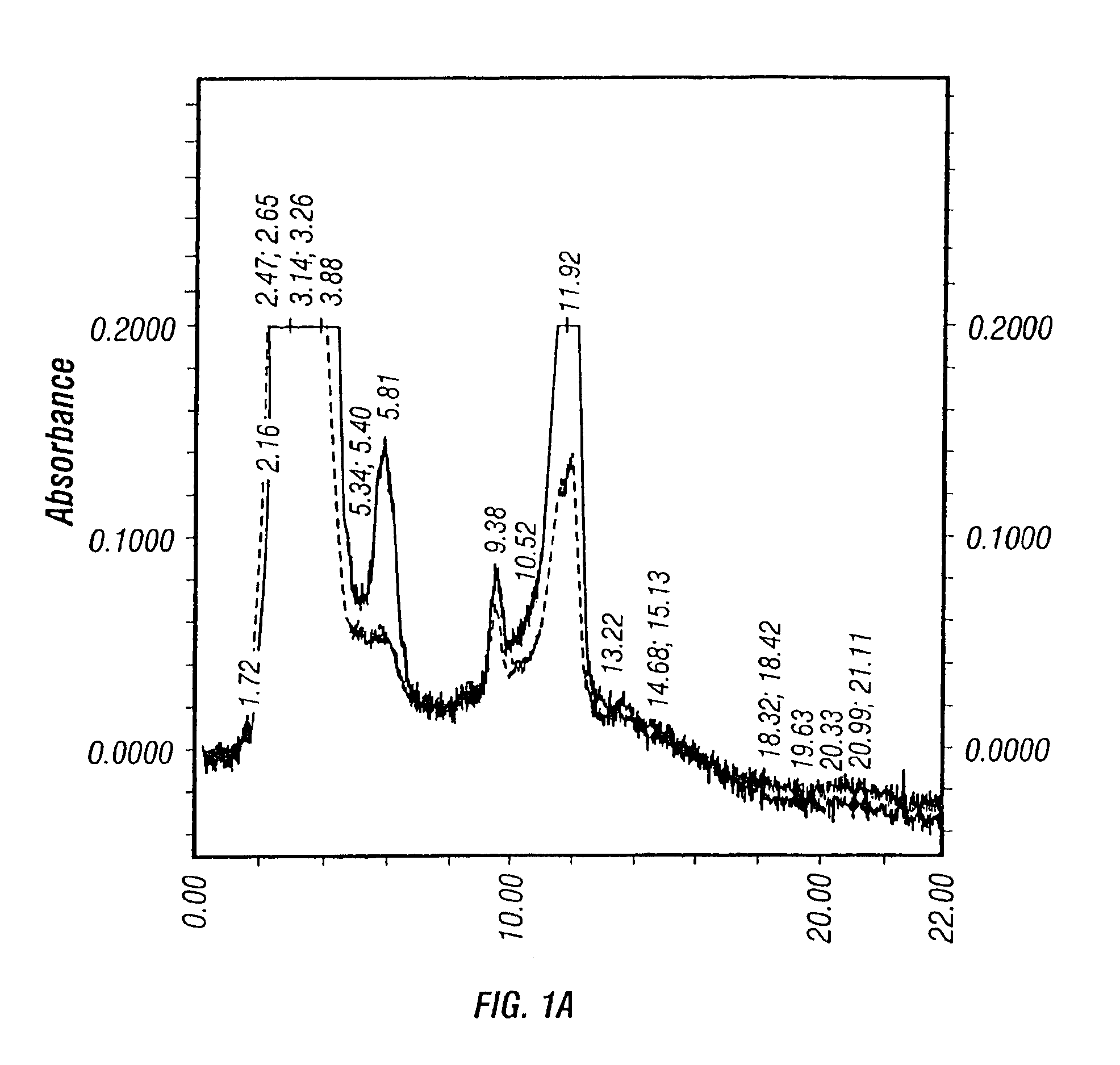
The present invention addresses the need to improve the yields of viral vectors when grown in cell culture systems. In particular, it has been demonstrated that for adenovirus, the use of low-medium perfusion rates in an attached cell culture system provides for improved yields. In other embodiments, the inventors have shown that there is improved Ad-p53 production cells grown in serum-free conditions, and in particular in serum-free suspension culture. Also important to the increase of yields is the use of detergent lysis. Combination of these aspects of the invention permits purification of virus by a single chromatography step that results in purified virus of the same quality as preparations from double CsCl banding using an ultracentrifuge.
Owner:JANSSEN VACCINES & PREVENTION BV
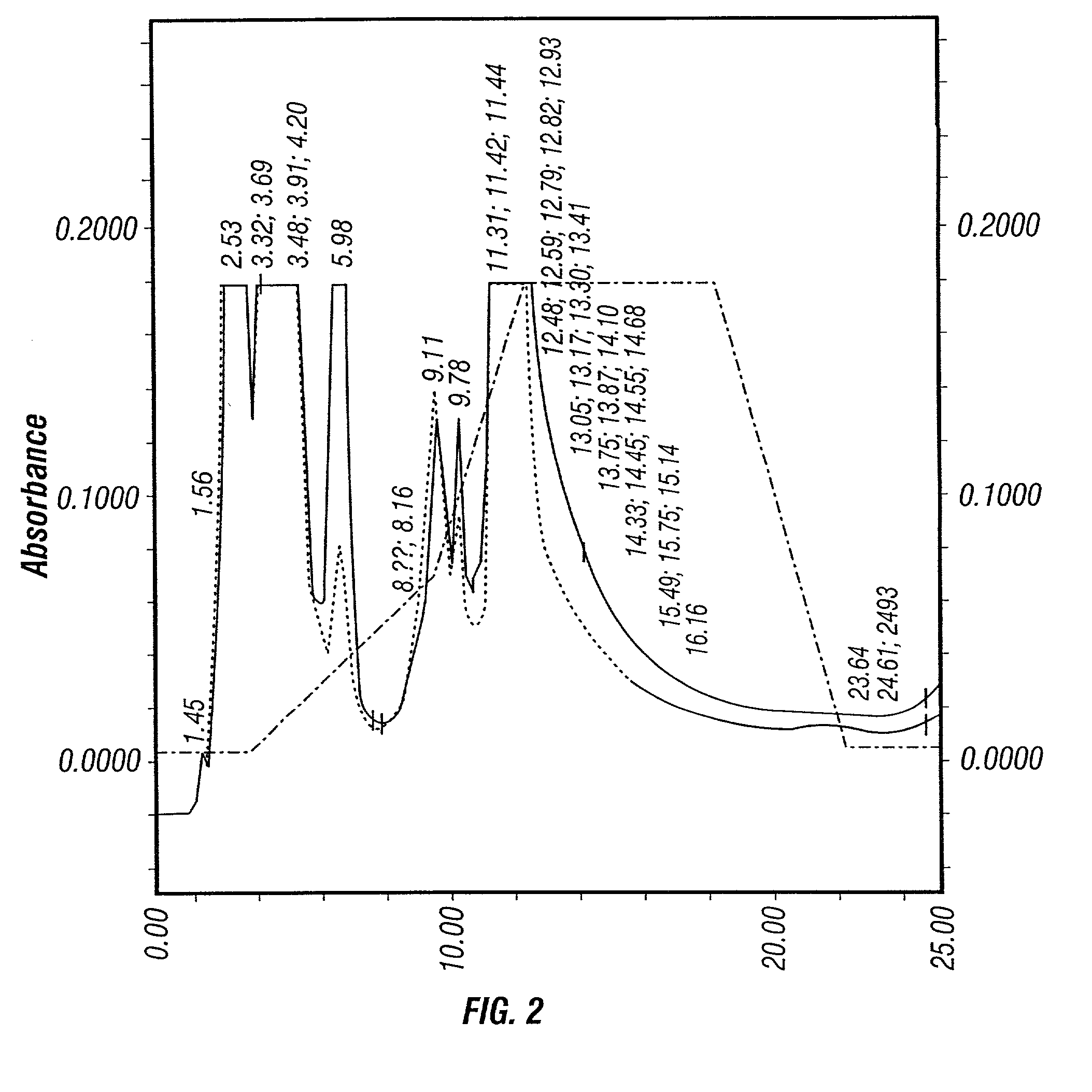
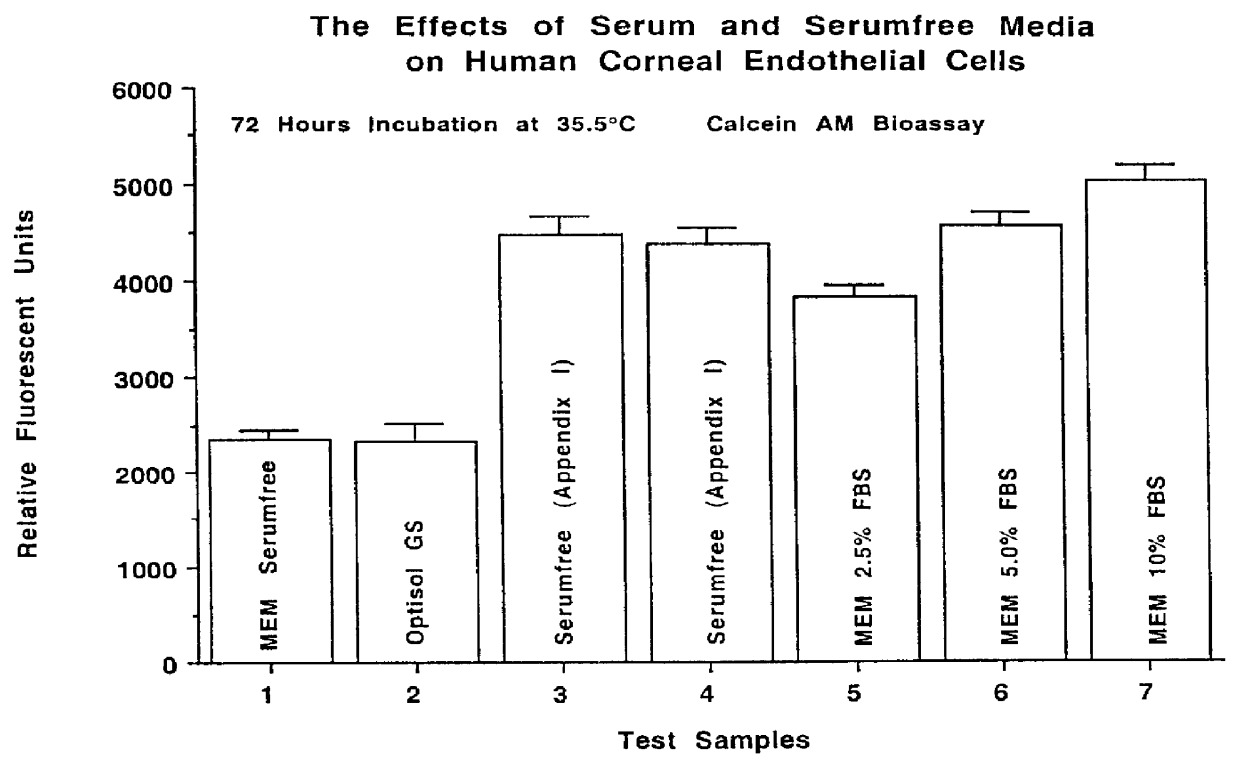
Defined serumfree medical solution for ophthalmology
A defined serumfree medical solution for applications in Ophthalmology, that contains one or more cell nutrient supplements, and a growth factor(s) which maintains and enhances the preservation of eye tissues, including human corneal, retinal and corneal epithelial tissues at low to physiological temperatures (2 DEG C. to 38 DEG C.). This solution is composed of a defined aqueous nutrient and electrolyte solution, supplemented with a glycosaminoglycan(s), a deturgescent agent(s), an energy source(s), a buffer system(s), an antioxidant(s), membrane stabilizing agents, an antibiotic(s) and / or antimycotic agent(s), ATP or energy precursors, nutrient cell supplements, coenzymes and enzyme supplements, nucleotide precursors, hormonal supplements, non-essential amino acids, trace minerals, trace elements and a growth factor(s).
