Cell culture environments for the serum-free expansion of mesenchymal stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
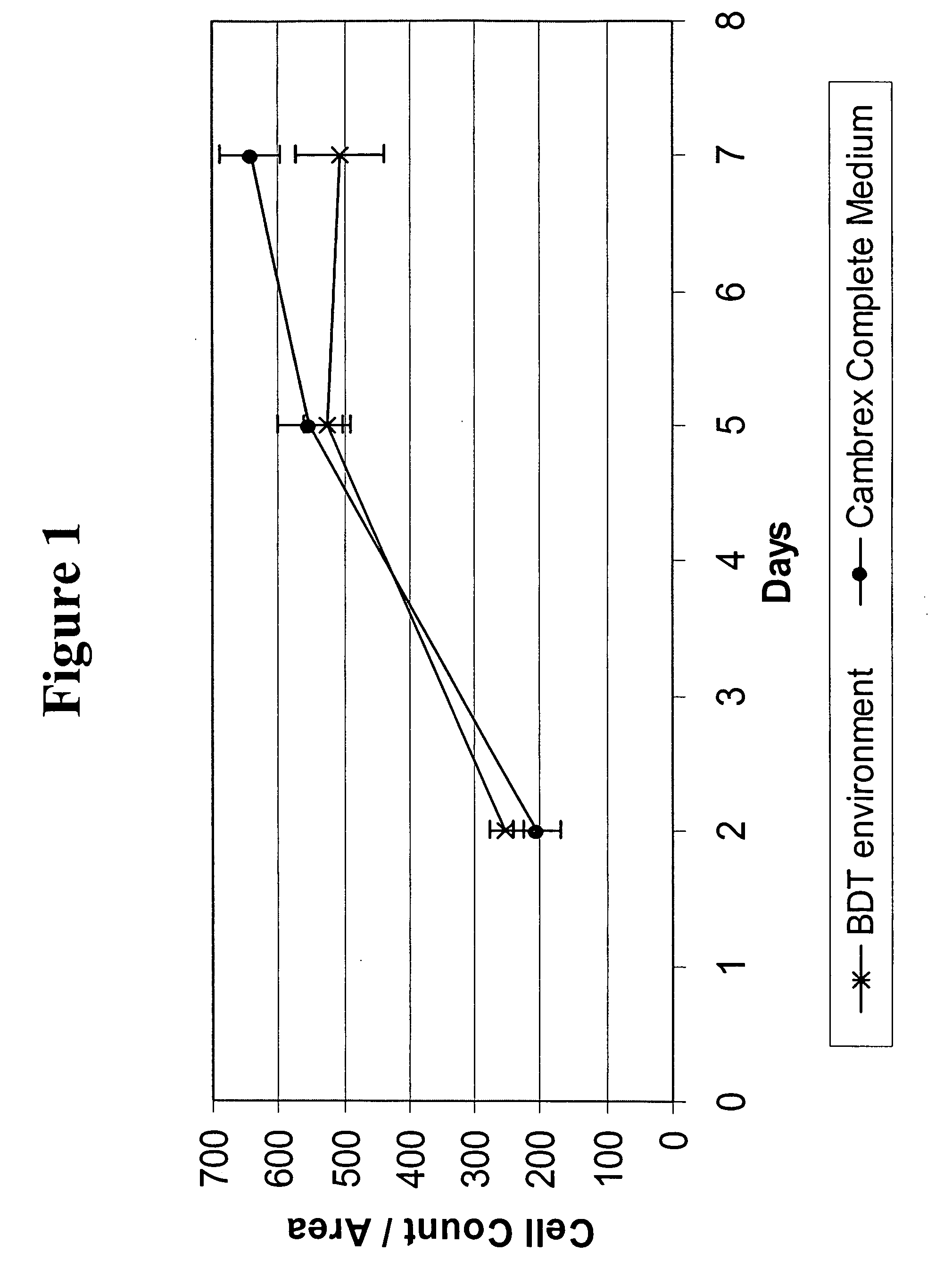
MSC Expansion in Various Serum-Free Media
[0109] MSCs along with complete growth medium were purchased from Cambrex Biosciences (Baltimore, Md.). Frozen cells were thawed and cultured following manufacturer's instruction. After reaching ˜90% confluency, MSCs were washed once with PBS, removed from the culture surface using Trypsin / EDTA and replated at a density of 1600 cells / well (for 96 well plates) or at a density of 50,000 cells / well (for 6-well plates) which corresponds to the manufacturers recommended seeding density of 5000 cells / cm2.