Owner:SKELNIK DEBRA L
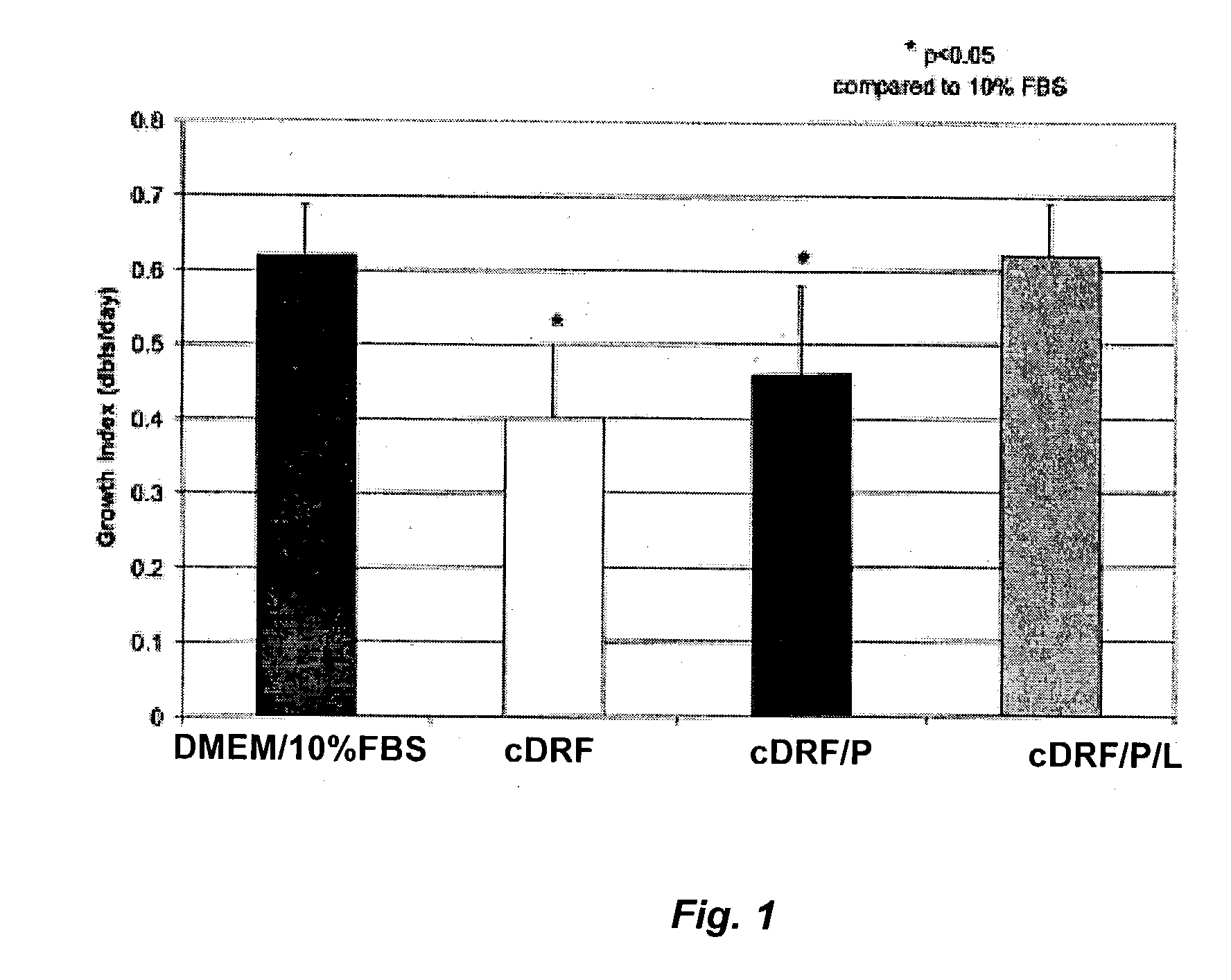
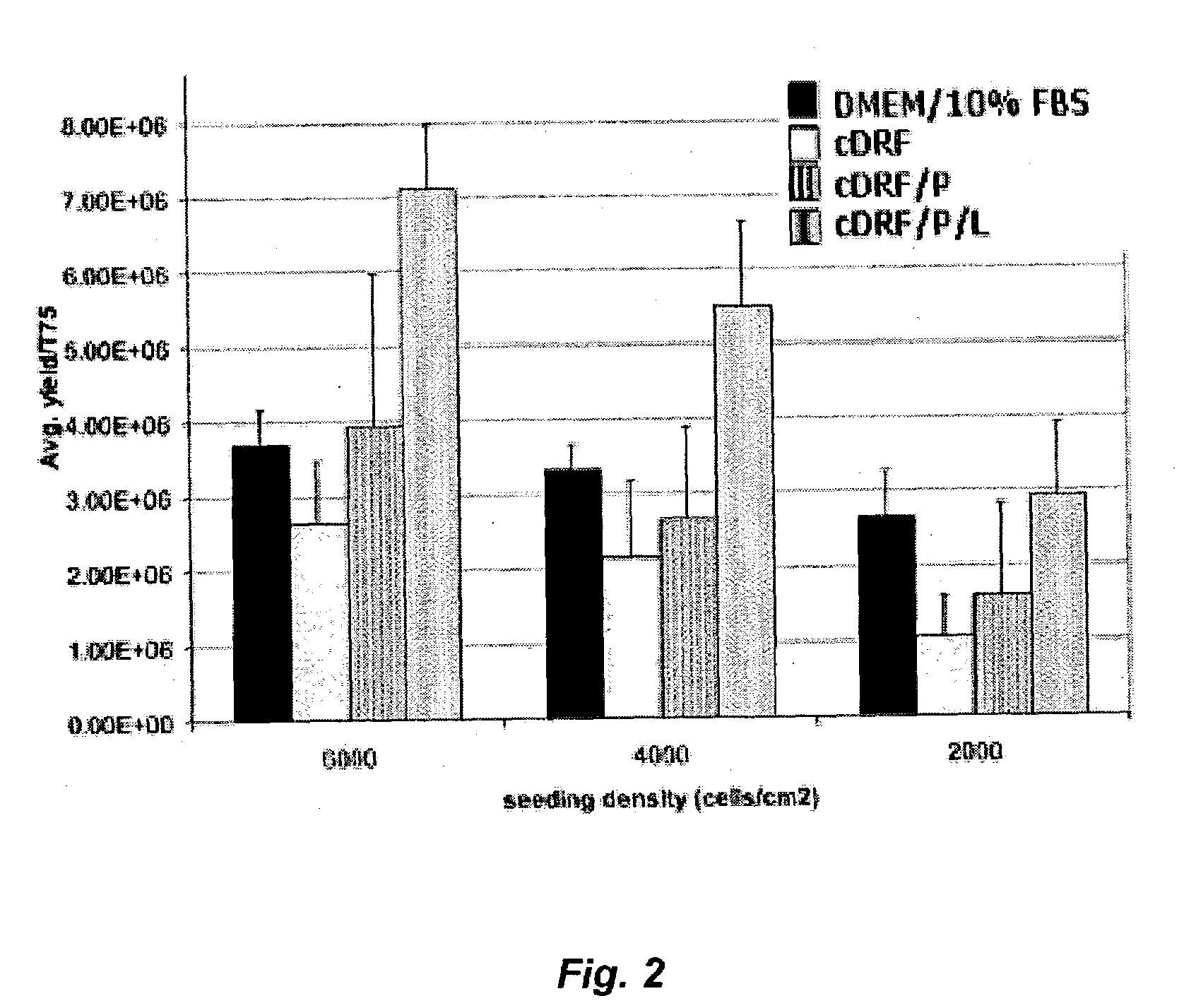
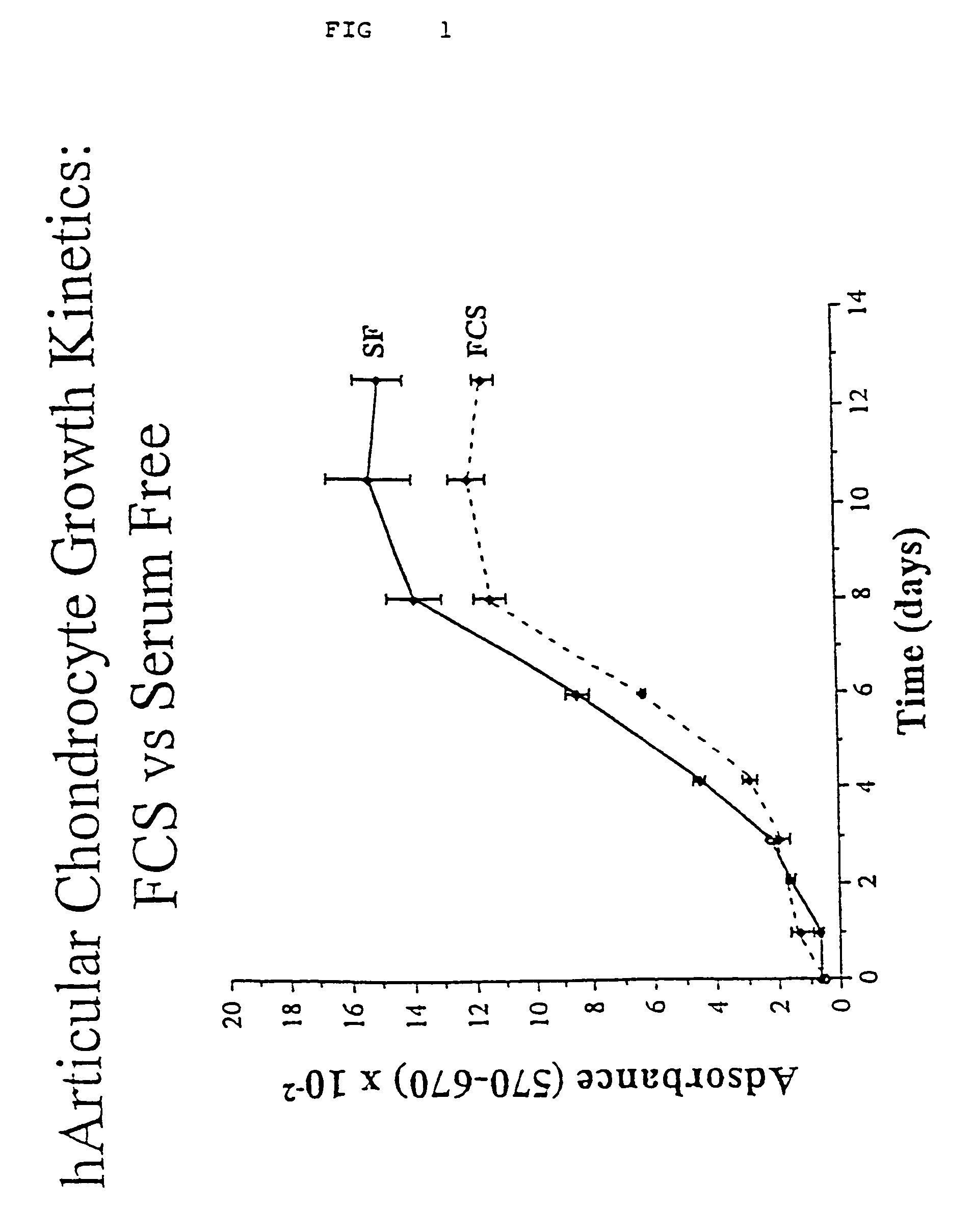
Serum-free media for chondrocytes and methods of use thereof
InactiveUS7169610B2Safe effective inexpensiveSafe and effective and inexpensiveCulture processArtificial cell constructsLipid formationSerum free media
The present invention provides defined serum-free cell culture media useful in culturing fibroblasts, especially articular chondrocytes, that avoids problems inherent in the use of serum-containing media. The defined media comprise platelet-derived growth factor (PDGF), and chemically defined lipids, or combinations of these compounds. In another aspect, the present invention also provides tissue culture methods that comprise incubating chondrocytes in the defined serum free media. The methods enhance attachment and proliferative expansion of chondrocytes seeded at low density while maintaining their redifferentiation potential.
Owner:GENZYME CORP
Mammalian cell lines for increasing longevity and protein yield from a cell culture
ActiveUS7537930B2Increase longevity and recombinant protein yieldIncreases lifespan and viabilityVirus peptidesApoptosis related proteinsSerum free mediaApoptosis
Owner:IMMUNOMEDICS INC
Methods for protein expression in mammalian cells in serum-free medium
ActiveUS7608425B2Increase longevity and recombinant protein yieldIncreases lifespan and viabilityPeptide/protein ingredientsApoptosis related proteinsSerum free mediaMammal
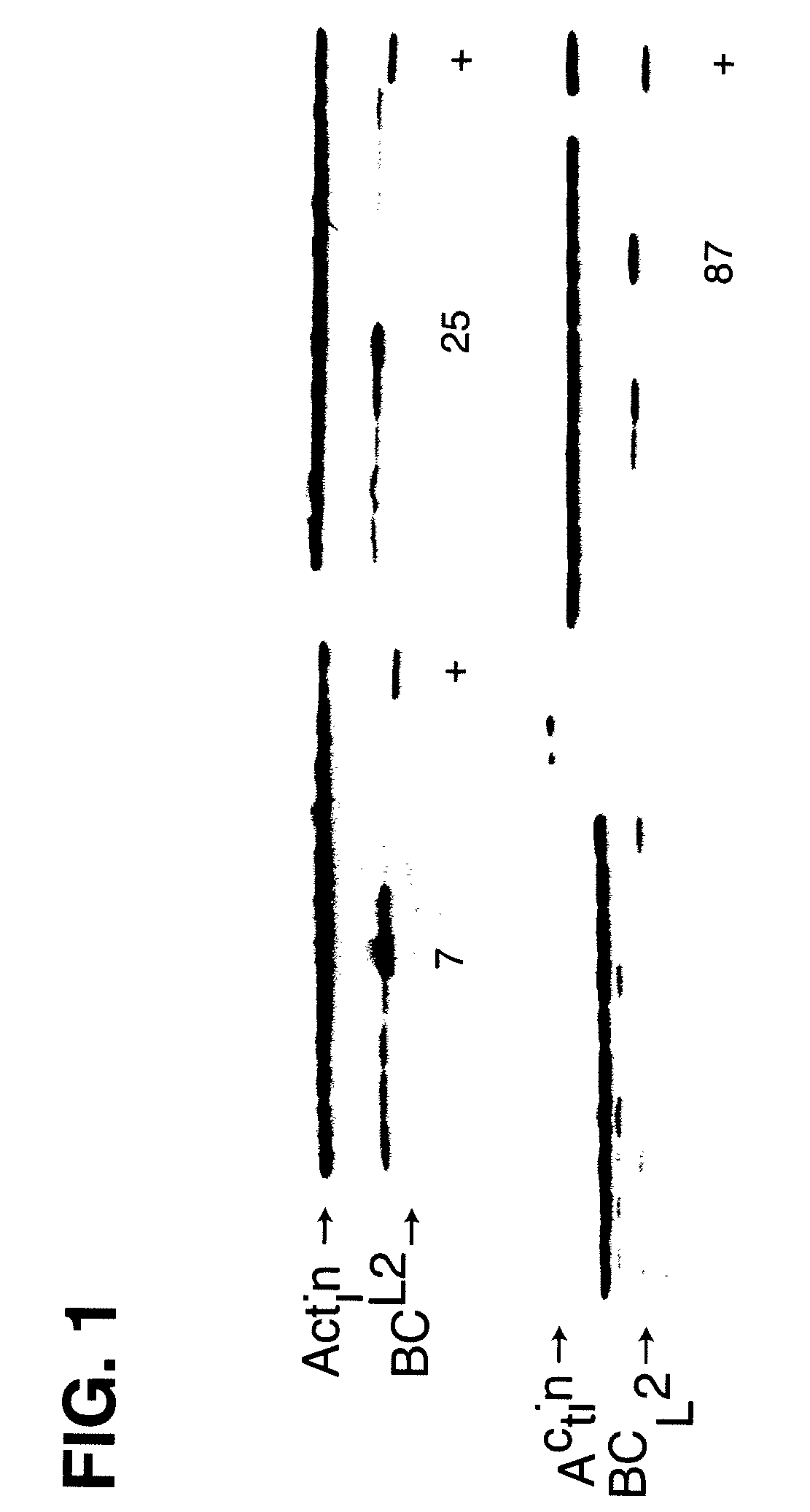
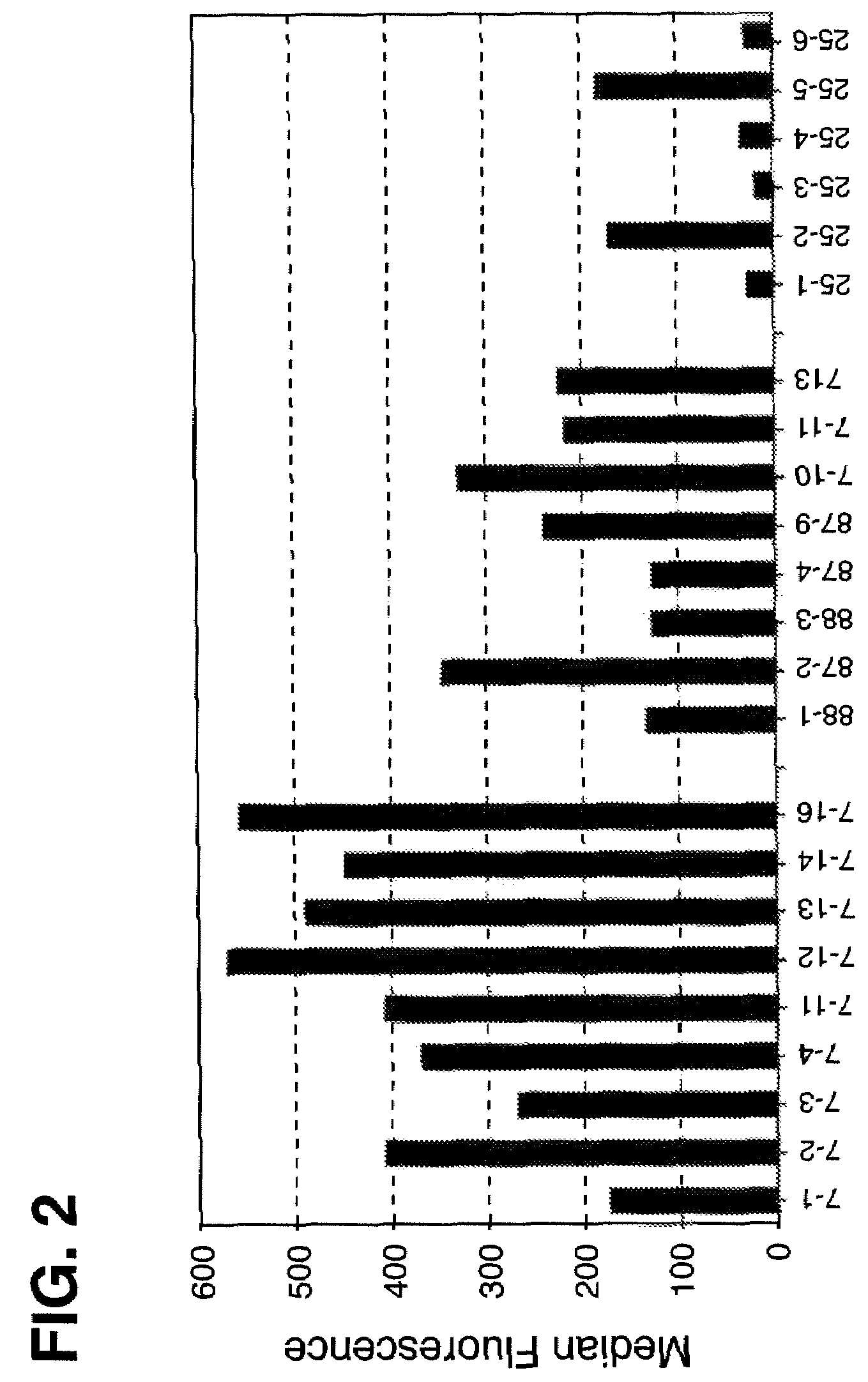
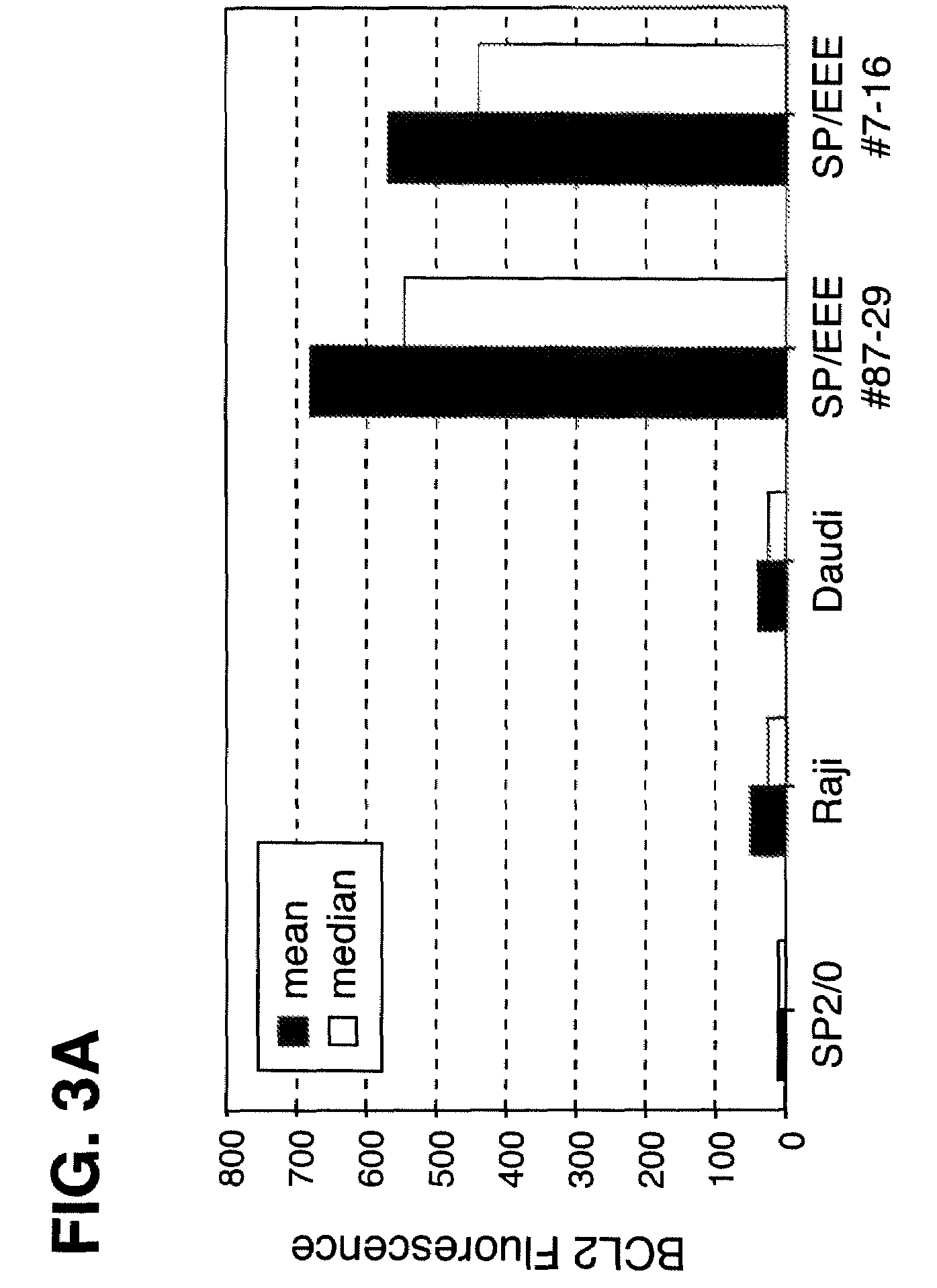
Disclosed are compositions and methods for increasing the longevity of a cell culture and permitting the increased production of proteins, preferably recombinant proteins, such as antibodies, peptides, enzymes, growth factors, interleukins, interferons, hormones, and vaccines. Cells transfected with an apoptosis-inhibiting gene or vector, such as a triple mutant Bcl-2 gene, can survive longer in culture, resulting in extension of the state and yield of protein biosynthesis. Such transfected cells exhibit maximal cell densities that equal or exceed the maximal density achieved by the parent cell lines. Transfected cells can also be pre-adapted for growth in serum-free medium, greatly decreasing the time required to obtain protein production in serum-free medium. In certain methods, the pre-adapted cells can be used for protein production following transfection under serum-free conditions. In preferred embodiments, the cells of use are SpESF or SpESF-X cells.