[0110] At day 6 or 7, cells were washed once with PBS, fixed using 4% paraformaldehyde for 15 minutes and then stained using DAPI according to manufacturer's suggestions (Molecular Probes, Eugene, Oreg.). For 96-well plate experiments, one image per well was taken at 4× magnification using Molecular Devices' Discovery-1 high content screening system. Cell nuclei enumeration was determined using Metamorph Image Analysis Software (Molecular Devices...
example 2
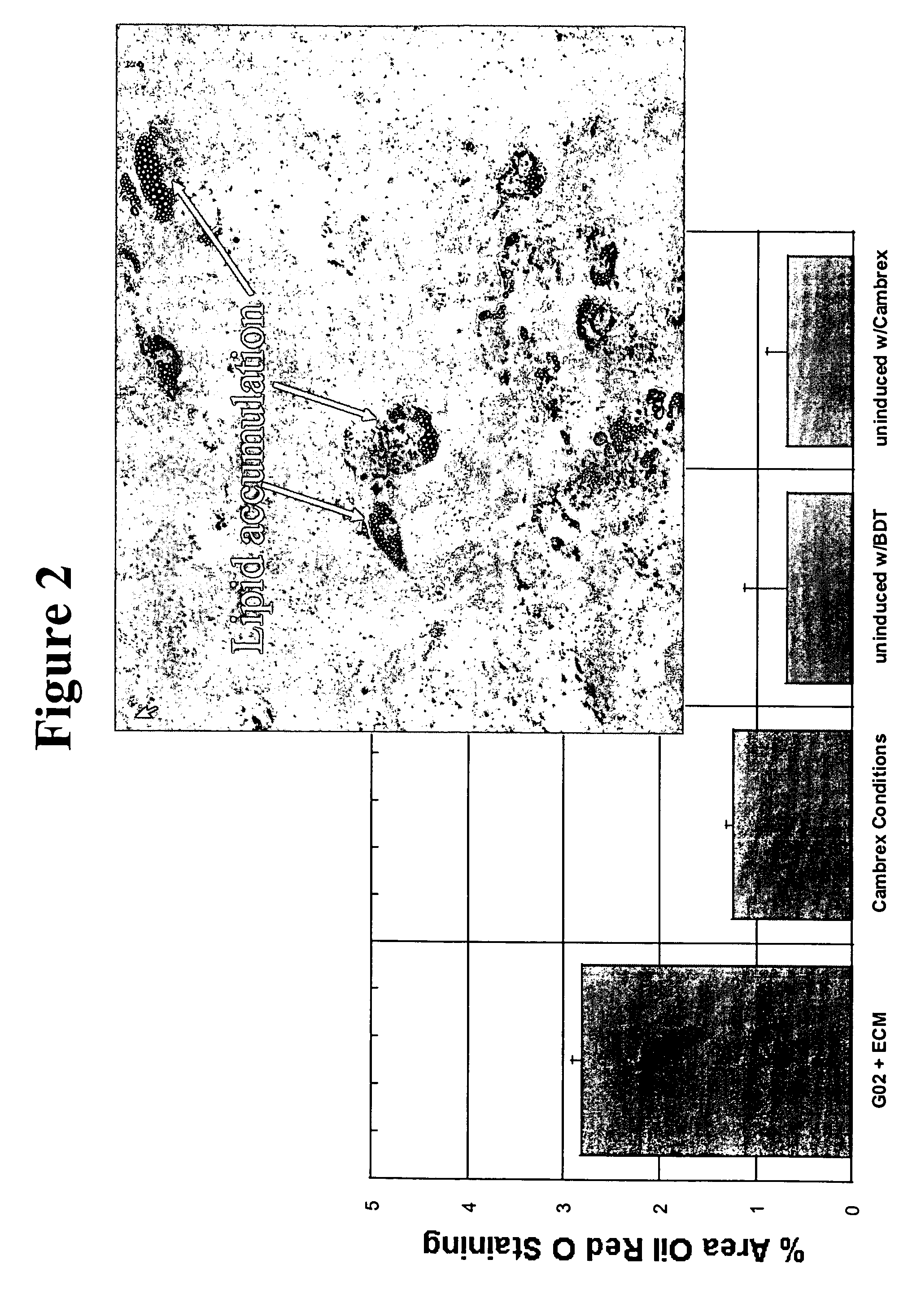
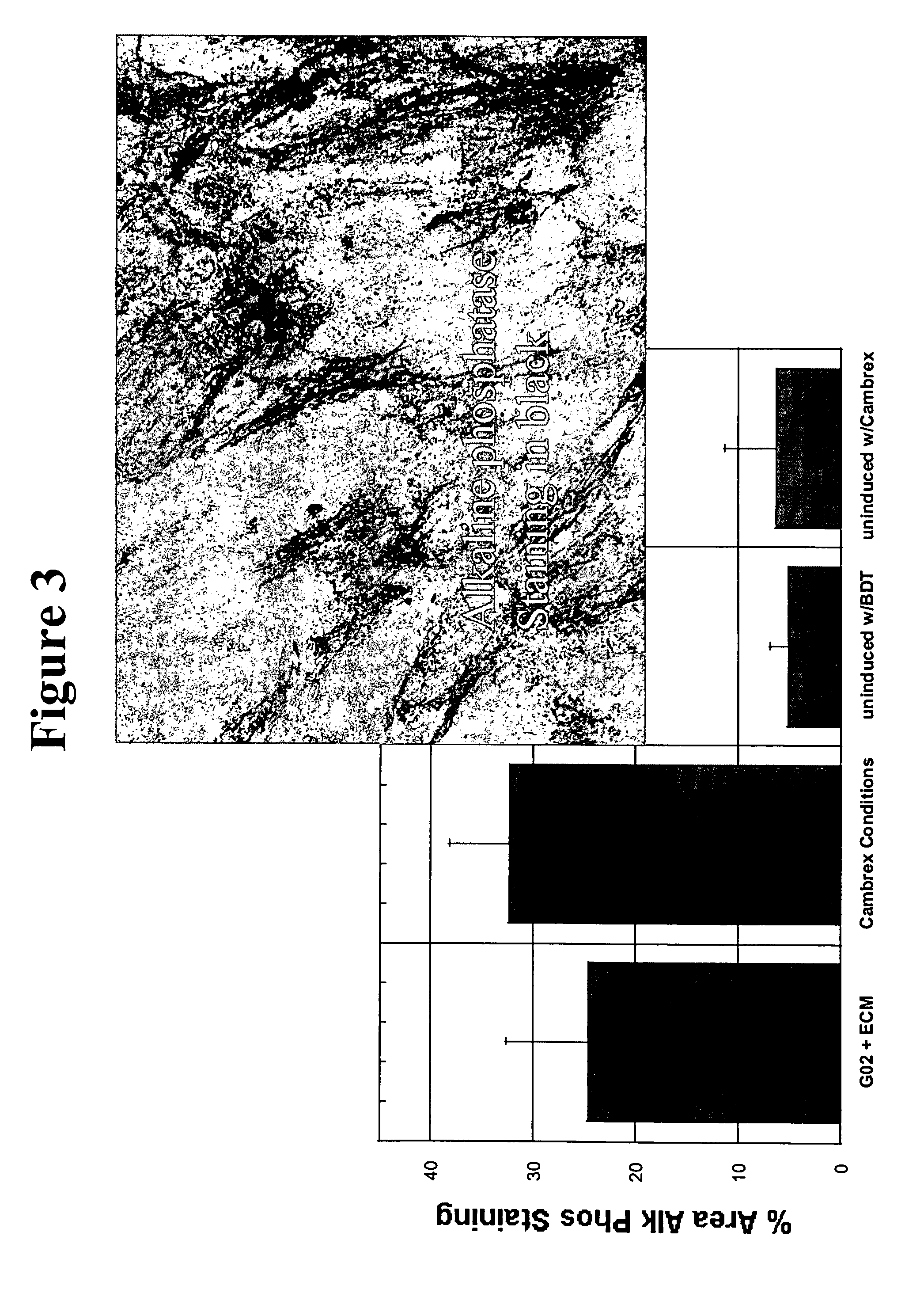
Differentiation Capacity and Surface Marker Characterization of G2 Expanded MSCs
[0113] MSCs expanded in serum-free media of the present invention on CAR Col 1 / FN surface for 7 days remained multipotent. Briefly, MSCs expanded in culture environments of the present invention were removed from the serum-free media and then cultured in either adipogenic (fat) or osteogenic (bone) induction media (per manufacturer's suggestions). MSCs expanded in the serum-free environments of the present invention were able to differentiate towards both adipogenic (see FIG. 2) and osteogenic (see FIG. 3) lineages. These results demonstrate that the serum-free environments of the present invention help to maintain stem cell pluripotency in expanded MSCs at least as well as the industry standard serum-containing media (Cambrex). This demonstrates that these cells are not committed towards specific lineages, and that therefore these MSCs may still be used for a variety or research or clinical application...
example 3
Comparison of Media and Cell Culture Surface Conditions
[0115] MSCs cultured in three different media (G2, base medium without G2 growth factors / cytokines, and in serum-containing complete medium) all expand better on a Col 1 / FN CAR surface as compared to TCPS surfaces (see FIG. 4). Also, MSCs cultured in G2 serum-free medium expand to equivalent levels as MSCs cultured in serum-containing medium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com