Owner:IMMUNOMEDICS INC
Method for the production and purification of adenoviral vectors
The present invention addresses the need to improve the yields of viral vectors when grown in cell culture systems. In particular, it has been demonstrated that for adenovirus, the use of low-medium perfusion rates in an attached cell culture system provides for improved yields. In other embodiments, the inventors have shown that there is improved Ad-p53 production with cells grown in serum-free conditions, and in particular in serum-free suspension culture. Also important to the increase of yields is the use of detergent lysis. Combination of these aspects of the invention permits purification of virus by a single chromatography step that results in purified virus of the same quality as preparations from double CsCl banding using an ultracentrifuge.
Owner:JANSSEN VACCINES & PREVENTION BV
Serum-free medium for mesenchymal stem cells
Serum-free media for growth and proliferation of chondrocytes and mesenchymal stem cells in culture are provided. A serum-free medium for growth of chondrocytes includes a serum-free composition comprising FGF-2, linoleic acid, ascorbic acid, B-mercaptoethanol, transferrin and dexamethasone. Further, the composition comprises EGF, PDGFbb, insulin and albumin. A method for growing chondrocytes in a serum free medium comprising the compostion is also provided. Also provided for mesenchymal stem cell growth, is a serum-free medium which includes a composition comprising FGF-2, LIF, SCF, pantotenate, biotin and selenium and method, therefore.
Owner:CONSORZIO PER LA GESTIONE DEL CENT DI BIOTECNOLOGIA AVANZATA +1
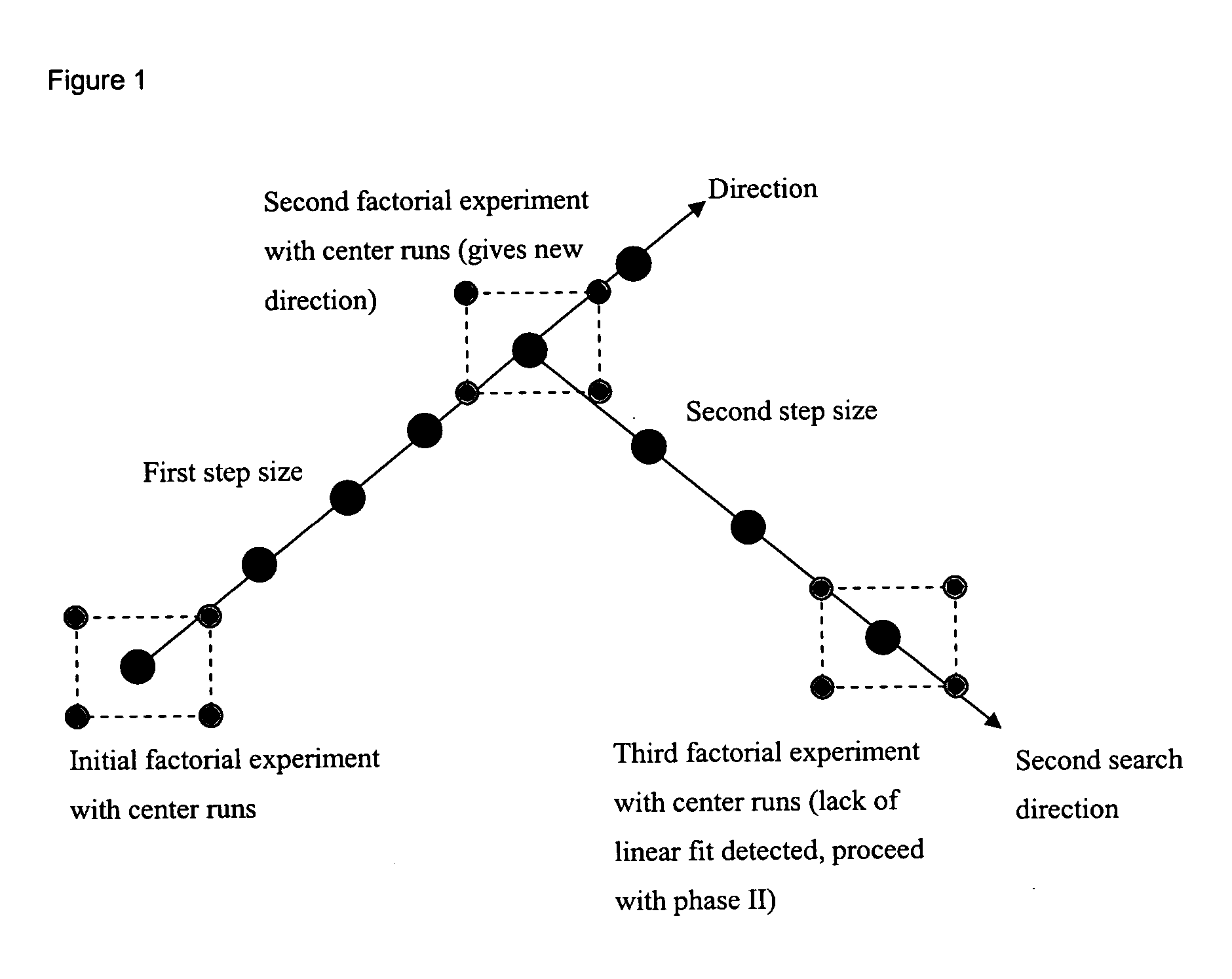
Optimizing culture medium for CD34<+> hematopoietic cell expansion
InactiveUS20050032122A1Simple compositionCulture processBlood/immune system cellsHematopoietic cellSerum free
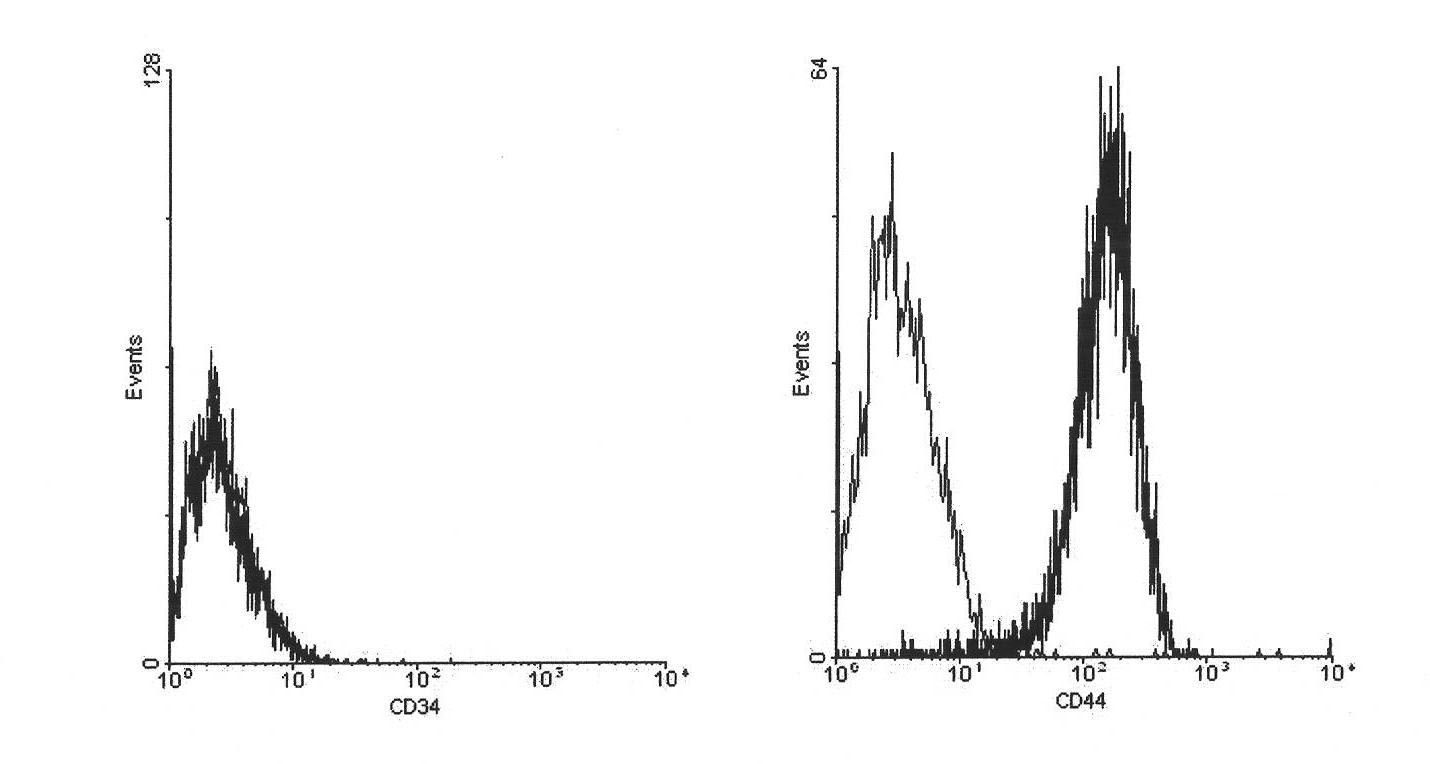
The present invention provides a method of determining the optimal composition of a serum-free, eukaryotic cell culture medium supplement, using 2-level factorial design and the deepest ascent method. The invention further provides a method of making a serum-free eukaryotic cell culture medium supplement and the generated thereof. The invention further provides a method of making a serum-free, eukaryotic cell culture medium and the medium generated thereof. The invention further provides a kit containing the medium of the invention. The invention also provides a method of expanding CD34<+> hematopoietic cells and a composition comprising CD34<+> hematopoietic cells in a serum-free, eukaryotic cell culture medium of the invention.
Owner:SINO CELL TECH
Cells for producing recombinant iduronate-2-sulfatase
ActiveUS20140004593A1Effective enzyme replacement therapyImprove the level ofAnimal cellsHydrolasesIduronate-2-sulfataseSerum free
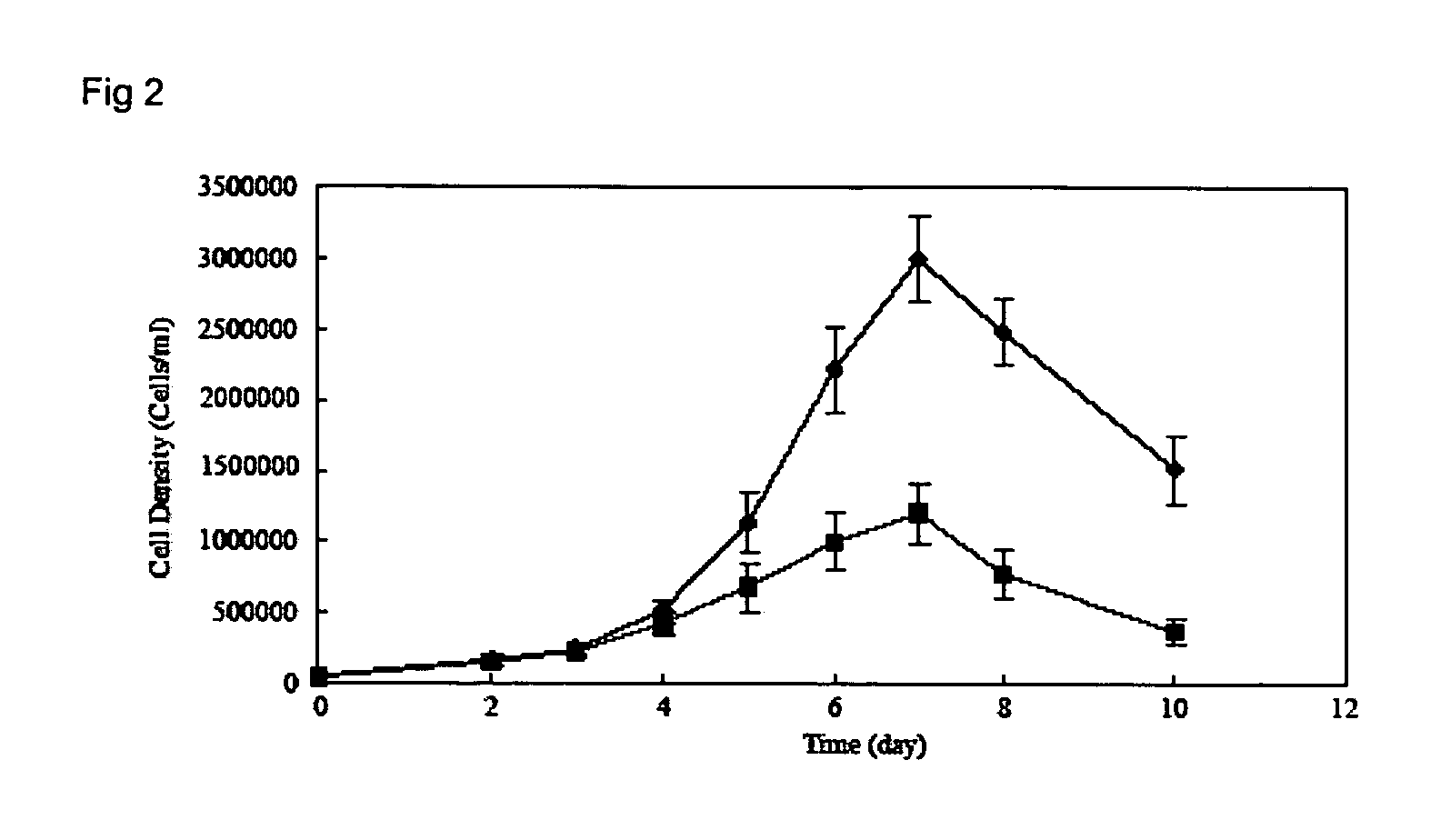
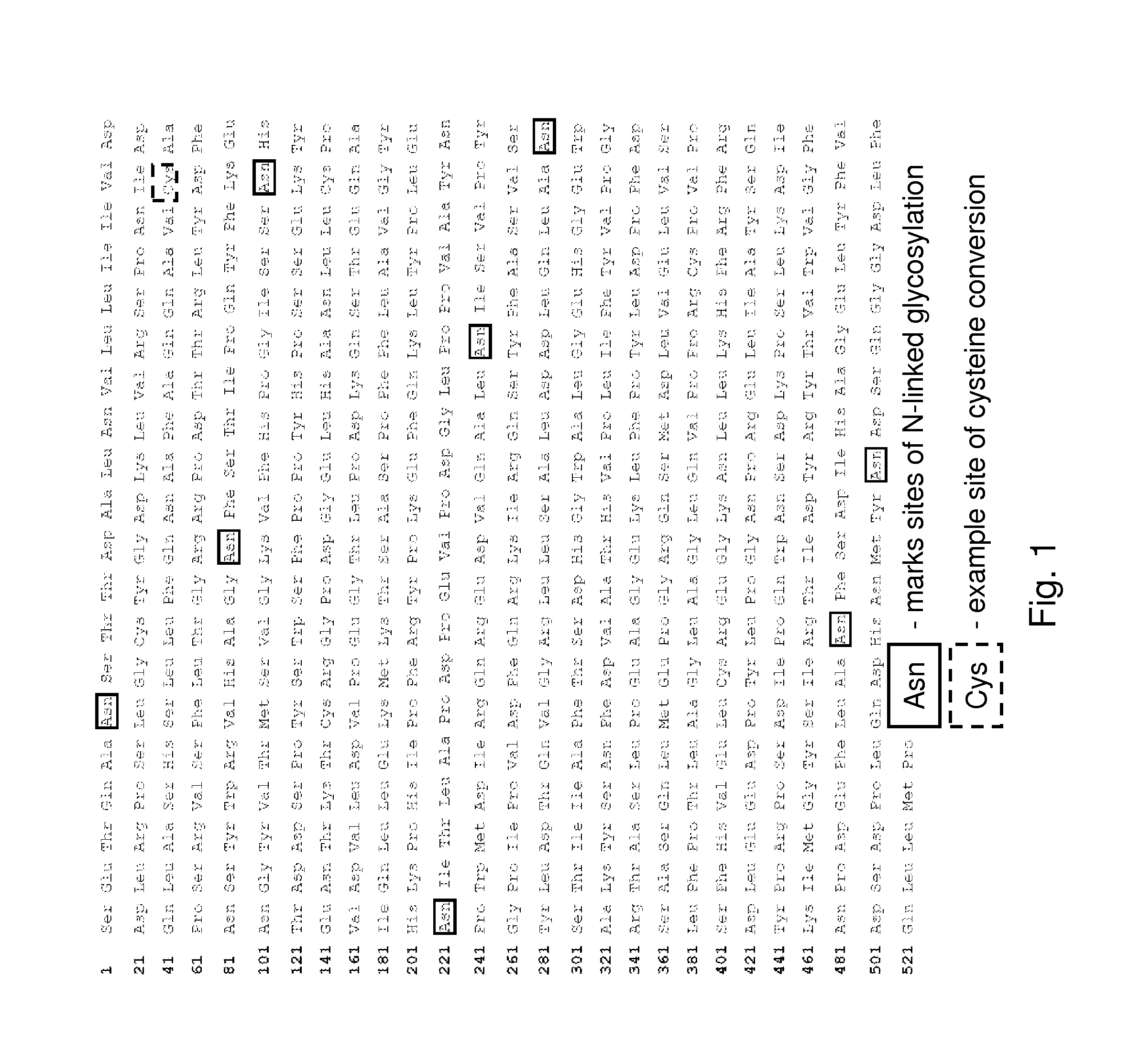
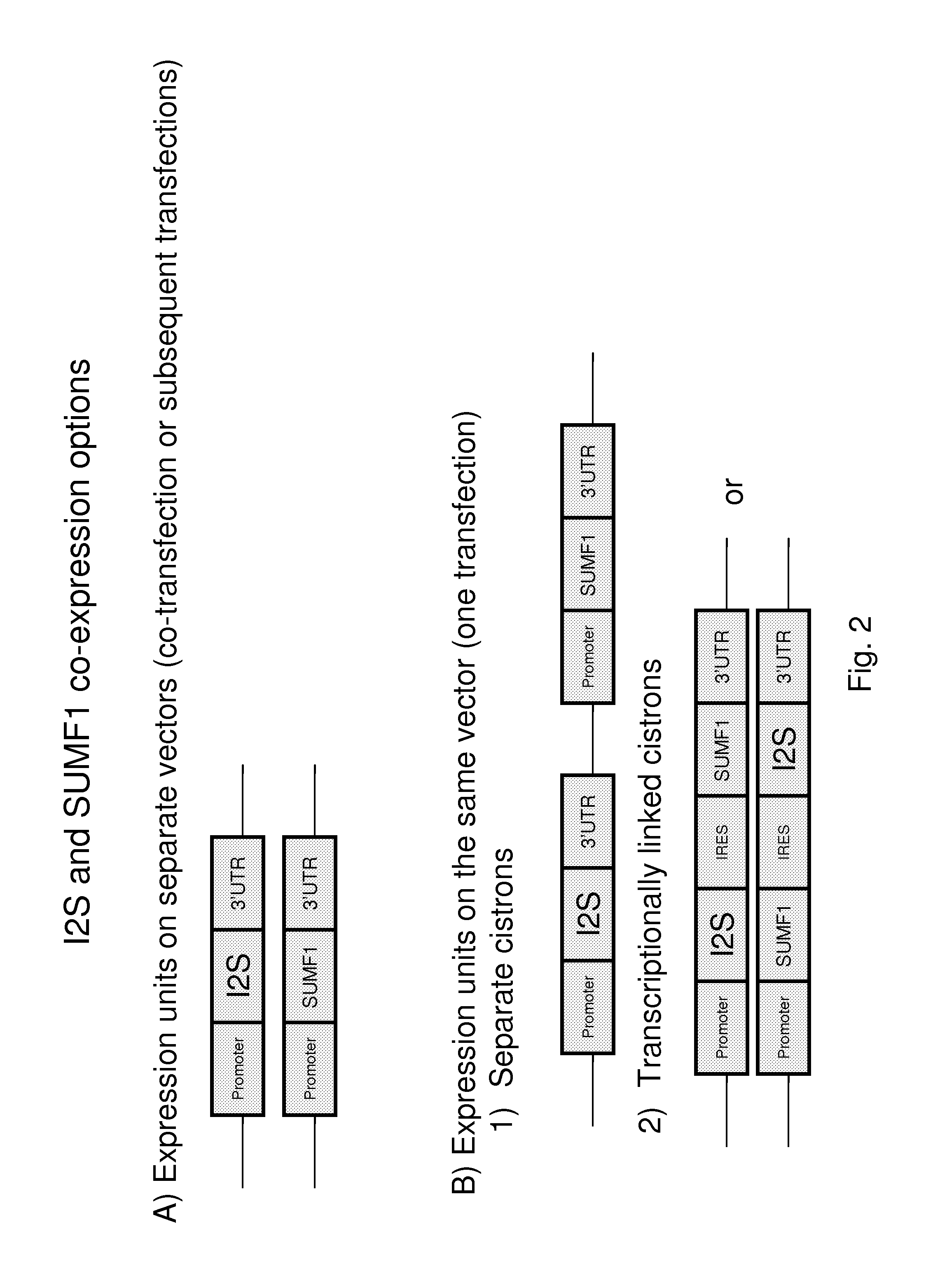
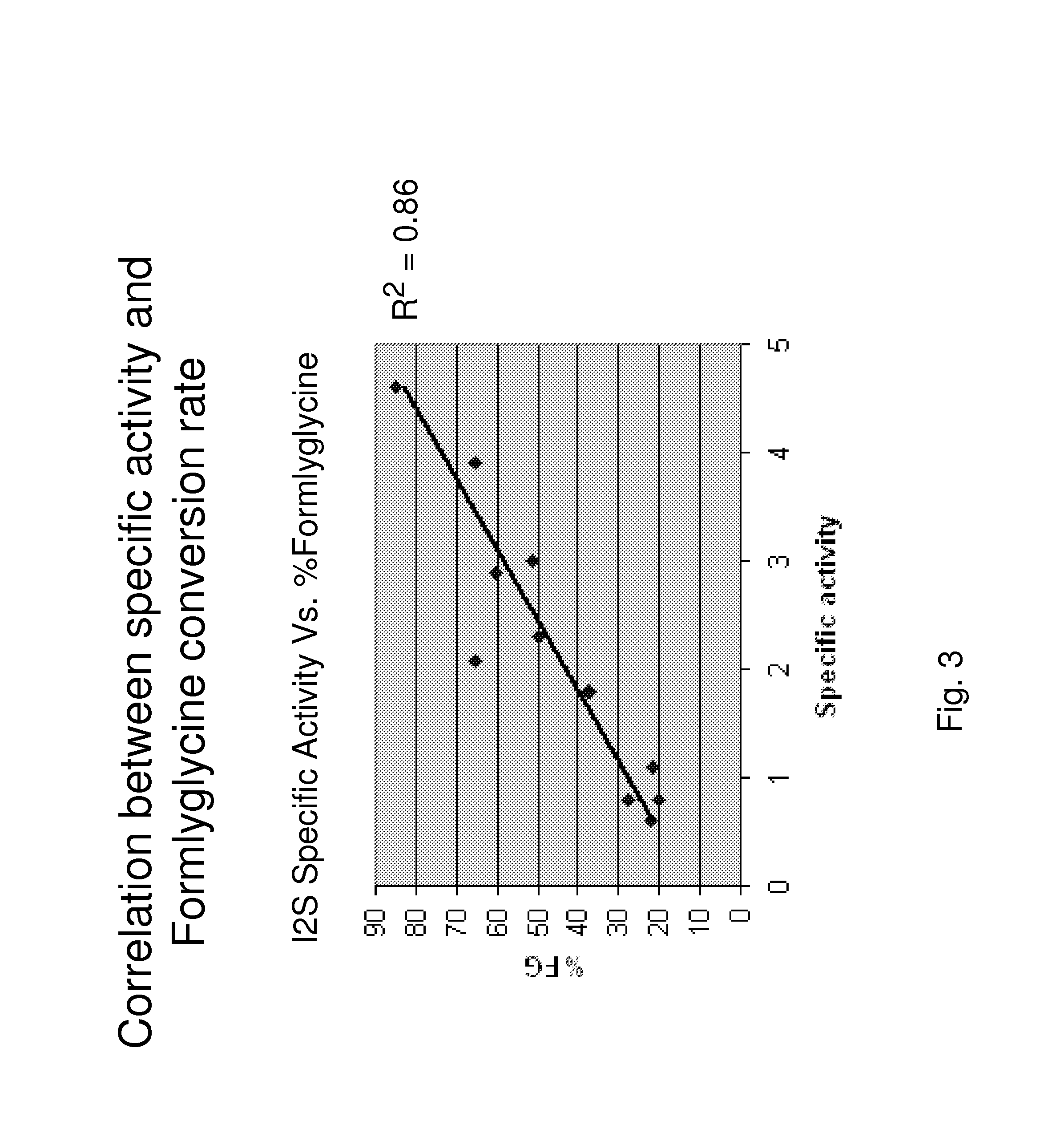
The present invention provides, among other things, methods and compositions for production of recombinant I2S protein with improved potency and activity using cells co-express I2S and FGE protein. In some embodiments, cells according to the present invention are engineered to simultaneously over-express recombinant I2S and FGE proteins. Cells according to the invention are adaptable to various cell culture conditions. In some embodiments, cells of the present invention adaptable to a large-scale suspension serum-free culture.
Owner:TAKEDA PHARMA CO LTD
Method for extracting original mesenchymal stem cells from placenta and serum-free amplification
InactiveCN102146359AImprove scalabilityConducive to clinical safety transplant applicationSkeletal/connective tissue cellsBlood serumSerum free
The invention belongs to the technical field of biomedicine and particularly discloses a method for extracting original mesenchymal stem cells from a placenta and serum-free amplification. The more original mesenchymal stem cells of the human placenta are extracted by using placenta tissues of the early aborted fetus, and a system of separating the mesenchymal stem cells from the placenta and amplification culture in vitro is built up. The genetic information and phenotype of the stem cells are more stable, the characteristics of the stem cells are more original, the cells can be effectively amplified, the product quality is improved, the stable and homogeneous mesenchymal stem cells of the placenta can be obtained, the method solves the problems that a large quantity of stem cells are needed for stem cell treatment, and the method can be used for treating various diseases.
Owner:王泰华
Serum-free medium for in vitro cultivation and amplification of mesenchymal stem cells
ActiveCN101412985AResidue reductionMaintain multilineage potentialSkeletal/connective tissue cellsSerum free mediaAntioxidant
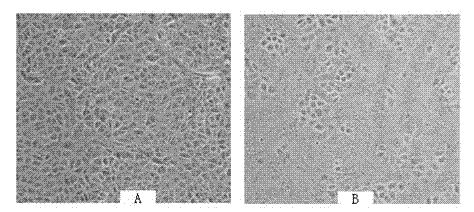
The present invention belongs to the field of biotechnology, and discloses a serum-free culture medium with specific chemical compositions for in vitro culture and amplification of bone marrow mesenchymal stem cells. By adding insulin, transferrin, ethanolamine, sodium selenite, growth factors, adherent factors, hormone, putrescine, inorganic salt, vitamin, albumin and antioxidant into a basic culture medium, the bone marrow mesenchymal stem cells can attach to the culture medium under a serum free condition, so the in vitro culture and amplification are realized, the potential of multi-directional differentiation is maintained, and the amplified cells can be induced to be osteoblast and lipocyte in vitro. The serum-free culture medium has the advantages that the clinic level cell products for human produced by the serum free culture medium can effectively avoid the potential risk of producing cell products by serum culture medium. The drawing appended is a photo of the confluence of the bone marrow mesenchymal stem cells cultured by the serum-free culture medium.
Owner:EAST CHINA UNIV OF SCI & TECH
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Human fat mesenchymal stem cell extract and lyophilized powder and application thereof
ActiveCN103784474ABiologically activeEasy to prepareCosmetic preparationsToilet preparationsBiotechnologyChemical products
The invention discloses a human fat mesenchymal stem cell extract and lyophilized powder and applications thereof. The human fat mesenchymal stem cell extract is prepared by the steps: collecting subcultured P3-P30 generations of human fat mesenchymal stem cells, culturing with cell growth liquid until 80% of cells are fused, removing culture liquid, washing the cells with a phosphate buffer solution for three times, adding into a serum-free culture solution, continuously culturing for 72 hours, collecting the serum-free culture solution, digesting uncollected cells with a digestion solution, crushing the cells with an ultrasonic crushing device, centrifuging, and then collecting supernatant; and mixing the collected serum-free culture solution and the collected supernatant, filtering, collecting filtrate, concentrating, and sterilizing. The human fat mesenchymal stem cell extract and the lyophilized powder thereof have multiple biological activities, can be used for preparing wound healing or cell repair medicaments and other biopharmaceutical products, and can also be used for preparing whitening products or skin anti-wrinkle products and other fine chemical products.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
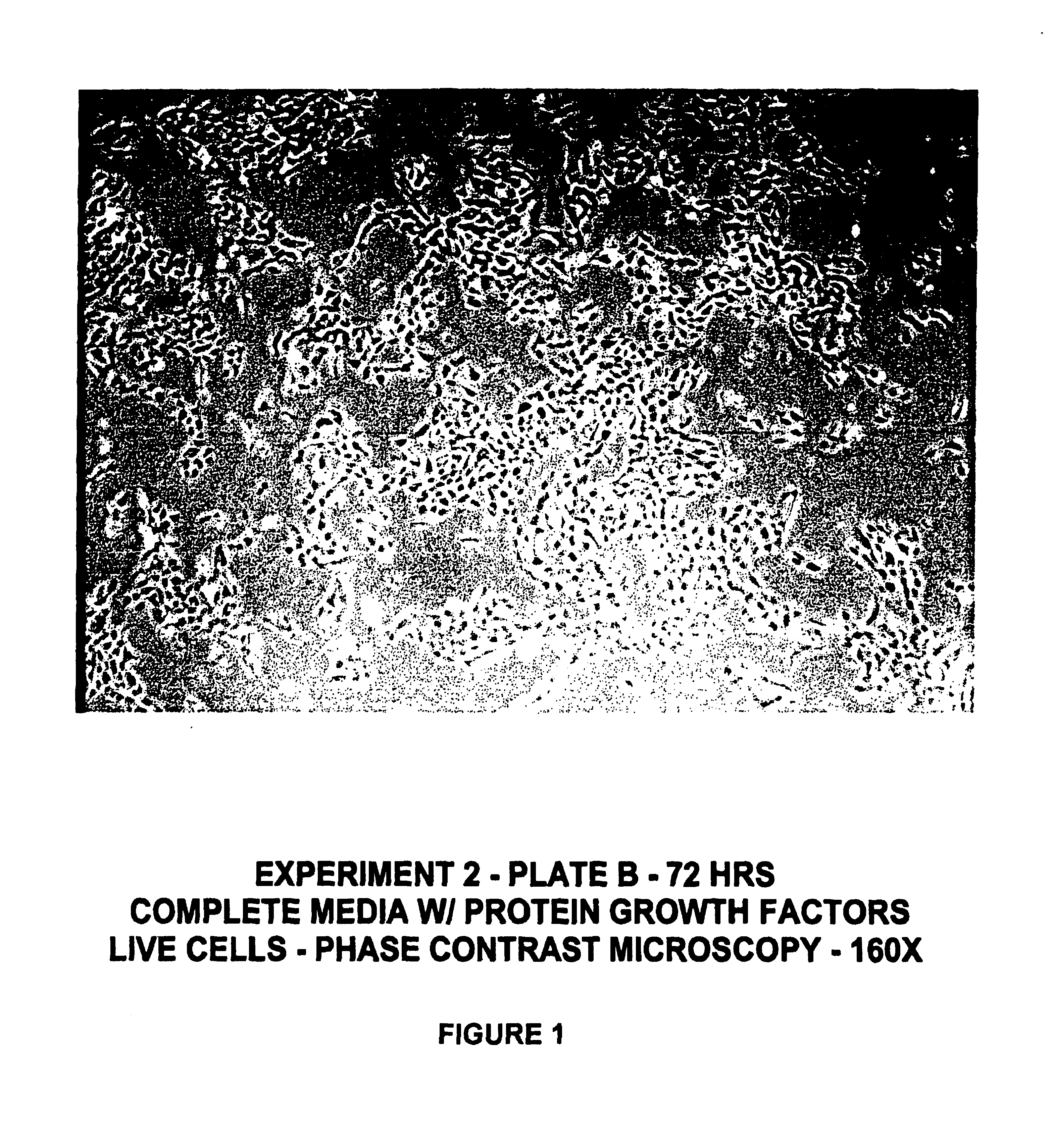
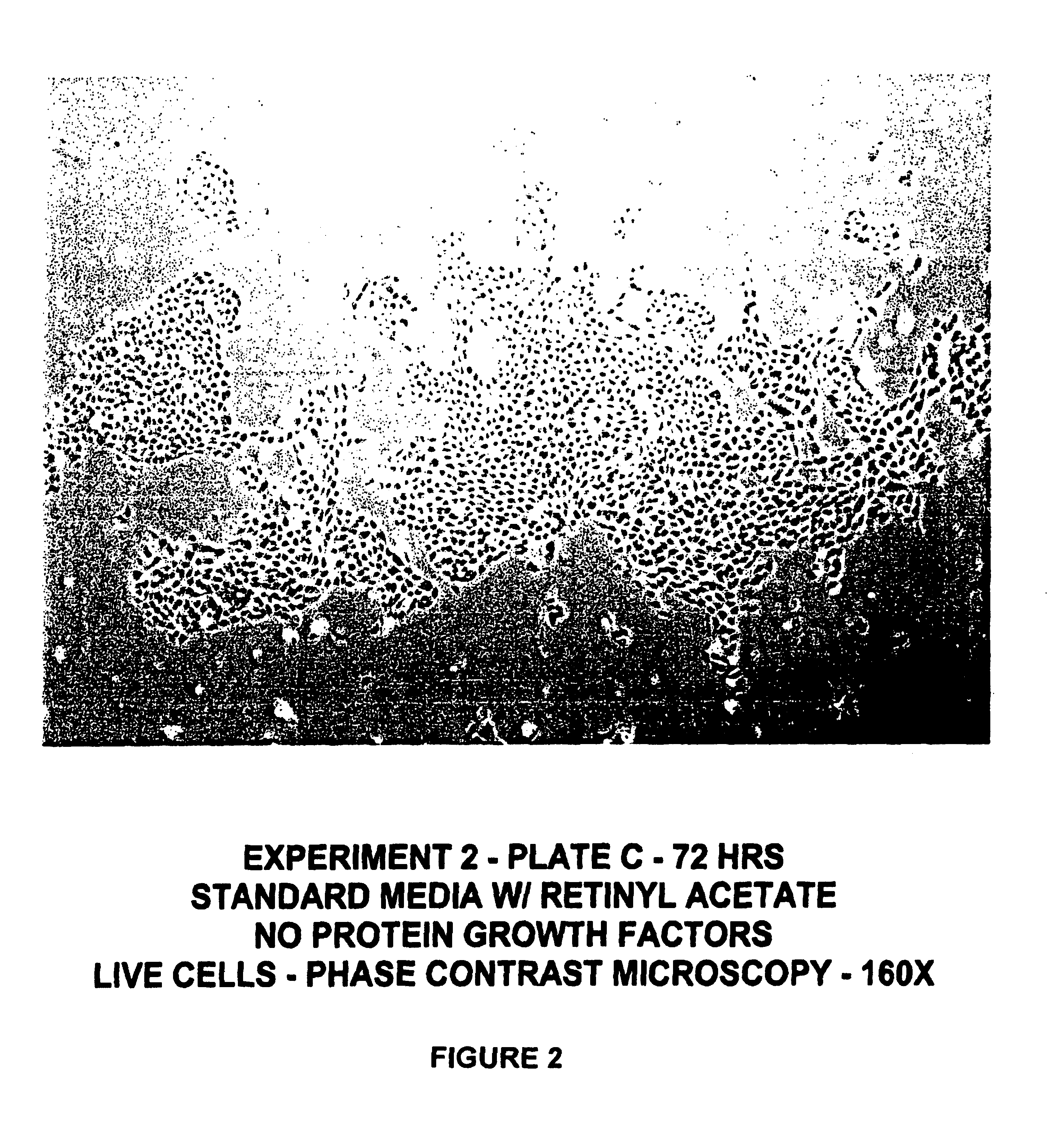
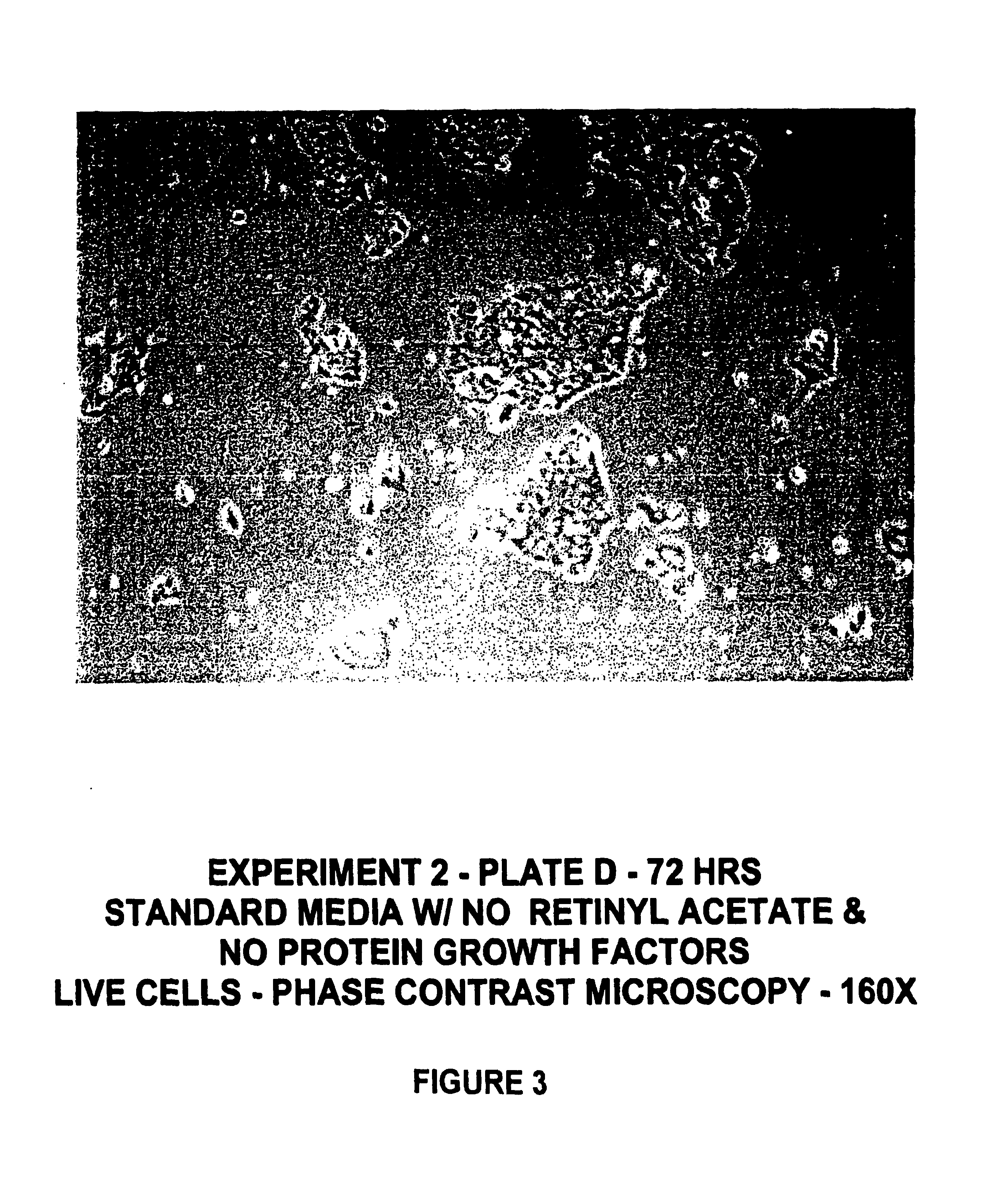
Protein-free defined media for the growth of normal human keratinocytes
Improvements are made to a novel media that replace the requirement for all protein growth factors by the addition to the medium of physiological concentrations of retinyl acetate. The media are serum-free, companion cell or feeder layer-free and organotypic, matrix free solutions for the cultivation of clonally competent basal keratinocytes. The media and methods are useful in the production of epidermal epithelial tissue that is suitable for skin grafting.
Owner:BIOPLAST MEDICAL
Generation of clonal mesenchymal progenitors and mesenchymal stem cell lines under serum-free conditions
ActiveUS20090081784A1Avoid difficult choicesHigh proliferation potentialVirusesGenetically modified cellsProgenitorSerum free
Methods for obtaining multipotent mesenchymal stem cells under serum-free conditions and methods for identifying multipotent mesenchymal progenitor cells are disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
Generation of clonal mesenchymal progenitors and mesenchymal stem cell lines under serum-free conditions
Methods for obtaining multipotent mesenchymal stem cells under serum-free conditions and methods for identifying multipotent mesenchymal progenitor cells are disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
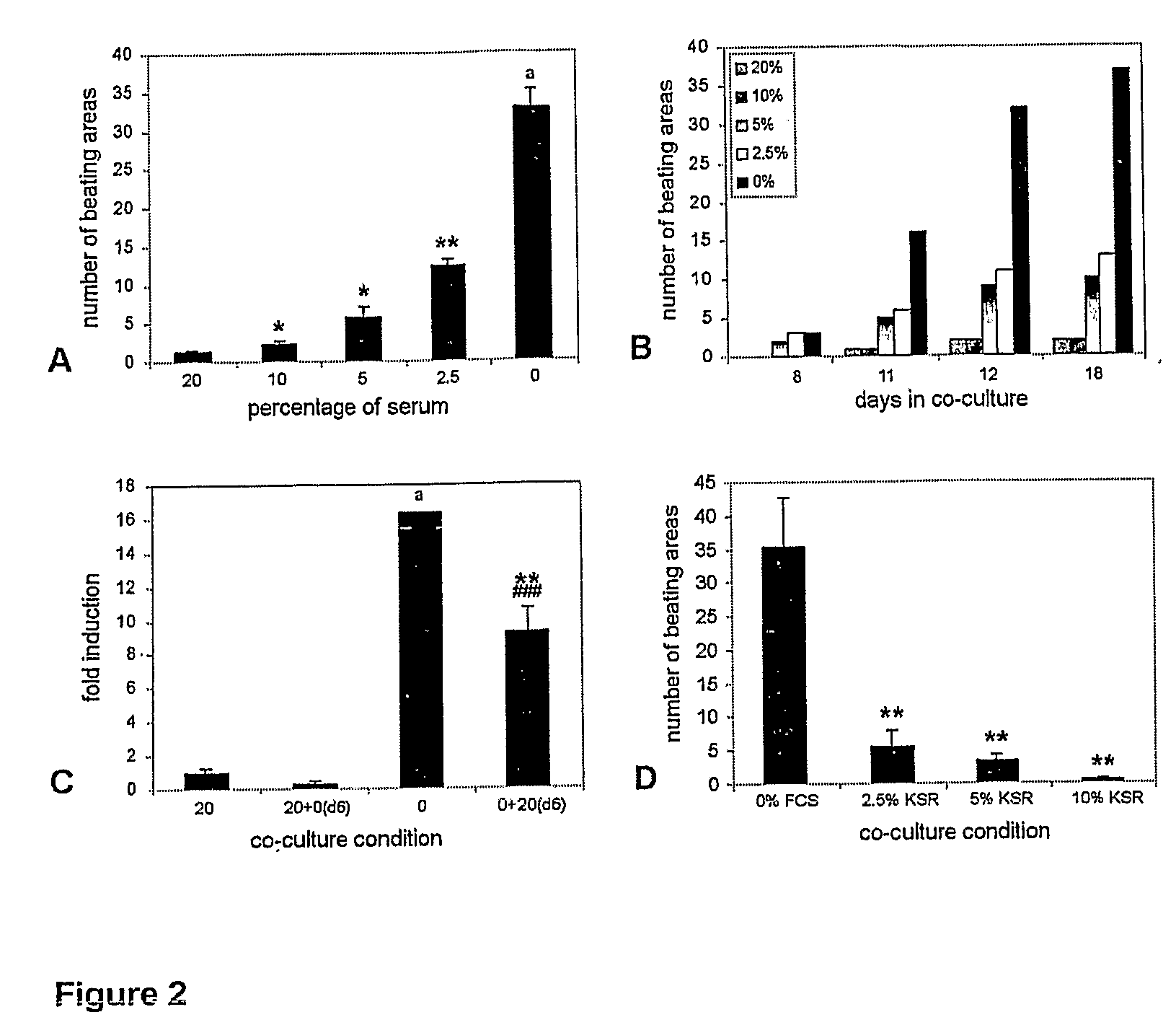
Differentiation of Human Embryonic Stem Cells and Cardiomyocytes and Cardiomyocyte Progenitors Derived Therefrom
The present invention provides a method to improve current culturing methods for the differentiation of cardiomyocytes from hES cells. The method includes culturing the hES cells in the presence of ascorbic acid or a derivative thereof. Preferably the culturing is conducted in serum free conditions. The invention also includes isolated cardiomyocytes and cardiac progenitors differentiated by the methods as well as the use of these cells in methods of treating and preventing cardiac diseases and conditions. Culture media and extracellular media are also provided which include ascorbic acid for the differentiation of hES cells to cardiomyocytes.
Owner:ES CELL INT
Method of Inducing the Differentiation of Embryonic Stem Cells Into Nerve by Serum-Free Suspension Culture
ActiveUS20080044901A1Effectively lead to differentiationEfficient inductionNervous system cellsEmbryonic cellsSerum free mediaNervous system
The present invention provides a clinically applicable method of inducing differentiation of embryonic stem cells, particularly a method of inducing differentiation of embryonic stem cells into forebrain neurons. More specifically, the present invention provides a method of inducing differentiation of embryonic stem cells, comprising culturing the embryonic stem cells as a floating aggregate in a serum-free medium, particularly a method of inducing differentiation of the embryonic stem cells into nervous system cells such as forebrain neurons and cerebellar neurons and sensory organ cells; a floating aggregate of embryonic stem cells obtained by culturing the embryonic stem cells as a floating aggregate in a serum-free medium; and cells derived from a floating aggregate of embryonic stem cells, particularly nervous system cells such as forebrain neurons and cerebellar neuron, sensory organ cells such as retinal precursor cells, and the like.
Owner:RIKEN
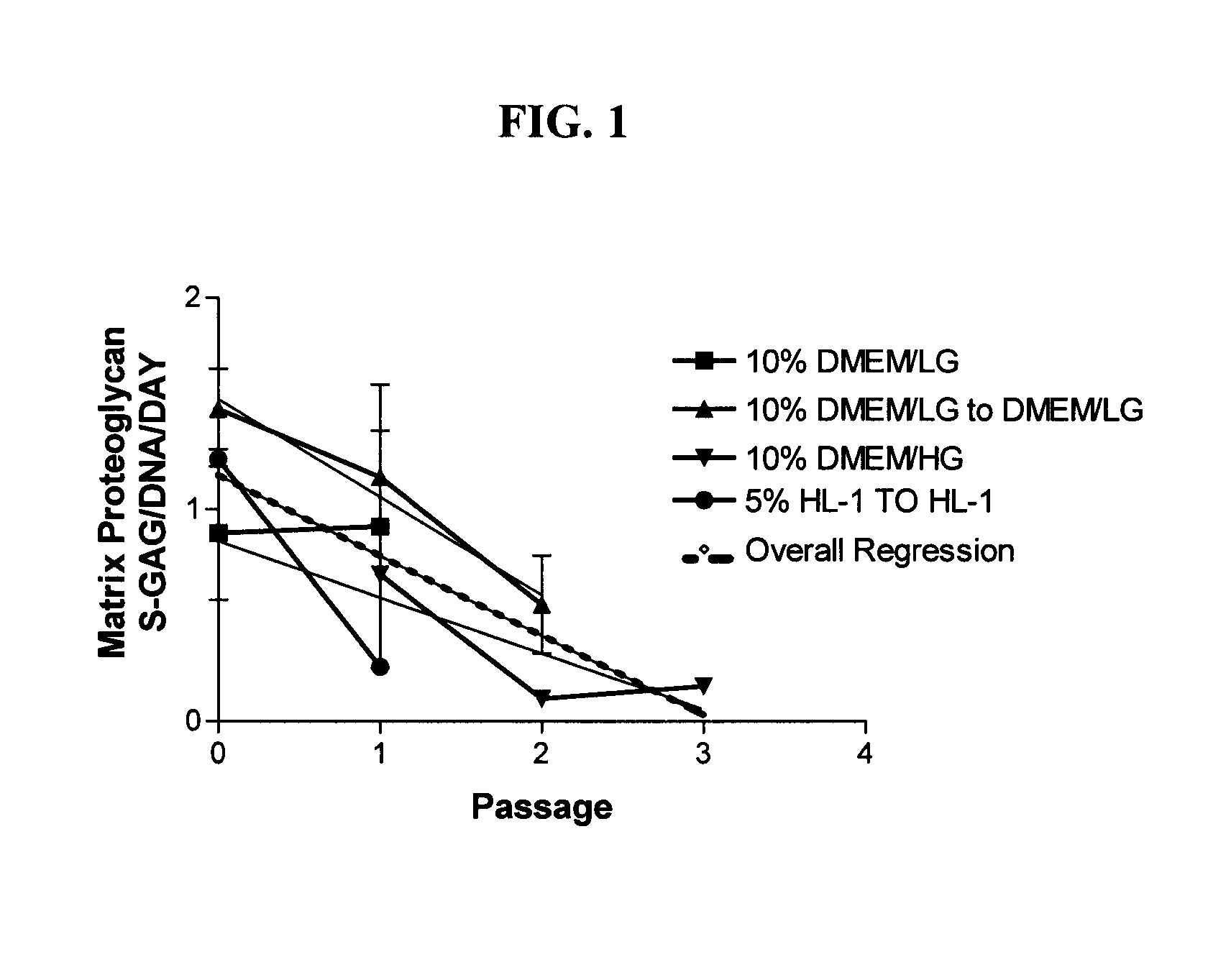
Method for Chondrocyte Expansion with Phenotype Retention
ActiveUS20080081369A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCell-Extracellular MatrixSerum free
The present invention provides a method for expanding a population of chondrocytes that maintains chondrocyte phenotype during the expansion by culturing the population in a defined serum-free expansion medium containing one or more cytokines and under low attachment conditions. The method further solves diffusion problems during the subsequent stage of extracellular matrix production by use of a perforated polycarbonate substrate that results in a randomly organized cultured neocartilage tissue. Chondrocytes expanded and cultured in this manner can be used in various medical applications to repair cartilaginous tissues that have been injured by trauma or disease.
Owner:ZIMMER INC
Serum-free cell culture fluid suitable for enriching and culturing tumour stem cells
The invention provides a serum-free cell culture fluid suitable for enriching and culturing tumour stem cells. DMEM / f12 serves as the basic culture fluid of the serum-free cell culture fluid and transferrin, insulin and other substances are also added as the components of the serum-free cell culture fluid. The serum-free cell culture fluid provided by the invention can efficiently enrich tumour stem cells from malignant tumour cell strains and tumour tissues. In addition, the serum-free cell culture fluid can promote stable growth of the tumour stem cells and maintain fine cell activity and physiological properties of the tumour stem cells, thus being very suitable for research fields related to tumour cells and tumour stem cells.
Owner:SUN YAT SEN UNIV
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Live attenuated influenza vaccine
InactiveUS7494659B2Simple and efficient processOvercomes drawbackSsRNA viruses negative-senseViral antigen ingredientsAntigenSerum free
The invention relates to a simple and efficient process for isolating viruses from various sources and for producing live attenuated influenza vaccines in a serum-free Vero cell culture under conditions where alterations in the surface antigens of the virus due to adpative selection are minimized or prevented. The process does not require purification of the virus-containing supernatant harvested from the cell culture nor post-incubation treatment of the viruses for HA activation. The invention further relates to influenza A and B master strain candidates and to vaccines made thereof.
Owner:POLYMUN SCI IMMUNBIOLOGISCHE FORSCHUNG
Porcine epidemic diarrhea virus, and culture method and application thereof
ActiveCN103756974APerfect infection monitoring systemMicroorganism based processesAntiviralsSerum freeTGE VACCINE
The invention discloses a porcine epidemic diarrhea virus, and a culture method and an application thereof. The porcine epidemic diarrhea virus is called as coronaviridae coronavirus porcine epidemic diarrhea virus GDSJ / 2012, is preserved in China center for type culture collection on May 15th, 2013, and has the preservation number of CCTCC NO:V201309. The culture of the virus strain requires a serum-free DMEM culture solution containing trypsin and magnesium chloride. The virus strain can be used for preparation of a PEDV diagnostic kit and a PEDV vaccine, has good immunogenicity, and can make up for the deficiency of few conventional vaccine types.
Owner:WENS FOODSTUFF GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com